Abstract
Introduction:
Melatonin has been considered a potent antioxidant that detoxifies a variety of reactive oxygen species in many pathophysiological states of eye. The present study was designed to determine the effects of Wi-Fi exposure on the lens oxidant, antioxidant redox systems, as well as the possible protective effects of melatonin on the lens injury induced by electromagnetic radiation (EMR).
Materials and Methods:
Thirty-two rats were used in the current study and they were randomly divided into four equal groups as follows: First and second groups were cage-control and sham-control rats. Rats in third group were exposed to Wi-Fi (2.45 GHz) for duration of 60 min/day for 30 days. As in the third group, the fourth group was treated with melatonin. The one-hour exposure to irradiation in second, third and fourth took place at noon each day.
Results:
Lipid peroxidation levels in the lens were slightly higher in third (Wi-Fi) group than in cage and sham control groups although their concentrations were significantly (P < 0.05) decreased by melatonin supplementation. Glutathione peroxidase (GSH-Px) activity was significantly (P < 0.05) lower in Wi-Fi group than in cage and sham control groups although GSH-Px (P < 0.01) and reduced glutathione (P < 0.05) values were significantly higher in Wi-Fi + melatonin group than in Wi-Fi group.
Conclusions:
There are poor oxidative toxic effects of one hour of Wi-Fi exposure on the lens in the animals. However, melatonin supplementation in the lens seems to have protective effects on the oxidant system by modulation of GSH-Px activity.
Keywords: Antioxidants, electromagnetic ration, glutathione, lens, oxidative stress
People are intensely exposed to electromagnetic radiation particularly for the last two decades. Electromagnetic radiation (EMR) signals consist of extremely low frequency fields (between 1Hz up to 100 kHz) and high frequency fields, in the band of the radio frequency fields (RF, 100 kHz-3GHz) and of the microwaves (MW, above 3 GHz). Besides their use for technological applications (e.g, power lines, mobile phones), the EMFs are today widely used in medicine for diagnostic and therapeutic purposes.[1,2] The World Health Organization Report (2007) and WHO Environmental Health Criteria Report (2007) issued precautions against EMR, considering the increased social and public interest in this subject, based on the epidemiological data, which associates the extra risk of amyotrophic lateral sclerosis, childhood leukemia, adult brain cancer, and miscarriage with EMR exposure of the power line radiation.[3]
The interaction of the biological system and EMR in the molecular level can result in change in cell cycle, induction of cell death, modification of protein expression and mainly oxidative stress.[4,5,6,7] EMR exposure of eye results in overproduction of reactive oxygen species (ROS), which can damage cellular components such as lipids, nucleic acids and proteins.[8] Lipid peroxidation in eye occurs because of elevated levels of ROS with the liberation of reactive aldehydes, such as malondialdehyde (MDA) and 4-hydroxy-2-nonenal.[9] ROS in eye can cause cellular damage by depleting enzymatic and/or non-enzymatic antioxidants triggering progressive dysfunction and eventually genotoxic events.[10,11] ROS are also increasingly generated in the tissues in many vision impairing and blinding diseases including cataracts, glaucoma and retinopathies. Oxidative stress induced by ROS is a major risk factor for senile cataract, particularly nuclear cataract.[9] The level of ROS in eye is usually regulated by intracellular antioxidant defense system. The antioxidant defense system is composed of antioxidant molecules such as reduced glutathione (GSH) and of a variety of antioxidant enzymes such as superoxide dismutase and glutathione peroxidase (GSH-Px).[12] The eye lens has also evolved a wide range of protective and repair systems to protect its components from oxidative stress, including high levels of reduced GSH and abundant antioxidant enzymes such as superoxide dismutase and catalase.[13]
Melatonin (N-acetyl-5-methoxytryptamine), also an indoleamine, is a neurohormone secreted primarily by the pineal gland and has an effect on most biological and physiological processes. The biosynthesis of melatonin from serotonin proceeds via two key enzymes, arylalkylamine N-acetyltransferase and hydroxyindole-O-methyltransferase (5-hydroxytrypamine).[14] Melatonin in the eye is synthesized in the crystalline lens cortical fiber cells, retinal photoreceptor cells and ciliary body. Additionally, specific melatonin receptors are found in the retina, coroid, ciliary body, lens, sclera, which are targets for melatonin.[15]
We have recently observed modulator role of melatonin on Wi-Fi-induced oxidative stress in brain and dorsal root ganglion of rats.[16] To our knowledge, there is no report whether Wi-Fi induce oxidative stress in lens of experimental animals and humans. The aim of the present study was to determine the effects of Wi-Fi frequency (2.45 GHz) exposure on the lens oxidant, antioxidant redox systems, as well as the possible protective effects of melatonin on the lens injury induced by the EMR.
Materials and Methods
Animals
In this study, 16 weeks old, 32 male albino rats of Wistar strain, weighing 220 ± 20 g were used. The study was approved by Ethical Committee of Suleyman Demirel University. Laboratory animals prepared by Ethical Committee of (Protocol Number; 2011-01). The rats were quarantined before irradiation, housed in individual stainless-steel cages in a pathogen-free environment in a windowless laboratory room with automatic temperature (22 ± 3°C) and lighting controls (12-h light/12-h dark) and fed standard laboratory chow and water ad libitum. Average light intensity was determined to be 4000 lux and humidity was 40 ± 10% in the laboratory. The authors confirm adherence to the Association for Research in Vision and Ophthalmology (ARVO) statement for the Use of Animals in Ophthalmic and Vision Research.
Experimental groups
After one week adaptation process, the animals were randomized into four groups of eight rats each. The rats in Group A1 (cage control) were not exposed to electromagnetic radiation (EMR) or did not receive melatonin. The rats in Group A2 (sham control) received an intraperitoneal injection of isotonic saline solution in the same volume and by the same scheme as that used in the group receiving melatonin without EMR exposure. Group B rats (the EMR group) were exposed to 1 hour of EMR from 9 am to 10 am for 30 days. Group C rats (treatment groups) were exposed to EMR in the same manner as Group B and received melatonin treatment. Melatonin was initially dissolved in 0.1 ml of dimethyl sulfoxide (DMSO) and then diluted with physiological saline solution. Melatonin was administered intraperitoneally at a dose of 10 μg/kg and in a 0.1 ml volume of DMSO. The injection was administered 24 hours after EMR exposure and continued for 30 days.
Exposure system and design
We used six rats in the exposure system in the same time [Fig. 1]. Details of exposure system have been described in detail elsewhere.[16,17] We used power values as 1 mW/m2 in the experiments as described below. A “SET ELECO” generator from Set Electronic Co, Istanbul (Turkey), provided with a half-wave dipole antenna system was used to irradiate the cells with a 2.45 GHz radio frequency with 217 Hz pulses. The electric field density was set at 11 V/m in order to get a 0.1 W/kg whole-body average specific absorption rate (SAR) and electromagnetic radiation values estimated for 2.45 GHz set up.
Figure 1.
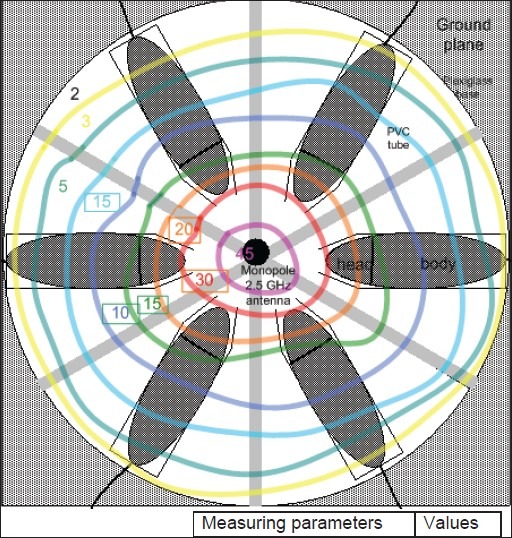
The EMR dose was calculated from the measured electric field density (V/m). SAR values were calculated by using electric properties of tissue sample and measured electric field intensities for every distance in certain frequency. These values were shown in Table 1.
Table 1.

The rats of Group A2 were placed in the cylindrical restrainer with the radio frequency source switched off during times similar to those used for irradiation. The A1 control animals were kept in their cage without any treatment or restraint of any kind.
Preparation of lens samples
Head of all animals were cut and the lens samples were dissected as described in own previous study.[18] The lens tissues were placed into glass bottles, labeled and stored in a deep freeze (−33°C) until processing (maximum 15 days). After weighing, The lens samples were placed on ice, and homogenized (2 minutes at 5000 rpm) in a five volumes (1:5, w/v) of ice-cold Tris-HCl buffer (50 mM, pH 7.4), by using a glass ultrasonic homogenizer (HD 2070, Bandelin Ultrasonic homogenizer, Bandelin Electronics GmbH and Co. KG, Berlin, Germany). All preparation procedures were performed on ice. The lens homogenate samples were used for immediate lipid peroxidation, GSH levels and enzyme activities.
Lipid peroxidation determinations
Lipid peroxidation levels in the lens homogenate were spectrophotometrically (at 532 nm) measured with the thiobarbituric-acid reaction by the method of Placer et al.[19]
Reduced glutathione (GSH), glutathione peroxidase (GSH-Px) and protein assays
The GSH content in the lens samples was spectrophotometrically measured at 412 nm using the method of Sedlak and Lindsay.[20] Standard curve of the GSH were prepared with different concentrations of GSH. GSH-Px activities of the lens homogenate were measured spectrophotometrically at 37 °C and 412 nm according to the Lawrence and Burk.[21] The protein content in the lens homogenate was measured by method of Lowry et al.,[22] with bovine serum albumin as the standard.
Statistical analyses
All results are expressed as means ± standard deviation (SD). To determine the effect of treatment, data were analyzed using analysis of Mann-Whitney U test. Pvalues of less than 0.05 were regarded as significant. Data was analyzed using the Statistical Package for the Social Sciences software (SPSS) statistical program (version 17.0 software, SPSS Inc. Chicago, IL, USA).
Results and Discussion
In this study, we found firstly in the literature that 2.45 GHz EMR, to which people are intensely Wi-Fi exposed in daily life, increased malondialdehyde (MDA), the indicator of lipid peroxidation and oxidative stress on the lens, and decreased antioxidants including GSH level and GSH-Px activity. Oxidative stress and thus, ROS, are believed to be a principal cause of noncongenital cataract. ROS have been implicated in the development of lens opacification, which has been demonstrated in both experimental animal models[18,23] and in cultured lens systems.[24,25] Cataract patients also exhibited elevated levels of oxidative stress.[18] ROS including superoxide and singlet oxygen can also be generated in the lens and aqueous humor, which explains why areas of the world with higher intensity of EMR irradiation have higher incidence of cataracts.[26]
Levels of lipid peroxidation are shown in [Fig. 2]. Mean values of cage control, sham control, Wi-Fi and Wi-Fi + melatonin groups as μM/g protein were 10.8, 10.7, 11.4 and 9.8, respectively. The lipid peroxidation levels were insignificantly higher in Wi-Fi group than in cage and sham control groups. However, lipid peroxidation levels were significantly (P < 0.05) lower in Wi-Fi + melatonin group than in Wi-Fi and control groups.
Figure 2.
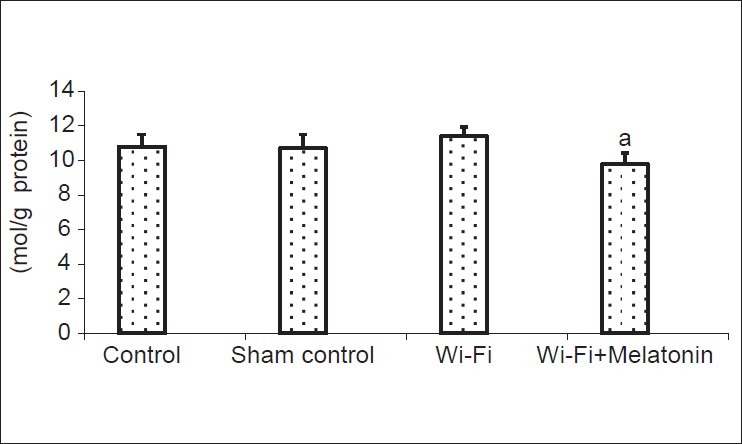
The effects of melatonin on lipid peroxidation levels on lens of Wi-Fi-induced EMR. (Mean ± SD). (mean ± SD).aP < 0.05 versus Wi-Fi and control groups
Levels of GSH are shown in [Fig. 3]. Mean values of cage control, sham control, Wi-Fi and Wi-Fi + melatonin groups as μM/g protein were 3.08, 3.12, 2.95 and 3.41, respectively. The GSH levels were insignificantly decreased in Wi-Fi group. However, GSH levels were significantly (P < 0.05) higher in Wi-Fi + melatonin group than in Wi-Fi group.
Figure 3.
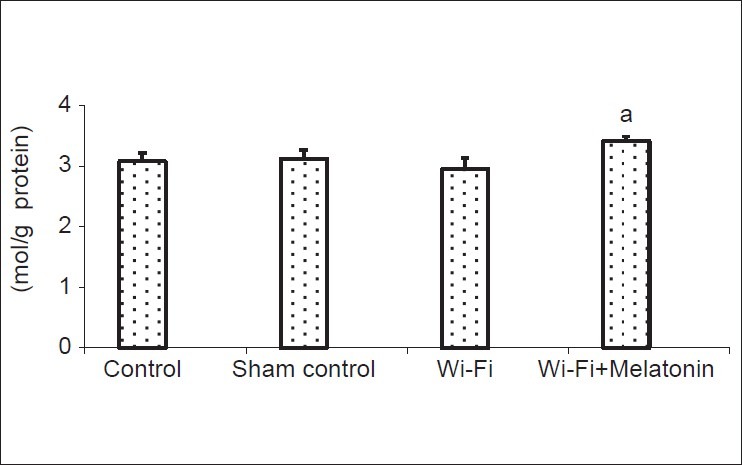
The effects of melatonin on reduced glutathione (GSH) levels on lens of Wi-Fi-induced EMR. (Mean ± SD). (mean ± SD).aP < 0.05 versus Wi-Fi group
Activities of GSH-Px are shown in [Fig. 4]. Mean values of cage control, sham control, Wi-Fi and Wi-Fi + melatonin groups as IU/g protein were 9.8, 10.4, 8.8 and 11.4, respectively. The GSH-Px activities were significantly (P < 0.05) lower in Wi-Fi group than in cage and sham control groups. The antioxidant enzyme activities were modulated by melatonin administration and their activities were significantly higher in Wi-Fi + melatonin group than in Wi-Fi (P < 0.01) and control groups (P < 0.05).
Figure 4.
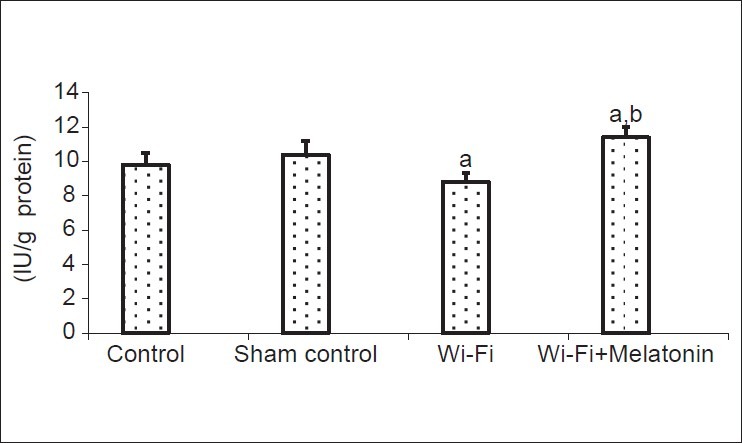
The effects of melatonin on reduced glutathione peroxidase (GSH-Px) activities on lens of 2.45 GHz induced EMR. (Mean ± SD). (mean ± SD).aP < 0.05 versus controls.bP < 0.01 versus Wi-Fi group
GSH is the most prevalent low molecular weight thiol containing antioxidant within mammalian cells. The GSH acts to protect cellular constituents from oxidative damage by directly reacting with oxidants or as the substrate for GSH-Px to scavenge peroxides.[27] It is believed that the high content of GSH in the lens protects thiols in structural proteins and enzymes for proper biological functions. The endogenous high level of GSH plays a crucial role as the first line of defense against exogenous and endogenous ROS and enables lens proteins to remain in a reduced state.[27] The ROS are continuously formed and are detoxified by superoxide dismutase, GSH-Px and catalase. Excessive production of free-radicals and the consumption of antioxidants lead to insufficient endogenous defense mechanisms. Lipid peroxidation values as MDA were increased in the lens samples by the Wi-Fi frequency exposure although GSH-Px activity was decreased by the exposure. The GSH-Px activity decreased in the lens samples because it may relate to the used antioxidants due to decrease ROS production and the compensatory in the antioxidant redox system.
Melatonin, as well as its metabolites, act directly to detoxify ROS such as hydroxyl radicals, peroxynitrite anion, singlet oxygen and reactive nitrogen species such as nitric oxide.[2,28] Melatonin possesses an electron-rich aromatic indole ring and acts as an electron-donor, thus having the ability to reduce and repair electrophilic radicals.[28] In addition, melatonin stimulates antioxidant enzymes such as superoxide dismutase and GSH-Px and they exert an antioxidative effect, indirectly.[27] Melatonin, as well as its metabolites, terminates the initiation and propagation of lipid peroxidation.[2] Melatonin is a stabilizer of cell membranes against oxidative stress.[28] In the current study we observed GSH and GSH-Px values were increased by melatonin administration although lipid peroxidation levels decreased due to its antioxidant protector effects and free radical scavenger effects. Consistent with results of the current study, studies using ultraviolet radiation[29] and ionize radiation[30] as the oxidant agent, in which antioxidant efficacy of melatonin on the lens was demonstrated, reported that oxidant-antioxidant indicators were similarly affected.
In conclusion, the results presented for Wi-Fi on lens are not consistent with a generalized antioxidant abnormality although melatonin induced modulator role on the over production of ROS. The beneficial effects of melatonin on antioxidant systems included regulation of the lipid peroxidation, GSH-Px and GSH values in the lens.
Acknowledgment
Authorship: MN formulated the present hypothesis. MN and LT were responsible for writing the report. SD was responsible for analysis of the data. ΦT and MCK made critical revision for the manuscript.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Consales C, Merla C, Marino C, Benassi B. Electromagnetic fields, oxidative stress, and neurodegeneration. Int J Cell Biol. 2012;2012:683897. doi: 10.1155/2012/683897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Espino J, Pariente JA, Rodríguez AB. Oxidative stress and immunosenescence: Therapeutic effects of melatonin. Oxid Med Cell Longev. 2012 doi: 10.1155/2012/670294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.The World Health Organization Report. Extremely low frequency fields. Environmental health criteria. 2007;238 Available from: http://www.who.int/peh-emf/publications/Complet_DEC_2007.pdf . [Google Scholar]
- 4.Li HW, Yao K, Jin HY, Sun LX, Lu DQ, Yu YB. Proteomic analysis of human lens epithelial cells exposed to microwaves. Jpn J Ophthalmol. 2007;51:412–6. doi: 10.1007/s10384-007-0483-9. [DOI] [PubMed] [Google Scholar]
- 5.Nazıroğlu M, Gümral N. Modulator effects of L-carnitine and selenium on wireless devices (2.45 GHz)-induced oxidative stress and electroencephalography records in brain of rat. Int J Radiat Biol. 2009;85:680–9. doi: 10.1080/09553000903009530. [DOI] [PubMed] [Google Scholar]
- 6.Türker Y, Nazıroğlu M, Gümral N, Celik O, Saygın M, Cömlekçi S, et al. Selenium and L-carnitine reduce oxidative stress in the heart of rat induced by 2.45-GHz radiation from wireless devices. Biol Trace Elem Res. 2011;143:1640–50. doi: 10.1007/s12011-011-8994-0. [DOI] [PubMed] [Google Scholar]
- 7.Nazıroğlu M, Yüksel M, Köse SA, Özkaya MO. Recent reports of Wi-Fi and mobile phone-induced radiation on oxidative stress and reproductive signaling pathways in females and males. J Membr Biol. 2013;246:869–75. doi: 10.1007/s00232-013-9597-9. [DOI] [PubMed] [Google Scholar]
- 8.Kocer I, Taysi S, Ertekin MV, Karslioglu I, Gepdiremen A, Sezen O, et al. The effect of L-carnitine in the prevention of ionizing radiation-induced cataracts: A rat model. Graefes Arch Clin Exp Ophthalmol. 2007;245:588–94. doi: 10.1007/s00417-005-0097-1. [DOI] [PubMed] [Google Scholar]
- 9.Kovacic P, Somanathan R. Unifying mechanism for eye toxicity: Electron transfer, reactive oxygen species, antioxidant benefits, cell signaling and cell membranes. Cell Membr Free Radic Res. 2008;2:56–69. [Google Scholar]
- 10.Özkaya D, Nazıroğlu M, Armağan A, Demirel A, Köroglu BK, Çolakoğlu N, et al. Dietary vitamin C and E modulates oxidative stress induced-kidney and lens injury in diabetic aged male rats through modulating glucose homeostasis and antioxidant systems. Cell Biochem Funct. 2011;29:287–93. doi: 10.1002/cbf.1749. [DOI] [PubMed] [Google Scholar]
- 11.Nazıroğlu M, Dilsiz N, Cay M. Protective role of intraperitoneally administered vitamins C and E and selenium on the levels of lipid peroxidation in the lens of rats made diabetic with streptozotocin. Biol Trace Elem Res. 1999;70:223–32. doi: 10.1007/BF02783831. [DOI] [PubMed] [Google Scholar]
- 12.Nordberg J, Arner ES. Reactive oxygen species, antioxidants, and the mammalian thioredoxin system. Free Radic Biol Med. 2001;31:1287–12. doi: 10.1016/s0891-5849(01)00724-9. [DOI] [PubMed] [Google Scholar]
- 13.Zheng Y, Liu Y, Ge J, Wang X, Liu L, Bu Z, et al. Resveratrol protects human lens epithelial cells against H2O2-induced oxidative stress by increasing catalase, SOD-1, and HO-1 expression. Mol Vis. 2010;16:1467–74. [PMC free article] [PubMed] [Google Scholar]
- 14.Itoh MT, Takahashi N, Abe M, Shimizu K. Expression and cellular localization of melatonin-synthesizing enzymes in the rat lens. J Pineal Res. 2007;42:92–6. doi: 10.1111/j.1600-079X.2006.00389.x. [DOI] [PubMed] [Google Scholar]
- 15.Alarma-Estrany P, Pintor J. Melatonin receptors in the eye: Location, second messengers and role in ocular physiology. Pharmacol Ther. 2007;113:507–22. doi: 10.1016/j.pharmthera.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 16.Nazıroğlu M, Çelik Ö, Özgül C, Çiğ B, Doğan S, Bal R, et al. Melatonin modulates wireless (2.45 GHz)-induced oxidative injury through TRPM2 and voltage gated Ca (2+) channels in brain and dorsal root ganglion in rat. Physiol Behav. 2012;105:683–92. doi: 10.1016/j.physbeh.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 17.Nazıroğlu M, Ciğ B, Doğan S, Uğuz AC, Dilek S, Faouzi D. 2.45-Gz wireless devices induce oxidative stress and proliferation through cytosolic Ca²⁺ influx in human leukemia cancer cells. Int J Radiat Biol. 2012;88:449–56. doi: 10.3109/09553002.2012.682192. [DOI] [PubMed] [Google Scholar]
- 18.Simþek M, Naziroğlu M, Erdinç A. Moderate exercise with a dietary vitamin C and E combination protects against streptozotocin-induced oxidative damage to the kidney and lens in pregnant rats. Exp Clin Endocrinol Diabetes. 2005;113:53–9. doi: 10.1055/s-2004-830528. [DOI] [PubMed] [Google Scholar]
- 19.Placer ZA, Cushman L, Johnson BC. Estimation of products of lipid peroxidation (malonyldialdehyde) in biological fluids. Anal Biochem. 1966;16:359–64. doi: 10.1016/0003-2697(66)90167-9. [DOI] [PubMed] [Google Scholar]
- 20.Sedlak J, Lindsay RH. Estimation of total, protein bound and non-protein sulfhydryl groups in tissue with Ellmann's reagent. Anal Biochem. 1968;25:192–205. doi: 10.1016/0003-2697(68)90092-4. [DOI] [PubMed] [Google Scholar]
- 21.Lawrence RA, Burk RF. Glutathione peroxidase activity in selenium-deficient rat liver. Biochem Biophys Res Commun. 1976;71:952–8. doi: 10.1016/0006-291x(76)90747-6. [DOI] [PubMed] [Google Scholar]
- 22.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin- Phenol reagent. J Biol Chem. 1951;193:265–75. [PubMed] [Google Scholar]
- 23.Truscott RJ. Age-related nuclear cataract-oxidation is the key. Exp Eye Res. 2005;80:709–25. doi: 10.1016/j.exer.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 24.Spector A, Wang GM, Wang RR, Li WC, Kuszak JR. A brief photochemically induced oxidative insult causes irreversible lens damage and cataract. I. Transparency and epithelial cell layer. Exp Eye Res. 1995;60:471–81. doi: 10.1016/s0014-4835(05)80062-4. [DOI] [PubMed] [Google Scholar]
- 25.Gupta SK, Trivedi D, Srivastava S, Joshi S, Halder N, Verma SD. Lycopene attenuates oxidative stress induced experimental cataract development: An in vitro and in vivo study. Nutrition. 2003;19:794–79. doi: 10.1016/s0899-9007(03)00140-0. [DOI] [PubMed] [Google Scholar]
- 26.Taylor HR, West SK, Rosenthal FS, Muñoz B, Newland HS, Abbey H, et al. Effect of ultraviolet radiation on cataract formation. N Engl J Med. 1999;319:1429–33. doi: 10.1056/NEJM198812013192201. [DOI] [PubMed] [Google Scholar]
- 27.Lou MF. Redox regulation in the lens. Prog Retin Eye Res. 2003;657:82. doi: 10.1016/s1350-9462(03)00050-8. [DOI] [PubMed] [Google Scholar]
- 28.Ekmekcioglu C. Melatonin receptors in humans: Biological role and clinical relevance. Biomed Pharmacother. 2006;60:97–108. doi: 10.1016/j.biopha.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 29.Bardak Y, Ozertürk Y, Ozgüner F, Durmuş M, Delibaş N. Effect of melatonin against oxidative stress in ultraviolet-B exposed rat lens. Curr Eye Res. 2000;20:225–30. [PubMed] [Google Scholar]
- 30.Karslioglu I, Ertekin MV, Taysi S, Koçer I, Sezen O, Gepdiremen A, et al. Radioprotective effects of melatonin on radiation-induced cataract. J Radiat Res. 2005;46:277–82. doi: 10.1269/jrr.46.277. [DOI] [PubMed] [Google Scholar]


