Abstract
Intraocular inflammatory eye disease is one of the important causes of ocular morbidity. Even though the prevalence of uveitis is less common in relation to diabetic retinopathy, glaucoma or age related macular degeneration, the complexity and heterogeneity of the disease makes it more unique. Putative uveitogenic retinal antigens incite innate immunity by the process of antigen mimicry and have been shown to be associated in patients with intraocular inflammatory disease by numerous experimental studies. Laboratory diagnostic tools to aid the etiologic association in intraocular inflammatory disease have evolved over the last two decades and we are entering into an era of molecular diagnostic tests. Sophisticated novel technologies such as multiplex bead assays to assess biological signatures have revolutionized the management of complex refractory uveitis. Nevertheless, there is still a long way to go to establish the causal relationship between these biomarkers and specific uveitic entities. Experimental studies have shown the supreme role of infliximab in the management of Behcet's disease. Despite significant experimental and case control studies, the deficiency of randomized clinical trials using these biologic agents has handicapped us in exploring them as a front line therapy in severe refractory uveitis. Studies still need to answer the safety of these potentially life threatening drugs in a selected group of patients and determine when to commence and for how long the treatment has to be given. This review article covers some basic concepts of cytokines in uveitis and their potential application for therapy in refractory uveitis.
Keywords: Aqueous humor, biologics, biological signatures, cytokines, intraocular inflammation, multiplex bead assays
Intraocular inflammation is one of the leading causes of world blindness next to cataract, glaucoma, diabetic retinopathy and age related macular degeneration. It accounts for 5 to 20% of blindness in the developed world and 25% in the developing world.[1]
Intraocular inflammation poses a significant therapeutic challenge given the heterogeneity of the uveitis spectrum along with the pressing need and increasing expectations for personalised care despite the varied immune etiology. The etiologic or stimulating trigger for most of uveitic entities is unknown. Uveitogenic proteins that can incite intraocular inflammation include rhodopsin, retinal arrestin, recoverin, phosducin, retinal pigment epithelium derived RPE-65 and inter-photoreceptor retinoid binding protein (IRBP). After the initial trigger, the immunogenic pathway is more of less similar for all types of intraocular inflammation.[2,3]
Conventional anti-inflammatory or immunosuppressive treatment though very effective in most of the uveitic entities is very non-specific and can frequently lead to systemic side effects. It can sometimes be ineffective for some recalcitrant severe uveitis. With the advent of experimental and cellular biology, several biomarkers are being identified. Many uveitic diseases are known to be strongly associated with particular HLA haplotypes. Recently, more and more uveitic entities are shown to have a significant association with specific cytokines. Cytokines have hence been recognised as biological markers for some intraocular inflammatory diseases and triggered a very strong drive towards personalised customised medicine. It has largely been supported by continued developmental of experimental models of autoimmune uveitis along with improved molecular biologic techniques.
This review attempts to present the current concepts of cytokines in uveitis and the practical application of the assessment of cytokines in serum and ocular fluid in inflammatory eye diseases and its use in the management of complex uveitis disorders. A systematic literature search was carried out using PubMed and Embase databases using the MeSH terms cytokines or chemokines or biologics or biomarkers in uveitis. All the results were filtered and the reports after year 2000 were taken into consideration for this review.
Biological Markers
The eye is an “immune privilege” organ and enjoys a unique ‘two edged sword’ relationship with the immune system. On one hand, the eye is protected from local inflammatory responses to environmental micro-organisms which would have had the potential to distort vision. On the other hand, immune privilege also leaves the eye vulnerable to an autoimmune attack by lymphocytes that have been primed elsewhere in the body by chance encounter with a self or with mimic antigens (Ags). Immune privilege is a complex phenomenon involving mechanical sequestration behind an efficient blood-retina barrier, active local inhibition by soluble and surface-bound molecules that actively inhibit activation and function of adaptive and innate immune cells, and also systemic regulation via induction of T regulatory cells.[2]
Cytokines and chemokines
The cluster of differentiation 4 (CD4) is a glycoprotein found on the surface of immune cells such as T helper cells, monocytes, macrophages, and dendritic cells. The CD4+ T helper cells are an essential part of the human immune system. They are called helper cells because one of their main roles is to send signals to other types of immune cells, including CD8 killer cells. Although posterior uveitis describes a range of different clinical entities, all forms are similar immunohistologically, characterized by an infiltration of mainly CD4+ T cells.[4] Cyclosporin A (CyA) can be effective in arresting the disease progression in many cases,[4] highlighting the importance of T cells. The ability to adaptively transfer disease using activated retinal antigen-specific CD4+ T cells in an experimental model is further evidence of CD4+ T cell-mediated processes inducing the irreversible destruction of the photoreceptor cells of the retina.[5] Therefore, posterior uveitis in humans is considered to be a T cell-mediated autoimmune disease, although the immunopathogenic mechanisms, the dynamics and precise contribution of leukocytes to these retinal diseases and the potential therapeutic benefit of modulating these cells are still unclear.
The CD4 interacts directly with MHC class II molecules on the surface of the antigen-presenting cell using its extracellular domain. Recognition of the MHC peptide complex by CD4+ T cells leads to secretion of cytokines. CD4+ T cells are differentiated on the basis of the type of the cytokines they secrete. Because CD4+ T cells help other cells through secretion of cytokines, they are also called ‘Th’ (T Helper) cells to differentiate them from cytotoxic CD8+ T cells activated by MHC class I peptide complex.
Classification
Originally, Th cells were divided into two subsets, a Th1 subset secreting Interferon -γ and Inter Leukin-2 responsible for cellular anti-viral immunity, and a Th2 subset secreting IL-4 required for blood borne parasitic responses.[6] CD4+ Th1 cells and IFN-γ are considered as the major effectors in the pathogenesis of experimental autoimmune uveitis (EAU).[6] The division was followed by an addition of a subset of regulatory CD4+ T cells that secrete IL-10 and Transforming Growth Factor-β.[7] However, the presence of inflammatory diseases in IFN-γ-deficient mice indicated existence of other Th cell subsets and led to the discovery of the Th17 subset secreting IL-17 and IL-23.[8] Recently, other Th cell subsets have been assigned on the basis of the secretion of IL-9 (Th9) or IL-21 (T follicular helper).[9]
Role as biological markers
During the course of an immune response, cellular communication, trafficking and polarization are achieved through the coordinated expression of cytokines and chemokines. Marked perturbation in these cytokine and chemokine networks are observed in many acute and chronic diseases. Cytokines play an important role in maintaining the inflammatory milleu in the body by maintaining lymphocyte homeostasis. Any up regulation of inflammatory cytokines or down regulation of regulatory cytokines play an important role in the pathophysiology of uveitis. Our knowledge and understanding about cytokines is largely based on animal models and experimental uveitis. There are numerous animal models of uveitis. Out of all the model of experimental associated uveitis, the mouse model using IRBP is the most common. The mouse model induced with IRBP most resembles the human model with inflammatory cell infiltrates in the retina, choroid, vitreous and damage to the photoreceptor layer.
Measurement of cytokines
The advent of bead based multi-detection assays has made it possible to detect multiple cytokine levels from a miniscule volume of aqueous fluid or serum.[10] A flow chart of multiplex cytokine assay is represented in Fig. 1. In a recent study by Iyer et al., aqueous humor fractions were analyzed for the concentration of 41 different cytokines, chemokines and growth factors thought to be involved in cytomegalovirus induced inflammation, with the FlexMAP 3D (Luminex; ) platform using the Milliplex Human Cytokine; kit.[11] These bead based assays have hence given us the insight into the interplay of different cytokines, chemokines and interleukins in the pathogenesis of many uveitic entities and hence provided us with the platform to embark on specific targeted therapy for some of these diseases [Fig. 2]. The major proinflammatory cytokines, their diagnostic utility in uveitis as biomarkers and tailored treatment of intraocular inflammation leveraging on the cytokine profile will be discussed later in this review.
Figure 1.
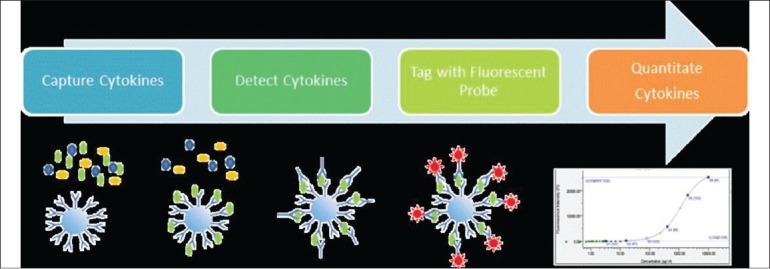
Process Flow in Multiplex Cytokine Analysis. Using a suspension phase array system (Luminex) cytokines are captured onto the surface of fluorescently coded microspheres. A biotinylated detector antibody is then bound in a sandwich configuration to the captured cytokine, washed and tagged. The amount of captured cytokine is then determined by measuring the amount of streptavidin conjugated flourescent probe complexed on the bead relative to a standard curve of pure cytokine
Figure 2.
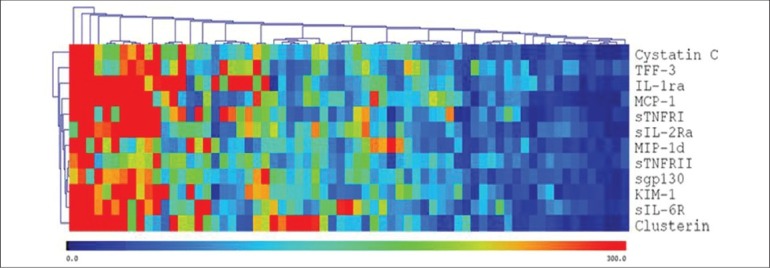
Multiplex Data Visualization. Multiplex proteomic data is organized via standard correlation clustering and presented in a heatmap visualization format. Patient samples are presented on the X-axis and clustered according to similarities in their pattern of cytokine expression
Aqueous humor for assessment of cytokines
Aqueous humor and vitreous humor are important for the maintenance of physiologic, nutritional and metabolic homeostasis within the anterior chamber and posterior chamber respectively. Components of both the aqueous humor and vitreous humor are a reflection of the state of intraocular tissues and show an altered biochemistry profile in inflammatory disorders. An anterior chamber paracentesis provides an aqueous humor sample with minimal risk as an outpatient procedure for the assessment of abnormal cytokines or inflammatory mediators in different intraocular inflammatory disorders with or without a systemic association.[10,12,13]
Cytokine Profile in Non Infectious Autoimmune Uveitis
An autoimmune etiology in uveitis is supported by strong HLA associations and by frequent responses to one or more retinal antigens. The identification of biomarkers in the aqueous humor can potentially provide us with an underlying cause in patients with “idiopathic” uveitis. A systematic review was conducted by Ooi et al., in 2006 on inflammatory cytokines in uveitis of various etiologies.[14] According to their review the various cytokines implicated in non- infectious autoimmune uveitis are tabulated in Table 1.
Table 1.
Cytokines and their association in various non-infectious inflammatory uveitic diseases (review of literature)
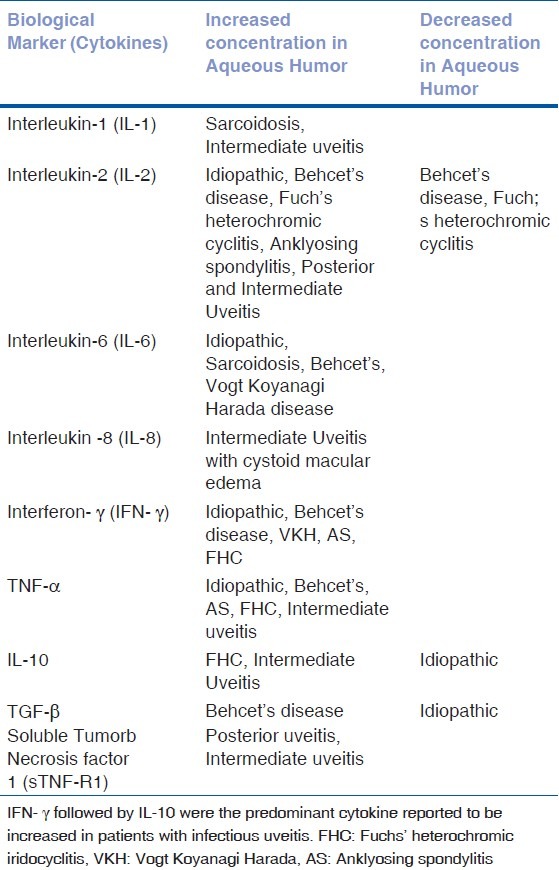
Idiopathic uveitis
The commonest form of uveitis and has been found to be associated with increased intraocular levels of IL-1β, IL-2, TNF-α, IFN- γ, IL-6, IL-8 and MCP-1.[12,13]
Behcet's disease
A systemic vasculitis with frequent ocular involvement in the form of uveitis and retinal vasculitis and has been associated with the HLA-B51 phenotype. Raised intraocular levels of the following immune factors have been found in patients with Behcet's disease-related uveitis: IL-2, IL-6, IFN-γ and TNF-α. In addition, a positive correlation was found between serum TNF-α levels and recurrent episodes of Behcet disease-related uveitis.[15]
Sarcoidosis
A granulomatous inflammatory disorder of unknown cause. The patients may have ocular involvement in the form of acute or chronic granulomatous uveitis and can involve the anterior, intermediate or posterior uveal layers. One study examining the aqueous immune profile of patients with sarcoidosis revealed elevated levels of IL-1α, IL-6 and IL-8.[14,16]
Vogt-Koyanagi-Harada (VKH) disease
A multisystem chronic granulomatous disorder which may manifest in the eye as a chronic, bilateral panuveitis. It is associated with HLA-DR1 and HLA-DR4 phenotype. Raised intraocular levels of the following immune factors have been identified thus far: IL-6, IL-8 and IFN- γ.[13]
Ankylosing spondylitis
A chronic inflammatory disorder of the axial skeleton with a strong association with HLA-B27 phenotype and may manifest in the eye as severe acute anterior uveitis. Reports have revealed elevated intraocular levels of IL-2, IFN- γ, IL-6 and TNF- α.[16]
Fuchs’ heterochromic iridocyclitis (FHC)
A chronic typically unilateral anterior uveitis syndrome with or without associated glaucoma. Intraocular levels of TGF- β was lower than that of controls while IL-8 and MCP-1 appeared to be raised (not statistically significant) compared to patients with idiopathic uveitis. One study found IFN-γ to be raised in FHC aqueous samples when compared to idiopathic uveitis with a larger number of FHC samples having higher levels of IL-10 (not statistically significant).[10]
Another study by Sijssens et al.,[17] was performed to evaluate the intraocular levels of 16 immune factors in adult and pediatric patients with uveitis.[2] This study's purpose was to distinguish the immune factor profiles occuring between 3 age groups - children (5-9 year olds), adolescents (10-18 year olds) and adults (>20 years old) - and did not distinguish immune factor profiles based on the etiology of uveitis. It revealed raised immune factor levels for IL-2, IL-12, IL-18, IFN-γ, TNF-α, IL-4, IL-13, and IL-10 response in children and adolesents when compared to samples obtained from adults. Adult patients with uveitis were found to have higher levels of IL-6 compared to children and adolescents and especially high levels were found in an adult patient with Behcet's disease and another patient with acute retinal necrosis (ARN).
Current and Potential ‘Tailored’ Treatment of Non-Infectious Uveitis Targeting Cytokines and Their Receptors
Due to the lack of an understanding of the pathophysiology of uveitis, conventional or established therapies of uveitis are largely based on non-specific immunosuppression. However, there is an urgent need to identify and target specific pro inflammatory cytokines responsible for specific uveitic entities for personalized therapy. Though treatment with specific therapy is idealistic, a limited knowledge in the understanding of the basic mechanisms of uveitis with the pleioptric nature of cytokines along with risk of inciting adverse reactions inhibits the physician in treating all patients with targeted therapy.
With the proteomic labelling, we should be able to target specific cytokine pathway and deliver targeted therapy for patients with intraocular inflammation. If corticosteroids revamped the treatment of intraocular inflammation in the early 1950s, immunosuppression has ushered in a new era of therapy for patients with severe intraocular inflammation and now with targeted therapy using biologics in specific uveitic entities we are probably embarking on much specialised stratified care.[4,5,12,18,19,20,21,22]
Biologic agents were first used for ocular inflammation in 1990s after the evolution of molecular and experimental biology. Most of these biologic agents are antidotes to inflammatory cytokines i.e. recombinant antibodies to cytokines or antagonists of inflammatory cytokines. That period also ushered in recombinant cytokines i.e. interferons. A key feature of biologics is specific targeted suppression of the immune effector responses that are responsible for damaging tissues.
There have been many reports on the use of biologics in ocular inflammatory eye diseases. In comparison to other systemic inflammatory diseases, there is still a scarcity of randomised controlled clinical trials for the use of this particular group of agents. Due to the heterogeneous nature of the disease, varied presentation and low prevalence, there are only case reports published so far.
Important points to consider before starting treatment with a biologic
The patient should be evaluated for tuberculosis infection by history, examination, chest X-ray, Mantoux test and Quantiferon Gold test.
Initial intravenous infusion is to be given in a facility with full resuscitation as fatal infusion reactions have been reported following severe cytokine release syndrome.
When switching between biologic therapies a washout period should be considered.
Biologics in Use for Uveitis
Anti TNF- α agents
First generation
Etanercept (Recombinant fusion protein)
Infliximab (mouse human chimeric monoclonal IgG1 antibody against TNF - α)
Adalimumab (a fully humanized monoclonal IgG1 antibody against TNF- α1)
Second generation
Certolizumab
Golimumab
Anti-interleukin therapies
Daclizumab (Anti IL-2 receptor humanized monoclonal antibody)
Anakinra (Anti IL-1 receptor antibody)
Gevokizumab (Anti IL-1β monoclonal antibodies)
Tocilizumab (Anti IL-6R monoclonal antibody)
Secukinumab (AIN 457) (Anti IL-17A antibody)
Rituximab (Anti CD20 monoclonal antibody)
Interferons
IFN α
Recombinant IFN α-2a (Roferon - A)
Recombinant IFN α-2b (Intron A)
Pegylated interferons
Fusion protein of CTLA-4
Abatacept
Etanercept
Etanercept is a soluble fusion protein and prevents both TNF-α and TNF-β from interacting with receptors. It consists of 2 dimmers of higher affinity type 2 TNF receptors. There are numerous reports on the use of etanercept for refractory cases of uveitis.[23,24,25,26] Etanercept has been studied extensively in patients with juvenile idiopathic arthritis, Behcet's disease and in pediatric non-infectious posterior uveitis. In numerous studies on the use of TNF-α inhibitors in uveitis, it was concluded that etanercept was less efficacious in inducing remission and also in preventing relapses as compare to infliximab.[23,24,26,27,28]
Infliximab
Infliximab is a chimeric IgG1 monoclonal antibody with the antigen-binding region derived from a mouse antibody and the constant region from a human antibody.[29] It binds to TNF-α with high affinity thereby blocking the binding of TNF-α to its receptor. One of the considerations in giving infliximab is that it can potentially induce antinuclear antibody (ANA) and anti-double stranded DNA on long term therapy.[30,31] Early monitoring and optimizing dose regimens can be useful in patients on long term Infliximab therapy. It has been shown to be superior to etanercept in preventing relapses and remission in various trials.[23,24,25] Side effects and dosing of this drug is listed in Table 2.
Table 2.
Dose, side effects and monitoring with biologics in patients with refractory uveitis
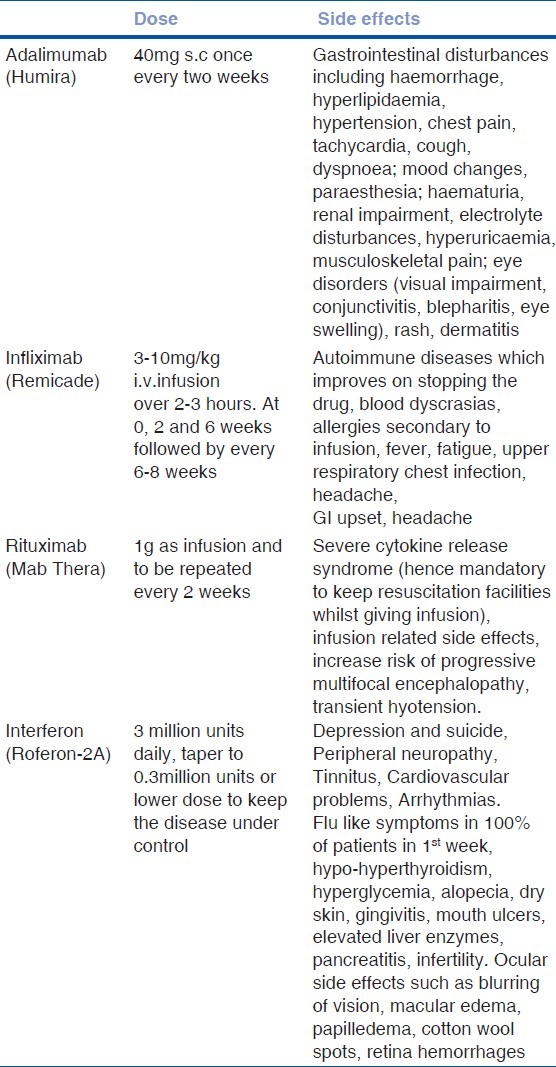
Adalimumab
Adalimumab is a fully humanized recombinant IgG1 monoclonal antibody with high binding to human TNF-α. Although there are wide variations in the pharmacokinetics of this biologic among patients, its distinct advantage is that it can be self-administered subcutaneously fortnightly. In a head to head study by Simonini et al., efficacy of infliximab was compared with adalimumab in pediatric non-infectious uveitis. As far as remission was concerned, both the agents were comparable but adalimumab was found to be superior to infliximab for treatment lasting over three years of duration.[32] Important side effects and dose for this drug has been listed in Table 2.
Golimumab
Golimumab is a novel fully humanized anti-TNFα monoclonal antibody that has been approved for the treatment of rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis. Along with other biologic agents this agent has also been used recently in patients with juvenile idiopathic arthritis associated uveitis. Overall, use of these drugs and other biologics has expanded the armanentrium of drugs which can be used in refractory uveitis. William et al., reported good outcomes using this agent in three patients with juvenile idiopathic arthritis associated anterior uveitis.[33]
Daclizumab
Daclizumab is a recombinant monoclonal antibody of the human IgG1 isotype composed of 90% human and 10% mouse antibody sequences that bind to CD25 with high affinity and inhibit IL-2-mediated responses of activated T cells. In a report by Nussenblatt and group, four of 39 patients developed solid malignant tumor while on daclizumab over a follow up period of 11 years.[34] It was hence withdrawn in 2009.
Anakinra
Anakinra is a recombinant non-glycosylated homologue of HuIL1Ra, a natural immunomodulating molecule, which competitively inhibits binding of IL1α and IL1β to the IL1 receptor type 1, which is expressed in a wide variety of tissues and organs. Teoh et al. has reported good response to this agent in IL-1 mediated inflammatory disorders (CINCA syndrome).[35] In addition, a clinical trial is underway at the National Institute of Arthritis and Musculoskeletal and Skin diseases (clinical trial reference number NCT01441076) for the use of anakinra in Behcet's disease.
Tocilizumab (Anti IL-6R monoclonal antibody)
Tocilizumab is a recombinant humanized monoclonal antibody and inhibits IL-6 mediated responses by binding to both membrane-bound and soluble IL-6 receptors with high affinity. It was also shown to be effective in treatment of refractory uveitis by Muselier et al.[36]
Secukinumab (AIN 457) (Anti IL-17A antibody)
Secukinumab is a fully humanized IgG1k monoclonal antibody neutralizing IL-17A. It has proved to be quite effective in the treatment of patients with anterior and posterior uveitis with no serious adverse effects.[37]
Rituximab (Anti CD20 monoclonal antibody)
Rituximab is a recombinant chimeric monoclonal antibody with binding efficacy to CD20. It works by blocking CD20-bearing
B cells. It was first used in the treatment of non Hodkgin's B cell lymphoma. It was subsequently used for rheumatoid arthritis, multiple sclerosis, systemic lupus erythematosus, and Wegener's granulomatosis. In addition, it was also used for the treatment of refractory uveitis.[38] Side effects and dosing of the rituxiamb are given in the Table 2.
Interferon-α
IFN-α is a type I interferon and has been used in the treatment of uveitis due to its antiproliferative, anti angiogenic and apoptotic effects. In addition, it has the ability to modulate immune responses, specifically activating dendritic, cytolytic T and NK cells. Interferon α-2A and Interferon α-2B are human recombinant interferons manufactured using recombinant DNA technology with E. coli to produce human proteins.
More recently, pegylated interferons have been used which have a longer duration of action allowing weekly administration (Pegasys, peginterferon α-2a; ViraferonPeg, peginterferon α-2b), but there is inadequate data to determine their efficacy.
Most of the biologics mainly Infliximab have been proven to be very effective in the treatment of severe refractory uveitis especially in patients with Behcet's disease. However, in view of the varied adverse effects, treatment with these agents has to be closely monitored and has to be given under supervision.[39] Details about the dosing and adverse effects are listed in Table 2.
Abatacept
It is a fusion protein that prevents antigen presenting cells from delivering the co-stimulatory signals to the T cells to fully activate them. There are case reports and case control studies reporting on the effectiveness of abatacept in the treatment of refractory uveitis in patients with juvenile idiopathic arthritis.[40]
Important consideration for patients on Biologics
Contraindicated in patients with tuberculosis or any active infection
Contraindicated in patients with pregnancy or breast feeding. Avoid pregnancy till 5 months after stopping last dose of biologics.
Rule out malignant conditions before starting biologics.
Monitor blood count regularly:- FBC, UandE's, LFTs and Glucose at baseline and subsequently at every 4 weeks for three months followed by every 6 weeks.
Patient to be advised to see a doctor if he develop fever, sore throat, bleeding.
As TNF-α agents can aggravate multiple sclerosis, rule out demyelinating disease before starting this agents in those set of patients.
-
Common side effects:
- Reduced immunity leading to increased risk of infection including flare up of latent tuberculosis.
- Worsening of heart failure if already present.
Limitations
Sensitivity and specificity of diagnostic tests in uveitis or intraocular inflammatory disease is always a point for concern. With the advent of molecular and experimental medicine, we have many more diagnostic markers. Though there is association of these biomarkers with some specific uveitis entities, there is no proven causal relationship as yet with any of these novel biomarkers. Whether it is the inflammatory cytokine causing the disease or is it the disease leading to release of a specific biomarker is yet to be determined in future research.
The cost of these sophisticated tests is a major logistic constraint in further establishing them as a front line investigative tool. This would prevent the treating physicians in customizing the tailored treatment for these complex disorders. In addition, targeted therapy with biologics is expensive and not without life threatening risks. Hence, it has to be delivered under the care of a specialist experienced with immunology and the pathophysiology of inflammatory diseases. Strict diligence with awareness of all the possible adverse effects will help in successfully rendering this specific therapy in refractory uveitis patients.
Conclusion
Cytokines are increasingly being recognised as biological markers in intraocular inflammatory diseases and has allowed us to identify and associate specific biologic signatures in these diseases. It has hence triggered a very strong drive towards customised ocular therapy. It has largely been supported by the development of experimental models of autoimmune uveitis along with improved molecular techniques.
Biologic therapies have been proven by different studies and randomized controlled trials to be effective in many systemic conditions and have subsequently revolutionized the treatment and prognosis of rheumatoid arthritis, juvenile idiopathic arthritis, seronegative arthropathies, and inflammatory bowel disease. However, due to the heterogeneity of many uveitic entities and the putative nature of the disease, similar clinical trials were not feasible in uveitis. However, there is level 1 evidence of the treatment benefit of infliximab in anterior uveitis associated with ankylosing spondylitis. There is increasing level 2 and 3 evidence for the efficacy of many biologic agents in refractory anterior and posterior uveitis, particularly Behcet's disease. With the discovery of newer disease specific cytokines we are embarking on international research networks and collaboration to facilitate randomized controlled trials comparing the efficacy of biologic therapies with each other and with traditional immunosuppressants. In future, evidence based medicine will pave way for informed clinical decisions on the optimal biologic and treatment regime for uveitis.
Acknowledgemement
The research was supported by the National Healthcare Group Eye Institute (NHGEI), National Institute for Health Research (NIHR) Biomedical Research Centre based at Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology. The views expressed are those of the author(s) and not necessarily those of the NHGEI, NHS, the NIHR or the Department of Health.
Footnotes
Source of Support: Dr Rupesh Agrawal is funded by National Medical Research Council (Singapore) Overseas research training fellowship at University College London & Moorfields Eye Hospital.
Conflict of Interest: None declared.
References
- 1.Gritz DC, Wong IG. Incidence and prevalence of uveitis in Northern California; The Northern California Epidemiology of Uveitis Study. Ophthalmology. 2004;111:491–500. doi: 10.1016/j.ophtha.2003.06.014. [DOI] [PubMed] [Google Scholar]
- 2.Caspi R. Autoimmunity in the immune privileged eye: Pathogenic and regulatory T cells. Immunol Res. 2008;42:41–50. doi: 10.1007/s12026-008-8031-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Luger D, Caspi RR. New perspectives on effector mechanisms in uveitis. Semin Immunopathol. 2008;30:135–43. doi: 10.1007/s00281-008-0108-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dick AD. Immune mechanisms of uveitis: Insights into disease pathogenesis and treatment. Int Ophthalmol Clin. 2000;40:1–18. doi: 10.1097/00004397-200004000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Dick AD, Carter DA. Cytokines and immunopathogenesis of intraocular posterior segment inflammation. Ocular Immunol Inflamm. 2003;11:17–28. doi: 10.1076/ocii.11.1.17.15575. [DOI] [PubMed] [Google Scholar]
- 6.Abbas AK, Murphy KM, Sher A. Functional diversity of helper T lymphocytes. Nature. 1996;383:787–93. doi: 10.1038/383787a0. [DOI] [PubMed] [Google Scholar]
- 7.Sakaguchi S. Naturally arising CD4+regulatory t cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol. 2004;22:531–62. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- 8.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 9.Akdis M. The cellular orchestra in skin allergy; are differences to lung and nose relevant? Curr Opin Allergy Clin Immunol. 2010;10:443–51. doi: 10.1097/ACI.0b013e32833d7d48. [DOI] [PubMed] [Google Scholar]
- 10.Curnow SJ, Falciani F, Durrani OM, Cheung CM, Ross EJ, Wloka K, et al. Multiplex bead immunoassay analysis of aqueous humor reveals distinct cytokine profiles in uveitis. Invest Ophthalmol Vis Sci. 2005;46:4251–9. doi: 10.1167/iovs.05-0444. [DOI] [PubMed] [Google Scholar]
- 11.Lane J, McLaren PJ, Dorrell L, Shianna KV, Stemke A, Pelak K, et al. A genome-wide association study of resistance to HIV infection in highly exposed uninfected individuals with hemophilia A. Hum Mol Genet. 2013;22:1903–10. doi: 10.1093/hmg/ddt033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Atan D, Fraser-Bell S, Plskova J, Kuffova L, Hogan A, Tufail A, et al. Cytokine polymorphism in noninfectious uveitis. Invest Ophthalmol Vis Sci. 2010;51:4133–42. doi: 10.1167/iovs.09-4583. [DOI] [PubMed] [Google Scholar]
- 13.El-Asrar AM, Struyf S, Kangave D, Al-Obeidan SS, Opdenakker G, Geboes K, et al. Cytokine profiles in aqueous humor of patients with different clinical entities of endogenous uveitis. Clin Immunol. 2011;139:177–84. doi: 10.1016/j.clim.2011.01.014. [DOI] [PubMed] [Google Scholar]
- 14.Ooi KG, Galatowicz G, Calder VL, Lightman SL. Cytokines and chemokines in uveitis: Is there a correlation with clinical phenotype. Clin Med Res? 2006;4:294–309. doi: 10.3121/cmr.4.4.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ahn JK, Yu HG, Chung H, Park YG. Intraocular cytokine environment in active Behcet uveitis. Am J Ophthalmol. 2006;142:429–34. doi: 10.1016/j.ajo.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 16.Ooi KG, Galatowicz G, Towler HM, Lightman SL, Calder VL. Multiplex cytokine detection versus ELISA for aqueous humor: IL-5, IL-10, and IFNgamma profiles in uveitis. Invest Ophthalmol Vis Sci. 2006;47:272–7. doi: 10.1167/iovs.05-0790. [DOI] [PubMed] [Google Scholar]
- 17.Sijssens KM, Rijkers GT, Rothova A, Stilma JS, de Boer JH. Distinct cytokine patterns in the aqueous humor of children, adolescents and adults with uveitis. Ocul Immunol Inflamm. 2008;16:211–6. doi: 10.1080/09273940802409969. [DOI] [PubMed] [Google Scholar]
- 18.Takeuchi H, Remington G. A systematic review of reported cases involving psychotic symptoms worsened by aripiprazole in schizophrenia or schizoaffective disorder. Psychopharmacology. 2013;228:175–85. doi: 10.1007/s00213-013-3154-1. [DOI] [PubMed] [Google Scholar]
- 19.Dick AD. Experimental approaches to specific immunotherapies in autoimmune disease: Future treatment of endogenous posterior uveitis? British J Ophthalmol. 1995;79:81–8. doi: 10.1136/bjo.79.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Imrie FR, Dick AD. Biologics in the treatment of uveitis. Curr Opin Ophthalmol. 2007;18:481–6. doi: 10.1097/ICU.0b013e3282f03d42. [DOI] [PubMed] [Google Scholar]
- 21.Sharma SM, Dick AD, Ramanan AV. Non-infectious pediatric uveitis: An update on immunomodulatory management. Paediatr Drugs. 2009;11:229–41. doi: 10.2165/00148581-200911040-00002. [DOI] [PubMed] [Google Scholar]
- 22.Willermain F, Rosenbaum JT, Bodaghi B, Rosenzweig HL, Childers S, Behrend T, et al. Interplay between innate and adaptive immunity in the development of non-infectious uveitis. Prog Retin Eye Res. 2012;31:182–94. doi: 10.1016/j.preteyeres.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith JR, Levinson RD, Holland GN, Jabs DA, Robinson MR, Whitcup SM, et al. Differential efficacy of tumor necrosis factor inhibition in the management of inflammatory eye disease and associated rheumatic disease. Arthritis Rheum. 2001;45:252–7. doi: 10.1002/1529-0131(200106)45:3<252::AID-ART257>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 24.Reiff A, Takei S, Sadeghi S, Stout A, Shaham B, Bernstein B, et al. Etanercept therapy in children with treatment-resistant uveitis. Arthritis Rheum. 2001;44:1411–5. doi: 10.1002/1529-0131(200106)44:6<1411::AID-ART235>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 25.Estrach C, Mpofu S, Moots RJ. Behcet's syndrome: Response to infliximab after failure of etanercept. Rheumatology. 2002;41:1213–4. [PubMed] [Google Scholar]
- 26.Saurenmann RK, Levin AV, Rose JB, Parker S, Rabinovitch T, Tyrrell PN, et al. Tumour necrosis factor alpha inhibitors in the treatment of childhood uveitis. Rheumatology. 2006;45:982–9. doi: 10.1093/rheumatology/kel030. [DOI] [PubMed] [Google Scholar]
- 27.Galor A, Perez VL, Hammel JP, Lowder CY. Differential effectiveness of etanercept and infliximab in the treatment of ocular inflammation. Ophthalmology. 2006;113:2317–23. doi: 10.1016/j.ophtha.2006.04.038. [DOI] [PubMed] [Google Scholar]
- 28.Tynjala P, Lindahl P, Honkanen V, Lahdenne P, Kotaniemi K. Infliximab and etanercept in the treatment of chronic uveitis associated with refractory juvenile idiopathic arthritis. Ann Rheum Dis. 2007;66:548–50. doi: 10.1136/ard.2006.058248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takeuchi M. A systematic review of biologics for the treatment of noninfectious uveitis. Immunotherapy. 2013;5:91–102. doi: 10.2217/imt.12.134. [DOI] [PubMed] [Google Scholar]
- 30.De Rycke L, Baeten D, Kruithof E, Van den Bosch F, Veys EM, De Keyser F. Infliximab, but not etanercept, induces IgM anti-double-stranded DNA autoantibodies as main antinuclear reactivity: Biologic and clinical implications in autoimmune arthritis. Arthritis Rheum. 2005;52:2192–201. doi: 10.1002/art.21190. [DOI] [PubMed] [Google Scholar]
- 31.Iwata D, Namba K, Mizuuchi K, Kitaichi N, Kase S, Takemoto Y, et al. Correlation between elevation of serum antinuclear antibody titer and decreased therapeutic efficacy in the treatment of Behcet's disease with infliximab. Graefes Arch Clin Exp Ophthalmol. 2012;250:1081–7. doi: 10.1007/s00417-011-1908-1. [DOI] [PubMed] [Google Scholar]
- 32.Simonini G, Taddio A, Cattalini M, Caputo R, De Libero C, Naviglio S, et al. Prevention of flare recurrences in childhood-refractory chronic uveitis: An open-label comparative study of adalimumab versus infliximab. Arthritis Care Res. 2011;63:612–8. doi: 10.1002/acr.20404. [DOI] [PubMed] [Google Scholar]
- 33.William M, Faez S, Papaliodis GN, Lobo AM. Golimumab for the treatment of refractory juvenile idiopathic arthritis-associated uveitis. J Ophthalmic Inflamm Infect. 2012;2:231–3. doi: 10.1007/s12348-012-0081-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wroblewski K, Sen HN, Yeh S, Faia L, Li Z, Sran P, et al. Long-term daclizumab therapy for the treatment of noninfectious ocular inflammatory disease. Can J Ophthalmol. 2011;46:322–8. doi: 10.1016/j.jcjo.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Teoh SC, Sharma S, Hogan A, Lee R, Ramanan AV, Dick AD. Tailoring biological treatment: Anakinra treatment of posterior uveitis associated with the CINCA syndrome. Br J Ophthalmol. 2007;91:263–4. doi: 10.1136/bjo.2006.0101477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Muselier A, Bielefeld P, Bidot S, Vinit J, Besancenot JF, Bron A. Efficacy of tocilizumab in two patients with anti-TNF-alpha refractory uveitis. Ocul Immunol Inflamm. 2011;19:382–3. doi: 10.3109/09273948.2011.606593. [DOI] [PubMed] [Google Scholar]
- 37.Hueber W, Patel DD, Dryja T, Wright AM, Koroleva I, Bruin G, et al. Effects of AIN457, a fully human antibody to interleukin-17A, on psoriasis, rheumatoid arthritis, and uveitis. Sci Transl Med. 2010;2:52ra72. doi: 10.1126/scitranslmed.3001107. [DOI] [PubMed] [Google Scholar]
- 38.Heiligenhaus A, Miserocchi E, Heinz C, Gerloni V, Kotaniemi K. Treatment of severe uveitis associated with juvenile idiopathic arthritis with anti-CD20 monoclonal antibody (rituximab) Rheumatology. 2011;50:1390–4. doi: 10.1093/rheumatology/ker107. [DOI] [PubMed] [Google Scholar]
- 39.Deuter CM, Zierhut M, Mohle A, Vonthein R, Stobiger N, Kotter I. Long-term remission after cessation of interferon-alpha treatment in patients with severe uveitis due to Behcet's disease. Arthritis Rheum. 2010;62:2796–805. doi: 10.1002/art.27581. [DOI] [PubMed] [Google Scholar]
- 40.Kenawy N, Cleary G, Mewar D, Beare N, Chandna A, Pearce I. Abatacept: A potential therapy in refractory cases of juvenile idiopathic arthritis-associated uveitis. Graefes Arch Clin Exp Ophthalmol. 2011;249:297–300. doi: 10.1007/s00417-010-1523-6. [DOI] [PubMed] [Google Scholar]


