Introduction
Obesity is currently a national and worldwide epidemic. Using the current NIH/WHO classification system, which groups individuals by body mass index (BMI) into five categories (Table 1), it was estimated that there were 1.6 billion overweight and 400 million obese adults worldwide in 2005, and that these numbers will rise to over 2.3 billion overweight and 700 million obese adults by 2015 [1]. In the United States alone, census bureau data suggests that there are currently about 134 million overweight adults, of which nearly 64 million can be considered obese; furthermore, almost 10 million Americans meet the criteria for morbid obesity with a BMI greater than 40 [2]. Obesity has been clearly recognized as a risk factor for adverse health outcomes in both chronic and acute disease [3, 4]. Following traumatic injury, obese patients have been found by many investigators to have increased rates of a variety of infectious and non-infectious complications with multiple organ failure being a common adverse outcome in this population of patients [4–13].
Table 1.
NIH/WHO Body Mass Index Classifications
| Class | Body Mass Index (kilogram/meter2) |
|---|---|
| Underweight | <18.5 |
| Normal Weight | 18.5–24.9 |
| Overweight | 25–29.9 |
| Obese | 30–39.9 |
| Morbidly Obese | ≥ 40 |
Interestingly, while the association of obesity with complications in the setting of traumatic injury has been well-defined, the underlying etiology for the development of complications remains ambiguous. Existing knowledge on the subject suggests a multifactorial causation, with immunologic dysfunction [14–18], anatomic and physiologic considerations [3, 19–22], and inadequate or inappropriate care [23], all potentially contributing to poorer outcomes. One specific area that warrants further investigation is that of resuscitation in the obese patient, and the initial and subsequent host response to injury. It has been noted previously that obesity is associated with vascular dysfunction, owing in part to abnormal circulating levels of insulin, aldosterone, cortisol, and leptin [24]. Furthermore, obesity has also been linked with left ventricular systolic and diastolic dysfunction, right ventricular diastolic dysfunction, and a cardiomyopathy syndrome [25]. These elements would suggest increased difficulty in resuscitation of the obese patient. In a study specifically examining the issue in trauma patients, Belzberg and colleagues confirmed hemodynamic abnormalities in obese patients following severe blunt trauma, including lower cardiac index and reduced tissue oxygenation when compared to nonobese patients [26]. Additionally, we have noted that morbidly obese patients demonstrate a slower return of base deficit to normal levels in the 24 hours following severe blunt trauma, and felt that this might be reflective of differential resuscitative practices and/or a differential host response to post-trauma resuscitation [27].
Given our findings and those of other investigators, we were interested in further characterizing resuscitative practices in blunt trauma patients and how these might differ with respect to BMI. Moreover, we wished to examine whether or not there were differences in the responses of patients of different body mass index to traditional resuscitative practices that might provide an explanation for the worse outcomes in these patients. We hypothesized that differences in resuscitative practice altered by BMI might provide an explanation for poorer outcomes and the increased risk of multiple organ failure in these high risk patients.
Methods
We performed a retrospective review of the prospectively collected information contained within the “Inflammation and the Host Response to Injury” database. The “Inflammation and the Host Response to Injury” Large Scale Collaborative Research Program was initiated in 2001 by the National Institute for General Medical Sciences and represents a multicenter collaborative effort between several major trauma centers across the United States. This "Glue Grant" has created a database containing outcome data from over 1000 subjects with severe blunt trauma, and in a subset of nearly 200 subjects, has collected serial genomic and proteomic data based on whole blood collections. A complete description of the goals of the grant, participating investigators and institutions, criteria for study enrollment, and study protocols may be found at the project website (http://www.gluegrant.org/). For the purposes of this effort, we focused on clinical outcomes data. In general, patients were reviewed if they met the inclusion and exclusion criteria for the epidemiological portion of the study. Briefly, enrolled patients were victims of severe blunt trauma arriving with an anticipated need for a blood transfusion accompanied with either a base deficit greater than or equal to six or systolic blood pressure less than 90 mmHg within 60 minutes of emergency department arrival. Patients were excluded from the study if they had isolated head injury or cervical spine injury with paralysis. For the purposes of this review, we restricted our analysis to adult patients (greater than 16 years of age) who had height and weight recorded for calculation of BMI. As we were interested in patient response to initial post-traumatic resuscitation, we excluded patients who expired within the first 48 hours. Additionally, given our previous finding of possible acid-base abnormalities, we excluded patients who might have had pre-existing difficulties with acid-base regulation, specifically omitting patients who had a known history of chronic pulmonary disease requiring oxygen or who had known history of chronic renal disease (defined as having a known baseline creatinine level of greater than 1.5). Patients were further excluded if their BMI put them in the category of “underweight” (BMI less than 18.5), as this population has health issues distinct from the ones of interest in this study.
Upon review of inclusion and exclusion criteria, patients were grouped into four groups based on BMI, “Normal” (18.5–24.9 kg/m2), “Overweight” (25–29.9 kg/m2), “Obese” (30–39.9 kg/m2), and “Morbidly Obese” (greater than 40 kg/m2). These groups were then compared on the basis of: demographic information; measures of injury severity and patient health; resuscitative volumes and compositions; traditional cardiovascular endpoints of resuscitation including heart rate, systolic blood pressure, central venous pressure, and pulmonary artery catheter measurements; and, metabolic indicators of resuscitation including base deficit, pH, and lactic acid. Patients were determined to have acidosis with a pH of less than 7.35. Primary metabolic acidosis was defined by a bicarbonate concentration of less than 22. Primary respiratory acidosis was defined by a pCO2 of ≥ 45. Metabolic acidosis was deemed to be present in the setting of respiratory acidosis if the bicarbonate concentration was less than would be expected utilizing the following formula: expected HCO3− = 24 + {(pCO2-40)/10}. Respiratory acidosis was deemed to be present in the setting of metabolic acidosis if the pCO2 was greater than 2 millimeters of mercury more than would be expected utilizing the following formula: expected pCO2 = 1.5 x [HCO3−] + 8. A mixed acidosis was determined to be present if features of both respiratory and metabolic acidosis were present. Lean body mass was calculated according to the method described by James (reviewed in [28]). Ideal body weight was calculated by the Devine formula (reviewed in [29]). Multiple organ failure (MOF) was used as an outcome measure, and was determined using the Marshall MOF Score [30]. For the purposes of this study, patients were defined as having MOF if their Marshall score was greater than or equal to five. Groups were compared using chi-square test for categorical variables and one way analysis of variance (for variables with normal distribution) or the Kruskal-Wallis analysis of variance (for variables with non-normal distribution) for continuous variables with significance determined at a p-value of less than 0.05. Post hoc pairwise comparisons were performed according to Dunn’s Method. Statistical analysis was completed using SigmaStat® 5.0 (SyStat Software Incorporated, San Jose, CA).
Results
General Patient Information
1,066 patient records were reviewed, with 877 patients eligible for final analysis based on inclusion and exclusion criteria (Figure 1). General characteristics of the 877 patients are seen in Table 2. Briefly, morbidly obese patients were older than patients in other groups and notably, developed multiple organ failure at a significantly greater rate. Mortality did not differ significantly between patient groups.
Figure 1.
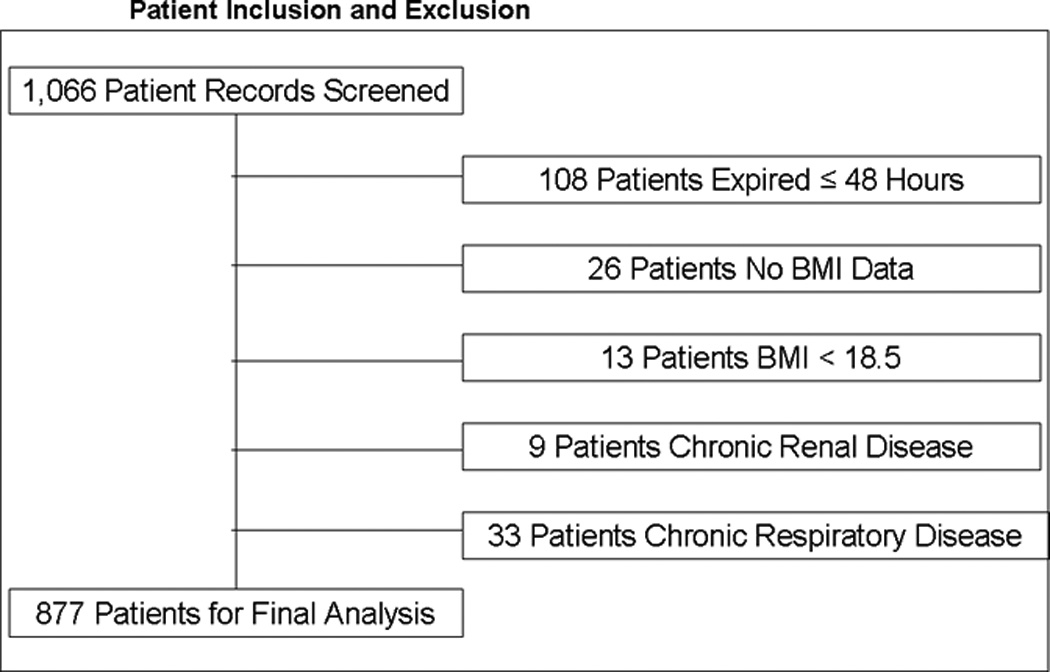
The breakdown of included and excluded patients is detailed, with 1,066 patients meeting inclusion criteria, and 189 patients excluded as shown.
Table 2.
General Study Population Information
| Normal (N=308) |
Overweight (N=292) |
Obese (N=222) |
Morbid (N=55) |
p-value | |
|---|---|---|---|---|---|
| Age, years (Median) | 35 | 40 | 45 | 47 | p<0.001 |
| Percent Male | 56.2% | 74.7% | 70.3% | 49.1% | p<0.001 |
| Injury Severity Score (Median) | 31 | 30 | 29 | 29 | p=0.260 |
| APACHE II Score (Median) | 28 | 28 | 29 | 31 | p=0.389 |
| Mortality | 11.7% | 7.9% | 11.3% | 14.5% | p=0.296 |
| Multiple Organ Failure (% Marshall Score ≥ 5) | 45.1% | 55.5% | 65.3% | 74.5% | p<0.001 |
Resuscitation Volume and Composition
We compared patients in terms of volume of fluid received, and the composition of this fluid volume. Figure 2A shows the total fluid volume received during the first 48 hours of resuscitation, with figures 2B and 2C depicting the blood product and crystalloid/colloid volumes received over that same time. Morbidly obese patients had received a greater total fluid resuscitation volume than normal weight patients by 48 hours; however, they did not differ from other groups, nor did they differ from normal weight patients at any other time points. Overweight patients received the greatest volume of blood product, and this significantly differed from normal weight patients between 13 and 18 hours after injury (p=0.048) and between 24 and 48 hours after injury (p=0.049). We normalized all fluid volumes by actual, lean, and ideal body masses to evaluate for occult differences with dependence on body mass (Figure 3). Unsurprisingly, normal and overweight patients received greater resuscitation volumes per kilogram of actual body mass (Panel A); interestingly though, there were no differences between the groups when volumes were adjusted for lean (Panel B) and ideal (Panel C) body mass.
Figure 2.
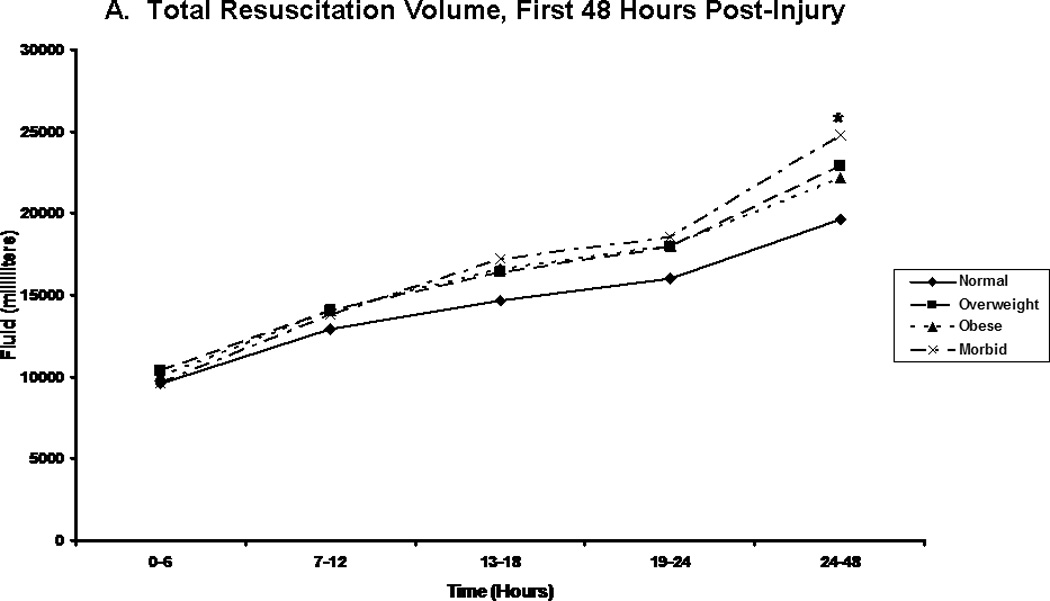
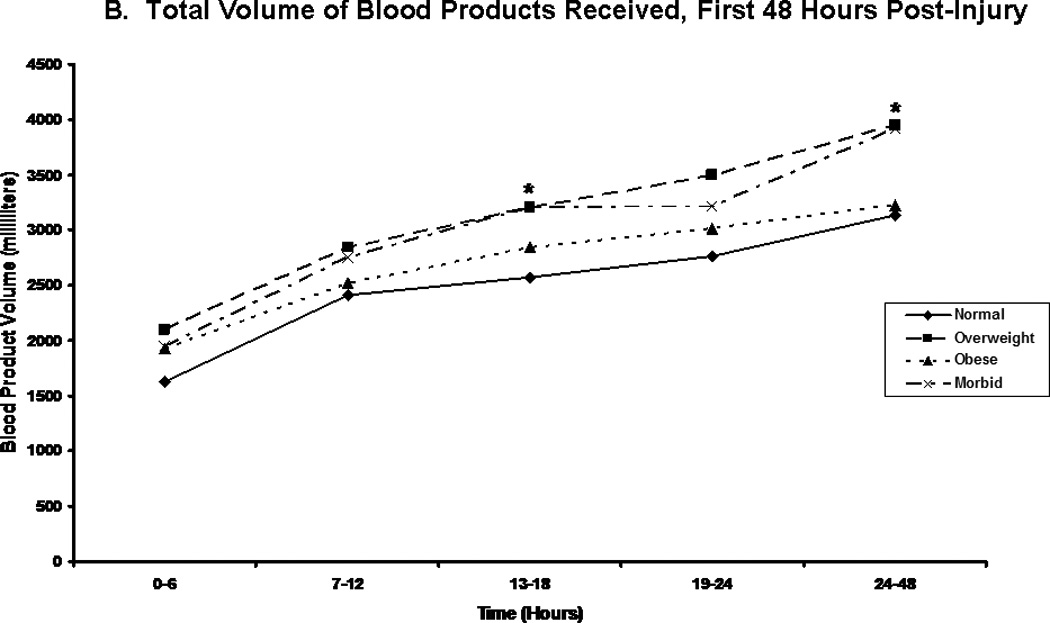
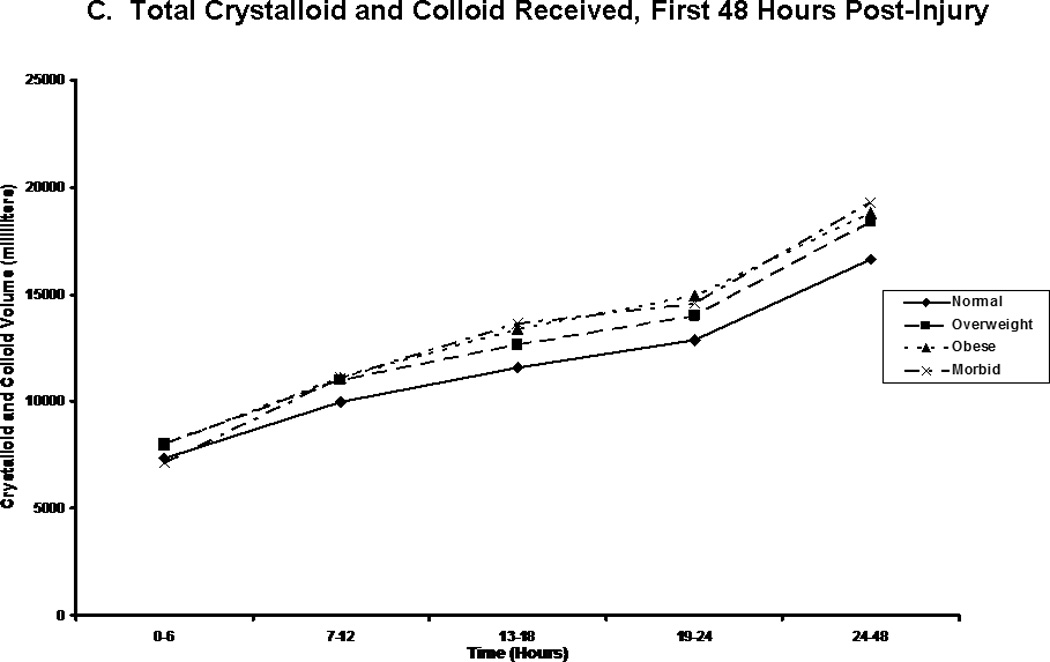
Post-injury fluid resuscitation volumes. Panel A depicts the total fluid volume received by patients in each body mass index classification over time during the first 48 hours following injury. Panel B depicts the total blood volume received by patients in each body mass index classification. Panel C depicts the total crystalloid volume received by patients in each body mass index classification. Values presented are medians. * indicates significantly greater volume received than normal weight patients at the specified time point.
Figure 3.
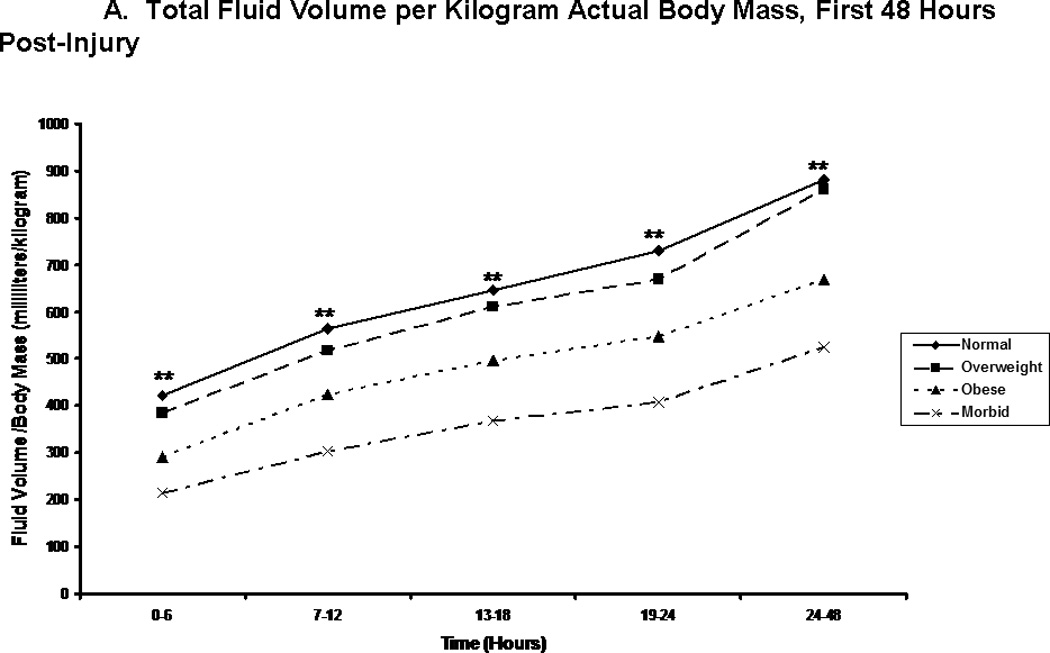
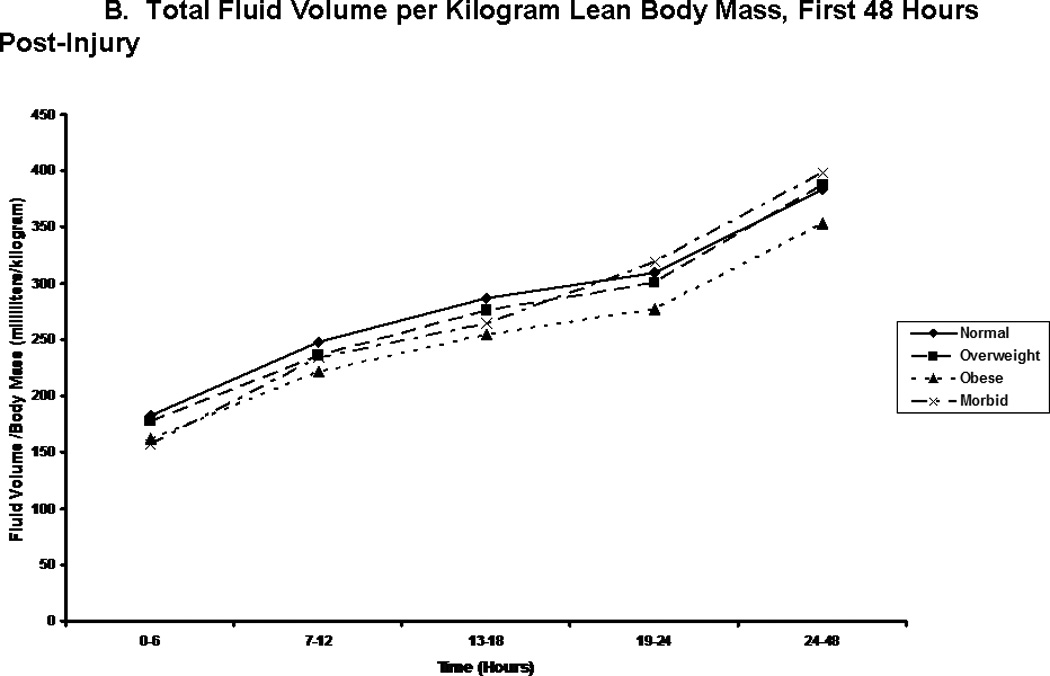
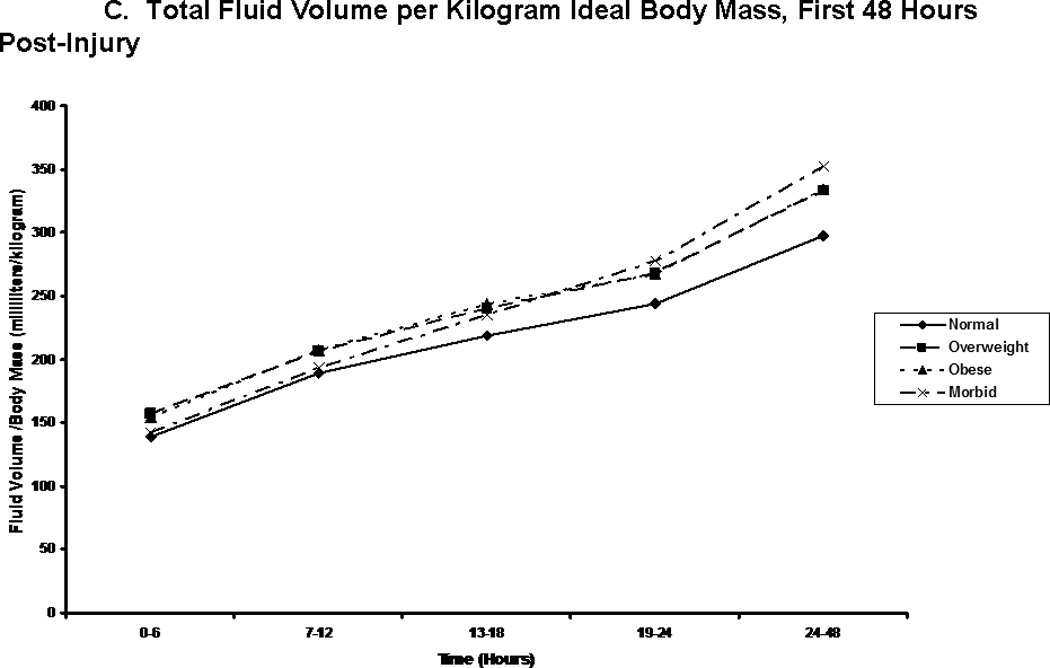
Total fluid volume per kilogram body weight. Panel A depicts the total fluid volume received by patient in each body mass index classification normalized by patients’ actual body mass over time during the first 48 hours following injury. Panel B depicts the total fluid volume received by patient in each body mass index classification normalized by patients’ estimated lean body mass over time during the first 48 hours following injury. Panel C depicts the total fluid volume received by patient in each body mass index classification normalized by patients’ calculated ideal body mass over time during the first 48 hours following injury. ** indicates significantly greater volume per body mass for normal and overweight patients when compared to obese and morbidly obese patients at the specified time point.
Cardiovascular Endpoints
Examination of traditional cardiovascular endpoints revealed few differences between groups. Mean arterial pressures were relatively similar, although curiously, the morbidly obese showed a wider range of blood pressure values, attaining greater highest mean arterial pressures, but also lower lowest mean arterial pressures in the first 24 hours than patients in the other groups (Table 3A). Normal weight patients had slightly lower lowest heart rates than other groups in the first 24 hours, but otherwise, the groups showed no differences in this metric (Table 3B). Conversely, significant differences were seen in central venous pressure readings between BMI groups (Figure 4). From 7 hours through the 48 hour mark, morbidly obese patients showed greater central venous pressures than normal weight patients, and in the second 24 hours, greater central venous pressures than overweight patients. Additionally, obese patients showed greater central venous pressures than normal weight patients from 13 hours through 48 hours. Finally, in a limited subset of patients, pulmonary artery catheter readings were obtained. In these patients, no differences were noted in cardiac index, mixed venous oxygen saturation, or pulmonary capillary wedge pressures (data not shown).
Table 3.
| A. Mean Arterial Pressures | |||||
|---|---|---|---|---|---|
| Normal | Overweight | Obese | Morbid | p-value | |
| Lowest, ED | 65 | 63 | 60 | 63 | p=0.082 |
| Highest, 0 – 24Hours | 113 | 117 | 118 | 118 | p=0.008 |
| Lowest, 0 – 24 Hours | 55 | 56 | 53 | 52 | p=0.004 |
| Lowest, 24 – 48 Hours | 77 | 79 | 77 | 78 | p=0.135 |
| B. Heart Rates | |||||
|---|---|---|---|---|---|
| Normal | Overweight | Obese | Morbid | p-value | |
| Highest, ED | 121 | 122 | 123 | 125 | p=0.609 |
| Highest, 0 – 24 Hours | 134 | 135 | 136 | 138 | p=0.628 |
| Lowest, 0 – 24 Hours | 75 | 78 | 81 | 80 | p=0.031 |
| Highest, 24 – 48 Hours | 102 | 104 | 105 | 107 | p=0.177 |
Figure 4.
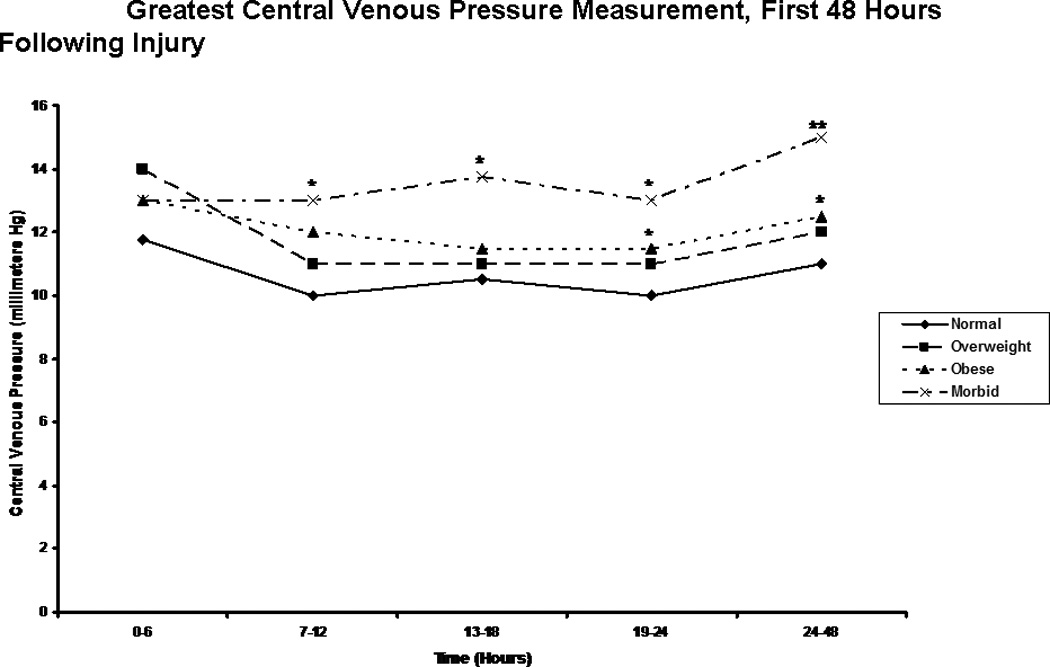
Central Venous Pressure readings. The figure depicts the median greatest central venous pressure measurements for patients in each body mass index classification during the first 48 hours following injury. * indicates a significantly greater central venous pressure reading than normal weight patients at the specified time point. ** indicates a significantly greater central venous pressure reading than normal weight and overweight patients at the specified time point.
Metabolic measurements and Endpoints
We next focused on laboratory measures and first found, as we had in our previous analysis, that the morbidly obese had a significantly slower resolution of base deficit when compared to patients in the other BMI classes (Figure 5). Perhaps more interestingly, though, when we compared groups on the basis of pH over time, we saw clear distinction between BMI classes, with the additional and significant finding of a persistent acidosis in the morbidly obese group extending through the first 48 hours (Figure 6). In order to better characterize the nature of this finding, we created composite blood gases for each BMI group for each 6 hour period studied, and determined the acid-base status (Table 4). This assessment revealed all groups to be in a state of mixed metabolic and respiratory acidosis during the first six hours following injury, followed by a period of metabolic acidosis in the second six hours. Beyond this point in time, the morbidly obese remained in metabolic acidosis through the 48 hours studied, while patients in the other groups resolved. This difference could not be explained solely by differences in lactic acid, as groups were similar in lactate content over all time periods studied with the exception of the six-hour time period between 13 and 18 hours, during which morbidly obese patients showed a significantly greater blood lactate concentration than did normal weight patients (Figure 7). As acidosis may be representative of cellular hypoxia due to a diminished oxygen carrying capacity, we assessed hemoglobin levels, finding that obese and morbidly obese patients showed greater lowest hemoglobin concentrations in the emergency department and in the first 24 hours following injury when compared to normal and overweight patients (Table 5). We were also concerned about the possibility that this phenomenon might be a reflection of early renal failure development; however, comparison of creatinine levels during the first 48 hours demonstrated that only normal weight patients differed significantly from the other groups, suggesting similar renal function between overweight, obese, and morbidly obese patients (Table 6).
Figure 5.
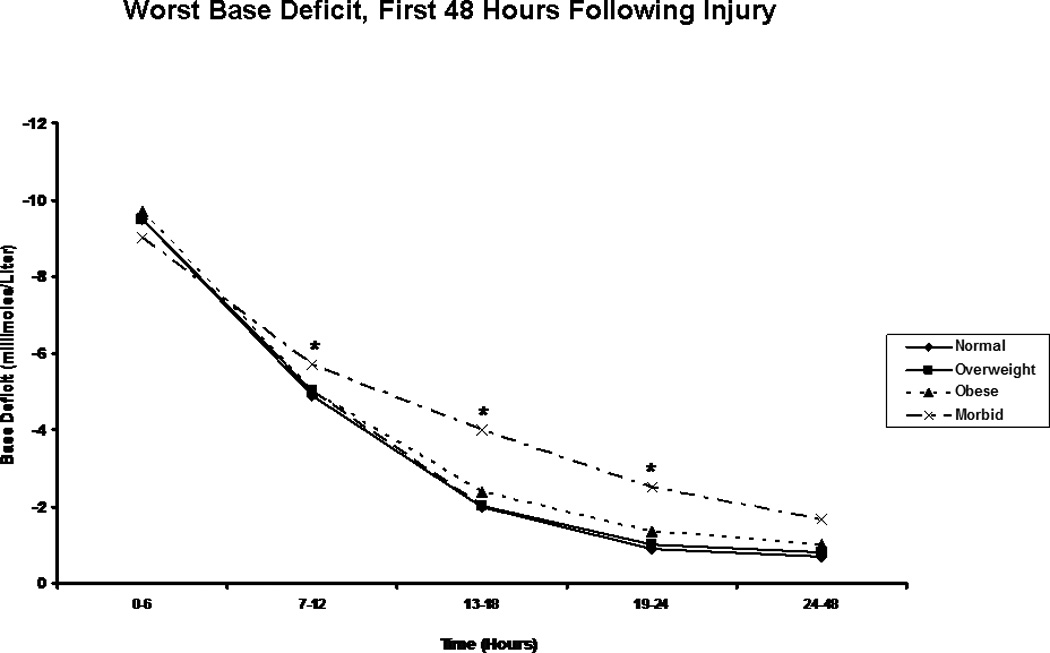
Worst Base Deficit. The figure depicts the median worst base deficit reading for patients in each body mass index classification during the first 48 hours following injury. * indicates a significantly greater base deficit than normal weight patients at the specified time point.
Figure 6.
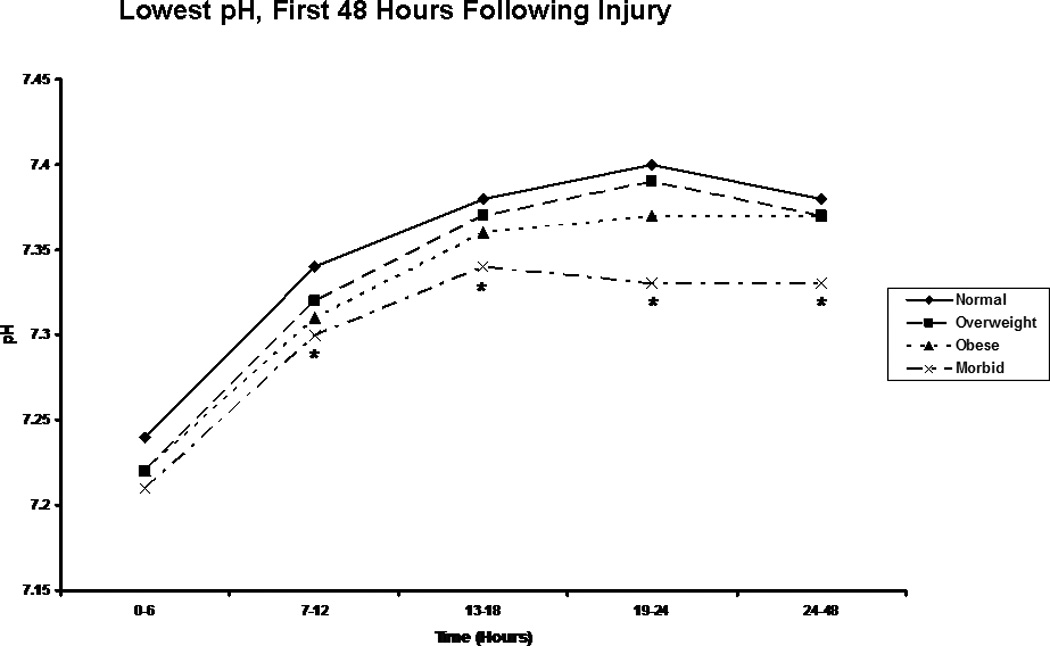
Lowest pH. The figure depicts the median worst pH reading for patients in each body mass index classification during the first 48 hours following injury. * indicates a significantly lower pH than normal weight patients at the specified time point.
Table 4.
Composite Acid-Base Status of Study Groups
| Time (Hours) | ||||||
|---|---|---|---|---|---|---|
| BMI Class | Parameter | 0–6 | 7–12 | 13–18 | 19–24 | 24–48 |
| Normal | pH | 7.24 | 7.34 | 7.38 | 7.40 | 7.38 |
| pCO2 | 39 | 38 | 38 | 39 | 40 | |
| pCO2 Exp | 34 | 38 | 41 | 43 | 44 | |
| HCO3 | 17 | 20 | 22 | 23 | 24 | |
| Acidosis | Mixed | Metabolic | None | None | None | |
| Overweight | pH | 7.22 | 7.32 | 7.37 | 7.39 | 7.37 |
| pCO2 | 41 | 40 | 40 | 39 | 42 | |
| pCO2 Exp | 34 | 38 | 41 | 43 | 44 | |
| HCO3 | 17 | 20 | 22 | 23 | 24 | |
| Acidosis | Mixed | Metabolic | None | None | None | |
| Obese | pH | 7.22 | 7.31 | 7.36 | 7.37 | 7.37 |
| pCO2 | 43 | 40 | 40 | 40 | 42 | |
| pCO2 Exp | 34 | 38 | 41 | 43 | 44 | |
| HCO3 | 17 | 20 | 22 | 23 | 24 | |
| Acidosis | Mixed | Metabolic | None | None | None | |
| Morbid | pH | 7.21 | 7.30 | 7.34 | 7.33 | 7.33 |
| pCO2 | 45 | 41 | 40 | 42 | 44 | |
| pCO2 Exp | 35 | 38 | 39 | 43 | 43 | |
| HCO3 | 18 | 20 | 21 | 23 | 23 | |
| Acidosis | Mixed | Metabolic | Metabolic | Metabolic | Metabolic | |
Figure 7.
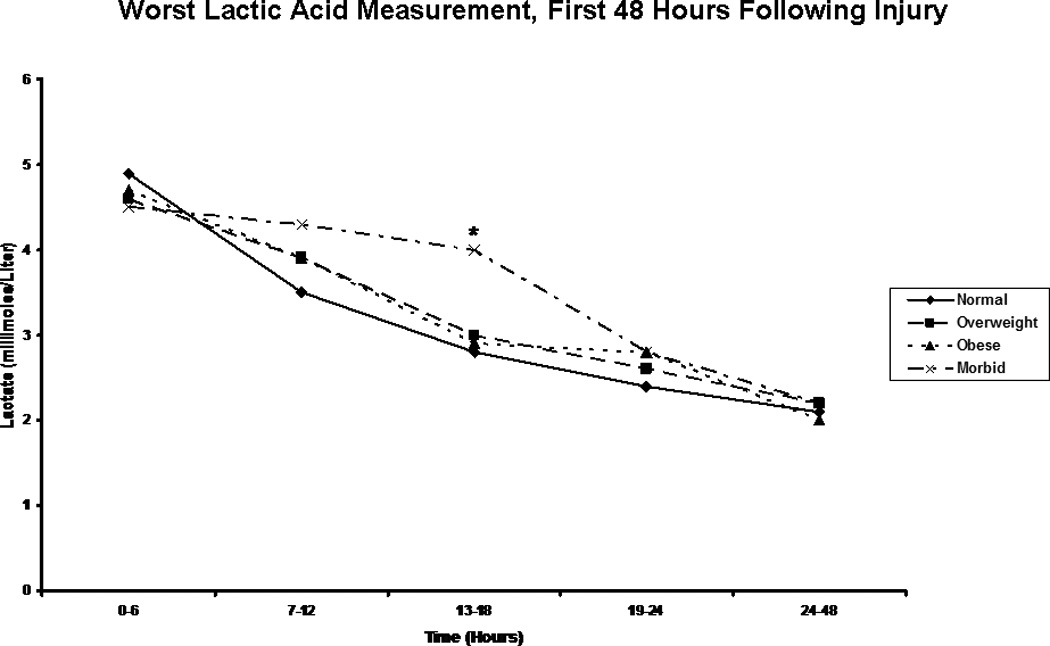
Highest Lactic Acid Level. The figure depicts the median worst lactic acid measurement for patients in each body mass index classification during the first 48 hours following injury. * indicates a significantly higher lactic acid level than normal weight patients at the specified time point.
Table 5.
Lowest Hemoglobin Concentrations of Study Groups
| Normal | Overweight | Obese | Morbid | p-value | |
|---|---|---|---|---|---|
| Emergency Department | 9.1 | 9.7 | 10.1 | 10.3 | p=0.009 |
| 0 – 24 Hours | 7.3 | 7.6 | 8.0 | 8.1 | p<0.001 |
| 24 – 48 Hours | 8.9 | 9.0 | 9.0 | 8.9 | p=0.634 |
Table 6.
Creatinine Concentrations of Study Groups
| Normal | Overweight | Obese | Morbid | p-value | |
|---|---|---|---|---|---|
| Creatinine, 0 – 24 Hours | 1.0 | 1.1 | 1.1 | 1.1 | p<0.001 |
| Creatinine, 24 – 48 Hours | 0.8 | 0.9 | 0.9 | 0.9 | p<0.001 |
Association of prolonged acidosis with multiple organ failure
We were interested in determining whether or not a relationship existed between the prolonged acidosis seen in the morbidly obese patient and the development of multiple organ failure. We first examined and classified patients by acid base status at 48 hours by looking at individual blood gas components for each patient. Unsurprisingly, a significantly greater proportion of morbidly obese patients were still in a state of metabolic acidosis at 48 hours when compared to patients in other BMI groups; interestingly, though, the proportion of patients in respiratory acidosis was similar (Table 7). When patients with persistent metabolic acidosis were compared in terms of proportion developing multiple organ failure, morbidly obese patients were once again found to have the highest proportion of patients with this complication (89%), and this difference was significant when compared to normal weight patients with (55%, p<0.001) or without (49%, p<0.001) persistent metabolic acidosis (Figure 8). Morbidly obese patients with persistent metabolic acidosis also showed a greater propensity toward multiple organ failure than did morbidly obese patients who resolved their acidosis (73%); however, this difference was not statistically significant.
Table 7.
Acid Base Status by BMI Class at 48 Hours Post-Injury
| Normal (N=253) |
Overweight (N=239) |
Obese (N=194) |
Morbid (N=45) |
p-value | |
|---|---|---|---|---|---|
| Metabolic | 15.8% | 22.6% | 16.5% | 40.0% | p<0.001 |
| Respiratory | 9.1% | 14.6% | 11.9% | 11.1% | p=0.299 |
| Resolved | 75.1% | 62.8% | 71.6% | 48.9% | p<0.001 |
Figure 8.
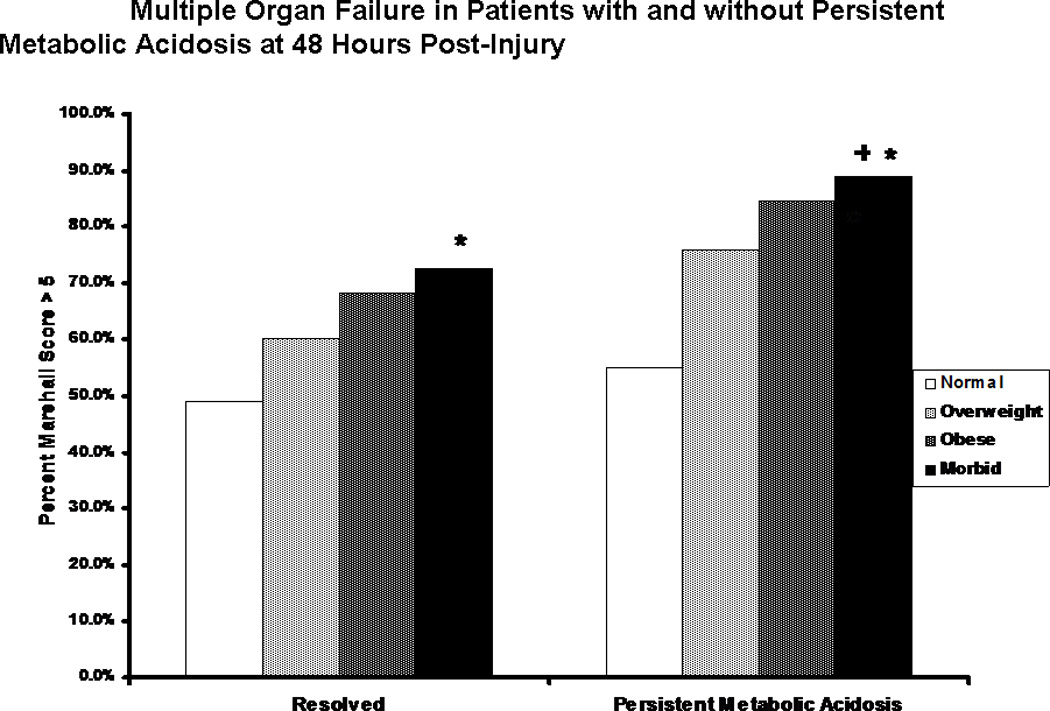
Multiple Organ Failure in patients with and without persistent metabolic acidosis. The proportion of patients developing multiple organ failure is shown for patients in each body mass index classification who showed (right) or did not show (left) persistent metabolic acidosis at 48 hours following injury. * indicates significantly greater proportion of patients developing multiple organ failure when compared to non-acidotic normal weight patients. + indicates significantly greater proportion of patients developing multiple organ failure when compared to acidotic normal weight patients.
Discussion
Resuscitation and differential responses of obese trauma patients have been addressed previously in the trauma literature. Belzberg and colleagues at Los Angeles County Trauma Center examined traditionally used hemodynamic parameters as well as transcutaneous oxygen and carbon dioxide measurement to assess resuscitation and outcome in obese trauma patients [26]. In their report, they evaluated 131 patients with a BMI greater than 30, demonstrating decreased cardiac index and reduced tissue oxygenation in 18 obese patients who expired when compared to 113 obese survivors. Furthermore, they demonstrated that the obese survivors showed overall lower arterial oxygen saturation, oxygen delivery, and tissue oxygenation but significantly higher heart rates than did 407 nonobese survivors. Their results suggest that obese patients would benefit from goal-directed resuscitative therapy; however, the authors indicate that the optimal physiologic endpoints have not yet been determined in this patient population, and that additional work is needed to define these endpoints and may need to include nontraditional monitoring techniques.
We concur, and feel that our work is supportive of the concepts brought forward by Belzberg et al. Through evaluation of traditional biomarkers, we have demonstrated that resuscitation in obese patients requires further scrutiny. Our analysis of traditionally used cardiovascular endpoints demonstrates relatively minimal differences between patients of different BMI classes, with the exception of central venous pressure readings, in which increasing BMI was found to be associated with greater pressure measurements. This interesting finding demonstrates the pitfalls of using traditional endpoints to measure resuscitation in this population of patients. Obese patients have been previously found to have alterations in stroke volume and cardiac output as a result of “obesity cardiomyopathy” [25], and a decrease in peripheral vascular resistance at baseline [24]. Furthermore, obesity decreases intrathoracic volumes with a simultaneous increase in intrathoracic pressure as a result of increased intraabdominal pressure [31]. All of these factors influence the central venous pressure measurement [32], provide plausible explanations for the potential fallaciously elevated levels that we have noted, and cast doubt on the usefulness of this measurement in obese trauma patients. Spurious elevations in central venous pressure due to these variations in cardiac and respiratory physiology and body habitus influence clinical decision-making, particularly with regard to patient volume status, and lead us to believe that the morbidly obese are routinely under resuscitated.
This notion is supported strongly in our data by the slower resolution of base deficit and pH in this patient population, and we feel that the resolution of pH in particular is suggestive of differential resuscitation, as we see clear BMI-related trajectories in this measurement over time. These differential changes in base deficit and pH are likely indicative of a state of prolonged cellular hypoxia following the acute insult brought on by resuscitation carried out to incorrect endpoints. While this is almost certainly true, it is curious that a traditionally used measure of cellular hypoxia, arterial lactic acid, only showed an intergroup difference at one time point during the first 48 hours of resuscitation. Our study and its conclusions are limited by the fact that the database utilized lacks information on patient cation and anion concentrations. This is potentially significant for two reasons: first, our findings may represent hyperchloremic metabolic acidosis; and second, it prevents us from calculating the strong ion difference. Although we can not definitively rule out the possibility of hyperchloremic metabolic acidosis, this is unlikely to be the reason in this particular study as patients were treated according to a standardized resuscitation protocol with similar crystalloid administration. Furthermore, we were unable to calculate the strong ion difference utilizing this data set and can not decisively state which components of acid-base balance account for the differences in resolution of pH; however, we speculate that the finding is due to differences in inorganic acid levels between the groups, as supported by the work of previous investigators indicating that metabolic acidosis is more dependent on these unmeasured inorganic acids than on lactic acid [33–35]. We are particularly intrigued by the recent work of Forni and colleagues and separately by Moviat et al and Bruegger et al, which suggests that the inorganic acids of greatest interest are Krebs cycle intermediates [33, 34, 36, 37], as this would be supportive of our previous finding that differences in citric acid cycle gene expression are seen in patients of different BMI [27]. This proposed mechanism would fit well into the picture of global metabolic dysfunction present in the morbidly obese, and represents a promising area for future metabolomic research that would complement studies of resuscitative adequacy in this patient population.
Regardless of the specific mechanism, we have also demonstrated that patients with prolonged acidosis develop multiple organ failure at a greater rate than do patients without this complication, with nearly 90% of morbidly obese patients having persistent metabolic acidosis developing multiple organ failure. This is in stark comparison to normal weight patients without persistent metabolic acidosis, of whom less than half developed MOF, and although not significant, represents an increase over morbidly obese patients not remaining in metabolic acidosis at 48 hours. This is not particularly surprising given the consequences of acidosis, including possible cardiorespiratory depression [38, 39], immune dysfunction [40–43], and further alterations of cellular metabolism [44, 45]; however, it does highlight the importance of determining the causes of acidosis, particularly in the high risk morbidly obese patient cohort.
Our major finding is a straightforward one: morbidly obese patients show prolonged metabolic acidosis in severe blunt trauma. This is a novel finding that we feel is symptomatic of suboptimal resuscitation dependent on inadequate endpoints combined with underlying metabolic abnormalities in this patient population. Given what is known about the abnormal physiology and biochemistry of the obese and morbidly obese patient, and about the causes and effects of acidosis, we feel this finding warrants further investigation, as it may represent a marker of organ dysfunction or a target for therapy in this high risk patient cohort.
Acknowledgments
RDW was supported by a T32 training grant (T32 CA106493-02) in surgical oncology from the National Cancer Institute, NIH. MJD was supported by a T32 training grant (T32 GM-08421) in trauma and burns from the National Institute of General Medical Sciences, NIH
The “Inflammation and the Host Response to Injury” project is fully funded by a grant (U54 GM062119-1) from the National Institute of General Medical Sciences awarded to Dr. Ronald G. Tompkins, Massachusetts General Hospital.
Contributor Information
Robert D. Winfield, Email: robert.winfield@surgery.ufl.edu.
Matthew J. Delano, Email: delanmj@surgery.ufl.edu.
Lawrence Lottenberg, Email: lawrence.lottenberg@surgery.ufl.edu.
Juan C. Cendan, Email: cendajc@surgery.ufl.edu.
Lyle L. Moldawer, Email: lyle.moldawer@surgery.ufl.edu.
Ronald V. Maier, Email: ronmaier@u.washington.edu.
References
- 1.World Health Organization. Obesity and Overweight, Fact Sheet No. 311. 2006 Sep; 2006 [cited 2007 November 6]; Available from: http://www.who.int/mediacentre/factsheets/fs311/en/index.html.
- 2.U.S. Department of Health and Human Services Weight Control Information Network. Statistics Related to Overweight and Obesity. 2007 [cited 2007 November 6]; Available from: http://win.niddk.nih.gov/statistics/index.htm.
- 3.Kopelman P. Health risks associated with overweight and obesity. Obes Rev. 2007;8(Suppl 1):13–17. doi: 10.1111/j.1467-789X.2007.00311.x. [DOI] [PubMed] [Google Scholar]
- 4.Bochicchio GV, Joshi M, Bochicchio K, et al. Impact of obesity in the critically ill trauma patient: a prospective study. J Am Coll Surg. 2006;203(4):533–538. doi: 10.1016/j.jamcollsurg.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 5.Brown CV, Neville AL, Rhee P, et al. The impact of obesity on the outcomes of 1,153 critically injured blunt trauma patients. J Trauma. 2005;59(5):1048–1051. doi: 10.1097/01.ta.0000189047.65630.c5. discussion 1051. [DOI] [PubMed] [Google Scholar]
- 6.Brown CV, Neville AL, Salim A, et al. The impact of obesity on severely injured children and adolescents. J Pediatr Surg. 2006;41(1):88–91. doi: 10.1016/j.jpedsurg.2005.10.012. discussion 88-91. [DOI] [PubMed] [Google Scholar]
- 7.Brown CV, Rhee P, Neville AL, et al. Obesity and traumatic brain injury. J Trauma. 2006;61(3):572–576. doi: 10.1097/01.ta.0000200842.19740.38. [DOI] [PubMed] [Google Scholar]
- 8.Byrnes MC, McDaniel MD, Moore MB, et al. The effect of obesity on outcomes among injured patients. J Trauma. 2005;58(2):232–237. doi: 10.1097/01.ta.0000152081.67588.10. [DOI] [PubMed] [Google Scholar]
- 9.Choban PS, Weireter LJ, Jr, Maynes C. Obesity and increased mortality in blunt trauma. J Trauma. 1991;31(9):1253–1257. doi: 10.1097/00005373-199109000-00009. [DOI] [PubMed] [Google Scholar]
- 10.Ciesla DJ, Moore EE, Johnson JL, et al. Obesity increases risk of organ failure after severe trauma. J Am Coll Surg. 2006;203(4):539–545. doi: 10.1016/j.jamcollsurg.2006.06.029. [DOI] [PubMed] [Google Scholar]
- 11.Duane TM, Dechert T, Aboutanos MB, et al. Obesity and outcomes after blunt trauma. J Trauma. 2006;61(5):1218–1221. doi: 10.1097/01.ta.0000241022.43088.e1. [DOI] [PubMed] [Google Scholar]
- 12.Neville AL, Brown CV, Weng J, et al. Obesity is an independent risk factor of mortality in severely injured blunt trauma patients. Arch Surg. 2004;139(9):983–987. doi: 10.1001/archsurg.139.9.983. [DOI] [PubMed] [Google Scholar]
- 13.Newell MA, Bard MR, Goettler CE, et al. Body mass index and outcomes in critically injured blunt trauma patients: weighing the impact. J Am Coll Surg. 2007;204(5):1056–1061. doi: 10.1016/j.jamcollsurg.2006.12.042. [DOI] [PubMed] [Google Scholar]
- 14.Axelsson J, Heimburger O, Lindholm B, et al. Adipose tissue and its relation to inflammation: the role of adipokines. J Ren Nutr. 2005;15(1):131–136. doi: 10.1053/j.jrn.2004.09.034. [DOI] [PubMed] [Google Scholar]
- 15.Hotamisligil GS, Arner P, Caro JF, et al. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J Clin Invest. 1995;95(5):2409–2415. doi: 10.1172/JCI117936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259(5091):87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 17.Rondinone CM. Adipocyte-derived hormones, cytokines, and mediators. Endocrine. 2006;29(1):81–90. doi: 10.1385/endo:29:1:81. [DOI] [PubMed] [Google Scholar]
- 18.Tilg H, Moschen AR. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol. 2006;6(10):772–783. doi: 10.1038/nri1937. [DOI] [PubMed] [Google Scholar]
- 19.Alaud-din A, Meterissian S, Lisbona R, et al. Assessment of cardiac function in patients who were morbidly obese. Surgery. 1990;108(4):809–818. discussion 818-20. [PubMed] [Google Scholar]
- 20.Qureshi K, Abrams GA. Metabolic liver disease of obesity and role of adipose tissue in the pathogenesis of nonalcoholic fatty liver disease. World J Gastroenterol. 2007;13(26):3540–3553. doi: 10.3748/wjg.v13.i26.3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith SC., Jr Multiple risk factors for cardiovascular disease and diabetes mellitus. Am J Med. 2007;120(3 Suppl 1):S3–S11. doi: 10.1016/j.amjmed.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 22.Vgontzas AN, Tan TL, Bixler EO, et al. Sleep apnea and sleep disruption in obese patients. Arch Intern Med. 1994;154(15):1705–1711. [PubMed] [Google Scholar]
- 23.Forman-Hoffman V, Little A, Wahls T. Barriers to obesity management: a pilot study of primary care clinicians. BMC Fam Pract. 2006;7:35. doi: 10.1186/1471-2296-7-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thakur V, Richards R, Reisin E. Obesity, hypertension, and the heart. Am J Med Sci. 2001;321(4):242–248. doi: 10.1097/00000441-200104000-00005. [DOI] [PubMed] [Google Scholar]
- 25.Wong C, Marwick TH. Obesity cardiomyopathy: pathogenesis and pathophysiology. Nat Clin Pract Cardiovasc Med. 2007;4(8):436–443. doi: 10.1038/ncpcardio0943. [DOI] [PubMed] [Google Scholar]
- 26.Belzberg H, Wo CC, Demetriades D, et al. Effects of age obesity on hemodynamics, tissue oxygenation, and outcome after trauma. J Trauma. 2007;62(5):1192–1200. doi: 10.1097/01.ta.0000219701.07295.b8. [DOI] [PubMed] [Google Scholar]
- 27.Winfield RD, Delano MJ, Dixon DJ, et al. Differences in outcome between obese and nonobese patients following severe blunt trauma are not consistent with an early inflammatory genomic response. Crit Care Med. 2009 doi: 10.1097/CCM.0b013e3181b08089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hallynck T, Pijck J, Soep H. Influence of age and lean body mass on the relation of creatinine clearance and drug half-life. Arch Int Pharmacodyn Ther. 1982;259(2):330–332. [PubMed] [Google Scholar]
- 29.Pai MP, Paloucek FP. The origin of the "ideal" body weight equations. Ann Pharmacother. 2000;34(9):1066–1069. doi: 10.1345/aph.19381. [DOI] [PubMed] [Google Scholar]
- 30.Marshall JC, Cook DJ, Christou NV, et al. Multiple organ dysfunction score: a reliable descriptor of a complex clinical outcome. Crit Care Med. 1995;23(10):1638–1652. doi: 10.1097/00003246-199510000-00007. [DOI] [PubMed] [Google Scholar]
- 31.Zerah F, Harf A, Perlemuter L, et al. Effects of obesity on respiratory resistance. Chest. 1993;103(5):1470–1476. doi: 10.1378/chest.103.5.1470. [DOI] [PubMed] [Google Scholar]
- 32.Magder S. Central venous pressure monitoring. Curr Opin Crit Care. 2006;12(3):219–227. doi: 10.1097/01.ccx.0000224866.01453.43. [DOI] [PubMed] [Google Scholar]
- 33.Moviat M, Terpstra AM, Ruitenbeek W, et al. Contribution of various metabolites to the "unmeasured" anions in critically ill patients with metabolic acidosis. Crit Care Med. 2008;36(3):752–758. doi: 10.1097/CCM.0B013E31816443CB. [DOI] [PubMed] [Google Scholar]
- 34.Moviat MA, Pickkers P, Ruitenbeek W, et al. The nature of unmeasured anions in critically ill patients. Crit Care. 2008;12(2):416. doi: 10.1186/cc6855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martin M, Murray J, Berne T, et al. Diagnosis of acid-base derangements and mortality prediction in the trauma intensive care unit: the physiochemical approach. J Trauma. 2005;58(2):238–243. doi: 10.1097/01.ta.0000152535.97968.4e. [DOI] [PubMed] [Google Scholar]
- 36.Forni LG, McKinnon W, Lord GA, et al. Circulating anions usually associated with the Krebs cycle in patients with metabolic acidosis. Crit Care. 2005;9(5):R591–R595. doi: 10.1186/cc3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bruegger D, Kemming GI, Jacob M, et al. Causes of metabolic acidosis in canine hemorrhagic shock: role of unmeasured ions. Crit Care. 2007;11(6):R130. doi: 10.1186/cc6200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Solaro RJ, Lee JA, Kentish JC, et al. Effects of acidosis on ventricular muscle from adult and neonatal rats. Circ Res. 1988;63(4):779–787. doi: 10.1161/01.res.63.4.779. [DOI] [PubMed] [Google Scholar]
- 39.Weiss J, Couper GS, Hiltbrand B, et al. Role of acidosis in early contractile dysfunction during ischemia: evidence from pHo measurements. Am J Physiol. 1984;247(5 Pt 2):H760–H767. doi: 10.1152/ajpheart.1984.247.5.H760. [DOI] [PubMed] [Google Scholar]
- 40.Kellum JA, Song M, Li J. Science review: extracellular acidosis and the immune response: clinical and physiologic implications. Crit Care. 2004;8(5):331–336. doi: 10.1186/cc2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kellum JA, Song M, Li J. Lactic and hydrochloric acids induce different patterns of inflammatory response in LPS-stimulated RAW 264.7 cells. Am J Physiol Regul Integr Comp Physiol. 2004;286(4):R686–R692. doi: 10.1152/ajpregu.00564.2003. [DOI] [PubMed] [Google Scholar]
- 42.Kellum JA, Song M, Venkataraman R. Effects of hyperchloremic acidosis on arterial pressure and circulating inflammatory molecules in experimental sepsis. Chest. 2004;125(1):243–248. doi: 10.1378/chest.125.1.243. [DOI] [PubMed] [Google Scholar]
- 43.Lardner A. The effects of extracellular pH on immune function. J Leukoc Biol. 2001;69(4):522–530. [PubMed] [Google Scholar]
- 44.Busa WB, Nuccitelli R. Metabolic regulation via intracellular pH. Am J Physiol. 1984;246(4 Pt 2):R409–R438. doi: 10.1152/ajpregu.1984.246.4.R409. [DOI] [PubMed] [Google Scholar]
- 45.Hollidge-Horvat MG, Parolin ML, Wong D, et al. Effect of induced metabolic acidosis on human skeletal muscle metabolism during exercise. Am J Physiol. 1999;277(4 Pt 1):E647–E658. doi: 10.1152/ajpendo.1999.277.4.E647. [DOI] [PubMed] [Google Scholar]


