Abstract
Background
FDA approval of long-acting injectable naltrexone (Vivitrol) for opioid dependence highlights the relevance of understanding mechanisms of antagonist treatment. Principles of learning suggest an antagonist works through extinguishing drug-seeking behavior, as episodes of drug use (“testing the blockade”) fail to produce reinforcement.
We hypothesized that opiate use would moderate the effect of naltrexone, specifically, that opiate-positive urines precede dropout in the placebo group, but not in the active-medication groups.
Methods
An 8-week, double-blind, placebo-controlled trial (N=57), compared the efficacy of low (192-mg) and high (384-mg) doses of a long-acting injectable naltrexone (Depotrex) with placebo (Comer et al., 2006). A Cox proportional hazard model was fit, modeling time-to-dropout as a function of treatment assignment and urine toxicology during treatment.
Results
Interaction of opiate urines with treatment group was significant. Opiate-positive urines predicted dropout on placebo and low-dose, but less so on high-dose naltrexone, where positive urines were more likely followed by sustained abstinence. Among patients with no opiate-positive urines, retention was higher in both low- and high-dose naltrexone conditions, compared to placebo.
Conclusions
Findings confirm that injection naltrexone produces extinction of drug-seeking behavior after episodes of opiate use. Adequate dosage appears important, as low-dose naltrexone resembled the placebo group; opiate positive urines were likely to be followed by dropout from treatment. The observation of high treatment retention among naltrexone-treated patients who do not test the blockade, suggests naltrexone may also exert direct effects on opiate-taking behavior that do not depend on extinction, perhaps by attenuating craving or normalizing dysregulated hedonic or neuroendocrine systems.
Keywords: Long-acting injectable naltrexone, Opiate dependence, Opioid antagonist, Treatment retention, Urine toxicology
1. INTRODUCTION
The opioid receptor antagonist naltrexone has been available in pill form since the 1980s for treatment of opiate dependence, but was largely ineffective due to poor adherence to daily pill regimen. The advent of long-acting injections (Comer et al., 2006) and implants (Hulse et al., 2009) of naltrexone circumvents the problem of adherence to daily pill taking, and has shown considerable promise as a treatment. A monthly intramuscular injection of naltrexone (trade name Vivitrol) has received FDA approval for treatment of opioid dependence, based on a pivotal 6-month placebo controlled trial, which showed that over 50% of opioid-dependent patients randomized to Vivitrol were retained in treatment and predominantly abstinent for the full 6 months (Krupitsky et al., 2011).
The advent of long-acting formulations of naltrexone makes it possible to address questions about the mechanism of action of antagonist treatment. The immediate pharmacological effect of naltrexone is potent blockade of the subjective and reinforcing effects of even substantial doses of opiates (Comer et al., 2002, Sullivan et al., 2006). Principles of learning implicated in the mechanisms of addiction suggest an antagonist would reduce opiate use through extinction, as episodes of drug use (“testing the blockade”) fail to produce reinforcement or other unconditioned responses. Clinical experience suggests some opiate-dependent patients treated with naltrexone do test the blockade repeatedly before establishing sustained abstinence. However, others seem to remain abstinent throughout, never testing the blockade, or report using opiates only once, suggesting the extinction of opiate use after one trial (typical report: “I tried heroin once, and nothing happened, so I realized I was wasting my money”). This raises questions regarding the mechanism of antagonist treatment for opiate dependence: 1) Does the effectiveness of antagonist treatment depend upon episodes of blocked use and consequent extinction? 2) Do some patients stop using opiates because of a placebo or expectancy effect--the expectation of blockade? Or, 3) Does naltrexone exert a direct effect on opiate use reduction that does not depend on extinction over episodes of use, by blocking the effect of conditioned cues or influencing the neural mechanisms of craving and relapse?
A previously completed double-blind, placebo-controlled trial, which demonstrated the efficacy of a long-acting injectable formulation of naltrexone (Comer et al., 2006), affords an opportunity to address these questions. Because patients and staff were blind to treatment condition, effects of expectancy should have occurred equally in the placebo and active medication groups. Urine was collected twice per week during the 8-week trial and tested for opiates. To examine the role of blocked use on the clinical effect of naltrexone, we employed data from this trial, using information on the presence/absence of one or more opiate-positive urine test results, treatment assignment (placebo, vs. low-dose naltrexone, vs. high-dose naltrexone), and retention in treatment. Primary outcome was retention in treatment since treatment dropout is most commonly associated with relapse to opiate use, and naltrexone was found to have a dose-dependent effect on treatment retention (Comer et al., 2006).
This analysis examines whether the treatment arms followed different patterns of survival for those who tested the opiate blockade compared to those who did not test. We considered the possibility of a dose-dependent therapeutic effect of naltrexone through a mechanism that is independent of its block of the opioid receptors. In the absence of any information as to whether, and to what degree, they could feel opiate effects during the study, non-testing participants who drop out and eventually relapse must be presumed to be influenced by other factors such as withdrawal or craving. If the naltrexone ‘non-testing’ groups had higher survival probabilities than the placebo ‘non-testing’ group, this outcome supports the possibility that naltrexone exerts a beneficial effect that does not rely on receptor blockade. We hypothesized that opiate use (positive urines) during treatment would moderate the favorable effect of naltrexone, a use-by-treatment interaction. Specifically, we expected that episodes of opiate use would precede dropout in the placebo group, but not in the high-dose naltrexone group, with the low-dose group showing an intermediate outcome.
2. METHODS
2.1 Participants
Details of the methods and sample have been reported previously (Comer et al., 2006). Briefly, participants were 57 men and women (age 18-59 years) meeting DSM-IV criteria for heroin dependence and seeking treatment at one of two university medical centers. Recruitment was through word-of-mouth and advertising in local newspapers. To be eligible, patients were required to be in good general health, based on psychiatric and medical history, physical examination, routine laboratory tests, and electrocardiogram. Patients were excluded from the study if they were dependent on methadone or on drugs other than heroin, nicotine, or caffeine (based on DSM-IV criteria), pregnant or lactating, unwilling to use a satisfactory method of birth control, currently diagnosed with major DSM-IV Axis I psychopathology that could interfere with study participation (e.g., mood disorder with functional impairment, schizophrenia), significant risk of suicide, or regular use of psychotropic medications. Acute or severe hepatitis was exclusionary, but patients with moderate liver enzyme elevations (SGOT or SGPT less than three times the upper end of the normal range) were eligible. The study was approved by the Institutional Review Boards of the New York State Psychiatric Institute and the University of Pennsylvania, and all participants gave written informed consent.
2.2 Study Design
This was a two-site, randomized, double-blind, placebo-controlled 8-week trial. Participants received an inpatient detoxification, followed by 3 days of ascending doses of oral naltrexone to ensure tolerability. Participants were then randomized to receive injections of either placebo, low dose-192 mg or high dose-384 mg of depot naltrexone (Depotrex; BIOTEK, Inc.) and discharged to outpatient treatment. Four weeks later, patients received a second injection. Depotrex is a subcutaneous injection of a suspension of polymer microspheres (Nuwayser et al., 1990), which at the 384 mg dose, produces a pharmacokinetic profile of naltrexone and the active metabolite 6-beta naltrexol (Comer et al., 2002) similar to that of Vivitrol (Dunbar et al., 2006), and produces dose-dependent blockade of the effects of up to 25 mg of intravenous heroin for at least 4 weeks (Comer et al., 2002). Participants were asked to attend the outpatient clinic twice per week during the 8-week outpatient trial. At each visit patients received manual-guided relapse prevention therapy, completed assessments, and provided a urine sample under staff observation, which was tested for opiates as well as other drugs.
2.3 Data Analysis
The primary outcome measure for the present analysis is retention in treatment, analyzed as a time-to-event (dropout) variable with survival analysis. Retention is arguably the most clinically meaningful outcome measure for this population, because dropout from treatment is the most common failure mode among outpatients under treatment for opiate dependence; patients who drop out of treatment have most likely relapsed. A Cox proportional hazard model was fit, modeling biweekly visits retained in treatment as a function of treatment assignment (placebo, low dose-192 mg naltrexone, high dose-384mg naltrexone), the presence of one or more positive urine toxicology results during treatment as a dichotomous covariate, and the interaction of treatment and urine toxicology. To assess the overall interaction, the type 3 test with 2 degrees of freedom was employed at the significance level of 0.05. Once the overall interaction was found significant, subgroup analysis was conducted at the significance level of 0.05. Proportional hazards assumption was assessed and validated in the final model. PROC PHREG in SAS was used to conduct these analyses (SAS, 2012).
While we originally (Comer et al. 2006) reported an N of 60 participants, three (3/60) randomized participants did not provide any urine data and were excluded from this analysis. In addition, one participant with consistently opiate-positive urines in the last visits of the study was counted as completing Visit 16 because we measured treatment retention regardless of urine result. These differences account for the fact that retention figures reported here are slightly higher than those reported in the original paper (Comer et al. 2006).
3. RESULTS
3.1 Participants
Of the sample randomized (N = 57), 77% were men, 37% were Caucasian, 35% were African-American, and 18% were Hispanic. The mean age was 41 (s.d. 11) years. The mean number of years of heroin use in this sample was 13.9 (s.d. 11.3). The distributions of sex, age, race and measures of lifetime and past 30-day drug use were not significantly different among the three treatment conditions.
3.2 Naltrexone, During-Treatment Opiate Use, and Retention in Treatment
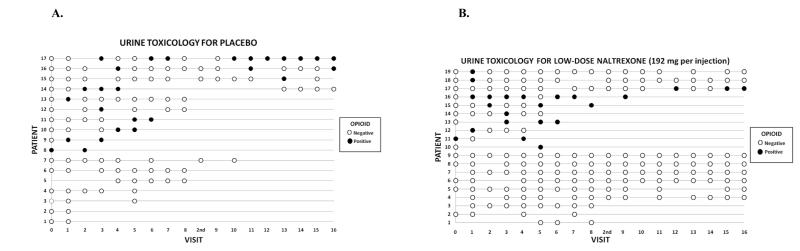
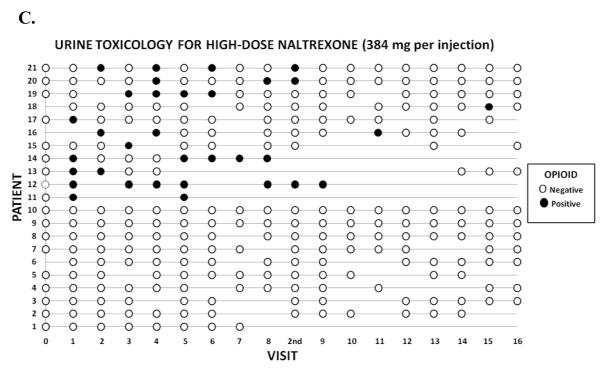
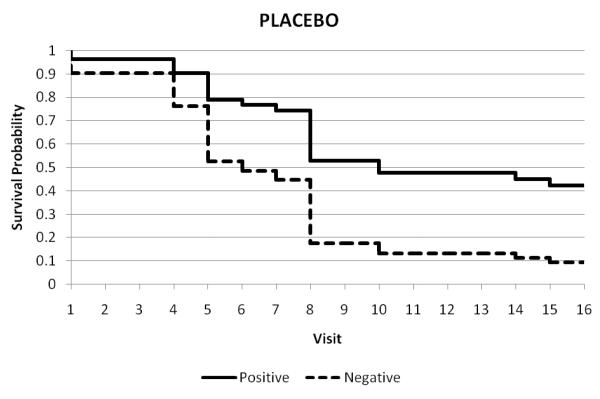
Figures 1A, 1B, and 1C display the raw data for the relationships between during-treatment opiate use and retention in treatment in the placebo (Figure 1A), low dose naltrexone-192mg (Figure 1B), and high dose naltrexone-384 mg (Figure 1C) conditions. Each row in the figures represents the data for a patient, and the columns represent each of the 16 twice-weekly clinic visits at which urine was collected across the 8-week trial. There was also an initial visit at the time of hospital discharge post-injection (Visit 0) and an extra visit (“2nd”) to receive the second depot naltrexone injection, for a total of 17 visits (Visits 1-8 and 9-16). Open circles represent visit dates when the patient attended the clinic and the urine toxicology was negative for opiates, indicating the patient has most likely been abstinent since the previous visit. Closed (darkened) circles represent visit dates when the patient attended the clinic and the urine toxicology was positive for opiates, indicating the patient used opiates in the several days prior to the visit. Empty points in the grid represent visits in which the patient either failed to attend or did not provide a urine sample. The patient-rows are ordered in such a way that patients who gave one or more opiate-positive urines are represented in the upper rows of each figure, followed by patients who gave no opiate-positive urines in the lower rows.
Figure 1A-C.
Graphical display of the treatment course of opiate-dependent patients (N = 57) in an 8-week, double-blind, placebo-controlled trial of a long-acting injection of naltrexone, comparing placebo (Figure 1A; N = 17), low dose-192 mg naltrexone injection (Figure 1B; N = 19), and high dose-384 mg naltrexone injection (Figure 1B; N = 21). Each row in the figures represents the data for a patient, and the columns represent each of the 16 twice-weekly clinic visits at which urine was collected across the 8-week trial, plus an initial visit at the time of hospital discharge post-injection (Visit 0) and an extra visit (“2nd”) to receive the second depot naltrexone injection, for a total of 17 visits (Visits 1-8 and 9-16). Open circles represent visits where the patient was present and gave a urine sample negative for opiates. Filled (darkened) circles represent visits where the patient was present and gave an opiate-positive urine sample. Blanks (no circle) indicate that patient was not present at that visit or did not provide a urine sample.
Table 1 shows the Cox model for retention in treatment. Patients were scored as dropouts at the time of their last visit to the clinic, with those who completed treatment (attended the last visit, Visit 16, at the end of week 8) censored at that point. The placebo non-user group is the reference group, and coefficients for the two medication non-user groups are calculated in relation to the placebo non-user group. The overall interaction was found significant (Wald X 22 = 6.40, p = 0.041). As can be seen, the model yields both a main effect of medication in the direction of lower risk of dropout on naltrexone non-users compared to placebo non-users, and an interaction between opiate urine toxicology and medication assignment. The interaction is located in the low dose-192 mg naltrexone condition, indicating that an opiate-positive urine predicts dropout similar to placebo in the low-dose naltrexone condition, but not in the high dose-384 mg naltrexone condition.
Table 1.
Cox proportional hazards regression model, modeling time to dropout from treatment, as a function of naltrexone treatment assignment.
| Coefficient | Standard Error |
Chi-Square | p-value | |
|---|---|---|---|---|
| Low dose | −1.87 | 0.70 | 7.15 | 0.0075 |
| High Dose | −2.43 | 0.81 | 8.95 | 0.0028 |
| Urine | −1.01 | 0.56 | 3.21 | 0.073 |
| Low dose * urine |
2.22 | 0.89 | 6.17 | 0.013 |
| High dose * urine |
1.37 | 1.07 | 1.63 | 0.20 |
Placebo (N = 17) is the reference group, against which the low dose-192 mg (N = 19), and high dose-384 mg (N = 21) conditions are contrasted. Urine toxicology, measured at twice-weekly clinic visits, was scored as a dichotomous covariate in this analysis as positive if one or more urines was positive for opioids during the trial, and negative otherwise (negative is the reference group). Values in the table are the regression coefficients for each term in the model, corresponding significance levels, and the point estimate of the hazard ration and its 95% confidence limits. The significant low-dose naltrexone-by-urine interaction term, indicates that the effect of low-dose naltrexone differs between patients with vs. without a positive urine; when urine is positive dropout is similar (and high) on low-dose naltrexone and placebo, while when urines are all negative, dropout rate is low on low-dose naltrexone (compared to high dropout on placebo) and approaches the low dropout rates for the high-dose naltrexone condition.
The pattern detected by the model is illustrated by the descriptive data in Figure 1. In the placebo group (Figure 1A), and the low-dose naltrexone group (Figure 1B) opiate-positive urines (filled circles) at any point in a patient’s course tend to be followed subsequently by missed visits and dropout from treatment, whereas in the high naltrexone dose group (Figure 1C), patients with opiate-positive urines (filled circles) more often continue to attend visits and complete treatment, converting to sustained negative urines over time. When the effects of an episode of opiate use are not blocked (placebo), or not fully blocked (low-dose naltrexone), use leads to dropout. In contrast, blocked (high-dose naltrexone) use does not lead to dropout, but rather extinction and sustained abstinence.
Inspection of Figure 1 reveals that 7/17 (41%) of placebo patients refrained from testing the blockade, while 9/19 (47%) in the low-dose group never tested, and 10/21 (48%) of the high-dose naltrexone group never had an opiate-positive urine. Thus, while a similar fraction of patients in the placebo and each naltrexone condition do not test the blockade, placebo patients with no positive urines had a significantly higher dropout rate (100%) than naltrexone patients with no positive urines (32%). This is consistent with the possibility that naltrexone may exert some direct effect in reducing the tendency to use opiates, that does not depend on episodes of blocked use and extinction. Given the small sizes of the subgroups of non-testing participants who dropped out in the placebo, low-, and high-dose groups (n=7, n=3, and n=3 respectively), we must exercise caution regarding the importance assigned to this small number of observations.
Figure 1 also reveals that an opiate-positive urine at the last visit before dropout is less clearly related to dropout than is an opiate-positive urine at any time prior to dropout. This finding suggests that dropout is a process, i.e. propensity to test the blockade, during which patients struggle with relapse. Thus there is some time between the warning sign of an opiate-positive urine, and the subsequent dropout; it is during this interval that clinical measures can be implemented to try to avoid dropout. These findings are consistent with data we have previously reported on the management of relapse (Sullivan et al., 2007). Yet injectable naltrexone at the full dose seems to block, or at least attenuate, the adverse prognostic effect of an opiate-positive urine.
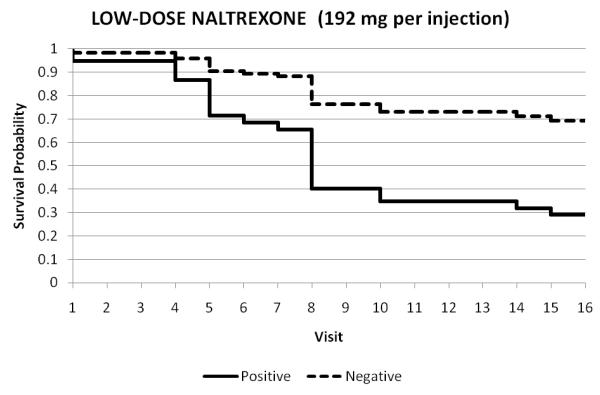
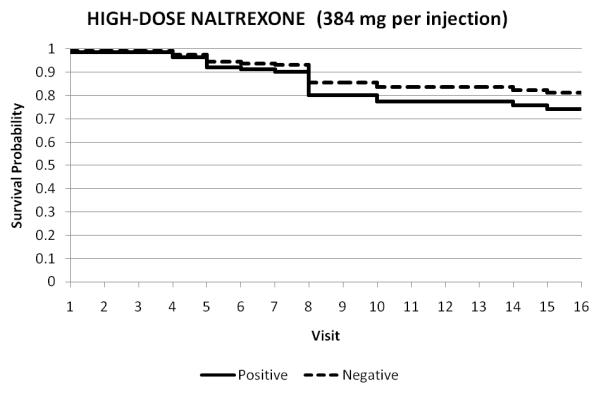
The modeled survival curves for the three treatment arms are represented in Figures 2A-C. These figures show retention under each treatment condition for those who tested the blockade vs. those who did not test. These survival data illustrate the interaction effect between opiate urine toxicology and treatment. Testing the opiate blockade resulted in lower retention at end of study for participants in either the placebo or low-dose (192-mg) naltrexone arms, but in the high-dose (384-mg) arm, the testing did not predict earlier dropout from treatment. The additional visit in Week 4 (2nd Depotrex administration) was not represented, as it was not included in measurement of retention across the 16 visits of this 8-week trial.
Figure 2A-C.
Treatment retention as a function of opiate status of urine toxicology throughout duration of trial: all opiate-negative (1) vs. any opiate-positive urine (0). Each figure represents the survival in twice weekly study visits over the 8-week trial for participants who tested the blockade (0) vs. those who did not test (1).
Testing the opiate blockade resulted in significantly lower retention at end of study for participants in either the placebo or low-dose (192-mg) naltrexone arms, but in the high-dose (384-mg) arm, the testing did not predict earlier dropout from treatment.
4. DISCUSSION
We used data from a placebo-controlled trial of a long-acting injectable formulation of naltrexone (Comer et al., 2006) to examine the mechanism of action of naltrexone as a treatment for opiate dependence. Urine toxicology for opiates, collected twice weekly, was examined as a covariate predicting dropout from treatment across the 8-week trial. As hypothesized, the interaction between opiate urine toxicology and treatment was significant. Opiate-positive urines predicted subsequent dropout from treatment in the placebo group and the low dose-192mg naltrexone group, while in the high-dose-384mg naltrexone condition, opiate-positive urines were less likely to lead to dropout; rather, in the high-dose naltrexone group patients tended to produce only one or a few positive urines, then achieve sustained abstinence. This finding is consistent with the expected mechanism of extinction through repeated trials of opiate use that are blocked by naltrexone--“testing the blockade.” It also suggests the importance of adequate dosage, as the low-dose naltrexone condition, which does not produce complete blockade (Comer et al., 2002), resembles the placebo group in that episodes of opiate use are likely to be followed by dropout. These survival data illustrate the interaction effect between opiate urine toxicology and treatment.
Perhaps the most interesting observation, however, was that naltrexone also appeared to exert a beneficial effect among patients who never gave a positive urine. Assuming this toxicology finding means that these patients used no opiates at all during the trial, the observation suggests that naltrexone exerts a beneficial effect that does not depend directly on episodes of blocked use and extinction. Since this trial was double-blinded, the findings would seem to rule out the explanation that this is an expectancy effect. If this result were merely expectancy (not using opiates because of expectation of blockade), then patients who never give an opiate- positive urine and are retained throughout the trial should also have been observed in the placebo group. It is also of interest that this effect seems less dependent on dose. Patients on low-dose naltrexone, who did not have positive urines, were retained equally well as those on high dose.
Attenuation of craving by naltrexone might explain a therapeutic effect that does not depend on episodes of blocked opiate use. Naltrexone reduced subjective craving for opioids compared to placebo in the pivotal placebo-controlled trial of the long-acting injection (Krupitsky et al., 2011) similar to that tested in the present trial. Cue-induced craving and drug seeking may represent conditioned drug-like effects mediated by the endogenous opioid system (Arnsten et al., 1981; Siegal and Ramos, 2002; Ghitza et al., 2010) that would be blocked by naltrexone. Similarly, prior studies examining naltrexone for the treatment of alcohol dependence also support its role in reducing craving (Richardson et al., 2008), and in particular cue-induced cravings (Ooteman et al., 2007, Monti et al., 1999).
Naltrexone might exert other beneficial effects on hedonic or regulatory systems that are dysregulated in opiate dependence. Kappa opioid receptors, which are dysphorigenic, are upregulated in opioid dependence (Al-Hasani et al., 2011). This might contribute to a lowered hedonic tone that promotes drug-seeking, and that might be normalized by the antagonist effect of naltrexone at kappa receptors. Some evidence (Anton et al., 2008) suggests that the beneficial effect of naltrexone on alcoholism depends on a functional variant of the mu opioid receptor (OPRM1). One hypothesis holds that this variant of the receptor is excessively sensitive to opioid agonist effects, and that the antagonist effect of naltrexone normalizes the system (Ray et al., 2007). An analogous normalization effect might partly explain the benefit of naltrexone in opiate dependence.
Concerns about treatment with naltrexone for opiate dependence have included the highlighted (Physician’s Desk Reference 2012) though rarely observed (Krupitsky et al., 2010) risk that patients will try to over-ride the blockade with escalating doses of opioids. A direct effect of naltrexone on craving or normalization of hedonic systems might help explain why such attempts to over-ride the blockade rarely occur.
Another concern has been that blockade of opioid receptors will result in chronic neuroendocrine dysregulation, including activation of the HPA axis (King et al., 2002), in contrast to agonist maintenance which has been shown to normalize neuro-endocrine status (Nava et al., 2006, Lorenzetti et al., 2010). Episodic oral naltrexone dosing has been shown to produce elevation of cortisol (Kosten et al., 1986a) and beta-endorphins (Kosten et al., 1986b). However, it has been conjectured that the stable naltrexone blood levels produced by a long-acting injection of naltrexone (as opposed to the fluctuating levels produced by daily doses of oral naltrexone) after some initial level of activation might eventually exert a normalizing effect on neuroendocrine systems (Kreek et al., 1984). This is based on prior studies showing that constant infusions of naloxone (in contrast to bolus doses) do not produce HPA activation (Delitala et al., 1982; Kreek et al., 1984). The present findings are reminiscent of the observation that among opiate-dependent patients initiated onto agonist maintenance with methadone, many showed no positive urines over 12 months, also consistent with rapid stabilization of opioid and related neuroendocrine systems (Kellogg et al., 2006).
Strengths of the present analysis include that the long-acting naltrexone formulation ensures a therapeutic naltrexone blood level for at least the month after injection and removes concerns about medication adherence. Another strength is the twice-weekly collection of urine, which lends itself to the analysis of the impact of opiate use.
A main weakness is that urine toxicology, collected every three to four days, may not have been sensitive to very small amounts of opiate use. Thus, some of the patients who appear never to have used, may have actually tested the blockade using very small amounts. Dropout from treatment is a meaningful outcome measure in that it reflects what happens in clinical practice, and it is a straightforward outcome with no missing data. Its drawback is that we are not able to confirm that the dropouts have relapsed, although relapse seems highly likely given all that is known about the natural history of opiate dependence and the high rate of relapse after exiting treatment (Weiss et al., 2011; Smyth et al., 2010, Unnithan et al., 1992, Gossop et al., 1989). The sample is small, and future trials of long-acting naltrexone should seek to measure day-to-day opioid use in an effort to replicate this analysis in larger samples, as well as making intensive efforts to locate and evaluate dropouts.
In summary, the present findings suggest that injection naltrexone works for some patients as a treatment for opiate dependence both through blockade of the acute reinforcing effects of opiates and subsequent extinction of drug taking behavior, and also perhaps through direct effects on neural mechanisms of craving or relapse, independent of episodes of use and extinction. Naltrexone might directly suppress opiate use by blocking conditioned cues. Or, the moderate steady blood levels of naltrexone, produced by the long-acting injection, may exert a normalizing effect on the opioid system, HPA axis and related neuro-endocrine systems. Adequate dose of naltrexone appears necessary among patients with positive urines, who test the blockade. However, among patients who do not test the blockade, lower doses may be effective. Given the high cost of injection naltrexone, and the burden of tolerability of repeated high volume injections, it might be worth pursuing future research testing the effectiveness of lower doses among selected patients. Future directions for research could also include more direct mechanistic studies in controlled human laboratory designs examining the impact of maintenance on long-acting naltrexone on responses to priming doses of opioids, environmental “external” cues, internal cues such as mood inductions, and stress responsiveness, as well as accompanying neuroendocrine measures.
Acknowledgments
The authors would like to thank Jesse Davidson for his technical assistance; Wendy Chang, MS, and Kenneth Carpenter, Ph.D. for reviewing an earlier draft of the manuscript; and Elie Nuwayser, Ph.D. and James Kerrigan, MS, of BIOTEK, Inc., for providing Depotrex medication and instructions on the proper methods of preparing, administering, and storing Depotrex, as well as their ongoing advice during the study.
This research was supported by center grants P50 DA09236 and P60 DA05186 from the National Institute on Drug Abuse (NIDA) (Bethesda, MD) and the VA/NIDA Interagency Agreement at Philadelphia VA Medical Center, and K24022412 (Dr Nunes). Biopharmaceutical Research Consultants Inc. received funding for data management and statistical support under contract N01DA-1-8817. Drs. Bisaga, Nunes, and Sullivan are currently participating in studies for which Alkermes is donating medication (injection naltrexone: Vivitrol). Dr. Comer has received an unrestricted educational grant from Reckitt.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: The authors alone are responsible for the content and writing of the paper and have no other conflicts of interest to report.
Contributor Information
Maria A. Sullivan, Columbia University and the New York State Psychiatric Institute 1051 Riverside Drive, Unit 120 New York, NY 10032, U.S.A. 212-543-6525 mas23@columbia.edu
Adam Bisaga, Columbia University and the New York State Psychiatric Institute 1051 Riverside Drive, Unit 120 New York, NY 10032.
John J. Mariani, Columbia University and the New York State Psychiatric Institute 1051 Riverside Drive, Unit 120 New York, NY 10032
Andrew Glass, Columbia University and the New York State Psychiatric Institute 1051 Riverside Drive, Unit 120 New York, NY 10032.
Frances R. Levin, Columbia University and the New York State Psychiatric Institute 1051 Riverside Drive, Unit 120 New York, NY 10032
Sandra D. Comer, Columbia University and the New York State Psychiatric Institute 1051 Riverside Drive, Unit 120 New York, NY 10032
Edward V. Nunes, Columbia University and the New York State Psychiatric Institute 1051 Riverside Drive, Unit 51 New York, NY 10032
REFERENCES
- Al-Hasani R, Burchas MR. Molecular mechanisms of opioid receptor-dependent signaling and behavior. Anesthesiology. 2011;115:1363–81. doi: 10.1097/ALN.0b013e318238bba6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alim TN, Tai B, Chiang CN, Green T, Rosse RB, Lindquist T, Deutsch SI. Tolerability study of a depot form of naltrexone substance abusers. In: Harris LS, editor. Problems of Drug Dependence 1994. vol 2. U.S. Government Printing Office; Washington, D.C.: 1995. p. 253. (NIDA Research Monograph No. 153). (NIH Publ. No. 95-3883) [Google Scholar]
- Amato L, Davoli M, Minozzi S, Ali R, Ferri M. Methadone at tapered doses for the management of opioid withdrawal. Cochrane Database Syst. Rev. 2005;20:CD003409. doi: 10.1002/14651858.CD003409.pub3. [DOI] [PubMed] [Google Scholar]
- Anton RF, Oroszi G, O’Malley S, Cooper D, Swift R, Pettinati H, Goldman D. An evaluation of mu-opioid receptor (OPRM1) as a predictor of naltrexone response in the treatment of alcohol dependence: results from the Combined Pharmacotherapies and Behavioral Interventions for Alcohol Dependence (COMBINE) study. Arch. Gen. Psychiatry. 2008;65:135–144. doi: 10.1001/archpsyc.65.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AF, Segal DS, Loughlin SE, Roberts DC. Evidence for an interaction of opioid and noradrenergic locus coeruleus systems in the regulation of environmental stimulus-directed behavior. Brain Res. 1981;222:351–363. doi: 10.1016/0006-8993(81)91038-6. [DOI] [PubMed] [Google Scholar]
- Callahan EJ, Rawson RA, McCleave B, Arias R, Glazer M, Liberman RP. The treatment of heroin addiction: naltrexone alone and with behavior therapy. Int. J. Addict. 1980;15:795–807. doi: 10.3109/10826088009040057. [DOI] [PubMed] [Google Scholar]
- Clarke TK, Krause K, Li T, Schumann G. As association of prodynorphine polymorphisms and opioid dependence in females in a Chinese population. Addict. Biol. 2009;14:366–370. doi: 10.1111/j.1369-1600.2009.00151.x. [DOI] [PubMed] [Google Scholar]
- Comer SD, Collins ED, Kleber HD, Nuwayser ES, Kerrigan JH, Fischman MW. Depot naltrexone: long-lasting antagonism of the effects of heroin in humans. Psychopharmacology. 2002;159:351–360. doi: 10.1007/s002130100909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comer SD, Sullivan MA, Yu E, Rothenberg JL, Kleber HD, Kampman K, Dackis C, O’Brien CP. Injectable, sustained-release naltrexone for the treatment of opioid dependence: a randomized, placebo-controlled trial. Arch. Gen. Psychiatry. 2006;63:210–218. doi: 10.1001/archpsyc.63.2.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delitala G, Giusti G, Rodriguez G. Growth hormone, prolactin and cortisol nyctohemera. Acta Endocrinol. 1982;100:321–326. doi: 10.1530/acta.0.1000321. [DOI] [PubMed] [Google Scholar]
- Dunbar JL, Turncliff RZ, Dong Q, Silverman BL, Ehrich EW, Lasseter KC. Single- and multiple-dose pharmacokinetics of long-acting injectable naltrexone. Alcohol. Clin. Exp. Res. 2006;30:480–490. doi: 10.1111/j.1530-0277.2006.00052.x. [DOI] [PubMed] [Google Scholar]
- Ghitza UE, Preston KL, Epstein DH, Kuwabara H, Endres CJ, Bencherif B, Boyd SJ, Copersino ML, Frost JJ, Gorelick DA. Brain mu-opioid receptor binding predicts treatment outcome in cocaine-abusing outpatients. Biol. Psychiatry. 2010;68:697–703. doi: 10.1016/j.biopsych.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossop M, Green L, Phillips G, Bradley B. Lapse, relapse, and survival among opiate addicts after treatment: a prospective follow-up study. Br. J. Psychiatry. 1989;154:348–353. doi: 10.1192/bjp.154.3.348. [DOI] [PubMed] [Google Scholar]
- Heishman SJ, Francis-Wood A, Keenan RM, Chiang CN, Terrill JB, Tai B, Henningfield JE. Safety and pharmacokinetics of a new formulation of naltrexone. In: Harris LS, editor. Problems of Drug Dependence 1993. vol 2. 1994. (NIDA Research Monograph No. 141). (NIH Publ. No. 94-3749) [Google Scholar]
- Hulse GK, Morris N, Arnold-Reed D, Tait RJ. Improving clinical outcomes in treating heroin dependence: randomized, controlled trial of oral or implant naltrexone. Arch. Gen. Psychiatry. 2009;66:1108–1115. doi: 10.1001/archgenpsychiatry.2009.130. [DOI] [PubMed] [Google Scholar]
- Johnson RE, Jaffe JH, Fudala PJ. A controlled trial of buprenorphine treatment for opioid dependence. JAMA. 1992;267:2750–2755. [PubMed] [Google Scholar]
- Kellogg S, Melia D, Khuri E, Lin A, Ho A, Kreek J. Adolescent and young adult heroin patients: drug use and success in methadone maintenance treatment. J, Addict. Dis. 2006;25:15–25. doi: 10.1300/J069v25n03_03. [DOI] [PubMed] [Google Scholar]
- King AC, Schluger J, Gunduz M, Borg L, Perret G, Ho A, Kreek MJ. Hypothalamic-pituitary-adrenocortical (HPA) axis response and biotransformation of oral naltrexone: preliminary examination of relationship to family history of alcoholism. Neuropsychopharmacology. 2002;26:778–788. doi: 10.1016/S0893-133X(01)00416-X. [DOI] [PubMed] [Google Scholar]
- Kosten TR, Kleber HD. Strategies to improve compliance with narcotic antagonists. Am. J. Drug Alcohol Abuse. 1984;10:249–266. doi: 10.3109/00952998409002784. [DOI] [PubMed] [Google Scholar]
- Kosten TR, Kreek MJ, Ragunath J, Kleber HD. Cortisol levels during chronic naltrexone maintenance treatment in ex-opiate addicts. Biol. Psychiatry. 1986a;21:217–220. doi: 10.1016/0006-3223(86)90150-2. [DOI] [PubMed] [Google Scholar]
- Kosten TR, Kreek MJ, Ragunath J, Kleber HD. A preliminary study of beta endorphin during chronic naltrexone maintenance treatment in ex-opiate addicts. Life Sci. 1986b;39:55–59. doi: 10.1016/0024-3205(86)90437-6. [DOI] [PubMed] [Google Scholar]
- Kranzler HR, Modesto-Lowe V, Nuwayser ES. Sustained-release naltrexone for alcoholism treatment: a preliminary study. Alcohol. Clin. Exp. Res. 1998;22:1074–1079. [PubMed] [Google Scholar]
- Kreek MJ, Schneider J, Ragunath J, Plevy S. Prolonged (24 hour) Infusion of the Opioid Antagonist Naloxone Does Not Significantly Alter Plasma Levels of Cortisol and ACTH in Humans. Proceedings of the 7th International Congress of Endocrinology; Elsevier Science; 1984. p. 1170. [Google Scholar]
- Krupitsky E, Nunes EV, Ling W, Illeperuma A, Gastfriend DR, Silverman BL. Injectable extended-release naltrexone for opioid dependence: a double-blind, placebo-controlled, multicentre randomised trial. Lancet. 2011;377:1506–1513. doi: 10.1016/S0140-6736(11)60358-9. [DOI] [PubMed] [Google Scholar]
- Lorenzetti V, Lubman DI, Velakoulis D, Yucel M. Pituitary gland volume among heroin users stabilized on substitution pharmacotherapy. Drug Alcohol Depend. 2010;110:164–166. doi: 10.1016/j.drugalcdep.2010.02.012. [DOI] [PubMed] [Google Scholar]
- Martin WR, Jasinski DR, Mansky PA. Naltrexone, an antagonist for the treatment of heroin dependence. Arch. Gen. Psychiatry. 1973;28:784–791. doi: 10.1001/archpsyc.1973.01750360022003. [DOI] [PubMed] [Google Scholar]
- Monti PS, Rohsenow DJ, Hutchison KE, Swift RM, Mueller TI, Colby SM, Brown RA, Gulliver SB, Godon A, Abrams DB. Naltrexone’s effect on cue-elicited craving among alcoholics in treatment. Alcohol. Clin. Exp. Res. 1999;23:1386–1394. [PubMed] [Google Scholar]
- Nava F, Caldiroli E, Premi S, Lucchini A. Relationship between plasma cortisol levels, withdrawal symptoms and craving in abstinent and treated heroin addicts. J. Addict. Dis. 2006;25:9–16. doi: 10.1300/J069v25n02_02. [DOI] [PubMed] [Google Scholar]
- Nosyk B, MacNab YC, Sun H, Fischer B, Marsh DC, Schechter MT, Anis AH. Proportional hazards frailty models for recurrent methadone maintenance treatment. Am. J. Epidemiol. 2009;170:783–792. doi: 10.1093/aje/kwp186. [DOI] [PubMed] [Google Scholar]
- Nuwayser ES, DeRoo DJ, Balskovich PD, Tsuk AG. Sustained release injectable naltrexone microcapsules. NIDA Res. Monogr. 1990;105:532–533. [PubMed] [Google Scholar]
- Ooteman W, Koeter MW, Verheul R, Schippers GM, van den Brink W. The effect of naltrexone and acamprosate on cue-induced craving, autonomic nervous system and neuroendocrine reactions to alcohol-related cues in alcoholics. Eur. Neuropsychopharmacol. 2007;17:558–566. doi: 10.1016/j.euroneuro.2007.02.012. [DOI] [PubMed] [Google Scholar]
- Ray LA, Hutchison KE. Effects of naltrexone on alcohol sensitivity and genetic moderators of medication response: a double-blind placebo-controlled study. Arch, Gen, Psychiatry. 2007;64:1069–1077. doi: 10.1001/archpsyc.64.9.1069. [DOI] [PubMed] [Google Scholar]
- Richardson K, Baillie A, Reid S, Morley K, Teesson M, Sannibale C, Weltman M, Haber P. Do acamprosate or naltrexone have an effect on daily drinking by reducing craving for alcohol? Addiction. 2008;103:953–959. doi: 10.1111/j.1360-0443.2008.02215.x. [DOI] [PubMed] [Google Scholar]
- Rothenberg JL, Sullivan MA, Church SH, Seracini A, Collins E, Kleber HD, Nunes EV. Behavioral naltrexone therapy: an integrated therapy for opiate dependence. J. Subst. Abuse Treat. 2002;23:351–360. doi: 10.1016/s0740-5472(02)00301-x. [DOI] [PubMed] [Google Scholar]
- SAS Institute . SAS version 9.3. SAS Institute; Cary, NC: 2012. [Google Scholar]
- Siegel S, Ramos BM. Applying laboratory research: drug anticipation and the treatment of drug addiction. Exp. Clin. Psychopharmacol. 2002;10:162–183. doi: 10.1037//1064-1297.10.3.162. [DOI] [PubMed] [Google Scholar]
- Smyth BP, Barry J, Keenan E, Ducray K. Lapse and relapse following inpatient treatment of opiate dependence. Ir. Med. J. 2010;103:176–179. [PubMed] [Google Scholar]
- Sullivan MA, Vosburg SK, Comer SD. Depot naltrexone: antagonism of the reinforcing, subjective, and physiological effects of heroin. Psychopharmacology. 2006;189:37–46. doi: 10.1007/s00213-006-0509-x. [DOI] [PubMed] [Google Scholar]
- Unnithan S, Gossop M, Strang J. Factors associated with relapse among opiate addicts in an out-patient detoxification programme. Br. J. Psychiatry. 1992;161:654–657. doi: 10.1192/bjp.161.5.654. [DOI] [PubMed] [Google Scholar]
- Wee S, Koob GF. The role of the dynorphin-kappa opioid system in the reinforcing effects of drugs of abuse. Psychopharmacology. 2010;201:121–135. doi: 10.1007/s00213-010-1825-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss RD, Potter JS, Provost SE, Huang Z, Jacobs P, Hasson A, Lindblad R, Connery HS, Prather K, Ling W. A multi-site, two-phase, Prescription Opioid Addiction Treatment Study (POATS): rationale, design, and methodology. Contemp. Clin. Trials. 2010;31:189–199. doi: 10.1016/j.cct.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss RD, Potter JS, Fiellin DA, Byrne M, Connery HS, Dickinson W, Gardin J, Griffin ML, Gourevitch MN, Haller DL, Hasson AL, Huang Z, Jacobs P, Kosinski AS, Lindblad R, McCance-Katz EF, Provost SE, Selzer J, Somoza EC, Sonne SC, Ling W. Adjunctive Counseling During Brief and Extended Buprenorphine-Naloxone Treatment for prescription opioid dependence: a 2-phase randomized controlled trial. Arch. Gen. Psychiatry. 2011;68:1238–46. doi: 10.1001/archgenpsychiatry.2011.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstock AR, Lintzeris N, Lea T. “Should I stay or should I go?” Coming off methadone and buprenorphine treatment. Int, J. Drug Policy. 2011;22:77–81. doi: 10.1016/j.drugpo.2010.08.001. [DOI] [PubMed] [Google Scholar]