Abstract
Introduction
Programmed cell death is well-orchestrated process regulated by multiple pro-apoptotic and anti-apoptotic genes, particularly those of the Bcl-2 gene family. These genes are well documented in cancer with aberrant expression being strongly associated with resistance to chemotherapy and radiation.
Areas covered
This review focuses on the resistance induced by the Bcl-2 family of anti-apoptotic proteins and current therapeutic interventions currently in preclinical or clinical trials that target this pathway. Major resistance mechanisms that are regulated by Bcl-2 family proteins and potential strategies to circumvent resistance are also examined. Although antisense and gene therapy strategies are used to nullify Bcl-2 family proteins, recent approaches use small molecule inhibitors and peptides. Structural similarity of the Bcl-2 family of proteins greatly favors development of inhibitors that target the BH3 domain, called BH3 mimetics.
Expert opinion
Strategies to specifically identify and inhibit critical determinants that promote therapy-resistance and tumor progression represent viable approaches for developing effective cancer therapies. From a clinical perspective, pretreatment with novel, potent Bcl-2 inhibitors either alone or in combination with conventional therapies hold significant promise for providing beneficial clinical outcomes. Identifying small molecule inhibitors with broader and higher affinities for inhibiting all of the Bcl-2 pro-survival proteins will facilitate development of superior cancer therapies.
Keywords: BH3 domain, apoptosis, Mcl-1, radiation resistance, chemotherapy resistance
1. Introduction
Continuous programmed cell death is essential for maintaining tissue homeostasis, and this process is positively or negatively regulated by specific genes 1. An imbalance can lead to severe tissue disturbances that can ultimately culminate in cancer. The BCL-2 (B-cell lymphoma-2) 2–4 gene was first discovered at the t (14; 18) chromosome translocation breakpoint in B-cell lymphomas. As a result of this translocation, immunoglobulin heavy chain gene promoter and enhancer in chromosome 14 drives the transcription of BCL-2, subsequently leading to constitutive expression of Bcl-2 in B-cell clones 3. Unlike previously identified oncogenes, Bcl-2 does not promote cell proliferation. Instead, overexpression of Bcl-2 inhibits cell death 5.
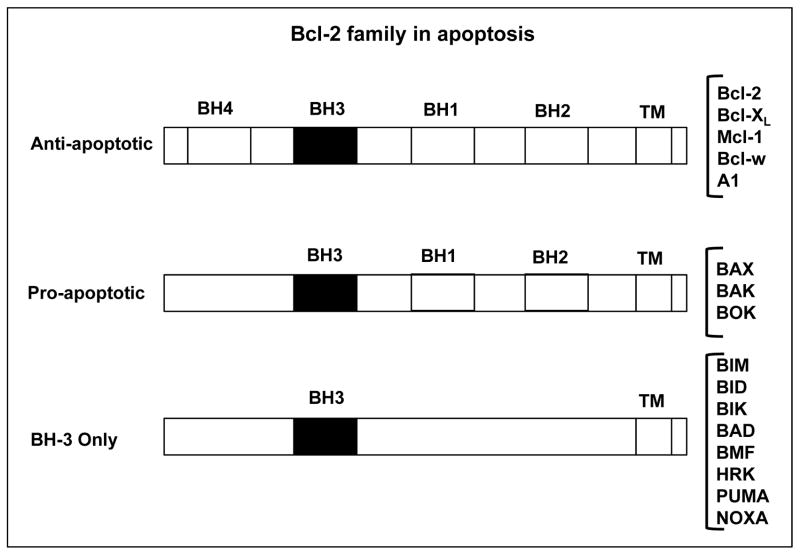
Over the years, the Bcl-2 family of proteins has expanded and now includes at least 12 predominantly expressed members including Bcl-2 itself. Functionally these molecules differ by either promoting or inhibiting apoptosis, thus establishing these molecules as pivotal determinants of whether a cell lives or dies. Based on their structure and function, the Bcl-2 family of proteins is further divided into three groups as listed in Figure 1. There are several pro-survival proteins, but 5 are well characterized including, Bcl-2, Bcl-XL, Bcl-w, Mcl-1 and A1, and three pro-apoptotic proteins, BAK, BAX and BOK, of which the first two are predominant and localized on the mitochondrial membrane. Upon receiving a death signal, oligomerization of BAK, BAX and BOK leads to formation of mitochondrial pores subsequently resulting in increased permeability of the mitochondrial membrane releasing cytochrome c (cyt c) into the cytosol ultimately leading to cell death. Both anti-apoptotic and pro-apoptotic proteins have a similar C-terminal membrane localization domain, three or four Bcl-2 homology domains (BH1, BH2, BH3 and BH4), and similar three-dimensional structures 6. However, the structural differences that apparently decide their mutually opposing roles are attributed to a few amino acids. There are eight members of another class of BH3-only pro-apoptotic proteins that lack all other Bcl-2 homology domains except BH3, named BIM, BID, BIK, BAD, BMF, HRK, PUMA and NOXA. All BH-3 only proteins also play pivotal roles by regulating the core Bcl-2 family proteins to promote apoptosis through binding via its BH-3 domain. The intrinsic apoptosis pathway starts with BH3-only protein induction or post-translational activation, which results in the inactivation of some BCL-2 family members. This relieves inhibition of BAX and BAK activation, which in turn promotes apoptosis. Some BH3-only proteins, such as BIM and PUMA, may also activate BAX and/or BAK 6.
Figure 1. Three subfamilies of Bcl-2 related proteins.
Family members sharing four bcl-2 homology (BH) domains are the multidomain proteins. These proteins share a common three-dimensional fold. Anti-apoptotic proteins are antagonists of BAX and BAK, in part by directly binding to them. BH-3 only proteins only have BH3 domain. They respond to stress and are natural antagonists of anti-apoptotic proteins.
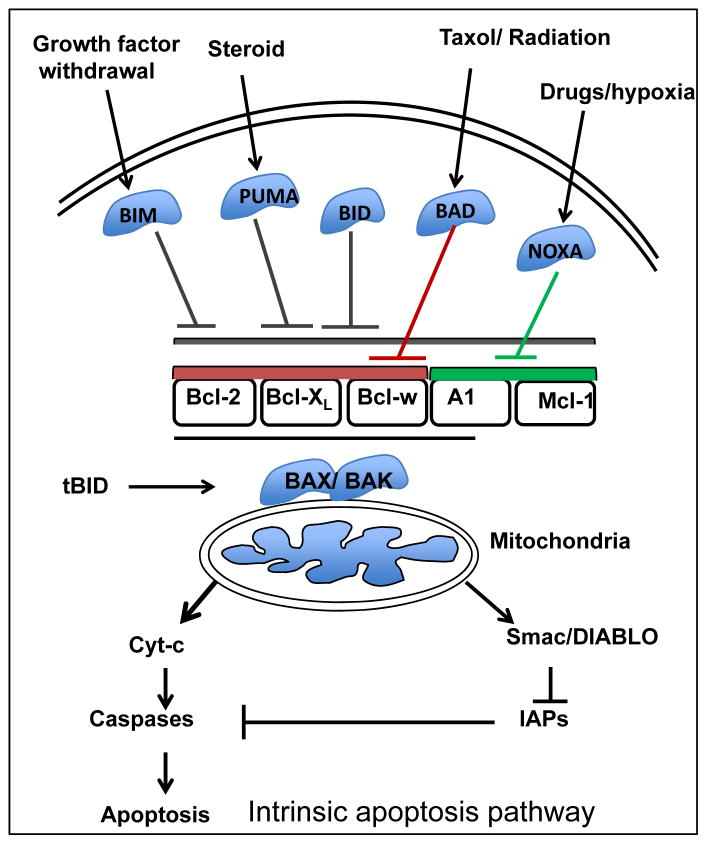
Apoptosis can be operationally divided into three stages. In the first stage, or initiation phase, the cells undergoing stress or DNA damage initiate a signaling cascade either through an intrinsic or extrinsic pathway. This is followed by the regulatory phase, where a sum of all of these signals is integrated to make the decision whether to undergo apoptosis or not. The third and final phase is the execution phase where caspases are cleaved and the cells are further engulfed by neighboring phagocytic cells 7. The Bcl-2 family of pro-apoptotic and anti-apoptotic proteins regulates the intrinsic pathway in the initiation phase leading to caspase-9 activation (Figure 2). BIM and PUMA bind to all five anti-apoptotic Bcl-2 family members. By contrast, NOXA only binds to Mcl-11 and A1, and BAD binds selectively to Bcl-w, Bcl-2 and Bcl-XL. BID binds avidly to Bcl-XL, BCL-w, Mcl-11 and A1, but only weakly to BCL-2. These binding specificities recapitulate the ability of these proteins to activate apoptosis. For example, BIM, BID or PUMA alone can induce apoptosis, whereas a combination of NOXA and BAD is required 6. On the other hand, the extrinsic pathway does not involve Bcl-2. Instead, the extrinsic pathway is triggered by ligation of death receptors, that are members of the tumor necrosis factor family (TNF) containing an intracellular death domain that can recruit and activate caspase-8 through the adapter protein Fas-associated death domain (FADD) at the cell surface 6. It is the interaction between the pro- and anti-apoptotic proteins mainly via their BH3 domains that determines cell fate. In a model suggested by Willis et al., Bcl-XL antagonizes the pro-apoptotic action of BAK or BAX by binding to their BH3 domains, and any member of the BH3-only proteins can relieve this interaction by binding to Bcl-XL8. Also, BH3-only proteins can interact directly with BAX and BAK leading to apoptosis induction 9. Bcl-XL and Mcl-1 directly target BAK, while BAX regulation also requires Bcl-2 10. Bcl-2 and Bcl-XL were shown to be involved in non-apoptotic cell death via their interaction with another BH3 domain containing protein Beclin-1 (BECN1). Binding of Bcl-2 or Bcl-XL with BECN1 inhibits autophagy, while release of this interaction induces autophagy 11, 12.
Figure 2. Pathway to apoptosis following various cellular insults.
Chemotherapy, radiation and other insults can initiate apoptosis through the mitochondrial intrinsic pathway. Pro-apoptotic proteins (BAD, BIM, BID) are activated and upon its activation mitochondria release cytochrome c into the cytosol. Cytochrome c binds with apoptotic protease activating factor-1 (APAF1) to activate initiator caspase-9 subsequently leading to apoptosis.
Although both pro-apoptotic and anti-apoptotic proteins share similar three-dimensional structure and BH domains, the specific difference in their amino acid sequences that determines their mutually opposing role is remains elusive. However, from a therapeutic standpoint, a BH3 mimetic can bind to pro-survival proteins to induce apoptosis and may also induce autophagy 13.
2. Role of the Bcl-2 family in mediating resistance of cancer cells to therapy
Impaired apoptosis is pivotal for tumor development, and altered expression of molecular determinants of apoptosis, such as the Bcl-2 family of proteins, has a detrimental outcome. Apoptosis resistance can lead to escape of tumor cells from immune-surveillance, and can eventually result in the expansion of a population of resistant neoplastic cells. Ranger et al. suggest that at least one Bcl-2 family pro-survival protein is required for the survival of every cell type 14. Other studies provide evidence that an appropriate balance between anti-apoptotic and pro-apoptotic molecules are required for tissue homeostasis 15. Although Bcl-2 was originally discovered as a gene translocated in B-cell malignancies, later studies showed that Bcl-2 and other Bcl-2 family pro-survival genes are transcriptionally upregulated by alternate mechanisms that do not involve chromosomal translocations. These observations expand the significance of Bcl-2 family pro-survival genes in multiple cancer indications 16, 17.
3. Resistance Mechanisms
Treatment of cancer with chemotherapy or radiation kills target cells primarily by induction of apoptosis. Anticancer drugs are classified as DNA-damaging agents, antimetabolites, mitotic inhibitors, nucleotide analogues or inhibitors of topoisomerases 18. Radiotherapy can induce cellular oxidative stress that culminates in tumor cell death 19. These approaches are effective in some patients; however, they are not very effective for others, mostly due to failure to activate apoptosis resulting in inherent resistance of some tumors to therapy, which represents a major clinical challenge. Tumor cells can acquire resistance to apoptosis by various mechanisms, including overexpression of anti-apoptotic genes, down-regulation or mutation of pro-apoptotic genes and alteration of p53 or the PI3K/AKT pathways 20.
3.1 Altered expression of pro-survival proteins
3.1.1 Bcl-2
Multiple studies have shown that high levels of BCL2 gene expression correlate with severity of malignancy of human tumors 21–23. Elevated expression of Bcl-2 in acute myeloid leukemia (AML) or increased Bcl-2/BAX ratio was shown to be associated with poor clinical response 22, 24, 25. Additionally, several studies have demonstrated a correlation between elevated Bcl-2 expression and poor prognosis in melanoma 26, breast 27, prostate 28, small cell lung 29, colorectal 30 and bladder cancers 31. Further studies using both in vitro and in vivo models have proven that higher Bcl-2 expression leads to resistance to chemotherapy and radiation 21, 22, 32. In follicular B-cell lymphoma, chromosomal translocation t (14, 18) places the BCL2 gene next to enhancer elements of the immunoglobulin heavy chain promoter locus leading to enhanced BCL2 gene expression 4, 33–35. This translocation is evident in 90% of follicular cell lymphomas and about 30% of diffuse large B cell lymphomas 36. Furthermore, overexpression of Bcl-2 has been demonstrated as a result of gene amplification 37, hyper-methylation of the BCL2 gene 38 or chromosomal deletions leading to loss of BCL2 targeting miRNAs such as miR-195, miR-24-2 and miR-365-2 39, 40. In addition, tumor associated viruses, such as Epstein-Barr virus (EBV) and human herpes virus 8 (HHV8 or Kaposi’s sarcoma-associated herpes virus), encode proteins that are homologues of Bcl-2, and elicit similar anti-apoptotic functions 41–43. Overexpression of the Bcl-2 family of pro-survival proteins by itself has not proven to be highly tumorigenic; however, in combination with additional synergistic mutation(s) this overexpression is profoundly detrimental to the host 53. Many follicular lymphomas with BCL2 gene translocations are relatively indolent 53, 54. Nevertheless, 8% of follicular lymphomas can progress to aggressive disease and those were shown to be associated with an additional MYC translocation 55. This correlation was first suggested using in vitro studies 5 and later confirmed in mice expressing both BCL2 and MYC transgenes 56. Further studies have provided convincing evidence that co-expression of Bcl-2 and c-myc makes cells resistant to therapy in multiple malignancies including lymphomas 57, breast 58 and pancreatic 59 cancers.
3.1.2 Bcl-XL
Besides Bcl-2, Bcl-XL has also been shown to confer resistance to multiple apoptosis-inducing pathways 44. Advanced and relapsed multiple myeloma (MM) are characterized by higher levels of Bcl-XL 45. Nagane et al. showed that constitutive activation of epidermal growth factor receptor (EGFR) tends to increase BCL-XL expression thereby leading to apoptosis resistance 46. Both Bcl-2 and Bcl-XL are overexpressed in close to 100% of hormone-refractory prostate cancers and are associated with therapy resistance leading to a very poor clinical outcome 47. Enhanced proliferation and survival of pancreatic cancers were also found to be due to Bcl-XL overexpression in conjunction with c-myc 59, 60. Studies also demonstrated that, lymphoid tumors with increased levels of Bcl-XL formed in MYC transgenic mice carried mutations in the p53 gene 61. Hence, mutations that enhance tumor growth along with suppression of apoptosis by overexpression of the Bcl-2 family of pro-survival genes lead to unfavorable clinical outcome.
Michaud et al. analyzed the levels of Bcl-2 and Bcl-XL in a panel of oropharyngeal squamous cell carcinomas (OPSCC) to determine any relationship with platinum-based therapy resistance 62. In OPSCC, Bcl-2 expression was associated with increased cisplatin resistance, while Bcl-XL expression failed to show any correlation. However, this association of Bcl-2 expression with cisplatin resistance was not observed in an H69 SCLC cell line 63.
3.1.3 Mcl-1
In leukemia patients, Mcl-1 expression was shown to be upregulated, particularly at relapse, indicating a possible enrichment of chemotherapy-resistant Mcl-1 expressing cells following a certain chemotherapy regimen 48. Expression of Mcl-1, but not of Bcl-2 or Bcl-XL, was shown to be associated with poor survival outcome in ovarian cancer patients 49 and in human cervical neoplasms IL-6 regulates the expression of Mcl-1 via a PI3K/Akt-dependent pathway and facilitates oncogenesis of cervical cancer by inhibiting cellular apoptosis 50. Additionally, Schwickart et al. demonstrated that in hematologic malignancies 51, the expression of deubiquitinase USP9X expression correlates with that of Mcl-1, and USP9X binds to Mcl-1, stabilizes it and thereby promotes Mcl-1 overexpression and cell survival. Recent studies showed that in B-cell malignancies, patients treated with the anti-CD20 antibody rituximab exhibited an increased level of Mcl-1 52. Mcl-1 downregulation using RNA interference sensitized these cells to rituximab treatment 52, underscoring the importance of Mcl-1 as a mediator of chemotherapy resistance.
Additionally, studies in a panel of lymphoma cell lines treated with protease inhibitors identified Mcl-1 increase and accumulation as an unwanted molecular consequence leading to apoptosis inhibition 64.
3.2 Inactivation of pro-apoptotic proteins
Besides overexpression of pro-survival genes, tumors acquire apoptosis resistance by down regulation or mutation of pro-apoptotic molecules. Two key events known to induce cancer cell survival and progression are loss of function of tumor suppressor genes such as p53 65 and mutation of pro-apoptotic proteins such as BAX, which are common in hematologic malignancies 66, 67. Since BH3 only proteins NOXA and PUMA are transcriptional targets of p53, loss of p53 by itself suppresses apoptosis induced by these proteins 68–70. Frame shift mutations causing loss of expression and mutation of BH domains of pro-apoptotic proteins that lead to loss of their function are common in colon cancers and result in apoptosis resistance 67.
To evade apoptosis, cancer cells are selected based on which specific group of Bcl-2 family proteins is altered. As suggested in a recent study, there are three types of apoptosis blockade 71. Class A blockade is due to a loss of function of the BH3-only activator proteins. Class B blockade constitutes failure to activate effectors due to loss or inactivation of BAX and BAK. Class C blockade is mainly due to increased expression of Bcl-2/Mcl-1. Based on this classification, BH3 profiling can be used to predict drug sensitivity and has been a useful tool in diffuse large B-cell lymphomas for stratifying drug sensitivity in patients 72.
3.3 Radiation-resistance mechanism
Tumor resistance to radiation is defined by the percentage of cells that are resistant after administering a total dose of radiation that can be safely delivered. Radiation induces oxidative stress and DNA damage resulting in apoptosis via p53- and BAX-dependent mechanisms. Bcl-2 expression has been implicated in radiation-resistance. Strasser and colleagues showed that Bcl-2 strongly induces clonogenic survival of lymphoma cells after irradiation 73. In contrast, Bcl-2 overexpressing PC-3 and LNCaP prostate cancer cells failed to induce radiation-resistance 74. However, both in vitro and in vivo approaches showed that cells exposed to lower levels of radiation induce Bcl-2 expression, as an adaptive measure to withstand environmental stress 75. Follow-up in vitro studies in pancreatic cancer cells proved that knockdown of Bcl-2 in these cells sensitized them to radiation resulting in effective cell death 75. Lee et al. investigated the role of Bcl-2 family of proteins in eliciting radiation-resistance in PANC-1 and AsPC-1 pancreatic cancer cells with mutated p53. Radiation resistant clones showed no change in Bcl-2, but displayed upregulation of Bcl-XL, emphasizing the importance of the Bcl-2 family proteins in inducing radiation-resistance. Elevated expression of Bcl-XL was detected in bladder carcinomas and squamous cell carcinomas of the oropharynx and it was shown to be associated with radiation-resistance 76,77.
3.4 Additional chemo-resistance and radiation-resistance mechanisms involving Bcl-2
Besides the mitochondrial pathway of apoptosis, Bcl-2 may block apoptosis via other mechanisms including inhibition of calcium mobilization 78, acting as an antioxidant to prevent free radical damage 79 and elevation of glutathione 63 (GSH 3) levels by preventing its depletion 80. Studies by Wright et al. showed that elevated Bcl-2 levels by virtue of increased GSH levels can prevent the activation of serine protease AP24 following treatment with TNF or UV light, which is capable of inducing nuclear fragmentation 81. This effect confers apoptosis resistance by GSH-induced elevated Bcl-2 expression.
4. Reversing resistance to chemotherapy and radiation by genetic and pharmacological inhibition of Bcl-2 family members
From a therapeutic standpoint, it is critical to understand that the process of apoptosis is intricately balanced, and perturbing the balance to induce apoptosis should be the goal of therapy. Accordingly, Bcl-2 interfering strategies have been accepted as a potential approach to induce apoptosis in cancer cells. Later studies shed light on a new concept that the apoptotic signal itself is induced in cancer cells even in the absence of chemotherapy, and abnormally higher expression of Bcl-2 proteins neutralize that apoptotic signal, making those cancer cells addicted to expression of Bcl-2 pro-survival proteins. Therefore, Bcl-2 inhibition strategies also present a form of ‘synthetic lethality’ that selectively kills cancer cells addicted to Bcl-2 expression for survival. For radio-sensitization as well, modifying the activity of cell survival genes such as Bcl-2 will be very beneficial 75.
Several strategies have been formulated over the years to target the Bcl-2 family of proteins including antisense oligonucleotides 82; peptides and small molecules inhibitors (SMIs) targeted toward apoptosis mediators. They are classified in Table 1.
Table 1.
Bcl-2-family targeting drugs
| Drug name | Drug class-mechanism of action | Drug targets | Stage of development | Ref (PMID #) |
|---|---|---|---|---|
| Cyclin-dependent kinase inhibitor | Flavopiridol | Cyclin dependent kinases and Mcl-1 | Clinical trials | 22374332 |
| SN-032 | CdKs 2,7,9 and MCL-1 | 22966018 | ||
| Sorafenib | B-RAF, PDGF, FLT, KIT, VEGF and Mcl-1 | 22698419 | ||
| Deubiquitinase inhibitor 78 | WP1130 | Bcr-Abl compartmentalization, inhibits USP9X (stabilize Mcl-1) | Pre-clinical | 17202319 |
| Antisense inhibitors | Oblimersen sodium | Bcl-2 | Phase III | 19738118 |
| BH3 mimetics | ABT-737 (AB-263) | Bcl-2, Bcl-XL, Bcl-w | Clinical trials | 22821746 |
| Gossypol (AT-101) | Bcl-2, Bcl-XL, Bcl-w, Mcl-1 | 21918390 | ||
| Apogossypol (ApoG2) | Bcl-2, Bcl-XL, Mcl-1 | 18769131 | ||
| Obatoclax (GX-15-070) | Bcl-2, Bcl-XL, Bcl-w, Mcl-1 Bcl-2 |
22333598 | ||
| HA-14 | Bcl-XL | 19228717 | ||
| BH3Is | Bcl-2, Bcl-XL, Mcl-1 | 16951185 | ||
| TW-37 | Bcl-2, Bcl-XL, Mcl-1, Bfl-1 | 21780116 | ||
| Sabutoclax (BI-97C1) | Bcl-2, Bcl-XL, Mcl-1, Bfl-1 | Preclinical | 22655238 | |
| BI-97D6 | Bcl-2, Bcl-XL, Mcl-1 | 22931411 22655238 |
||
| BH-3 M6 | Bcl-2, Bcl-XL, Mcl-1 | 21148306 |
Abbreviations: CdK- cyclin-dependent kinase; PDGF: platelet derived growth factor; VEGF-Vascular endothelial growth factor
4.1 Bcl-2 inhibition strategies
Recombinant adenovirus encoding BAX was the first to be introduced in its class. However, this approach was not successful due to toxicity affecting healthy cells 83. Recently, microRNAs, a novel class of gene regulators, regulating Bcl-2 expression have been identified. Using computational and experimental approaches, Sing et al. showed that miR-195, miR-24-2 and miR-365-2 act as negative regulators of Bcl-2 by binding to the 3’-UTR of the BCL2 gene 40. Overexpression of these miRNAs alone resulted in increased apoptosis and also augmented etoposide-induced apoptosis in MCF-7 breast cancer cells, suggesting that miRNA-based Bcl-2 inhibition strategies hold promise in the future.
4.2 Mcl-1 targeting
Recently, many researchers have recognized Mcl-1 as a critical player in apoptosis-resistance in multiple cancers. Despite the fact that Mcl-1 is structurally similar to other Bcl-2 family of proteins, there are significant differences in its BH3 binding groove, which weakens the affinity of other BH3 mimetics to target Mcl-1. Previous studies using phage display libraries have analyzed the binding requirements of the Mcl-1 binding groove 84. Cyclin dependent kinase inhibitors including Flavopiridol, and SNS-032 were shown to transcriptionally suppress the expression of several genes including Mcl-1. Based on that premise, Flavopiridol has been used for treating high-risk CLL patients demonstrating promising results 85 and synergism when treated with a proteasome inhibitor. Sorafenib, originally developed as a B-raf inhibitor, is suggested to decrease Mcl-1 translation, resulting in increased apoptosis in leukemia cells 86. A unique characteristic of Mcl-1 is that it has an extremely short half-life due to ubiquitin-mediated proteosomal degradation as compared to other Bcl-2 family proteins. This gives an added advantage for therapeutic exploitation 87. A recent study showed that USP9X, a deubiquitinase, modified the expression of Mcl-1, and its knockdown lead to rapid degradation of Mcl-1 51. WP1130, a small molecule inhibitor for Bcr-Abl, has shown inhibitory effects on USP9X. When used to treat CML, WP1130 led to decreased levels of Mcl-1 and removal of Bcr-Abl eventually leading to apoptosis 88. In total, these data highlight the value of Mcl-1 inhibition as a potential tumor inhibition strategy.
5. Targeting Bcl-2 family members for therapy
Targeting the mitochondrial-mediated apoptosis pathway might facilitate the cytotoxic effects of chemotherapy as well as radiotherapy. Tumors that are resistant to apoptosis due to aberrant expression of Bcl-2 family proteins, when treated with DNA intercalating agents or radiotherapy, may survive and induce further genetic instability instead of undergoing apoptosis 89. Therefore, treating cells with drugs modulating the Bcl-2 pathway is critical in achieving desired clinical outcomes. Multiple strategies have been established to target the Bcl-2 pathway, including antisense-mediated inhibition, peptide inhibitors and small molecule inhibitors.
5.1 Antisense oligonucleotides (ASOs) and antibodies
The principle of antisense strategy is that introduction of a single oligonucleotide strand complementary to the target sequence of a chosen mRNA leads to formation of a DNA heteroduplex, which is vulnerable to destruction by RNAse H, ultimately resulting in reduced levels of the target mRNA 90.
5.1.1 Oblimersen (G-3139)
One of the first drugs developed for inhibiting the Bcl-2 family was Oblimersen sodium; an antisense modified 18-mer oligonucleotide with a phosphorothioate backbone complementary to the BCL2 gene. Although trials initiated by Gentra for this agent showed moderate success in treating low-grade lymphoid malignancies, it failed to get FDA approval, since it did not show substantial survival advantage in clinical trials in melanoma, multiple myeloma and chronic myelocytic leukemia 91. A Phase II study in myeloid leukemia showed robust intracellular accumulation of G-3139 in bone marrow leading to Bcl-2 downregulation 92. A recent report showed that a combinatorial approach with dacarbazene in melanoma patients improved survival at 24 months and significantly increased progression-free survival 13. Use of ASOs is limited due to disadvantages such as DNAse-mediated degradation, which is resolved by phosphorothioate modifications, and non-specific binding. Also, with the relatively short half-life of ASOs, a complete suppression of the target gene is less likely to be achieved.
A similar strategy was employed in developing Bcl-XL ASOs 93. As a single agent, Bcl-XL ASOs also suffered a similar inconclusive fate as Bcl-2 ASOs. Nevertheless, an antisense approach that targets both Bcl-2 and Bcl-XL had augmented therapeutic value 94. Mcl-1 antisense strategy also showed promising results in vitro in multiple cancer indications, and hepatocellular carcinomas (HCC) displayed increased sensitivity to cisplatin treatment 82.
Besides ASOs, other approaches were also employed to inhibit the expression of Bcl-2. An antibody targeting Bcl-2 was developed and was shown to increase drug-induced cytotoxicity in breast and other cancers 95. Furthermore Bcl-2 targeting ribozymes were developed that showed promise in inhibiting myeloid leukemia growth 96. Like ASOs, antibodies and ribozymes suffered from a lack of stability thus limiting its use in the clinic.
5.2 Peptide and peptidomimetics
Given the limited success of approaches to reduce the expression of the Bcl-2 family of anti-apoptotic proteins, a different approach was introduced to antagonize Bcl-2 function, rather than changing its levels. Understanding the crystal structure of all eight BH3-only proteins revealed the relationship of each of these proteins with its pro-survival counterparts. Based on the mechanism of apoptosis induction explained earlier, BAX and BAK need to be activated as a result of either BH3-only proteins binding directly to them or BH3-only proteins binding with anti-apoptotic proteins resulting in release of pro-apoptotic proteins. BH1-BH3 domains of Bcl-XL form a hydrophobic groove 97 where the α-helix of a BH3-only protein can bind. Sattler et al. 98 first tested the concept of whether a BAX-BH3 peptide that can bind to the hydrophobic groove of Bcl-XL could inhibit its anti-apoptotic function. Results from this study provided the proof-of-concept for designing numerous specific antagonists that are referred to as BH3 mimetics, which, when bound to the hydrophobic crevice on Bcl-2 or Bcl-XL, impair their function and induce apoptosis. Over the years several short peptides representing the BH3 domain of BH3-only proteins have been designed.. Recently, Zhang and colleagues discovered that nuclear receptor Nur77 is capable of binding between the BH3 and BH4 domains, unmasking the BH3 domain and leading to a functional switch of Bcl-2 from an anti-apoptotic to a pro-apoptotic protein 101. This strategy might help construct newer derivatives holding promise. BIM-BH3 peptide is a novel hydrocarbon labeled-peptide modeled to target the BH3 domain of BIM, inhibiting Bcl-2-BIM interactions. This stapled peptide is highlighted for its selective activation of cell death in hematologic tumors and AML xenografts 102.
5.3 Small molecule inhibitors
Small molecules are organic molecules of low molecular weight (less than 750 Daltons). Compared to ASOs and peptides, their relatively smaller size and lower manufacturing costs make small molecule inhibitors (SMIs) a more attractive treatment strategy. SMIs are developed based on the fact that the BH3 binding hydrophobic cleft in Bcl-2 is critical for its anti-apoptotic function, and blocking that groove, using SMIs might inhibit its heterodimerization, thus disarming the anti-apoptotic function of Bcl-2 and tipping the balance towards apoptosis. Various screening strategies have been undertaken over the years in an attempt to find SMIs against the bcl-2 family of proteins. Natural products such as actimycin A, celerythrin and purpurogallin, and some compounds derived from tea and cotton extracts have demonstrated efficacy as antitumor agents by modulating the Bcl-2 family of anti-apoptotic proteins 99, 103. Although the advent of SMIs resolved most of the problems associated with antisense therapy, newer problems arose, such as non-specific binding to catalytic subunits of other oncoproteins leading to off-target effects. Following are some of the SMIs in clinical and preclinical stages of development.
5.3.1 Gossypol
Gossypol is a natural polyphenol isolated from cottonseeds 104. Gossypol, also known as BL-193, was used as a contraceptive and for anticancer studies since 1980s. Subsequently, more effective isoforms such as (−)-BL-193, (+)-BL-193 and (±)-BL-193, were developed, with the -(−) form being the more potent one 105. Using nuclear magnetic resonance (NMR), the mechanism of (−)-BL-193 action was resolved showing that BL-193 binds to the hydrophobic groove of Bcl-2 and Bcl-XL 106. Moreover, gossypol was shown to induce DNA breakage in the presence of metal ions such as copper 104. Gossypol is presently in clinical trials as a single agent and in combination (Table 2).
Table 2.
Completed Combination clinical trials
| Bcl-2 inhibitor | Other drug | Tumor type | Phase |
|---|---|---|---|
| Flavopriridol | Cytarabine, Mitoxantrone | AML | Phase II |
| Vironostat | Advanced adult solid tumors | Phase I | |
| Oxaliplatin, Fluorouracil, Leucovorin | Advanced adult solid tumors | Phase I | |
|
| |||
| Oblimersen | Dacarbazine | Advanced Melanoma | Phase III |
| Fludarabine/Rituximab, Cyclophosphamide | CLL | Phase III | |
| Dexamethasone | MM | Phase III | |
| Ara C/ Daunorubicin | AML | Phase II | |
| Carboplatin/ etoposide | SCLC | Phase II | |
| Docetaxel | HR-Prostate cancer | Phase II | |
| IFNα | Renal cancer | Phase I/II | |
| Doxorubicin | Hepatocellular | Phase I/II | |
|
| |||
| AT101 | Temozolomide | Brain and CNS tumors | Phase I |
| Lenalidomide | ALL, chronic B-cell Leukemia | Phase I/II | |
| Rituximab | CLL, Follicular lymphoma | Phase II | |
| Docetaxel | Prostate Cancer, NSCLC | Phase II | |
| Erlotinib | EGFR mutant lung cancer | Phase II | |
| Topotecan | SCLC | Phase II | |
|
| |||
| Gossypol | Paclitaxel, Carboplatin | Lymphoma | Phase I |
| Cisplatin, Etoposide | SCLC | Phase I/II | |
| Docetaxel, Prednisone | HR-Prostate cancer | Phase I | |
|
| |||
| ABT-737 | Platinum | Ovarian cancer | |
|
| |||
| Obatoclax | Etoposide | Extensive stage-SCLC MCL, Hodgkin’s or Non | Phase I/II |
| Bortezomib | Hodgkins lymphoma, MM | Phase II | |
| Docetaxel | NSCLC | Phase II | |
| Rituximab, Bendamustine | Lymphoma | Phase II | |
This data is adopted from www.clinicaltrials.gov.
Abbreviations: AML Acute Myelocytic leukemia; CLL Chronic Lymphocytic leukemia; MM Multiple Myeloma; SCLC small cell lung cancer; HR hormone refractory; NSCLC- Non-Small cell Lung cancer; CNS-Central Nervous System; MCL-Mantle cell lymphoma.
5.3.2 TW37
TW37 is a second-generation benzenesulphonyl derivative of gossypol 107. TW37 has an added advantage that it shows higher affinity to Bcl-2, Bcl-XL and also Mcl-1, as compared to other BH-3 binding SMIs. TW-37 induced apoptosis in a de novo chemoresistant WSU-DLCL2 lymphoma cell line 108. Moreover, pre-exposure of lymphoma cells to TW-37 significantly enhanced the killing effect of the cyclophosphamide-doxorubicin-vincristine-prednisone (CHOP) regimen.108, 109.
5.3.3 ApoG2
Apogossypolone is a third generation gossypol derivative, which is also effective in targeting Mcl-1. ApoG2 was developed by Ascenta to reduce toxicity and non-specific reactivity. In preclinical studies, ApoG2 induced apoptosis and displayed promising results in different types of lymphomas 110, 111.
5.3.4 ABT-737
The first in class of SMIs eliciting cancer-specific killing is ABT-737, developed by Abbott Laboratories. Combining structure-activity relationship (SAR) by NMR with structure-based drug design and chemical synthesis led to the discovery of the lead compound ABT-737 that mimicked the BH3 domain of BAD and bound selectively to Bcl-2, Bcl-XL and Bcl-w. ABT-737 was ineffective at activating apoptosis in cells doubly deficient in BAX and BAK, suggesting its activity is mediated through Bcl-2 112. ABT-737 was tested in multiple myeloma (MM) and was shown to abrogate the viability of bortezomib, dexamethasone and thalidomide-refractory patients 113. Acquired resistance to ABT-737 was shown to be associated with increased Mcl-1 expression and reduced expression of its primary target Bcl-2 or Bcl-2 heterodimers 114.
5.3.5 ABT-263
ABT-263 is an orally available derivative of ABT-737 that can bind to serum proteins, resulting in longer half-life, and inhibit Bcl-2, Bcl-XL and Bcl-w. Given its effectiveness as a single agent in preclinical studies, ABT-263 is currently being evaluated in clinical trials for SCLCs and leukemia 115. Using a systems biology approach, Tahir et al. correlated Bcl-2 expression as a function of ABT-263 sensitivity in 36 SCLC and 31 leukemia/ lymphoma cell lines 116, and generated an ABT-263 sensitivity signature based on this data.
5.3.6. AT-101
AT-101 is the negative (−) enantiomer of gossypol and a potent inhibitor of the antiapoptotic Bcl-2 family members, Bcl-2, Bcl-XL and Mcl-1, with an IC50 of 1-10 μM. AT-101 was effective against B-cell lymphomas 117. In mantle cell lymphoma, AT-101 was synergistic when sequentially combined with carfilzomib, etoposide, oxorubicin, and 4-hydroxycyclophosphamide 117. In diffuse large B-cell lymphoma, AT-101 displayed a synergistic effect when sequentially combined with 4-HC 117. AT-101 is currently in phase II clinical trials as a single agent.
5.3.7 Obatoclax
Obatoclax is a synthetic derivative of prodiginines developed by Gemin X (GX015-070), presently Cephalon. Although it is a pan Bcl-2 inhibitor, its binding affinity to Bcl-2 family is less than ABT-737 118, 119. However, Obatoclax was shown to inhibit Mcl-1 and antagonize Mcl-1-mediated resistance, a characteristic not displayed by ABT-737, by interfering with direct interaction between Mcl-1 and BAK 120. Unlike other BH3 mimetics, Obatoclax does not entirely depend on BAX and BAK for apoptosis induction 121. A recent study showed that Obatoclax could induce Bax-mediated apoptosis in cholangiocarcinoma 122. At a lower concentration, Obatoclax induced a S/G2 cell-cycle block, while at a higher concentration it induced apoptosis in CD34+ AML progenitor cells 121, and its effect was more pronounced when combined with AraC. Similarly, in esophageal cancers, Obatoclax showed synergism with carboplatin and 5-flurouracil (5-FU) 123. Another recent study showed that Obatoclax could overcome glucocorticoid resistance in acute lymphoblastic leukemia (ALL) via induction of apoptosis and autophagy 124. However, toxicity may restrict the use of Obatoclax in humans. In a phase I dose escalation study, Obatoclax showed neuropsychiatric toxicities with 1-hour infusions, although 3-hour infusions improved the clinical efficacy and considerably reduced toxicity, suggesting 3-hour infusions as clinically accepted standard of care125. Furthermore, a phase III trial (NCT01563601) for Obatoclax in combination with Carboplatin and Etoposide compared with chemotherapy arm alone is underway in naïve patients with advanced-stage small cell lung cancer.
5.3.8 HA14-1
Wang et al, using in silico screens, developed a new compound HA14-1 as a small molecule antagonist for Bcl-2 proteins 96. It was shown to disrupt the interaction of BAK-BH3-domain peptides with Bcl-2 and Bcl-XL proteins, and strongly inhibit Bcl-2-BAX interactions 126. In MDA-MB-231 breast cancer cells, HA14-1 was shown to act as a chemosensitizer by enhancing the apoptotic effects of cisplatin 127.
5.3.9 Sabutoclax (BI-97C1)
Sabutoclax is a new gossypol derivative originally identified to bind Bcl-XL with low binding affinity. Additionally, it was shown to bind Bcl-2, Mcl-1 and Bfl-1 128 and failed to display toxicity against BAX/BAK double knock out mouse embryonic fibroblasts (MEF), suggesting lack of significant off-target effects compared to its predecessors. Sabutoclax induced apoptosis in diffuse large B-cell lymphoma cells, which were resistant to ABT-737 129. Further testing showed affinities for specific proteins such as Bcl-2 (IC50=0.32 μM), Mcl-1 (IC50=0.20 μM) and Bfl (IC50=0.32 μM) 130. Wei et al. tested the efficacy of BI-97C1 in Mcl-1 overexpressing M2182 prostate cancer cells and observed 60% tumor xenograft inhibition 105.
Our previous results showed that melanoma differentiation associated gene-7/interleukin-24 (mda-7/IL-24) 131 induces cancer-specific apoptosis in multiple cancers and suppression of Mcl-1 augments its cell killing effects 132–134. In a recent study, we showed that Sabutoclax, but not ABT-737, sensitizes mda-7/IL-24-induced apoptosis in prostate cancer cells, confirming the Mcl-1 suppressing role of Sabutoclax 132, 133. Also, a combinatorial approach of Sabutoclax and an adenovirus delivering mda-7/IL-24 induced autophagy that facilitated NOXA and Bim-induced and Bax/BAK-mediated apoptosis.
5.3.10 BI-97D6
In a recent study, we reported the synthesis of a series of ApoG2 derivatives, particularly a chiral compound (±) BI-97D6 that can potently inhibit Bcl-XL, Bcl-2, Mcl-1and Bfl-1 with IC50 values of 76 ± 5, 31 ± 2, 25 ± 8, and 122 ± 28 nM, respectively 129. In the same study we report that, BI97D6 could induce apoptosis in a panel of prostate, lung cancer and lymphoma cell lines, while displaying little toxicity in BAX/BAK deficient cells, suggesting its mode of action is predominantly dependent on the Bcl-2 pathway.
5.3.11 BH3-M6
This is the most recent BH3 mimetic that was designed as a pan Bcl-2 antagonist that inhibits binding of Bcl-XL, Bcl-2 and Mcl-1 to multi-protein domains of BAX or BAK 135. In a recent study Kazi and colleagues showed that, at higher concentrations (25–50 μM), BH3-M6 induced apoptosis in the lung adenocarcinoma cell line A549, and also sensitized cells to apoptosis induced by proteasome inhibitor CEP-1612 135.
6. Combination therapies
Both chemotherapy and radiation-therapy agents are intended to induce apoptosis in cancer cells. However, studies over time proved that these approaches alone exert limited success in the clinic. This is primarily due to the innate ability of cancer cells to acquire resistance based on the mechanisms already discussed. Therefore, tailoring cancer treatment by combining strategies to inhibit anti-apoptotic mediators along with conventional therapies would in principle be a promising approach. Bcl-2 family targeting strategies sensitize cancer cells to be vulnerable to conventional chemotherapeutics, especially those cancers that are resistant due to genetic complexity Furthermore, the effect of Bcl-2 inhibitors in combination with immunotherapy has been evaluated 136. Farsi et al. showed a significant difference in tumor burden in preclinical studies when Obatoclax was treated in combination with recombinant vaccine 137, 138. Several trials that employ this strategy are ongoing and completed studies are listed in Table 2.
7. Expert opinion
Studies over the years in multiple cancer subtypes have provided compelling evidence of the importance of overexpression of anti-apoptotic proteins Bcl-1, Bcl-XL and Mcl-1 in regulating apoptotic-resistance following chemotherapy or radiation-therapy. Overexpression of these proteins or loss of function of pro-apoptotic proteins augments therapy-resistance and further generates genetically unstable cells. There is a pressing mandate to develop novel strategies and drugs using existing tactics to circumvent this observed resistance in multiple cancers. Understanding the regulation of the Bcl-2 family of pro-survival genes and underlying resistance mechanisms and their tissue dynamics will greatly facilitate the development of effective strategies to inhibit Bcl-2-mediated resistance. By inhibiting the Bcl-2 family of pro-survival proteins, the ultimate goal is to eliminate the surviving cancer cells that are primarily apoptosis-resistant or make these cells more vulnerable to conventional therapies when pretreated or used in combination with these agents. Additional critical questions remain that need to be answered in this field and generating a clearer picture of apoptosis-resistance will be useful for developing more efficacious approaches. Although both pro-apoptotic and anti-apoptotic proteins share similar 3-dimensional structure and BH domains, the clear difference in their amino acid sequence that determines mutually opposing roles remains elusive. Better understanding of these amino acid residues and how these proteins interact will aid in developing strategies to modify pro-survival proteins to assume the function of pro-apoptotic proteins.
A major weakness of current Bcl-2 family targeting therapy strategies is their inability to target the complete repertoire of anti-apoptotic proteins with the same affinity, making them effective in killing only those cells that depend on the primary target of the drug as the driver of resistance. Although the significance of Mcl-1 as an oncogene and anti-apoptotic gene has received increasing experimental support, most of the Bcl-2 inhibitors to date are weak Mcl-1 inhibitors. Therefore, treating with those agents may not provide any therapeutic advantage for cancers whose main driver is Mcl-1. Pancreatic cancer is a typical example and conventional chemotherapy and radiotherapy along with Bcl-2 drugs are ineffective in inducing apoptosis in pancreatic cancers. Therefore, we have been employing novel SMIs that can target Mcl-1 as well as Bcl-2 and Bcl-XL to treat therapy-resistant cancers such as pancreatic cancer. Besides Obatoclax, using Sabutoclax or BI-97D6 with their broader spectrum of activity holds promise as a single agent, and more robustly, in combination with other conventional and non-conventional therapeutic agents. Given the heterogeneity of cancers, researchers are recognizing the importance of developing targeting strategies that cover all the Bcl-2 family pro-survival proteins to avoid developing resistance to this targeted therapy. Since Mcl-1 is a major driver of resistance, more potent Mcl-1 targeting strategies could become a mainstay therapy in complex tumors that are resistant to most forms of chemotherapy and radiotherapy such as pancreatic cancers that overexpress Mcl-1 132, 139. Besides Mcl-1, other potential mediators in the apoptotic machinery need to be explored and targeted. Moreover, use of Bcl-2 inhibitors as neoadjuvant therapies in radiation-resistant tumors such as pancreatic cancers, could yield clinical advantage and benefit. High throughput screening technologies coupled with bioinformatic approaches will continue to be employed in the coming years to develop novel drugs against crucial players in cancer development and progression, such as Mcl-1 139. In our opinion, future development of more targeted inhibitors that have higher and broader affinities for all the major players of the Bcl-2 family, combined with personalized approaches targeting other cancer-regulated pathways, will greatly improve patient survival and limit or prevent tumor recurrence
Article Highlight Box.
Bcl-2 family of proteins are functionally involved in either promoting or inhibiting apoptosis, thereby establishing these molecules as pivotal determinants of whether a cell lives or dies.
An appropriate balance between anti-apoptotic and pro-apoptotic molecules is required for tissue homeostasis.
Tumors acquire apoptosis resistance by aberrant expression of bcl-2 family of proteins, mainly by upregulation of pro-survival molecules and down regulation or mutation of pro-apoptotic molecules.
Genetic or pharmacological targeting of Bcl-2 family proteins is a potential strategy to reverse apoptosis resistance to radiation and/or chemotherapy.
Among the strategies developed, BH-3 mimetics hold the most promise and provide an exciting opportunity for cancer therapeutics.
Combining Bcl-2 family targeting strategies has been shown to sensitize cancer cells to conventional chemotherapies and immunotherapies, especially in those cancers that are resistant due to genetic complexity.
Acknowledgments
Support was provided by National Institutes of Health grants 1R01 CA097318, 1R01 CA127641 and the National Foundation for Cancer Research (NFCR) to PBF; 1R01 CA108520 (PD); National Institutes of Health Grant R01 CA138540 and the James S. McDonnell Foundation; National Foundation for Cancer Research (NFCR) (PBF); Samuel Waxman Cancer Research Foundation (SWCRF) (PBF and DS); Army DoD W81XWH-11-1-0480 (PBF and XYW); National Institutes of Health grant R01 CA149668 (J.C.R., M.P.); National Institutes of Health grant R01 CA168517 (MP); and California Institute for Regenerative Medicine grant TR2-01789 (M.P.). DS is a Harrison Scholar and a Blick Scholar in the VCU Massey Cancer Center (MCC) and VCU School of Medicine (SOM), respectively. PBF holds the Thelma Newmeyer Corman Chair in Cancer Research in the VCU MCC and VCU SOM. DS and PBF are SWCRF Investigators.
Footnotes
Conflict of interests: None of the authors have any financial conflict of interest.
References
Papers that are noted are of * interest to readers or ** considerable interest to readers.
- 1.Ellis HM, Horvitz HR. Genetic control of programmed cell death in the nematode C. elegans. Cell. 1986 Mar 28;44(6):817–29. doi: 10.1016/0092-8674(86)90004-8. [DOI] [PubMed] [Google Scholar]
- 2*.Bakshi A, Jensen JP, Goldman P, et al. Cloning the cromosomal break point of the t(14,18) human lymphomas, clustering around JH on chromosome 14 and near a transcriptional unit. Cell. 1985;41(18) doi: 10.1016/s0092-8674(85)80070-2. First demonstration of significance of Bcl-2 family of proteins. [DOI] [PubMed] [Google Scholar]
- 3.Cleary ML, Smith SD, Sklar J. Cloning and structural analysis of cDNAs for bcl-2 and a hybrid bcl-2/immunoglobulin transcript resulting from the t(14;18) translocation. Cell. 1986 Oct 10;47(1):19–28. doi: 10.1016/0092-8674(86)90362-4. [DOI] [PubMed] [Google Scholar]
- 4.Tsujimoto Y, Cossman J, Jaffe E, Croce CM. Involvement of the bcl-2 gene in human follicular lymphoma. Science. 1985 Jun 21;228(4706):1440–3. doi: 10.1126/science.3874430. [DOI] [PubMed] [Google Scholar]
- 5.Vaux DL, Cory S, Adams JM. Bcl-2 gene promotes haemopoietic cell survival and cooperates with c-myc to immortalize pre-B cells. Nature. 1988 Sep 29;335(6189):440–2. doi: 10.1038/335440a0. [DOI] [PubMed] [Google Scholar]
- 6.Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nature reviews Molecular cell biology. 2008 Jan;9(1):47–59. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]
- 7.Hakem R, Hakem A, Duncan GS, Henderson JT, Woo M, Soengas MS, et al. Differential requirement for caspase 9 in apoptotic pathways in vivo. Cell. 1998 Aug 7;94(3):339–52. doi: 10.1016/s0092-8674(00)81477-4. [DOI] [PubMed] [Google Scholar]
- 8.Willis SN, Fletcher JI, Kaufmann T, van Delft MF, Chen L, Czabotar PE, et al. Apoptosis initiated when BH3 ligands engage multiple Bcl-2 homologs, not Bax or Bak. Science. 2007 Feb 9;315(5813):856–9. doi: 10.1126/science.1133289. [DOI] [PubMed] [Google Scholar]
- 9.Lindsten T, Thompson CB. Cell death in the absence of Bax and Bak. Cell death and differentiation. 2006 Aug;13(8):1272–6. doi: 10.1038/sj.cdd.4401953. [DOI] [PubMed] [Google Scholar]
- 10.Ackler S, Mitten MJ, Foster K, Oleksijew A, Refici M, Tahir SK, et al. The Bcl-2 inhibitor ABT-263 enhances the response of multiple chemotherapeutic regimens in hematologic tumors in vivo. Cancer chemotherapy and pharmacology. 2010 Oct;66(5):869–80. doi: 10.1007/s00280-009-1232-1. [DOI] [PubMed] [Google Scholar]
- 11.Oberstein A, Jeffrey PD, Shi Y. Crystal structure of the Bcl-XL-Beclin 1 peptide complex: Beclin 1 is a novel BH3-only protein. The Journal of biological chemistry. 2007 Apr 27;282(17):13123–32. doi: 10.1074/jbc.M700492200. [DOI] [PubMed] [Google Scholar]
- 12.Maiuri MC, Le Toumelin G, Criollo A, Rain JC, Gautier F, Juin P, et al. Functional and physical interaction between Bcl-X(L) and a BH3-like domain in Beclin-1. The EMBO journal. 2007 May 16;26(10):2527–39. doi: 10.1038/sj.emboj.7601689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bedikian AY, Millward M, Pehamberger H, Conry R, Gore M, Trefzer U, et al. Bcl-2 antisense (oblimersen sodium) plus dacarbazine in patients with advanced melanoma: the Oblimersen Melanoma Study Group. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2006 Oct 10;24(29):4738–45. doi: 10.1200/JCO.2006.06.0483. [DOI] [PubMed] [Google Scholar]
- 14.Ranger AM, Malynn BA, Korsmeyer SJ. Mouse models of cell death. Nature genetics. 2001 Jun;28(2):113–8. doi: 10.1038/88815. [DOI] [PubMed] [Google Scholar]
- 15.Leibowitz B, Yu J. Mitochondrial signaling in cell death via the Bcl-2 family. Cancer biology & therapy. 2010 Mar;9(6):417–22. doi: 10.4161/cbt.9.6.11392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Green DR, Chipuk JE. p53 and metabolism: Inside the TIGAR. Cell. 2006 Jul 14;126(1):30–2. doi: 10.1016/j.cell.2006.06.032. [DOI] [PubMed] [Google Scholar]
- 17.Look AT. Oncogenic transcription factors in the human acute leukemias. Science. 1997 Nov 7;278(5340):1059–64. doi: 10.1126/science.278.5340.1059. [DOI] [PubMed] [Google Scholar]
- 18.Stahnke K, Fulda S, Friesen C, Strauss G, Debatin KM. Activation of apoptosis pathways in peripheral blood lymphocytes by in vivo chemotherapy. Blood. 2001 Nov 15;98(10):3066–73. doi: 10.1182/blood.v98.10.3066. [DOI] [PubMed] [Google Scholar]
- 19.Herr I, Debatin KM. Cellular stress response and apoptosis in cancer therapy. Blood. 2001 Nov 1;98(9):2603–14. doi: 10.1182/blood.v98.9.2603. [DOI] [PubMed] [Google Scholar]
- 20.Igney FH, Krammer PH. Death and anti-death: tumour resistance to apoptosis. Nature reviews Cancer. 2002 Apr;2(4):277–88. doi: 10.1038/nrc776. [DOI] [PubMed] [Google Scholar]
- 21.Weller M, Malipiero U, Aguzzi A, Reed JC, Fontana A. Protooncogene bcl-2 gene transfer abrogates Fas/APO-1 antibody-mediated apoptosis of human malignant glioma cells and confers resistance to chemotherapeutic drugs and therapeutic irradiation. The Journal of clinical investigation. 1995 Jun;95(6):2633–43. doi: 10.1172/JCI117965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Campos L, Rouault JP, Sabido O, Oriol P, Roubi N, Vasselon C, et al. High expression of bcl-2 protein in acute myeloid leukemia cells is associated with poor response to chemotherapy. Blood. 1993 Jun 1;81(11):3091–6. [PubMed] [Google Scholar]
- 23.Hermine O, Haioun C, Lepage E, d’Agay MF, Briere J, Lavignac C, et al. Prognostic significance of bcl-2 protein expression in aggressive non-Hodgkin’s lymphoma. Groupe d’Etude des Lymphomes de l’Adulte (GELA) Blood. 1996 Jan 1;87(1):265–72. [PubMed] [Google Scholar]
- 24.Bincoletto C, Saad ST, da Silva ES, Queiroz ML. Haematopoietic response and bcl-2 expression in patients with acute myeloid leukaemia. European journal of haematology. 1999 Jan;62(1):38–42. doi: 10.1111/j.1600-0609.1999.tb01112.x. [DOI] [PubMed] [Google Scholar]
- 25.Karakas T, Maurer U, Weidmann E, Miething CC, Hoelzer D, Bergmann L. High expression of bcl-2 mRNA as a determinant of poor prognosis in acute myeloid leukemia. Annals of oncology: official journal of the European Society for Medical Oncology / ESMO. 1998 Feb;9(2):159–65. doi: 10.1023/a:1008255511404. [DOI] [PubMed] [Google Scholar]
- 26.Grover R, Wilson GD. Bcl-2 expression in malignant melanoma and its prognostic significance. European journal of surgical oncology: the journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology. 1996 Aug;22(4):347–9. doi: 10.1016/s0748-7983(96)90176-6. [DOI] [PubMed] [Google Scholar]
- 27.Joensuu H, Pylkkanen L, Toikkanen S. Bcl-2 protein expression and long-term survival in breast cancer. The American journal of pathology. 1994 Nov;145(5):1191–8. [PMC free article] [PubMed] [Google Scholar]
- 28.McDonnell TJ, Troncoso P, Brisbay SM, Logothetis C, Chung LW, Hsieh JT, et al. Expression of the protooncogene bcl-2 in the prostate and its association with emergence of androgen-independent prostate cancer. Cancer research. 1992 Dec 15;52(24):6940–4. [PubMed] [Google Scholar]
- 29.Jiang SX, Sato Y, Kuwao S, Kameya T. Expression of bcl-2 oncogene protein is prevalent in small cell lung carcinomas. The Journal of pathology. 1995 Oct;177(2):135–8. doi: 10.1002/path.1711770206. [DOI] [PubMed] [Google Scholar]
- 30.Sinicrope FA, Hart J, Michelassi F, Lee JJ. Prognostic value of bcl-2 oncoprotein expression in stage II colon carcinoma. Clinical cancer research: an official journal of the American Association for Cancer Research. 1995 Oct;1(10):1103–10. [PubMed] [Google Scholar]
- 31.Gazzaniga P, Gradilone A, Vercillo R, Gandini O, Silvestri I, Napolitano M, et al. Bcl-2/bax mRNA expression ratio as prognostic factor in low-grade urinary bladder cancer. International journal of cancer Journal international du cancer. 1996 Apr 22;69(2):100–4. doi: 10.1002/(SICI)1097-0215(19960422)69:2<100::AID-IJC5>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 32.Miyashita T, Reed JC. bcl-2 gene transfer increases relative resistance of S49.1 and WEHI7.2 lymphoid cells to cell death and DNA fragmentation induced by glucocorticoids and multiple chemotherapeutic drugs. Cancer research. 1992 Oct 1;52(19):5407–11. [PubMed] [Google Scholar]
- 33.Fulda S, Friesen C, Los M, Scaffidi C, Mier W, Benedict M, et al. Betulinic acid triggers CD95 (APO-1/Fas)- and p53-independent apoptosis via activation of caspases in neuroectodermal tumors. Cancer research. 1997 Nov 1;57(21):4956–64. [PubMed] [Google Scholar]
- 34.McDonnell TJ, Deane N, Platt FM, Nunez G, Jaeger U, McKearn JP, et al. bcl-2-immunoglobulin transgenic mice demonstrate extended B cell survival and follicular lymphoproliferation. Cell. 1989 Apr 7;57(1):79–88. doi: 10.1016/0092-8674(89)90174-8. [DOI] [PubMed] [Google Scholar]
- 35*.Reed JC, Cuddy M, Slabiak T, Croce CM, Nowell PC. Oncogenic potential of bcl-2 demonstrated by gene transfer. Nature. 1988 Nov 17;336(6196):259–61. doi: 10.1038/336259a0. First report on Bcl-2 targettig approach. [DOI] [PubMed] [Google Scholar]
- 36.Weiss LM, Warnke RA, Sklar J, Cleary ML. Molecular analysis of the t(14;18) chromosomal translocation in malignant lymphomas. The New England journal of medicine. 1987 Nov 5;317(19):1185–9. doi: 10.1056/NEJM198711053171904. [DOI] [PubMed] [Google Scholar]
- 37.Rao PH, Houldsworth J, Dyomina K, Parsa NZ, Cigudosa JC, Louie DC, et al. Chromosomal and gene amplification in diffuse large B-cell lymphoma. Blood. 1998 Jul 1;92(1):234–40. [PubMed] [Google Scholar]
- 38.Hanada M, Delia D, Aiello A, Stadtmauer E, Reed JC. bcl-2 gene hypomethylation and high-level expression in B-cell chronic lymphocytic leukemia. Blood. 1993 Sep 15;82(6):1820–8. [PubMed] [Google Scholar]
- 39.Cimmino A, Calin GA, Fabbri M, Iorio MV, Ferracin M, Shimizu M, et al. miR-15 and miR-16 induce apoptosis by targeting BCL2. Proceedings of the National Academy of Sciences of the United States of America. 2005 Sep 27;102(39):13944–9. doi: 10.1073/pnas.0506654102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Singh R, Saini N. Downregulation of BCL2 by miRNAs augments drug-induced apoptosis - a combined computational and experimental approach. Journal of cell science. 2012 Mar 15;125(Pt 6):1568–78. doi: 10.1242/jcs.095976. [DOI] [PubMed] [Google Scholar]
- 41.Henderson S, Huen D, Rowe M, Dawson C, Johnson G, Rickinson A. Epstein-Barr virus-coded BHRF1 protein, a viral homologue of Bcl-2, protects human B cells from programmed cell death. Proceedings of the National Academy of Sciences of the United States of America. 1993 Sep 15;90(18):8479–83. doi: 10.1073/pnas.90.18.8479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tarodi B, Subramanian T, Chinnadurai G. Epstein-Barr virus BHRF1 protein protects against cell death induced by DNA-damaging agents and heterologous viral infection. Virology. 1994 Jun;201(2):404–7. doi: 10.1006/viro.1994.1309. [DOI] [PubMed] [Google Scholar]
- 43.Sarid R, Sato T, Bohenzky RA, Russo JJ, Chang Y. Kaposi’s sarcoma-associated herpesvirus encodes a functional bcl-2 homologue. Nature medicine. 1997 Mar;3(3):293–8. doi: 10.1038/nm0397-293. [DOI] [PubMed] [Google Scholar]
- 44.Boise LH, Gonzalez-Garcia M, Postema CE, Ding L, Lindsten T, Turka LA, et al. bcl-x, a bcl-2-related gene that functions as a dominant regulator of apoptotic cell death. Cell. 1993 Aug 27;74(4):597–608. doi: 10.1016/0092-8674(93)90508-n. [DOI] [PubMed] [Google Scholar]
- 45.Kirsh EJ, Baunoch DA, Stadler WM. Expression of bcl-2 and bcl-X in bladder cancer. The Journal of urology. 1998 Apr;159(4):1348–53. [PubMed] [Google Scholar]
- 46.Nagane M, Levitzki A, Gazit A, Cavenee WK, Huang HJ. Drug resistance of human glioblastoma cells conferred by a tumor-specific mutant epidermal growth factor receptor through modulation of Bcl-XL and caspase-3-like proteases. Proceedings of the National Academy of Sciences of the United States of America. 1998 May 12;95(10):5724–9. doi: 10.1073/pnas.95.10.5724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Castilla C, Congregado B, Chinchon D, Torrubia FJ, Japon MA, Saez C. Bcl-xL is overexpressed in hormone-resistant prostate cancer and promotes survival of LNCaP cells via interaction with proapoptotic Bak. Endocrinology. 2006 Oct;147(10):4960–7. doi: 10.1210/en.2006-0502. [DOI] [PubMed] [Google Scholar]
- 48.Kaufmann SH, Karp JE, Svingen PA, Krajewski S, Burke PJ, Gore SD, et al. Elevated expression of the apoptotic regulator Mcl-1 at the time of leukemic relapse. Blood. 1998 Feb 1;91(3):991–1000. [PubMed] [Google Scholar]
- 49.Shigemasa K, Katoh O, Shiroyama Y, Mihara S, Mukai K, Nagai N, et al. Increased MCL-1 expression is associated with poor prognosis in ovarian carcinomas. Japanese journal of cancer research: Gann. 2002 May;93(5):542–50. doi: 10.1111/j.1349-7006.2002.tb01289.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wei LH, Kuo ML, Chen CA, Chou CH, Cheng WF, Chang MC, et al. The anti-apoptotic role of interleukin-6 in human cervical cancer is mediated by up-regulation of Mcl-1 through a PI 3-K/Akt pathway. Oncogene. 2001 Sep 13;20(41):5799–809. doi: 10.1038/sj.onc.1204733. [DOI] [PubMed] [Google Scholar]
- 51.Schwickart M, Huang X, Lill JR, Liu J, Ferrando R, French DM, et al. Deubiquitinase USP9X stabilizes MCL1 and promotes tumour cell survival. Nature. 2010 Jan 7;463(7277):103–7. doi: 10.1038/nature08646. [DOI] [PubMed] [Google Scholar]
- 52.Hussain S, Datta S, Roy RK, Pal AK. Surface plasmon resonance in nanocrystalline gold-copper alloy films. Journal of nanoscience and nanotechnology. 2007 Dec;7(12):4486–93. doi: 10.1166/jnn.2007.907. [DOI] [PubMed] [Google Scholar]
- 53.Liu Y, Hernandez AM, Shibata D, Cortopassi GA. BCL2 translocation frequency rises with age in humans. Proceedings of the National Academy of Sciences of the United States of America. 1994 Sep 13;91(19):8910–4. doi: 10.1073/pnas.91.19.8910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zelenetz AD, Chen TT, Levy R. Clonal expansion in follicular lymphoma occurs subsequent to antigenic selection. The Journal of experimental medicine. 1992 Oct 1;176(4):1137–48. doi: 10.1084/jem.176.4.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55*.Yano T, Jaffe ES, Longo DL, Raffeld M. MYC rearrangements in histologically progressed follicular lymphomas. Blood. 1992 Aug 1;80(3):758–67. Initial report on a Bcl-2 transgenic mice developed that forms tumors. [PubMed] [Google Scholar]
- 56.Strasser A, Harris AW, Bath ML, Cory S. Novel primitive lymphoid tumours induced in transgenic mice by cooperation between myc and bcl-2. Nature. 1990 Nov 22;348(6299):331–3. doi: 10.1038/348331a0. [DOI] [PubMed] [Google Scholar]
- 57.Strasser A, Elefanty AG, Harris AW, Cory S. Progenitor tumours from Emu-bcl-2-myc transgenic mice have lymphomyeloid differentiation potential and reveal developmental differences in cell survival. The EMBO journal. 1996 Aug 1;15(15):3823–34. [PMC free article] [PubMed] [Google Scholar]
- 58.Jager R, Herzer U, Schenkel J, Weiher H. Overexpression of Bcl-2 inhibits alveolar cell apoptosis during involution and accelerates c-myc-induced tumorigenesis of the mammary gland in transgenic mice. Oncogene. 1997 Oct 9;15(15):1787–95. doi: 10.1038/sj.onc.1201353. [DOI] [PubMed] [Google Scholar]
- 59.Naik P, Karrim J, Hanahan D. The rise and fall of apoptosis during multistage tumorigenesis: down-modulation contributes to tumor progression from angiogenic progenitors. Genes & development. 1996 Sep 1;10(17):2105–16. doi: 10.1101/gad.10.17.2105. [DOI] [PubMed] [Google Scholar]
- 60.Pelengaris S, Khan M, Evan GI. Suppression of Myc-induced apoptosis in beta cells exposes multiple oncogenic properties of Myc and triggers carcinogenic progression. Cell. 2002 May 3;109(3):321–34. doi: 10.1016/s0092-8674(02)00738-9. [DOI] [PubMed] [Google Scholar]
- 61.Eischen CM, Woo D, Roussel MF, Cleveland JL. Apoptosis triggered by Myc-induced suppression of Bcl-X(L) or Bcl-2 is bypassed during lymphomagenesis. Molecular and cellular biology. 2001 Aug;21(15):5063–70. doi: 10.1128/MCB.21.15.5063-5070.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Michaud WA, Nichols AC, Mroz EA, Faquin WC, Clark JR, Begum S, et al. Bcl-2 blocks cisplatin-induced apoptosis and predicts poor outcome following chemoradiation treatment in advanced oropharyngeal squamous cell carcinoma. Clinical cancer research: an official journal of the American Association for Cancer Research. 2009 Mar 1;15(5):1645–54. doi: 10.1158/1078-0432.CCR-08-2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kumar Biswas S, Huang J, Persaud S, Basu A. Down-regulation of Bcl-2 is associated with cisplatin resistance in human small cell lung cancer H69 cells. Molecular cancer therapeutics. 2004 Mar;3(3):327–34. [PubMed] [Google Scholar]
- 64.Nencioni A, Hua F, Dillon CP, Yokoo R, Scheiermann C, Cardone MH, et al. Evidence for a protective role of Mcl-1 in proteasome inhibitor-induced apoptosis. Blood. 2005 Apr 15;105(8):3255–62. doi: 10.1182/blood-2004-10-3984. [DOI] [PubMed] [Google Scholar]
- 65.Greenblatt MS, Bennett WP, Hollstein M, Harris CC. Mutations in the p53 tumor suppressor gene: clues to cancer etiology and molecular pathogenesis. Cancer research. 1994 Sep 15;54(18):4855–78. [PubMed] [Google Scholar]
- 66.Meijerink JP, Raemaekers JM, Mensink EJ. New type of t(14;18) in a non-Hodgkin’s lymphoma provides insight in molecular events in early B-cell differentiation. British journal of haematology. 1995 Nov;91(3):630–9. doi: 10.1111/j.1365-2141.1995.tb05359.x. [DOI] [PubMed] [Google Scholar]
- 67.Rampino N, Yamamoto H, Ionov Y, Li Y, Sawai H, Reed JC, et al. Somatic frameshift mutations in the BAX gene in colon cancers of the microsatellite mutator phenotype. Science. 1997 Feb 14;275(5302):967–9. doi: 10.1126/science.275.5302.967. [DOI] [PubMed] [Google Scholar]
- 68.Oda E, Ohki R, Murasawa H, Nemoto J, Shibue T, Yamashita T, et al. Noxa, a BH3-only member of the Bcl-2 family and candidate mediator of p53-induced apoptosis. Science. 2000 May 12;288(5468):1053–8. doi: 10.1126/science.288.5468.1053. [DOI] [PubMed] [Google Scholar]
- 69.Nakano K, Vousden KH. PUMA, a novel proapoptotic gene, is induced by p53. Molecular cell. 2001 Mar;7(3):683–94. doi: 10.1016/s1097-2765(01)00214-3. [DOI] [PubMed] [Google Scholar]
- 70.Shibue T, Takeda K, Oda E, Tanaka H, Murasawa H, Takaoka A, et al. Integral role of Noxa in p53-mediated apoptotic response. Genes & development. 2003 Sep 15;17(18):2233–8. doi: 10.1101/gad.1103603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chonghaile TN, Letai A. Mimicking the BH3 domain to kill cancer cells. Oncogene. 2008 Dec;27(Suppl 1):S149–57. doi: 10.1038/onc.2009.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Del Gaizo Moore V, Brown JR, Certo M, Love TM, Novina CD, Letai A. Chronic lymphocytic leukemia requires BCL2 to sequester prodeath BIM, explaining sensitivity to BCL2 antagonist ABT-737. The Journal of clinical investigation. 2007 Jan;117(1):112–21. doi: 10.1172/JCI28281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Strasser A, Harris AW, Jacks T, Cory S. DNA damage can induce apoptosis in proliferating lymphoid cells via p53-independent mechanisms inhibitable by Bcl-2. Cell. 1994 Oct 21;79(2):329–39. doi: 10.1016/0092-8674(94)90201-1. [DOI] [PubMed] [Google Scholar]
- 74.Kyprianou N, King ED, Bradbury D, Rhee JG. bcl-2 over-expression delays radiation-induced apoptosis without affecting the clonogenic survival of human prostate cancer cells. International journal of cancer Journal international du cancer. 1997 Jan 27;70(3):341–8. doi: 10.1002/(sici)1097-0215(19970127)70:3<341::aid-ijc16>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 75.Rosser CJ, Gaar M, Porvasnik S. Molecular fingerprinting of radiation resistant tumors: can we apprehend and rehabilitate the suspects? BMC cancer. 2009;9:225. doi: 10.1186/1471-2407-9-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Aebersold DM, Kollar A, Beer KT, Laissue J, Greiner RH, Djonov V. Involvement of the hepatocyte growth factor/scatter factor receptor c-met and of Bcl-xL in the resistance of oropharyngeal cancer to ionizing radiation. International journal of cancer Journal international du cancer. 2001 Feb 20;96(1):41–54. doi: 10.1002/1097-0215(20010220)96:1<41::aid-ijc5>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 77.Lee JU, Hosotani R, Wada M, Doi R, Kosiba T, Fujimoto K, et al. Role of Bcl-2 family proteins (Bax, Bcl-2 and Bcl-X) on cellular susceptibility to radiation in pancreatic cancer cells. Eur J Cancer. 1999 Sep;35(9):1374–80. doi: 10.1016/s0959-8049(99)00134-3. [DOI] [PubMed] [Google Scholar]
- 78.Lam M, Dubyak G, Chen L, Nunez G, Miesfeld RL, Distelhorst CW. Evidence that BCL-2 represses apoptosis by regulating endoplasmic reticulum-associated Ca2+ fluxes. Proceedings of the National Academy of Sciences of the United States of America. 1994 Jul 5;91(14):6569–73. doi: 10.1073/pnas.91.14.6569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hockenbery DM, Oltvai ZN, Yin XM, Milliman CL, Korsmeyer SJ. Bcl-2 functions in an antioxidant pathway to prevent apoptosis. Cell. 1993 Oct 22;75(2):241–51. doi: 10.1016/0092-8674(93)80066-n. [DOI] [PubMed] [Google Scholar]
- 80.Ellerby LM, Ellerby HM, Park SM, Holleran AL, Murphy AN, Fiskum G, et al. Shift of the cellular oxidation-reduction potential in neural cells expressing Bcl-2. Journal of neurochemistry. 1996 Sep;67(3):1259–67. doi: 10.1046/j.1471-4159.1996.67031259.x. [DOI] [PubMed] [Google Scholar]
- 81.Wright SC, Wang H, Wei QS, Kinder DH, Larrick JW. Bcl-2-mediated resistance to apoptosis is associated with glutathione-induced inhibition of AP24 activation of nuclear DNA fragmentation. Cancer research. 1998 Dec 1;58(23):5570–6. [PubMed] [Google Scholar]
- 82.Sieghart W, Losert D, Strommer S, Cejka D, Schmid K, Rasoul-Rockenschaub S, et al. Mcl-1 overexpression in hepatocellular carcinoma: a potential target for antisense therapy. Journal of hepatology. 2006 Jan;44(1):151–7. doi: 10.1016/j.jhep.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 83.Kagawa S, Pearson SA, Ji L, Xu K, McDonnell TJ, Swisher SG, et al. A binary adenoviral vector system for expressing high levels of the proapoptotic gene bax. Gene therapy. 2000 Jan;7(1):75–9. doi: 10.1038/sj.gt.3301048. [DOI] [PubMed] [Google Scholar]
- 84.Lee EF, Czabotar PE, Yang H, Sleebs BE, Lessene G, Colman PM, et al. Conformational changes in Bcl-2 pro-survival proteins determine their capacity to bind ligands. The Journal of biological chemistry. 2009 Oct 30;284(44):30508–17. doi: 10.1074/jbc.M109.040725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kitada S, Zapata JM, Andreeff M, Reed JC. Protein kinase inhibitors flavopiridol and 7-hydroxy-staurosporine down-regulate antiapoptosis proteins in B-cell chronic lymphocytic leukemia. Blood. 2000 Jul 15;96(2):393–7. [PubMed] [Google Scholar]
- 86.Rahmani M, Davis EM, Crabtree TR, Habibi JR, Nguyen TK, Dent P, et al. The kinase inhibitor sorafenib induces cell death through a process involving induction of endoplasmic reticulum stress. Molecular and cellular biology. 2007 Aug;27(15):5499–513. doi: 10.1128/MCB.01080-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhong Q, Gao W, Du F, Wang X. Mule/ARF-BP1, a BH3-only E3 ubiquitin ligase, catalyzes the polyubiquitination of Mcl-1 and regulates apoptosis. Cell. 2005 Jul 1;121(7):1085–95. doi: 10.1016/j.cell.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 88.Sun H, Kapuria V, Peterson LF, Fang D, Bornmann WG, Bartholomeusz G, et al. Bcr-Abl ubiquitination and Usp9x inhibition block kinase signaling and promote CML cell apoptosis. Blood. 2011 Mar 17;117(11):3151–62. doi: 10.1182/blood-2010-03-276477. [DOI] [PubMed] [Google Scholar]
- 89.Johnstone RW, Ruefli AA, Lowe SW. Apoptosis: a link between cancer genetics and chemotherapy. Cell. 2002 Jan 25;108(2):153–64. doi: 10.1016/s0092-8674(02)00625-6. [DOI] [PubMed] [Google Scholar]
- 90.Olie RA, Zangemeister-Wittke U. Targeting tumor cell resistance to apoptosis induction with antisense oligonucleotides: progress and therapeutic potential. Drug resistance updates: reviews and commentaries in antimicrobial and anticancer chemotherapy. 2001 Feb;4(1):9–15. doi: 10.1054/drup.2001.0181. [DOI] [PubMed] [Google Scholar]
- 91.O’Brien S, Moore JO, Boyd TE, Larratt LM, Skotnicki AB, Koziner B, et al. 5-year survival in patients with relapsed or refractory chronic lymphocytic leukemia in a randomized, phase III trial of fludarabine plus cyclophosphamide with or without oblimersen. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2009 Nov 1;27(31):5208–12. doi: 10.1200/JCO.2009.22.5748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dai G, Wei X, Liu Z, Liu S, Marcucci G, Chan KK. Characterization and quantification of Bcl-2 antisense G3139 and metabolites in plasma and urine by ion-pair reversed phase HPLC coupled with electrospray ion-trap mass spectrometry. Journal of chromatography B, Analytical technologies in the biomedical and life sciences. 2005 Oct 25;825(2):201–13. doi: 10.1016/j.jchromb.2005.05.049. [DOI] [PubMed] [Google Scholar]
- 93.Heere-Ress E, Thallinger C, Lucas T, Schlagbauer-Wadl H, Wacheck V, Monia BP, et al. Bcl-X(L) is a chemoresistance factor in human melanoma cells that can be inhibited by antisense therapy. International journal of cancer Journal international du cancer. 2002 May 1;99(1):29–34. doi: 10.1002/ijc.10248. [DOI] [PubMed] [Google Scholar]
- 94*.Zangemeister-Wittke U, Leech SH, Olie RA, Simoes-Wust AP, Gautschi O, Luedke GH, et al. A novel bispecific antisense oligonucleotide inhibiting both bcl-2 and bcl-xL expression efficiently induces apoptosis in tumor cells. Clinical cancer research: an official journal of the American Association for Cancer Research. 2000 Jun;6(6):2547–55. An antisense approach to inhibit Bcl-2 and Bcl-XL. [PubMed] [Google Scholar]
- 95.Piche A, Grim J, Rancourt C, Gomez-Navarro J, Reed JC, Curiel DT. Modulation of Bcl-2 protein levels by an intracellular anti-Bcl-2 single-chain antibody increases drug-induced cytotoxicity in the breast cancer cell line MCF-7. Cancer research. 1998 May 15;58(10):2134–40. [PubMed] [Google Scholar]
- 96.Wang JL, Zhang ZJ, Choksi S, Shan S, Lu Z, Croce CM, et al. Cell permeable Bcl-2 binding peptides: a chemical approach to apoptosis induction in tumor cells. Cancer research. 2000 Mar 15;60(6):1498–502. [PubMed] [Google Scholar]
- 97.Chang BS, Minn AJ, Muchmore SW, Fesik SW, Thompson CB. Identification of a novel regulatory domain in Bcl-X(L) and Bcl-2. The EMBO journal. 1997 Mar 3;16(5):968–77. doi: 10.1093/emboj/16.5.968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98*.Sattler M, Liang H, Nettesheim D, Meadows RP, Harlan JE, Eberstadt M, et al. Structure of Bcl-xL-Bak peptide complex: recognition between regulators of apoptosis. Science. 1997 Feb 14;275(5302):983–6. doi: 10.1126/science.275.5302.983. Representation of peptidomimetics as a novel strategy to target Bcl-2 family. [DOI] [PubMed] [Google Scholar]
- 99.Kitada S, Leone M, Sareth S, Zhai D, Reed JC, Pellecchia M. Discovery, characterization, and structure-activity relationships studies of proapoptotic polyphenols targeting B-cell lymphocyte/leukemia-2 proteins. Journal of medicinal chemistry. 2003 Sep 25;46(20):4259–64. doi: 10.1021/jm030190z. [DOI] [PubMed] [Google Scholar]
- 100.Nakashima T, Miura M, Hara M. Tetrocarcin A inhibits mitochondrial functions of Bcl-2 and suppresses its anti-apoptotic activity. Cancer research. 2000 Mar 1;60(5):1229–35. [PubMed] [Google Scholar]
- 101.Kolluri SK, Zhu X, Zhou X, Lin B, Chen Y, Sun K, et al. A short Nur77-derived peptide converts Bcl-2 from a protector to a killer. Cancer cell. 2008 Oct 7;14(4):285–98. doi: 10.1016/j.ccr.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Labelle JL, Katz SG, Bird GH, Gavathiotis E, Stewart ML, Lawrence C, et al. A stapled BIM peptide overcomes apoptotic resistance in hematologic cancers. The Journal of clinical investigation. 2012 Jun 1;122(6):2018–31. doi: 10.1172/JCI46231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tzung SP, Kim KM, Basanez G, Giedt CD, Simon J, Zimmerberg J, et al. Antimycin A mimics a cell-death-inducing Bcl-2 homology domain 3. Nature cell biology. 2001 Feb;3(2):183–91. doi: 10.1038/35055095. [DOI] [PubMed] [Google Scholar]
- 104.Zaidi R, Hadi SM. Complexes involving gossypol, DNA and Cu(II) Biochemistry international. 1992 Dec;28(6):1135–43. [PubMed] [Google Scholar]
- 105.Wei J, Kitada S, Stebbins JL, Placzek W, Zhai D, Wu B, et al. Synthesis and biological evaluation of Apogossypolone derivatives as pan-active inhibitors of antiapoptotic B-cell lymphoma/leukemia-2 (Bcl-2) family proteins. Journal of medicinal chemistry. 2010 Nov 25;53(22):8000–11. doi: 10.1021/jm100746q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wang G, Nikolovska-Coleska Z, Yang CY, Wang R, Tang G, Guo J, et al. Structure-based design of potent small-molecule inhibitors of anti-apoptotic Bcl-2 proteins. Journal of medicinal chemistry. 2006 Oct 19;49(21):6139–42. doi: 10.1021/jm060460o. [DOI] [PubMed] [Google Scholar]
- 107.Wang Z, Song W, Aboukameel A, Mohammad M, Wang G, Banerjee S, et al. TW-37, a small-molecule inhibitor of Bcl-2, inhibits cell growth and invasion in pancreatic cancer. International journal of cancer Journal international du cancer. 2008 Aug 15;123(4):958–66. doi: 10.1002/ijc.23610. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 108.Mohammad RM, Goustin AS, Aboukameel A, Chen B, Banerjee S, Wang G, et al. Preclinical studies of TW-37, a new nonpeptidic small-molecule inhibitor of Bcl-2, in diffuse large cell lymphoma xenograft model reveal drug action on both Bcl-2 and Mcl-1. Clinical cancer research: an official journal of the American Association for Cancer Research. 2007 Apr 1;13(7):2226–35. doi: 10.1158/1078-0432.CCR-06-1574. [DOI] [PubMed] [Google Scholar]
- 109.Zeitlin BD, Joo E, Dong Z, Warner K, Wang G, Nikolovska-Coleska Z, et al. Antiangiogenic effect of TW37, a small-molecule inhibitor of Bcl-2. Cancer research. 2006 Sep 1;66(17):8698–706. doi: 10.1158/0008-5472.CAN-05-3691. [DOI] [PubMed] [Google Scholar]
- 110.Hu ZY, Sun J, Zhu XF, Yang D, Zeng YX. ApoG2 induces cell cycle arrest of nasopharyngeal carcinoma cells by suppressing the c-Myc signaling pathway. Journal of translational medicine. 2009;7:74. doi: 10.1186/1479-5876-7-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sun J, Li ZM, Hu ZY, Zeng ZL, Yang DJ, Jiang WQ. Apogossypolone inhibits cell growth by inducing cell cycle arrest in U937 cells. Oncology reports. 2009 Jul;22(1):193–8. doi: 10.3892/or_00000424. [DOI] [PubMed] [Google Scholar]
- 112.van Delft MF, Wei AH, Mason KD, Vandenberg CJ, Chen L, Czabotar PE, et al. The BH3 mimetic ABT-737 targets selective Bcl-2 proteins and efficiently induces apoptosis via Bak/Bax if Mcl-1 is neutralized. Cancer cell. 2006 Nov;10(5):389–99. doi: 10.1016/j.ccr.2006.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chauhan D, Velankar M, Brahmandam M, Hideshima T, Podar K, Richardson P, et al. A novel Bcl-2/Bcl-X(L)/Bcl-w inhibitor ABT-737 as therapy in multiple myeloma. Oncogene. 2007 Apr 5;26(16):2374–80. doi: 10.1038/sj.onc.1210028. [DOI] [PubMed] [Google Scholar]
- 114.Hann CL, Daniel VC, Sugar EA, Dobromilskaya I, Murphy SC, Cope L, et al. Therapeutic efficacy of ABT-737, a selective inhibitor of BCL-2, in small cell lung cancer. Cancer research. 2008 Apr 1;68(7):2321–8. doi: 10.1158/0008-5472.CAN-07-5031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Park CM, Bruncko M, Adickes J, Bauch J, Ding H, Kunzer A, et al. Discovery of an orally bioavailable small molecule inhibitor of prosurvival B-cell lymphoma 2 proteins. Journal of medicinal chemistry. 2008 Nov 13;51(21):6902–15. doi: 10.1021/jm800669s. [DOI] [PubMed] [Google Scholar]
- 116.Tahir SK, Wass J, Joseph MK, Devanarayan V, Hessler P, Zhang H, et al. Identification of expression signatures predictive of sensitivity to the Bcl-2 family member inhibitor ABT-263 in small cell lung carcinoma and leukemia/lymphoma cell lines. Molecular cancer therapeutics. 2010 Mar;9(3):545–57. doi: 10.1158/1535-7163.MCT-09-0651. [DOI] [PubMed] [Google Scholar]
- 117.Paoluzzi L, Gonen M, Gardner JR, Mastrella J, Yang D, Holmlund J, et al. Targeting Bcl-2 family members with the BH3 mimetic AT-101 markedly enhances the therapeutic effects of chemotherapeutic agents in in vitro and in vivo models of B-cell lymphoma. Blood. 2008 Jun 1;111(11):5350–8. doi: 10.1182/blood-2007-12-129833. [DOI] [PubMed] [Google Scholar]
- 118**.Reed JC. Apoptosis-targeted therapies for cancer. Cancer cell. 2003 Jan;3(1):17–22. doi: 10.1016/s1535-6108(02)00241-6. A comprehensive review on Bcl-2 targeting therapies. [DOI] [PubMed] [Google Scholar]
- 119.Trudel S, Stewart AK, Li Z, Shu Y, Liang SB, Trieu Y, et al. The Bcl-2 family protein inhibitor, ABT-737, has substantial antimyeloma activity and shows synergistic effect with dexamethasone and melphalan. Clinical cancer research: an official journal of the American Association for Cancer Research. 2007 Jan 15;13(2 Pt 1):621–9. doi: 10.1158/1078-0432.CCR-06-1526. [DOI] [PubMed] [Google Scholar]
- 120.Nguyen M, Marcellus RC, Roulston A, Watson M, Serfass L, Murthy Madiraju SR, et al. Small molecule obatoclax (GX15-070) antagonizes MCL-1 and overcomes MCL-1-mediated resistance to apoptosis. Proceedings of the National Academy of Sciences of the United States of America. 2007 Dec 4;104(49):19512–7. doi: 10.1073/pnas.0709443104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Konopleva M, Watt J, Contractor R, Tsao T, Harris D, Estrov Z, et al. Mechanisms of antileukemic activity of the novel Bcl-2 homology domain-3 mimetic GX15-070 (obatoclax) Cancer research. 2008 May 1;68(9):3413–20. doi: 10.1158/0008-5472.CAN-07-1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Smoot RL, Blechacz BR, Werneburg NW, Bronk SF, Sinicrope FA, Sirica AE, et al. A Bax-mediated mechanism for obatoclax-induced apoptosis of cholangiocarcinoma cells. Cancer research. 2010 Mar 1;70(5):1960–9. doi: 10.1158/0008-5472.CAN-09-3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Pan J, Cheng C, Verstovsek S, Chen Q, Jin Y, Cao Q. The BH3-mimetic GX15-070 induces autophagy, potentiates the cytotoxicity of carboplatin and 5-fluorouracil in esophageal carcinoma cells. Cancer letters. 2010 Jul 28;293(2):167–74. doi: 10.1016/j.canlet.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 124.Heidari N, Hicks MA, Harada H. GX15–070 (obatoclax) overcomes glucocorticoid resistance in acute lymphoblastic leukemia through induction of apoptosis and autophagy. Cell death & disease. 2010;1:e76. doi: 10.1038/cddis.2010.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hwang JJ, Kuruvilla J, Mendelson D, Pishvaian MJ, Deeken JF, Siu LL, et al. Phase I dose finding studies of obatoclax (GX15–070), a small molecule pan-BCL-2 family antagonist, in patients with advanced solid tumors or lymphoma. Clinical cancer research: an official journal of the American Association for Cancer Research. 2010 Aug 1;16(15):4038–45. doi: 10.1158/1078-0432.CCR-10-0822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Manero F, Gautier F, Gallenne T, Cauquil N, Gree D, Cartron PF, et al. The small organic compound HA14–1 prevents Bcl-2 interaction with Bax to sensitize malignant glioma cells to induction of cell death. Cancer research. 2006 Mar 1;66(5):2757–64. doi: 10.1158/0008-5472.CAN-05-2097. [DOI] [PubMed] [Google Scholar]
- 127.Tian D, Das SG, Doshi JM, Peng J, Lin J, Xing C. sHA 14–1, a stable and ROS-free antagonist against anti-apoptotic Bcl-2 proteins, bypasses drug resistances and synergizes cancer therapies in human leukemia cell. Cancer letters. 2008 Feb 8;259(2):198–208. doi: 10.1016/j.canlet.2007.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wei J, Kitada S, Rega MF, Emdadi A, Yuan H, Cellitti J, et al. Apogossypol derivatives as antagonists of antiapoptotic Bcl-2 family proteins. Molecular cancer therapeutics. 2009 Apr;8(4):904–13. doi: 10.1158/1535-7163.MCT-08-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129*.Wei J, Stebbins JL, Kitada S, Dash R, Zhai D, Placzek WJ, et al. An optically pure apogossypolone derivative as potent pan-active inhibitor of anti-apoptotic bcl-2 family proteins. Frontiers in oncology. 2011;1:28. doi: 10.3389/fonc.2011.00028. First report on BI-97D6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130*.Wei J, Stebbins JL, Kitada S, Dash R, Placzek W, Rega MF, et al. BI-97C1, an optically pure Apogossypol derivative as pan-active inhibitor of antiapoptotic B-cell lymphoma/leukemia-2 (Bcl-2) family proteins. Journal of medicinal chemistry. 2010 May 27;53(10):4166–76. doi: 10.1021/jm1001265. First report on BI-97C1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131**.Fisher PB. Is mda-7/IL-24 a “magic bullet” for cancer? Cancer research. 2005 Nov 15;65(22):10128–38. doi: 10.1158/0008-5472.CAN-05-3127. Review on mda-7/IL-24, a protein capable of inducing cancer specific cell death. [DOI] [PubMed] [Google Scholar]
- 132**.Quinn BA, Dash R, Azab B, Sarkar S, Das SK, Kumar S, et al. Targeting Mcl-1 for the therapy of cancer. Expert opinion on investigational drugs. 2011 Oct;20(10):1397–411. doi: 10.1517/13543784.2011.609167. A comprehensive report on Mcl-1 targetting therapies. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133**.Dash R, Azab B, Quinn BA, Shen X, Wang XY, Sas SK, et al. Apogossypol derivative BI-97C1 (Sabutoclax) targeting Mcl-1 sensitizes prostate cancer cells to mda-7/IL-24-mediated toxicity. Proceedings of the national academy of sciences USA. 2011 May 24;108(21):8785–90. doi: 10.1073/pnas.1100769108. Demonstration that inhibiting Mcl-1 selectively sensitizes prostate cancer cells to mda-7/IL-24-mediated toxicity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Azab B, Dash R, Das SK, Bhutia SK, Shen XN, Quinn BA, et al. Enhanced delivery of mda-7/IL-24 using a serotype chimeric adenovirus (Ad.5/3) in combination with the Apogossypol derivative BI-97C1 (Sabutoclax) improves therapeutic efficacy in low CAR colorectal cancer cells. Journal of cellular physiology. 2012 May;227(5):2145–53. doi: 10.1002/jcp.22947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kazi A, Sun J, Doi K, Sung SS, Takahashi Y, Yin H, et al. The BH3 alpha-helical mimic BH3-M6 disrupts Bcl-X(L), Bcl-2, and MCL-1 protein-protein interactions with Bax, Bak, Bad, or Bim and induces apoptosis in a Bax- and Bim-dependent manner. The Journal of biological chemistry. 2011 Mar 18;286(11):9382–92. doi: 10.1074/jbc.M110.203638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hodge JW, Ardiani A, Farsaci B, Kwilas AR, Gameiro SR. The tipping point for combination therapy: cancer vaccines with radiation, chemotherapy, or targeted small molecule inhibitors. Seminars oncology. 2012 Jun;39(3):323–39. doi: 10.1053/j.seminoncol.2012.02.006. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Farsaci B, Sabzevari H, Higgins JP, DiBari MG, Takai S, Schlom J, et al. Effect of a small molecule BCL-2 inhibitor on immune function and use with a recombinant vaccine. International J Cancer. 2010 Oct 1;127(7):1603–13. doi: 10.1002/ijc.25177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Vanneman M, Dranoff G. Combining immunotherapy and targeted therapies in cancer treatment. Nature reviews cancer. 2012 Mar 22;12(4):237–51. doi: 10.1038/nrc3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139*.Hedvat M, Emdad L, Das SK, Kim K, Dasgupta S, Thomas S, et al. Selected approaches for rational drug design and high throughput screening to identify anti-cancer molecules. Anticancer Agents Medicinal Chemistry. 2012 Aug 23; doi: 10.2174/187152012803529709. in press Report on novel drug design and screening approaches. [DOI] [PMC free article] [PubMed] [Google Scholar]