Abstract
HIV replication is unrestrained in vivo in the vast majority of infected subjects, and the ability of some rare individuals to control this virus is poorly understood. Standard immunogenicity assays for detecting HIV-1-specific CD8+ T-cell responses, such as IFN-γ ELISpot and intracellular cytokine staining, generally fail to correlate with in vivo inhibition of HIV replication. Several viral inhibition assays, which measure the effectiveness of CD8+ T-cell responses in suppressing HIV replication in vitro, have been described; but most depend on in vitro expansion of CD8+ T cells, and some show inhibitory activity in HIV-negative individuals. We have optimized an assay to assess the suppressive capability of CD8+ T cells directly ex vivo, eliminating the potential for altering their function through activation or expansion prior to assay setup, and thereby enhancing the assay’s sensitivity by avoiding non-specific inhibition. With this method, the ability of ex vivo CD8+ T cells to control HIV-1 replication in vitro can be quantified over several orders of magnitude. Specifically, our assay can be used to better define the antiviral function of CD8+ T cells induced by vaccination, and can provide insight into their ability to control viral replication if HIV infection occurs post-vaccination.
Keywords: HIV suppression, CD8 T cells, Viral inhibition, Vaccine-induced T-cell responses
1. Introduction
In the last decade, the development of novel technologies has led to the extensive characterization of HIV-specific CD8+ T-cell responses. Several phenotypic and functional markers, such as perforin expression and the ability to secrete a number of cytokines, have been described to correlate with disease progression (Betts et al., 2006; Burgers et al., 2009; Hersperger et al., 2010). However, standard immunogenicity assays for detecting CD8+ T-cell responses, such as IFN-γ ELISpot and intracellular cytokine staining (ICS), fail to adequately predict inhibition of HIV replication in vivo, since the release of IFN-γ or TNF-α by ex vivo CD8+ T cells in response to peptide stimulation does not necessarily indicate effective viral control (Cao et al., 2003; Yang, 2003; Lieberman, 2004; Pantaleo and Koup, 2004; Gray et al., 2009). A more precise evaluation of candidate HIV vaccines is required, and it is critical to develop functional assays to characterize CD8+ T-cell responses in addition to standard immunogenicity assays. With this goal, we have optimized a viral inhibition assay (VIA) that allows us to quantify the ability of CD8+ T cells to mediate inhibition of HIV-1 replication ex vivo, which may better reflect their anti-viral function in vivo.
In vitro inhibition of viral replication has been demonstrated since the late 1980s (Walker et al., 1986; Brinchmann et al., 1990; Wiviott et al., 1990; Mackewicz and Levy, 1992; Chen et al., 1993; Toso et al., 1995; Yang et al., 1997), and has been attributed to both cytolytic and soluble factors. More recent studies have built on this to develop a reproducible and quantitative assay that reflects the capacity of CD8+ T cells to mediate inhibition of viral replication in vitro (Saez-Cirion et al., 2007; Migueles et al., 2008; Freel et al., 2010; Julg et al., 2010; Spentzou et al., 2010). However, issues have arisen with these assays such as high background, most notably increased levels of viral suppression in samples from low-risk individuals not expected to show a biological response (Spentzou et al., 2010). In addition, it is unclear which separation and stimulation techniques are best for the acquisition of pure and stable cell cultures. Some protocols separate both effector (CD8+ T cells) and target (CD4+ T cells) on the same day (Saez-Cirion et al., 2007), leading to a prolonged culture of effector cells in the absence of any stimulus or maintenance while target cells are prepared. Others stimulate peripheral blood mononuclear cell (PBMC) samples for 2–3 days and separate the individual populations on the day of assay setup (Fauce et al., 2007; Freel et al., 2010; Julg et al., 2010; Spentzou et al., 2010), therefore artificially activating the effector cells, leading to increased non-specific inhibition of viral replication in the assay. The assay we have developed addresses these issues as well as optimizes the protocol to provide results that are effective and accurate with low background and low variability. Moreover, when availability is limited, input cell numbers can be reduced 4-fold without dramatically affecting the accuracy of the assay. The methods described here include infection of CD4+ T-cell targets, separation of effector and target cells, and stimulation of these populations for the setup of the assay. We have improved the dynamic range and sensitivity of the ELISA p24-antigen detection procedure. The benefit of increased sensitivity is most valuable when screening samples from vaccine recipients with very low levels of suppression of HIV-1 replication. This assay provides insight not only into the suppressive capabilities of CD8+ T cells from infected subjects, but also into the effectiveness of vaccine-induced CD8+ T-cell responses in healthy volunteers.
2. Materials and Methods
2.1 Study samples
All subjects were enrolled at the Seattle HIV Vaccine Trials Unit, and peripheral blood mononuclear cells (PBMC) were prepared as previously described (Bull et al., 2007). Unvaccinated HIV-seronegative control PBMC samples were obtained from volunteers in the Seattle Assay Control (SAC) cohort (Frahm et al., 2012), as were HIV-seropositive samples from individuals on treatment (Walsh et al., 2013). Long Term Non-Progressors (LTNP) had documented HIV infection for ≥10 years and maintained CD4+ T-cell counts >350 cells/μl over years of observation in the absence of antiretroviral treatment (Malhotra et al., 2001). Study participants enrolled in HIV Vaccine Trials Network protocols were healthy, HIV-1-uninfected adults. All cohorts enrolled men and women ≥18 years old. All participants provided informed written consent prior to enrollment, and all protocols were approved by the relevant Institutional Review Boards.
2.2 Viruses
The primary HIV-1 strain used in the VIA was BaL, a laboratory-adapted CCR5-tropic clade B isolate (Gartner et al., 1986). In addition, isolates from subtype A (93RW024 (Gao et al., 1994)), subtype B (SF162 (Cheng-Mayer and Levy, 1988) and US4 (Michael et al., 1999)), subtype C (94IN_20635_4 (Lole et al., 1999)), subtype D (93UG065 (Gao et al., 1994)), and circulating recombinant forms CRF01_AE (CM240 (Michael et al., 1999)) and CRF02_AG (HIV-1 DJ263 (Michael et al., 1999)) were used to assess cross-recognition of various HIV-1 clades. All viruses were obtained from the NIH AIDS Research and Reference Reagent Program (Bethesda, MD). The titer of each viral isolate was determined by end-point dilution and defined as the 50% tissue culture infectious dose (TCID50). Briefly, PBMC from five donors were depleted of CD8+ T cells using EasySep Human CD8 Positive Selection Kit (StemCell Technologies, Vancouver, BC), then 1×106 cells from each donor were stimulated with 1μg/mL of PHA (Murex, New York, NY) for three days. The stimulated CD8+ T-cell-depleted PBMC were then pooled and infected for four hours with 10-fold serial dilutions of virus stock. Following infection, cells were re-suspended in R10 medium [RPMI 1640 (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (FBS; Gemini Bio-Products, Sacramento, CA), penicillin/streptomycin (Invitrogen), and L-glutamine (Invitrogen)] at a concentration of 1×106 cells/ml, and 1×105 cells were then dispensed into designated wells of a 96-well plate containing 100 μl R10-50 [R10 supplemented with 50 U/ml of IL-2 (Roche Applied Science, Indianapolis, IN)]. On day 4, 100 μl of supernatant was removed and replaced with fresh R10-50. The supernatant was harvested at day 7 and p24 was measured from five replicates with 10 dilutions each using a p24 ELISA kit following manufacturer instructions (PerkinElmer, Waltham, MA). A well was scored positive if the background-subtracted p24 levels from three or more replicates was greater than 30 pg/ml. The TCID50 was calculated according to the Spearman-Karber formula.
Infectious Molecular Clones (IMCs) expressing Renilla luciferase were used in a later optimization step, these are described below (section 2.9).
2.3 Generation of autologous CD4+ T-cell targets (day -4)
PBMC from each donor were thawed in R10, counted and re-suspended at 5×107 cells/ml in RoboSep Buffer (StemCell Technologies) for magnetic separation of CD4+ T cells using the RoboSep cell separation instrument (StemCell Technologies), or manually following the manufacturer’s protocol. Separation was performed by negative selection using the StemCell CD4+ enrichment cocktail (StemCell Technologies), which depletes CD8+ T cells, natural killer cells, B cells, macrophages, monocytes, and dendritic cells (see Supplementary Figure 1 for purity). Separated CD4+ T cells were re-suspended at 1×106 cells/ml in R10-50 and stimulated for four days at 37°C/5% CO2 with 1 μg/ml PHA (Remel, Lenexa, KS). For experiments using CD3/CD28 antibodies as a stimulation method, cells were thawed and separated as described above and cultured with 30 μg/ml anti-CD3 and 1 μg/ml anti-CD28 (both from MabTech, Mariemont, OH).
2.4 Infection of autologous CD4+ T-cell targets (day 0)
After stimulation for 4 days, CD4+ T cells were infected with exogenous HIV-1 at a multiplicity of infection (MOI) of 0.01 using a magnetofection procedure following manufacturer instructions. Exogenous virus (5×103 virus particles) was mixed with 5 μl magnetic beads (Oz Bioscience, Boca Raton, FL) and 45 μl R10 media and then incubated for 15 minutes at room temperature. This mixture was then added to stimulated CD4+ T cells (5×105/well) in a 96-well plate. The plate was spun in a centrifuge for 2 minutes at 1600 rpm with no brake, placed over a magnet and incubated at 37°C for 15 minutes. Following incubation, each participant’s cells were re-suspended, pooled, counted, and adjusted to a concentration of 2×106 cells/ml in R10-50 for use in the assay.
2.5 Isolation of autologous CD8+ T-cell effectors (day 0)
On the day of assay setup thawed PBMC were re-suspended at 5×107 cells/ml in RoboSep Buffer for magnetic separation of CD8+ T cells using the RoboSep instrument or manually. Separation was performed by negative selection using the Stem Cell CD8+ enrichment cocktail (StemCell Technologies), which depletes CD4+ T cells, natural killer cells, B cells, macrophages, monocytes, and dendritic cells. Separated CD8+ T cells were re-suspended at 4×106 cells/ml in R10-50.
2.6 Viral Inhibition Assay setup (day 0)
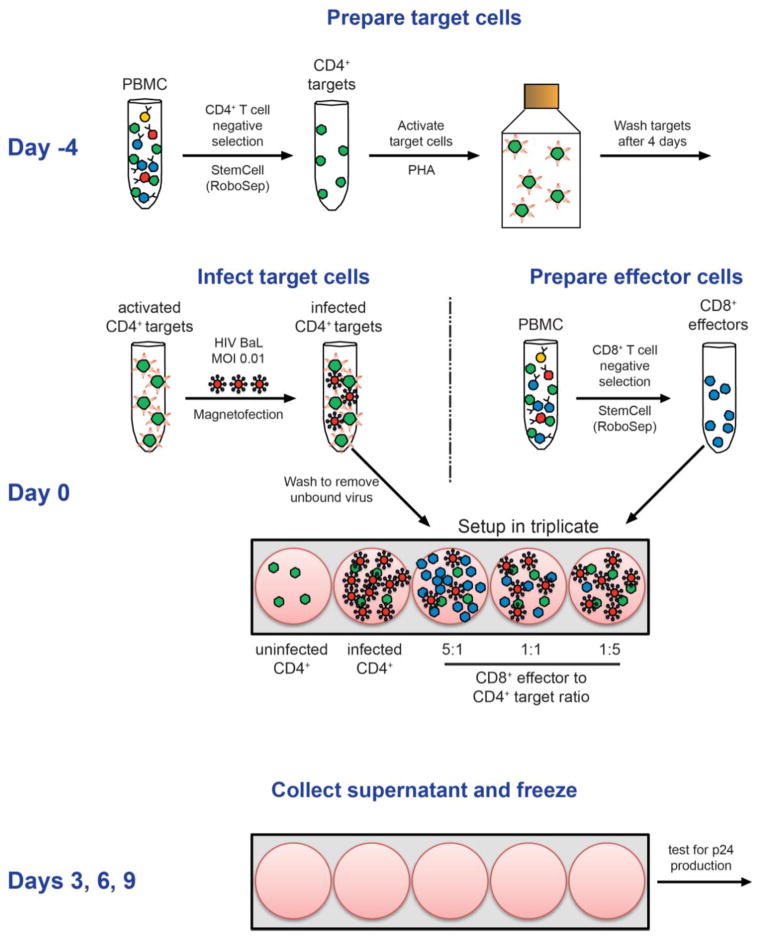
As detailed in Figure 1, CD8+ T cells (effectors) were added to infected CD4+ T cells (targets) in a 96-well plate at corresponding effector to target ratios (E:T) of 5:1, 1:1, and 1:5, keeping the number of targets constant at 1×105 cells/well. Infectivity controls consisted of infected CD4+ T-cell targets without added CD8+ T-cell effectors. Uninfected controls consisted of uninfected target cells without added effectors. Cultures were set up in triplicate and incubated at 37°C/5% CO2 for nine days. Supernatant was collected at days 3, 6, and 9 and analyzed for p24 production using a standard p24 ELISA.
Figure 1. Overview of VIA method.
A schematic of the VIA procedure from thawing and stimulation of CD4+ T cells (Day -4) to day of co-culture setup (Day 0). One vial of PBMC is thawed to separate CD4+ targets, which are stimulated with PHA for 4 days. On Day 0, target cells are infected with HIV-1 at an MOI of 0.01. In addition, effector cells (CD8+ T cells) are thawed and separated from a new vial of PBMC, and then combined in triplicate with target cells at various E:T ratios. Control wells consisting of uninfected or infected CD4+ T cells only are also included. Supernatants are collected and frozen at days 3, 6, and 9 post-infection and tested for p24 production.
2.7 p24 ELISA
ELISA was performed to detect HIV-1 p24 core antigen production using a kit according to manufacturer instructions (PerkinElmer). Because very high p24 levels can be generated, each sample was tested in triplicate at a 1:10, 1:100, 1:1,000, and 1:10,000 dilution to ensure that the readings were within the linear range of detection. The absorbance of each well was determined at 490 nm (with a reference filter at 600 nm) using a Spectramax microplate reader (Molecular Devices, Sunnyvale, CA) and calibrated against the absorbance of an HIV-1 p24 antigen standard or standard curve. The resulting data were analyzed using Microsoft Excel (Microsoft Corporation, Redmond, WA) and GraphPad Prism (GraphPad Software, La Jolla, CA).
2.8 Assessment of viral inhibition
Viral inhibition was assessed as the difference in log10 p24 production in infected target cells in the absence and presence of effectors (the “Δ log inhibition”). Concentrations of Gag p24 (in pg/ml) from the lowest dilution of supernatant that was found within the OD range of the standard curve were averaged across triplicates and log10-transformed. Values in the presence of effector cells at each E:T ratio were subtracted from values of infected target cells in the absence of effector cells. We believe that presenting data as Δ log inhibition values gives a more clear and concise picture of the suppressive capabilities of the effector cells being tested in the assay as it accounts for the variability in levels of p24 in the infected-only controls.
To validate the results from the p24 ELISA, a confirmatory intracellular flow cytometric-based assay that measures p24 production within CD4+ T cells was developed. After removal of cell culture supernatants at day six post-infection, cells were harvested and stained with the following antibody panel: CD3-ECD (BD Biosciences), CD4-Alexa680 (BD Biosciences), CD8-PerCP-Cy5.5 (Beckman Coulter), and KC57-PE (detects Gag p24; Beckman Coulter). Samples were collected on an LSRII flow cytometer (BD Biosciences) and analyzed using FlowJo (TreeStar Software, Ashland, OR). The percentage of p24 positive cells was determined for each E:T ratio and for the CD4+ only infected control.
2.9 Viral inhibition using Renilla-luciferase expressing infectious molecular clones
In an effort to decrease the cost associated with p24 ELISAs, a luciferase assay was compared to the p24 ELISA in 16 individuals. The plasmid pNL-LucR.T2A.BaL.ecto (Edmonds et al., 2010) was kindly provided by Dr. Christina Ochsenbauer (University of Alabama at Birmingham). Virus was prepared by transfection of HEK293/T17 cells using FugeneHD from Promega (Madison, WI) and was titered by infection of TZM-bl cells. CD4+ T cells were separated and stimulated as described in section 2.3. On day 0, an MOI of 0.05 was used to infect activated CD4 cells and the VIA was set up as described in sections 2.4 to 2.6. We determined that 3 days was the optimal incubation period to give a reproducible and reliable luciferase response using the Renilla-Glo Luciferase Assay System (Promega) in a time-course experiment. Prior to testing for luciferase production, 100 μl of supernatant was pulled from each well and frozen at −80°C for p24 ELISA testing.
The cells were centrifuged at 860× g for 3 minutes; flicking the plate and blotting on gauze removed the remaining supernatant. In order to remove any remaining traces of media that may interfere with the lysis of the cells, wells were washed with 100 μl 1× PBS. The cells were lysed using 1× Glo lysis buffer (Promega) for 5 minutes at room temperature. The lysed cell solution was transferred to a 96-well microlite-2 plate (Thermo Scientific, Waltham, MA) and 100 μl of Renilla-Glo luciferase assay substrate, diluted 1:100 with Renilla-Glo luciferase assay buffer, was added to each test well for 10 minutes at room temperature. In order to monitor the degradation of signal over time, we also tested 2 μg/ml recombinant Renilla rentiformis luciferase (RayBiotech Inc., Norcross, GA) in duplicate on each side of the plate, as well as a negative control of 1× PBS in duplicate. Luminescence in Relative Light Units (RLUs) was measured on a Dynex MLX luminometer (Chantilly, VA) using Revelation software.
2.10 Statistical analyses
Comparisons between different study populations were done using GraphPad Prism. The Mann-Whitney test was used for comparison of two groups, one-way ANOVA was used for comparisons across all groups, and correlations were analyzed using Spearman rank correlations.
3. Results
3.1 Cell separation and stimulation (day -4)
The overall procedure of the viral inhibition assay is summarized in Figure 1. To begin optimization of the assay, pure cultures of target (CD4+ T cells) and effector (CD8+ T cells) cells that could actively function in vitro were required. Using one uninfected donor and one LTNP, various manual and automated isolation procedures were evaluated, including depletion of superfluous cells via a bi-specific antibody, positive selection of T-cell subsets, magnetic separation using negative selection antibody cocktails, and machine separation using the RoboSep instrument in conjunction with negative selection antibody cocktails. Negative selection produces pure cell cultures that are not attached to magnetized beads, thus providing a clear and easy readout on cell counts and cell types. As shown in Figure 2A, p24 production by infected CD4+ T cells was comparable following isolation with the RoboSep or manual separation, similar to what has been reported for other assays (Saez-Cirion et al., 2010). The RoboSep instrument permits the concurrent separation of four samples and is a more streamlined approach, thereby increasing throughput and reducing the possibility of operator error. Therefore, we utilized the RoboSep instrument in conjunction with negative selection cell separation reagents when isolating all cell populations. To explore alternative isolation procedures, anti-CD3/CD8 and anti-CD3/CD4 bi-specific antibody stimulation to concurrently isolate and stimulate target and effector cells were tested, but found to be less consistent and less effective (data not shown).
Figure 2. Comparison of cell separation, stimulation, and infection techniques.
A. Comparison of RoboSep (gray) and manual (black) separation techniques for samples from two donors. Purified CD4+ T cells were stimulated with PHA for four days and infected with virus after separation. Supernatants were collected on day 6 and p24 levels (pg/ml) were determined using ELISA. B. Comparison of PHA (black) and anti-CD3/CD28 (white) stimulation techniques for CD4+ T cells from two donors. CD4+ T cells were separated manually and stimulated with PHA or anti-CD3/CD28 for four days, infected and assayed for p24 production as described above. C. Effect of CD8+ T-cell preparation on viral inhibition. PBMC from one donor were enriched for CD8+ T cells either on Day 0 (squares), or on Day -4 using positive selection and rested in R10 at 37°C until combining them with infected target cells (circles). Alternatively, cells remaining after isolation of target cells using the CD4 enrichment kit on Day -4 (i.e., CD8+ T cells, NK cells, B cells, macrophages, monocytes and DCs) were cultured in R10 media and then on Day 0 of assay setup enriched for CD8+ T cells for use as effector cells (triangles). Separated CD8+ T cells were added to target cells at E:T ratios of 5:1, 1:1, and 1:5 and run alongside controls of uninfected and infected target cells only. Supernatants were collected on Day 6 and p24 levels (pg/ml) were determined using ELISA.
Using one uninfected donor and one LTNP, two activation techniques were evaluated to achieve activation of target CD4+ T cells; stimulation with PHA and anti-CD3/CD28 antibodies. As shown in Figure 2B, PHA and anti-CD3/CD28 antibody activation produced CD4+ target cells that were capable of HIV-1 infection at comparable levels; PHA was determined to be a more cost-effective to use and was selected for the assay. It is important to note that neither stimulation procedure affects viral infectivity or levels of effector cell suppression.
The timing regarding isolation of effector cells from PBMC varies in the literature, as effector CD8+ T cells can either be separated and rested for the duration of CD4+ T-cell stimulation or separated on the day of assay setup (Freel et al., 2010; Saez-Cirion et al., 2010; Spentzou et al., 2010). We compared several options for isolating effector cells from one LTNP donor and determined that better discrimination of viral inhibition levels was obtained by thawing a new vial of cells for separation of CD8+ T cells on the day of assay setup (Figure 2C). Although this requires a second vial of cells, it avoids resting the effector cells in the absence of any stimulus, potentially leading to reduced cell numbers and functionality after four days; or stimulation of effector cells, leading to increased non-specific viral inhibition by CD8+ T cells from HIV-negative donors due to the release of antiviral mediators by the activated CD8+ T cells.
3.2 Infection procedure (day 0)
Infection of CD4+ T cells with HIV-1BaL was also optimized. Three infection procedures were compared: a 4-hour incubation (Julg et al., 2010; Spentzou et al., 2010), spinning virus onto the cells (“spinoculation”) (Forestell et al., 1996), and magnetofection (Sacha et al., 2007). While successful for other groups (Edgar, 2004; Saez-Cirion et al., 2007), spinoculation failed to achieve similar efficiency as compared to the other two methods in our hands and was not investigated further. However, spinoculation may be considered as an alternative infection technique if the other two methods are not available. The 4-hour incubation and magnetofection were similar in efficiency (data not shown); magnetofection was selected because it greatly streamlines the assay, does not rely on long setup and incubation times, and decreases toxicity and issues with low cell yields (Migueles et al., 2008). To standardize infection of target cells during magnetofection, we increased the number of cells in each well and moved the infection procedure to a 96-well plate (previously used 24-well plate), allowing for higher cell numbers (5×105/well) to be infected at the same time due to their closer proximity and the smaller volume of media needed. Along with the infection protocol, we established that a multiplicity of infection (MOI) of 0.01 was sufficient for infection with HIV-1BaL and greatly reduced the amount of virus needed for the assay. In previous experiments we determined that an MOI of 0.01 achieved similar levels of infection as an MOI of 0.05, but any further decrease in MOI leads to some decrease in the infection levels of the CD4+ T cells (data not shown).
3.3 Effector to Target ratios
To further explore the robustness of the VIA, we determined whether higher E:T ratios could be used, such as a 10:1, 25:1, or 50:1.
Since E:T ratios above 5:1 did not yield additional suppression among an uninfected, a vaccinated, and a LTNP donor (Supplementary Figure 2), these higher E:T ratios were not pursued further. In addition to a wide range of E:T ratios, the assay can also be used with smaller cell numbers while maintaining similar levels of viral inhibition, as shown using a LTNP donor (Figure 3). Smaller cell numbers result in decreased p24 production, but still maintain the same trends found at each E:T ratio for higher cell numbers.
Figure 3. Effect of CD4+ target cell titration on consistency of viral inhibition and resulting p24 production.
CD4+ targets from one LTNP were plated in triplicate at various concentrations (1×105 cells [triangle, typical assay setup], 5×104 [diamond], 2.5×104 [star], 1.25×104 [square], and 6.25×103 [circle]) with corresponding ratios of CD8+ effectors (5:1, 1:1 and 1:5; filled symbols) along with uninfected controls (open symbols). Aside from differing the concentration of CD4+ targets, the inhibition assay was performed as shown in Figure 1.
The use of low input cell numbers is particularly useful when working with limited samples. Furthermore, the assay can be modified to keep the number of CD8+ T cells per well constant while adjusting the number of CD4+ T cells to produce different E:T ratios (Supplementary Figure 3), although this leads to some loss of sensitivity. However, we determined that utilizing a fixed number of CD4+ T cells and adjusting the number of effectors provides a more streamlined approach, improves the ease of assay setup, as well as supplies a lower number of targets to ensure accurate results. As shown in Supplementary Figure 2, the lowest number of CD4+ T cells tested is 10,000 cells per condition, which provides similar results to our standard number of 100,000 CD4+ T cells, and may be used for precious samples or in instances when cell numbers are low.
3.4 Virus detection
To optimize the p24 ELISA, we defined positivity cutoffs, determined the standard curve, and established the number of replicates needed in order to provide confidence in our results. Additionally, we determined the optimal day post-infection to test supernatants and the dilutions of cell culture supernatant necessary to remain within the limit of detection provided by the p24 ELISA kit.
As shown in Supplementary Figure 4A, the 4-point standard curve (12.5 pg/ml to 100 pg/ml) provided by the kit does not provide accurate readouts at the lower and upper end of the absorbance range. Thus, we moved to a 6-point curve with values ranging from 6.25 pg/ml to 200 pg/ml, and included a negative control consisting of media alone, which was used as a zero value (Supplementary Figure 4B). These additions improved the sensitivity of the assay and permitted detection of absorbances (OD) from 0 to approximately 2.75. Beyond an absorbance of 2.75 the curve plateaus and is unreliable (Supplementary Figure 4C). As a result, any OD readings above the 200 pg/ml standard curve OD reading were considered to be past the limit of quantification. Similarly, we chose the lowest positive value (6.25 pg/ml) in the standard curve as our limit of detection for the assay since values below that level are unreliable.
Finding the peak measurement of HIV-1 Gag p24 production in collected supernatants is critical for this assay. At days 2 and 4, the levels of p24 expressed by infected CD4+ cells, as represented by samples from two uninfected donors, are continuously increasing, reaching a maximum by day 6 (Figure 4). We therefore used supernatants collected from all donors at day 6 post infection to determine viral inhibition.
Figure 4. Kinetics of p24 production.
Target cells from two donors (different symbols represent different donors) with no effector cells added were infected and supernatants were collected at days 2, 4, 6, 8, and 10 post-infection. Production of p24 increases through day 6 before declining at later time points.
Often p24 in the supernatant would exceed the upper limit of quantification when supernatants were assayed directly from cell culture. Therefore, a set of dilutions was necessary to detect values that fall within the OD range of the standard curve. We tested two-fold serial dilutions from 1:10 to 1:10,240 for the 5:1 E:T ratio and the infected only control in one LTNP. The Δ log inhibition was calculated for all dilutions (Supplementary Figure 5). Dilutions between 1:10 and 1:320 showed no inhibition due to OD values being above the upper limit of detection on the standard curve. Measurable inhibition only became detectable above the 1:320 dilution, once p24 concentrations in the 5:1 E:T sample were reduced sufficiently so as to be within the detectable range of the ELISA. The Δ log inhibition then increases clearly up to the 1:2560 dilution, at which point the concentration of p24 within the CD4 only infected control supernatant is also within the detectable range of the ELISA. From this we determined that to obtain accurate Δ log inhibition values, dilutions of 1:10, 1:100, 1:1,000, and 1:10,000 were required. Uninfected controls were only diluted 1:10 since very little to no virus was produced in these wells. Infected-only controls were measured at only at the 1:10,000 dilution because all lower dilutions were consistently beyond the upper limit of detection. Each sample was run in a minimum of two replicates, with triplicates performed when cell numbers permitted. For analysis, the lowest dilution that gave a reading within the range of the standard curve (≤2.75 OD) was used to calculate p24 production.
In addition to the p24 ELISA testing, a comparison was set up with a luciferase assay. Eight LTNPs were compared to eight HIV negative participants using both assays. P24 ELISA testing was completed in a separate experiment because the optimal signal for the ELISA is at day 6 post infection, while the optimal signal for the luciferase assay is at day 3 and cells are harvested at that time. A strong correlation was found between the two assays (p=0.0003, Spearman r=0.79; Figure 5). Both assays showed a significant difference between the LTNPs and HIV negative participants (p<0.001). The luciferase assay registered more nonspecific inhibition than the p24 ELISA at the effector to target ratio of 5:1 and 1:1, but at the 1:5 ratio there was very limited nonspecific inhibition found in either assay.
Figure 5. Comparison of p24 ELISA and luciferase assay readout.
Δ log inhibition of p24 ELISA and luciferase assay at the 5:1 E:T ratio. Eight LTNPs (closed squares) and eight HIV negative donors (open triangles) were compared. Supernatants for the p24 ELISA were harvested six days post infection. Cells for the luciferase assay were harvested three days post infection.
3.5 Assay precision
To explore assay precision, four blinded donors varying from high to no viral inhibition were tested using our standard protocol. Donors assayed were infected (A), vaccinated (B), uninfected (C), and a LTNP (D). Each donor was assayed in triplicate by three technicians. At the 5:1 E:T ratio, the assay is reproducible when considering technician and donor differences, and can be easily adapted and executed by multiple individuals yielding similar results (Figure 6A). While there are some differences in inhibition among the three technicians, the trends remain the same for each donor and effectively identifies the infected, vaccinated, uninfected, and LTNP participants. The main difference observed in the assay was found in the infected donor, which may indicate that varied results for the assay may be detected in those individuals on treatment. This may be due to technical issues, or could be due to inherent donor traits. As seen in Figure 6B, the Δ log inhibition for the 5:1 E:T ratios is consistent across technicians and across experiments.
Figure 6. Assay precision.
A. p24 values for four donors (A–D) run by three operators (differentiated by different symbols) in triplicate. Each data point represents a single test. The levels of p24 (pg/ml) at the 5:1 E:T ratio are reported. B. Δ log inhibition at the 5:1 E:T ratio of the same donors shown in part A.. Each data point represents the mean of triplicate runs by one operator (differentiated by different symbols).
3.6 Assay performance in different cohorts
To further investigate the reliability of the assay, 20 HIV negative donors, 20 HIV positive donors on treatment, and 18 LTNPs were analyzed. Our data show clear differences between all groups (p<0.0001, Kruskal-Wallis test, Figure 7), indicating that the assay is able to distinguish between CTL-mediated inhibition in uninfected donors compared to infected donors, and more importantly, in donors with varying viral loads and treatment conditions. It is clear from these results that the assay is able to detect specific variances in each group, as shown by the greatest inhibition of viral replication by LTNPs, followed by donors on treatment, and lastly, very little to no inhibition in uninfected controls. This data shows that our assay is able to detect differences across a broad range of values in those on treatment compared to those controlling virus naturally. Additionally, donor differences point to important and distinguishable differences in individuals with very different disease progression profiles.
Figure 7. Assay performance in different cohorts.

A. Twenty HIV negative donors (open triangles), 20 HIV positive donors on treatment (closed diamonds), and 18 LTNPs (closed squares) were analyzed. Δ log inhibition for each data point and the mean inhibition of each donor group at the 5:1 E:T ratio is shown.
3.6 Inhibition of multiple virus subtypes
In addition to HIV-1BaL, we wanted to determine the extent to which CD8+ T cells from an individual would respond to different viral strains and subtypes. Viruses from subtype A (93RW024), subtype B (SF162 and US4), subtype C (94IN_20635_4) subtype D (93UG065) and circulating recombinant forms CRF01_AE (90TH_CM240) and CRF02_AG (DJ263) were assayed using eight individual donors, and suppression was calculated as Δ log inhibition using the 5:1 E:T ratio. As seen in Figure 8, the assay is readily capable of determining the suppressive ability of CD8+ T cells when exposed to many virus subtypes, but magnitudes of inhibition vary between subtypes which may lead to future applicable conclusions about disease progression or vaccine profiles.
Figure 8. Robustness of the VIA in response to infection with different viral subtypes.
Eight donors with varying levels of virus inhibition (A–B HIV−, C–D vaccine recipients, E–F HIV+ on ART, G–H LTNP) were infected with seven different virus strains: 93RW024 (subtype A, red), SF162 (subtype B, green), BaL (subtype B, teal), US4 (subtype B, blue), 94IN20635-4 (subtype C, yellow), 93UG065 (subtype D, purple), and 90TH_CM240 (subtype 01_AE, orange). Δ log inhibition at the 5:1 E:T ratio is shown.
4. Discussion
The quantitative assay we have developed to assess the ability of CD8+ T cells to control viral replication in vitro is reliable, reproducible, and accurate. The main benefit of this assay is the ability to quantify direct ex vivo CD8+ HIV-1-suppressive activity in culture, which can provide insight into the functional activity of CD8+ T cells in vivo. This assay has utility in measuring antiviral effector function of vaccine-elicited CD8+ T cells, as well as the suppressive capabilities of CD8+ T cells relative to control of viral replication in vivo. Results from the recent RV144 HIV vaccine trial in Thailand (Rerks-Ngarm et al., 2009) have highlighted the importance of elucidating results from vaccine trials where immunogenicity results are not notable or not measured, but effects on acquisition are apparent (Vaccari et al., 2010). This assay may provide further insight into the benefits of vaccines in vivo and how the immune system responds to vaccination and infection. In addition, this assay can be used to measure the protective qualities of CD8+ T cells within HIV-infected cohorts or from other HIV vaccine trials, such as the Step study, where high CD8+ T-cell response rates were observed based on cytokine secretion but no effect on acquisition or post-infection virus control was detected (McElrath et al., 2008).
While our assay is similar to other viral inhibition assays, its strength lies in the extremely low background level and the ability to detect even slight suppressive capabilities of CD8+ T cells due to the wide dynamic range of the assay, which covers several logs of virus production through the use of a commercially available p24 ELISA kit. One caveat is the expense associated with the kit, especially since multiple dilutions need to be tested; we are investigating the luciferase assay as an alternative measure of viral quantification that will be more cost-effective but provide an equally sensitive readout. Our protocol also eliminates the risk of cross-contamination by dendritic cells, macrophages, natural killer cells, and other immune cells, as well as over-stimulation of target and effector cells due to the cell separation and stimulation techniques, which is important when working with an assay that requires multiple days of tissue culture. The assay also remains robust over a wide range of input cell numbers and with infection by a variety of viral isolates. Furthermore, additional isolates or more clinically relevant isolates may be used in this assay to evaluate the robustness of T-cell responses in infected individuals.
We have tested a variety of procedures for the various steps within the assay, which provide a more streamlined, accurate, and reproducible analytical tool. While some variation in the production of HIV from infected CD4+ T cells occurs across donors, these differences are minor in comparison to the wide dynamic range of the assay and are unlikely to affect its sensitivity; we have yet to explore whether the observed differences are of a technical nature or represent biological variability, possibly due to the presence of antiviral CD4+ T-cell responses. Additionally, results from the assay reveal detectable and distinguishable differences in individuals with varying levels of infection, indicating the VIA assay is a useful tool for detecting differences in vaccine recipients. We have ensured robustness and reproducibility in the assay by modifying and optimizing steps such as infection of target cells, timing of supernatant collection, dilution of supernatant stocks to provide an accurate range for the ELISA assay, and development of an effective ELISA standard curve. In addition, correlations between suppressive capabilities of CD8+ T cells and CD4+ T-cell counts and viral load in individuals who are infected with HIV, as well as with other assay readouts (such as cytokine production or proliferative capacity) can be explored.
While this assay alone will not provide insight into the complete suppressive capabilities of CD8+ T cells in relation to HIV infection or prevention, it can be used to provide quantitative results from many population cohorts. Besides testing the suppressive capabilities of vaccine-induced T cells in vaccine recipients, it can be used to study differences in the inhibitory capacity of cells from long-term non-progressors and/or elite controllers, discordant couples, and infected individuals on and off treatment. These findings will prove beneficial as we work to further identify which level of inhibition is meaningful for a vaccine to elicit, thereby providing a readout that may correlate with protection from HIV acquisition or disease progression.
Supplementary Material
Highlights.
We optimized an assay to determine suppression of viral replication in vitro
Our assay uses ex vivo effector T cells rather than cell lines
Background suppression levels are very low
Low levels of inhibition such as those induced by vaccination can be measured
Acknowledgments
Research reported in this publication was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under award number UM1AI068618. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
We thank Dr. Boris Jülg for sharing his protocol and supporting assay development.
We thank all study volunteers and the Seattle HIV Vaccine Trials Unit for providing samples; Stephen Voght for scientific discussion and assistance with preparation of the manuscript; and the James B. Pendleton Charitable Trust for their generous equipment donation.
The following reagents were obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH: HIV-1 Ba-L from Dr. Suzanne Gartner, Dr. Mikulas Popovic and Dr. Robert Gallo; HIV-1 93RW024 and HIV-1 93UG065 from The UNAIDS Network for HIV Isolation and Characterization; HIV-1 SF162 from Dr. Jay Levy; HIV-1 US4 (GS 007), HIV-1 CM240 (GS 022) and HIV-1 DJ263 (GS 003) from Dr. Nelson Michael, and HIV-1 94IN_20635_4 from Drs. Smita Kulkarni, Ramesh S. Paranjape and Deepak Gadkari.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Betts MR, Nason MC, West SM, De Rosa SC, Migueles SA, Abraham J, Lederman MM, Benito JM, Goepfert PA, Connors M, Roederer M, Koup RA. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T-cells. Blood. 2006;107:4781–9. doi: 10.1182/blood-2005-12-4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinchmann JE, Gaudernack G, Vartdal F. CD8+ T cells inhibit HIV replication in naturally infected CD4+ T cells. Evidence for a soluble inhibitor. J Immunol. 1990;144:2961–6. [PubMed] [Google Scholar]
- Bull M, Lee D, Stucky J, Chiu YL, Rubin A, Horton H, McElrath MJ. Defining blood processing parameters for optimal detection of cryopreserved antigen-specific responses for HIV vaccine trials. J Immunol Methods. 2007;322:57–69. doi: 10.1016/j.jim.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgers WA, Riou C, Mlotshwa M, Maenetje P, de Assis Rosa D, Brenchley J, Mlisana K, Douek DC, Koup R, Roederer M, de Bruyn G, Karim SA, Williamson C, Gray CM. Association of HIV-specific and total CD8+ T memory phenotypes in subtype C HIV-1 infection with viral set point. J Immunol. 2009;182:4751–61. doi: 10.4049/jimmunol.0803801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J, McNevin J, Holte S, Fink L, Corey L, McElrath MJ. Comprehensive analysis of human immunodeficiency virus type 1 (HIV-1)-specific gamma interferon-secreting CD8+ T cells in primary HIV-1 infection. J Virol. 2003;77:6867–78. doi: 10.1128/JVI.77.12.6867-6878.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CH, Weinhold KJ, Bartlett JA, Bolognesi DP, Greenberg ML. CD8+ T lymphocyte-mediated inhibition of HIV-1 long terminal repeat transcription: a novel antiviral mechanism. AIDS research and human retroviruses. 1993;9:1079–86. doi: 10.1089/aid.1993.9.1079. [DOI] [PubMed] [Google Scholar]
- Cheng-Mayer C, Levy JA. Distinct biological and serological properties of human immunodeficiency viruses from the brain. Annals of neurology. 1988;23(Suppl):S58–61. doi: 10.1002/ana.410230716. [DOI] [PubMed] [Google Scholar]
- Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic acids research. 2004;32:1792–7. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmonds TG, Ding H, Yuan X, Wei Q, Smith KS, Conway JA, Wieczorek L, Brown B, Polonis V, West JT, Montefiori DC, Kappes JC, Ochsenbauer C. Replication competent molecular clones of HIV-1 expressing Renilla luciferase facilitate the analysis of antibody inhibition in PBMC. Virology. 2010;408:1–13. doi: 10.1016/j.virol.2010.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauce SR, Yang OO, Effros RB. Autologous CD4/CD8 co-culture assay: a physiologically-relevant composite measure of CD8+ T lymphocyte function in HIV-infected persons. J Immunol Methods. 2007;327:75–81. doi: 10.1016/j.jim.2007.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forestell SP, Dando JS, Bohnlein E, Rigg RJ. Improved detection of replication-competent retrovirus. Journal of virological methods. 1996;60:171–8. doi: 10.1016/0166-0934(96)02052-6. [DOI] [PubMed] [Google Scholar]
- Frahm N, DeCamp AC, Friedrich DP, Carter DK, Defawe OD, Kublin JG, Casimiro DR, Duerr A, Robertson MN, Buchbinder SP, Huang Y, Spies GA, De Rosa SC, McElrath MJ. Human adenovirus-specific T cells modulate HIV-specific T cell responses to an Ad5-vectored HIV-1 vaccine. The Journal of clinical investigation. 2012;122:359–67. doi: 10.1172/JCI60202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freel SA, Lamoreaux L, Chattopadhyay PK, Saunders K, Zarkowsky D, Overman RG, Ochsenbauer C, Edmonds TG, Kappes JC, Cunningham CK, Denny TN, Weinhold KJ, Ferrari G, Haynes BF, Koup RA, Graham BS, Roederer M, Tomaras GD. Phenotypic and functional profile of HIV-inhibitory CD8 T cells elicited by natural infection and heterologous prime/boost vaccination. J Virol. 2010;84:4998–5006. doi: 10.1128/JVI.00138-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao F, Yue L, Craig S, Thornton CL, Robertson DL, McCutchan FE, Bradac JA, Sharp PM, Hahn BH. Genetic variation of HIV type 1 in four World Health Organization-sponsored vaccine evaluation sites: generation of functional envelope (glycoprotein 160) clones representative of sequence subtypes A, B, C, and E. WHO Network for HIV Isolation and Characterization. AIDS research and human retroviruses. 1994;10:1359–68. doi: 10.1089/aid.1994.10.1359. [DOI] [PubMed] [Google Scholar]
- Gartner S, Markovits P, Markovitz DM, Kaplan MH, Gallo RC, Popovic M. The role of mononuclear phagocytes in HTLV-III/LAV infection. Science. 1986;233:215–9. doi: 10.1126/science.3014648. [DOI] [PubMed] [Google Scholar]
- Gray CM, Mlotshwa M, Riou C, Mathebula T, de Assis Rosa D, Mashishi T, Seoighe C, Ngandu N, van Loggerenberg F, Morris L, Mlisana K, Williamson C, Karim SA. Human immunodeficiency virus-specific gamma interferon enzyme-linked immunospot assay responses targeting specific regions of the proteome during primary subtype C infection are poor predictors of the course of viremia and set point. J Virol. 2009;83:470–8. doi: 10.1128/JVI.01678-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hersperger AR, Pereyra F, Nason M, Demers K, Sheth P, Shin LY, Kovacs CM, Rodriguez B, Sieg SF, Teixeira-Johnson L, Gudonis D, Goepfert PA, Lederman MM, Frank I, Makedonas G, Kaul R, Walker BD, Betts MR. Perforin expression directly ex vivo by HIV-specific CD8 T-cells is a correlate of HIV elite control. PLoS pathogens. 2010;6:e1000917. doi: 10.1371/journal.ppat.1000917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julg B, Williams KL, Reddy S, Bishop K, Qi Y, Carrington M, Goulder PJ, Ndung’u T, Walker BD. Enhanced anti-HIV functional activity associated with Gag-specific CD8 T-cell responses. J Virol. 2010;84:5540–9. doi: 10.1128/JVI.02031-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman J. Tracking the killers: how should we measure CD8 T cells in HIV infection? AIDS. 2004;18:1489–93. doi: 10.1097/01.aids.0000131320.75396.4d. [DOI] [PubMed] [Google Scholar]
- Lole K, Bollinger R, Paranjape R, Gadkari D, Kulkarni S, Novak N, Ingersoll R, Sheppard H, Ray S. Full-length human immunodeficiency virus type 1 genomes from subtype C-infected seroconverters in India, with evidence of intersubtype recombination. J Virology. 1999;73:152–160. doi: 10.1128/jvi.73.1.152-160.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackewicz C, Levy JA. CD8+ cell anti-HIV activity: nonlytic suppression of virus replication. AIDS research and human retroviruses. 1992;8:1039–50. doi: 10.1089/aid.1992.8.1039. [DOI] [PubMed] [Google Scholar]
- Malhotra U, Holte S, Dutta S, Berrey MM, Delpit E, Koelle DM, Sette A, Corey L, McElrath MJ. Role for HLA class II molecules in HIV-1 suppression and cellular immunity following antiretroviral treatment. The Journal of clinical investigation. 2001;107:505–17. doi: 10.1172/JCI11275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElrath MJ, De Rosa SC, Moodie Z, Dubey S, Kierstead L, Janes H, Defawe OD, Carter DK, Hural J, Akondy R, Buchbinder SP, Robertson MN, Mehrotra DV, Self SG, Corey L, Shiver JW, Casimiro DR. HIV-1 vaccine-induced immunity in the test-of-concept Step Study: a case-cohort analysis. Lancet. 2008 doi: 10.1016/S0140-6736(08)61592-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael NL, Herman SA, Kwok S, Dreyer K, Wang J, Christopherson C, Spadoro JP, Young KK, Polonis V, McCutchan FE, Carr J, Mascola JR, Jagodzinski LL, Robb ML. Development of calibrated viral load standards for group M subtypes of human immunodeficiency virus type 1 and performance of an improved AMPLICOR HIV-1 MONITOR test with isolates of diverse subtypes. Journal of clinical microbiology. 1999;37:2557–63. doi: 10.1128/jcm.37.8.2557-2563.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migueles SA, Osborne CM, Royce C, Compton AA, Joshi RP, Weeks KA, Rood JE, Berkley AM, Sacha JB, Cogliano-Shutta NA, Lloyd M, Roby G, Kwan R, McLaughlin M, Stallings S, Rehm C, O’Shea MA, Mican J, Packard BZ, Komoriya A, Palmer S, Wiegand AP, Maldarelli F, Coffin JM, Mellors JW, Hallahan CW, Follman DA, Connors M. Lytic granule loading of CD8+ T cells is required for HIV-infected cell elimination associated with immune control. Immunity. 2008;29:1009–21. doi: 10.1016/j.immuni.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantaleo G, Koup RA. Correlates of immune protection in HIV-1 infection: what we know, what we don’t know, what we should know. Nature medicine. 2004;10:806–10. doi: 10.1038/nm0804-806. [DOI] [PubMed] [Google Scholar]
- Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, Paris R, Premsri N, Namwat C, de Souza M, Adams E, Benenson M, Gurunathan S, Tartaglia J, McNeil JG, Francis DP, Stablein D, Birx DL, Chunsuttiwat S, Khamboonruang C, Thongcharoen P, Robb ML, Michael NL, Kunasol P, Kim JH. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. The New England journal of medicine. 2009;361:2209–20. doi: 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]
- Sacha JB, Chung C, Rakasz EG, Spencer SP, Jonas AK, Bean AT, Lee W, Burwitz BJ, Stephany JJ, Loffredo JT, Allison DB, Adnan S, Hoji A, Wilson NA, Friedrich TC, Lifson JD, Yang OO, Watkins DI. Gag-specific CD8+ T lymphocytes recognize infected cells before AIDS-virus integration and viral protein expression. J Immunol. 2007;178:2746–54. doi: 10.4049/jimmunol.178.5.2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saez-Cirion A, Lacabaratz C, Lambotte O, Versmisse P, Urrutia A, Boufassa F, Barre-Sinoussi F, Delfraissy JF, Sinet M, Pancino G, Venet A. HIV controllers exhibit potent CD8 T cell capacity to suppress HIV infection ex vivo and peculiar cytotoxic T lymphocyte activation phenotype. Proc Natl Acad Sci U S A. 2007;104:6776–81. doi: 10.1073/pnas.0611244104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saez-Cirion A, Shin SY, Versmisse P, Barre-Sinoussi F, Pancino G. Ex vivo T cell-based HIV suppression assay to evaluate HIV-specific CD8+ T-cell responses. Nature protocols. 2010;5:1033–41. doi: 10.1038/nprot.2010.73. [DOI] [PubMed] [Google Scholar]
- Spentzou A, Bergin P, Gill D, Cheeseman H, Ashraf A, Kaltsidis H, Cashin-Cox M, Anjarwalla I, Steel A, Higgs C, Pozniak A, Piechocka-Trocha A, Wong J, Anzala O, Karita E, Dally L, Gotch F, Walker B, Gilmour J, Hayes P. Viral inhibition assay: a CD8 T cell neutralization assay for use in clinical trials of HIV-1 vaccine candidates. The Journal of infectious diseases. 2010;201:720–9. doi: 10.1086/650492. [DOI] [PubMed] [Google Scholar]
- Toso JF, Chen CH, Mohr JR, Piglia L, Oei C, Ferrari G, Greenberg ML, Weinhold KJ. Oligoclonal CD8 lymphocytes from persons with asymptomatic human immunodeficiency virus (HIV) type 1 infection inhibit HIV-1 replication. The Journal of infectious diseases. 1995;172:964–73. doi: 10.1093/infdis/172.4.964. [DOI] [PubMed] [Google Scholar]
- Vaccari M, Poonam P, Franchini G. Phase III HIV vaccine trial in Thailand: a step toward a protective vaccine for HIV. Expert Rev Vaccines. 2010;9:997–1005. doi: 10.1586/erv.10.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker CM, Moody DJ, Stites DP, Levy JA. CD8+ lymphocytes can control HIV infection in vitro by suppressing virus replication. Science. 1986;234:1563–6. doi: 10.1126/science.2431484. [DOI] [PubMed] [Google Scholar]
- Walsh PN, Friedrich DP, Williams JA, Smith RJ, Stewart TL, Carter DK, Liao HX, McElrath MJ, Frahm N, Network NHVT. Optimization and qualification of a memory B-cell ELISpot for the detection of vaccine-induced memory responses in HIV vaccine trials. J Immunol Methods. 2013;394:84–93. doi: 10.1016/j.jim.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiviott LD, Walker CM, Levy JA. CD8+ lymphocytes suppress HIV production by autologous CD4+ cells without eliminating the infected cells from culture. Cellular immunology. 1990;128:628–34. doi: 10.1016/0008-8749(90)90054-u. [DOI] [PubMed] [Google Scholar]
- Yang OO. Will we be able to ‘spot’ an effective HIV-1 vaccine? Trends Immunol. 2003;24:67–72. doi: 10.1016/s1471-4906(02)00034-0. [DOI] [PubMed] [Google Scholar]
- Yang OO, Kalams SA, Trocha A, Cao H, Luster A, Johnson RP, Walker BD. Suppression of human immunodeficiency virus type 1 replication by CD8+ cells: evidence for HLA class I-restricted triggering of cytolytic and noncytolytic mechanisms. J Virol. 1997;71:3120–8. doi: 10.1128/jvi.71.4.3120-3128.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.