Abstract
Objective
To systematically review factors associated with HIV disease progression among illicit drug users, focusing on exposures exogenous to individuals that likely shape access and adherence to HIV treatment.
Design
A systematic review of peer-reviewed English-language studies among HIV-seropositive illicit drug users with at least one of these endpoint of interest: a diagnosis of AIDS; death; changes/differences in CD4 cell counts; or changes/differences in plasma HIV-1 RNA levels.
Methods
Articles were included if they reported factors associated with an outcome of interest among a group of illicit drug users. Studies were identified, screened and selected using systematic methods.
Results
Of 2,668 studies matching the search criteria, 58 (2%) met the inclusion criteria, all but one from North America or Western Europe. Overall, 41 (71%) studies contained significant individual-level clinical characteristics or behaviours (e.g., illicit drug use) associated with disease progression. Fifteen studies (26%) identified significant social, physical, economic or policy-level exposures, including incarceration, housing status or lack of legal income.
Conclusion
While past studies demonstrate important environmental exposures that appear to shape access to care and subsequent disease progression, the limited literature to examine these factors demonstrates the need for future research to consider risk environment characteristics and the role they may play in shaping health outcomes from HIV infection among drug users through determining access and adherence to evidence-based care. (198 words)
Keywords: Antiretroviral therapy, CD4, drug users, pathogenesis, progression, risk factors, viral load
INTRODUCTION
Highly active antiretroviral therapy (HAART) has resulted in steep declines in HIV-related morbidity and mortality [1]. With appropriate levels of adherence to prescribed therapies, engagement in HAART has been shown to reliably suppress plasma HIV RNA, delay disease progression and dramatically improve survival [2, 3].
Unfortunately, the full clinical benefits of HAART have not been seen among all HIV-seropositive groups. Uptake of HAART is lower among individuals who use drugs [4, 5]; compared to individuals in other risk categories, they exhibit higher rates of sub-optimal treatment outcomes [1, 6, 7]. For example, in a multi-centre study of individuals beginning HAART, injection drug users (IDU) experienced mortality rates approximately five times higher than individuals infected through sexual contact [8]. A large multi-centre study including over 7500 seroconverters from Europe, Australia and Canada found worsening disparities in progression rates between illicit drug users and members of other exposure groups in the HAART era, suggesting inferior treatment uptake and adherence patterns [9]. As IDU can benefit from HAART at similar rates as non-IDU given adequate compliance to therapeutic regimens [10], investigations of sub-optimal outcomes have largely focused on individual-level barriers and facilitators of HAART access and adherence [11–14], including psychological co-morbidities and drug use patterns. Although proximate (i.e., behavioural and drug-related) patterns of exposure to HAART have been shown to be strongly associated with HIV disease outcomes [3], the relationships between external exposures and disease progression among drug users are unclear.
In recent years, efforts to model and address the negative sequelae of illicit drug use, including accidental overdose death, soft tissue damage and infection with blood-borne pathogens, have expanded beyond proximate causes to include contextual determinants [15–17]. Specifically, the risk environment conceptual framework describes how the interactions between social, political, economic and physical determinants at the macro- and micro-environmental levels facilitate or constrain individual behaviours and structure the risk of drug-related harms [15, 16]. In line with previous works [15, 16], we have chosen to define social- and structural-level exposures as those external to individuals which interact with individual-level characteristics and behaviours to determine HIV-related vulnerabilities. While high-profile reviews have recently applied the risk environment framework to HIV transmission patterns [17], we are unaware of the framework being applied to an examination of factors associated with HIV disease progression. In light of this, and recent high-profile calls for analyses of HIV treatment outcomes among drug users that include broader social- and structural-level exposures [11, 18], we sought to conduct a systematic review explicitly informed by the risk environment framework of the scientific literature on HIV disease progression among illicit drug users.
METHODS
Search strategy
We used an a priori-defined search strategy based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [19]. We searched the EBM, EMBASE, MEDLINE, PubMed and Science Citation Index electronic databases to identify relevant studies published in peer-reviewed journals between 1 January 1996 and 1 November 2010. Articles were selected for further review if they had at least one match in each of three sets of keywords or search terms: Illicit drug use (i.e., “heroin”, “crack”, “amphetamines”, “cocaine”, “injection drug user”, “illicit drug user”); disease progression (i.e., “viral suppression”, “viral load”, “cd4”, “death”); and HIV/AIDS. When possible, filters were used to exclude case reports, case series, reviews and other non-eligible study types. Only studies among human subjects were included. We also reviewed the citation lists of included studies for eligible studies.
Inclusion and exclusion criteria
Studies were included if they were conducted among HIV-seropositive individuals who were current or former illicit drug users or contained eligible analyses among strata of current or former drug users. Eligible study endpoints were: Change or difference in CD4 cell count or percentage; change or difference in plasma HIV-1 RNA viral load (PVL); incidence or prevalence of AIDS, as defined by the United States Centers for Disease Control diagnostic guidelines; and death, including all-cause, pre-AIDS, HIV-related and infectious disease-related mortality. To be included, studies had to include analyses of factors associated with these outcomes of interest, with significance assessed through appropriate statistical tests or the estimation of effect measures and confidence intervals. Studies were ineligible if they were written in a language other than English or were not published in a peer-reviewed journal.
Search protocol
One author (M-JSM) conducted the database search and entered study abstracts matching the keywords and criteria into a search database. After removing duplicates, studies clearly not meeting the criteria were excluded from further review. Full text versions of all remaining potentially eligible articles were retrieved and independently reviewed by two authors (M-JSM and BDM). Each author marked each remaining study “included” or “excluded”; any discrepancies were discussed by the authors until a consensus was reached.
RESULTS
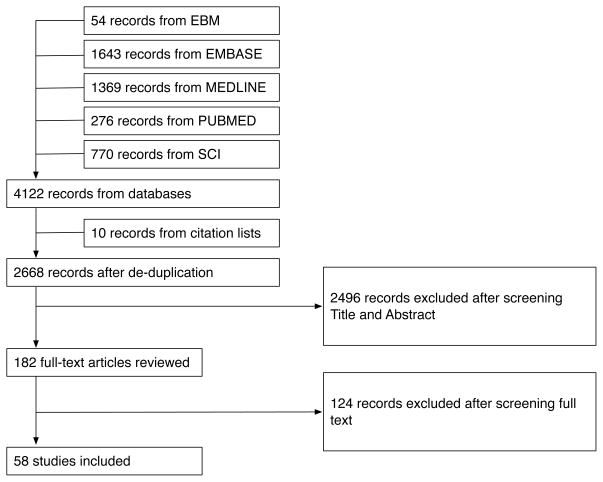
Four thousand one hundred twenty-two records matched all search criteria and were retrieved from electronic databases; 10 articles were identified following manual searching of reference lists. Following removal of duplicate records, 2668 studies remained eligible for review. After screening citation data and abstracts, the full-text version of 182 reports (6.8%) were assessed by both M-JSM and BDM. Of these, 56 (2.1%) are included in this report [20–77]. Figure 1 presents the results of the acquisition, screening and selection process.
Figure 1.
Flowchart of study acquisition, screening and selection process
Table 1 presents details of the included studies stratified by endpoint, setting and sample type. Of the 56 articles, 16 (29%) included an analysis of factors associated with time to AIDS diagnosis among HIV-seropositive drug users. Death was an outcome of interest in 23 (41%) studies. Changes or differences in CD4 cell count was an endpoint in 16 (29%) studies. In 15 (27%) studies, changes or differences in PVL was an endpoint. All but one study (in Thailand [51]) was conducted among HIV-seropositive drug users in Western settings. The plurality (27, 48%) occurred in the United States or Canada; 21 (38%) in Western Europe countries; and 7 (13.8%) in multi-national settings. The mean study sample size was 363 individuals (inter-quartile range [IQR]: 125 – 524) and the median follow-up time was 44 months (IQR: 30 – 61). Twenty-one studies (38%), all from North America, recruited participants from community settings; of the remainder, 14 (25%) recruited individuals from hospital settings; 12 (21%) from drug treatment settings; two (4%) used population-based data; and seven (13%) employed analytic samples constituted using multiple recruitment strategies.
Table 1.
Descriptive summary of reviewed studies on HIV disease progression among illicit drug users
| AIDS | Mortality | CD4 | PVL | All | |
|---|---|---|---|---|---|
| All | 16 (100%) [32, 35, 36, 39, 49, 57, 58, 62, 63, 67, 69–71, 73–75] | 23 (100%) [20, 24, 26, 27, 34, 37, 39, 42, 48, 52, 53, 57–59, 62–64, 67, 68, 70–73] | 16 (100%) [23, 25, 26, 30, 33, 38, 43, 45, 47, 51, 61, 62, 65, 67, 69, 77] | 15 (100%) [21, 28, 29, 31, 40, 41, 44, 47, 50, 54–56, 60, 66, 76] | 58 (100%) [20–77] |
| Setting | |||||
| North America | 6 (38%) [32, 36, 39, 73–75] | 9 (39%) [24, 27, 34, 39, 42, 53, 68, 72, 73] | 6 (38%) [23, 30, 33, 45, 47, 77] | 9 (60%) [21, 31, 40, 41, 44, 47, 54–56] | 27 (47%) [21, 23, 24, 27, 30–34, 36, 39–42, 44, 45, 47, 53–56, 68, 72–75, 77] |
| Western Europe | 4 (25%) [35, 49, 62, 67] | 10 (43%) [20, 22, 26, 37, 48, 52, 59, 62, 63, 67] | 6 (38%) [25, 26, 38, 61, 62, 67] | 6 (40%) [28, 29, 50, 60, 66, 76] | 21 (36%) [20, 22, 25, 26, 28, 29, 35, 37, 38, 48–50, 52, 59–62, 64, 66, 67, 76] |
| Asia | 0 (0%) | 0 (0%) | 1 (6%) [51] | 0 (0%) | 1 (2%) [51] |
| Multi-centre | 6 (38%) [57, 58, 63, 69–71] | 5 (22%) [57, 58, 63, 70, 71] | 2 (13%) [43, 69] | 0 (0%) | 7 (12%) [43, 57, 58, 63, 69–71] |
| Sample | |||||
| Community | 4 (25%) [32, 36, 39, 73] | 7 (30%) [24, 34, 39, 53, 68, 72, 73] | 6 (38%) [23, 30, 43, 45, 47, 77] | 7 (47%) [31, 40, 41, 44, 47, 55, 56] | 21 (36%) [23, 24, 30–32, 34, 36, 39–41, 43–45, 47, 53, 55, 56, 68, 72, 73, 77] |
| Clinical/Treatment | 4 (25%) [49, 67, 74, 75] | 10 (43%) [20, 22, 26, 27, 37, 42, 48, 59, 64, 67] | 7 (44%) [25, 26, 33, 38, 51, 61, 67] | 7 (47%) [21, 28, 29, 50, 60, 66, 76] | 25 (43%) [20–22, 25–29, 33, 37, 38, 42, 48–51, 59–61, 64, 66, 67, 74–76] |
| Other | 8 (50%) [35, 57, 58, 62, 63, 69–71] | 7 (30%) [52, 57, 58, 62, 63, 70, 71] | 2 (13%) [62, 69] | 1 (7%) [54] | 10 (17%) [35, 52, 54, 57, 58, 62, 63, 69–71] |
| Sample size, median (IQR) | 600 (504 – 761) | 487 (126 – 686) | 238 (128 – 259) | 189 (106 – 246) | 363 (125 – 524) |
| Follow-up months, median (IQR) | 61 (48 – 84) | 56 (41 – 81) | 45 (30 – 57) | 12 (12 – 24) | 44 (30 – 61) |
Progression to AIDS
The associations identified in this review, stratified by clinical endpoint and the risk environment framework, are presented in Table 2. Sixteen studies compared rates of disease progression among IDU by modeling the time to a diagnosis of AIDS. In the period preceding the widespread availability of HAART among drug-using populations, several studies [39, 57, 62, 63, 67] used samples of individuals with well-estimated HIV seroconversion dates to assess factors possibly associated with the natural history of HIV infection. Studies confirmed the well-established prognostic value of host immunologic [32, 36, 49, 63, 73–75] and virologic characteristics [32, 73] observed in other risk categories. In the pre-HAART era, no study found strong evidence of an effect of illicit drug use on clinical progression [58, 75]. For example, in a multi-centre study of IDU in Italy and the United States [58], participants in Baltimore, Maryland, who were mostly poly-drug injectors, and participants in Italy, who were mostly cocaine injectors, did not exhibit different rates of AIDS, as might be expected if drug use accelerated disease progression. In addition, neither age at first injection nor length of injection career was associated with time to AIDS in a pooled analysis of all participants [58]. Although no study could be found assessing the direct effect of access and adherence to HAART on time to AIDS among IDU, studies using calendar time as a proxy measure of the general availability of HAART provide weak evidence of the benefit of HAART on progression to AIDS [57, 69–71].
TABLE 2.
Summary of reviewed studies
| Article | Location | Cohort | Recruitment and follow-up | Sample | Drug use | HIV treatment | Outcome |
|---|---|---|---|---|---|---|---|
| Page et al., 1996 [53] | Miami, Florida, United States | Community-recruited from non-treatment settings, 1987–1988; followed-up until 1995 | 116 current IDU, 32 (28%) female, 108 (93%) black, 80 (69%) aged 30–40 years | 48 (41%) at least daily heroin | 14 (12%) exposed to AZT | Time to death (all-cause) | |
| Brown et al., 1996 [27] | New York City, New York, United States | Recruited from 6 methadone maintenance clinics, 1988 – 1991; followed-up until 1993 | 328 heterosexuals with history of IDU, 202 (62%) male, 191 (58%) African American, 186 (57%) age 30–39 | 165 (50%) injection drugs in previous 3 months, 122 (37%) injection heroin in last three month | Proportion exposed to zidovudine and PCP prophylaxis not reported | Time to death (all-cause) | |
| German AIDS Study Group [37] | Germany | Recruited from 20 HIV/AIDS treatment centres, 1982 – 1993 | 1554 IDU, 59% male, average age 31 years | Not reported | All exposed to antiretrovirals, 88% zidovudine | Time to death (all-cause) | |
| Brettle et al., 1996 [26] | Edinburgh, Scotland | Edinburgh City Hospital cohort | Recruited from hospital, 1985 – 1994 | 260 IDU estimated to have seroconverted between 1983 and 1985, 180 (69%) male, 188 (72%) < 25 years | Not reported | Proportion exposed to zidovudine not reported | Time to death (all-cause); time to AIDS [CDC 1987 definition]; time to CDC IV [CDC 1986 definition] |
| Crum et al., 1996 [30] | Baltimore, Maryland, United States | AIDS Link to the Intravenous Experience (ALIVE) | Recruited from community settings, 1988 – 1989 | 188 IDU seroconverting ± 1 year from November 1993, 74% men, 92% African-American | 35% > 10 years injection history at baseline; 16% no injection in last six months | 29% zidovudine use | Change in CD4+%, change in CD8+% |
| Hershow et al., 1996 [39] | Chicago, Illinois; Baltimore, Maryland; Bronx, New York; Rome, Italy | AIDS Outreach Intervention Project (Chicago); ALIVE (Baltimore); Bronx HERO (Bronx); Italian Seroconversion Study (Rome) | Recruited from community and treatment settings | 370 IDU with incident HIV infection, 27% female, mean age 32 years at seroconversion | Not reported | 23% ever exposed to zidovudine | Time to death from AIDS-defining condition; time to AIDS [CDC 1987] |
| Ferrando et al., 1996 [33] | San Francisco, California, United States | Recruited at MMT clinic in 1991 | 57 IDU on MMT and zidovudine treatment, 42% female, 52% Caucasian | 60% cocaine dependence, 36% alcohol dependence, 11% amphetamine dependence | 100% exposed to zidovudine | CD4+ cell count change | |
| Montella et al., 1997 [49] | Rome, Italy | Italian Seroconversion Study | Recruited from hospital setting, 1985 – 1991; followed prospectively to 1993 | 549 IDU seroconverting 1985 – 1991, 140 female, | 100% history of heroin injection | Not reported | Time to AIDS [CDC 1987] |
| Prins et al., 1997 [63] | Spain, Scotland, Netherlands, Switzerland, France, Austria | European Seroconverter Study | Recruited from community and treatment settings, 1982 – 1988; | 664 IDU with documented seroconversion, 221 (33%) female, mean age 25 years at seroconversion | Not reported | Not reported | Time to death, pre-AIDS |
| Radkowski et al., 1997 [65] | Poland | Recruited from methadone clinic (cases) and detoxification ward (controls) | 56 IDU, 73% female | Not reported | Not reported | Change in CD4+ cell counts | |
| Lyles et al., 1997 [45] | Baltimore, Maryland, United States | ALIVE | Recruited from community settings, 1988 – 1989 | 605 IDU recruited before 1993, 78% male, 97% African American | 71% current user, 33% inject < 1 day | Not reported | Difference in CD4+ cell count pairs |
| Baum et al., 1997 [24] | Miami, Florida, United States | Community recruited from non-treatment settings, 1987–1988; followed-up until 1995 | 125 IDU, 34% women, 88% African American | 77% positive urine screen for cocaine use during study | Proportion exposed to zidovudine not reported | Time to death, HIV-related causes | |
| Marmor et al., 1997 [46] | New York City, New York, United States | Recruited from hospital-based methadone programme, 1990 –1991, followed until 1993 | 133 IDU, 78% male, 47% Hispanic | 11 (9%) = 1 injection/day; 78 (61%) < 1 injection/day | Not reported | Time to death (all-cause) | |
| Vlahov et al., 1998 [73] | Baltimore, Maryland, United States | ALIVE | Recruited from community settings, 1988 – 1989; followed until 1996 | 522 IDU with incidence seroconversion, 80% male, 96% African-American | Not reported | 6% on zidovudine at baseline; 47% exposed to monotherapy during follow-up | Time to death (infectious disease-related); time to AIDS diagnosis |
| Haydon et al., 1998 [38] | Edinburgh, Scotland | Edinburgh IDU cohort | Recruitment from clinical settings, 1985 onwards | 240 IDU with known HCV serostatus, 163 (68%) male, | Not reported | Not reported | Time to death (all-cause); time to CDC Stage IV [undefined definition date] and time to AIDS [CDC 1997 definition] |
| Zhang et al., 1998 [77] | Baltimore, Maryland, United States | ALIVE | Recruited from community settings, 1988 – 1989 | 170 IDU, 31 (17%) women, 6 (3%) | 156 (89%) current IDU | Not reported | CD4+ cell count |
| Webber et al., 1998 [74] | New York City, New York, United States | Bronx HERO | Recruited from clinic-based methadone treatment programme, 1985, followed until 1997 | 524 IDU, 42% female, 64% Hispanic | Approximately 77% reported any injection at each follow-up | Approximately 60% exposed to zidovudine; 80% exposed to PCP prophylaxis | Time to AIDS-defining condition [CDC 1993 definition] |
| Farzadegan et al., 1998 [32] | Baltimore, Maryland, United States | ALIVE | Recruited from community settings, 1988 – 1989 and 1992 – 1993, followed until 1996 | 812 IDU, 24% female, 96% African American | 80% current drug use at baseline | 10% zidovudine use in the previous six months at baseline | Time to AIDS [CDC 1993 definition] |
| Webber et al., 1999 [75] | New York City, New York, United States | Bronx HERO | Recruited from clinic-based methadone treatment programme, 1985, followed until 1997 | 524 IDU, 302 (58%) male, 63% Hispanic | 93% self-reported illicit drug use during follow-up, 77% self-reported injection | Proportion exposed to zidovudine at baseline and during follow-up not reported | Time to AIDS [CDC 1993 definition] |
| Pezzotti et al., 1999 [58] | Baltimore, Maryland, United States and Italy | ALIVE and ISS | Community and hospital recruitment | 1003 IDU with estimated seroconversion 1988 – 1996, 28% female, 77% non-black | 21% > 10 years injection career; | Not reported | Time to AIDS [CDC 1993 definition], time to death (infectious-disease related) |
| Krol et al., 1999 [43] | Baltimore, Maryland, United States and Amsterdam, Netherland | ALIVE and the Amsterdam Cohort Study among Drug Users | Community-recruited | 287 IDU with estimated seroconversion date, 72% male, 73% non-White | 81% self-report injection drug use; 37% cocaine injectors | Not reported | Decline in CD4+ cell counts |
| Schinkel et al., 1999 [67] | Amsterdam, Netherlands | Amsterdam Cohort Study Among Drug Users | Recruited from 1985 until 1997 | 108 IDU with incidence seroconversions, 60% male, 89% White race | Not reported | Not reported | Time to AIDS [CDC 1987 and 1993 definitions], time to death (all-cause), time to CD4+ cell count < 200 |
| Carrieri et al., 1999 [28] | Marseille, Nice and Paris, France | MANIF | Recruited from clinical settings, October 1995 – October 1996 | 108 IDU, 34% female, average 33 years old | 39% injected morphine/heroin in previous six months at baseline, 25% in maintenance therapy | Not reported | HIV-1 RNA viral load |
| Prazuck et al., 1999 [61] | Villeneuve-Saint-Georges, France | Recruited from HIV clinical service, 1991 – 1996; followed for 481 months | 12 IDU, 100% male | 100% active IDU | 4 (33%) ever exposed to zidovudine | Differences in CD4+ cell count | |
| Prins et al., 1999 [62] | Spain, Scotland, Netherlands, Switzerland, France, Austria | European Seroconverter Study | Recruitment from community and clinical settings, 1982 – 1988; followed until 1995 | 664 IDU, 221 (33%) female | Not reported | Not reported | Time to death (AIDS-related); time to immunosuppression; time to AIDS [CDC 1987 definition |
| Piketty et al., 1999 [59] | Paris, France | Recruited from clinic setting, 1989 – 1992; followed until 1996 | 124 IDU, 91 (73%) male | 68% reported daily injection at baseline; 57% reported daily injecting at study end | 35% received antiretroviral therapy during follow-up | Time to AIDS-defining event [CDC, 1992]; time to death (all-cause) | |
| Shor-Posner et al., 2000 [68] | Miami, Florida, United States | Miami HIV-1-Infected Drug Abusers Cohorts (MIDAS) | Recruited from community clinic and followed from 1992 – 1996 | 125 IDU, 66% male, 89% African-American | 50% positive for poly-drug use by urine toxicology; 55% self-report daily use of illicit drugs | 8% ART-exposed | Time to death (all-cause) |
| Ajello et al., 2000 [20] | Unreported | Recruitment setting and method unreported; study period 1993 – 1995 | 21 non-IDU non-HIV; 47 HIV-seronegative IDU and 101 HIV-seropositive IDU | Not reported | Not reported | Difference in CD4+ cell count | |
| Carrieri et al., 2000 [29] | Paris, Nice and Marseille, France | MANIF | Recruited from clinical settings, October 1995 – October 1996, followed until 1998 | 103 HAART-treated patients, 20 on buprenorphine and 83 ex-IDU | Not reported | All participants initiated HAART at baseline; adherence not reported | Difference in plasma HIV RNA load |
| Prins et al., 2000 [64] | Amsterdam, Netherlands and London, England | Amsterdam Cohort Studies on HIV Infection and AIDS; Royal Free Hospital cohort | Recruited from clinical settings prior to December, 1985; followed until January 1, 1998 | 111 men with haemophilia; 118 IDU; 158 men who have sex with men; all with well-estimated seroconversion date | Not reported | Not reported | Time to death (pre-AIDS); time to AIDS |
| Pradier et al., 2001 [60] | Paris, Nice and Marseille, France | MANIF | Recruited from clinical settings, October 1995 – October 1996, followed until 1998 | 119 IDU prescribed HAART with complete adherence evaluation, 65% male | 5.9% active IDU at baseline | All prescribed HAART; 28.6% < 100% adherence during follow-up | Change in plasma HIV RNA load; change in CD4+ cell count |
| Moreno et al., 2001 [50] | Madrid, Spain | Recruited from specialist clinical setting and followed-up from October 1996 – October 1998 | 54 (26.0%) IDU on methadone and 154 (74%) IDU not on methadone; all prescribe HAART | 10 (18%) in methadone group reported illicit drug use during follow-up | All prescribed HAART; 63% had > 90% adherence during study | Changes in plasma HI RNA load; change in CD4+ cell count | |
| Barber et al., 2001 [22] | Lleida, Catalonia, Spain | Lleida AIDS Cohort | Recruited from specialist clinical setting and followed-up until March 1999 | 185 IDU with seroconversion date between 1982 and 1991; 100% Caucasian, 75% male | Not reported | 154 (74.0%) received ART at some point during study | Time to AIDS [CDC 1987] and time to AIDS [CDC 1993] |
| Zaccarelli et al., 2002 [76] | Rome, Italy | Recruited from outpatient clinic and followed-up from October 1998 – December 1999 | 80 IDU; 58 (72.5%) male, mean age 37 | 20% reported active injection drug use | 100% treated with combination antiretroviral therapy | Time to virologic failure defined as two observations > 500 copies/mL | |
| Arnsten et al., 2002 [21] | Bronx, New York City, New York | HERO | Recruited from substance abuse treatment clinic and followed-up from July 1998 to April 2000 | 80 IDU on antiretroviral therapy 51 (60%) male, 19 (23%) African American | 32 (38%) self-reported heroin or cocaine use during study | 100% on ART; 59 (69%) had regimens including a protease inhibitor | Plasma HIV RNA suppression |
| Perez-Hoyos et al., 2003 [57] | Valencia and Barcelona, Spain | GEMES | Recruited from clinical settings and followed until January 2000 | 830 IDU with well-estimated seroconversion dates in the 1980s | Not reported | Approximately one-third accessed ART during study period | Time to AIDS; time to death (all-cause) |
| Golub et al., 2003 [36] | Baltimore, Maryland, United States | ALIVE | Recruited from community settings; followed-up until December, 1999 | 451 IDU without AIDS diagnosis [CDC 1993]; 76.3% men, 95.8% African-American | Not reported | Not reported | Time to AIDS [CDC 1993]; time to death (all-cause) |
| Palepu et al., 2003a [56] | Vancouver, Canada | Drug Treatment Programme | Recruited from treatment registry and followed-up until December, 2000 | 174 IDU on HAART | Not reported | Not reported | Likelihood of plasma HIV RNA suppression |
| Palepu et al., 2003b [54] | Vancouver, Canada | Barriers to antiretroviral therapy (BART) | Recruited from community settings and followed-up from May 1996 to July 2001 | 234 IDU on HAART; 38.0% female | 65% injected heroin and/or cocaine daily over study period | 100% initiated HAART at baseline | Likelihood of plasma HIV RNA suppression |
| van Asten et al., 2003 [71] | Valencia, Spain; Edinburgh, Scotland; Amsterdam, Netherlands; Geneva, Switzerland; Glasgow, Scotland; Paris, France; Innsbruck, Austria | European Seroconverter Study | Recruited from clinical settings from 1982 to 1988 and followed until January 1998 | 751 IDU with well-estimated date of seroconversion; 33% female, median age 26 years at seroconversion | Not reported | Not reported | Time to AIDS [CDC 1993 definition] |
| Nguyen et al., 2004 [51] | Bangkok, Thailand | Recruited from drug treatment settings between 1995 – 1998 | 130 IDU with incident seroconversion; mean age 31 years, 89.2% male | Not reported | Not reported | Time to CD4+ cell count < 200 cells | |
| de la Hera et al., 2004 [35] | Valencia and Barcelona, Spain | GEMES | Recruited from clinical settings and followed until March 2001 | 929 IDU with well-estimated seroconversion dates; 24.7% women | Not reported | 337 (36.3%) prescribed HAART during study | Time to AIDS; time to death (all-cause) |
| van Asten and Prins, 2004 [69] | Valencia, Spain; Edinburgh, Scotland; Amsterdam, Netherlands; Geneva, Switzerland; Glasgow, Scotland; Paris, France; Innsbruck, Austria | European Seroconverter Study and Italian Seroconverter Study | Recruited from clinical settings from 1982 to 1988 and followed until January 1998 | 126 IDU co-infected with hepatitis C; 40 (32%) female | Not reported | 75% had exposure to HAART by the end of the study period | Time to AIDS; time to death (pre-AIDS) |
| van Asten et al., 2005 [70] | Valencia, Spain; Edinburgh, Scotland; Amsterdam, Netherlands; Geneva, Switzerland; Glasgow, Scotland; Paris, France; Innsbruck, Austria | European Seroconverter Study | Recruited from clinical settings from 1982 to 1988 and followed until January 2002 | 790 IDU with well-estimated date of seroconversion, 68% male | Not reported | 227 (42.8%) in the HAART era exposed to HAART | Time to AIDS; time to all-cause death |
| Galai et al., 2005 [34] | Baltimore, Maryland, United States | ALIVE | Recruited from community settings and followed-up until December, 2000 | 1030 IDU with CD4+ cell counts < 500 cells | Approximately 30% inject > 1 time/day | Approximately 5% exposed to HAART | Time to death (HIV related) |
| Bouhnik et al., 2005 [25] | Paris, Nice and Marseille, France | MANIF 2000 | Recruited from clinical settings and followed until 2000 | 243 IDU initiating HAART with CD4+ cell count > 200 cells | 17% active injectors at baseline | All initiated HAART | Time to death (AIDS-related) or CD4+ cell count < 200 cells |
| Vlahov et al., 2005 [72] | Baltimore, Maryland, United States | ALIVE | Recruited from community settings and followed until December 2002 | 665 IDU with CD4+ cell count < 200 cells; 94.6% Black, 74.6% male | 63.7% injected illicit drugs during study | 62.1% ever exposed to HAART during study | Time to death (HIV-related) |
| Kohli et al., 2005 [42] | Bronx, New York City, New York, United States | HERO | Recruited from methadone treatment clinics from 1996 to 2001 | 398 IDU, 237 (59.5%) male, 267 (67.1%) Hispanic | 185 (46.5%) any illicit drug use in previous six months at baseline | 165 (46.4%) exposed to HAART at baseline | Time to death (all-cause) |
| Knowlton et al., 2006 [40] | Baltimore, Maryland; Miami, Florida; New York City, New York; San Francisco, California, United States | Interventions for Seropositive Injectors — Research and Evaluation (INSPIRE) | Recruited from community settings and followed up from 2000 to 2004 | 1113 IDU on HAART; 156 (34%) female, 315 (69%) non-Hispanic black | 432 (91%) self-report illicit drug use | All on HAART; 42% self-reported taking prescribed medications | Likelihood of HIV RNA viral load suppression |
| Palepu et al., 2006 [55] | Vancouver, Canada | BART | Recruited from community settings and followed-up from May 1996 to November 2004 | 278 IDU on HAART; 160 (57.6%) male | Approximately 50% self-reported daily cocaine injection at baseline | All on HAART; 129 (46.4%) =95% adherent by pharmacy refill at baseline | Likelihood of HIV RNA viral load suppression |
| Duncan et al., 2007 [31] | Miami, Florida, United States | Recruited from community settings | 80 individuals, 100% self-reported crack cocaine smoking; 100% African-American women | Participants self-reported approximately 22 days of crack use in previous month | None exposed to treatment at recruitment | Change in CD4+ cell counts | |
| Mehta et al., 2007 [47] | Baltimore, Maryland, United States | ALIVE | Recruited from community settings and followed until 2004 | 258 IDU initiating HAART between 1996 and 2002; 29% female and 95% African American | 42.6% any injection drug use at HAART initiation | All individuals initiated HAART; 101 “consistent use” | Change in CD4+ cell count; change in plasma HIV RNA load |
| Knowlton et al., 2007 [41] | Baltimore, Maryland; Miami, Florida; New York City, New York; San Francisco, California, United States | INSPIRE | Recruited from community settings and followed from 2001 to 2005 | 133 IDU on HAART for at least 12 months with viral load improvements since initiation | 109 (82%) any injection drug use during study period | 104% adherence = 90% | Likelihood of achieving or remaining undetectable HIV RNA load |
| Michel et al., 2009 [48] | Paris, Nice and Marseille, France | MANIF 2000 | Recruited from clinical settings between 1995 and 1997 and followed until January 2005 | 294 IDU on HAART with at least two study interviews | 100 (34%) reported being in opioid substitution; 179 (60.9%) reported no heroin use in previous six months | All on HAART | Time to death |
| Baum et al., 2009 [23] | Miami, Florida, United States | Recruited from homeless shelter between 2002 and 2005 | 222 IDU, 77% black, 73% male | 50% crack cocaine use at baseline, 6% heroin use | Approximately 65% on ART during study | Time to CD4+ cell count = 200 cells | |
| Roux et al., 2009 [66] | Paris, Nice and Marseille, France | MANIF 2000 | Recruited from clinical settings between 1995 – 1996 | 113 IDU, 30 (26.5%) women | 30 (26.5%) heroin, 26 (23.0%) cocaine during previous six months | 59 (52.2) 100% adherence to HAART by self-report | Likelihood of plasma HIV RNA load suppression |
| Krusi et al., 2010 [44] | Vancouver, Canada | AIDS Care Cohort to evaluate Exposure to Survival Services | Recruited from community settings and followed up from 1996 to 2007 | 381 participants initiating ART, 157 (41.2%) women | 323 (84.8%) self-report injection drug use during previous six months at baseline | 135 (39.6%) < 95% adherent to ART by pharmacy refill first six months after baseline | Likelihood of plasma HIV RNA load suppression |
| Omland et al., 2010 [52] | Denmark | Danish HIV Cohort Study | Recruited from specialized clinical settings and followed from January 1995 to December 2006 | 392 IDU with HCV co-infection, 245 (62.5%) male | Not reported | 160 (40.8%) on HAART | Time to death (all-cause) |
Mortality
Survival of HIV-seropositive IDU was investigated in 23 studies, in which 11 (20%) [20, 22, 26, 27, 37, 48, 53, 57, 67, 68, 70] modeled all-cause mortality, 6 (11%) [24, 34, 39, 59, 62, 72] modeled HIV- or AIDS-related mortality, 2 (4%) [58, 73] modeled infectious disease-related mortality and 3 (5%) [63, 64, 71] modeled time to pre-AIDS mortality. Unsurprisingly, both HIV-related and all-cause mortality rates were high in studies of untreated IDU populations, approximating 50 per 1000 person-years [24, 27, 42, 53, 59, 62, 63, 72, 73]. Studies among HAART-naïve samples [20, 24, 26, 27, 37, 39, 53, 58, 59, 62–64, 67, 68, 73] found little evidence of unique clinical or biological correlates of survival among IDU. As with analyses of time to AIDS, studies of the relationship between patterns of illicit drug use and HIV-related death were contradictory. In two studies of community-recruited injection drug users in Baltimore [34, 72], cocaine use was associated with lower rates of death; drug use was not associated with survival in other analyses [42, 53]. More recently, studies conducted in the wake of HAART uptake among IDU [34, 42, 52, 57, 70–72] have confirmed its beneficial impact on survival. Although based on self-reported data on exposure to medication, individuals treated with HAART had sharply reduced relative hazards of death compared to antiretroviral-naïve participants in a study of 665 community-recruited IDU followed from 1988 to 2002 [72]. In contrast to the well-described relationships between endogenous factors and survival, few associations with social- or structural-level factors were observed. In an early study of the relationship between HIV treatment and opioid substitution therapy, engagement in MMT at baseline was predictive of survival among IDU in Germany [37]. Lack of legal income at baseline was the strongest predictor of shortened survival in a small study among Parisian IDU after adjustment for age, CD4 cell count, p24 antigenemia, age and baseline drug use [59].
Immunologic changes
Immunologic status as measured by changes or differences in counts of circulating CD4 cells was a focus of 16 studies. Only weak evidence was found for a relationship between illicit drug use patterns and immunologic progression [43, 45, 47, 61, 65]. Only one study identified an association between immunosuppression and social- or structural-level factors [47]. In Mehta et al.’s study of HAART initiators in Baltimore, individuals reporting recent incarceration had significantly lower adjusted odds of CD4 cell count improvements [47].
Virologic changes
Differences or changes in plasma viral load were assessed in fifteen studies. In studies of drug-using individuals on HAART [21, 31, 41, 55, 66], drug use was not a major predictor of elevated viral loads or treatment failure in four of five studies [31, 41, 55, 66]. Conversely, access to substitution therapy was strongly associated with optimal virologic response in studies of community-recruited drug users in France [66] and Canada [55]. Five studies [29, 44, 47, 50, 76] observed virologic trajectories following the initiation of HAART. Notably, in three [44, 47, 50] of four studies assessing them [44, 47, 50, 76], drug use patterns were not associated with lower relative hazards of suppression. A number of social and structural factors emerged as determinants of PVL [40, 41, 44, 56, 66]. Two studies by Knowlton et al. identified microsocial factors, such as social support and the quality of communication with medical care-givers, as positively associated with PVL suppression [40, 41]. In Vancouver, Canada, Palepu et al. found that being incarcerated in the six months prior to follow-up was a barrier to virologic suppression among drug users in a setting of universal access to HIV care [56]. Similarly, in a multi-centre study in the United States [40], individuals reporting stable housing environments had over three times higher odds of suppression after adjustment for a range of individuals and inter-personal factors.
DISCUSSION
Consistent with existing critiques of the scientific literature on HIV among drug users [11, 18], the major finding of this review is that few studies of disease progression among illicit drug users included measures of exposures at the social and structural levels. While a strong majority of these studies confirmed endogenous host and viral characteristics associated with the natural history of HIV infection as well as treatment outcomes, only a minority of studies identified associations between physical, social, political or economic factors and disease progression. In this group, Knowlton et al.’s studies [40, 41] are a notable example. In their study of individual-, social- and structural-level exposures on the likelihood of viral suppression among drug users on HAART [40], high social support, good communication with healthcare providers and stable housing were independent predictors of suppression. In two studies, incarceration was associated with poorer immunologic [47] and virologic [56] response following HAART initiation. This result stands in contrast to many prison-based trials of ART delivery, which have produced high levels of adherence to treatment [78, 79]. However, the inferior responses to ART identified in this review likely stem from treatment interruptions caused by movement between correctional and community environments [80, 81].
Notably, many of the social and structural risk factors for disease progression in this review — specifically incarceration [47, 56], poor housing status [40] and lack of legal income [59] — have been identified as important determinants of vulnerability to HIV infection in past descriptions of the risk environment framework [16, 17]. Thus, future analyses of HIV treatment outcomes might consider using this conceptual framework to model the disease progression process in drug users. More specifically, the evidence gathered in this review suggests that broader social and structural forces produce HIV disease outcomes through the mechanisms of access and adherence to antiretroviral therapy and related evidence-based treatments for individuals who use illicit drugs. Thus, future research could be informed by the risk environment framework to investigate the setting-specific social and structural determinants of treatment access and adherence.
This review found only weak evidence of a direct relationship between illicit drug use and disease progression. It is noteworthy that all studies reporting this association among groups of ART-treated participants did not include robust measures of patient adherence. Our finding stands in sharp contrast to numerous laboratory studies that have found important associations between illicit drugs and relevant virologic or immunologic functioning [82–86]. For example, exposure to morphine has been shown to up-regulate HIV replication in vitro [83]; cocaine use has been shown to impair immunologic performance in both murine and human subjects [84, 85]. However, these molecular-level effects were not clearly reproduced in studies of untreated human subjects in this review. In groups of drug users surveyed before the widespread use of HAART, illicit drug use was associated with disease progression in some [28, 43, 75] but not other [27, 43, 45, 58] studies. In addition, it is possible that the effect of illicit drug use is over-estimated if confounding by factors common to both drug use and HAART adherence is not considered. For example, while Weber et al. estimated that crack cocaine users had a faster time to AIDS diagnosis, their multivariate model did not include information on exposures likely to be associated with crack cocaine use and HIV-related morbidity, such as poorer access to healthcare, unstable housing or nutritional deficiencies. Among HAART-treated groups of drug users, the effect of illicit drugs on disease progression is thought to be mediated through lower levels of adherence to therapy. Although many studies are limited by poor or incomparable measures of drug use [23], stronger support for this hypothesis was found in this review [21, 24, 55, 76]. For example, frequent heroin use was univariately associated with lower odds of viral suppression in Palepu et al.’s 2006 study [55] of HIV-seropositive drug users in Vancouver; in a multivariate model including ART adherence, this association was not statistically significant, suggesting a mediating relationship. Nevertheless, it should be remembered that these studies largely fail to include any measurement of social or structural factors which might account for some of the effect of illicit drug use on non-adherence, such as higher levels of incarceration, poor housing status and physical and psychological co-morbidities. Among these studies, only Baum et al. [23] reported an independent effect for crack cocaine use on both CD4 cell decline and PVL after accounting for exposure to ART. In their short-term longitudinal study of 222 active illicit drug users in Miami, Florida, ongoing crack cocaine use was marginally associated with a faster rate of progression to CD4 < 200 cells in a multivariate model including baseline CD4+ cell count and HAART exposure but no measure of social or structural vulnerability [23]. However, it is unlikely their self-reported measure of HAART use adequately captured exposure to treatment as it did not predict PVL suppression in a univariate analysis. Also of note is a recent analysis using data from a long-running community-recruited cohort of HIV-seropositive IDU which failed to find a relationship between patterns of ongoing illicit drug use and viral suppression following HAART initiation [44].
The two main findings of this review — the strong focus, to date, on individual-level factors and the moderate and likely mediated associations between patterns of illicit drug use and disease progression — should be considered in light of the urgent need for interventions to improve HIV treatment outcomes among drug users. While the medical management of HIV-seropositive drug users in the clinical setting can be complex [87], clinical trials have proven directly administered antiretroviral therapy (DAART) twinned with opioid substitution therapy is effective at improving treatment outcomes [88–93]. This review suggests that the emerging evidence of relationships between exogenous factors and disease progression might provide useful new targets for clinical and community-based interventions, for example among drug users at risk of incarceration or homelessness, to support required levels of adherence among marginalized, drug-using individuals.
Limitations common to many of these studies should be mentioned in order to contextualize the findings. Most notably, although the most recent estimates suggest that close to 100 countries in the Americas, Europe, Africa and Asia are home to HIV-seropositive illicit drug users [94], these studies only drew from seropositive groups in a small minority of countries in western Europe, the United States and Canada. Notably, the only study including non-Western HIV-seropositive illicit drug users identified a novel host genotype associated with swifter CD4+ cell decline among untreated drug users. While this review has focused on social- and structural-level factors, the presence of immunologic polymorphisms among drug users has not been well evaluated. More generally, the patterns of disease progression among HIV-seropositive drug users in the countries with the largest ongoing HIV outbreaks outside sub-Saharan Africa [18] — Russia, China, Ukraine, Vietnam and Malaysia — have not been evaluated. A future study could investigate if this deficit is a result of our reliance on English-language studies or reflects a gap in the scientific literature. A further limitation is the dependence on samples of drug users drawn from treatment settings (25, 45%).
To conclude, this review of disease progression among illicit drug users found that most studies concentrated on individual-level host and viral characteristics. Although few considered the broader physical, social, political and economic determinants of disease production or treatment outcomes, some studies did identify important associations with factors including incarceration, housing status and engagement in opioid substitution therapies. Although many studies focused on the effect of drug use patterns, weak and contradictory evidence was observed to support the hypothesis that drug use is directly related to disease progression. In light of this review, future research and interventions should consider the risk environment framework when seeking to reduce HIV-related morbidity and mortality among drug users.
Table 3.
Factors associated with HIV disease progression among illicit drug users
| AIDS | Mortality | CD4+ cell count | HIV RNA viral load | ||
|---|---|---|---|---|---|
| EXOGENOUS | |||||
| Macro- | Physical | Study site [39, 62, 63, 70], study year [35, 70, 74] | HAART1 era [34, 72], study year [57, 70], study site [39, 62, 70] | ||
| Social | |||||
| Policy | |||||
| Economic | |||||
| Micro | Physical | Incarceration [47] | Housing [40], incarceration [56] | ||
| Social | Social support [40, 41], patient-provider communication [40] | ||||
| Policy | MMT2 [37] | MMT2 [44, 66], Retention in OST3 [66] | |||
| Economic | Lack of legal income [59] | ||||
| ENDOGENOUS | |||||
| Co-morbidities | Crack use [75], psychological distress [36] | Anemia [72], cocaine use [72], selenium deficiency [24, 68], withdrawal symptoms [48], STD [34], recent hospitalization [34, 72], serum thiol [46] | CES-D4 score [25], HCV5 genotype [69], Syringe borrowing [43], Active injection drug use [47, 61], Injection heroin use [43], Injection drug use duration [43], Illicit drug use duration [45], illicit drug use [65] | Alcohol use [56], cocaine use [21], crack use [31], illicit drug use [76], injection drug use [28] | |
| Pharmacotherapies | HAART1 use [34, 42, 72], ART6 use[37], PCP7 prophylaxis,[34] | HAART1 non-adherence [25] | ART6 regimen [54, 56, 60], HAART1 adherence [50, 55, 56, 60, 66, 76], time since ART6 initiation [21, 54–56] | ||
| HIV-related morbidity | Thrush [36, 49], HIV symptoms [75] | Thrush [72], AIDS diagnosis [34, 46] | AIDS diagnosis [50] | ||
| Virologic characteristics | Viral load [32, 73] | Viral load [48, 73] | Viral load [77] | Viral load [54, 56, 60, 76] | |
| Immunologic characteristics | CD4+ cell count [32, 36, 49, 63, 73–75], IgA8 level [49] | CD4+ cell count [27, 37, 42, 53, 63, 64, 68, 72, 73], IgA8 level [53], IgG9 level [53], sTNFR-II10 level [20] | CD4+ cell count [25] | CD4+ cell count [28, 40, 41, 44, 60] | |
| Genetic characteristics | HLA11 genotype [26] | CCR512 haplotype [51], HLA11 haplotype [26] | |||
| Host characteristics | Age [49, 74], age at seroconversion [62], time since seroconversion [62], gender [57] | Age [37, 48], BMI [68], gender [37, 57], time since seroconversion [59, 63], age at seroconversion [59] | Age [28, 47, 55], race [41], gender [21] | ||
Highly active antiretroviral therapy;
Methadone maintenance therapy;
Opioid substitution therapy;
Centre for Epidemiologic Studies Depression Scale;
Hepatitis C virus;
Antiretroviral therapy;
Pneumocsystis carinii pneumonia;
Immunoglobulin A;
Immunoglobulin G;
Soluble tumour necrosis factor;
Human leukocyte antigen;
CC Chemokine receptor 5
Acknowledgments
The authors thank the study participants for their contribution to the research as well as current and past researchers and staff. The authors would specifically like to thank Deborah Graham, Tricia Collingham, Caitlin Johnston, Steve Kain, and Calvin Lai for their research and administrative assistance. The study was supported by the US National Institutes of Health (R01DA021525) and the Canadian Institutes of Health Research (MOP-79297, RAA-79918). Both K.S. and T.K. are supported by Michael Smith Foundation for Health Research Scholar Awards and the Canadian Institutes of Health Research New Investigator Awards. K.S. is also partially supported by US National Institutes of Health (R01DA028648).
References
- 1.Zwahlen M, Harris R, May M, Hogg R, Costagliola D, de Wolf F, et al. Mortality of HIV-infected patients starting potent antiretroviral therapy: comparison with the general population in nine industrialized countries. Int J Epidemiol. 2009;38:1624–1633. doi: 10.1093/ije/dyp306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Palella FJ, Jr, Delaney KM, Moorman AC, Loveless MO, Fuhrer J, Satten GA, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998;338:853–860. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 3.Hogg RS, Heath KV, Yip B, Craib KJ, O’Shaughnessy MV, Schechter MT, et al. Improved survival among HIV-infected individuals following initiation of antiretroviral therapy. JAMA. 1998;279:450–454. doi: 10.1001/jama.279.6.450. [DOI] [PubMed] [Google Scholar]
- 4.Strathdee SA, Palepu A, Cornelisse PG, Yip B, O’Shaughnessy MV, Montaner JS, et al. Barriers to use of free antiretroviral therapy in injection drug users. JAMA. 1998;280:547–549. doi: 10.1001/jama.280.6.547. [DOI] [PubMed] [Google Scholar]
- 5.Wood E, Montaner JS, Tyndall MW, Schechter MT, O’Shaughnessy MV, Hogg RS. Prevalence and correlates of untreated human immunodeficiency virus type 1 infection among persons who have died in the era of modern antiretroviral therapy. J Infect Dis. 2003;188:1164–1170. doi: 10.1086/378703. [DOI] [PubMed] [Google Scholar]
- 6.Wood E, Montaner JS, Yip B, Tyndall MW, Schechter MT, O’Shaughnessy MV, et al. Adherence to antiretroviral therapy and CD4 T-cell count responses among HIV-infected injection drug users. Antivir Ther. 2004;9:229–235. [PubMed] [Google Scholar]
- 7.Weber R, Huber M, Rickenbach M, Furrer H, Elzi L, Hirschel B, et al. Uptake of and virological response to antiretroviral therapy among HIV-infected former and current injecting drug users and persons in an opiate substitution treatment programme: the Swiss HIV Cohort Study. HIV Med. 2009;10:407–416. doi: 10.1111/j.1468-1293.2009.00701.x. [DOI] [PubMed] [Google Scholar]
- 8.Zwahlen M, Harris R, May M, Hogg R, Costagliola D, de Wolf F, et al. Mortality of HIV-infected patients starting potent antiretroviral therapy: comparison with the general population in nine industrialized countries. In. Int J Epidemiol. 2009:1624–1633. doi: 10.1093/ije/dyp306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Porter K, Babiker A, Bhaskaran K, Darbyshire J, Pezzotti P, Walker AS. Determinants of survival following HIV-1 seroconversion after the introduction of HAART. Lancet. 2003;362:1267–1274. doi: 10.1016/s0140-6736(03)14570-9. [DOI] [PubMed] [Google Scholar]
- 10.Wood E, Montaner JSG, Yip B, Tyndall MW, Schechter MT, O’Shaughnessy MV, et al. Adherence and plasma HIV RNA responses to highly active antiretroviral therapy among HIV-1 infected injection drug users. In. Cmaj. 2003:656–661. [PMC free article] [PubMed] [Google Scholar]
- 11.Krüsi A, Wood E, Montaner J, Kerr T. Social and structural determinants of HAART access and adherence among injection drug users. Int J Drug Pol. 2010:4–9. doi: 10.1016/j.drugpo.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 12.Malta M, Magnanini M, Strathdee SA, Bastos F. Adherence to Antiretroviral Therapy Among HIV-Infected Drug Users: A Meta-Analysis. AIDS & Behavior. 2008 doi: 10.1007/s10461-008-9489-7. [DOI] [PubMed] [Google Scholar]
- 13.Malta M, Strathdee SA, Magnanini MMF, Bastos FI. Adherence to antiretroviral therapy for human immunodeficiency virus/acquired immune deficiency syndrome among drug users: a systematic review. In. Addiction. 2008:1242–1257. doi: 10.1111/j.1360-0443.2008.02269.x. [DOI] [PubMed] [Google Scholar]
- 14.Wood E, Kerr T, Tyndall M, Montaner J. A review of barriers and facilitators of HIV treatment among injection drug users. AIDS. 2008:1247–1256. doi: 10.1097/QAD.0b013e3282fbd1ed. [DOI] [PubMed] [Google Scholar]
- 15.Rhodes T. The ‘risk environment’: a framework for understanding and reducing drug-related harm. International journal of drug policy. 2002;13:85–94. [Google Scholar]
- 16.Rhodes T, Singer M, Bourgois P, Friedman SR, Strathdee SA. The social structural production of HIV risk among injecting drug users. Soc Sci Med. 2005;61:1026–1044. doi: 10.1016/j.socscimed.2004.12.024. [DOI] [PubMed] [Google Scholar]
- 17.Strathdee SA, Hallett TB, Bobrova N, Rhodes T, Booth R, Abdool R, et al. HIV and risk environment for injecting drug users: the past, present, and future. Lancet. 2010;376:268–284. doi: 10.1016/S0140-6736(10)60743-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wolfe D, Carrieri MP, Shepard D. Treatment and care for injecting drug users with HIV infection: a review of barriers and ways forward. Lancet. 2010;376:355–366. doi: 10.1016/S0140-6736(10)60832-X. [DOI] [PubMed] [Google Scholar]
- 19.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62:1006–1012. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 20.Ajello F, La Licata R, Lodato M, Vitale F, Bonura F, Valenti R, et al. Soluble tumor necrosis factor alpha receptors (sTNF-Rs) in HIV-1-infected intravenous drug users: change in circulating sTNF-R type II level and survival for AIDS patients. Eur J Epidemiol. 2000;16:209–216. doi: 10.1023/a:1007632617516. [DOI] [PubMed] [Google Scholar]
- 21.Arnsten JH, Demas PA, Grant RW, Gourevitch MN, Farzadegan H, Howard AA, et al. Impact of active drug use on antiretroviral therapy adherence and viral suppression in HIV-infected drug users. J Gen Intern Med. 2002;17:377–381. doi: 10.1046/j.1525-1497.2002.10644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barber Y, Rubio C, Fernández E, Rubio M, Fibla J. Host genetic background at CCR5 chemokine receptor and vitamin D receptor loci and human immunodeficiency virus (HIV) type 1 disease progression among HIV-seropositive injection drug users. J Infect Dis. 2001;184:1279–1288. doi: 10.1086/324000. [DOI] [PubMed] [Google Scholar]
- 23.Baum MK, Rafie C, Lai S, Sales S, Page B, Campa A. Crack-cocaine use accelerates HIV disease progression in a cohort of HIV-positive drug users. J Acquir Immune Defic Syndr. 2009;50:93–99. doi: 10.1097/QAI.0b013e3181900129. [DOI] [PubMed] [Google Scholar]
- 24.Baum MK, Shor-Posner G, Lai S, Zhang G, Lai H, Fletcher MA, et al. High risk of HIV-related mortality is associated with selenium deficiency. J Acquir Immune Defic Syndr Hum Retrovirol. 1997;15:370–374. doi: 10.1097/00042560-199708150-00007. [DOI] [PubMed] [Google Scholar]
- 25.Bouhnik A-D, Préau M, Vincent E, Carrieri MP, Gallais H, Lepeu G, et al. Depression and clinical progression in HIV-infected drug users treated with highly active antiretroviral therapy. Antivir Ther (Lond) 2005;10:53–61. [PubMed] [Google Scholar]
- 26.Brettle RP, McNeil AJ, Burns S, Gore SM, Bird AG, Yap PL, et al. Progression of HIV: follow-up of Edinburgh injecting drug users with narrow seroconversion intervals in 1983–1985. AIDS. 1996;10:419–430. [PubMed] [Google Scholar]
- 27.Brown LS, Siddiqui NS, Chu AF. Natural history of HIV-1 infection and predictors of survival in a cohort of HIV-1 seropositive injecting drug users. J Natl Med Assoc. 1996;88:37–42. [PMC free article] [PubMed] [Google Scholar]
- 28.Carrieri MP, Tamalet C, Vlahov D, Yahi N, Chesney M, Moatti JP. Relationship between HIV-1 viral load and continued drug use in untreated infected injection drug users. Addiction biology. 1999;4:197–202. doi: 10.1080/13556219971704. [DOI] [PubMed] [Google Scholar]
- 29.Carrieri MP, Vlahov D, Dellamonica P, Gallais H, Lepeu G, Spire B, et al. Use of buprenorphine in HIV-infected injection drug users: negligible impact on virologic response to HAART. The Manif-2000 Study Group. Drug Alcohol Depend. 2000;60:51–54. doi: 10.1016/s0376-8716(99)00136-2. [DOI] [PubMed] [Google Scholar]
- 30.Crum RM, Galai N, Cohn S, Celentano DD, Vlahov D. Alcohol use and T-lymphocyte subsets among injection drug users with HIV-1 infection: a prospective analysis. Alcohol Clin Exp Res. 1996;20:364–371. doi: 10.1111/j.1530-0277.1996.tb01654.x. [DOI] [PubMed] [Google Scholar]
- 31.Duncan R, Shapshak P, Page JB, Chiappelli F, McCoy CB, Messiah SE. Crack cocaine: effect modifier of RNA viral load and CD4 count in HIV infected African American women. Front Biosci. 2007;12:1488–1495. doi: 10.2741/2162. [DOI] [PubMed] [Google Scholar]
- 32.Farzadegan H, Hoover DR, Astemborski J, Lyles CM, Margolick JB, Markham RB, et al. Sex differences in HIV-1 viral load and progression to AIDS. Lancet. 1998;352:1510–1514. doi: 10.1016/S0140-6736(98)02372-1. [DOI] [PubMed] [Google Scholar]
- 33.Ferrando SJ, Wall TL, Batki SL, Sorensen JL. Psychiatric morbidity, illicit drug use and adherence to zidovudine (AZT) among injection drug users with HIV disease. Am J Drug Alcohol Abuse. 1996;22:475–487. doi: 10.3109/00952999609001674. [DOI] [PubMed] [Google Scholar]
- 34.Galai N, Vlahov D, Bareta JC, Wang C, Cohn S, Sterling TR. Prognostic factors for survival differ according to CD4+ cell count among HIV-infected injection drug users: pre-HAART and HAART eras. J Acquir Immune Defic Syndr. 2005;38:74–81. doi: 10.1097/00126334-200501010-00014. [DOI] [PubMed] [Google Scholar]
- 35.García de la Hera M, Ferreros I, del Amo J, García de Olalla P, Pérez Hoyos S, Muga R, et al. Gender differences in progression to AIDS and death from HIV seroconversion in a cohort of injecting drug users from 1986 to 2001. J Epidemiol Community Health. 2004;58:944–950. doi: 10.1136/jech.2003.017475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Golub ET, Astemborski JA, Hoover DR, Anthony JC, Vlahov D, Strathdee SA. Psychological distress and progression to AIDS in a cohort of injection drug users. J Acquir Immune Defic Syndr. 2003;32:429–434. doi: 10.1097/00126334-200304010-00013. [DOI] [PubMed] [Google Scholar]
- 37.German AIDS Study Group. Observational analysis of German injecting drug users (IDU): survival with and without methadone maintenance treatment. German AIDS Study Group GASG/IdkF. Eur J Med Res. 1996;1:209–214. [PubMed] [Google Scholar]
- 38.Haydon GH, Flegg PJ, Blair CS, Brettle RP, Burns SM, Hayes PC. The impact of chronic hepatitis C virus infection on HIV disease and progression in intravenous drug users. Eur J Gastroenterol Hepatol. 1998;10:485–489. doi: 10.1097/00042737-199806000-00009. [DOI] [PubMed] [Google Scholar]
- 39.Hershow RC, Galai N, Fukuda K, Graber J, Vlahov D, Rezza G, et al. An international collaborative study of the effects of coinfection with human T-lymphotropic virus type II on human immunodeficiency virus type 1 disease progression in injection drug users. J Infect Dis. 1996;174:309–317. doi: 10.1093/infdis/174.2.309. [DOI] [PubMed] [Google Scholar]
- 40.Knowlton A, Arnsten J, Eldred L, Wilkinson J, Gourevitch M, Shade S, et al. Individual, interpersonal, and structural correlates of effective HAART use among urban active injection drug users. J Acquir Immune Defic Syndr. 2006;41:486–492. doi: 10.1097/01.qai.0000186392.26334.e3. [DOI] [PubMed] [Google Scholar]
- 41.Knowlton AR, Arnsten JH, Gourevitch MN, Eldred L, Wilkinson JD, Rose CD, et al. Microsocial environmental influences on highly active antiretroviral therapy outcomes among active injection drug users: the role of informal caregiving and household factors. J Acquir Immune Defic Syndr. 2007;46 (Suppl 2):S110–119. doi: 10.1097/QAI.0b013e31815767f8. [DOI] [PubMed] [Google Scholar]
- 42.Kohli R, Lo Y, Howard AA, Buono D, Floris-Moore M, Klein RS, et al. Mortality in an urban cohort of HIV-infected and at-risk drug users in the era of highly active antiretroviral therapy. Clin Infect Dis. 2005;41:864–872. doi: 10.1086/432883. [DOI] [PubMed] [Google Scholar]
- 43.Krol A, Flynn C, Vlahov D, Miedema F, Coutinho RA, van Ameijden EJ. New evidence to reconcile in vitro and epidemiologic data on the possible role of heroin on CD4+ decline among HIV-infected injecting drug users. Drug Alcohol Depend. 1999;54:145–154. doi: 10.1016/s0376-8716(98)00158-6. [DOI] [PubMed] [Google Scholar]
- 44.Krüsi A, Milloy M-J, Kerr T, Zhang R, Guillemi S, Hogg R, et al. Ongoing drug use and outcomes from highly active antiretroviral therapy among injection drug users in a Canadian setting. Antivir Ther (Lond) 2010;15:789–796. doi: 10.3851/IMP1614. [DOI] [PubMed] [Google Scholar]
- 45.Lyles CM, Margolick JB, Astemborski J, Graham NM, Anthony JC, Hoover DR, et al. The influence of drug use patterns on the rate of CD4+ lymphocyte decline among HIV-1-infected injecting drug users. AIDS. 1997;11:1255–1262. doi: 10.1097/00002030-199710000-00009. [DOI] [PubMed] [Google Scholar]
- 46.Marmor M, Alcabes P, Titus S, Frenkel K, Krasinski K, Penn A, et al. Low serum thiol levels predict shorter times-to-death among HIV-infected injecting drug users. AIDS. 1997;11:1389–1393. doi: 10.1097/00002030-199711000-00014. [DOI] [PubMed] [Google Scholar]
- 47.Mehta SH, Lucas G, Astemborski J, Kirk GD, Vlahov D, Galai N. Early immunologic and virologic responses to highly active antiretroviral therapy and subsequent disease progression among HIV-infected injection drug users. AIDS Care. 2007;19:637–645. doi: 10.1080/09540120701235644. [DOI] [PubMed] [Google Scholar]
- 48.Michel L, Giorgi R, Villes V, Poizot-Martin I, Dellamonica P, Spire B, et al. Withdrawal symptoms as a predictor of mortality in patients HIV-infected through drug use and receiving highly active antiretroviral therapy (HAART) Drug Alcohol Depend. 2009;99:96–104. doi: 10.1016/j.drugalcdep.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 49.Montella F, Pezzotti P, Di Sora F, Recchia O, Lauria F, Rezza G. Improving the prognostic value of CD4+ count using IgA and clinical signs in HIV-seropositive i.v. drug users. Infection. 1997;25:117–120. doi: 10.1007/BF02113591. [DOI] [PubMed] [Google Scholar]
- 50.Moreno A, Perez-Elías MJ, Casado JL, Muñoz V, Antela A, Dronda F, et al. Long-term outcomes of protease inhibitor-based therapy in antiretroviral treatment-naive HIV-infected injection drug users on methadone maintenance programmes. AIDS. 2001;15:1068–1070. doi: 10.1097/00002030-200105250-00021. [DOI] [PubMed] [Google Scholar]
- 51.Nguyen L, Li M, Chaowanachan T, Hu DJ, Vanichseni S, Mock PA, et al. CCR5 promoter human haplogroups associated with HIV-1 disease progression in Thai injection drug users. AIDS. 2004;18:1327–1333. doi: 10.1097/00002030-200406180-00012. [DOI] [PubMed] [Google Scholar]
- 52.Omland LH, Jepsen P, Weis N, Christensen PB, Laursen AL, Nielsen H, et al. Mortality in HIV-infected injection drug users with active vs cleared hepatitis C virus-infection: a population-based cohort study. J Viral Hepat. 2010;17:261–268. doi: 10.1111/j.1365-2893.2009.01175.x. [DOI] [PubMed] [Google Scholar]
- 53.Page JB, Lai S, Fletcher MA, Patarca R, Smith PC, Lai HC, et al. Predictors of survival in human immunodeficiency virus type 1-seropositive intravenous drug users. Clin Diagn Lab Immunol. 1996;3:51–60. doi: 10.1128/cdli.3.1.51-60.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Palepu A, Tyndall M, Yip B, O’Shaughnessy MV, Hogg RS, Montaner JSG. Impaired virologic response to highly active antiretroviral therapy associated with ongoing injection drug use. J Acquir Immune Defic Syndr. 2003;32:522–526. doi: 10.1097/00126334-200304150-00009. [DOI] [PubMed] [Google Scholar]
- 55.Palepu A, Tyndall MW, Joy R, Kerr T, Wood E, Press N, et al. Antiretroviral adherence and HIV treatment outcomes among HIV/HCV co-infected injection drug users: the role of methadone maintenance therapy. Drug Alcohol Depend. 2006;84:188–194. doi: 10.1016/j.drugalcdep.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 56.Palepu A, Tyndall MW, Li K, Yip B, O’Shaughnessy MV, Schechter MT, et al. Alcohol use and incarceration adversely affect HIV-1 RNA suppression among injection drug users starting antiretroviral therapy. J Urban Health. 2003;80:667–675. doi: 10.1093/jurban/jtg073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pérez-Hoyos S, del Amo J, Muga R, del Romero J, García de Olalla P, Guerrero R, et al. Effectiveness of highly active antiretroviral therapy in Spanish cohorts of HIV seroconverters: differences by transmission category. AIDS. 2003;17:353–359. doi: 10.1097/00002030-200302140-00009. [DOI] [PubMed] [Google Scholar]
- 58.Pezzotti P, Galai N, Vlahov D, Rezza G, Lyles CM, Astemborski J. Direct comparison of time to AIDS and infectious disease death between HIV seroconverter injection drug users in Italy and the United States: results from the ALIVE and ISS studies. AIDS Link to Intravenous Experiences. Italian Seroconversion Study. J Acquir Immune Defic Syndr Hum Retrovirol. 1999;20:275–282. doi: 10.1097/00042560-199903010-00010. [DOI] [PubMed] [Google Scholar]
- 59.Piketty C, Castiel P, Giral P, Lhomme JP, Boubilley D, Olievenstein C, et al. Lack of legal income is strongly associated with an increased risk of AIDS and death in HIV-infected injecting drug users. AIDS Care. 1999;11:429–436. doi: 10.1080/09540129947802. [DOI] [PubMed] [Google Scholar]
- 60.Pradier C, Carrieri P, Bentz L, Spire B, Dellamonica P, Moreau J, et al. Impact of short-term adherence on virological and immunological success of HAART: a case study among French HIV-infected IDUs. Int J STD AIDS. 2001;12:324–328. doi: 10.1258/0956462011923165. [DOI] [PubMed] [Google Scholar]
- 61.Prazuck T, Malkin JE, Belec L, Chamaret S, Semaille C, Alcais A, et al. ‘Brown sugar’ heroin intoxication and improvement of surrogate immunologic markers in HIV infection. Clin Microbiol Infect. 1999;5:244–252. doi: 10.1111/j.1469-0691.1999.tb00136.x. [DOI] [PubMed] [Google Scholar]
- 62.Prins M, Brettle RP, Robertson JR, Hernández Aguado I, Broers B, Carré N, et al. Geographical variation in disease progression in HIV-1 seroconverted injecting drug users in Europe? Int J Epidemiol. 1999;28:541–549. doi: 10.1093/ije/28.3.541. [DOI] [PubMed] [Google Scholar]
- 63.Prins M, Hernández Aguado IH, Brettle RP, Robertson JR, Broers B, Carré N, et al. Pre-AIDS mortality from natural causes associated with HIV disease progression: evidence from the European Seroconverter Study among injecting drug users. AIDS. 1997;11:1747–1756. doi: 10.1097/00002030-199714000-00012. [DOI] [PubMed] [Google Scholar]
- 64.Prins M, Sabin CA, Lee CA, Devereux H, Coutinho RA. Pre-AIDS mortality and its association with HIV disease progression in haemophilic men, injecting drug users and homosexual men. AIDS. 2000;14:1829–1837. doi: 10.1097/00002030-200008180-00019. [DOI] [PubMed] [Google Scholar]
- 65.Radkowski M, Werezynska T, Laskus T, Kaminska E, Kuligowska S. Effect of cessation of drug misuse on the immune status of HIV-infected injecting drug users. Addiction biology. 1997;2:95–99. doi: 10.1080/13556219772903. [DOI] [PubMed] [Google Scholar]
- 66.Roux P, Carrieri MP, Cohen J, Ravaux I, Poizot-Martin I, Dellamonica P, et al. Retention in opioid substitution treatment: a major predictor of long-term virological success for HIV-infected injection drug users receiving antiretroviral treatment. Clin Infect Dis. 2009;49:1433–1440. doi: 10.1086/630209. [DOI] [PubMed] [Google Scholar]
- 67.Schinkel J, Langendam MW, Coutinho RA, Krol A, Brouwer M, Schuitemaker H. No evidence for an effect of the CCR5 delta32/+ and CCR2b 64I/+ mutations on human immunodeficiency virus (HIV)-1 disease progression among HIV-1-infected injecting drug users. J Infect Dis. 1999;179:825–831. doi: 10.1086/314658. [DOI] [PubMed] [Google Scholar]
- 68.Shor-Posner G, Campa A, Zhang G, Persaud N, Miguez-Burbano MJ, Quesada J, et al. When obesity is desirable: a longitudinal study of the Miami HIV-1-infected drug abusers (MIDAS) cohort. J Acquir Immune Defic Syndr. 2000;23:81–88. doi: 10.1097/00126334-200001010-00011. [DOI] [PubMed] [Google Scholar]
- 69.van Asten L, Prins M. Infection with concurrent multiple hepatitis C virus genotypes is associated with faster HIV disease progression. AIDS. 2004;18:2319–2324. doi: 10.1097/00002030-200411190-00013. [DOI] [PubMed] [Google Scholar]
- 70.van Asten L, Zangerle R, Hernández Aguado I, Boufassa F, Broers B, Brettle RP, et al. Do HIV disease progression and HAART response vary among injecting drug users in Europe? Eur J Epidemiol. 2005;20:795–804. doi: 10.1007/s10654-005-1049-0. [DOI] [PubMed] [Google Scholar]
- 71.van Asten LC, Boufassa F, Schiffer V, Brettle RP, Robertson JR, Hernández Aguado I, et al. Limited effect of highly active antiretroviral therapy among HIV-positive injecting drug users on the population level. Eur J Public Health. 2003;13:347–349. doi: 10.1093/eurpub/13.4.347. [DOI] [PubMed] [Google Scholar]
- 72.Vlahov D, Galai N, Safaeian M, Galea S, Kirk GD, Lucas GM, et al. Effectiveness of highly active antiretroviral therapy among injection drug users with late-stage human immunodeficiency virus infection. Am J Epidemiol. 2005;161:999–1012. doi: 10.1093/aje/kwi133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vlahov D, Graham N, Hoover D, Flynn C, Bartlett JG, Margolick JB, et al. Prognostic indicators for AIDS and infectious disease death in HIV-infected injection drug users: plasma viral load and CD4+ cell count. JAMA. 1998;279:35–40. doi: 10.1001/jama.279.1.35. [DOI] [PubMed] [Google Scholar]
- 74.Webber MP, Schoenbaum EE, Gourevitch MN, Buono D, Chang CJ, Klein RS. Temporal trends in the progression of human immunodeficiency virus disease in a cohort of drug users. Epidemiology. 1998;9:613–617. [PubMed] [Google Scholar]
- 75.Webber MP, Schoenbaum EE, Gourevitch MN, Buono D, Klein RS. A prospective study of HIV disease progression in female and male drug users. AIDS. 1999;13:257–262. doi: 10.1097/00002030-199902040-00014. [DOI] [PubMed] [Google Scholar]
- 76.Zaccarelli M, Barracchini A, De Longis P, Perno CF, Soldani F, Liuzzi G, et al. Factors related to virologic failure among HIV-positive injecting drug users treated with combination antiretroviral therapy including two nucleoside reverse transcriptase inhibitors and nevirapine. AIDS Patient Care STDS. 2002;16:67–73. doi: 10.1089/10872910252806117. [DOI] [PubMed] [Google Scholar]
- 77.Zhang H, Flynn C, Nelson KE, Chen W, Kawalski R, Vlahov D. HIV/HTLV-II coinfection and CD4+ cell count controlling for plasma HIV viral load in injection drug users in Baltimore. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;18:397–398. doi: 10.1097/00042560-199808010-00018. [DOI] [PubMed] [Google Scholar]
- 78.Babudieri S, Aceti A, D’Offizi GP, Carbonara S, Starnini G. Directly observed therapy to treat HIV infection in prisoners. JAMA. 2000;284:179–180. doi: 10.1001/jama.284.2.179. [DOI] [PubMed] [Google Scholar]
- 79.Altice FL, Mostashari F, Friedland GH. Trust and the acceptance of and adherence to antiretroviral therapy. J Acquir Immune Defic Syndr. 2001;28:47–58. doi: 10.1097/00042560-200109010-00008. [DOI] [PubMed] [Google Scholar]
- 80.Small W, Wood E, Betteridge G, Montaner J, Kerr T. The impact of incarceration upon adherence to HIV treatment among HIV-positive injection drug users: a qualitative study. AIDS Care. 2009;21:708–714. doi: 10.1080/09540120802511869. [DOI] [PubMed] [Google Scholar]
- 81.Baillargeon J, Giordano TP, Rich JD, Wu ZH, Wells K, Pollock BH, et al. Accessing antiretroviral therapy following release from prison. JAMA. 2009;301:848–857. doi: 10.1001/jama.2009.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mientjes GH, Miedema F, van Ameijden EJ, van den Hoek AA, Schellekens PT, Roos MT, et al. Frequent injecting impairs lymphocyte reactivity in HIV-positive and HIV-negative drug users. AIDS. 1991;5:35–41. doi: 10.1097/00002030-199101000-00005. [DOI] [PubMed] [Google Scholar]
- 83.Peterson PK, Sharp BM, Gekker G, Portoghese PS, Sannerud K, Balfour HH., Jr Morphine promotes the growth of HIV-1 in human peripheral blood mononuclear cell cocultures. AIDS. 1990;4:869–873. doi: 10.1097/00002030-199009000-00006. [DOI] [PubMed] [Google Scholar]
- 84.Lopez MC, Colombo LL, Huang DS, Wang Y, Watson RR. Modification of thymic cell subsets induced by long-term cocaine administration during a murine retroviral infection producing AIDS. Clin Immunol Immunopathol. 1992;65:45–52. doi: 10.1016/0090-1229(92)90246-k. [DOI] [PubMed] [Google Scholar]
- 85.Donahoe RM, Nicholson JK, Madden JJ, Donahoe F, Shafer DA, Gordon D, et al. Coordinate and independent effects of heroin, cocaine, and alcohol abuse on T-cell E-rosette formation and antigenic marker expression. Clin Immunol Immunopathol. 1986;41:254–264. doi: 10.1016/0090-1229(86)90109-1. [DOI] [PubMed] [Google Scholar]
- 86.Brown SM, Stimmel B, Taub RN, Kochwa S, Rosenfield RE. Immunologic dysfunction in heroin addicts. Arch Intern Med. 1974;134:1001–1006. [PubMed] [Google Scholar]
- 87.Altice FL, Kamarulzaman A, Soriano VV, Schechter M, Friedland GH. Treatment of medical, psychiatric, and substance-use comorbidities in people infected with HIV who use drugs. Lancet. 2010;376:367–387. doi: 10.1016/S0140-6736(10)60829-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Macalino GE, Hogan JW, Mitty JA, Bazerman LB, Delong AK, Loewenthal H, et al. A randomized clinical trial of community-based directly observed therapy as an adherence intervention for HAART among substance users. AIDS. 2007;21:1473–1477. doi: 10.1097/QAD.0b013e32811ebf68. [DOI] [PubMed] [Google Scholar]
- 89.Altice FL, Maru DS, Bruce RD, Springer SA, Friedland GH. Superiority of directly administered antiretroviral therapy over self-administered therapy among HIV-infected drug users: a prospective, randomized, controlled trial. Clin Infect Dis. 2007;45:770–778. doi: 10.1086/521166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Maru DS, Bruce RD, Walton M, Springer SA, Altice FL. Persistence of virological benefits following directly administered antiretroviral therapy among drug users: results from a randomized controlled trial. J Acquir Immune Defic Syndr. 2009;50:176–181. doi: 10.1097/QAI.0b013e3181938e7e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Berg KM, Litwin A, Li X, Heo M, Arnsten JH. Directly observed antiretroviral therapy improves adherence and viral load in drug users attending methadone maintenance clinics: a randomized controlled trial. Drug Alcohol Depend. 2011;113:192–199. doi: 10.1016/j.drugalcdep.2010.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lucas GM, Mullen BA, Weidle PJ, Hader S, McCaul ME, Moore RD. Directly administered antiretroviral therapy in methadone clinics is associated with improved HIV treatment outcomes, compared with outcomes among concurrent comparison groups. Clin Infect Dis. 2006;42:1628–1635. doi: 10.1086/503905. [DOI] [PubMed] [Google Scholar]
- 93.Lucas GM, Weidle PJ, Hader S, Moore RD. Directly administered antiretroviral therapy in an urban methadone maintenance clinic: a nonrandomized comparative study. Clin Infect Dis. 2004;38 (Suppl 5):S409–413. doi: 10.1086/421405. [DOI] [PubMed] [Google Scholar]
- 94.Mathers BM, Degenhardt L, Ali H, Wiessing L, Hickman M, Mattick RP, et al. HIV prevention, treatment, and care services for people who inject drugs: a systematic review of global, regional, and national coverage. Lancet. 2010;375:1014–1028. doi: 10.1016/S0140-6736(10)60232-2. [DOI] [PubMed] [Google Scholar]