Abstract
Background
Huang-Lian-Jie-Du-Tang (HLJDT) is the classical traditional Chinese recipe for heat clearance and detoxification and is used in diabetic patients in the clinical practice of traditional Chinese medicine.
Objective
The aim of this study was to evaluate the protective effects of long-term treatment with HLJDT on vascular endothelial function in rats with type 2 diabetes mellitus (T2DM).
Methods
The male T2DM model rats were induced by intraperitoneal injection of low-dose streptozotocin plus a high-fat and high-calorie laboratory diet. The T2DM animals were randomly divided into the T2DM model group, the low-dose HLJDT group (0.42 g/kg/d), and the high-dose HLJDT group (1.25 g/kg/d).
Results
Administration of HLJDT (0.42 or 1.25 g/kg/d) for 8 weeks decreased the levels of serum fasting blood glucose, malondialdehyde, and vascular tissue interleukin 6 but raised the level of serum superoxide dismutase compared with the T2DM model group in a dose-dependent manner. In addition, HLJDT treatment restored the impaired endothelial-dependent vascular relaxation in aortic preparations from the T2DM model group in a dose-dependent manner.
Conclusions
Early and long-term treatments with HLJDT could have anti-inflammatory, antioxidant properties and could protect vascular endothelium from the cardiovascular complications associated with T2DM.
Key words: aorta, Huang-Lian-Jie-Du-Tang, oxidative stress, type 2 diabetes mellitus rats, vasodilation
Introduction
The prevalence of type 2 diabetes mellitus (T2DM) is dramatically increasing throughout the world, by 9.7% in China in 2009. There are approximately 100 million people with diabetes.1 Cardiovascular complications are currently the major causes of morbidity and mortality in patients with T2DM.
Huang-Lian-Jie-Du-Tang (HLJDT) is a famous traditional Chinese recipe that has been used to treat toxic heat syndromes and infectious diseases. Recently, the benefits of HLJDT on diabetes have been noted when it was used in diabetic patients.2 Several studies have indicated that rats with T2DM given HLJDT had reduced levels of serum fasting blood glucose (FBG), serum fasting insulin, serum total cholesterol, triglycerides, angiotensin II, and von Willebrand factor and increased levels of high-density lipoproteins and nitric oxide.3 It is well-known that vascular endothelial cell dysfunction is considered important in the progression of diabetic vascular complications.4 However, the effect of HLJDT on blood vessel function has not been reported. It was also reported that endothelial injury involves inflammation and oxidative stress.5,6 An elevated level of interleukin 6 (IL-6) (a marker of inflammation) predicts the development of T2DM.7 Malondialdehyde (MDA) is the end product of the major reactions leading to significant oxidation of polyunsaturated fatty acids in cellular membranes and, thus, serves as a reliable marker of oxidative stress.8 Superoxide dismutase (SOD), an endogenous oxygen free radical scavenger, plays a major role in oxidation and antioxidant balance. Superoxide dismutase activity plays a vital role in the regulation of oxidative status in T2DM.9
In the present study, we investigated whether HLJDT treatment improved endothelial function by observing the impact of HLJDT on acetylcholine-induced endothelial diastolic function in the thoracic aortic ring. In rats, T2DM was induced by a high-fat diet and intraperitoneal injection of streptozotocin, and HLJDT was administrated for 8 weeks. We measured the levels of serum MDA, SOD, and vascular tissue IL-6 to investigate whether the protective effect of HLJDT on endothelial function may be due to antioxidant or anti-inflammatory pathway activation.
Materials and Methods
Animals
All the experiments were performed in accordance with the Guidelines for the Care and Use of Laboratory Animals published by the US National Institutes of Health and were approved by the Institutional Animal Care and Use Committee of Guangzhou Medical University, Guangzhou, People's Republic of China. Healthy male Wistar rats (215–265 g) were purchased from Guangdong Province Medicine Experimental Animal Center (Guangzhou, Guangdong, China) and were housed 4 to a cage with free access to standard rat chow and water. After 2 weeks of adaptive feeding, the rats were injected with streptozotocin, 30 mg/kg IP. The control rats were injected with vehicle (0.1 M citrate buffer). Next, an oral glucose tolerance test (OGTT) was performed on the third day. Twenty-four rats showing abnormal OGTT results were selected and randomly divided into 3 groups: the diabetic model group (MOD), the low-dose HLJDT group (0.42 g/kg/d raw herb), and the high-dose HLJDT group (1.25 g/kg/d raw herb). The HLJDT suspension was intragastrically administrated to the rats every morning between 8:30 and 9:30, and the rats were fed a high-fat and high-calorie diet by adding sucrose, refined lard, milk powder, cholalic acid, and eggs into basic forage in the proportion of 30:20:4:2:1:63 according to the previous report.3,10 In addition, a control group (CON) was fed a basic diet throughout the experimental period. Except for fasting for 12 hours before the OGTT or before humane killing of the animal, all the rats had free access to food and water. The body weight of the animals was monitored weekly to observe the changes and adjust the dose of drugs for administration. At the end of the 8 weeks, the animals were humanely killed under anesthesia with pentobarbital, 45 mg/kg IP.
Drug Administration
The HLJDT was prepared by using an age-old recipe, a prescription of 4 medicinal herbs consisting of the crude drugs Coptidis rhizoma, Scutellariae radix, Phellodendri cortex chinensis, and Gardeniae fructus in a weight ratio of 3:2:2:3, respectively, as described previously.11 Coptidis rhizoma is dry rhizome of the plant Coptis chinensis Franch (family Ranunculaceae), Scutellariae radix is dry roots of the plant Scutellaria baicalensis Georgi (family Labiatae), Phellodendri cortex chinensis is dry yellow bark of the plant Phellodendron chinense Schneid (family Rutaceae), and Gardeniae fructus is the dry ripe fruit of the plant Gardenia jasminoides Ellis (family Rubiaceae). The raw material was provided by the First Affiliated Hospital of Guangzhou Medical University and was identified by associate professor Jianyie Zhang of the Pharmacy Department of Guangzhou Medical University. All other reagents were purchased from Sigma-Aldrich (St. Louis, Missouri), except where otherwise specified.
HPLC Analysis
The content of berberine in HLJDT was investigated by HPLC. The HPLC analysis was performed using a Waters 2695 liquid chromatograph (Shimadzu, Kyoto, Japan) connected to a model 2998 diode array detector and controlled by LC Driver Ver.2.0 for Waters Empower software. The chromatographic separation was performed using an Ultimate Capcell Pak C18 column (250 × 4.6 mm, 5-μm inside diameter; Shiseido, Japan) with a column temperature of 35°C and a UV wavelength of 345 nm. Standard of berberine was obtained from the National Institute for Control of Pharmaceutical and Biology Products (Beijing, China). Acetonitrile was of HPLC grade (Merck, Darmstadt, Germany), and all other chemicals were of analytical reagent grade. The mobile phase was composed of 2 parts: (1) 0.05 mol potassium dihydrogen phosphate in 1 L of HPLC-grade water and (2) chromatographically pure acetonitrile; parts 1 and 2 were mixed in the ratio of 52:48 (v/v), respectively. After 4 g of SDS was added to every 1 L of mixed solution, the pH of the solution was adjusted to 4.0 using phosphoric acid and was degassed by helium gas. The flow rate was set at 1 mL/min, and the injection volume was 5 μL. As shown in Figure 1, berberine was comprehensively separated, and its retention time was at 39 minutes. The content of berberine in the HLJDT extract was 18 mg · g.
Figure 1.
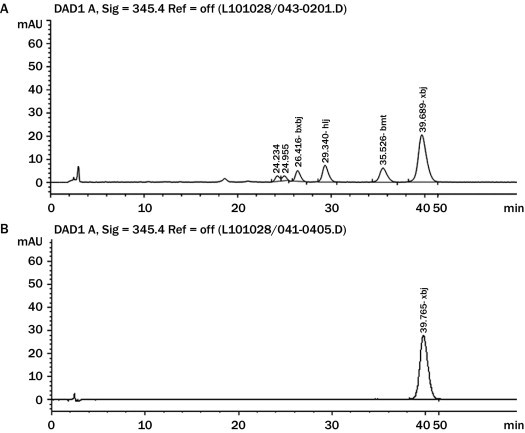
The chromatographic fingerprint of Huang-Lian-Jie-Du-Tang (HLJDT). The marked peak of HLJDT at 39.689 minutes is (A) berberine, according to (B) the standard berberine.
Measurement of Serum OGTT and FBG Level
The OGTT was conducted in the overnight-fasted animals by orally administering 2.5 g/kg glucose (Figure 2A). Blood samples were collected from the tail vein immediately before (0 hours) and 1 and 2 hours after gastric perfusion. Levels of FBG were measured using glucose meters and test strips (Johnson & Johnson Ltd, New Brunswick, New Jersey). Values are presented as the average of 2 separate measurements.
Figure 2.
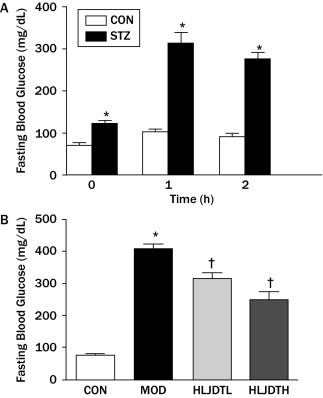
(A) Oral glucose tolerance test. CON = control group; STZ = streptozotocin-treated group. Values are presented as mean (SEM) (n = 8–24 in each group). (B) Effects of Huang-Lian-Jie-Du-Tang (HLJDT) on serum fasting blood glucose levels in rats with type 2 diabetes mellitus. CON = control group; HLJDTH = high-dose HLJDT (1.25 g/kg/d); HLJDTL = low-dose HLJDT (0.42 g/kg/d); MOD = type 2 diabetic model group. Values are presented as mean (SEM) (n = 8 in each group). *P < 0.05 versus CON; †P < 0.05 versus MOD.
Measurement of Levels of Serum MDA, SOD, and Vascular Tissue IL-6
Blood samples were collected from eyes under anesthesia by pentobarbital, 30 mg/kg; serum was separated by centrifugation at 3000g for 15 minutes (Microfuge 22R; Beckman Coulter Inc, Brea, California) for the determination of MDA and SOD levels (kits from Jiancheng Bioengineering Research Institute, Nanjing, China). The content of MDA was detected by the thiobarbituric acid reagent method. The SOD activity in serum was detected by the xanthine oxidase method according to the manufacturer's instructions. The thoracic aorta was rapidly isolated and was carefully cleaned of fat and loose connective tissue. Part of the thoracic aorta was homogenized in the lysis buffer, and homogenates were centrifuged at 4000g for 10 minutes at 4°C. The supernatant was taken for the assays of IL-6 level using an enzyme-linked immunosorbent assay kit (R&D Systems, Minneapolis, Minnesota) following the manufacturer's instructions. Protein concentration was determined by the Coomassie Brilliant Blue method using bovine serum albumin as a standard. All the samples were assayed in triplicate.
Evaluation of Vasorelaxant Activity in Aorta
Sections of the thoracic aorta segments were carefully sectioned into 3-mm-long rings. Arterial rings were connected to isometric transducers (ADInstruments Inc, Colorado Springs, Colorado) and were suspended in organ chambers filled with 10 mL of gassed (95% oxygen and 5% carbon dioxide) Krebs solution (pH 7.4, 37°C) composed of 118.3 mM sodium chloride, 4.7 mM potassium chloride, 2.5 mM calcium chloride, 1.2 mM magnesium sulfate, 1.2 mM potassium dihydrogen phosphate, 25.0 mM sodium bicarbonate, and 11.1 mM glucose. Isometric tension was recorded continuously. Arteries were allowed to equilibrate for 30 minutes and then were gradually stretched to 2.5 g of tension over 40 minutes. After 2 challenges with 60 mM potassium chloride, 10 μM indomethacin was added to eliminate the possible influence of endogenous cyclooxygenase before the submaximal contractions induced by phenylephrine (10–6 M). Vascular rings in which the maximal constrictions in response to phenylephrine (10–6 M) did not differ significantly were selected for relaxation experiments. After a stable contraction plateau, acetylcholine (10–9–10–4 M)-induced relaxations were conducted respectively. In another set, similar concentrations of sodium nitroprusside ranging from 10–9 to 10–6 M were added. The relaxations are expressed as a percentage of maximal constriction induced by phenylephrine (10–6 M).
Statistical Analysis
All the values are expressed as mean (SEM). Statistical significance was determined by the t test for unpaired observations and the Mann-Whitney rank sum test. One-way ANOVA was performed for multiple comparisons between groups. In all the comparisons, the difference was considered statistically significant at P < 0.05.
Results
Effects of Long-term Treatment With HLJDT on Body Weight and FBG Level
After continuous administration of HLJDT for 8 weeks, the general condition of the rats improved, as seen by burnished hair, increased activity, and decreased 24-hour urine volume (rats padding by day 2 times instead of 3 times a week). Body weight increased with age. Although weight grew more slowly in the MOD group than in the CON and HLJDT groups, there was no significant difference between them (data not shown). The FBG values in the MOD group were significantly higher than those in the CON group (P < 0.05). However, the FBG level was significantly decreased in the HLJDT group compared with the MOD group (Figure 2B).
Effects of Long-term Treatment With HLJDT on MDA Content and SOD Activity in Serum
Serum MDA content was significantly increased and SOD activity was significantly decreased in the MOD group compared with the CON group (P < 0.05). However, serum MDA content was significantly reduced in the HLJDT groups compared with the MOD group (Figure 3). In addition, the serum SOD level was significantly increased in the HLJDT groups compared with the MOD group in a dose-dependent manner (Figure 4).
Figure 3.
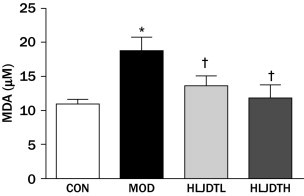
Effects of Huang-Lian-Jie-Du-Tang (HLJDT) on the level of serum malondialdehyde (MDA) in rats with type 2 diabetic mellitus. CON = control group; HLJDTH = high-dose HLJDT (1.25 g/kg/d); HLJDTL = low-dose HLJDT (0.42 g/kg/d); MOD = type 2 diabetic model group. Values are presented as mean (SEM) (n = 8 in each group). *P < 0.05 versus CON; †P < 0.05 versus MOD.
Figure 4.
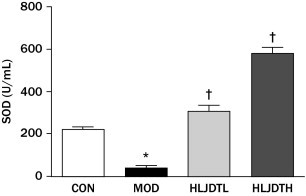
Effects of Huang-Lian-Jie-Du-Tang (HLJDT) on the level of serum superoxide dismutase (SOD) in rats with type 2 diabetes mellitus. CON = control group; HLJDTH = high-dose HLJDT (1.25 g/kg/d); HLJDTL = low-dose HLJDT (0.42 g/kg/d); MOD = type 2 diabetic model group. Values are presented as mean (SEM) (n = 8 in each group). *P < 0.05 versus CON; †P < 0.05 versus MOD.
Effects of Long-term Treatment With HLJDT on the IL-6 Level in the Aorta
The vascular tissue IL-6 level was significantly increased in the MOD group compared with the CON group (P < 0.05) but was reversed by HLJDT treatment in a dose-dependent manner (Figure 5).
Figure 5.
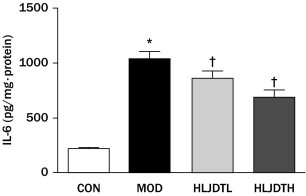
Effects of Huang-Lian-Jie-Du-Tang (HLJDT) on the level of interleukin 6 (IL-6) from aorta tissue in rats with type 2 diabetes mellitus. CON = control group; HLJDTH = high-dose HLJDT (1.25 g/kg/d); HLJDTL = low-dose HLJDT (0.42 g/kg/d); MOD = type 2 diabetic model group. Values are presented as mean (SEM) (n = 8 in each group). *P < 0.05 versus CON; †P < 0.05 versus MOD.
Effects of Long-term Treatment With HLJDT on Relaxation of Aortic Rings
Acetylcholine caused a concentration-dependent relaxation of phenylephrine-precontracted aortic preparations in all the groups. Acetylcholine-induced relaxation (10–9–10–4 M) was significantly decreased in the MOD group compared with the CON group. However, HLJDT treatment significantly increased acetylcholine-induced relaxation (10–9–10–4 M) compared with that in the MOD group in a dose-dependent manner (Figure 6). In addition, sodium nitroprusside–induced relaxation in the aorta did not significantly differ in all the groups (data not shown).
Figure 6.
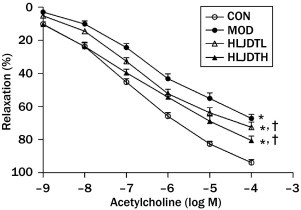
Effects of long-term administration of Huang-Lian-Jie-Du-Tang (HLJDT) on acetylcholine-induced relaxation in endothelium-intact thoracic aorta from rats with type 2 diabetes mellitus. CON = control group; HLJDTH = high-dose HLJDT (1.25 g/kg·d); HLJDTL = low-dose HLJDT (0.42 g/kg/d); MOD = type 2 diabetic model group. Values are presented as mean (SEM) (n = 8 in each group). *P < 0.05, CON compared with MOD; †P < 0.05, HLJDT compared with MOD.
Discussion
Diabetes mellitus has become a major public health problem in the 21st century. Taking a global perspective, T2DM accounts for 90% to 95% of all diagnosed DM in adults,12 and strategies aimed at the prevention and treatment of diabetes are needed. The diabetic model induced by lower-dose streptozotocin injection plus high-fat chow feeding was recognized as T2DM due to changes in the levels of glucose, lipids, and insulin in blood serum.10,13
In this study, we established the model of T2DM (MOD). We observed that levels of FBG, vascular tissue inflammatory cytokine IL-6, and serum MDA increased but the level of SOD decreased in MOD. In addition, endothelium-dependent vascular dilation mediated by acetylcholine in aortic preparations reduced in MOD. These results indicated that the rats developed endothelial injury, vascular inflammation, and oxidative stress in the large blood vessels. It is well-known that functional alterations in the vascular endothelium occur in many diseases, including diabetes. In the present study, we found that the acetylcholine-induced relaxation in the MOD group was significantly improved by long-term treatment with HLJDT in a dose-dependent manner. The relaxation in response to sodium nitroprusside, a nitric oxide donor, did not significantly differ between the CON and HLJDT groups. Sodium nitroprusside induced the vascular relaxation via a mechanism that does not depend on the endothelium.14 Thus, the results suggested that the endothelium-independent vascular dilation in the aorta was not affected by HLJDT treatment. We also found that MOD increased the MDA level in addition to the FBG level, decreased the SOD level, and increased the IL-6 level, which were reversed by HLJDT treatment, suggesting that HLJDT had antioxidant and anti-inflammatory effects, resulting in the improvement of MOD.
Diabetes mellitus is categorized as Xiaokezheng according to its clinical manifestations in traditional Chinese medicine (TCM). Based on the theory of TCM, the main site of the lesion is in the lung, stomach, or kidney, and the basic pathogenesis is associated with overconsumption of Yin (body fluids), causing excessive “heat” of tissues in patients with DM. Some Chinese scholars have also proposed a theory that T2DM results from “toxicity.”15 This “poison” means that there are excessive harmful substances in the body, such as “sugar poison” (too much sugar) and too many “inflammatory actors” and “oxygen free radicals.” The existence of poisons in the body becomes an important factor in the long-term complications associated with T2DM. The HLJDT is the classical TCM of heat clearance and detoxification (Arcane Essentials from the Imperial Library). The King drug, Coptis of HLJDT, can purge the sthenic heart-fire to eliminate the “hot poison”; the minister herb Radix Scutellariae can purge Shang Jiao; the adjunctive drug amur corktree bark can purge Xia Jiao; and Fructus Gardeniae can purge the triple Jiao and reduce heat to remove the evil heat out of the urine. These components of HLJDT can be used to enhance the efficacy of one another according to the “differentiation treatment” of TCM. Therefore, application of HLJDT from the poison on T2DM is also consistent with the principles of differentiation treatment of TCM.
As is well-known, DM is characterized by elevated blood sugar levels and is often accompanied by sustained cardiovascular complications of metabolic diseases. Macrovascular and microvascular diseases are currently the principal causes of morbidity and mortality in patients with T2DM; dysfunction of the vascular endothelial cell is considered an initiating factor, and it plays a critical role in the development of diabetic vascular complications.4 However, the etiology of endothelial dysfunction in T2DM is still unknown. Hyperglycemia is clearly recognized as the primary culprit in the pathogenesis of diabetic complications. A considerable body of evidence implicates oxidative stress and the massive inflammatory cytokine production as important pathogenic elements in diabetic endothelial dysfunction.5–8,16
Compiled by the famous Tang dynasty doctor Wang Tao, HLJDT has been traditionally used to treat inflammation, Chinese toxic heat syndromes, and infectious diseases.17 Also, HLJDT is commonly prescribed in Japan for treating cerebrovascular disease.11 It is composed of Rhizoma coptidis, Radix scutellariae, Cortex phellodendri, and Fructus gardenia.11,17 Chinese medicine prescription research notes that the major component of HLJDT is Rhizoma coptidis, the active ingredient of which is berberine. In the present study, HPLC analysis also revealed that the main component in HLJDT is berberine. Berberine, a natural plant alkaloid, is well-known for its anti-inflammatory activity. Currently, research suggests that berberine decreases serum lipid and glucose levels.18 Furthermore, animal experiments also show that berberine benefits diabetes.19–25 Radix scutellariae of HLJDT is composed of a variety of flavonoids. The main components of flavonoids are baicalin, baicalein, and wogonoside, which have antioxidant26 and antihyperglycemic27 properties. Cortex phellodendri of HLJDT contains berberine, which can strengthen the role of Coptis. The main active ingredient of Fructus gardeniae of HLJDT is geniposide. Geniposide includes a variety of iridoid glycosides (also known as genipin l-glucoside), which are therapeutic for diabetes.28 Accordingly, many ingredients in HLJDT have protective effects against the impairment of T2DM. The mixture of these ingredients may exert synergistic effects because the pharmacologic activity of HLJDT is performed by multilevel, multitarget, multichannel actions and the prescription plays an integrated role in T2DM. Moreover, there were no obvious adverse effects reported with HLJDT long-term clinical application.
Conclusions
The present results suggest that long-term administration of HLJDT could have protective effects on endothelium in T2DM. The antioxidant properties coupled with attenuation of inflammatory mediators may contribute to the protection afforded by HLJDT. These findings indicate that HLJDT could have a positive effect on diabetic vascular disease and could be used to prevent and treat the vascular complications of T2DM.
Conflicts of Interest
The authors have indicated that they have no conflicts of interest regarding the content of this article.
Acknowledgments
This work was supported in part by the Traditional Chinese Medicine Administration Research Foundation of Guangdong Province (No. 2007134 to Dr. Yi), the Research Foundation of Guangdong Provincial Science and Technology Department (No. 2012B031800235 to Dr. Yi), and the National Natural Science Foundation of China (No. 30572188 to J.-D. Luo). We thank associate professor Jianyie Zhang for his kind identification of medicinal herbs and Xiaoqian Wu for her guidance with article submission. Drs. Yi and Hou contributed to the literature search. Dr. Chen contributed to the figure creation. Dr. Yi contributed to the study design. Drs. He, K.-F. Luo, Zhang, Liu, Xue, Hou, and Chen contributed to the data collection and data interpretation. Drs. Yi, Liu, Xue, and J.-D. Luo contributed to the writing.
References
- 1.Yang W., Lu J., Weng J. Prevalence of diabetes among men and women in China. N Engl J Med. 2010;362:1090–1101. doi: 10.1056/NEJMoa0908292. [DOI] [PubMed] [Google Scholar]
- 2.Tian W.H., Lu B., Hu X.J. Huanglian Jiedu decoction treated the diabetes 32 cases. Shaanxi J Trad Chin Med. 2008;29:1603–1605. [Google Scholar]
- 3.Xiao Y.L., Lu F.E., Xu L.J. Protective effects of Huanglian Jiedu decoction on vascular endothelial function in type 2 diabetic rats [in Chinese] Zhongguo Zhong Yao Za Zhi. 2005;30:1767–1770. [PubMed] [Google Scholar]
- 4.De Vriese A.S., Verbeuren T.J., Van de Voorde J. Endothelial dysfunction in diabetes. Br J Pharmacol. 2000;130:963–974. doi: 10.1038/sj.bjp.0703393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hartge M.M., Unger T., Kintscher U. The endothelium and vascular inflammation in diabetes. Diab Vasc Dis Res. 2007;4:84–88. doi: 10.3132/dvdr.2007.025. [DOI] [PubMed] [Google Scholar]
- 6.Ceriello A., Motz E. Is oxidative stress the pathogenic mechanism underlying insulin resistance, diabetes, and cardiovascular disease?: The common soil hypothesis revisited. Arterioscler Thromb Vasc Biol. 2004;24:816–823. doi: 10.1161/01.ATV.0000122852.22604.78. [DOI] [PubMed] [Google Scholar]
- 7.Pradhan A.D., Manson J.E., Rifai N. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001;286:327–334. doi: 10.1001/jama.286.3.327. [DOI] [PubMed] [Google Scholar]
- 8.Rossi R., Dalle-Donne I., Milzani A., Giustarini D. Oxidized forms of glutathione in peripheral blood as biomarkers of oxidative stress. Clin Chem. 2006;52:1406–1414. doi: 10.1373/clinchem.2006.067793. [DOI] [PubMed] [Google Scholar]
- 9.Johansen J.S., Harris A.K., Rychly D.J., Ergul A. Oxidative stress and the use of antioxidants in diabetes: linking basic science to clinical practice. Cardiovasc Diabetol. 2005;4:5. doi: 10.1186/1475-2840-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Srinivasan K., Viswanad B., Asrat L. Combination of high-fat diet-fed and low-dose streptozotocin-treated rat: a model for type 2 diabetes and pharmacological screening. Pharmacol Res. 2005;52:313–320. doi: 10.1016/j.phrs.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 11.Xu J., Murakami Y., Matsumoto K. Protective effect of Oren-gedoku-to (Huang-Lian-Jie-Du-Tang) against impairment of learning and memory induced by transient cerebral ischemia in mice. J Ethnopharmacol. 2000;73:405–413. doi: 10.1016/s0378-8741(00)00303-2. [DOI] [PubMed] [Google Scholar]
- 12.Roger V.L., Go A.S., Lloyd-Jones D.M. Heart disease and stroke statistics: 2012 update: a report from the American Heart Association. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen G., Lu F.E., Xu L.J. Effects of Huanglian Jiedu Decoction on glucose transporter 4 in target tissues of type 2 diabetic rats [in Chinese] Zhong Xi Yi Jie He Xue Bao. 2007;5:412–415. [PubMed] [Google Scholar]
- 14.Rapoport R.M., Draznin M.B., Murad F. Endothelium-dependent relaxation in rat aorta may be mediated through cyclic GMP-dependent protein phosphorylation. Nature. 1983;306:174–176. doi: 10.1038/306174a0. [DOI] [PubMed] [Google Scholar]
- 15.Lu F.E., Wang Z.M., Guo A.Q. Exploration on the Hypothesis of Treating Diabetes Mellitus with the Detoxification Principle of Traditional Chinese Medicine. Chin J Bas Med in Trad Chin Med. 2002;8:335–337. [Google Scholar]
- 16.Sjoholm A., Nystrom T. Endothelial inflammation in insulin resistance. Lancet. 2005;365:610–612. doi: 10.1016/S0140-6736(05)17912-4. [DOI] [PubMed] [Google Scholar]
- 17.Lu J., Wang J.S., Kong L.Y. Anti-inflammatory effects of Huang-Lian-Jie-Du decoction, its two fractions and four typical compounds. J Ethnopharmacol. 2011;134:911–918. doi: 10.1016/j.jep.2011.01.049. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Y., Li X., Zou D. Treatment of type 2 diabetes and dyslipidemia with the natural plant alkaloid berberine. J Clin Endocrinol Metab. 2008;93:2559–2565. doi: 10.1210/jc.2007-2404. [DOI] [PubMed] [Google Scholar]
- 19.Chen C., Zhang Y., Huang C. Berberine inhibits PTP1B activity and mimics insulin action. Biochem Biophys Res Commun. 2010;397:543–547. doi: 10.1016/j.bbrc.2010.05.153. [DOI] [PubMed] [Google Scholar]
- 20.Lee Y.S., Kim W.S., Kim K.H. Berberine, a natural plant product, activates AMP-activated protein kinase with beneficial metabolic effects in diabetic and insulin-resistant states. Diabetes. 2006;55:2256–2264. doi: 10.2337/db06-0006. [DOI] [PubMed] [Google Scholar]
- 21.Zhou J., Zhou S., Tang J. Protective effect of berberine on beta cells in streptozotocin- and high-carbohydrate/high-fat diet-induced diabetic rats. Eur J Pharmacol. 2009;606:262–268. doi: 10.1016/j.ejphar.2008.12.056. [DOI] [PubMed] [Google Scholar]
- 22.Yu Y., Liu L., Wang X. Modulation of glucagon-like peptide-1 release by berberine: in vivo and in vitro studies. Biochem Pharmacol. 2010;79:1000–1006. doi: 10.1016/j.bcp.2009.11.017. [DOI] [PubMed] [Google Scholar]
- 23.Cok A., Plaisier C., Salie M.J. Berberine acutely activates the glucose transport activity of GLUT1. Biochimie. 2011;93:1187–1192. doi: 10.1016/j.biochi.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yin J., Gao Z., Liu D. Berberine improves glucose metabolism through induction of glycolysis. Am J Physiol Endocrinol Metab. 2008;294:E148–E156. doi: 10.1152/ajpendo.00211.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu L., Yu Y.L., Yang J.S. Berberine suppresses intestinal disaccharidases with beneficial metabolic effects in diabetic states, evidences from in vivo and in vitro study. Naunyn Schmiedebergs Arch Pharmacol. 2010;381:371–381. doi: 10.1007/s00210-010-0502-0. [DOI] [PubMed] [Google Scholar]
- 26.Li J., Zhang M., Chao J., Shuang S. Preparation and characterization of the inclusion complex of Baicalin (BG) with beta-CD and HP-beta-CD in solution: an antioxidant ability study. Spectrochim Acta A Mol Biomol Spectrosc. 2009;73:752–756. doi: 10.1016/j.saa.2009.03.025. [DOI] [PubMed] [Google Scholar]
- 27.Li H.T., Wu X.D., Davey A.K., Wang J. Antihyperglycemic effects of baicalin on streptozotocin - nicotinamide induced diabetic rats. Phytother Res. 2011;25:189–194. doi: 10.1002/ptr.3238. [DOI] [PubMed] [Google Scholar]
- 28.Zhang C.Y., Parton L.E., Ye C.P. Genipin inhibits UCP2-mediated proton leak and acutely reverses obesity- and high glucose-induced beta cell dysfunction in isolated pancreatic islets. Cell Metab. 2006;3:417–427. doi: 10.1016/j.cmet.2006.04.010. [DOI] [PubMed] [Google Scholar]


