Abstract
Methamphetamine (METH) and 3,4-methylenedioxymethamphetamine (MDMA, ecstasy) are amphetamine derivatives with high abuse liability. These amphetamine-related drugs of abuse mediate their effects through the acute activation of both dopaminergic and serotonergic neurons. Long-term abuse of these amphetamine derivatives, however, results in damage to both dopaminergic and serotonergic terminals throughout the brain. This toxicity is mediated in part by oxidative stress, metabolic compromise, and inflammation. The overall objective of this review is to highlight experimental evidence that METH and MDMA increase oxidative stress, produce mitochondrial dysfunction, and increase inflammation that converge and culminate in the long-term toxicity to dopaminergic and serotonergic neurons.
Keywords: methamphetamine, MDMA, oxidative stress, inflammation, mitochondrial dysfunction, matrix metalloproteinase
Introduction
The amphetamine derivatives methamphetamine (METH) and 3,4-methylenedioxymethamphetamine (MDMA, ecstasy) are highly abused drugs with potent stimulating effects in the central nervous system. The acute central and peripheral effects of both METH and MDMA are illustrated by increases in euphoria and mood, decreases in appetite, increases in alertness, and hyperthermia. Many of these acute effects are believed to play a role in the high abuse liability of these amphetamine-related drugs of abuse. The high abuse potential of these substituted amphetamines is supported by current statistics reported in the 2006 National Survey on Drug Use and Health wherein it was estimated that 500,000 to 700,000 individuals age 12 and older had used METH and MDMA within the past month. The survey also highlighted the profound comorbidity between chronic substance abuse and treatment for psychiatric disorders. Additionally, a 2005 study by the Drug Abuse Warning Network reported that 138,950 emergency room visits were attributed to amphetamine-related drugs of abuse, thus highlighting the potentially harmful effects associated with the use of METH and MDMA. Taken together, these epidemiological studies illustrate the health-related risks of the amphetamine compounds and the importance for determining how these drugs function acutely and how their chronic use may have long-term consequences.
Basic neuropharmacology of METH and MDMA
The primary acute neuropharmacological actions of METH and MDMA are to increase the release of both dopamine (DA) and serotonin (5HT) from nerve terminals in the central nervous system. The mechanism by which amphetamine-related compounds enhance the release of DA and 5HT is mediated, in part, by their interaction with dopamine and serotonin transporters (DAT and SERT, respectively) and the subsequent entry into dopaminergic and serotonergic nerve terminals. Upon entry into the terminal, amphetamines disrupt the sequestration of neurotransmitters by the vesicles resulting in increased levels of neurotransmitter in the cytosol and subsequent reverse transport out of the nerve terminal (Sulzer et al. 2005). The specificity of amphetamine compounds for dopaminergic versus serotonergic terminals is dependent upon their structure. The parent compound amphetamine has a much greater affinity for DAT versus SERT. The addition of the methyl group to the terminal amine of amphetamine enhances the affinity of METH for SERT. Finally, the addition of the methylenedioxy group imparts an even greater relative affinity of MDMA for SERT versus DAT when compared to either amphetamine or METH (Rothman and Baumann 2003). The greater affinity for SERT compared to DAT results in a greater acute increase in 5HT relative to DA release. This effect is more pronounced after MDMA compared to METH. In contrast, amphetamine has relatively greater effects on DA compared to 5HT release.
In addition to increases in both DA and 5HT, amphetamine-related drugs of abuse also increase extracellular levels of glutamate in both the striatum and hippocampus (Rocher and Gardier 2001; Nash and Yamamoto 1992; Cunningham et al. 2004). Despite the actions of METH and MDMA at DAT and SERT, there is no indication that increases in glutamate are mediated by a direct interaction with the plasmalemmal glutamate transporter (Kokoshka et al. 1998). Although the mechanisms of glutamate release are not fully characterized, the increase in glutamate in the striatum after high doses of METH is mediated via a polysynaptic pathway within the basal ganglia and is a consequence of METH-induced DA release (Mark et al. 2004). Despite an incomplete understanding of how METH and MDMA enhance glutamate release, the fact remains that glutamate, in addition to DA and 5HT, are enhanced after the administration of amphetamine derivatives. These acute increases in DA, 5HT, and glutamate are important for both the acute effects and long-term consequences of amphetamine-related drugs of abuse which will be addressed in this review.
Neurotoxicity profile of METH and MDMA
Table 1 summarizes the neurotoxicity profiles of METH and MDMA. The long-term consequences of METH and MDMA use are manifested by a variety of different markers. In preclinical studies, the toxicity of METH and MDMA is evidenced by decreases in markers of dopaminergic and serotonergic neurons. Specifically, METH produces long-lasting decreases in tyrosine hydroxylase immunoreactive fibers and activity (the rate-limiting enzyme for DA synthesis), dopamine reuptake sites, and DA tissue content within the striatum while sparing other DA-rich areas (Ricaurte et al. 1980; Wagner et al. 1980; Hotchkiss and Gibb 1980; Ryan et al. 1988). This METH toxicity to DA neurons occurs in both rodents and nonhuman primates and has been shown to persist for up to 4 years after drug administration in nonhuman primates (Seiden et al. 1976; Bittner et al. 1981; Woolverton et al. 1989). METH also is toxic to 5HT terminals in multiple brain regions including the striatum, hippocampus, and frontal cortex (Ricaurte et al. 1980; Seiden et al. 1988). In contrast, MDMA has a more selective toxicity for 5HT neurons in most species. Specifically, MDMA decreases tryptophan hydroxylase activity, serotonin reuptake sites, 5HT tissue content, and the density of fine axon terminals of 5HT neurons in numerous brain regions in rodents (Stone et al. 1986; Battaglia et al. 1987; Ricaurte et al. 1985; O’Hearn et al. 1988). MDMA toxicity to 5HT terminals is also evident in nonhuman primates as indicated by decreases in 5HT content and damage to 5HT nerve fibers (Ricaurte et al. 1988).
Table 1.
Neurotoxicity profile of methamphetamine and MDMA
| Methamphetamine | MDMA |
|---|---|
| Decreases in markers of DA and 5HT terminals: tyrosine and tryptophan hydroxylase, DAT and SERT, DA and 5HT tissue content |
Decreases in markers of 5HT terminals: tryptophan hydroxylase, SERT, 5HT tissue content |
| Increases in Fluoro-Jade staining | Increases in Fluoro-Jade staining |
| Increases in GFAP | Increases in GFAP |
Long-term deficits in markers of dopaminergic and serotonergic neurons are not restricted to rodent and nonhuman primate models but are also evident in human METH and MDMA abusers. Clinical studies using functional magnetic resonance imaging to measure changes in DAT levels in human METH abusers indicate that chronic use of METH results in decreased DAT in the striatum that persists for up to 3 years following abstinence (McCann et al. 1998). The decreases in DAT have been correlated with deficits in working memory associated with chronic METH abuse (McCann et al. 2008). These working memory deficits associated with METH abuse have also been associated with abnormalities in brain regions other than the striatum. For example, decreases in hippocampal volume were observed that correlated with decreases in memory performance on a word recall test of individuals that used METH for an average of 10 years (Thompson et al. 2004). In addition to the hippocampal structural abnormalities and striatal dopaminergic deficits, abstinent METH users also display global decreases in SERT in brain regions associated with an aggressive phenotype (Sekine et al. 2006). Similarly, wide-spread decreases in SERT are also observed in abstinent MDMA users (McCann et al. 2005) and have been linked to abnormalities in cognitive function and impulsive behaviors (McCann et al. 1999, 2000).
Although many clinical and preclinical studies have focused on specific markers of dopaminergic and serotonergic terminal degeneration after the administration of METH and MDMA, other markers are also important to the neurotoxicity profile of these substituted amphetamines. Degenerating axons and cell bodies have been observed after METH and MDMA. Increases in silver staining of degenerating nerve fibers are observed after high-dose METH (Ricaurte et al. 1982) as well as increases in Fluoro-Jade staining indicative of neuronal degeneration after exposure to either METH or MDMA (Eisch et al. 1998; Schmued 2003; Ricaurte et al. 1982). Morphological changes to glia have also been demonstrated that occur apart from the markers of axonal and cell body degeneration after METH and MDMA. These changes are most reliably illustrated by increases in glial fibrillary fluorescent protein (GFAP) which is used as a marker for central nervous system injury and toxicity. GFAP is increased in the striatum after METH and MDMA (O’Callaghan and Sriram 2005; O’Callaghan and Miller 1994; Pu and Vorhees 1993; Hess et al. 1990). Striatal microglia are also activated (Orio et al. 2004; Escubedo et al. 1998) and are only observed after exposure to the amphetamine derivatives that are neurotoxic to DA and 5HT nerve terminals (Thomas et al. 2004). This is noteworthy in light of the toxicity of amphetamine-related drugs of abuse because activated microglia may increase the levels of proinflammatory cytokines. In fact, METH and MDMA increase the levels tumor necrosis factor (TNF)-α and interleukin (IL)-1β (Orio et al. 2004; Sriram et al. 2006). Therefore, inflammatory processes may play a role in mediating toxicity of the substituted amphetamines based on the strong link between microglial activation, increases in GFAP, and toxicity to dopaminergic and serotonergic neurons.
Mechanisms of toxicity
Although numerous mechanisms have been suggested to be responsible for METH and MDMA toxicity, the purpose of this review is to highlight the individual and combined roles of oxidative stress, metabolic compromise, and inflammation. Experimental data for increases in markers of free radical production, mitochondrial dysfunction, and microglial activation–inflammatory cytokine production are evident and have been linked to the acute increases in DA and glutamate produced by METH and MDMA administration. The remainder of this review will focus on how these markers of oxidative stress, metabolic compromise, and inflammation mediate the long-term toxicity produced by the amphetamine-related drugs of abuse, METH and MDMA. Table 2 summarizes the mechanisms underlying the toxic effects of these drugs.
Table 2.
Mechanisms of toxicity of methamphetamine and MDMA
| Toxicity | Mechanisms |
|---|---|
| Free radical production and oxidative stress | Increases in 2,3-DHBA formation |
| Increases in lipid peroxidation and protein oxidation | |
| Protection against toxicity by free radical spin trapping agents and antioxidants | |
| Increases in spectrin and tau proteolysis | |
| Increases in nitrosylated proteins | |
| Protection against toxicity by NOS and ONOO− inhibitors | |
| Metabolic compromise | Acute increases and long-term decreases in glucose metabolism |
| Decreases in ATP | |
| Decreases in ETC complex activities | |
| Protection against toxicity by energy supplementation | |
| Inflammation | Increases in microglial activation |
| Increases in proinflammatory cytokine production | |
| Protection against toxicity by anti-inflammatory drugs |
Free radical production and oxidative stress
The most direct evidence of increases in free radical production after METH and MDMA administration is the increase in salicylate trapping of hydroxyl radicals and the subsequent rise in the formation of 2,3-dihydroxybenzoic acid (2,3-DHBA) within the striatum (Yamamoto and Zhu 1998; Shankaran et al. 1999; Giovanni et al. 1995). These increases in free radical production consequently result in increases in markers of lipid peroxidation and protein oxidation, namely malonyldialdehyde and protein carbonyl formation (Yamamoto and Zhu 1998; Gluck et al. 2001; Sprague and Nichols 1995b). The importance of these acute increases in free radical production in mediating long-term dopaminergic and serotonergic depletions is illustrated in numerous studies investigating the protective role of free radical spin trapping agents and antioxidants. For example, the antioxidant spin trapping agent phenylbutylnitrone protects against METH-induced DA depletions in the striatum and MDMA-induced 5HT depletions in the hippocampus and cortex (Yamamoto and Zhu 1998; Colado and Green 1995). The ability of the antioxidants, ascorbate and vitamin E, to attenuate DA and 5HT depletions in the striatum after high-dose METH was one of the first indications that METH toxicity occurs through oxidative stress (Wagner et al. 1985; De Vito and Wagner 1989). Subsequent experiments showed that ascorbate also blocked MDMA toxicity to 5HT neurons in the striatum (Gudelsky 1996). Numerous other antioxidants including selenium and N-acetylcysteine have been shown to be protective against METH and MDMA toxicity (Imam et al. 1999; Fukami et al. 2004; Sanchez et al. 2003). The role of oxidative stress in mediating METH and MDMA toxicity is further illustrated by decreases in the activity of the endogenous antioxidants glutathione peroxidase, catalase, and superoxide dismutase observed after either METH or MDMA administration (Jayanthi et al. 1998, 1999). Conversely, transgenic mice overexpressing Cu–Zn superoxide dismutase are relatively resistant to the neurotoxic effects of METH (Cadet et al. 1994, 1995).
Although decreases in endogenous antioxidant enzyme capacity may mediate increased free radical production, acute increases in DA have been shown to play a predominant role in the increases in oxidative stress associated with METH and MDMA administration. The generation of free radical species subsequent to increases in DA is mainly derived from auto-oxidation of DA or enzymatic oxidation by monoamine oxidase (MAO) to result in the production of superoxide and hydrogen peroxide. The production of hydrogen peroxide from the enzymatic metabolism of DA may also be catalyzed by iron to form hydroxyl radicals via the Fenton reaction (Olanow 1992; Cohen 1987). Additionally, cytotoxic quinone formation can occur following the auto-oxidation of DA and is an important mediator of DA toxicity (Fornstedt et al. 1989; Stokes et al. 1999). A supporting role of DA and dopamine-derived free radical species and cytotoxic quinones has also been proposed to mediate METH toxicity (LaVoie and Hastings 1999). Decreased synthesis of DA or blockade of DAT protects against METH toxicity (Marek et al. 1990; Schmidt et al. 1985). Additionally, removal of iron by the iron chelator deferoxamine attenuates the long-term decreases in DA tissue content in the striatum after METH administration (Yamamoto and Zhu 1998).
DA is also an important mediator of MDMA toxicity. MDMA-induced 5HT depletions are exacerbated by enhancing DA concentrations via pretreatment with the DA precursor l-3,4-dihydroxyphenylalanine (DOPA; Schmidt et al. 1991). Conversely, inhibition of DA synthesis, depletion of DA stores with reserpine, or destruction of DA neurons attenuates MDMA-induced damage to 5HT neurons (Stone et al. 1988; Schmidt et al. 1990). Similarly, pretreatment with the DA uptake inhibitor mazindol attenuates MDMA-induced increases in extracellular DA, increases in 2,3-DHBA formation, and depletions in 5HT (Shankaran et al. 1999). Moreover, inhibition of DA metabolism is protective as evidenced by the blockade of MDMA toxicity via inhibition or gene knockdown of MAO (Sprague and Nichols 1995a; Falk et al. 2002).
Despite a clear role of DA in mediating MDMA toxicity, there are also caveats associated with how DA may mediate MDMA-induced depletions in 5HT in brain areas that are sparsely innervated by DA. In this regard, a recent study by Breier et al. implicated a role for the DA precursor tyrosine in mediating MDMA toxicity (Breier et al. 2006). The novel findings from this study are twofold. First, tyrosine levels increase in the brain after MDMA administration. Second, tyrosine can be converted to DA within 5HT terminals via nonenzymatic hydroxylation to DOPA followed by DA via aromatic amino acid decarboxylase (AADC). This conversion of tyrosine to DA can directly mediate toxicity as blockade of AADC blocks MDMA-induced 5HT depletions.
In addition to a prominent involvement of DA-mediated oxidative stress in METH and MDMA toxicity, acute increases in glutamate also contribute to METH- and MDMA-induced oxidative stress and toxicity. Acute increases in glutamate may activate both ionotropic and metabotropic glutamate receptors and increase intracellular calcium levels. Increases in calcium may lead to activation of a variety of proteases and kinases that result in breakdown of cytoskeletal proteins and the formation of reactive oxygen species (Sattler and Tymianski 2000, 2001). One specific protease activated by the increases in intracellular calcium is calpain which degrades the cytoskeletal membrane protein, spectrin (Siman and Noszek 1988). Along these lines, high-dose METH administration increased striatal spectrin proteolysis that was blocked by pretreatment with the AMPA receptor antagonist GYKI (Staszewski and Yamamoto 2006). Breakdown of other cytoskeletal proteins that are vulnerable to proteolysis by calpain, such as the microtubule-binding protein tau, is also observed in the hippocampus after METH administration (Warren et al. 2005). Calpain-induced breakdown of tau and spectrin is also observed in primary cortical cultures after both METH and MDMA application (Warren et al. 2007). Despite these increases in in vitro markers of tau and spectrin breakdown after exposure to METH or MDMA, in vivo evidence for cleaved tau formation is observed only after neurotoxic doses of METH but not MDMA (Straiko et al. 2007).
Despite these differences between METH and MDMA on calpain-mediated cytoskeletal protein breakdown, other downstream effects of calpain activation may occur in relation to METH- and MDMA-induced increases in oxidative stress. Glutamate-mediated calpain activation also catalyzes the conversion of xanthine dehydrogenase to xanthine oxidase. Xanthine oxidase promotes the catabolism of xanthine and hypoxanthine to uric acid, yielding oxygen free radicals in the process (Dykens et al. 1987). Additional free radical species may be produced by activation of NMDA receptors resulting in stimulation of phospholipase A2 and the release of arachidonic acid. Lipoxygenase and cyclooxygenase (COX) conversion of arachidonic acid to eicosanoids may also increase oxygen free radicals (Dumuis et al. 1988; Lazarewicz et al. 1988). In light of the glutamate-receptor-mediated increases in oxygen free radical production and the role of free radical species in METH and MDMA toxicity, pretreatment with the NMDA receptor antagonist MK-801 protects against METH and MDMA toxicity to DA and 5HT terminals, respectively (Farfel et al. 1992). Caveats to these studies exist however, such that NMDA receptor antagonism also blocks METH- and MDMA-induced hyperthermia, a factor also important in mediating toxicity (Farfel and Seiden 1995a, b; Bowyer et al. 1994). Despite the difficulties in delineating the role of NMDA receptors versus hyperthermia in mediating METH and MDMA toxicity, other glutamate receptor systems have been implicated. For example, pretreatment with the metabotropic GluR5 antagonists MPEP and SIB-1893 attenuate METH toxicity without altering METH-induced hyperthermia (Battaglia et al. 2002). These GluR5 antagonists also attenuate METH-induced increases in 2,3-DHBA formation, thus associating glutamate receptor activation, oxidative stress, and METH toxicity. Moreover, the blockade of METH-induced glutamate release in the striatum is also protective against toxicity (Mark et al. 2004).
Excitotoxicity produced by METH and MDMA also may occur via the production of nitric oxide (NO). NO formed from the activation of nitric oxide synthase (NOS) following glutamate-mediated calcium entry through NMDA receptors has been shown to be neurotoxic (Dawson and Dawson 1996). NO can react with superoxide to form the oxidant, peroxynitrite (ONOO−; Radi et al. 1991; Lafon-Cazal et al. 1993). In fact, METH-induced DA toxicity can be blocked by the NOS inhibitor 7-nitroindazole and the peroxynitrite decomposition catalyst FeTPPS (Imam et al. 2000; Itzhak and Ali 1996). Similar effects are observed with regards to MDMA toxicity such that decreasing NO via inhibition of NOS and decreasing ONOO− by pretreatment with a peroxynitrite decomposition catalyst attenuate MDMA-induced depletions in striatal 5HT (Darvesh et al. 2005). The mechanism through which NO and ONOO− mediate the toxicity of substituted amphetamines is not completely understood; however, one suggested mechanism is through their ability to oxidatively modify both tyrosine and cysteine residues on proteins (Stamler and Hausladen 1998). In this regard, an overall increase in nitration of tyrosine residues, as evidenced by 3-nitrotyrosine formation, is evident in the striatum after high-dose METH and MDMA administration (Quinton and Yamamoto 2006). An increase in NO- and ONOO−-mediated nitration of proteins is important in light of the fact that tyrosine hydroxylase and tryptophan hydroxylase, biosynthetic enzymes of DA and 5HT, respectively, are readily nitrated by both NO and ONOO− (Kuhn and Arthur 1997; Kuhn and Geddes 1999; Kuhn et al. 1999). Therefore, the oxidation and nitration of proteins associated with DA and 5HT terminals may be important mediators of METH and MDMA toxicity. In fact, some of the first evidence for the role of oxidation and nitration of proteins in METH and MDMA toxicity stemmed from the observation that MDMA produced an inactivation of tryptophan hydroxylase that was reversed by sulfhydryl-reducing compounds (Stone et al. 1989; Gibb et al. 1990). Subsequently, METH- and MDMA-induced modifications to both tyrosine and tryptophan hydroxylase have been demonstrated and illustrate a specific target for substituted amphetamine-induced increases in reactive oxygen and reactive nitrogen species (RNS; Kuhn and Geddes 2000). More direct evidence for the role of protein nitration in toxicity was recently presented in a study by Eyerman and Yamamoto (2007) which illustrated that the immunoreactivity of the vesicular monoamine transporter 2 (VMAT2), the protein responsible for the uptake of DA into vesicles, was decreased and nitrosylated as early as 1 h after repeated high-dose METH. Both the increases in nitrosylation and decreases in protein were blocked by prior administration of the NOS inhibitor, S-methyl-l-thiocitrulline. These alterations in the VMAT2 protein are important in light of the fact that VMAT2 plays a permissive role in METH toxicity to DA terminals (Fumagalli et al. 1999; Fleckenstein et al. 2000). Additionally, the study by Eyerman and Yamamoto (2007) provides one example of how glutamate and dopamine interact and converge to mediate METH toxicity such that glutamate-mediated nitration of VMAT2 decreases the sequestration of DA, thus elevating cytosolic DA concentrations and DA-derived oxidative species and increasing the vulnerability of the DA terminal to oxidative stress.
Metabolic compromise
Alterations in brain energy utilization are evident after both METH and MDMA administration. Studies in rodent models using 2-deoxyglucose illustrate acute increases in glucose metabolism in brain areas of the extrapyramidal motor system after intravenous administration of METH (Pontieri et al. 1990). Similar increases in cerebral glucose utilization are observed after MDMA administration in comparable brain areas associated with locomotion, such as the globus pallidus and striatum (Quate et al. 2004). Additionally, increases in extracellular glucose concentrations in the striatum with accompanying decreases in brain glycogen are observed acutely after MDMA administration and are suggestive of an increase in the use of cerebral energy (Darvesh et al. 2002). Despite these acute increases in energy utilization after METH and MDMA administration, long-term decreases in cerebral glucose metabolism occur for up to 60 days in rats after high-dose METH and illustrate that initial increases in energy utilization are accompanied by long-term impairments in energy metabolism (Huang et al. 1999). In addition to alterations in glucose metabolism, other markers indicative of acute energy use are evident. For example, decreases in levels of adenosine triphosphate (ATP) are observed acutely in the striatum and hippocampus after high-dose METH and MDMA administration (Chan et al. 1994; Darvesh and Gudelsky 2005). The decreases in ATP after METH administration are potentiated by inhibiting glucose uptake and utilization suggesting that acute changes in glucose utilization are important in the maintenance of sufficient ATP levels within the brain. Additionally, the potentiated decreases in ATP produced by inhibition of glucose uptake and utilization are associated with a potentiation of long-term decreases in DA tissue content in the striatum and implicate a role of energy impairment in METH-induced dopaminergic toxicity (Chan et al. 1994).
A role for energy impairment in METH and MDMA toxicity is further evidenced by studies that demonstrate the potentiation of both METH and MDMA toxicity by the coadministration of metabolic inhibitors. For example, central administration of METH, which alone does not produce toxicity, synergizes with the mitochondrial complex II inhibitor malonate to deplete striatal dopamine (Burrows et al. 2000b). Similarly, coinfusion of MDMA and malonate synergistically depletes striatal 5HT. Despite the fact that systemic MDMA produces long-term depletions of 5HT but is not toxic to dopaminergic neurons, it is interesting to note that, when combined with malonate, MDMA depletes levels of DA in the striatum (Nixdorf et al. 2001). Conversely, METH and MDMA toxicity is attenuated by supplementation with energy substrates. For example, augmentation of energy metabolism via intrastriatal infusion with either ubiquinone or nicotinamide can block METH-induced depletions in striatal DA content (Stephans et al. 1998). Similarly, perfusion of both ubiquinone and nicotinamide attenuates MDMA-induced depletions in 5HT in the striatum and hippocampus (Darvesh and Gudelsky 2005). Overall, these studies illustrate the role of metabolic compromise in mediating the toxicity of amphetamine-related drugs of abuse.
In addition to the aforementioned studies implicating energy dysregulation in toxicity after METH and MDMA administration, other studies have directly illustrated alterations in electron transport chain (ETC) activity after high-dose METH and MDMA administration. As the ETC is the main source of ATP within the brain, alterations in ETC activity may be in part responsible for the decreased levels of ATP seen following METH and MDMA. The first investigation of METH- and MDMA-induced alterations in the function of specific ETC complexes was by Burrows et al. (2000a, b), illustrating that both METH and MDMA decrease levels of cytochrome oxidase, a marker of ETC complex IV activity (Burrows et al. 2000a). Subsequent studies have also investigated alterations in other mitochondrial electron transport chain complexes. Specifically, a decrease in complex II–III but not I–III activity was observed in the striatum at both 1 and 24 h after METH (Brown et al. 2005). This decrease in activity was not a direct effect of METH as in vitro incubation of a mitochondrial preparation with METH did not alter complex II–III activity at levels of METH similar to those observed in the brain after high-dose administration. The decreases in complex II activity, however, were dependent on METH-induced increases in glutamate as evidenced by the ability of the NMDA antagonist, MK-801, to block the METH-induced decreases in complex II–III activity. In addition to glutamate, a role for the downstream production of ONOO− was also demonstrated. In vitro application of ONOO− decreased complex II activity and the ONOO− scavenger blocked METH-induced decreases in complex II–III activity, implicating a role of both glutamate and glutamate-mediated ONOO− production in METH-induced decreases in mitochondrial activity. In addition to the mitochondrial effects of METH, reductions in both complex I and complex II ETC activity are also observed 12 h after high-dose MDMA administration (Quinton and Yamamoto 2006). Despite these decreases in activity, the responsible mechanisms are unknown; however, similar to METH, increases in either reactive oxygen or nitrogen species may also play an important role in mediating the MDMA-induced decreases in mitochondrial ETC activity.
Inflammation
The role of inflammation in the toxicity of amphetamine-related drugs of abuse is not as well defined as that of oxidative stress and metabolic compromise, but increases in neuroinflammation after both METH and MDMA administration are noted by an increase in the activation of microglia (Orio et al. 2004; Escubedo et al. 1998). The activation of microglia results in the secretion of a variety of reactive species including proinflammatory cytokines, prostaglandins, nitric oxide, and superoxide, all of which can damage neuronal tissue (Kreutzberg 1996; Hanisch 2002). The importance of microglial activation in mediating METH and MDMA toxicity is highlighted by the fact that only the neurotoxic amphetamines produce microglial activation (Thomas et al. 2004). Additional support for the role of microglial activation in METH toxicity is illustrated by the observation that microglial activation precedes the appearance of damage to dopaminergic neurons in the striatum produced by METH (LaVoie et al. 2004).
One mechanism by which microglial activation may contribute to neurotoxicity is via increases in the expression of cytokines such as IL-1β, IL-6, and TNF-α that initiate and promote neuroinflammation (Streit et al. 1999; Stoll and Jander 1999). In fact, a single high dose of METH increases striatal mRNA levels of IL-1α, IL-6, and TNF-α and hypothalamic mRNA levels of IL-1β (Sriram et al. 2006; Yamaguchi et al. 1991). The importance of these increases in cytokine production in relation to METH toxicity are illustrated by an attenuation of toxicity to both dopaminergic and serotonergic neurons in IL-6 knockout mice (Ladenheim et al. 2000). Despite this attenuation, an exacerbation of METH toxicity is observed in TNF-α knockout mice with an accompanying attenuation of toxicity by exogenous administration of TNF-α (Nakajima et al. 2004). The protective effects of exogenous TNF-α, however, were due in part to increases in vesicular DA uptake by TNF-α that counteracted the METH-induced decreases in vesicular uptake, thus resulting in an overall reduction in METH-induced increases in free cytosolic DA. Despite the increase in levels of proinflammatory cytokines, the precise role of these increases in proinflammatory cytokine production in mediating toxicity to METH is still under investigation. Similarly, very few studies have illustrated a role for proinflammatory cytokines in the toxicity of MDMA. The only report of increases in proinflammatory cytokine production after MDMA administration is a significant increase in IL-1β after a single dose of MDMA (Orio et al. 2004). Moreover, the intracerebroventricular administration of IL-1β potentiated MDMA-induced 5HT toxicity (O’Shea et al. 2005).
In addition to the paucity of research on the role of proinflammatory cytokines in mediating METH and MDMA toxicity, the role of anti-inflammatory drugs in blocking toxicity of amphetamine-related drugs of abuse is controversial and not well understood. For example, the nonsteroidal anti-inflammatory drug ketoprofen attenuated METH-induced increases in microglia activation and decreases in striatal DAT (Asanuma et al. 2003) but was ineffective at blocking METH-induced decreases in striatal DA tissue content (Thomas and Kuhn 2005a). In fact, pretreatment with the COX-2 inhibitor celecoxib potentiated the decrease in METH-induced striatal DA depletions (Zhang et al. 2007), a result that is in accord with the decrease in COX-2 expression measured at 24 h after METH (Kita et al. 2000). Similar variations are observed with administration of the anti-inflammatory drug, minocycline which attenuates METH-induced microglial activation but does not block METH-induced increases in TNF-α or striatal DA toxicity (Sriram et al. 2006). Despite the inability of minocycline to block METH toxicity and the conflicting results with COX-2 inhibitors, COX-2 knockout mice are resistant to METH toxicity (Thomas and Kuhn 2005a) and minocycline does block MDMA-induced toxicity to 5HT in the striatum and hippocampus (Zhang et al. 2006).
Although there are conflicting data regarding the roles of specific cytokines in mediating METH and MDMA toxicity, it is well accepted that microglia are activated after administration of neurotoxic amphetamine derivatives. The mechanism of microglial activation is not well characterized but there appear to be roles for both DA and glutamate in mediating METH-induced microglial activation. The role of DA has not been tested directly; however, DA quinone formation is increased after high-dose METH (LaVoie and Hastings 1999) and DA quinones can activate microglia (Kuhn et al. 2006). In addition, microglia are also activated by METH-induced glutamate efflux that, in turn, can be blocked by the NMDA receptor antagonist, MK-801 (Thomas and Kuhn 2005b). Overall, given the paucity of research on mechanisms of microglial activation and given the controversial data regarding the role of cytokine activation in METH and MDMA toxicity, future studies are needed to further characterize the mechanism of METH- and MDMA-induced increases in microglial activation and to delineate the contribution of not only cytokines but other factors secreted from activated microglia.
Interactions between oxidative stress, metabolic compromise, and inflammation
Oxidative stress, metabolic compromise, and inflammation not only mediate toxicity individually but also interact collectively to perpetuate METH and MDMA toxicity. There are multiple interactions and a few examples are highlighted. With regards to the interaction between oxidative stress and metabolic compromise, mitochondria are known to be the primary source of intracellular reactive oxygen species generation (Chance et al. 1979). Not only do mitochondria produce a large amount of ROS under normal conditions but inhibition of mitochondrial ETC function with the complex II–III inhibitor 3-nitropropionic acid increases ROS production (Schulz et al. 1996). As both METH and MDMA decrease the function of complex II–III activity, decreased mitochondrial function may result in an enhancement of oxidative stress. Moreover, decreased levels of ATP could disrupt membrane potential, decrease glutamate uptake, and depolarize the cell to cause the release of glutamate and further potentiate the increases in extracellular glutamate concentrations (Lipton and Rosenberg 1994). This enhancement in extracellular glutamate could subsequently result in increases in ROS and RNS production via NMDA receptor activation. Increases in ROS and RNS could then feedback to produce a greater decrease in glutamate uptake resulting in a feed forward production of oxidative stress (Volterra et al. 1994).
Oxidative stress and metabolic compromise are also mediators of inflammation. The contribution of oxidative stress to inflammation is illustrated by a decrease in the inflammatory response to ischemia after pretreatment with antioxidants (Bemeur et al. 2005; Bowler et al. 2002). This attenuation of inflammation by antioxidants is also observed in mice challenged with lipopolysaccharide (LPS; Shen et al. 2005). Similarly, disruption of mitochondrial function with 3-nitroproprionic acid activates microglia (Ryu et al. 2003) and inhibition of complex I mitochondrial function with rotenone activates microglia and promotes TNF-α production (Liu et al. 2006; Sherer et al. 2003). Although these effects of mitochondrial inhibition on microglial activation may be mediated via secondary increases in ROS, the importance of both oxidative stress and metabolic compromise in mediating inflammatory responses is clear.
The bidirectional interactions between inflammation, oxidative stress, and metabolic compromise are further supported by the findings that inflammation enhances oxidative stress and metabolic dysfunction. The induction of inflammation by LPS increases oxidative stress in both the rat heart and brain (Ben Shaul et al. 2001; Zujovic et al. 2001) as well as in primary rat microglial cell cultures stimulated with LPS in vitro (Candelario-Jalil et al. 2007). Furthermore, increases in lipid peroxidation, decreases in antioxidant enzymes, as well as decreases in mitochondrial membrane potential and redox activity are observed in vivo following an LPS challenge to mice (Noble et al. 2007) or to microglial cell lines in vitro (Moss and Bates 2001). The effects of ROS also can directly affect the mitochondria as evidenced by increases in protein carbonyl formation, lipid peroxidation, and tyrosine nitration of mitochondria 3 days after LPS challenge (Hunter et al. 2007). Moreover, the LPS-induced increases in ROS can be attenuated by the mitochondrial specific antioxidant, MitoQ (Barhoumi et al. 2004). Therefore, the importance of inflammation in mediating both oxidative stress and metabolic compromise is clearly evident.
The interactions between oxidative stress and metabolic compromise are important in light of the similarities between the mechanisms of substituted amphetamine toxicity and LPS-induced inflammation, given the fact that both amphetamines and LPS-induced inflammation increase oxidative stress and produce mitochondrial dysfunction. Despite these similarities, the toxicity profile between amphetamines and LPS is distinct in that LPS-induced inflammatory responses decrease markers of DA cell bodies in the substantia nigra, whereas METH is toxic to dopaminergic terminals in the striatum (Hunter et al. 2007; Ricaurte et al. 1982). Despite these distinctions between the toxicity profiles of LPS and METH, both inflammation and amphetamine-related drugs of abuse produce neurodegeneration to the nigrostriatal DA system via a mechanism involving oxidative stress and metabolic compromise.
The matrix metalloproteinases: convergence of oxidative stress, metabolic compromise, and inflammation
The matrix metalloproteinases (MMPs) are proteolytic enzymes that are involved in cleavage and remodeling of the extracellular matrix. The MMP family consists of more than 18 members that have similar N-terminal domains and require Zn2+ for their enzymatic activity (Ethell and Ethell 2007). Although most of the MMPs are not constitutively expressed, MMPs have been shown to be activated in numerous disease states and after a variety of insults. As MMPs can degrade the extracellular matrix, regulation of their activity is essential. The regulation of MMP activity can occur via induction of gene transcription, proenzyme activation, and the action of endogenous tissue inhibitors of metalloproteinases (Yong et al. 1998). Of specific interest are MMP-2 and MMP-9, gelatinases that are capable of cleaving gelatin, collagens IV and V, and fibronectin (Yong et al. 2001). With regards to their expression, MMP-2 is constitutively expressed and is normally present in brain tissue and cerebrospinal fluid, whereas MMP-9 is present at low levels but can be upregulated in many disease states (Rosenberg and Mun-Bryce 2004). Both MMP-2 and MMP-9 have been implicated in cerebral ischemia, kainate-induced neuronal injury, and remodeling associated with hippocampal long-term potentiation and memory (Szklarczyk et al. 2002; Lo et al. 2002; Nagy et al. 2006).
Although numerous mechanisms mediate the activation of MMP-2 and MMP-9, their activation by oxidative stress, metabolic compromise, and inflammation is of particular interest. The contribution of oxidative stress to MMP-2 and MMP-9 activation is illustrated by increases in MMP-2 and MMP-9 activity in brain microvascular endothelial cells following treatment with donors of nitric oxide, superoxide, and peroxynitrite (Haorah et al. 2007). The importance of oxidative stress and free-radical-induced increases in MMP-2 and MMP-9 activity have also been observed in numerous other systems, including superoxide dismutase knockout mice that demonstrate increased MMP-2 and MMP-9 activity in response to cerebral ischemia (Jian and Rosenberg 2005; Gasche et al. 2001). Similar free-radical-mediated increases in pro forms of MMP-2 and MMP-9 and active MMP-9 are observed in the striatum of mice after injection of the complex II mitochondrial inhibitor 3-nitropropionic acid (Kim et al. 2003). Induction of inflammatory responses and cytokine production also produces activation of both MMP-2 and MMP-9 in vitro and in vivo. In vitro stimulation of rat brain endothelial cells with LPS results in the induction of MMP-9 (Harkness et al. 2000) and stimulation of astrocyte cultures with LPS, IL-1β, or TNF-α for 24 h results in an increased expression of MMP-9 mRNA and activity of MMP-2 and MMP-9 (Gottschall et al. 1995; Gottschall and Yu 1995; Wells et al. 1996). Mixed cultures of astrocytes and microglia also produce an active form of MMP-9 when stimulated by LPS (Colton et al. 1993). Additionally, LPS and IL-1 increase the activity of both MMP-2 and MMP-9 in cultured microglia (Rosenberg et al. 2001). There also is an increased production of MMP-9 in brain after intracerebral injection of LPS or TNF-α (Rosenberg et al. 1995; Mun-Bryce and Rosenberg 1998). Overall, these data support a clear role for oxidative stress, mitochondrial inhibition, and inflammatory responses in producing activation of MMP-2 and MMP-9.
Similar to the increases in oxidative stress, decreases in mitochondrial function, and increases in inflammatory responses that occur after the administration of amphetamine-related drugs of abuse, MMP-2 and MMP-9 are also activated by METH and MDMA. Our laboratory recently illustrated an increase in both pro and active forms of MMP-9 in the hippocampus 24 h following high-dose METH administration (7.5 mg/kg q 2 h × 4). The effects of METH on the active form of MMP-2 were not investigated, although no changes in pro MMP-2 were detected (Fazo et al. 2008). Based on the evidence that METH-induced increases in oxidative stress, decreases in mitochondrial function, and increases in inflammation play important roles in toxicity, it could be hypothesized that increases in MMP activation after METH administration may play a role in METH-induced toxicity.
One possible mechanism by which increased activity of MMP-2 and MMP-9 may mediate METH toxicity is via an increase in blood brain barrier (BBB) permeability. In fact, a single high-dose challenge of METH can produce increase markers of BBB permeability that, in turn, may be important in mediating METH toxicity to dopamine terminals (Bowyer and Ali 2006). Although the precise mechanism of BBB permeability is unknown, MMP activation may play a role. The potential role of MMPs in BBB disruption is further illustrated by an increase in BBB permeability following induction of MMP-9 with LPS. This increase in BBB permeability is blocked by the MMP inhibitor BB-1101 (Mun-Bryce and Rosenberg 1998). Additional support is illustrated by the action of MMPs on proteins associated with BBB integrity. Specifically, degradation of extracellular matrix proteins and phosphorylation of tight junction proteins occur in brain microvascular endothelial cells following MMP-2 and MMP-9 stimulation by ROS (Haorah et al. 2007). Given the ability of MMPs to modify the BBB and the fact that METH increases BBB permeability, it could be hypothesized that METH-induced increases in BBB permeability are mediated by MMP-2 and MMP-9 activation via a mechanism involving increases in oxidative stress and inflammation.
Although no studies to date have investigated the hypothesized role of MMPs in mediating METH-induced BBB permeability and toxicity, a few studies have linked MMPs to the rewarding effects of METH, namely METH-induced behavior sensitization and conditioned place preference (CPP). For example, repeated low doses of METH that result in behavioral sensitization induce the expression of MMP-2 and MMP-9 in the frontal cortex and nucleus accumbens. The importance of these increases in MMP-2 and MMP-9 in mediating the rewarding effects of METH are illustrated by a decrease in CPP in both MMP-2 and MMP-9 knockout mice compared to wild-type mice (Mizoguchi et al. 2007b). Further support is provided by studies showing that MMP-2 and MMP-9 knockout mice demonstrate an attenuation of METH-induced DA release in the nucleus accumbens (Mizoguchi et al. 2007b). Given that dopaminergic transmission in the nucleus accumbens is important in mediating the rewarding effects of drugs of abuse, a decrease in METH-induced CPP may occur through an attenuation of METH-induced DA release in the nucleus accumbens. In addition, MMPs are important mediators of learning and memory and MMP-9 knockout mice display impairments in long-term potentiation and hippocampal-dependent memory (Nagy et al. 2006). Since learning and memory are important in the development of drug dependence that occurs with the chronic administration of drugs of abuse, MMP-2 and MMP-9 may play an essential role in the development of METH-induced sensitization and CPP through their effects on learning and memory (Mizoguchi et al. 2008).
Despite the limited research on the role of MMPs in mediating the toxicity to drugs of abuse, there is evidence that MMP-2 and MMP-9 are involved in drug reward such that inhibition of MMP-2 and MMP-9 attenuates the rewarding effects of chronic low-dose METH (Mizoguchi et al. 2007a). Therefore, based on this and emerging evidence that MMPs are activated after high-dose METH administration and given the rationale for their hypothesized role in mediating BBB permeability, future studies are required to further elucidate the role of MMPs in mediating the abuse liability and toxicity of high-dose METH administration.
Conclusion
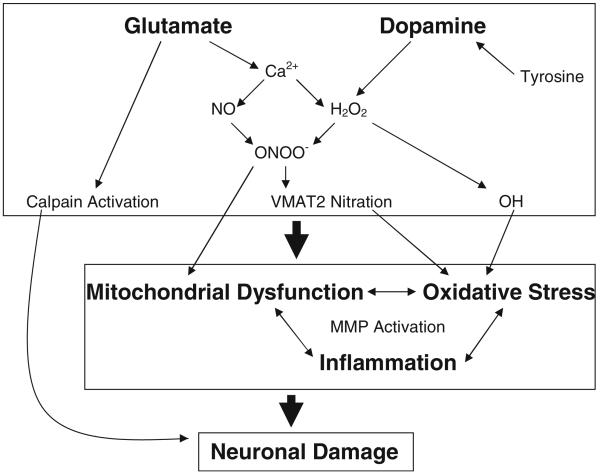
In conclusion, this review highlights the mechanisms of toxicity produced by the amphetamine class of drugs of abuse, with a specific focus on oxidative stress, metabolic compromise, and inflammation. As illustrated in Fig. 1, an overall schematic representation of the mechanisms of METH and MDMA toxicity is presented. The acute increases in glutamate and DA observed during METH and MDMA administration produce an increase in both ROS and RNS that results in increases in oxidative stress, mitochondrial dysfunction, and inflammatory responses. These increases in oxidative stress, mitochondrial dysfunction, and inflammation interact synergistically to promote neuronal damage. These increases are also hypothesized to contribute to an increase in MMP activation which can, via disruption of the BBB, promote toxicity to dopaminergic and serotonergic neurons.
Fig. 1.
Summary of the mechanisms mediating the toxicity of amphetamine-related drugs of abuse. Acute increases in glutamate may increase Ca2+ influx resulting in NOS activation and the production of NO which can produce ONOO− via a reaction with superoxide. Similarly, acute increases in dopamine may be enzymatically metabolized to form H2O2 which can be further converted via the Fenton reaction to hydroxyl radicals (OH). In addition to OH directly increasing oxidative stress, the production of ONOO− can modify proteins such as VMAT2 which may compromise its function and increase the availability of extracellular dopamine to undergo enzymatic metabolism or auto-oxidation to further increase oxidative stress. The production of ONOO− may also directly inhibit mitochondrial function which may further propagate increases in oxidative stress. The increases in both mitochondrial dysfunction and oxidative stress may also influence and enhance inflammatory responses that further interact to increase MMP activation. The increases in mitochondrial dysfunction, oxidative stress, and inflammation may result in neuronal damage to dopaminergic and serotonergic terminals which may or may not be dependent on MMP activation. Calpain activation following acute increases in glutamate may also produce neuronal damage
Contributor Information
Bryan K. Yamamoto, Department of Pharmacology and Experimental Therapeutics, Laboratory of Neurochemistry, Boston University School of Medicine, L-613, 715 Albany St., Boston, MA 02118, USA; Department of Neurosciences, University of Toledo Health Science Campus, 3000 Arlington Avenue, Toledo, OH 43614, USA
Jamie Raudensky, Department of Pharmacology and Experimental Therapeutics, Laboratory of Neurochemistry, Boston University School of Medicine, L-613, 715 Albany St., Boston, MA 02118, USA.
References
- Asanuma M, Tsuji T, Miyazaki I, Miyoshi K, Ogawa N. Methamphetamine-induced neurotoxicity in mouse brain is attenuated by ketoprofen, a non-steroidal anti-inflammatory drug. Neurosci Lett. 2003;352:13–16. doi: 10.1016/j.neulet.2003.08.015. doi:10.1016/j.neulet.2003.08.015. [DOI] [PubMed] [Google Scholar]
- Barhoumi R, Faske J, Liu X, Tjalkens RB. Manganese potentiates lipopolysaccharide-induced expression of NOS2 in C6 glioma cells through mitochondrial-dependent activation of nuclear factor kappa B. Brain Res Mol Brain Res. 2004;122:167–179. doi: 10.1016/j.molbrainres.2003.12.009. doi:10.1016/j.molbrainres.2003.12.009. [DOI] [PubMed] [Google Scholar]
- Battaglia G, Yeh SY, O’Hearn E, Molliver ME, Kuhar MJ, De Souza EB. 3,4-Methylenedioxymethamphetamine and 3,4-methylenedioxyamphetamine destroy serotonin terminals in rat brain: quantification of neurodegeneration by measurement of [3H] paroxetine-labeled serotonin uptake sites. J Pharmacol Exp Ther. 1987;242:911–916. [PubMed] [Google Scholar]
- Battaglia G, Fornai F, Busceti CL, Aloisi G, Cerrito F, de Blasi A, et al. Selective blockade of mGlu5 metabotropic glutamate receptors is protective against methamphetamine neurotoxicity. J Neurosci. 2002;22:2135–2141. doi: 10.1523/JNEUROSCI.22-06-02135.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bemeur C, Ste-Marie L, Desjardins P, Vachon L, Butterworth RF, Hazell AS, et al. Dehydroascorbic acid normalizes several markers of oxidative stress and inflammation in acute hyperglycemic focal cerebral ischemia in the rat. Neurochem Int. 2005;46:399–407. doi: 10.1016/j.neuint.2004.11.007. doi:10.1016/j.neuint.2004.11.007. [DOI] [PubMed] [Google Scholar]
- Ben Shaul V, Lomnitski L, Nyska A, Zurovsky Y, Bergman M, Grossman S. The effect of natural antioxidants, NAO and apocynin, on oxidative stress in the rat heart following LPS challenge. Toxicol Lett. 2001;123:1–10. doi: 10.1016/s0378-4274(01)00369-1. doi:10.1016/S0378-4274(01)00369-1. [DOI] [PubMed] [Google Scholar]
- Bittner SE, Wagner GC, Aigner TG, Seiden LS. Effects of a high-dose treatment of methamphetamine on caudate dopamine and anorexia in rats. Pharmacol Biochem Behav. 1981;14:481–486. doi: 10.1016/0091-3057(81)90306-3. doi:10.1016/0091-3057(81)90306-3. [DOI] [PubMed] [Google Scholar]
- Bowler RP, Sheng H, Enghild JJ, Pearlstein RD, Warner DS, Crapo JD. A catalytic antioxidant (AEOL 10150) attenuates expression of inflammatory genes in stroke. Free Radic Biol Med. 2002;33:1141–1152. doi: 10.1016/s0891-5849(02)01008-0. doi:10.1016/S0891-5849(02)01008-0. [DOI] [PubMed] [Google Scholar]
- Bowyer JF, Ali S. High doses of methamphetamine that cause disruption of the blood-brain barrier in limbic regions produce extensive neuronal degeneration in mouse hippocampus. Synapse. 2006;60:521–532. doi: 10.1002/syn.20324. doi:10.1002/syn.20324. [DOI] [PubMed] [Google Scholar]
- Bowyer JF, Davies DL, Schmued L, Broening HW, Newport GD, Slikker W, Jr, et al. Further studies of the role of hyperthermia in methamphetamine neurotoxicity. J Pharmacol Exp Ther. 1994;268:1571–1580. [PubMed] [Google Scholar]
- Breier JM, Bankson MG, Yamamoto BK. L-tyrosine contributes to (+)-3,4-methylenedioxymethamphetamine-induced serotonin depletions. J Neurosci. 2006;26:290–299. doi: 10.1523/JNEUROSCI.3353-05.2006. doi:10.1523/JNEUROSCI.3353-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JM, Quinton MS, Yamamoto BK. Methamphetamine-induced inhibition of mitochondrial complex II: roles of glutamate and peroxynitrite. J Neurochem. 2005;95:429–436. doi: 10.1111/j.1471-4159.2005.03379.x. doi:10.1111/j.1471-4159.2005.03379.x. [DOI] [PubMed] [Google Scholar]
- Burrows KB, Gudelsky G, Yamamoto BK. Rapid and transient inhibition of mitochondrial function following methamphetamine or 3,4-methylenedioxymethamphetamine administration. Eur J Pharmacol. 2000a;398:11–18. doi: 10.1016/s0014-2999(00)00264-8. doi:10.1016/S0014-2999(00)00264-8. [DOI] [PubMed] [Google Scholar]
- Burrows KB, Nixdorf WL, Yamamoto BK. Central administration of methamphetamine synergizes with metabolic inhibition to deplete striatal monoamines. J Pharmacol Exp Ther. 2000b;292:853–860. [PubMed] [Google Scholar]
- Cadet JL, Sheng P, Ali S, Rothman R, Carlson E, Epstein C. Attenuation of methamphetamine-induced neurotoxicity in copper/zinc superoxide dismutase transgenic mice. J Neurochem. 1994;62:380–383. doi: 10.1046/j.1471-4159.1994.62010380.x. [DOI] [PubMed] [Google Scholar]
- Cadet JL, Ladenheim B, Hirata H, Rothman RB, Ali S, Carlson E, et al. Superoxide radicals mediate the biochemical effects of methylenedioxymethamphetamine (MDMA): evidence from using CuZn-superoxide dismutase transgenic mice. Synapse. 1995;21:169–176. doi: 10.1002/syn.890210210. doi:10.1002/syn.890210210. [DOI] [PubMed] [Google Scholar]
- Candelario-Jalil E, de Oliveira AC, Graf S, Bhatia HS, Hull M, Munoz E, et al. Resveratrol potently reduces prostaglandin E2 production and free radical formation in lipopolysaccharide-activated primary rat microglia. J Neuroinflammation. 2007;4:25. doi: 10.1186/1742-2094-4-25. doi:10.1186/1742-2094-4-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan P, Di Monte DA, Luo JJ, DeLanney LE, Irwin I, Langston JW. Rapid ATP loss caused by methamphetamine in the mouse striatum: relationship between energy impairment and dopaminergic neurotoxicity. J Neurochem. 1994;62:2484–2487. doi: 10.1046/j.1471-4159.1994.62062484.x. [DOI] [PubMed] [Google Scholar]
- Chance B, Sies H, Boveris A. Hydroperoxide metabolism in mammalian organs. Physiol Rev. 1979;59:527–605. doi: 10.1152/physrev.1979.59.3.527. [DOI] [PubMed] [Google Scholar]
- Cohen G. Monoamine oxidase, hydrogen peroxide, and Parkinson’s disease. Adv Neurol. 1987;45:119–125. [PubMed] [Google Scholar]
- Colado MI, Green AR. The spin trap reagent alpha-phenyl-N-tert-butyl nitrone prevents ‘ecstasy’-induced neurodegeneration of 5-hydroxytryptamine neurones. Eur J Pharmacol. 1995;280:343–346. doi: 10.1016/0014-2999(95)00298-y. doi:10.1016/0014-2999(95)00298-Y. [DOI] [PubMed] [Google Scholar]
- Colton CA, Keri JE, Chen WT, Monsky WL. Protease production by cultured microglia: substrate gel analysis and immobilized matrix degradation. J Neurosci Res. 1993;35:297–304. doi: 10.1002/jnr.490350309. doi:10.1002/jnr.490350309. [DOI] [PubMed] [Google Scholar]
- Cunningham J, Raudensky J, Gudelsky G, Tonkiss J, Yamamoto BK. Acute and long-term neurobiological effects of MDMA (ecstasy) on the hippocampus. Society for Neuroscience; Washington, DC: 2004. 2004. Program No.689.2.2004 Abstract Viewer/Itinerary Planner. [Google Scholar]
- Darvesh AS, Gudelsky GA. Evidence for a role of energy dysregulation in the MDMA-induced depletion of brain 5-HT. Brain Res. 2005;1056:168–175. doi: 10.1016/j.brainres.2005.07.009. doi:10.1016/j.brainres.2005.07.009. [DOI] [PubMed] [Google Scholar]
- Darvesh AS, Shankaran M, Gudelsky GA. 3,4-Methylenedioxymethamphetamine produces glycogenolysis and increases the extracellular concentration of glucose in the rat brain. J Pharmacol Exp Ther. 2002;301:138–144. doi: 10.1124/jpet.301.1.138. doi:10.1124/jpet.301.1.138. [DOI] [PubMed] [Google Scholar]
- Darvesh AS, Yamamoto BK, Gudelsky GA. Evidence for the involvement of nitric oxide in 3,4-methylenedioxymethamphetamine-induced serotonin depletion in the rat brain. J Pharmacol Exp Ther. 2005;312:694–701. doi: 10.1124/jpet.104.074849. doi:10.1124/jpet.104.074849. [DOI] [PubMed] [Google Scholar]
- Dawson VL, Dawson TM. Nitric oxide neurotoxicity. J Chem Neuroanat. 1996;10:179–190. doi: 10.1016/0891-0618(96)00148-2. doi:10.1016/0891-0618(96)00148-2. [DOI] [PubMed] [Google Scholar]
- De Vito MJ, Wagner GC. Methamphetamine-induced neuronal damage: a possible role for free radicals. Neuropharmacology. 1989;28:1145–1150. doi: 10.1016/0028-3908(89)90130-5. doi:10.1016/0028-3908(89)90130-5. [DOI] [PubMed] [Google Scholar]
- Dumuis A, Sebben M, Haynes L, Pin JP, Bockaert J. NMDA receptors activate the arachidonic acid cascade system in striatal neurons. Nature. 1988;336:68–70. doi: 10.1038/336068a0. doi:10.1038/336068a0. [DOI] [PubMed] [Google Scholar]
- Dykens JA, Stern A, Trenkner E. Mechanism of kainate toxicity to cerebellar neurons in vitro is analogous to reperfusion tissue injury. J Neurochem. 1987;49:1222–1228. doi: 10.1111/j.1471-4159.1987.tb10014.x. doi:10.1111/j.1471-4159.1987.tb10014.x. [DOI] [PubMed] [Google Scholar]
- Eisch AJ, Schmued LC, Marshall JF. Characterizing cortical neuron injury with Fluoro-Jade labeling after a neurotoxic regimen of methamphetamine. Synapse. 1998;30:329–333. doi: 10.1002/(SICI)1098-2396(199811)30:3<329::AID-SYN10>3.0.CO;2-V. doi:10.1002/(SICI)1098-2396(199811)30:3<329::AID-SYN10>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Escubedo E, Guitart L, Sureda FX, Jimenez A, Pubill D, Pallas M, et al. Microgliosis and down-regulation of adenosine transporter induced by methamphetamine in rats. Brain Res. 1998;814:120–126. doi: 10.1016/s0006-8993(98)01065-8. doi:10.1016/S0006-8993(98)01065-8. [DOI] [PubMed] [Google Scholar]
- Ethell IM, Ethell DW. Matrix metalloproteinases in brain development and remodeling: synaptic functions and targets. J Neurosci Res. 2007;85:2813–2823. doi: 10.1002/jnr.21273. doi:10.1002/jnr.21273. [DOI] [PubMed] [Google Scholar]
- Eyerman DJ, Yamamoto BK. A rapid oxidation and persistent decrease in the vesicular monoamine transporter 2 after methamphetamine. J Neurochem. 2007;103:1219–1227. doi: 10.1111/j.1471-4159.2007.04837.x. [DOI] [PubMed] [Google Scholar]
- Falk EM, Cook VJ, Nichols DE, Sprague JE. An antisense oligonucleotide targeted at MAO-B attenuates rat striatal serotonergic neurotoxicity induced by MDMA. Pharmacol Biochem Behav. 2002;72:617–622. doi: 10.1016/s0091-3057(02)00728-1. doi:10.1016/S0091-3057(02)00728-1. [DOI] [PubMed] [Google Scholar]
- Farfel GM, Seiden LS. Role of hypothermia in the mechanism of protection against serotonergic toxicity. I. Experiments using 3,4-methylenedioxymethamphetamine, dizocilpine, CGS 19755 and NBQX. J Pharmacol Exp Ther. 1995a;272:860–867. [PubMed] [Google Scholar]
- Farfel GM, Seiden LS. Role of hypothermia in the mechanism of protection against serotonergic toxicity. II. Experiments with methamphetamine, p-chloroamphetamine, fenfluramine, dizocilpine and dextromethorphan. J Pharmacol Exp Ther. 1995b;272:868–875. [PubMed] [Google Scholar]
- Farfel GM, Vosmer GL, Seiden LS. The N-methyl-d-aspartate antagonist MK-801 protects against serotonin depletions induced by methamphetamine, 3,4-methylenedioxymethamphetamine and p-chloroamphetamine. Brain Res. 1992;595:121–127. doi: 10.1016/0006-8993(92)91460-v. doi:10.1016/0006-8993(92)91460-V. [DOI] [PubMed] [Google Scholar]
- Fazo N, Raudensky J, Yamamoto BK. Methamphetamine neurotoxicity: possible roles for matrix metalloproteinases. Society on NeuroImmune Pharmacology; 2008. Poster #W8. [Google Scholar]
- Fleckenstein AE, Gibb JW, Hanson GR. Differential effects of stimulants on monoaminergic transporters: pharmacological consequences and implications for neurotoxicity. Eur J Pharmacol. 2000;406:1–13. doi: 10.1016/s0014-2999(00)00639-7. doi:10.1016/S0014-2999(00)00639-7. [DOI] [PubMed] [Google Scholar]
- Fornstedt B, Brun A, Rosengren E, Carlsson A. The apparent autoxidation rate of catechols in dopamine-rich regions of human brains increases with the degree of depigmentation of substantia nigra. J Neural Transm Park Dis Dement Sect. 1989;1:279–295. doi: 10.1007/BF02263482. doi:10.1007/BF02263482. [DOI] [PubMed] [Google Scholar]
- Fukami G, Hashimoto K, Koike K, Okamura N, Shimizu E, Iyo M. Effect of antioxidant N-acetyl-l-cysteine on behavioral changes and neurotoxicity in rats after administration of methamphetamine. Brain Res. 2004;1016:90–95. doi: 10.1016/j.brainres.2004.04.072. doi:10.1016/j.brainres.2004.04.072. [DOI] [PubMed] [Google Scholar]
- Fumagalli F, Gainetdinov RR, Wang YM, Valenzano KJ, Miller GW, Caron MG. Increased methamphetamine neurotoxicity in heterozygous vesicular monoamine transporter 2 knock-out mice. J Neurosci. 1999;19:2424–2431. doi: 10.1523/JNEUROSCI.19-07-02424.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasche Y, Copin JC, Sugawara T, Fujimura M, Chan PH. Matrix metalloproteinase inhibition prevents oxidative stress-associated blood-brain barrier disruption after transient focal cerebral ischemia. J Cereb Blood Flow Metab. 2001;21:1393–1400. doi: 10.1097/00004647-200112000-00003. doi:10.1097/00004647-200112000-00003. [DOI] [PubMed] [Google Scholar]
- Gibb JW, Johnson M, Hanson GR. Neurochemical basis of neurotoxicity. Neurotoxicology. 1990;11:317–321. [PubMed] [Google Scholar]
- Giovanni A, Liang LP, Hastings TG, Zigmond MJ. Estimating hydroxyl radical content in rat brain using systemic and intraventricular salicylate: impact of methamphetamine. J Neurochem. 1995;64:1819–1825. doi: 10.1046/j.1471-4159.1995.64041819.x. [DOI] [PubMed] [Google Scholar]
- Gluck MR, Moy LY, Jayatilleke E, Hogan KA, Manzino L, Sonsalla PK. Parallel increases in lipid and protein oxidative markers in several mouse brain regions after methamphetamine treatment. J Neurochem. 2001;79:152–160. doi: 10.1046/j.1471-4159.2001.00549.x. doi:10.1046/j.1471-4159.2001.00549.x. [DOI] [PubMed] [Google Scholar]
- Gottschall PE, Yu X. Cytokines regulate gelatinase A and B (matrix metalloproteinase 2 and 9) activity in cultured rat astrocytes. J Neurochem. 1995;64:1513–1520. doi: 10.1046/j.1471-4159.1995.64041513.x. [DOI] [PubMed] [Google Scholar]
- Gottschall PE, Yu X, Bing B. Increased production of gelatinase B (matrix metalloproteinase-9) and interleukin-6 by activated rat microglia in culture. J Neurosci Res. 1995;42:335–342. doi: 10.1002/jnr.490420307. doi:10.1002/jnr.490420307. [DOI] [PubMed] [Google Scholar]
- Gudelsky GA. Effect of ascorbate and cysteine on the 3,4-methylenedioxymethamphetamine-induced depletion of brain serotonin. J Neural Transm. 1996;103:1397–1404. doi: 10.1007/BF01271253. doi:10.1007/BF01271253. [DOI] [PubMed] [Google Scholar]
- Hanisch UK. Microglia as a source and target of cytokines. Glia. 2002;40:140–155. doi: 10.1002/glia.10161. doi:10.1002/glia.10161. [DOI] [PubMed] [Google Scholar]
- Haorah J, Ramirez SH, Schall K, Smith D, Pandya R, Persidsky Y. Oxidative stress activates protein tyrosine kinase and matrix metalloproteinases leading to blood-brain barrier dysfunction. J Neurochem. 2007;101:566–576. doi: 10.1111/j.1471-4159.2006.04393.x. doi:10.1111/j.1471-4159.2006.04393.x. [DOI] [PubMed] [Google Scholar]
- Harkness KA, Adamson P, Sussman JD, Davies-Jones GA, Greenwood J, Woodroofe MN. Dexamethasone regulation of matrix metalloproteinase expression in CNS vascular endothelium. Brain. 2000;123(Pt 4):698–709. doi: 10.1093/brain/123.4.698. doi:10.1093/brain/123.4.698. [DOI] [PubMed] [Google Scholar]
- Hess A, Desiderio C, McAuliffe WG. Acute neuropathological changes in the caudate nucleus caused by MPTP and methamphetamine: immunohistochemical studies. J Neurocytol. 1990;19:338–342. doi: 10.1007/BF01188403. doi:10.1007/BF01188403. [DOI] [PubMed] [Google Scholar]
- Hotchkiss AJ, Gibb JW. Long-term effects of multiple doses of methamphetamine on tryptophan hydroxylase and tyrosine hydroxylase activity in rat brain. J Pharmacol Exp Ther. 1980;214:257–262. [PubMed] [Google Scholar]
- Huang YH, Tsai SJ, Su TW, Sim CB. Effects of repeated high-dose methamphetamine on local cerebral glucose utilization in rats. Neuropsychopharmacology. 1999;21:427–434. doi: 10.1016/S0893-133X(99)00029-9. doi:10.1016/S0893-133X(99)00029-9. [DOI] [PubMed] [Google Scholar]
- Hunter RL, Dragicevic N, Seifert K, Choi DY, Liu M, Kim HC, et al. Inflammation induces mitochondrial dysfunction and dopaminergic neurodegeneration in the nigrostriatal system. J Neurochem. 2007;100:1375–1386. doi: 10.1111/j.1471-4159.2006.04327.x. doi:10.1111/j.1471-4159.2006.04327.x. [DOI] [PubMed] [Google Scholar]
- Imam SZ, Crow JP, Newport GD, Islam F, Slikker W, Jr, Ali SF. Methamphetamine generates peroxynitrite and produces dopaminergic neurotoxicity in mice: protective effects of peroxynitrite decomposition catalyst. Brain Res. 1999;837:15–21. doi: 10.1016/s0006-8993(99)01663-7. doi:10.1016/S0006-8993(99)01663-7. [DOI] [PubMed] [Google Scholar]
- Imam SZ, Islam F, Itzhak Y, Slikker W, Jr, Ali SF. Prevention of dopaminergic neurotoxicity by targeting nitric oxide and peroxynitrite: implications for the prevention of methamphetamine-induced neurotoxic damage. Ann N Y Acad Sci. 2000;914:157–171. doi: 10.1111/j.1749-6632.2000.tb05193.x. [DOI] [PubMed] [Google Scholar]
- Itzhak Y, Ali SF. The neuronal nitric oxide synthase inhibitor, 7-nitroindazole, protects against methamphetamine-induced neurotoxicity in vivo. J Neurochem. 1996;67:1770–1773. doi: 10.1046/j.1471-4159.1996.67041770.x. [DOI] [PubMed] [Google Scholar]
- Jayanthi S, Ladenheim B, Cadet JL. Methamphetamine-induced changes in antioxidant enzymes and lipid peroxidation in copper/zinc-superoxide dismutase transgenic mice. Ann N Y Acad Sci. 1998;844:92–102. doi:10.1111/j.1749-6632.1998.tb08224.x. [PubMed] [Google Scholar]
- Jayanthi S, Ladenheim B, Andrews AM, Cadet JL. Over-expression of human copper/zinc superoxide dismutase in transgenic mice attenuates oxidative stress caused by methylenedioxymethamphetamine (ecstasy) Neuroscience. 1999;91:1379–1387. doi: 10.1016/s0306-4522(98)00698-8. doi:10.1016/S0306-4522(98)00698-8. [DOI] [PubMed] [Google Scholar]
- Jian LK, Rosenberg GA. Matrix metalloproteinases and free radicals in cerebral ischemia. Free Radic Biol Med. 2005;39:71–80. doi: 10.1016/j.freeradbiomed.2005.03.033. doi:10.1016/j.freeradbiomed.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Kim GW, Gasche Y, Grzeschik S, Copin JC, Maier CM, Chan PH. Neurodegeneration in striatum induced by the mitochondrial toxin 3-nitropropionic acid: role of matrix metalloproteinase-9 in early blood–brain barrier disruption? J Neurosci. 2003;23:8733–8742. doi: 10.1523/JNEUROSCI.23-25-08733.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kita T, Shimada K, Mastunari Y, Wagner GC, Kubo K, Nakashima T. Methamphetamine-induced striatal dopamine neurotoxicity and cyclooxygenase-2 protein expression in BALN/c mice. Neuropharmacology. 2000;39:399–406. doi: 10.1016/s0028-3908(99)00175-6. [DOI] [PubMed] [Google Scholar]
- Kokoshka JM, Metzger RR, Wilkins DG, Gibb JW, Hanson GR, Fleckenstein AE. Methamphetamine treatment rapidly inhibits serotonin, but not glutamate, transporters in rat brain. Brain Res. 1998;799:78–83. doi: 10.1016/s0006-8993(98)00472-7. doi:10.1016/S0006-8993(98)00472-7. [DOI] [PubMed] [Google Scholar]
- Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 1996;19:312–318. doi: 10.1016/0166-2236(96)10049-7. doi:10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- Kuhn DM, Arthur RE., Jr Inactivation of tryptophan hydroxylase by nitric oxide: enhancement by tetrahydrobiopterin. J Neurochem. 1997;68:1495–1502. doi: 10.1046/j.1471-4159.1997.68041495.x. [DOI] [PubMed] [Google Scholar]
- Kuhn DM, Geddes TJ. Peroxynitrite inactivates tryptophan hydroxylase via sulfhydryl oxidation. Coincident nitration of enzyme tyrosyl residues has minimal impact on catalytic activity. J Biol Chem. 1999;274:29726–29732. doi: 10.1074/jbc.274.42.29726. doi:10.1074/jbc.274.42.29726. [DOI] [PubMed] [Google Scholar]
- Kuhn DM, Geddes TJ. Molecular footprints of neurotoxic amphetamine action. Ann N Y Acad Sci. 2000;914:92–103. doi: 10.1111/j.1749-6632.2000.tb05187.x. [DOI] [PubMed] [Google Scholar]
- Kuhn DM, Aretha CW, Geddes TJ. Peroxynitrite inactivation of tyrosine hydroxylase: mediation by sulfhydryl oxidation, not tyrosine nitration. J Neurosci. 1999;19:10289–10294. doi: 10.1523/JNEUROSCI.19-23-10289.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn DM, Francescutti-Verbeem DM, Thomas DM. Dopamine quinones activate microglia and induce a neurotoxic gene expression profile: relationship to methamphetamine-induced nerve ending damage. Ann N Y Acad Sci. 2006;1074:31–41. doi: 10.1196/annals.1369.003. doi:10.1196/annals.1369.003. [DOI] [PubMed] [Google Scholar]
- Ladenheim B, Krasnova IN, Deng X, Oyler JM, Polettini A, Moran TH, et al. Methamphetamine-induced neurotoxicity is attenuated in transgenic mice with a null mutation for interleukin-6. Mol Pharmacol. 2000;58:1247–1256. doi: 10.1124/mol.58.6.1247. [DOI] [PubMed] [Google Scholar]
- Lafon-Cazal M, Pietri S, Culcasi M, Bockaert J. NMDA-dependent superoxide production and neurotoxicity. Nature. 1993;364:535–537. doi: 10.1038/364535a0. doi:10.1038/364535a0. [DOI] [PubMed] [Google Scholar]
- LaVoie MJ, Hastings TJ. Dopamine quinone formation and protein modification associated with the striatal neurotoxicity of methamphetamine: evidence against a role for extra cellular dopamine. J Neurosci. 1999;19:1484–1491. doi: 10.1523/JNEUROSCI.19-04-01484.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaVoie MJ, Card JP, Hastings TG. Microglial activation precedes dopamine terminal pathology in methamphetamine-induced neurotoxicity. Exp Neurol. 2004;187:47–57. doi: 10.1016/j.expneurol.2004.01.010. doi:10.1016/j.expneurol.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Lazarewicz JW, Wroblewski JT, Palmer ME, Costa E. Activation of N-methyl-d-aspartate-sensitive glutamate receptors stimulates arachidonic acid release in primary cultures of cerebellar granule cells. Neuropharmacology. 1988;27:765–769. doi: 10.1016/0028-3908(88)90088-3. doi:10.1016/0028-3908(88)90088-3. [DOI] [PubMed] [Google Scholar]
- Lipton SA, Rosenberg PA. Excitatory amino acids as a final common pathway for neurologic disorders. N Engl J Med. 1994;330:613–622. doi: 10.1056/NEJM199403033300907. doi:10.1056/NEJM199403033300907. [DOI] [PubMed] [Google Scholar]
- Liu X, Wu JY, Zhou F, Sun XL, Yao HH, Yang Y, et al. The regulation of rotenone-induced inflammatory factor production by ATP-sensitive potassium channel expressed in BV-2 cells. Neurosci Lett. 2006;394:131–135. doi: 10.1016/j.neulet.2005.10.018. doi:10.1016/j.neulet.2005.10.018. [DOI] [PubMed] [Google Scholar]
- Lo EH, Wang X, Cuzner ML. Extracellular proteolysis in brain injury and inflammation: role for plasminogen activators and matrix metalloproteinases. J Neurosci Res. 2002;69:1–9. doi: 10.1002/jnr.10270. doi:10.1002/jnr.10270. [DOI] [PubMed] [Google Scholar]
- Marek GJ, Vosmer G, Seiden LS. Dopamine uptake inhibitors block long-term neurotoxic effects of methamphetamine upon dopaminergic neurons. Brain Res. 1990;513:274–279. doi: 10.1016/0006-8993(90)90467-p. doi:10.1016/0006-8993(90)90467-P. [DOI] [PubMed] [Google Scholar]
- Mark KA, Soghomonian JJ, Yamamoto BK. High-dose methamphetamine acutely activates the striatonigral pathway to increase striatal glutamate and mediate long-term dopamine toxicity. J Neurosci. 2004;24:11449–11456. doi: 10.1523/JNEUROSCI.3597-04.2004. doi:10.1523/JNEUROSCI.3597-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann UD, Wong DF, Yokoi F, Villemagne V, Dannals RF, Ricaurte GA. Reduced striatal dopamine transporter density in abstinent methamphetamine and methcathinone users: evidence from positron emission tomography studies with [11C]WIN-35,428. J Neurosci. 1998;18:8417–8422. doi: 10.1523/JNEUROSCI.18-20-08417.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann UD, Mertl M, Eligulashvili V, Ricaurte GA. Cognitive performance in (+/−) 3,4-methylenedioxymethamphetamine (MDMA, “ecstasy”) users: a controlled study. Psychopharmacology (Berl) 1999;143:417–425. doi: 10.1007/s002130050967. doi:10.1007/s002130050967. [DOI] [PubMed] [Google Scholar]
- McCann UD, Eligulashvili V, Ricaurte GA. (+/−)3,4-Methylenedioxymethamphetamine (‘ecstasy’)-induced serotonin neurotoxicity: clinical studies. Neuropsychobiology. 2000;42:11–16. doi: 10.1159/000026665. doi:10.1159/000026665. [DOI] [PubMed] [Google Scholar]
- McCann UD, Szabo Z, Seckin E, Rosenblatt P, Mathews WB, Ravert HT, et al. Quantitative PET studies of the serotonin transporter in MDMA users and controls using [11C]McN5652 and [11C]DASB. Neuropsychopharmacology. 2005;30:1741–1750. doi: 10.1038/sj.npp.1300736. doi:10.1038/sj.npp.1300736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann UD, Kuwabara H, Kumar A, Palermo M, Abbey R, Brasic J, et al. Persistent cognitive and dopamine transporter deficits in abstinent methamphetamine users. Synapse. 2008;62:91–100. doi: 10.1002/syn.20471. doi:10.1002/syn.20471. [DOI] [PubMed] [Google Scholar]
- Mizoguchi H, Yamada K, Mouri A, Niwa M, Mizuno T, Noda Y, et al. Role of matrix metalloproteinase and tissue inhibitor of MMP in methamphetamine-induced behavioral sensitization and reward: implications for dopamine receptor down-regulation and dopamine release. J Neurochem. 2007a;102:1548–1560. doi: 10.1111/j.1471-4159.2007.04623.x. doi:10.1111/j.1471-4159.2007.04623.x. [DOI] [PubMed] [Google Scholar]
- Mizoguchi H, Yamada K, Niwa M, Mouri A, Mizuno T, Noda Y, et al. Reduction of methamphetamine-induced sensitization and reward in matrix metalloproteinase-2 and -9-deficient mice. J Neurochem. 2007b;100:1579–1588. doi: 10.1111/j.1471-4159.2006.04288.x. [DOI] [PubMed] [Google Scholar]
- Mizoguchi H, Yamada K, Nabeshima T. Neuropsychotoxicity of abused drugs: involvement of matrix metalloproteinase-2 and -9 and tissue inhibitor of matrix metalloproteinase-2 in methamphetamine-induced behavioral sensitization and reward in rodents. J Pharmacol Sci. 2008;106:9–14. doi: 10.1254/jphs.fm0070139. doi:10.1254/jphs.FM0070139. [DOI] [PubMed] [Google Scholar]
- Moss DW, Bates TE. Activation of murine microglial cell lines by lipopolysaccharide and interferon-gamma causes NO-mediated decreases in mitochondrial and cellular function. Eur J Neurosci. 2001;13:529–538. doi: 10.1046/j.1460-9568.2001.01418.x. doi:10.1046/j.1460-9568.2001.01418.x. [DOI] [PubMed] [Google Scholar]
- Mun-Bryce S, Rosenberg GA. Gelatinase B modulates selective opening of the blood-brain barrier during inflammation. Am J Physiol. 1998;274:R1203–R1211. doi: 10.1152/ajpregu.1998.274.5.R1203. [DOI] [PubMed] [Google Scholar]
- Nagy V, Bozdagi O, Matynia A, Balcerzyk M, Okulski P, Dzwonek J, et al. Matrix metalloproteinase-9 is required for hippocampal late-phase long-term potentiation and memory. J Neurosci. 2006;26:1923–1934. doi: 10.1523/JNEUROSCI.4359-05.2006. doi:10.1523/JNEUROSCI.4359-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima A, Yamada K, Nagai T, Uchiyama T, Miyamoto Y, Mamiya T, et al. Role of tumor necrosis factor-alpha in methamphetamine-induced drug dependence and neurotoxicity. J Neurosci. 2004;24:2212–2225. doi: 10.1523/JNEUROSCI.4847-03.2004. doi:10.1523/JNEUROSCI.4847-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash JF, Yamamoto BK. Methamphetamine neurotoxicity and striatal glutamate release: comparison to 3,4-methylenedioxymethamphetamine. Brain Res. 1992;581:237–243. doi: 10.1016/0006-8993(92)90713-j. doi:10.1016/0006-8993(92)90713-J. [DOI] [PubMed] [Google Scholar]
- Nixdorf WL, Burrows KB, Gudelsky GA, Yamamoto BK. Enhancement of 3,4-methylenedioxymethamphetamine neurotoxicity by the energy inhibitor malonate. J Neurochem. 2001;77:647–654. doi: 10.1046/j.1471-4159.2001.00262.x. doi:10.1046/j.1471-4159.2001.00262.x. [DOI] [PubMed] [Google Scholar]
- Noble F, Rubira E, Boulanouar M, Palmier B, Plotkine M, Warnet JM, et al. Acute systemic inflammation induces central mitochondrial damage and mnesic deficit in adult Swiss mice. Neurosci Lett. 2007;424:106–110. doi: 10.1016/j.neulet.2007.07.005. doi:10.1016/j.neulet.2007.07.005. [DOI] [PubMed] [Google Scholar]
- O’Callaghan JP, Miller DB. Neurotoxicity profiles of substituted amphetamines in the C57BL/6J mouse. J Pharmacol Exp Ther. 1994;270:741–751. [PubMed] [Google Scholar]
- O’Callaghan JP, Sriram K. Glial fibrillary acidic protein and related glial proteins as biomarkers of neurotoxicity. Expert Opin Drug Saf. 2005;4:433–442. doi: 10.1517/14740338.4.3.433. doi:10.1517/14740338.4.3.433. [DOI] [PubMed] [Google Scholar]
- O’Hearn E, Battaglia G, De Souza EB, Kuhar MJ, Molliver ME. Methylenedioxyamphetamine (MDA) and methylenedioxymethamphetamine (MDMA) cause selective ablation of serotonergic axon terminals in forebrain: immunocytochemical evidence for neurotoxicity. J Neurosci. 1988;8:2788–2803. doi: 10.1523/JNEUROSCI.08-08-02788.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olanow CW. An introduction to the free radical hypothesis in Parkinson’s disease. Ann Neurol. 1992;32(Suppl):S2–S9. doi: 10.1002/ana.410320703. doi:10.1002/ana.410320703. [DOI] [PubMed] [Google Scholar]
- Orio L, O’Shea E, Sanchez V, Pradillo JM, Escobedo I, Camarero J, et al. 3,4-Methylenedioxymethamphetamine increases interleukin-1beta levels and activates microglia in rat brain: studies on the relationship with acute hyperthermia and 5-HT depletion. J Neurochem. 2004;89:1445–1453. doi: 10.1111/j.1471-4159.2004.02443.x. doi:10.1111/j.1471-4159.2004.02443.x. [DOI] [PubMed] [Google Scholar]
- O’Shea E, Sanchez V, Orio L, Escobedo I, Green AR, Colado MI. 3,4-Methylenedioxymethamphetamine increases pro-interleukin-1beta production and caspase-1 protease activity in frontal cortex, but not in hypothalamus, of Dark Agouti rats: role of interleukin-1beta in neurotoxicity. Neuroscience. 2005;135:1095–1105. doi: 10.1016/j.neuroscience.2005.06.084. doi:10.1016/j.neuroscience.2005.06.084. [DOI] [PubMed] [Google Scholar]
- Pontieri FE, Crane AM, Seiden LS, Kleven MS, Porrino LJ. Metabolic mapping of the effects of intravenous methamphetamine administration in freely moving rats. Psychopharmacology (Berl) 1990;102:175–182. doi: 10.1007/BF02245919. doi:10.1007/BF02245919. [DOI] [PubMed] [Google Scholar]
- Pu C, Vorhees CV. Developmental dissociation of methamphetamine-induced depletion of dopaminergic terminals and astrocyte reaction in rat striatum. Brain Res Dev Brain Res. 1993;72:325–328. doi: 10.1016/0165-3806(93)90201-k. doi:10.1016/0165-3806(93)90201-K. [DOI] [PubMed] [Google Scholar]
- Quate L, McBean DE, Ritchie IM, Olverman HJ, Kelly PA. Acute methylenedioxymethamphetamine administration: effects on local cerebral blood flow and glucose utilisation in the Dark Agouti rat. Psychopharmacology (Berl) 2004;173:287–295. doi: 10.1007/s00213-004-1784-z. doi:10.1007/s00213-004-1784-z. [DOI] [PubMed] [Google Scholar]
- Quinton MS, Yamamoto BK. Causes and consequences of methamphetamine and MDMA toxicity. AAPS J. 2006;8:E337–E347. doi: 10.1007/BF02854904. doi:10.1208/aapsj080238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radi R, Beckman JS, Bush KM, Freeman BA. Peroxynitrite oxidation of sulfhydryls. The cytotoxic potential of superoxide and nitric oxide. J Biol Chem. 1991;266:4244–4250. [PubMed] [Google Scholar]
- Ricaurte GA, Schuster CR, Seiden LS. Long-term effects of repeated methylamphetamine administration on dopamine and serotonin neurons in the rat brain: a regional study. Brain Res. 1980;193:153–163. doi: 10.1016/0006-8993(80)90952-x. doi:10.1016/0006-8993(80)90952-X. [DOI] [PubMed] [Google Scholar]
- Ricaurte GA, Guillery RW, Seiden LS, Schuster CR, Moore RY. Dopamine nerve terminal degeneration produced by high doses of methylamphetamine in the rat brain. Brain Res. 1982;235:93–103. doi: 10.1016/0006-8993(82)90198-6. doi:10.1016/0006-8993(82)90198-6. [DOI] [PubMed] [Google Scholar]
- Ricaurte G, Bryan G, Strauss L, Seiden L, Schuster C. Hallucinogenic amphetamine selectively destroys brain serotonin nerve terminals. Science. 1985;229:986–988. doi: 10.1126/science.4023719. doi:10.1126/science.4023719. [DOI] [PubMed] [Google Scholar]
- Ricaurte GA, Forno LS, Wilson MA, DeLanney LE, Irwin I, Molliver ME, et al. (+/−)3,4-Methylenedioxymethamphetamine selectively damages central serotonergic neurons in nonhuman primates. JAMA. 1988;260:51–55. doi:10.1001/jama.260.1.51. [PubMed] [Google Scholar]
- Rocher C, Gardier AM. Effects of repeated systemic administration of d-fenfluramine on serotonin and glutamate release in rat ventral hippocampus: comparison with methamphetamine using in vivo microdialysis. Naunyn Schmiedebergs Arch Pharmacol. 2001;363:422–428. doi: 10.1007/s002100000381. doi:10.1007/s002100000381. [DOI] [PubMed] [Google Scholar]
- Rosenberg GA, Mun-Bryce S. Matrix metalloproteinases in neuroinflammation and cerebral ischemia. Ernst Schering Res Found Workshop. 2004;47:1–16. doi: 10.1007/978-3-662-05426-0_1. [DOI] [PubMed] [Google Scholar]
- Rosenberg GA, Estrada EY, Dencoff JE, Stetler-Stevenson WG. Tumor necrosis factor-alpha-induced gelatinase B causes delayed opening of the blood-brain barrier: an expanded therapeutic window. Brain Res. 1995;703:151–155. doi: 10.1016/0006-8993(95)01089-0. doi:10.1016/0006-8993(95)01089-0. [DOI] [PubMed] [Google Scholar]
- Rosenberg GA, Cunningham LA, Wallace J, Alexander S, Estrada EY, Grossetete M, et al. Immunohistochemistry of matrix metalloproteinases in reperfusion injury to rat brain: activation of MMP-9 linked to stromelysin-1 and microglia in cell cultures. Brain Res. 2001;893:104–112. doi: 10.1016/s0006-8993(00)03294-7. doi:10.1016/S0006-8993(00)03294-7. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Baumann MH. Monoamine transporters and psychostimulant drugs. Eur J Pharmacol. 2003;479:23–40. doi: 10.1016/j.ejphar.2003.08.054. doi:10.1016/j.ejphar.2003.08.054. [DOI] [PubMed] [Google Scholar]
- Ryan LJ, Martone ME, Linder JC, Groves PM. Continuous amphetamine administration induces tyrosine hydroxylase immunoreactive patches in the adult rat neostriatum. Brain Res Bull. 1988;21:133–137. doi: 10.1016/0361-9230(88)90129-3. doi:10.1016/0361-9230(88)90129-3. [DOI] [PubMed] [Google Scholar]
- Ryu JK, Nagai A, Kim J, Lee MC, McLarnon JG, Kim SU. Microglial activation and cell death induced by the mitochondrial toxin 3-nitropropionic acid: in vitro and in vivo studies. Neurobiol Dis. 2003;12:121–132. doi: 10.1016/s0969-9961(03)00002-0. doi:10.1016/S0969-9961(03)00002-0. [DOI] [PubMed] [Google Scholar]
- Sanchez V, Camarero J, O’Shea E, Green AR, Colado MI. Differential effect of dietary selenium on the long-term neurotoxicity induced by MDMA in mice and rats. Neuropharmacology. 2003;44:449–461. doi: 10.1016/s0028-3908(02)00411-2. doi:10.1016/S0028-3908(02)00411-2. [DOI] [PubMed] [Google Scholar]
- Sattler R, Tymianski M. Molecular mechanisms of calcium-dependent excitotoxicity. J Mol Med. 2000;78:3–13. doi: 10.1007/s001090000077. doi:10.1007/s001090000077. [DOI] [PubMed] [Google Scholar]
- Sattler R, Tymianski M. Molecular mechanisms of glutamate receptor-mediated excitotoxic neuronal cell death. Mol Neurobiol. 2001;24:107–129. doi: 10.1385/MN:24:1-3:107. doi:10.1385/MN:24:1-3:107. [DOI] [PubMed] [Google Scholar]
- Schmidt CJ, Ritter JK, Sonsalla PK, Hanson GR, Gibb JW. Role of dopamine in the neurotoxic effects of methamphetamine. J Pharmacol Exp Ther. 1985;233:539–544. [PubMed] [Google Scholar]
- Schmidt CJ, Black CK, Taylor VL. Antagonism of the neurotoxicity due to a single administration of methylenedioxymethamphetamine. Eur J Pharmacol. 1990;181:59–70. doi: 10.1016/0014-2999(90)90245-2. doi:10.1016/0014-2999(90)90245-2. [DOI] [PubMed] [Google Scholar]
- Schmidt CJ, Black CK, Taylor VL. l-DOPA potentiation of the serotonergic deficits due to a single administration of 3,4-methylenedioxymethamphetamine, p-chloroamphetamine or methamphetamine to rats. Eur J Pharmacol. 1991;203:41–49. doi: 10.1016/0014-2999(91)90788-r. doi:10.1016/0014-2999(91)90788-R. [DOI] [PubMed] [Google Scholar]
- Schmued LC. Demonstration and localization of neuronal degeneration in the rat forebrain following a single exposure to MDMA. Brain Res. 2003;974:127–133. doi: 10.1016/s0006-8993(03)02563-0. doi:10.1016/S0006-8993(03)02563-0. [DOI] [PubMed] [Google Scholar]
- Schulz JB, Henshaw DR, MacGarvey U, Beal MF. Involvement of oxidative stress in 3-nitropropionic acid neurotoxicity. Neurochem Int. 1996;29:167–171. doi: 10.1016/0197-0186(95)00122-0. doi:10.1016/0197-0186(95)00122-0. [DOI] [PubMed] [Google Scholar]
- Seiden LS, Fischman MW, Schuster CR. Long-term methamphetamine induced changes in brain catecholamines in tolerant rhesus monkeys. Drug Alcohol Depend. 1976;1:215–219. doi: 10.1016/0376-8716(76)90030-2. doi:10.1016/0376-8716(76)90030-2. [DOI] [PubMed] [Google Scholar]
- Seiden LS, Commins DL, Vosmer G, Axt K, Marek G. Neurotoxicity in dopamine and 5-hydroxytryptamine terminal fields: a regional analysis in nigrostriatal and mesolimbic projections. Ann N Y Acad Sci. 1988;537:161–172. doi: 10.1111/j.1749-6632.1988.tb42104.x. doi:10.1111/j.1749-6632.1988.tb42104.x. [DOI] [PubMed] [Google Scholar]
- Sekine Y, Ouchi Y, Takei N, Yoshikawa E, Nakamura K, Futatsubashi M, et al. Brain serotonin transporter density and aggression in abstinent methamphetamine abusers. Arch Gen Psychiatry. 2006;63:90–100. doi: 10.1001/archpsyc.63.1.90. doi:10.1001/archpsyc.63.1.90. [DOI] [PubMed] [Google Scholar]
- Shankaran M, Yamamoto BK, Gudelsky GA. Mazindol attenuates the 3,4-methylenedioxymethamphetamine-induced formation of hydroxyl radicals and long-term depletion of serotonin in the striatum. J Neurochem. 1999;72:2516–2522. doi: 10.1046/j.1471-4159.1999.0722516.x. doi:10.1046/j.1471-4159.1999.0722516.x. [DOI] [PubMed] [Google Scholar]
- Shen KP, Lo YC, Yang RC, Liu HW, Chen IJ, Wu BN. Antioxidant eugenosedin-A protects against lipopolysaccharide-induced hypotension, hyperglycaemia and cytokine immunoreactivity in rats and mice. J Pharm Pharmacol. 2005;57:117–125. doi: 10.1211/0022357055137. doi:10.1211/0022357055137. [DOI] [PubMed] [Google Scholar]
- Sherer TB, Betarbet R, Kim JH, Greenamyre JT. Selective microglial activation in the rat rotenone model of Parkinson’s disease. Neurosci Lett. 2003;341:87–90. doi: 10.1016/s0304-3940(03)00172-1. doi:10.1016/S0304-3940(03)00172-1. [DOI] [PubMed] [Google Scholar]
- Siman R, Noszek JC. Excitatory amino acids activate calpain I and induce structural protein breakdown in vivo. Neuron. 1988;1:279–287. doi: 10.1016/0896-6273(88)90076-1. doi:10.1016/0896-6273(88)90076-1. [DOI] [PubMed] [Google Scholar]
- Sprague JE, Nichols DE. Inhibition of MAO-B protects against MDMA-induced neurotoxicity in the striatum. Psychopharmacology (Berl) 1995a;118:357–359. doi: 10.1007/BF02245967. doi:10.1007/BF02245967. [DOI] [PubMed] [Google Scholar]
- Sprague JE, Nichols DE. The monoamine oxidase-B inhibitor l-deprenyl protects against 3,4-methylenedioxymethamphetamine-induced lipid peroxidation and long-term serotonergic deficits. J Pharmacol Exp Ther. 1995b;273:667–673. [PubMed] [Google Scholar]
- Sriram K, Miller DB, O’Callaghan JP. Minocycline attenuates microglial activation but fails to mitigate striatal dopaminergic neurotoxicity: role of tumor necrosis factor-alpha. J Neurochem. 2006;96:706–718. doi: 10.1111/j.1471-4159.2005.03566.x. doi:10.1111/j.1471-4159.2005.03566.x. [DOI] [PubMed] [Google Scholar]
- Stamler JS, Hausladen A. Oxidative modifications in nitrosative stress. Nat Struct Biol. 1998;5:247–249. doi: 10.1038/nsb0498-247. doi:10.1038/nsb0498-247. [DOI] [PubMed] [Google Scholar]
- Staszewski RD, Yamamoto BK. Methamphetamine-induced spectrin proteolysis in the rat striatum. J Neurochem. 2006;96:1267–1276. doi: 10.1111/j.1471-4159.2005.03618.x. doi:10.1111/j.1471-4159.2005.03618.x. [DOI] [PubMed] [Google Scholar]
- Stephans SE, Whittingham TS, Douglas AJ, Lust WD, Yamamoto BK. Substrates of energy metabolism attenuate methamphetamine-induced neurotoxicity in striatum. J Neurochem. 1998;71:613–621. doi: 10.1046/j.1471-4159.1998.71020613.x. [DOI] [PubMed] [Google Scholar]
- Stokes AH, Hastings TG, Vrana KE. Cytotoxic and genotoxic potential of dopamine. J Neurosci Res. 1999;55:659–665. doi: 10.1002/(SICI)1097-4547(19990315)55:6<659::AID-JNR1>3.0.CO;2-C. doi:10.1002/(SICI)1097-4547(19990315)55:6<659::AID-JNR1<3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Stoll G, Jander S. The role of microglia and macrophages in the pathophysiology of the CNS. Prog Neurobiol. 1999;58:233–247. doi: 10.1016/s0301-0082(98)00083-5. doi:10.1016/S0301-0082(98)00083-5. [DOI] [PubMed] [Google Scholar]
- Stone DM, Stahl DC, Hanson GR, Gibb JW. The effects of 3,4-methylenedioxymethamphetamine (MDMA) and 3,4-methylenedioxyamphetamine (MDA) on monoaminergic systems in the rat brain. Eur J Pharmacol. 1986;128:41–48. doi: 10.1016/0014-2999(86)90555-8. doi:10.1016/0014-2999(86)90555-8. [DOI] [PubMed] [Google Scholar]
- Stone DM, Johnson M, Hanson GR, Gibb JW. Role of endogenous dopamine in the central serotonergic deficits induced by 3,4-methylenedioxymethamphetamine. J Pharmacol Exp Ther. 1988;247:79–87. [PubMed] [Google Scholar]
- Stone DM, Johnson M, Hanson GR, Gibb JW. Acute inactivation of tryptophan hydroxylase by amphetamine analogs involves the oxidation of sulfhydryl sites. Eur J Pharmacol. 1989;172:93–97. doi: 10.1016/0922-4106(89)90048-5. doi:10.1016/0922-4106(89)90048-5. [DOI] [PubMed] [Google Scholar]
- Straiko MM, Coolen LM, Zemlan FP, Gudelsky GA. The effect of amphetamine analogs on cleaved microtubule-associated protein-tau formation in the rat brain. Neuroscience. 2007;144:223–231. doi: 10.1016/j.neuroscience.2006.08.073. doi:10.1016/j.neuroscience.2006.08.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streit WJ, Walter SA, Pennell NA. Reactive microgliosis. Prog Neurobiol. 1999;57:563–581. doi: 10.1016/s0301-0082(98)00069-0. doi:10.1016/S0301-0082(98)00069-0. [DOI] [PubMed] [Google Scholar]
- Sulzer D, Sonders MS, Poulsen NW, Galli A. Mechanisms of neurotransmitter release by amphetamines: a review. Prog Neurobiol. 2005;75:406–433. doi: 10.1016/j.pneurobio.2005.04.003. doi:10.1016/j.pneurobio.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Szklarczyk A, Lapinska J, Rylski M, McKay RD, Kaczmarek L. Matrix metalloproteinase-9 undergoes expression and activation during dendritic remodeling in adult hippocampus. J Neurosci. 2002;22:920–930. doi: 10.1523/JNEUROSCI.22-03-00920.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas DM, Kuhn DM. Cyclooxygenase-2 is an obligatory factor in methamphetamine-induced neurotoxicity. J Pharmacol Exp Ther. 2005a;313:870–876. doi: 10.1124/jpet.104.080242. doi:10.1124/jpet.104.080242. [DOI] [PubMed] [Google Scholar]
- Thomas DM, Kuhn DM. MK-801 and dextromethorphan block microglial activation and protect against methamphetamine-induced neurotoxicity. Brain Res. 2005b;1050:190–198. doi: 10.1016/j.brainres.2005.05.049. doi:10.1016/j.brainres.2005.05.049. [DOI] [PubMed] [Google Scholar]
- Thomas DM, Dowgiert J, Geddes TJ, Francescutti-Verbeem D, Liu X, Kuhn DM. Microglial activation is a pharmacologically specific marker for the neurotoxic amphetamines. Neurosci Lett. 2004;367:349–354. doi: 10.1016/j.neulet.2004.06.065. doi:10.1016/j.neulet.2004.06.065. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Hayashi KM, Simon SL, Geaga JA, Hong MS, Sui Y, et al. Structural abnormalities in the brains of human subjects who use methamphetamine. J Neurosci. 2004;24:6028–6036. doi: 10.1523/JNEUROSCI.0713-04.2004. doi:10.1523/JNEUROSCI.0713-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volterra A, Trotti D, Tromba C, Floridi S, Racagni G. Glutamate uptake inhibition by oxygen free radicals in rat cortical astrocytes. J Neurosci. 1994;14:2924–2932. doi: 10.1523/JNEUROSCI.14-05-02924.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner GC, Ricaurte GA, Seiden LS, Schuster CR, Miller RJ, Westley J. Long-lasting depletions of striatal dopamine and loss of dopamine uptake sites following repeated administration of methamphetamine. Brain Res. 1980;181:151–160. doi: 10.1016/0006-8993(80)91265-2. doi:10.1016/0006-8993(80)91265-2. [DOI] [PubMed] [Google Scholar]
- Wagner GC, Carelli RM, Jarvis MF. Pretreatment with ascorbic acid attenuates the neurotoxic effects of methamphetamine in rats. Res Commun Chem Pathol Pharmacol. 1985;47:221–228. [PubMed] [Google Scholar]
- Warren MW, Kobeissy FH, Liu MC, Hayes RL, Gold MS, Wang KK. Concurrent calpain and caspase-3 mediated proteolysis of alpha II-spectrin and tau in rat brain after methamphetamine exposure: a similar profile to traumatic brain injury. Life Sci. 2005;78:301–309. doi: 10.1016/j.lfs.2005.04.058. doi:10.1016/j.lfs.2005.04.058. [DOI] [PubMed] [Google Scholar]
- Warren MW, Zheng W, Kobeissy FH, Cheng LM, Hayes RL, Gold MS, et al. Calpain- and caspase-mediated alpha II-spectrin and tau proteolysis in rat cerebrocortical neuronal cultures after ecstasy or methamphetamine exposure. Int J Neuropsychopharmacol. 2007;10:479–489. doi: 10.1017/S1461145706007061. doi:10.1017/S1461145706007061. [DOI] [PubMed] [Google Scholar]
- Wells GM, Catlin G, Cossins JA, Mangan M, Ward GA, Miller KM, et al. Quantitation of matrix metalloproteinases in cultured rat astrocytes using the polymerase chain reaction with a multicompetitor cDNA standard. Glia. 1996;18:332–340. doi:10.1002/(SICI)1098-1136(199612)18:4<332::AID-GLIA7<3.0.CO;2-Z. [PubMed] [Google Scholar]
- Woolverton WL, Ricaurte GA, Forno LS, Seiden LS. Long-term effects of chronic methamphetamine administration in rhesus monkeys. Brain Res. 1989;486:73–78. doi: 10.1016/0006-8993(89)91279-1. doi:10.1016/0006-8993(89)91279-1. [DOI] [PubMed] [Google Scholar]
- Yamaguchi T, Kuraishi Y, Minami M, Nakai S, Hirai Y, Satoh M. Methamphetamine-induced expression of interleukin-1 beta mRNA in the rat hypothalamus. Neurosci Lett. 1991;128:90–92. doi: 10.1016/0304-3940(91)90766-m. doi:10.1016/0304-3940(91)90766-M. [DOI] [PubMed] [Google Scholar]
- Yamamoto BK, Zhu W. The effects of methamphetamine on the production of free radicals and oxidative stress. J Pharmacol Exp Ther. 1998;287:107–114. [PubMed] [Google Scholar]
- Yong VW, Krekoski CA, Forsyth PA, Bell R, Edwards DR. Matrix metalloproteinases and diseases of the CNS. Trends Neurosci. 1998;21:75–80. doi: 10.1016/s0166-2236(97)01169-7. doi:10.1016/S0166-2236(97)01169-7. [DOI] [PubMed] [Google Scholar]
- Yong VW, Power C, Forsyth P, Edwards DR. Metalloproteinases in biology and pathology of the nervous system. Nat Rev Neurosci. 2001;2:502–511. doi: 10.1038/35081571. doi:10.1038/35081571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Shirayama Y, Shimizu E, Iyo M, Hashimoto K. Protective effects of minocycline on 3,4-methylenedioxymethamphetamine-induced neurotoxicity in serotonergic and dopaminergic neurons of mouse brain. Eur J Pharmacol. 2006;544:1–9. doi: 10.1016/j.ejphar.2006.05.047. doi:10.1016/j.ejphar.2006.05.047. [DOI] [PubMed] [Google Scholar]
- Zhang X, Dong F, Mayer GE, Bruch DC, Ren J, Culver B. Selective inhibition of cyclooxygenase-2 exacerbates methamphetamine-induced dopamine depletion in the striatum in rats. Neuroscience. 2007;150:950–958. doi: 10.1016/j.neuroscience.2007.09.059. doi:10.1016/j.neuroscience.2007.09.059. [DOI] [PubMed] [Google Scholar]
- Zujovic V, Schussler N, Jourdain D, Duverger D, Taupin V. In vivo neutralization of endogenous brain fractalkine increases hippocampal TNF alpha and 8-isoprostane production induced by intracerebroventricular injection of LPS. J Neuroimmunol. 2001;115:135–143. doi: 10.1016/s0165-5728(01)00259-4. doi:10.1016/S0165-5728(01)00259-4. [DOI] [PubMed] [Google Scholar]