Abstract
BACKGROUND
Diabetes is a risk factor for dementia. It is unknown whether higher glucose levels increase the risk of dementia in people without diabetes.
METHODS
We used 35,264 clinical measurements of glucose levels and 10,208 measurements of glycated hemoglobin levels from 2067 participants without dementia to examine the relationship between glucose levels and the risk of dementia. Participants were from the Adult Changes in Thought study and included 839 men and 1228 women whose mean age at baseline was 76 years; 232 participants had diabetes, and 1835 did not. We fit Cox regression models, stratified according to diabetes status and adjusted for age, sex, study cohort, educational level, level of exercise, blood pressure, and status with respect to coronary and cerebrovascular diseases, atrial fibrillation, smoking, and treatment for hypertension.
RESULTS
During a median follow-up of 6.8 years, dementia developed in 524 participants (74 with diabetes and 450 without). Among participants without diabetes, higher average glucose levels within the preceding 5 years were related to an increased risk of dementia (P = 0.01); with a glucose level of 115 mg per deciliter (6.4 mmol per liter) as compared with 100 mg per deciliter (5.5 mmol per liter), the adjusted hazard ratio for dementia was 1.18 (95% confidence interval [CI], 1.04 to 1.33). Among participants with diabetes, higher average glucose levels were also related to an increased risk of dementia (P = 0.002); with a glucose level of 190 mg per deciliter (10.5 mmol per liter) as compared with 160 mg per deciliter (8.9 mmol per liter), the adjusted hazard ratio was 1.40 (95% CI, 1.12 to 1.76).
CONCLUSIONS
Our results suggest that higher glucose levels may be a risk factor for dementia, even among persons without diabetes. (Funded by the National Institutes of Health.)
With the aging of the population, dementia has become a major threat to public health worldwide.1 The rate of obesity is also increasing, with a parallel increase in the rate of diabetes.2 The results of studies assessing the association between obesity or diabetes and the risk of dementia have been mixed.3,4 It is imperative to understand the potential consequences of the obesity and diabetes epidemics for the incidence of dementia.5 Any effects that obesity has on the risk of dementia are likely to include effects on metabolism. We evaluated extensive longitudinal clinical data from a prospective cohort with research-quality case ascertainment to test the hypothesis that glucose levels are associated with the risk of dementia.
METHODS
PARTICIPANTS
The Adult Changes in Thought (ACT) study6 initially included 2581 randomly selected dementia-free members of the Group Health Cooperative (hereafter referred to as Group Health), a health care system in Washington State. Participants had to be 65 years of age or older at the time of e4nrollment, which occurred from 1994 through 1996. An additional 811 participants were enrolled between 2000 and 2002. Participants were invited to return at 2-year intervals for the purpose of identifying incident cases of dementia. The sample for the current study was limited to 2067 participants who had at least one follow-up visit, had been enrolled in Group Health for at least 5 years before study entry, and had at least five measurements of glucose or glycated hemoglobin (measured as hemoglobin A1C or as total glycated hemoglobin, with the latter measurement reflecting an older hemoglobin assay) over the course of 2 or more years before study entry. The demographic characteristics of the ACT study participants who were included in the current study and those who were excluded were similar, although several clinical characteristics were more common among participants in the current study (see Table S1 in the Supplementary Appendix, available with the full text of this article at NEJM.org).
STUDY OVERSIGHT
The study procedures were approved by the institutional review boards of Group Health and the University of Washington, and participants provided written informed consent. The first three authors vouch for the accuracy of the study and the completeness of the data and analysis. Author responsibilities are discussed in the Methods S7 section in the Supplementary Appendix.
IDENTIFICATION OF DEMENTIA
Study participants were assessed for dementia every 2 years with the use of the Cognitive Abilities Screening Instrument, for which scores range from 0 to 100 and higher scores indicate better cognitive functioning.7 Patients with scores of 85 or less underwent further clinical and psychometric evaluation, including a battery of neuropsychological tests (see the Methods S1 section in the Supplementary Appendix). The results of these evaluations and laboratory testing and imaging records were then reviewed in a consensus conference. Diagnoses of dementia8 and of probable or possible Alzheimer’s disease9 were made on the basis of research criteria. Dementia-free participants continued with scheduled follow-up visits. The incidence date for dementia was recorded as the halfway point between the study visit at which dementia was diagnosed and the previous visit.6
RISK FACTORS ASSESSED
Glucose Levels
Clinical data, including measurements of fasting glucose, random measurements of glucose, and measurements of glycated hemoglobin, were captured as computerized laboratory data from 1988 onward. We transformed values for total glycated hemoglobin to hemoglobin A1C values using this formula: hemoglobin A1C = (0.6 × total glycated hemoglobin) + 1.7. We then transformed the calculated hemoglobin A1C values to daily average glucose values with this formula: daily average glucose = (28.7 × hemoglobin A1C) − 46.7.10 We combined the recorded glucose values and daily average glucose values derived from glycated hemoglobin values using a hierarchical Bayesian framework (see the Methods S2 section in the Supplementary Appendix) to compute a time-varying estimate of the average glucose level for each participant. This approach creates an estimate of glucose level, weighted by the precision of the measures for glucose and glycated hemoglobin and stabilized with the use of a shrinkage factor to account for the instability of the estimation for participants with relatively few observations. We computed average glucose levels for each participant at study baseline and subsequently in 5-year rolling windows. Our approach to measurement was closely correlated with a simpler way of estimating glucose exposure (see the Methods S3 section and Fig. S6 in the Supplementary Appendix). The analysis included data from study participants for all time frames in which at least one measurement of glucose or glycated hemoglobin was available. Our secondary analyses explicitly considered more recent exposure (average glucose level in the preceding 5 years) as compared with more distant exposure (average glucose level in the period between 5 and 8 years earlier).
Diabetes
We classified participants as having treated diabetes on the basis of diabetes-related medication data from Group Health pharmacy records (Table S2 in the Supplementary Appendix). At least two filled prescriptions per year were required for the classification, with the onset date for treated diabetes defined as the date when the second prescription was filled. Once a participant was classified as having treated diabetes, the classification was retained for the remainder of the study.
Apolipoprotein E Genotype
Data on apolipoprotein E (APOE) genotype were available for 1818 participants (88%). APOE status was determined with the use of published methods11,12 and categorized as the presence or absence of any ε4 alleles.
Other Risk Factors
Risk factors with the potential to confound the relation between glucose levels and dementia were defined with the use of ACT study and Group Health data sources (see the Methods S4 section in the Supplementary Appendix). Exercise level was assessed with the use of questions about types of physical activity and the number of times each was performed in a week. These numbers were totaled, and those who exercised 3 or more days per week were categorized as having regular exercise, as previously reported.13 At each study visit, a research staff member administered a questionnaire that asked participants about their smoking status and whether a doctor had told them they had coronary artery disease, cerebrovascular disease, or hypertension. Blood pressure, measured while the participant was seated, was determined as the average of two measurements on the left arm, with a 5-minute rest period between measurements. Atrial fibrillation was determined with the use of codes 427.3, 427.31, and 427.32 from the International Classification of Diseases, 9th Revision, in accordance with procedures at Group Health. Treatment for hypertension was determined on the basis of Group Health pharmacy data (Table S3 in the Supplementary Appendix).
STATISTICAL ANALYSIS
We used stratified Cox regression models with empirical standard errors to examine the relation between glucose level and incidence of dementia. Age was used as the time axis. Stratification was based on status with respect to diabetes and cerebrovascular disease, which allowed for different baseline hazard functions across these strata in the estimation of model parameters. We controlled for age at study entry, study cohort, sex, educational level, exercise level, blood pressure, and status with respect to coronary artery disease, atrial fibrillation, smoking, and treatment for hypertension.
Glucose levels were incorporated in models with the use of natural cubic splines14 (see the Methods S8 section in the Supplementary Appendix) to allow for a nonlinear association between glycemia and risk of dementia as measured by the log hazard. Separate splines were used in accordance with diabetes status. The statistical significance (at the 0.05 level) of the association between glycemia and risk of dementia was estimated with the use of two-sided Wald tests of the composite hypotheses that all model parameters associated with the splines were equal to 0 (omnibus tests; α = 0.05). We assessed the proportional hazards of covariate effects by testing for interactions with (log) time and plotting Schoenfeld residuals.15 All statistical analyses were performed with the use of SAS software, version 9.2 (SAS Institute), and R, version 2.15.1 (R Foundation for Statistical Computing).
We performed several sensitivity analyses, testing for interactions with glucose levels according to sex and age at study entry, investigating clinical data from participants whose data were particularly influential on model results, contracting or expanding the window for calculating the average glucose level (2 or 8 years rather than 5 years), adjusting for the presence of one or more APOE ε4 alleles, changing the parameters of the prior distribution within the Bayesian framework for exposure computation (see the Methods S5 section in the Supplementary Appendix), and making additional modifications to our glucose exposure model to account for prandial status when that was indicated (see the Methods S6 section in the Supplementary Appendix).
RESULTS
BASELINE CHARACTERISTICS
The baseline characteristics of the 2067 study participants are presented in Table 1. There were 35,264 values available for fasting and random glucose levels and 10,208 values available for glycated hemoglobin levels (total glycated hemoglobin or hemoglobin A1C). During the 5 years preceding study enrollment, the median glucose level for participants without diabetes was 101 mg per deciliter (interquartile range, 96 to 108 [5.6 mmol per liter; interquartile range, 5.3 to 6.0]), and the median level for those with diabetes was 175 mg per deciliter (interquartile range, 153 to 198 [9.7 mmol per liter; interquartile range, 8.5 to 11.0]). Distributions of glucose levels throughout the study period are summarized in Table S4 and Figure S1 in the Supplementary Appendix.
Table 1.
Baseline Characteristics of the Study Participants.
| Characteristic | Participants with Diabetes (N = 232) | Participants without Diabetes (N = 1835) |
|---|---|---|
| Member of original study cohort, 1994–1996 — no. (%) | 157 (67.7) | 1344 (73.2) |
| Age — yr | ||
| Median | 74 | 76 |
| Interquartile range | 71–78 | 71–81 |
| Female sex — no. (%) | 120 (51.7) | 1108 (60.4) |
| Race — no. (%)* | ||
| White | 190 (81.9) | 1673 (91.2) |
| Black | 28 (12.1) | 72 (3.9) |
| Asian | 9 (3.9) | 58 (3.2) |
| Other | 5 (2.2) | 32 (1.7) |
| Education beyond high school — no. (%) | 121 (52.2) | 1122 (61.1) |
| Apolipoprotein E ε4 allele — no. (%)† | 45 (22.2) | 416 (25.8) |
| Atrial fibrillation — no. (%) | 32 (13.8) | 201 (11.0) |
| Treated for hypertension — no. (%) | 189 (81.5) | 1248 (68.0) |
| Mean blood pressure — mm Hg | ||
| Systolic | ||
| Median | 143 | 139 |
| Interquartile range | 129–159 | 126–155 |
| Diastolic‡ | ||
| Median | 73 | 75 |
| Interquartile range | 66–80 | 68–82 |
| Coronary artery disease — no. (%)§ | 76 (32.8) | 434 (23.8) |
| Cerebrovascular disease — no. (%)¶ | 36 (15.7) | 207 (11.3) |
| Congestive heart failure — no. (%)|| | 15 (6.5) | 89 (4.9) |
| Regular exercise — no. (%)** | 162 (69.8) | 1300 (70.8) |
| Smoking status — no. (%)†† | ||
| Current smoker | 6 (2.6) | 98 (5.3) |
| Former smoker | 125 (53.9) | 853 (46.5) |
| Never smoked | 101 (43.5) | 883 (48.1) |
| Self-rated health fair or poor — no. (%) | 80 (34.5) | 344 (18.7) |
Race was self-reported.
Data on apolipoprotein E genotype were missing for 29 people with diabetes (12.5%) and 220 people without diabetes (12.0%).
Data on diastolic pressure were missing for 8 people with diabetes (3.4%) and 29 people without diabetes (1.6%).
Data on coronary artery disease were missing for 12 people without diabetes (0.7%).
Data on cerebrovascular disease were missing for 3 people with diabetes (1.3%) and 9 people without diabetes (0.5%).
Data on congestive heart failure were missing for 4 people without diabetes (0.2%).
Regular exercise was defined as 3 or more “activity days” per week, defined as days in which at least 15 minutes of exercise such as walking, bicycling, or swimming was performed. See the Supplementary Appendix for further details.
Data on smoking were missing for 1 person without diabetes (0.1%).
DEMENTIA, ALZHEIMER’S DISEASE, AND GLYCEMIA
Over a median follow-up period of 6.8 years, dementia developed in 524 of the 2067 participants (25.4%), including 450 of the 1724 participants who did not have diabetes at the end of follow-up (26.1%) and 74 of the 343 participants who had diabetes at the end of follow-up (21.6%). A total of 403 participants (19.5%) had probable or possible Alzheimer’s disease at the end of follow-up, 55 (2.7%) had dementia from vascular disease, and 66 (3.2%) had dementia from other causes (Table S5 in the Supplementary Appendix).
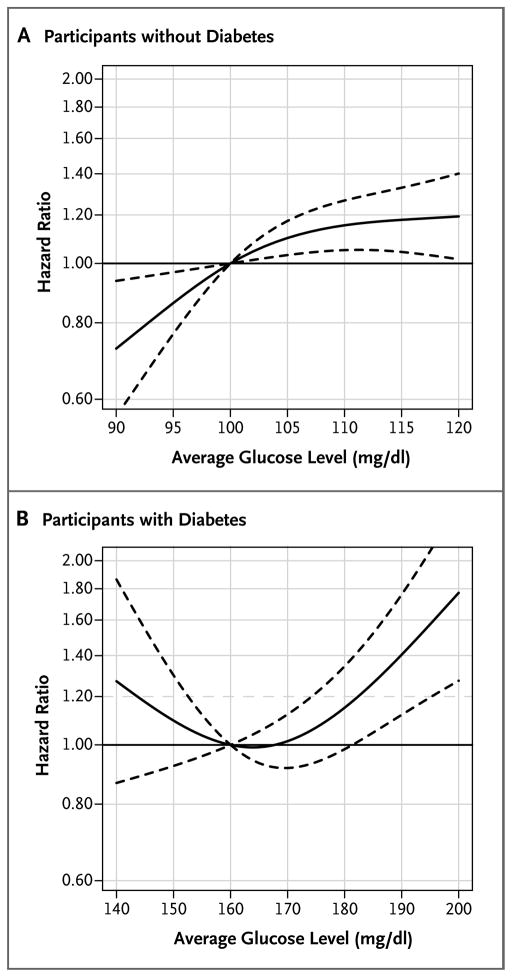
Associations between average glucose levels in the preceding 5 years and the development of dementia are shown in Table 2 and Figure 1. Among participants without diabetes, the risk of dementia increased with increasing glucose levels (P = 0.01 for the omnibus test). For an average glucose level of 115 mg per deciliter (6.4 mmol per liter), as compared with 100 mg per deciliter (5.5 mmol per liter), the adjusted hazard ratio for dementia was 1.18 (95% confidence interval [CI], 1.04 to 1.33). Among participants with diabetes, those with the highest levels of glucose had an increased risk of dementia (P = 0.002). For an average glucose level of 190 mg per deciliter (10.5 mmol per liter), as compared with 160 mg per deciliter (8.9 mmol per liter), the adjusted hazard ratio for dementia was 1.40 (95% CI, 1.12 to 1.76).
Table 2.
Risk of Incident Dementia Associated with Average Glucose Level over the Preceding 5 Years among Participants without Diabetes and Those with Diabetes.*
| Average Glucose Level | Hazard Ratio for Dementia (95% CI) |
|---|---|
| Participants without diabetes | |
| 95 mg/dl | 0.86 (0.77–0.97) |
| 100 mg/dl | 1.00 |
| 105 mg/dl | 1.10 (1.03–1.17) |
| 110 mg/dl | 1.15 (1.05–1.27) |
| 115 mg/dl | 1.18 (1.04–1.33) |
| P value | 0.01 |
| Participants with diabetes | |
| 150 mg/dl | 1.10 (0.92–1.30) |
| 160 mg/dl | 1.00 |
| 170 mg/dl | 1.01 (0.92–1.12) |
| 180 mg/dl | 1.15 (0.98–1.34) |
| 190 mg/dl | 1.40 (1.12–1.76) |
| P value | 0.002 |
Estimates were adjusted for age at study entry, study cohort (original cohort enrolled in 1994 through 1996 versus expansion cohort enrolled in 2000 through 2002), sex, educational level, and time-varying measures of exercise level, average systolic and diastolic blood pressure, and status with respect to coronary artery disease, cerebrovascular disease, atrial fibrillation, treatment for hypertension, and smoking. The risk of dementia associated with different glucose levels was modeled with the use of natural cubic splines. The P values are based on omnibus tests of the composite hypothesis that all regression model parameters corresponding to the spline were equal to zero. As such, these P values reflect the significance for tests of the null hypothesis that there was no association between glucose level and risk of dementia, with distinct values for participants with diabetes and those without diabetes. To convert the values for glucose to millimoles per liter, multiply by 0.05551.
Figure 1. Risk of Incident Dementia Associated with the Average Glucose Level during the Preceding 5 Years, According to the Presence or Absence of Diabetes.
Solid curves represent estimates of the hazard ratios for the risk of incident dementia across average glucose levels relative to a reference level of 100 mg per deciliter for participants without diabetes (Panel A) and 160 mg per deciliter for participants with diabetes (Panel B). The dashed lines represent pointwise 95% confidence intervals. To convert the values for glucose to millimoles per liter, multiply by 0.05551.
Table 3 shows the results of analyses of the risk of dementia associated with glucose levels averaged over the preceding 5 years or the period between 5 and 8 years earlier. Average glucose levels were highly correlated for the two time periods (r = 0.85). Including glucose levels for both periods in regression models resulted in somewhat attenuated estimates of associations between more recent elevations in glucose levels and risk of dementia.
Table 3.
Risk of Incident Dementia among Participants without and Those with Diabetes, According to Glucose Values Averaged over the Preceding 5 Years and the Period between 5 and 8 Years Earlier.*
| Average Glucose Level | Hazard Ratio for Dementia (95% CI) |
|---|---|
| Participants without diabetes | |
| Preceding 5 yr | |
| 95 mg/dl | 0.88 (077–1.00) |
| 100 mg/dl | 1.00 |
| 105 mg/dl | 1.09 (1.01–1.17) |
| 110 mg/dl | 1.14 (1.01–1.27) |
| 115 mg/dl | 1.16 (1.00–1.35) |
| P value | 0.09 |
| Between 5 and 8 yr earlier | |
| 95 mg/dl | 0.94 (0.81–1.08) |
| 100 mg/dl | 1.00 |
| 105 mg/dl | 1.03 (0.95–1.10) |
| 110 mg/dl | 1.02 (0.91–1.15) |
| 115 mg/dl | 1.01 (0.85–1.20) |
| P value | 0.65 |
| Participants with diabetes | |
| Preceding 5 yr | |
| 150 mg/dl | 1.15 (0.94–1.42) |
| 160 mg/dl | 1.00 |
| 170 mg/dl | 0.96 (0.85–1.10) |
| 180 mg/dl | 1.04 (0.84–1.28) |
| 190 mg/dl | 1.20 (0.89–1.62) |
| P value | 0.06 |
| Between 5 and 8 yr earlier | |
| 150 mg/dl | 0.92 (0.79–1.07) |
| 160 mg/dl | 1.00 |
| 170 mg/dl | 1.06 (0.96–1.18) |
| 180 mg/dl | 1.10 (0.91–1.34) |
| 190 mg/dl | 1.13 (0.85–1.50) |
| P value | 0.52 |
Estimates were adjusted for age at study entry, study cohort, sex, educational level, time-varying measures of exercise level, average systolic and diastolic blood pressure, and status with respect to coronary artery disease, cerebrovascular disease, atrial fibrillation, treatment for hypertension, and smoking. The associations between glucose levels and the risk of dementia were modeled with the use of natural cubic splines, with separate splines for distant levels (5 to 8 years) and recent levels (up to 5 years). The P values are based on omnibus tests of the composite hypothesis that all regression model parameters corresponding to the spline were equal to zero. As such, these P values reflect the significance for tests of the null hypothesis that there was no association between glucose level and risk of dementia, with distinct values for participants with diabetes and those without diabetes.
SENSITIVITY ANALYSES
There was no evidence of effect modification according to sex for participants without diabetes (P = 0.86 for interaction) or for participants with diabetes (P = 0.72 for interaction). Similarly, there was no evidence of effect modification according to age at study entry among participants without diabetes (P = 0.84). However, there was a suggestion of possible effect modification according to age at study entry among participants with diabetes, but the effect was not significant (P = 0.13). We estimated the hazard ratios for study entry at 70 to 78 years of age for participants with diabetes (Fig. S2 in the Supplementary Appendix). An increased risk associated with both higher and lower glucose levels appeared to be especially prominent among participants who were older at study entry.
Among people without diabetes, no individual participant had data that had a particularly marked influence on model parameter estimates (see the Results S1 section and Fig. S3 in the Supplementary Appendix). Some people with diabetes did have data that had a marked influence on model parameter estimates, and we reviewed their medical records. We repeated our primary analyses after excluding data from one participant with acromegaly (Fig. S4 in the Supplementary Appendix) and after excluding data from that participant and two other participants, each of whom had an atypical natural history of type 2 diabetes (Fig. S5 in the Supplementary Appendix). The exclusion of these data resulted in the near elimination of the suggestion of elevated risk at the lowest glucose levels.
Additional adjustment for the APOE genotype did not change our findings (Table S6 in the Supplementary Appendix). Point estimates were similar when 2-year windows of glucose exposure were used rather than 5-year windows, although the risk of dementia was significant only for participants with diabetes when the 2-year window of glucose exposure was used (Table S7 in the Supplementary Appendix). Results were similar when exposure was estimated assuming more dispersed or less dispersed prior distributions for glucose and hemoglobin A1C (Table S8 in the Supplementary Appendix). Results were similar when we accounted for the differences between fasting and random glucose levels (Table S9 in the Supplementary Appendix).
DISCUSSION
In this prospective, community-based cohort study, we found that higher glucose levels were associated with an increased risk of dementia in populations without and with diabetes. The findings were consistent across a variety of sensitivity analyses. These data suggest that higher levels of glucose may have deleterious effects on the aging brain. Our findings underscore the potential consequences of temporal trends in obesity and diabetes5 and suggest the need for interventions that reduce glucose levels.
Most studies that have investigated associations between glucose metabolism and the risk of dementia have focused on diabetes itself, and they have yielded inconsistent results.4 Other studies have measured levels of glycated hemoglobin16–19 or assessed the results of glucose tolerance tests.20–22 Many of these studies have shown relationships between elevated levels of glycated hemoglobin or postprandial (but not fasting) glucose levels and dementia-related outcomes, such as changes in hippocampal volume on neuroimaging or rates of cognitive decline. To our knowledge, no prior study has evaluated glucose levels as a time-varying phenomenon. Most of the previous studies used categorical exposure variables, such as the presence or absence of diabetes or normal versus impaired glucose tolerance.
In contrast, we used a hierarchical Bayesian model to develop a time-varying estimate of glucose levels (see the Methods S2 section in the Supplementary Appendix). This approach enabled us to incorporate clinically obtained measurements of random and fasting blood glucose and glycated hemoglobin in a single composite estimate of glucose exposure. The extensive clinical laboratory data available and the long-term follow-up of the cohort, in which there were hundreds of cases of dementia, afforded us the opportunity to evaluate the hazards associated with glucose levels using a spline model, which allowed us to evaluate risk across the entire spectrum of observed glucose levels. We found a monotonically increasing association between the glucose level and the risk of dementia among people without diabetes, which suggests that any incremental increase in glucose levels is associated with an increased risk of dementia. We found the same relationship between glycemia and risk of dementia among people with diabetes at the higher end of the range of glucose levels. We also found an inverse association between glucose level and risk of dementia among people with diabetes who had relatively low levels of glucose, although this association appeared to be driven by glucose levels in three participants with atypical courses of type 2 diabetes. Our findings were consistent across many sensitivity analyses, reinforcing our confidence in their reliability.
Higher glucose levels may contribute to an increased risk of dementia through several potential mechanisms, including acute and chronic hyperglycemia and insulin resistance23 and increased microvascular disease of the central nervous system.24–28 Although the development of dementia in people with diabetes could have led to a deterioration in selfcare, which in turn may have led to increased glucose levels, the similar relationship between glycemia and dementia in people without diabetes suggests a different causal relationship. The underlying mechanisms of the association between elevated glucose levels and dementia need to be clarified in future studies.
There are several causes of dementia, including Alzheimer’s disease, vascular disease, Lewy-body disease, and combinations of these disorders.29 It is difficult to discriminate reliably among these causes, so we chose to focus this assessment on overall dementia.
The strengths of this study include the prospective community-based design, the large sample with minimal attrition, access to extensive clinical laboratory and medical records, prospective ascertainment of cases of dementia with widely used research criteria, and careful sensitivity analyses. Several limitations should be acknowledged. The possibility of confounding by unmeasured or unknown factors cannot be excluded. We were limited to available clinical laboratory measurements obtained at irregular intervals for estimates of glucose levels. Measurements of glucose and glycated hemoglobin were numerous, with an average of 17 measurements of blood glucose and 5 measurements of glycated hemoglobin available per person. We noted large differences in glycemia between people with and those without diabetes. We stratified our analyses according to diabetes status, which was determined on the basis of whether a person was receiving diabetes-related medications. Diabetes was almost certainly present for several years before the initial prescription of such medications, which means that some of the higher glucose values observed among people who were classified as not having diabetes probably reflected diabetes that had not yet been treated with diabetes-related medications. We found that increased risk was associated with higher glucose levels even at the lowest end of the glucose spectrum among people who had not received a diagnosis of diabetes, for whom increased risk was not likely to be a result of undiagnosed diabetes. Our results may not be generalizable to other ethnic groups. Many of our covariates were obtained by self-report.
In conclusion, our data provided evidence that higher glucose levels are associated with an increased risk of dementia.
Supplementary Material
Acknowledgments
Supported by a grant (U01 AG 06781, to Dr. Larson) from the National Institutes of Health.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Reitz C, Brayne C, Mayeux R. Epidemiology of Alzheimer disease. Nat Rev Neurol. 2011;7:137–52. doi: 10.1038/nrneurol.2011.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lam DW, LeRoith D. The worldwide diabetes epidemic. Curr Opin Endocrinol Diabetes Obes. 2012;19:93–6. doi: 10.1097/MED.0b013e328350583a. [DOI] [PubMed] [Google Scholar]
- 3.Gorelick PB, Scuteri A, Black SE, et al. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42:2672–713. doi: 10.1161/STR.0b013e3182299496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kloppenborg RP, van den Berg E, Kappelle LJ, Biessels GJ. Diabetes and other vascular risk factors for dementia: which factor matters most? A systematic review. Eur J Pharmacol. 2008;585:97–108. doi: 10.1016/j.ejphar.2008.02.049. [DOI] [PubMed] [Google Scholar]
- 5.Arterburn DE, Crane PK, Sullivan SD. The coming epidemic of obesity in elderly Americans. J Am Geriatr Soc. 2004;52:1907–12. doi: 10.1111/j.1532-5415.2004.52517.x. [DOI] [PubMed] [Google Scholar]
- 6.Kukull WA, Higdon R, Bowen JD, et al. Dementia and Alzheimer disease incidence: a prospective cohort study. Arch Neurol. 2002;59:1737–46. doi: 10.1001/archneur.59.11.1737. [DOI] [PubMed] [Google Scholar]
- 7.Teng EL, Hasegawa K, Homma A, et al. The Cognitive Abilities Screening Instrument (CASI): a practical test for cross-cultural epidemiological studies of dementia. Int Psychogeriatr. 1994;6:45–58. doi: 10.1017/s1041610294001602. [DOI] [PubMed] [Google Scholar]
- 8.Diagnostic and statistical manual of mental disorders. 4. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 9.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–44. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 10.Nathan DM, Kuenen J, Borg R, Zheng H, Schoenfeld D, Heine RJ. Translating the A1C assay into estimated average glucose values. Diabetes Care. 2008;31:1473–8. doi: 10.2337/dc08-0545. Erratum, Diabetes Care 2009;32:207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hixson JE, Vernier DT. Restriction isotyping of human apolipoprotein E by gene amplification and cleavage with HhaI. J Lipid Res. 1990;31:545–8. [PubMed] [Google Scholar]
- 12.Emi M, Wu LL, Robertson MA, et al. Genotyping and sequence analysis of apolipoprotein E isoforms. Genomics. 1988;3:373–9. doi: 10.1016/0888-7543(88)90130-9. [DOI] [PubMed] [Google Scholar]
- 13.Larson EB, Wang L, Bowen JD, et al. Exercise is associated with reduced risk for incident dementia among persons 65 years of age and older. Ann Intern Med. 2006;144:73–81. doi: 10.7326/0003-4819-144-2-200601170-00004. [DOI] [PubMed] [Google Scholar]
- 14.Hastie T, Tibshirani RJ, Freedman J. Elements of statistical learning. New York: Springer; 2001. [Google Scholar]
- 15.Kalbfleisch JD, Prentice RL. Statistical analysis of failure time data. 2. Hoboken, NJ: John Wiley; 2002. [Google Scholar]
- 16.Yaffe K, Blackwell T, Whitmer RA, Krueger K, Barrett Connor E. Glycosylated hemoglobin level and development of mild cognitive impairment or dementia in older women. J Nutr Health Aging. 2006;10:293–5. [PubMed] [Google Scholar]
- 17.Ravona-Springer R, Moshier E, Schmeidler J, et al. Changes in glycemic control are associated with changes in cognition in non-diabetic elderly. J Alzheimers Dis. 2012;30:299–309. doi: 10.3233/JAD-2012-120106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Christman AL, Matsushita K, Gottesman RF, et al. Glycated haemoglobin and cognitive decline: the Atherosclerosis Risk in Communities (ARIC) study. Diabetologia. 2011;54:1645–52. doi: 10.1007/s00125-011-2095-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Enzinger C, Fazekas F, Matthews PM, et al. Risk factors for progression of brain atrophy in aging: six-year follow-up of normal subjects. Neurology. 2005;64:1704–11. doi: 10.1212/01.WNL.0000161871.83614.BB. [DOI] [PubMed] [Google Scholar]
- 20.Ohara T, Doi Y, Ninomiya T, et al. Glucose tolerance status and risk of dementia in the community: the Hisayama study. Neurology. 2011;77:1126–34. doi: 10.1212/WNL.0b013e31822f0435. [DOI] [PubMed] [Google Scholar]
- 21.van den Berg E, de Craen AJ, Biessels GJ, Gussekloo J, Westendorp RG. The impact of diabetes mellitus on cognitive decline in the oldest of the old: a prospective population-based study. Diabetologia. 2006;49:2015–23. doi: 10.1007/s00125-006-0333-1. [DOI] [PubMed] [Google Scholar]
- 22.Rönnemaa E, Zethelius B, Sundelöf J, et al. Glucose metabolism and the risk of Alzheimer’s disease and dementia: a population-based 12 year follow-up study in 71-year-old men. Diabetologia. 2009;52:1504–10. doi: 10.1007/s00125-009-1393-9. [DOI] [PubMed] [Google Scholar]
- 23.Strachan MW. RD Lawrence Lecture 2010: the brain as a target organ in Type 2 diabetes: exploring the links with cognitive impairment and dementia. Diabet Med. 2011;28:141–7. doi: 10.1111/j.1464-5491.2010.03199.x. [DOI] [PubMed] [Google Scholar]
- 24.Pandini G, Pace V, Copani A, Squatrito S, Milardi D, Vigneri R. Insulin has multiple antiamyloidogenic effects on human neuronal cells. Endocrinology. 2013;154:375–87. doi: 10.1210/en.2012-1661. [DOI] [PubMed] [Google Scholar]
- 25.Bartl J, Meyer A, Brendler S, Riederer P, Grünblatt E. Different effects of soluble and aggregated amyloid beta42 on gene/protein expression and enzyme activity involved in insulin and APP pathways. J Neural Transm. 2013;120:113–20. doi: 10.1007/s00702-012-0852-5. [DOI] [PubMed] [Google Scholar]
- 26.Nalivaeva NN, Beckett C, Belyaev ND, Turner AJ. Are amyloid-degrading enzymes viable therapeutic targets in Alzheimer’s disease? J Neurochem. 2012;120(Suppl 1):167–85. doi: 10.1111/j.1471-4159.2011.07510.x. [DOI] [PubMed] [Google Scholar]
- 27.Candeias E, Duarte AI, Carvalho C, et al. The impairment of insulin signaling in Alzheimer’s disease. IUBMB Life. 2012;64:951–7. doi: 10.1002/iub.1098. [DOI] [PubMed] [Google Scholar]
- 28.Correia SC, Santos RX, Carvalho C, et al. Insulin signaling, glucose metabolism and mitochondria: major players in Alzheimer’s disease and diabetes interrelation. Brain Res. 2012;1441:64–78. doi: 10.1016/j.brainres.2011.12.063. [DOI] [PubMed] [Google Scholar]
- 29.Sonnen JA, Larson EB, Crane PK, et al. Pathological correlates of dementia in a longitudinal, population-based sample of aging. Ann Neurol. 2007;62:406–13. doi: 10.1002/ana.21208. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.