Abstract
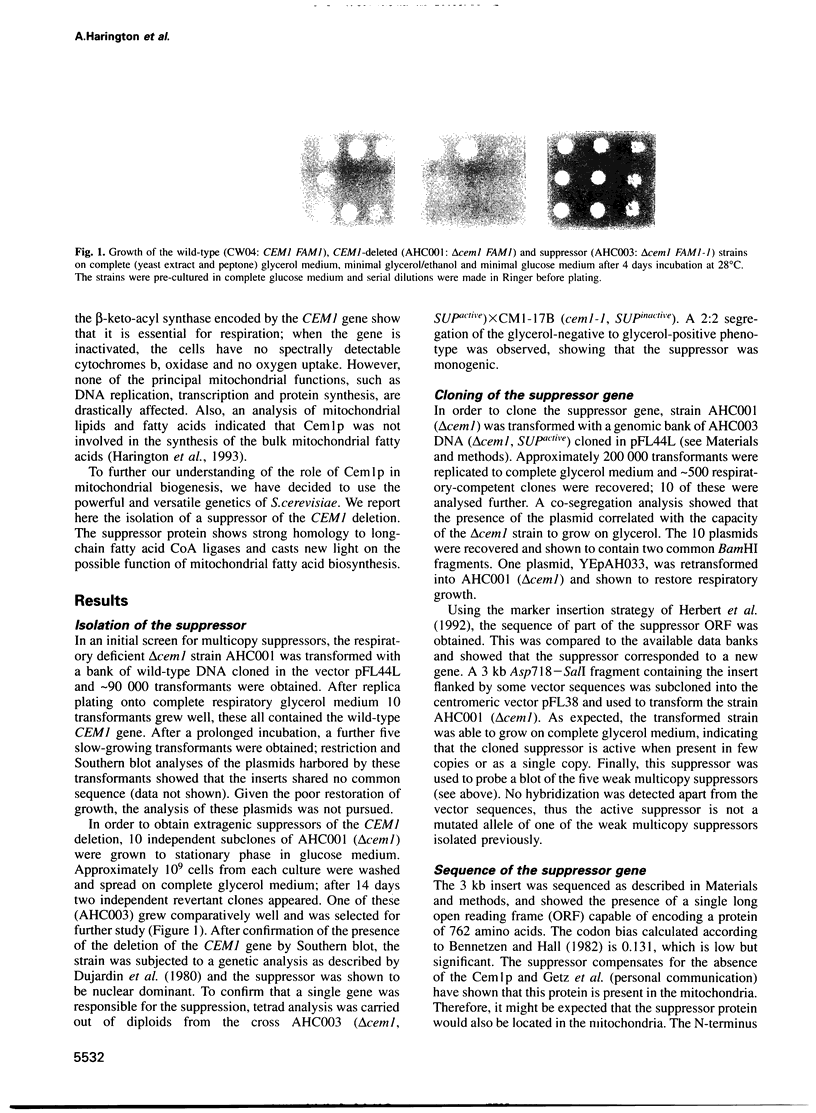
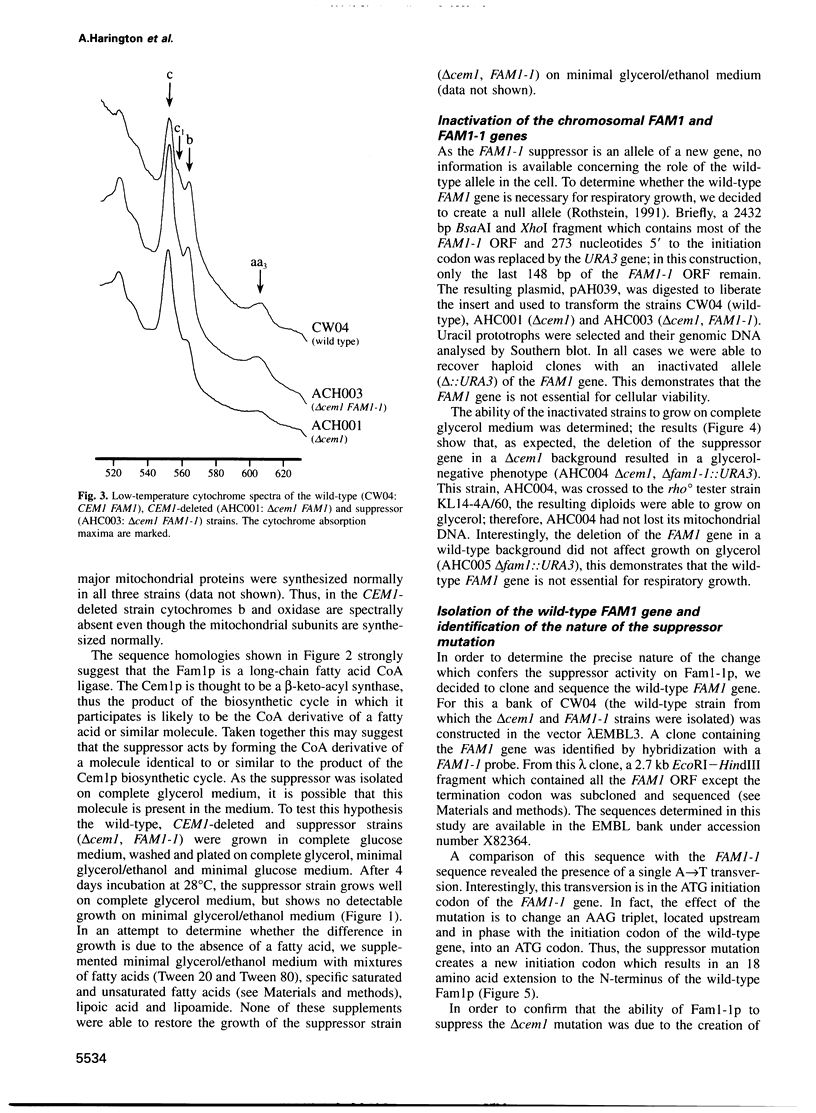
We have isolated an extragenic suppressor, FAM1-1, which is able to restore respiratory growth to a deletion of the CEM1 gene (mitochondrial beta-keto-acyl synthase). The sequence of the suppressor strongly suggests that it encodes a long-chain fatty acid CoA ligase (fatty-acyl-CoA synthetase). We have also cloned and sequenced the wild-type FAM1 gene, which is devoid of suppressor activity. The comparison of the two sequences shows that the suppressor mutation is an A-->T transversion, which creates a new initiation codon and adds 18 amino acids to the N-terminus of the protein. This extension has all the characteristics of a mitochondrial targeting sequence, whilst the N-terminus of the wild-type protein has none of these characteristics. In vitro mitochondrial import experiments show that the N-terminal half of the suppressor protein, but not of the wild-type, is transported into mitochondria. Thus, we hypothesize that the suppressor acts by changing the subcellular localization of the protein and relocating at least some of the enzyme from the cytosol to the mitochondria. These results support the hypothesis that some form of fatty acid synthesis, specific for the mitochondria, is essential for the function of the organelle.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altschul S. F., Gish W., Miller W., Myers E. W., Lipman D. J. Basic local alignment search tool. J Mol Biol. 1990 Oct 5;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Bennetzen J. L., Hall B. D. Codon selection in yeast. J Biol Chem. 1982 Mar 25;257(6):3026–3031. [PubMed] [Google Scholar]
- Boeke J. D., LaCroute F., Fink G. R. A positive selection for mutants lacking orotidine-5'-phosphate decarboxylase activity in yeast: 5-fluoro-orotic acid resistance. Mol Gen Genet. 1984;197(2):345–346. doi: 10.1007/BF00330984. [DOI] [PubMed] [Google Scholar]
- Brody S., Mikolajczyk S. Neurospora mitochondria contain an acyl-carrier protein. Eur J Biochem. 1988 Apr 15;173(2):353–359. doi: 10.1111/j.1432-1033.1988.tb14005.x. [DOI] [PubMed] [Google Scholar]
- Chen D. C., Yang B. C., Kuo T. T. One-step transformation of yeast in stationary phase. Curr Genet. 1992 Jan;21(1):83–84. doi: 10.1007/BF00318659. [DOI] [PubMed] [Google Scholar]
- Chuman L., Brody S. Acyl carrier protein is present in the mitochondria of plants and eucaryotic micro-organisms. Eur J Biochem. 1989 Oct 1;184(3):643–649. doi: 10.1111/j.1432-1033.1989.tb15061.x. [DOI] [PubMed] [Google Scholar]
- Claisse M. L., Pajot P. F. Presence of cytochrome c1 in cytoplasmic "petite" mutants of Saccharomyces cerevisiae. Eur J Biochem. 1974 Nov 1;49(1):49–59. doi: 10.1111/j.1432-1033.1974.tb03810.x. [DOI] [PubMed] [Google Scholar]
- Claisse M. L., Slonimski P. P., Johnson J., Mahler H. R. Mutations within an intron and its flanking sites: patterns of novel polypeptides generated by mutants in one segment of the cob-box region of yeast mitochondrial DNA. Mol Gen Genet. 1980 Feb;177(3):375–387. doi: 10.1007/BF00271476. [DOI] [PubMed] [Google Scholar]
- Daum G. Lipids of mitochondria. Biochim Biophys Acta. 1985 Jun 12;822(1):1–42. doi: 10.1016/0304-4157(85)90002-4. [DOI] [PubMed] [Google Scholar]
- Davis N. K., Chater K. F. Spore colour in Streptomyces coelicolor A3(2) involves the developmentally regulated synthesis of a compound biosynthetically related to polyketide antibiotics. Mol Microbiol. 1990 Oct;4(10):1679–1691. doi: 10.1111/j.1365-2958.1990.tb00545.x. [DOI] [PubMed] [Google Scholar]
- Dower W. J., Miller J. F., Ragsdale C. W. High efficiency transformation of E. coli by high voltage electroporation. Nucleic Acids Res. 1988 Jul 11;16(13):6127–6145. doi: 10.1093/nar/16.13.6127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dujardin G., Pajot P., Groudinsky O., Slonimski P. P. Long range control circuits within mitochondria and between nucleus and mitochondria. I. Methodology and phenomenology of suppressors. Mol Gen Genet. 1980;179(3):469–482. doi: 10.1007/BF00271736. [DOI] [PubMed] [Google Scholar]
- Duronio R. J., Knoll L. J., Gordon J. I. Isolation of a Saccharomyces cerevisiae long chain fatty acyl:CoA synthetase gene (FAA1) and assessment of its role in protein N-myristoylation. J Cell Biol. 1992 May;117(3):515–529. doi: 10.1083/jcb.117.3.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grivell L. A. Nucleo-mitochondrial interactions in yeast mitochondrial biogenesis. Eur J Biochem. 1989 Jul 1;182(3):477–493. doi: 10.1111/j.1432-1033.1989.tb14854.x. [DOI] [PubMed] [Google Scholar]
- Harington A., Herbert C. J., Tung B., Getz G. S., Slonimski P. P. Identification of a new nuclear gene (CEM1) encoding a protein homologous to a beta-keto-acyl synthase which is essential for mitochondrial respiration in Saccharomyces cerevisiae. Mol Microbiol. 1993 Aug;9(3):545–555. doi: 10.1111/j.1365-2958.1993.tb01715.x. [DOI] [PubMed] [Google Scholar]
- Herbert C. J., Macadre C., Bécam A. M., Lazowska J., Slonimski P. P. The MRS1 gene of S. douglasii: co-evolution of mitochondrial introns and specific splicing proteins encoded by nuclear genes. Gene Expr. 1992;2(3):203–214. [PMC free article] [PubMed] [Google Scholar]
- Higgins D. G., Sharp P. M. CLUSTAL: a package for performing multiple sequence alignment on a microcomputer. Gene. 1988 Dec 15;73(1):237–244. doi: 10.1016/0378-1119(88)90330-7. [DOI] [PubMed] [Google Scholar]
- Hopwood D. A., Sherman D. H. Molecular genetics of polyketides and its comparison to fatty acid biosynthesis. Annu Rev Genet. 1990;24:37–66. doi: 10.1146/annurev.ge.24.120190.000345. [DOI] [PubMed] [Google Scholar]
- James G., Olson E. N. Fatty acylated proteins as components of intracellular signaling pathways. Biochemistry. 1990 Mar 20;29(11):2623–2634. doi: 10.1021/bi00463a001. [DOI] [PubMed] [Google Scholar]
- Kamiryo T., Parthasarathy S., Numa S. Evidence that acyl coenzyme A synthetase activity is required for repression of yeast acetyl coenzyme A carboxylase by exogenous fatty acids. Proc Natl Acad Sci U S A. 1976 Feb;73(2):386–390. doi: 10.1073/pnas.73.2.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipman D. J., Pearson W. R. Rapid and sensitive protein similarity searches. Science. 1985 Mar 22;227(4693):1435–1441. doi: 10.1126/science.2983426. [DOI] [PubMed] [Google Scholar]
- Mikolajczyk S., Brody S. De novo fatty acid synthesis mediated by acyl-carrier protein in Neurospora crassa mitochondria. Eur J Biochem. 1990 Jan 26;187(2):431–437. doi: 10.1111/j.1432-1033.1990.tb15322.x. [DOI] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothstein R. Targeting, disruption, replacement, and allele rescue: integrative DNA transformation in yeast. Methods Enzymol. 1991;194:281–301. doi: 10.1016/0076-6879(91)94022-5. [DOI] [PubMed] [Google Scholar]
- Runswick M. J., Fearnley I. M., Skehel J. M., Walker J. E. Presence of an acyl carrier protein in NADH:ubiquinone oxidoreductase from bovine heart mitochondria. FEBS Lett. 1991 Jul 29;286(1-2):121–124. doi: 10.1016/0014-5793(91)80955-3. [DOI] [PubMed] [Google Scholar]
- Sackmann U., Zensen R., Röhlen D., Jahnke U., Weiss H. The acyl-carrier protein in Neurospora crassa mitochondria is a subunit of NADH:ubiquinone reductase (complex I). Eur J Biochem. 1991 Sep 1;200(2):463–469. doi: 10.1111/j.1432-1033.1991.tb16205.x. [DOI] [PubMed] [Google Scholar]
- Schwarz E., Seytter T., Guiard B., Neupert W. Targeting of cytochrome b2 into the mitochondrial intermembrane space: specific recognition of the sorting signal. EMBO J. 1993 Jun;12(6):2295–2302. doi: 10.1002/j.1460-2075.1993.tb05883.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schüller H. J., Hahn A., Tröster F., Schütz A., Schweizer E. Coordinate genetic control of yeast fatty acid synthase genes FAS1 and FAS2 by an upstream activation site common to genes involved in membrane lipid biosynthesis. EMBO J. 1992 Jan;11(1):107–114. doi: 10.1002/j.1460-2075.1992.tb05033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slonimski P. P., Brouillet S. A data-base of chromosome III of Saccharomyces cerevisiae. Yeast. 1993 Sep;9(9):941–1029. doi: 10.1002/yea.320090902. [DOI] [PubMed] [Google Scholar]
- Spaink H. P., Sheeley D. M., van Brussel A. A., Glushka J., York W. S., Tak T., Geiger O., Kennedy E. P., Reinhold V. N., Lugtenberg B. J. A novel highly unsaturated fatty acid moiety of lipo-oligosaccharide signals determines host specificity of Rhizobium. Nature. 1991 Nov 14;354(6349):125–130. doi: 10.1038/354125a0. [DOI] [PubMed] [Google Scholar]
- Towler D. A., Gordon J. I., Adams S. P., Glaser L. The biology and enzymology of eukaryotic protein acylation. Annu Rev Biochem. 1988;57:69–99. doi: 10.1146/annurev.bi.57.070188.000441. [DOI] [PubMed] [Google Scholar]
- Tzagoloff A., Dieckmann C. L. PET genes of Saccharomyces cerevisiae. Microbiol Rev. 1990 Sep;54(3):211–225. doi: 10.1128/mr.54.3.211-225.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zensen R., Husmann H., Schneider R., Peine T., Weiss H. De novo synthesis and desaturation of fatty acids at the mitochondrial acyl-carrier protein, a subunit of NADH:ubiquinone oxidoreductase in Neurospora crassa. FEBS Lett. 1992 Sep 28;310(2):179–181. doi: 10.1016/0014-5793(92)81324-f. [DOI] [PubMed] [Google Scholar]






