Abstract
Prior to the advent of synthetic nematocides, natural products such as seaweed were used to control nematode infestations. The nematocidal agent in seaweed is betaine, an amino acid that functions as an osmolyte and methyl donor. However, the molecular mechanisms of betaine toxicity are unknown. Here, we identify the betaine transporter SNF-3 and a betaine receptor ACR-23 in the nematode C. elegans. Mutating snf-3 in a sensitized background causes the animals to be hypercontracted and paralyzed, presumably because of excess extracellular betaine. These behavioral defects are suppressed by mutations in acr-23, which encodes a ligand-gated cation channel of the cys-loop family. ACR-23 is activated by betaine and functions in the mechanosensory neurons to maintain basal levels of locomotion. However, overactivation of the receptor by excess betaine or by the allosteric modulator monepantel causes hypercontraction and death of the nematode. Thus, monepantel targets a betaine signaling pathway in nematodes.
Keywords: betaine receptor, SNF-3, betaine transporter, ACR-23, phospholipase Cβ, egl-8, C. elegans
Introduction
Humans have long competed with parasitic nematodes for crops, livestock, and personal health. Early farmers dealt with nematode infestations by using natural pesticides such as wormwood, tobacco, and algae (seaweed), which contain natural phytochemicals that interfere with parasitic nematode development1–3. Seaweed extracts are rich in betaines (δ-aminovaleric betaine, γ-amino-butyric betaine, and glycine betaine), and direct application of individual betaines to plants suppresses nematode growth as effectively as algal extract4.
Glycine betaine, or simply ‘betaine’, is a ubiquitous non-canonical amino acid that acts as an osmolyte or as a methyl donor. In addition to its role in metabolism, betaine may play specific roles in the mammalian nervous system. Betaine has anti-epileptic properties 5–7 and the transporter BGT-1 is localized to dendritic spines and astrocytes in the mammalian brain8.
In nematodes betaine arrests larval development 2–4. However, the molecular target of betaine is not known. The targets of synthetic anthelmintics are often ligand-gated ion channels. The imidazothiazoles directly activate acetylcholine–gated ion channels, which stimulates muscle contraction and paralyzes the worm9,10. The macrocyclic lactones open glutamate-gated chloride channels and inhibit pharyngeal pumping 11–13. Amino-acetonitrile derivatives (AADs) are a recently discovered class of compounds that target a nematode-specific ion channel, called ACR-23 in C. elegans14. ACR-23 belongs to the nicotinic acetylcholine receptor subfamily, but its homolog in parasitic nematodes, MPTL-1, is not gated by acetylcholine or choline15. The endogenous ligand and biological function of ACR-23 remain to be characterized.
In this manuscript, we study the role of betaine in the nematode C. elegans, and demonstrate that ACR-23 is a betaine receptor. First, we characterize the betaine transporter SNF-3 and show that mutations in this transporter are subviable in a sensitized background lacking phospholipase Cβ. Second, we demonstrate that mutations in acr-23 suppress this lethality, suggesting that excess betaine is acting via ACR-23. Third, we demonstrate that ACR-23 is gated by betaine and potentiated by the AAD monepantel. Thus, ancient and modern anthelmintics act on the same target: a betaine-activated ion channel that is only found in nematodes.
Results
An enhancer screen identifies a betaine transporter
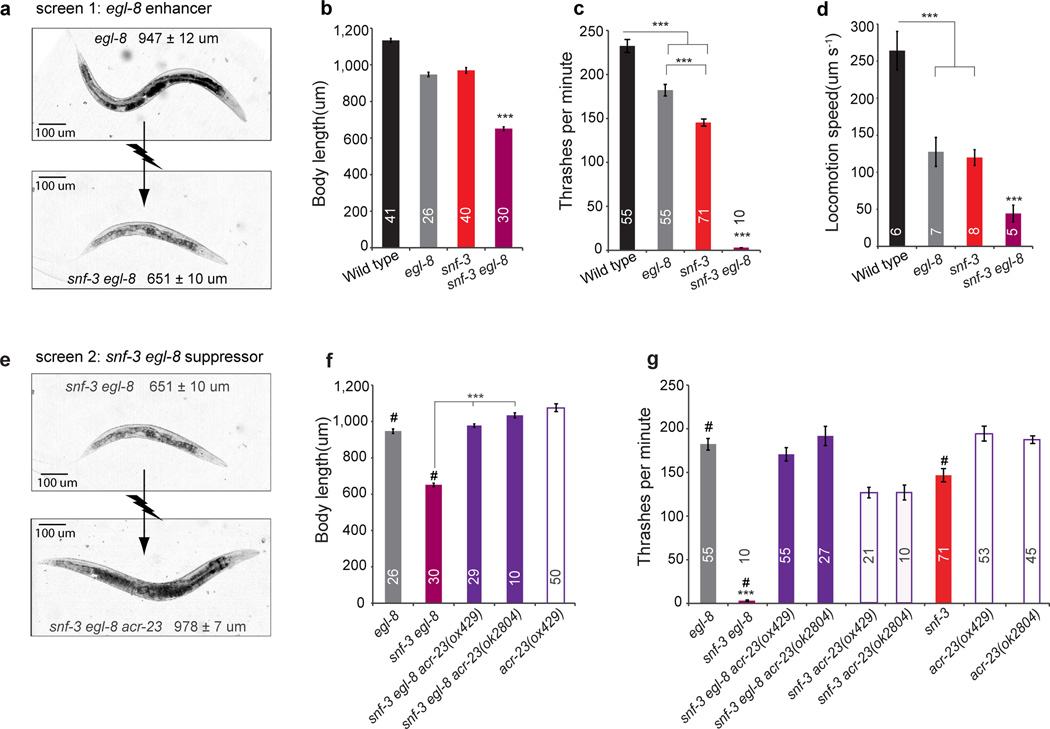
We were able to identify the betaine pathway inadvertently while studying phospholipase Cβ function. In the nematode C. elegans, mutants lacking the only phospholipase Cβ gene (egl-8) exhibit subtle behavioral defects in locomotion and egg-laying, in contrast to the near lethal phenotype of mutants lacking Gαq, the upstream activator of phospholipase Cβ 16,17. This modest loss-of-function phenotype of egl-8 suggests the existence of parallel pathways to inositol signaling that modulate locomotion. To uncover these pathways, we performed an F2 enhancer screen in an egl-8 mutant background. We screened 1669 haploid genomes and identified a single mutation that caused a synthetic phenotype in the presence of an egl-8 mutation. Mapping, rescue and sequencing experiments demonstrated that the enhancer was an allele of the snf-3 gene (sodium neurotransmitter symporter family-3, Supplementary Fig. S1a). The snf-3 egl-8 double mutants exhibit severe synthetic phenotypes (Fig. 1a–d): the strain is subviable, uncoordinated, and dumpy. The dumpy phenotype is caused by hypercontraction of the muscle since it can be suppressed by mutations in the contractile apparatus unc-22 (twitchin) and unc-54 (myosin) (data not shown)9. The hypercontracted phenotype of snf-3 egl-8 double mutants is fully synthetic: neither single mutant is hypercontracted (snf-3 egl-8 vs egl-8 in Fig. 1a–b, and snf-3 in Supplementary Fig. S1c). The uncoordinated phenotype is also more severe than that of either single mutant (Fig. 1c–d). Multiple allelic combinations exhibit the same synthetic hypercontracted phenotype (data not shown), demonstrating that the phenotypes are not due to background mutations.
Figure 1. Two genetics screens reveal a betaine receptor.
(a) snf-3 was identified as an enhancer of the phosopholipase Cβ mutation egl-8(sa47). Images of adult hermaphrodites of the phosopholipase Cβ mutant egl-8(sa47) and the snf-3(ox354) egl-8(sa47) double mutant that was isolated in the screen. Mean lengths of 26 (egl-8) and 30 (snf-3; egl-8) adults are shown. (b) Hypercontracted phenotype. Mean body length of young adult animals in micrometers ± s.e.m. *** p< 0.001 relative to wild type and single mutants. Genotypes are wild-type, egl 8(sa47), snf-3(ox354), and snf-3(ox354) egl-8(sa47). (c) Quantification of locomotion in liquid using a thrashing assay (thrashes ± s.e.m. *** p< 0.001). (d) Average locomotion speed over a 5-minute period on an agar plate without food (speed ± s.e.m. *** p< 0.001). Speed was determined using an automated worm tracking system. (e) acr-23 was isolated as a suppressor of the snf-3 egl-8 hypercontracted phenotype. Images of adult hermaphrodites of the snf-3(ox354) egl-8(sa47) double mutant (n = 30) and the snf-3(ox354) egl-8(sa47) acr-23(ox429) triple mutant (n = 29). (f, g) Quantification of the hypercontracted (f) and locomotion (g) phenotypes. Data represent the mean ± s.e.m. *** p< 0.001 compared to other mutants. For all histograms, the number of animals tested is shown inside each bar. Statistical significance was determined using one-way ANOVA followed by Tukey post-hoc comparisons. #, data are the same as Fig. 1b,c.
snf-3 encodes a neurotransmitter transporter of the solute carrier 6 (SLC6) gene family. Members of this transporter family clear neurotransmitters from the synaptic cleft, including the neurotransmitters serotonin, dopamine, norepinephrine, GABA and glycine. SNF-3 is orthologous to the vertebrate betaine/GABA transporter (BGT1 or SLC6A12). Although we do not fully understand the genetic interaction between the betaine transporter and PLCβ, the synthetic phenotype provided a selection to identify the betaine receptor.
Hypercontraction in snf-3 egl-8 is mediated by acr-23
Mutations in SLC6 transporters often result in constitutive signaling because the neurotransmitter cannot be cleared from the synaptic cleft18,19. For example, mice lacking the dopamine transporter are hyperactive20, while worms lacking the same gene have defects in their locomotory behaviors21,22. These defects are consistent with the role of dopamine in the control of locomotion.
To determine whether the phenotype of snf-3 egl-8 mutants was caused by constitutive betaine signaling, we screened for suppressors of the snf-3 synthetic phenotype. Specifically, we mutagenized snf-3 egl-8 double mutants with ENU and screened for suppressors of the hypercontracted phenotype (Fig. 1e, screen 2). We screened 37,000 haploid genomes and obtained 35 suppressors. The phenotypes of the triple mutants fall into three broad classes: those that resemble egl-8 single mutants (snf-3 specific suppressors), those that resemble snf-3 mutants (egl-8 specific suppressors) and those with novel phenotypes. The strongest snf-3-specific suppressor was the mutation ox429. We identified the suppressor mutation by genome resequencing and by transgenic rescue. ox429 is an allele of the gene acr-23 (acetylcholine receptor like-23) (Supplementary Fig. S2a). acr-23 encodes a nematode-specific ligand-gated channel subunit and is predicted to function as a cation channel based on its sequence homology to acetylcholine receptors (Supplementary Fig. S2c). The only known function of ACR-23 is that it imparts sensitivity to the new class of anthelmintic drugs known as AADs. Genetic screens for AAD-resistance in C. elegans identified 27 independent mutations in a single target, acr-2314 and mutations in related receptor subunits within the same subfamily do not confer resistance to AAD23.
acr-23(ox429) fully suppresses the hypercontracted (Fig. 1f) and uncoordinated phenotypes (Fig 1g) of the snf-3 egl-8 double mutants. For example, the double mutants are completely paralyzed in liquid (Fig. 1g, 0 % of wild-type rate), whereas the snf-3 egl-8 acr-23 triple mutants thrash actively (Fig. 1g, 45% of wild-type rate). A deletion allele acr-23(ok2804) also suppresses the double mutants (Fig. 1f–g). The triple mutants still exhibit egl-8 phenotypes including lethargy (Fig. 1g), constipation (data not shown), and a mild egg-laying defect (data not shown), suggesting that acr-23 mutations specifically suppress snf-3, but not egl-8 pathways.
SNF-3 is a Na+/Cl−-dependent betaine transporter
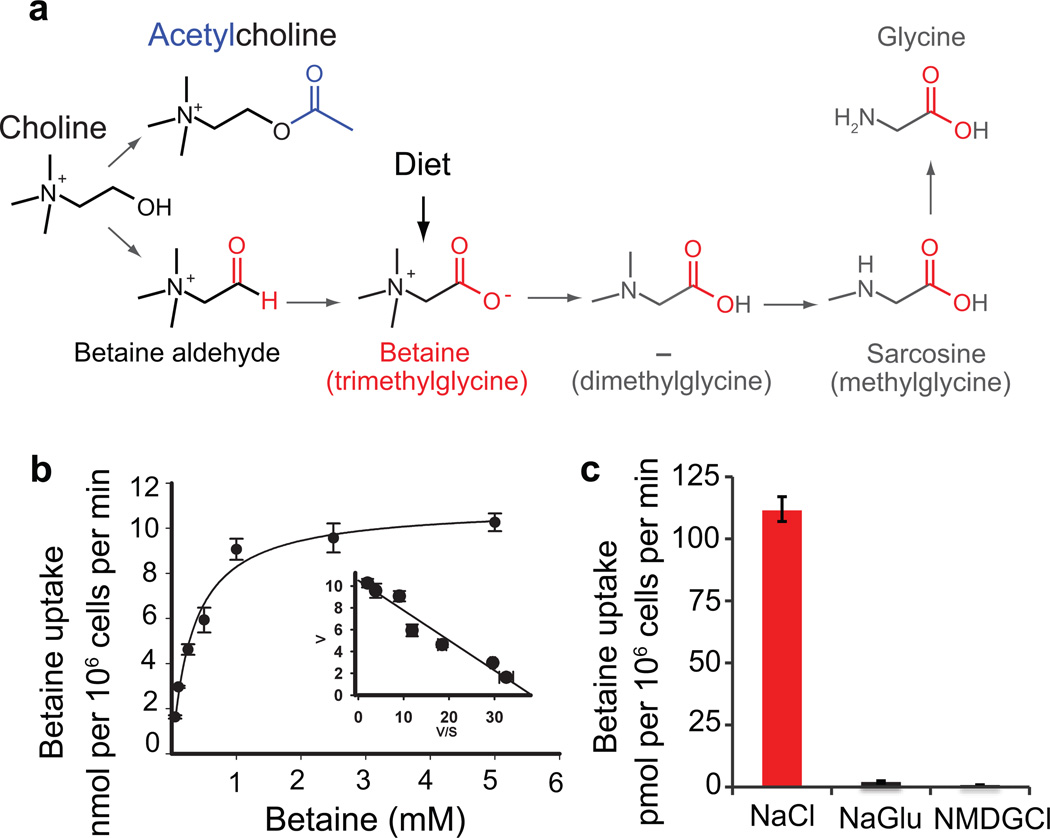
The vertebrate ortholog of SNF-3, betaine/GABA transporter 1 (BGT1 or SLC6A12) has two substrates, betaine and GABA. However, it has a higher affinity for GABA (Km= 93 µM) than betaine (Km= 398 µM)24,25. By contrast, we find that SNF-3 transports betaine but not GABA. Betaine is a noncanonical amino acid that is present in all organisms (Fig. 2a). It acts as an osmolyte to maintain cell volume during stress26 and as a methyl donor in the conversion of homocysteine to methionine. Betaine is acquired either by dietary uptake using a plasma membrane transporter or is generated as an oxidation product of choline27. Ultimately, betaine is metabolized to glycine.
Figure 2. SNF-3 is a sodium chloride-dependent betaine transporter.
(a) Betaine synthesis pathway. Betaine (N, N, N-trimethylglycine) is either acquired via diet or generated in two steps by oxidation of choline. (b) Saturation curve of betaine uptake by SNF-3 in HRPE cells. Error bars represent mean ± s.e.m. Each experiment was performed in triplicate. Inset: Eadie-Hofstee conversion of the same saturation data (v/s): [betaine uptake velocity (v) versus betaine uptake velocity/betaine concentration (v/s)]. Km was calculated using the Eadie-Hofstee equation. (c) SNF-3-mediated uptake of betaine is dependent on Na+ and Cl− ions. HRPE cells were incubated with 2.5 µM betaine for 15 minutes in assay buffer containing either NaCl, NaGlu or NMDGCl. Gluconate replaced chloride ions (NaGlu), and sodium ions were replaced by N-methyl-D-glucamine (NMDGCl). Error bars represent mean ± s.e.m. Each experiment was performed in triplicate.
To rapidly screen for the relevant substrate, we expressed SNF-3 in Xenopus oocytes and applied potential transport molecules. Because SLC6 transporters co-transport ions along with substrate molecules, they are electrogenic. We tested candidate substrates and found that only betaine induced a specific current (Supplementary Fig. S3a). GABA, the betaine metabolites dimethyl-glycine, N-methylglycine, glycine and other related compounds, are not substrates of the SNF-3 transporter.
To characterize the transport kinetics of SNF-3, we measured the accumulation of radio-labeled betaine in Human Retinal Pigment Epithelial (HRPE) cells, which do not express an endogenous betaine transporter. The expression of SNF-3 increased betaine uptake greater than 100-fold (108 ± 3 pmol/106 cells/min) over control cells (0.8 ± 0.1 pmol/106 cells/min). SNF-3-mediated transport was saturable with a Km of 320 ± 50 µM (Fig. 2b); this value is similar to the affinity of BGT-1 for betaine (398 µM)24. As expected for protein-mediated transport, activity is saturable over a range of concentrations (Fig. 2b and Supplementary Fig. S3b) and voltages (Supplementary Fig. S3c). Like other SLC6 transporters, SNF-3 requires sodium and chloride to move substrates into cells against their chemical gradient. The absence of sodium (in NMDG chloride solution) or the absence of chloride (sodium gluconate solution) abolished betaine uptake (Fig. 2c and Supplementary Fig. S3d), and transport is voltage-dependent (Supplementary Fig. S3e). The Michaelis constant of SNF-3 for sodium ( ) was 37 ± 1 mM with a Hill coefficient of 2.2 ± 0.1 (Supplementary Fig. S3f), and the corresponding value for chloride () was 12 ± 1 mM with a Hill coefficient of 1.0 ± 0.1 (Supplementary Fig. S3g). Thus, these data demonstrate that SNF-3 functions as a betaine transporter with a unidirectional influx of 1 betaine: 2 Na+: 1 Cl− per transport cycle.
In contrast to BGT1, SNF-3 does not transport GABA in our biochemical assays, and this lack of GABA transport activity has also been demonstrated functionally. In the nematode, the GABA transporter is encoded by snf-1128. Overexpression of the SNF-3 transporter was unable to compensate for the absence of the GABA transporter SNF-11 even when overexpressed under the snf-11 promoter, indicating that SNF-3 cannot transport GABA in vivo 28. The presence of a dedicated transporter suggests that betaine may play a specific role in cell signaling.
SNF-3 is required for betaine clearance
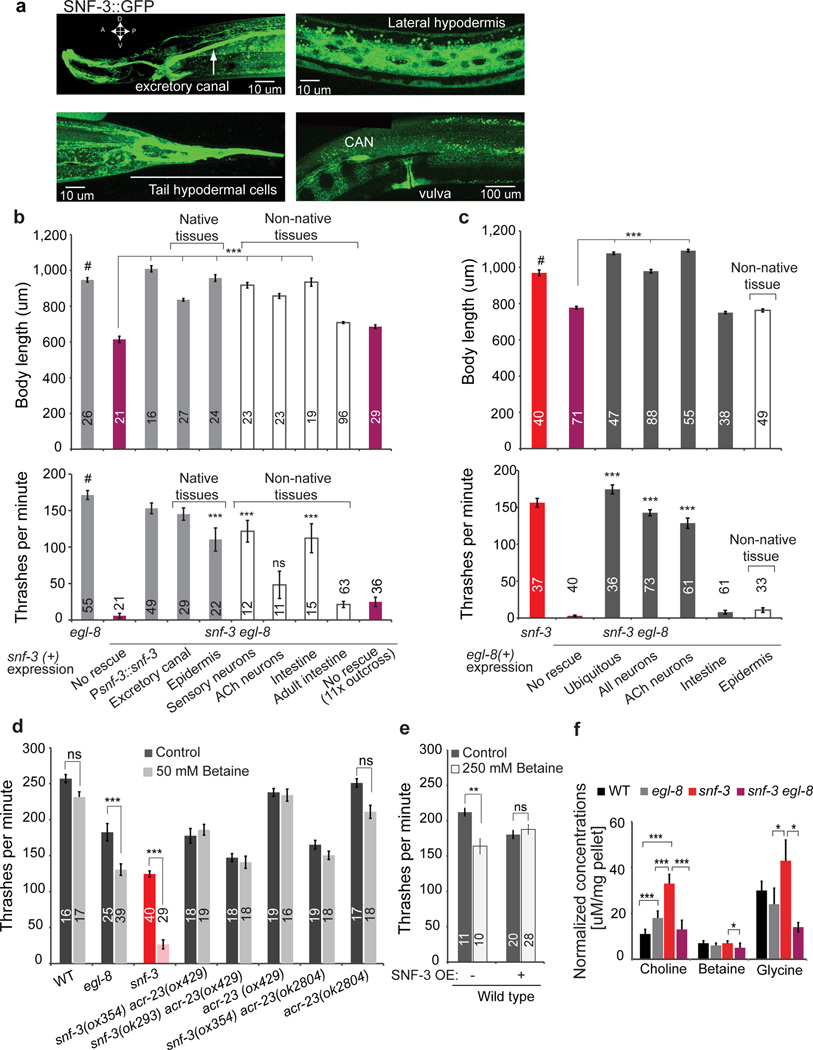
Members of the SLC6 transporter family clear neurotransmitters from synapses and the extrasynaptic space, and thereby limit their activity. To determine where SNF-3 functions, we examined snf-3 expression using a functional SNF-3::GFP transgene (Supplementary Fig. S1b). Transgenic worms displayed strong expression in the excretory canal, tail hypodermal cells, epidermis, and vulval epithelia cells (Fig. 3a). SNF-3 was also expressed in some neurons, including the excretory canal-associated neuron (CAN, Fig. 3a), and some sensory neurons in the head including ILs, OLs, ADE and AQR (Supplementary Fig. 2d–e). We were able to rescue the hypercontraction and the locomotory phenotypes of snf-3 egl-8 double mutants by expressing wild-type SNF-3 using the native snf-3 promoter or using tissue-specific promoters for the excretory canal or skin (Pglt-3 and Ppdi-2, respectively; Fig. 3b). Interestingly, expressing SNF-3 in tissues that do not normally express the gene, such as the intestine, chemosensory neurons or acetylcholine motor neurons, also rescued the hypercontracted phenotype (Fig. 3b). Rescue requires expression during larval development: expression of SNF-3 in the intestine during larval development (Pvha-6), but not in the adult (Pvit-2; Fig. 3b) rescued the hypercontracted phenotype. This stage-dependent rescue is consistent with the retarded larval development of snf-3 mutants (Supplementary Fig. S2e). The non-cell autonomous rescue of the snf-3 egl-8 phenotypes suggests that the hypercontracted and locomotory phenotypes are caused by a lack of betaine clearance; presumably any tissue – not just the epidermis and excretory canal – can remove the excess betaine.
Figure 3. SNF-3 is required for betaine clearance.
(a) Expression pattern of SNF-3::GFP. Images show the head, tail, lateral midbody and vulva region of a transgenic hermaphrodite expressing GFP fused to genomic SNF-3 (Psnf-3:: SNF-3- GFP::snf-3 utr). All images are adult hermaphrodites; anterior is to the left. (b) snf-3 tissue-specific rescue of the hypercontracted (above) and locomotion (below) phenotype. Above, expression of SNF-3 cDNA in excretory canal (Pglt-3), skin (Ppdi-2), intestine (Pvha-6), chemosensory neurons (Podr-4), or acetylcholine neurons (Punc-17) rescues the body morphology of snf-3 egl-8 double mutants to normal (egl-8 phenotype). Expression in the adult intestine (Pvit-2) did not rescue the phenotypes. The error bars represent the mean body length ± s.e.m. *** p< 0.001 compared to the snf-3 egl-8 double mutants. The differences between the egl-8 control and rescue of the snf-3 egl-8 double mutant by expression of snf-3(+) in the excretory canal (p=0.0088) or in the acetylcholine motor neurons (p=0.0031) are statistically significant. Below, for the locomotion phenotype, the differences between egl-8 control and rescue in the skin (p=0.0003) and intestine (p=0.0048) rescue are statistically significant. Expression of SNF-3 in the acetylcholine motor neurons did not rescue snf-3 egl-8 locomotory defects (p = 0.3702), possibly because SNF-3 activity is electrogenic and interferes with motor neuron function. ns means not statistically significant. ‘Native tissues’ are cells that express the snf-3 gene; ‘non-native tissues’ are cells in which snf-3 gene expression was not detected. 11X outcrossed snf-3 egl-8 is EG7513. The error bars represent thrashes ± s.e.m. *** p< 0.001 compared to the snf-3 egl-8 double mutants. The number of animals tested is shown inside each bar. (c) Tissue-specific rescue of the egl-8 hypercontracted (top) and locomotion (bottom) phenotypes. egl-8 cDNA was expressed in all cells (Pdpy-30), neurons (Prab-3), acetylcholine neurons (Punc-17), intestine (Pvha-6), or epidermis (Pdpy-7). Expression of EGL-8 in acetylcholine neurons (Punc-17) generates transgenic animals that are long and loopy-coilers. The number of animals tested is shown inside each bar. The error bars represent the means ± s.e.m. *** p< 0.001. Statistical significance was determined using one-way ANOVA followed by Tukey post-hoc comparisons. (d) Exogenous betaine slows locomotion. (e) Overexpression of SNF-3 confers resistance to excess betaine. For panels d and e the number of animals tested is shown inside each bar. The error bars represent the means ± s.e.m. Statistical significance was determined using one-way ANOVA followed by Tukey post-hoc comparisons. *** p< 0.001, ** p< 0.01, ns (not significant ) p > 0.05. (f) Average concentrations of total betaine in whole animals. These metabolites were identified using Proton NMR spectroscopy. Statistical significance was determined using a one-way ANOVA on each metabolite with Bonferroni correction. n= 8000 animals per strain. ns, p >0.05, * p< 0.05, ** p< 0.01, *** p< 0.001. Alleles are egl-8(sa47) and snf-3(ox354), unless indicated otherwise.
In contrast to snf-3, the phospholipase Cβ gene egl-8 was required in the nervous system, but not epidermis (Fig. 3c). Further analyses of EGL-8 revealed that the synthetic hypercontraction is caused by a defect in acetylcholine neurons (Punc-17). Expression in sensory neurons (data not shown), or ventral cord motor neurons (data not shown) did not rescue the double-mutant phenotypes. Thus, the functional requirements for EGL-8 and SNF-3 are in separate tissues: the transporter SNF-3 functions primarily in the epidermis to clear betaine from the extracellular space, whereas EGL-8 is required in the nervous system to modulate neuronal activity.
Exogenous betaine paralyzes snf-3 mutants
These data suggest that the phenotypes caused by snf-3 mutants are due to excess betaine in the extracellular space. If true, then high levels of exogenous betaine should mimic snf-3 phenotypes. We grew C. elegans on different concentrations of betaine and tested their locomotion in liquid. Wild-type worms were not affected by 50 mM betaine (Fig. 3d). egl-8 mutants were hypersensitive to 50 mM betaine and became sluggish in liquid, but they did not become hypercontracted. snf-3 mutants were strongly hypersensitive to 50 mM betaine and were paralyzed in liquid. The toxic effect of betaine is mediated by ACR-23 since snf-3 acr-23 double mutants were resistant to exogenous betaine (Fig. 3d). Wild-type animals grown at a higher concentration of betaine (250 mM) exhibited slowed swimming in the presence of betaine (Fig. 3e no array), whereas animals overexpressing the SNF-3 transporter were resistant to 250 mM betaine (Fig. 3e). These data suggest that excess betaine suppresses locomotion and that the SNF-3 betaine transporter clears betaine from the extracellular space.
snf-3 mutants exhibit locomotory phenotypes even if excess betaine is not applied (Fig. 1c–d), suggesting that betaine may already be present in worms. To determine betaine levels in C. elegans, we performed proton-NMR spectroscopic analysis on the worm metabolome. Betaine was present in extracts of worms at 7.0 ± 1.0 µM/mg of dry pellet (Fig. 3f), whereas the concentrations of GABA and acetylcholine were below detection (data not shown). Total betaine content did not change in snf-3 mutants but a redistribution to the extracellular space would not be detectable in assays of whole worms. Interestingly, concentrations of the metabolites choline and glycine were higher in snf-3 mutants (Fig. 3f). Levels of betaine in C. elegans are controlled in part by diet29. To confirm that our E. coli strains released betaine in the media, we measured the concentration of betaine in the media. We found that the bacterial media contained betaine at 2.3 ± 0.5 mM/ml, demonstrating that betaine is readily available from food.
ACR-23 forms a homomeric betaine receptor
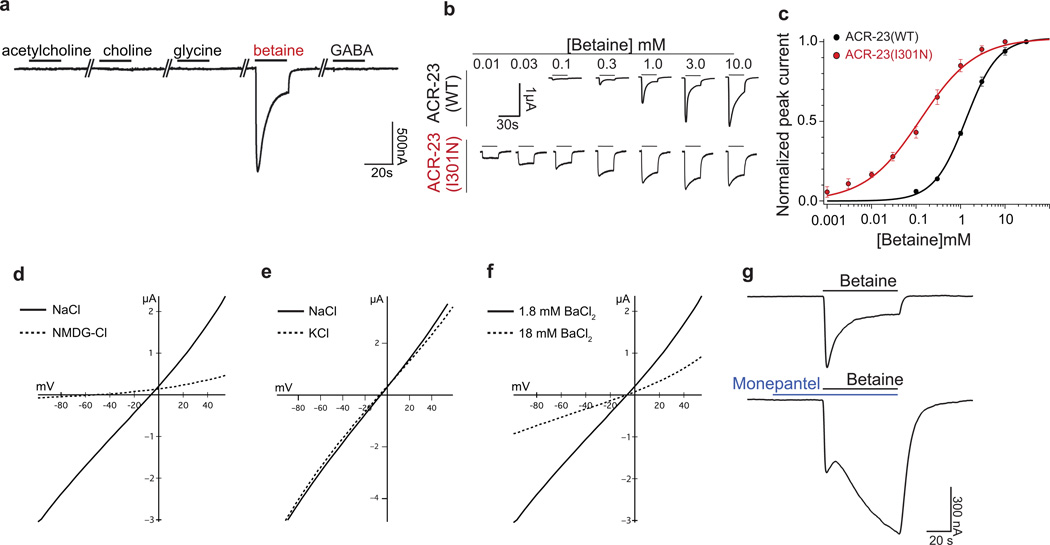
To test whether betaine acts directly on the ACR-23 receptor, we expressed ACR-23 in Xenopus oocytes and examined its response to various ligands. ACR-23 forms an ion channel that is activated by betaine, but not by acetylcholine, choline, glycine, or GABA (Fig. 4a). The sensitivity of ACR-23 for betaine is relatively weak; the EC50 for betaine is 1.4 mM with a Hill coefficient of 1.2 ± 0.1 (Fig. 4b–c) compared to an EC50 of 40 µM for GABA receptors that function at the C. elegans neuromuscular junction30. Although we could not confirm the EC50 by recording from larval muscles in vivo, the sensitivity of the channel to betaine is consistent with the high Km of the betaine transporter (~0.3 mM) and the concentrations of betaine found in extracellular space (~0.2 mM in mammals)24.
Figure 4. ACR-23 is a betaine-activated ion channel.
(a) Xenopus laevis oocytes expressing ACR-23 respond to a 20 s pulse of 1mM betaine, but not 1mM acetylcholine, choline, glycine, or GABA. The mean betaine-evoked response = 1.3 ± 0.13 µA for oocytes injected with 0.1 ng cRNA (n = 9 oocytes). (b) Representative betaine-evoked currents from oocytes expressing ACR-23 or ACR-23(I301N) stimulated with the indicated betaine concentration. (c) Betaine sensitivity of the wild-type ACR-23 channel (black line, n = 6 oocytes) and ACR-23(I301N) gain-of-function channel (red line, n = 5). EC50 is 1.4 mM for the wild type and 166 µM for ACR-23(I301N) (p = 0.0017). Error bars are means ± s.e.m. Statistical significance was determined using two-tailed unpaired Student’s t-test with Welch’s correction. (d,e,f) ACR-23 is a monovalent cation channel. Insets indicate predicted ion flux under physiological conditions. (d) ACR-23 is permeable to sodium. Representative I-V curves in modified Ringer’s solution containing Na+ (n = 9) or NMDG (n = 8). (e) ACR-23 is potassium permeable. Representative I-V curves in modified Ringer’s solution containing Na+ (n = 7) or K+ (n = 7). (f) ACR-23 is not permeable to the divalent cation Ba2+. The reversal potential of betaine-evoked currents was not altered when extracellular Ba2+ concentration was increased 10-fold (n = 9). (g) Monepantel allosterically modulates ACR-23. A representative trace shows current induced by 1mM betaine in the absence (above) or presence (below) of 300 pM monepantel in oocytes expressing ACR-23 (n = 11).
ACR-23 is likely to be a non-selective monovalent cation channel. Like other cation channels, ACR-23 lacks a proline at the -2’ position and has a glutamate at the -1’ position of the TM2 pore-forming helix31 (Supplementary Fig. S2c). Betaine-induced inward currents reverse at −9.4 ± 0.9 mV (Fig. 4d), which is consistent with a non-selective cation channel. To demonstrate cation permeability, we replaced Na+ in the extracellular solution with the large impermeant cation N-methyl-D-glucamine (NMDG). NMDG largely abolished betaine-evoked inward currents and shifted the reversal potential to −42.8 ± 5.6 mV, indicating that monovalent cations contribute substantially to the ACR-23 current (Fig. 4d). To determine whether the ACR-23 channel is permeable to K+ ions, we replaced external NaCl with KCl. These conditions did not cause any shift in the reversal potential (ΔErev= 0.6 ± 0.7 mV, Fig. 4e), indicating that the channel is also permeable to K+ ions and exhibits no selectivity between Na+ and K+ (PNa/PK = 1.0). Two experiments demonstrate that ACR-23 exhibits little or no divalent cation permeability. First, we tested whether betaine-evoked currents could activate the endogenous Ca2+-activated chloride channels in Xenopus oocytes, which can be activated by calcium but not barium influx through calcium-permeable ion channels32. However, replacement of extracellular Ca2+ with Ba2+ did not alter betaine-evoked currents in oocytes (data not shown). Second, increasing the concentration of Ba2+ ten-fold failed to elicit a significant shift in betaine-evoked reversal potential (ΔErev= 1.1 ± 1.1 mV, Fig. 4f). Thus, ACR-23 forms a homomeric betaine-gated ion channel that is non-selective for monovalent cations.
ACR-23 was first isolated as the target of the novel class of anthelmintic drugs called amino-acetonitrile derivatives (AADs), commercially available as Zolvix containing the AAD monepantel14. However, the pharmacological effects of this drug on the ACR-23 receptor have never been tested because the endogenous ligand of ACR-23 was not known15. To determine the effects of monepantel on ACR-23, we applied 300 pM monepantel (diluted Zolvix) to ACR-23-expressing oocytes. Monepantel did not elicit any current by itself, but rather potentiated betaine-induced currents (Fig. 4g). Recently it was observed that one thousand-fold higher concentrations of monepantel (300 nM) can act as an agonist of ACR-2333. At 300pM monepantel does not activate the ion channel, and has no effect on the size of the initial betaine-induced current, however, it blocks the desensitization of the ACR-23 receptor (Fig. 4g). Sixty seconds after the initial application of betaine, the current amplitude was 5.3 ± 0.9 times larger in the presence of monepantel than in its absence. Thus, monepantel functions as a positive allosteric modulator of betaine gating. Allosteric modulators, such as benzodiazepines and barbiturates that positively enhance GABA transmission, alter the response of the receptor to its natural ligand without competing for the binding site.
ACR-23 acts in the nervous system to regulate locomotion
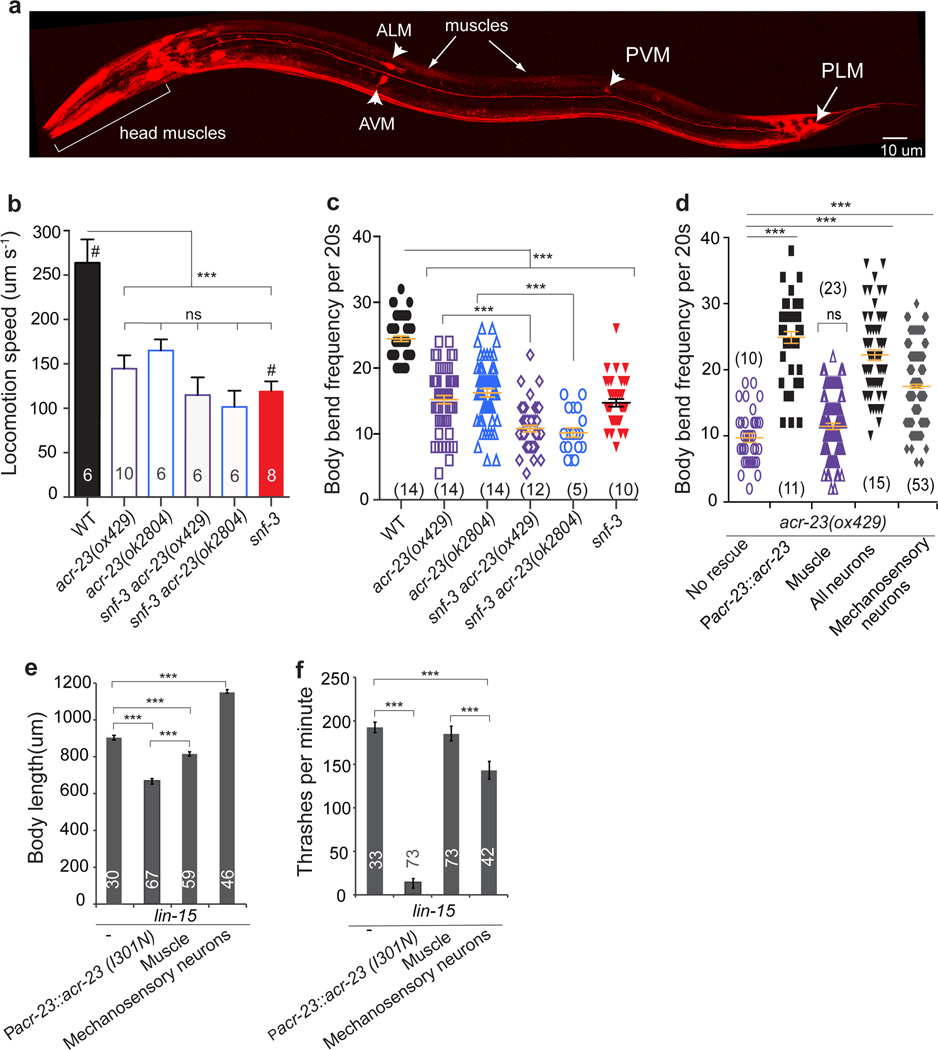
To determine where ACR-23 functions during locomotion, we characterized the expression pattern of the acr-23 gene using transcriptional mCherry reporter constructs (Fig. 5a and Supplementary Fig. S2b). acr-23 is expressed strongly in the six mechanosensory neurons, multiple interneurons, and in body muscles. Muscle expression is higher in larvae and weak in adults. Expression of acr-23 in the larval body muscles is consistent with the hypercontraction phenotype observed in snf-3 egl-8 double mutants.
Figure 5. ACR-23 is required for sustained locomotion.
(a) ACR-23 expression pattern. A transgenic hermaphrodite expressing mCherry driven by the acr-23 promoter (Pacr-23::mCherry::let-858utr). The strongest expression in the adult is in the mechanosensory neurons ALM, PLM, AVM and PVM (arrow heads) and head muscles (bracket). Expression is also seen in interneurons and body muscle. (b) Locomotion speed over a 5-minute period on an agar plate without food. Speed was determined using an automated worm tracking system. #, data from Fig. 1d. *** p< 0.001 ns, not significant (p>0.05). (c) Rate of body bends of animals on an agar plate. Each animal was tested 4 times in the absence of food. *** p< 0.001. The number of animals tested is shown at the bottom of each column. The error bars represent the means ± s.e.m. (d) Neuronal expression rescues acr-23 crawling defects. *** p< 0.001. The error bars represent the means ± s.e.m. The number of animals tested is shown at the bottom of each column. Each animal was tested 4 times in the absence of food. ns, not significant relative to no rescue. (e,f) ACR-23 activation reproduces snf-3; egl-8 double mutant phenotypes. Quantification of the hypercontracted (e), and locomotion (f) phenotypes. *** p< 0.001. The number of animals tested is shown inside each bar. The error bars represent the means ± s.e.m. Statistical significance was determined using one-way ANOVA followed by Tukey post-hoc comparisons.
The lack of expression in motor neurons was surprising because mutants lacking acr-23 (Supplementary Fig. S1c) exhibit mild yet significant swimming defects (Fig. 1g), are lethargic when crawling on an agar plate (Fig. 5b,c) and their movement is interrupted by frequent pauses. snf-3 mutants and snf-3 acr-23 double mutants are equally sluggish (Fig. 1g and Fig. 5b–c), suggesting that the effect of betaine on locomotion is mediated by different receptors.
To determine where ACR-23 functions during locomotion, we rescued the mutant using tissue-specific promoters. Expression in the nervous system rescued the locomotory phenotypes of acr-23 mutants (Fig. 5d). Expression in the mechanosensory neurons also partially restored motor activity (Fig. 5d), consistent with the known role for these cells in controlling basal levels of locomotion34. Thus, like egl-8, acr-23 is also required in the nervous system. These data suggest that phospholipase Cβ is limiting ACR-23 function in the nervous system, either directly or indirectly.
Gain-of-function ACR-23 resembles snf-3 egl-8 double mutants
Together these data suggest that an increase in betaine levels in the snf-3 egl-8 double mutants inappropriately activates ACR-23 and leads to hypercontraction. To demonstrate that increased current via ACR-23 alone was capable of generating the hypercontracted phenotype, we generated an ACR-23 receptor with higher ligand sensitivity. The second transmembrane domain forms the pore in ligand-gated ion channels and the residues are numbered 0’ to 19’ by convention35. Mutations at the 13’ position of the pore are known to increase the ligand sensitivity and conductance of cys-loop ion channels, probably by affecting transitions to the open state31. We engineered a variant at the 13’ position, ACR-23(I301N), based on known gain-of-function mutations in a related receptors36 (Supplementary Fig. S2c). Similar to other receptors31, ACR-23 (I301N) was more sensitive to betaine and the channel desensitized less than the wild-type receptor (Fig. 4c). The EC50 for the receptor decreased 8-fold from 1400 µM to 166 µM (Fig. 4c).
This hypersensitive variant of ACR-23 in transgenic animals recapitulated the snf-3 egl-8 double mutant phenotype. Most ACR-23(I301N) transgenic worms died during larval development. Those that escaped lethality and reached adulthood were severely hypercontracted (Supplementary Fig. S2d and Fig. 5e) and uncoordinated (Fig. 5f), closely resembling the phenotype of snf-3 egl-8 mutants. We also evaluated the tissue-specific effects of the gain-of-function ACR-23 (I301N) mutation. Expressing ACR-23 (I301N) in body muscles alone generated hypercontracted animals that only exhibited modest locomotion defects (Fig. 5e–f and Supplementary Fig. S2e). Conversely, expression of ACR-23(I301N) in the mechanosensory neurons resulted in morphologically wild-type animals that exhibited uncoordinated thrashing in liquid (Fig. 5e–f and Supplementary Fig. S2d). These data are consistent with ACR-23 acting in part via the nematode somatosensory circuit that modulates locomotion.
Discussion
Using two forward genetic screens, we uncovered the molecular pathway that leads to betaine toxicity in nematodes. ACR-23 is a betaine-gated cation channel expressed in body muscle and neurons. The betaine transporter SNF-3 removes betaine from the extracellular space (Supplementary Fig. 4). In the absence of snf-3 betaine can accumulate in the extracellular space, and snf-3 mutants exhibit subtle locomotory phenotypes. These phenotypes curiously are exacerbated by mutations in phospholipase Cβ. In the snf-3 egl-8 double mutant, constitutive activation of ACR-23 causes hypercontraction, paralysis and sometimes death, due to a combination of effects on the nervous system and muscle. Why phospholipase Cβ mutations are synthetic with mutations in the transporter is not clear; however, one possibility is that phospholipase Cβ is required to downregulate ACR-23 itself or the activity of the neurons in which it functions. Thus, the elimination of the receptor by mutation restores muscle function and locomotion, reversing the effect of the snf-3 egl-8 mutations.
In addition to its natural ligand betaine, ACR-23 is also the target of a novel class of anthelmintic drugs called amino-acetonitrile derivatives (AADs), commercially available as Zolvix, which contains the active compound monepantel14. Monepantel induces muscle hypercontraction, spasmodic pharyngeal contraction, paralysis, and death in C. elegans14. These characteristics are common to other anthelmintic drugs that activate ligand-gated ion channels in the nematode. Unlike these receptor agonists, monepantel acts as an allosteric modulator of ACR-23, potentiating betaine signaling during development and resulting in nematode death. A drawback of monepantel is that some of the genera lacking the acr-23 gene include parasitic nematodes23, such as Strongyloides and Ascaris, which represent a major public health concern in developing countries. Unlike acr-23, SNF-3 has an ortholog in most parasitic nematodes species with a sequenced genome. Thus, the betaine-SNF-3 pathway offers a unique set of targets that can be used to improve AAD efficiency, or develop new anthelmintic drugs.
What then is the normal role of betaine in the nematode? Betaine signaling is required in C. elegans for basal levels of locomotion: acr-23 mutants are sluggish when crawling on agar. The effect on locomotion is mediated in part via ACR-23 receptors expressed in the mechanosensory neurons. The mechanosensory neurons innervate the locomotory command neurons to stimulate touch-induced movement as well as spontaneous levels of locomotion37,38. The mechanosensory neuron dendritic processes run adjacent to the epidermis and in some cases are completely embedded in the epidermal cells expressing the betaine clearance transporter SNF-3. We cannot exclude the possibility that betaine is acting as a conventional neurotransmitter, that is, that it is released onto neurons and muscles at synapses. However, it is also possible that betaine is released by the epidermis rather than by neurons. Thus, in this model the skin may both release betaine onto the mechanosensory processes and clear it from the space between the skin and dendrite, acting as both source and sink for this novel neurotransmitter.
Betaine has been shown to have anticonvulsant properties in vertebrate brain5–7, but its mechanisms of action have not been elucidated. While acr-23 is not conserved in the vertebrate, snf-3 and phospholipase Cβ are conserved and expressed in the vertebrate nervous system. The mammalian nervous system contains many uncharacterized ligand-gated ion channels and G-protein coupled receptors. Some of these could potentially mediate betaine anticonvulsive properties.
Methods
Genetic Screens
Enhancer screen
We mutagenized EG4094 egl-8(sa47) V; oxEx771[B0348 egl-8(+); rol-6(sd); Pmyo-2::GFP] with 20nM ENU. B0348 is a cosmid that rescues egl-8, the gene encoding phospholipase-C β. We singled individual F1 and F2 offspring carrying the plcβ rescuing extrachromosomal array, oxEx771[B0348 (egl-8+; rol-6(sd); Pmyo-2::GFP]. We screened F3 offspring for the presence of an enhancer by looking for novel phenotypes in non-GFP expressing siblings (plcβ mutant background), not observed in GFP expressing siblings (plcβ rescued). We screened 1669 haploid genomes and isolated snf-3(ox354) from this screen.
We mapped snf-3(ox354) to the left arm of chromosome II using single nucleotide polymorphism mapping39. The region was further narrowed by rescuing snf-3 using standard microinjection transgenic techniques40. snf-3(ox354) was rescued by injecting snf-3(ox354) egl-8(sa47) animals with a pool of cosmids F45D11, M01D1 and C07D2 along with 2 ng µl-1 Pmyo-2::GFP co-injection marker. Each cosmid was injected at 20 ng µl-1. Cosmid C07D2 alone was sufficient to rescue the snf-3(ox354) enhancer defects. We were able to rescue snf-3(ox354) by injecting 4 overlapping PCR fragments from wild-type genomic DNA of the T13B5.1 gene. To identify the molecular lesion in snf-3(ox354), we sequenced snf-3 PCR fragments generated from snf-3(ox354) egl-8(sa47) double mutants animals.
Suppressor screen
We mutagenized EG7081 snf-3(ox354); egl-8(sa47) with 20 nM ENU. We screened the F2 progeny for non-hypercontracted and non-uncoordinated animals. We screened a total of 37,000 haploid genomes. We isolated acr-23(ox429) from this screen.
To identify the mutation ox429, we resequenced the genome of EG6501 snf-3(ox354) egl-8(sa47) acr-23(ox429) using an Illumina Genome Analyzer II (GAII). The molecular lesion in acr-23(ox429) was confirmed using traditional Sanger sequencing. To rescue acr-23(ox429), we injected snf-3 egl-8 acr-23 triple mutants (EG6501) with either 4 overlapping PCR fragments with a 1kb overlap (25ng µl-1 each) of acr-23 genomic DNA or a full-length acr-23 gene with 2 ng µl-1 Pmyo-2::mCherry co-injection marker. From injections of these two constructs, we obtained over 10 transgenic lines with the restoration of the hypercontracted phenotype observed in simple snf-3 egl-8 double mutants. Most of these transgenic strains were very sick and hard to maintain. We further confirmed that ox429 was an allele of acr-23 by crossing a null allele of acr-23(ok2804) into snf-3 egl-8 double mutants to generate snf-3 egl-8 acr-23(ok2804) (EG6544).
Molecular biology
snf-3
To create a full-length genomic snf-3 DNA plasmid, we excised a 15kb genomic fragment of snf-3 from C07D2 using StuI and restriction digest and ligated it into pLITMUS38 (pADA49). To generate a fluorescently tagged SNF-3, we cut the GFP with MluI from the pPD114.24 vector (Andy Fire), and ligated it into an MluI site in the largest intron in frame with the gene to generate pADA65. The GFP tag is located in the extracellular loop between transmembrane domain 9 and 10. 30ng µl-1 of pADA65 was injected in snf-3(ox354) egl-8(sa47) along with 2 ng µl-1 Pmyo-3::mCherry and Punc-122::GFP co-injection marker. Multiple transgenic lines were obtained and they fully rescued snf-3(ox354) enhancer defects (strain EG4769 carries oxEx1067). We also tagged SNF-3 at the N-terminus by ligating Psnf-3 and the snf-3 coding region into the GFP vector pPD117.01 (Andy Fire) to generate pADA73. 10ng µl-1 of pADA73 was injected into lin-15(n765ts) along with 2 ng µl-1 Pmyo-3::mCherry co-injection marker and lin-15(+)(pL15EK). We generated EG6888 from these injections. To visualize neurons expressing SNF-3::GFP, we subjected EG6888 to RNAi feeding plates against GFP (pPD128.110). For tissue-specific rescue, we used the MultiSite Gateway Pro 3-fragment recombination technology (Invitrogen, Grand Island, NY; catalog no.12537-023). All constructs containing a promoter were inserted into the pDONR™P4-P1 vector (slot 1). These promoters contained the initiating start codon (ATG). snf-3 cDNA, lacking the ATG, was inserted into the pDONR™221 vector (slot 2). The C-terminal tag fluorescent protein (GFP or mCherry) followed by the 3’UTRs of unc-54 or let-858 gene were inserted into pDONR™P2R-P3 vector (slot 3). The final recombination product was inserted into pDEST™R4-R3 vector.
egl-8
To create rescuing constructs for egl-8, we used MultiSite Gateway Technology. egl-8 cDNA without an ATG was inserted into the pDONR™221 vector (slot 2).
acr-23
To create a full-length genomic acr-23 construct, we divided the genomic region into three fragments that were inserted into different Multisite Gateway pDONR vectors. A 2.9kb promoter and the full genomic region except for the last two exons of the gene were inserted into the pDONR™P4-P1 vector. The 4kb intron and exon 8 were inserted into the pDONR™221 vector, and the last exon and the 3’UTR (252bases) were inserted into pDONR™P2R-P3 vector. These three fragments were recombined with pDEST™R4-R3 vector to create pADA246. To create pASP268, we inserted mCherry in operon between the last exon of the gene and the 3’UTR in the pDONR™P2R-P3 vector using Gibson Assembly Cloning technology (New England Biolab, Ipswich, MA). The intergenic region of the gdp-2 gdp-3 was used to express mCherry from an operon. All the constructs used for tissue specific rescues were created using the MultiSite Gateway Technology. An acr-23 cDNA containing an ATG was inserted into the pDONR™221 vector. In this case, the corresponding promoters (Pmyo-3, Prab-3 and Pmec-7) in slot 1 lack ATG.
Confocal microscopy
We acquired images of fluorescently-tagged fusion proteins in living C. elegans with a 63X 1.4NA oil objective on a Pascal LSM5 confocal microscope (Carl Zeiss, Inc).
Body length assay
We selected L4 worms the day before the assay for each genotype studied. On the day of the experiment, we anesthetized adult worms with 15 mM sodium azide in M9 solution on freshly made 2% agarose pads. We used a plan-Neofluar 10x 0.3 NA objective on a Pascal LSM5 confocal microscope. The size of the worm was determined using a build-in function on the LSM5 image browser.
Behavioral assays
Growth assay
We plated 10 L4 larvae on NGM plates 12 hr before we started the experiment. We transferred the adults every 6.5 hours (at room temperature) or overnight 12 hours (at 15 °C). After the animals were transferred, we counted the number of eggs laid. We monitored hatching rate and development rate to adulthood. Adulthood was determined by the presence of a vulva. The percentage of animals was normalized to each plate.
Betaine assay
We top-spreaded NGM plates with betaine to obtain a final concentration of 50mM and 250mM. These plates were allowed to dry at room temperature for 24 hours and then seeded with the E. coli strain OP50. On the day of the experiment, we added 3 L4 worms to each plate. The plates were coded so the experimenter was blind to betaine concentrations and genotype of the strains. The first and second generations progeny were then tested in a thrashing assay. For the snf-3 overexpression line (EG8093), the array oxEx1208 was crossed out from strain EG5051 and crossed twice against N2.
Thrashing assay for locomotion
Single young adult worms were placed in a microtiter well in a 96-well plate without agar, containing 150 µl water. Assays in M9 yielded similar results (not shown). The animal was allowed to acclimate for 2 min and we counted thrashes for 60 sec. One thrash reflects the bending of the body from the midbody toward one side of the animal and back again. The value obtained is doubled to reflect the true number of bends.
Crawling assay for locomotion
We transferred well-fed young adult animals to a food-free NGM plate (100mm). Animals were allowed to acclimate for 5 minutes, then we counted the number of body bends generated by each animal every 20 seconds. Each animal was tested 4 times.
Crawling speed assay
The speed of the worm was determined using an automated worm tracking and analysis system. A single young adult animal was transferred to to a food-free NGM plate (100mm) placed on a motorized microscope stage (OptiScan™ ES111, Prior Scientific, Inc., Rockland, MA). After 1 minute acclimation period, the worm was imaged at 5 frames per second for 5 minutes using a VGA FireWire camera (XCD-V60, Sony, Tokyo, Japan) mounted on a Leica MS5 stereomicroscope (Wetzlar, Germany). A custom MATLAB program41 (The MathWorks, Inc., Natick, MA) was used to control the camera and the motorized stage and to determine the mean locomotion speed. The speed was calculated based on the distance traveled by the worm over 5 minutes.
Statistical Analysis
Statistical analyses were performed using Kaleidagraph 3.6, and GraphPad Prism 6. All grouped data are reported as means ± s.e.m. Statistical significance between genotypes was determined using one-way ANOVA followed by Tukey post-hoc comparisons. We used two-tailed unpaired Student’s t-test with Welch’s correction to determine the difference between wild-type and the gain-of-function (Fig. 4c). Logarithmic normal distribution was assumed for betaine EC50 values, so we use the pEC50 values for statistical analysis. p < 0.05 was considered statistically significant. No statistical methods were used to predetermine sample sizes, but our sample sizes are similar to those reported in previous publications in the field (thrash assay42, speed41, body bends21, and body length43). Data distributions were assumed to be normal, but this was not formally tested. Data collection and analysis were not always performed blind, but we specifically state which experiments were performed blind in the method section. All the data were collected and processed side by side in a randomized manner.
SNF-3 transport assay in HRPE cells
HRPE cells were transfected using a vaccinia virus expressing SNF-3 cDNA as described previously44,45. HRPE cells grown in 24-well plates were infected with a recombinant vaccinia virus (VTF7–3). This virus carries the gene for T7 RNA polymerase in its genome. The virus was allowed to adsorb onto the cells for 30 min at 37°C with gentle shaking of the plate. Cells were then transfected with the plasmid DNA (empty vector pSPORT1 or SNF-3 cDNA construct) using the lipofection procedure (Invitrogen). The cells were incubated at 37 °C for 12 h and then assayed for transport activity. The uptake of [14C]betaine (2.5 µM) was determined at 37 °C. In most experiments, the media was 25 mM Hepes/Tris, pH 7.5, 140 mM NaCl, 5.4 mM KCl, 1.8 mM CaCl2, 0.8 mM MgSO4, and 5 mM glucose. In experiments in which the cation and anion dependence of the transport process was investigated, NaCl was replaced isoosmotically by sodium gluconate or N-methyl-D-glucamine (NMDG) chloride. Uptake measurements were routinely made in parallel in control cells transfected with vector alone and in cells transfected with the vector-cDNA construct. The uptake activity in cDNA-transfected cells was adjusted for the endogenous activity measured in control cells to calculate the cDNA-specific activity. Experiments were performed in triplicate, and each experiment was repeated at least three times. Results are presented as means ± s.e. The kinetic parameters, Michaelis-Menten constant (Km) and maximal velocity (Vmax), were calculated by fitting the data to a Michaelis-Menten equation describing a single saturable transport system. Analysis was done by nonlinear regression, and the resultant values for the kinetic parameters were confirmed by linear regression.
Electrophysiological studies in Xenopus oocytes
SNF-3 analysis
The procedures for cRNA in vitro transcription, Xenopus laevis oocyte isolation, microinjection, superfusion, voltage clamping and data analysis have been described previously45,46. Briefly, the plasmid SNF-3::pSPORT1 was linearized with NotI and transcribed in vitro to cRNA using the mMESSAGE mMACHINE RNA transcription kit (Ambion, Austin, TX). The expression of SNF-3 was initially detected by comparing the uptake of (2.5 µM) [14C]-betaine (55 mCi/mmol, American Radiolabeled Chemicals, St. Louis, MO) in water-injected oocytes versus SNF-3-injected oocytes. The electrophysiological characteristics of the heterologously expressed SNF-3 were then studied using a GeneClamp 500 (Axon Instruments). Kinetic parameters for the saturable transport of SNF-3 were calculated using the Michaelis-Menten equation. Data were analyzed by nonlinear regression and confirmed by linear regression.
ACR-23 analysis
ACR-23 analysis was performed as described previously47. To generate plasmid constructs for Xenopus oocyte expression, we subcloned full-length error-free ACR-23 cDNAs and ACR-23 gain-of-function (ACR-23(I301N)) into the pSGEM expression vector using the SLIC cloning technique48 to produce pADA206 and pADA278. RNAs were prepared using the T7 mMessage mMachine kit (Ambion). Capped ACR-23 (0.1–5 ng total) or ACR-23(I301N) (0.5–2.5 ng total) RNAs were injected into Xenopus oocytes. 1– 4 days post-injection, two-electrode voltage clamp recordings were performed. Voltage was clamped at −60 mV. The standard bath solution was Ringer's: 115 mM NaCl, 2.5 mM KCl, 1.8 mM CaCl2, 10 mM Hepes, pH 7.2 (NaOH). Each oocyte was subjected to 20 s applications of agonist with 2 minutes wash between test applications. Expression of ACR-23(I301N) was very toxic to Xenopus oocytes. We only used healthy oocytes with low channel expression for further analysis of this receptor. Eight oocytes were used to characterize the betaine concentration-response relationship of ACR-23(I301N), but individual oocytes were not tested through the full range of active concentrations. Three oocytes were tested at 0.001– 0.003 mM, 5 were tested at 0.01– 0.3 mM, and all were tested at 1–10 mM. Curves were fit to data from the 5 oocytes with data in the linear response range to perform statistical analysis of EC50 values.
Ion selectivity
For voltage-ramp experiments, CaCl2 was replaced by BaCl2 in the extracellular solutions in order to eliminate possible contributions from the endogenous Ca2+-activated chloride channel. To assay sodium permeability, we substituted 115 mM N-methyl-D-glucamine (NMDG) chloride for NaCl. Thus Na-free solution contains 115 mM NMDG, 2.5 mM KCl, 1.8 mM BaCl2, and 10 mM Hepes, pH 7.2 (HCl). To compare sodium and potassium permeability, NaCl was replaced with equimolar KCl: 115 mM KCl, 2.5 mM NaCl, 1.8 mM BaCl2, 10 mM Hepes, pH 7.2 (KOH). To assay barium permeability, BaCl2 was increased tenfold: 115 mM NaCl, 18 mM BaCl2, 2.5 mM KCl, 10 mM HEPES, pH 7.2 (NaOH). The osmolarity of each solution was adjusted to 300 mOsm with sucrose. Reversal potentials were measured using 2 s voltage ramps (−100 mV to +60 mV) during sustained betaine application. Reversal potential shifts were similar at various time points after initiating betaine perfusion. Ramps at 20 s were analyzed for the potassium substitution experiment because of changing rectification characteristics at later time points, while ramps at 60 s were used for all other analyses. An agar bridge was used to minimize changes in junction potentials when switching solutions. Small residual voltage offsets due to differing ion composition in perfusion buffers were measured relative to standard solution before each voltage ramp recording, and were subtracted from the amplifier command voltages during data analysis. Data analysis was performed using Igor software (WaveMetrix). All chemicals were purchased from Sigma Chemical Co. (St. Louis, MO).
Monepantel
Commercially available Zolvix (25 mg/ml monepantel, Novartis) was diluted in DMSO to make a 10 mM stock solution of monepantel. The stock solution was diluted in water to make a 10 µM working stock in 0.1% DMSO. Final concentrations were achieved by diluting in Ringer’s to make the experimental perfusion solutions. 1 mM betaine was applied for 60 s either alone or in the presence of 0.3 nM monepantel following a 40 s pre-application of monepantel alone. All perfusion buffers were supplemented with 0.1% DMSO. Peak current magnitudes in three 10 s time windows were compared in the presence and absence of monepantel: (1) immediately after applying monepantel alone, (2) immediately after initiating betaine application, and (3) 50 s after sustained betaine application.
NMR spectroscopy
Culture of nematodes
Strain genotypes were blinded to the experimenter. Early embryos were isolated by alkaline hypochlorite treatment and plated after synchronization on NGM plates seeded with HB101 bacteria as a food source at 20 °C. For each biological replicate, ~8000 animals were harvested, washed twice with M9, twice with water and stored at −80 °C until extraction. Eight samples were grown for each strain.
Extraction procedure for metabolites
Metabolites from whole nematodes were extracted using a methanol–chloroform procedure. 600 µl of a methanol–chloroform mix (2:1 v:v) was added to the frozen nematodes. Samples were homogenised using a Polytron and sonicated for 15 min. 200 µl each of chloroform and water were added, the samples centrifuged and the aqueous layer separated from the lipid one. The procedure was repeated twice. The aqueous layer was dried overnight in an evacuated centrifuge.
Analysis of aqueous extracts
The dried extracts were rehydrated in 600 µl D2O, containing 0.05 mM sodium-3-(tri-methylsilyl)-2,2,3,3-tetradeuteriopropionate (TSP) (Cambridge Isotope Laboratories, MA, USA) as an internal standard. The samples were analyzed using an AVANCE II+ NMR spectrometer operating at 500.13 MHz for the 1H frequency (Bruker, Germany) using a 5 mm TXI probe. Spectra were collected using a solvent suppression pulse sequence based on a one-dimensional NOESY pulse sequence to saturate the residual 1H water signal (relaxation delay = 2 s, t1 increment = 3 µs, mixing time = 150 ms, solvent presaturation applied during the relaxation time and the mixing time). 196 transients were collected into 16 K data points over a spectral width of 12 ppm at 27 °C for the adult samples.
Analysis of the metabolic profile of the bacteria
400 µl of the bacteria (HB101) broth used to feed worms was mixed with 600 µl of water and centrifuged. The supernatant was collected and dried in an evacuated centrifuge. The dried samples were rehydrated in 600 µl D2O, containing 0.05 mM sodium-3-(tri-methylsilyl)-2,2,3,3-tetradeuteriopropionate (TSP) (Cambridge Isotope Laboratories, MA, USA) as an internal standard and analyzed by NMR spectroscopy in the same way as the extracts described above. 128 transients were collected for these samples.
Data processing
NMR spectra were processed using an ACD one-dimensional NMR processor (vers. 13, ACD, Toronto, Canada). Free induction decays were Fourier transformed following multiplication by a line broadening of 1 Hz, and referenced to TSP at 0.0 ppm. Spectra were phased and baseline corrected manually. Each spectrum was integrated using 0.02 ppm integral regions between 0.5 and 4.5, and 5.5–9.5 ppm. Each spectral region was normalized to a total integral value of 1000. In addition the resonances of selected metabolites (betaine, choline and glycine) were integrated using the software package Chenomx vs. 7.1. The integrals were normalized to total pellet dry weight.
Multivariate analysis of metabolic profiles
Each set of metabolic profiles obtained were analysed by multivariate analysis. Datasets were imported into SIMCA-P 12.0 (Umetrics, Umeå, Sweden) for processing using PCA and PLS-DA (a regression extension of PCA used for supervised classification). Proton NMR data were Pareto scaled, in which each variable was centered and multiplied by 1/(Sk)1/2 where Sk is the standard deviation of the variable.
Transgenic worms
The plasmids used in this study were generated using standard molecular biology techniques and Gateway technology (Invitrogen). All transgenic strains were generated by standard microinjection techniques40.
Strains
The Bristol N2 strain was used as the wild type; worms were raised at room temperature (22 °C) on NGM plates seeded with OP50 E. coli. All other strains generated for this work are listed in Table 1.
Supplementary Material
Acknowledgements
We thank the following people for their help. Erik M. Peden for comments and input on the manuscript; Jim Rand and Greg Mullen and the Caenorhabditis Genetics Center for providing strains, M. Wayne Davis and Colin Thacker for Illumina sequencing analysis, Andy Fire for providing the GFP expression vectors, Zhao-Wen Wang for the worm tracker, Hsiao-Fen Han, Jann W. Gardner and Dane Maxfield for help with locomotion assays. We also thank Robin Gasser for his generous gift of Zolvix. This work was supported by grants from the National Institutes of Health, AG22468 (YJF), NS034307 (EMJ) and NRSA F32GM084596 (ASP); and the National Science Foundation grant IOS-0920069 (EMJ). EMJ is an investigator of the Howard Hughes Medical Institute.
Footnotes
Author Contributions
A.S.P. designed and performed genetics screens, behavior analyses and wrote the paper. Y.J.F., G.J. and V.G. designed and performed the physiological analyses of the betaine transporter. P.M. performed the physiological analyses of the betaine receptor. J.L.G and C.C. measured betaine concentrations in the worm using NMR spectroscopy. E.A.M. and K.J.M. helped J.L.G with nematode cultures. E.M.J. provided guidance and edited the paper.
Competing financial interests
The authors declare no competing financial interests.
Reference
- 1.Morgan K, Tarjan A. Management of Sting Nematode on Centipede grass with Kelp Extracts. Proceedings of the Florida State Horticultural Society Soc. 1980;93:97–99. [Google Scholar]
- 2.Wu Y, Jenkins T, Blunden G, Whapham CA, Hankins SD. The role of betaines in alkaline extracts of Ascophyllum nodosum in the reduction of Meloidogyne javanica and M. incognita infestations of tomato plants. Fundamental and applied nematology. 1997;20:99–102. [Google Scholar]
- 3.Whapham CA, Jenkins T, Blunden G, Hankins SD. The role of seaweed extracts, Ascophyllum nodosum, in the reduction in fecundity of Meloidogyne javanica. Fundamental and applied nematology. 1994;17:181–183. [Google Scholar]
- 4.Wu Y, Jenkins T, Blunden G, von Mende N, Hankins S. Suppression of fecundity of the root-knot nematode, Meloidogyne javanica, in monoxenic cultures of Arabidopsis thaliana treated with an alkaline extract of Ascophyllum nodosum. Journal of Applied Phycology. 1998;10:91–94. [Google Scholar]
- 5.Ghoz EH, Freed WJ. Effects of betaine on seizures in the rat. Pharmacol. Biochem. Behav. 1985;22:635–640. doi: 10.1016/0091-3057(85)90287-4. [DOI] [PubMed] [Google Scholar]
- 6.Freed WJ, Gillin JC, Wyatt RJ. Anticonvulsant properties of betaine. Epilepsia. 1979;20:209–213. doi: 10.1111/j.1528-1157.1979.tb04797.x. [DOI] [PubMed] [Google Scholar]
- 7.Wuerthele SE, Yasuda RP, Freed WJ, Hoffer BJ. The effect of local application of homocysteine on neuronal activity in the central nervous system of the rat. Life Sci. 1982;31:2683–2691. doi: 10.1016/0024-3205(82)90712-3. [DOI] [PubMed] [Google Scholar]
- 8.Zhu X-M, Ong W-Y. A light and electron microscopic study of betaine/GABA transporter distribution in the monkey cerebral neocortex and hippocampus. J. Neurocytol. 2004;33:233–240. doi: 10.1023/b:neur.0000030698.66675.90. [DOI] [PubMed] [Google Scholar]
- 9.Lewis JA, Wu CH, Berg H, Levine JH. The genetics of levamisole resistance in the nematode Caenorhabditis elegans. Genetics. 1980;95:905–928. doi: 10.1093/genetics/95.4.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lewis JA, Wu CH, Levine JH, Berg H. Levamisole-resistant mutants of the nematode Caenorhabditis elegans appear to lack pharmacological acetylcholine receptors. Neuroscience. 1980;5:967–989. doi: 10.1016/0306-4522(80)90180-3. [DOI] [PubMed] [Google Scholar]
- 11.Dent JA, Davis MW, Avery L. avr-15 encodes a chloride channel subunit that mediates inhibitory glutamatergic neurotransmission and ivermectin sensitivity in Caenorhabditis elegans. EMBO J. 1997;16:5867–5879. doi: 10.1093/emboj/16.19.5867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arena JP, Liu KK, Paress PS, Schaeffer JM, Cully DF. Expression of a glutamate-activated chloride current in Xenopus oocytes injected with Caenorhabditis elegans RNA: evidence for modulation by avermectin. Brain Res. Mol. Brain Res. 1992;15:339–348. doi: 10.1016/0169-328x(92)90127-w. [DOI] [PubMed] [Google Scholar]
- 13.Cully DF, et al. Cloning of an avermectin-sensitive glutamate-gated chloride channel from Caenorhabditis elegans. Nature. 1994;371:707–711. doi: 10.1038/371707a0. [DOI] [PubMed] [Google Scholar]
- 14.Kaminsky R, et al. A new class of anthelmintics effective against drug-resistant nematodes. Nature. 2008;452:176–180. doi: 10.1038/nature06722. [DOI] [PubMed] [Google Scholar]
- 15.Rufener L, Baur R, Kaminsky R, Maser P, Sigel E. Monepantel allosterically activates DEG-3/DES-2 channels of the gastrointestinal nematode Haemonchus contortus. Mol Pharmacol. 2010 doi: 10.1124/mol.110.066498. [DOI] [PubMed] [Google Scholar]
- 16.Lackner MR, Nurrish SJ, Kaplan JM. Facilitation of synaptic transmission by EGL-30 Gqalpha and EGL-8 PLCbeta: DAG binding to UNC-13 is required to stimulate acetylcholine release. Neuron. 1999;24:335–346. doi: 10.1016/s0896-6273(00)80848-x. [DOI] [PubMed] [Google Scholar]
- 17.Miller KG, Emerson MD, Rand JB. Goalpha and diacylglycerol kinase negatively regulate the Gqalpha pathway in C. elegans. Neuron. 1999;24:323–333. doi: 10.1016/s0896-6273(00)80847-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Torres GE, Gainetdinov RR, Caron MG. Plasma membrane monoamine transporters: structure, regulation and function. Nat Rev Neurosci. 2003;4:13–25. doi: 10.1038/nrn1008. [DOI] [PubMed] [Google Scholar]
- 19.Hahn MK, Blakely RD. The Functional Impact of SLC6 Transporter Genetic Variation. Annu. Rev. Pharmacol. Toxicol. 2007;47:401–441. doi: 10.1146/annurev.pharmtox.47.120505.105242. [DOI] [PubMed] [Google Scholar]
- 20.Giros B, Jaber M, Jones SR, Wightman RM, Caron MG. Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature. 1996;379:606–612. doi: 10.1038/379606a0. [DOI] [PubMed] [Google Scholar]
- 21.Sawin ER, Ranganathan R, Horvitz HR. C. elegans Locomotory Rate Is Modulated by the Environment through a Dopaminergic Pathway and by Experience through a Serotonergic Pathway. Neuron. 2000;26:619–631. doi: 10.1016/s0896-6273(00)81199-x. [DOI] [PubMed] [Google Scholar]
- 22.McDonald PW, et al. Vigorous Motor Activity in Caenorhabditis elegans Requires Efficient Clearance of Dopamine Mediated by Synaptic Localization of the Dopamine Transporter DAT-1. J. Neurosci. 2007;27:14216–14227. doi: 10.1523/JNEUROSCI.2992-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rufener L, Keiser J, Kaminsky R, Mäser P, Nilsson D. Phylogenomics of ligand-gated ion channels predicts monepantel effect. PLoS Pathog. 2010;6 doi: 10.1371/journal.ppat.1001091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamauchi A, et al. Cloning of a Na+- and Cl-dependent betaine transporter that is regulated by hypertonicity. Journal of Biological Chemistry. 1992;267:649–652. [PubMed] [Google Scholar]
- 25.Borden LA, Smith KE, Gustafson EL, Branchek TA, Weinshank RL. Cloning and expression of a betaine/GABA transporter from human brain. J. Neurochem. 1995;64:977–984. doi: 10.1046/j.1471-4159.1995.64030977.x. [DOI] [PubMed] [Google Scholar]
- 26.Hoffmann EK, Lambert IH, Pedersen SF. Physiology of cell volume regulation in vertebrates. Physiol. Rev. 2009;89:193–277. doi: 10.1152/physrev.00037.2007. [DOI] [PubMed] [Google Scholar]
- 27.Craig SA. Betaine in human nutrition. The American Journal of Clinical Nutrition. 2004;80:539–549. doi: 10.1093/ajcn/80.3.539. [DOI] [PubMed] [Google Scholar]
- 28.Mullen GP, et al. The Caenorhabditis elegans snf-11 gene encodes a sodium-dependent GABA transporter required for clearance of synaptic GABA. Mol. Biol. Cell. 2006;17:3021–3030. doi: 10.1091/mbc.E06-02-0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reinke SN, Hu X, Sykes BD, Lemire BD. Caenorhabditis elegans diet significantly affects metabolic profile, mitochondrial DNA levels, lifespan and brood size. Mol. Genet. Metab. 2010;100:274–282. doi: 10.1016/j.ymgme.2010.03.013. [DOI] [PubMed] [Google Scholar]
- 30.Bamber BA, Beg AA, Twyman RE, Jorgensen EM. The Caenorhabditis elegans unc-49 Locus Encodes Multiple Subunits of a Heteromultimeric GABA Receptor. J. Neurosci. 1999;19:5348–5359. doi: 10.1523/JNEUROSCI.19-13-05348.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Galzi J-L, et al. Mutations in the channel domain of a neuronal nicotinic receptor convert ion selectivity from cationic to anionic. Nature. 1992;359:500–505. doi: 10.1038/359500a0. [DOI] [PubMed] [Google Scholar]
- 32.Vernino S, Amador M, Luetje CW, Patrick J, Dani JA. Calcium modulation and high calcium permeability of neuronal nicotinic acetylcholine receptors. Neuron. 1992;8:127–134. doi: 10.1016/0896-6273(92)90114-s. [DOI] [PubMed] [Google Scholar]
- 33.Rufener L, et al. acr-23 Encodes a Monepantel-Sensitive Channel in Caenorhabditis elegans. PLoS Pathog. 2013;9:e1003524. doi: 10.1371/journal.ppat.1003524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chalfie M, Sulston J. Developmental genetics of the mechanosensory neurons of Caenorhabditis elegans. Dev. Biol. 1981;82:358–370. doi: 10.1016/0012-1606(81)90459-0. [DOI] [PubMed] [Google Scholar]
- 35.Keramidas A, Moorhouse AJ, Schofield PR, Barry PH. Ligand-gated ion channels: mechanisms underlying ion selectivity. Prog. Biophys. Mol. Biol. 2004;86:161–204. doi: 10.1016/j.pbiomolbio.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 36.Treinin M, Chalfie M. A mutated acetylcholine receptor subunit causes neuronal degeneration in C. elegans. Neuron. 1995;14:871–877. doi: 10.1016/0896-6273(95)90231-7. [DOI] [PubMed] [Google Scholar]
- 37.Riddle DL, Blumenthal T, Meyer BJ, Priess JR. Mechanosensory Control of Locomotion. 1997 . At< http://www.ncbi.nlm.nih.gov/books/NBK20005/>. [Google Scholar]
- 38.Chalfie M, et al. The neural circuit for touch sensitivity in Caenorhabditis elegans. J. Neurosci. 1985;5:956–964. doi: 10.1523/JNEUROSCI.05-04-00956.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Davis MW, et al. Rapid single nucleotide polymorphism mapping in C. elegans. BMC Genomics. 2005;6:118. doi: 10.1186/1471-2164-6-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mello CC, Kramer JM, Stinchcomb D, Ambros V. Efficient gene transfer in C.elegans: Extrachromosomal maintenance and integration of transforming sequences. EMBO Journal. 1991;10:3959–3970. doi: 10.1002/j.1460-2075.1991.tb04966.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen B, et al. α-Catulin CTN-1 is required for BK channel subcellular localization in C. elegans body-wall muscle cells. EMBO J. 2010;29:3184–3195. doi: 10.1038/emboj.2010.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miller KG, et al. A genetic selection for Caenorhabditis elegans synaptic transmission mutants. Proc. Natl. Acad. Sci. U.S.A. 1996;93:12593–12598. doi: 10.1073/pnas.93.22.12593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hammarlund M, Davis WS, Jorgensen EM. Mutations in β-Spectrin Disrupt Axon Outgrowth and Sarcomere Structure. J Cell Biol. 2000;149:931–942. doi: 10.1083/jcb.149.4.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fei YJ, Fujita T, Lapp DF, Ganapathy V, Leibach FH. Two oligopeptide transporters from Caenorhabditis elegans: molecular cloning and functional expression. Biochem J. 1998;332:565–572. doi: 10.1042/bj3320565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jiang G, et al. A Na+/Cl- -coupled GABA transporter, GAT-1, from Caenorhabditis elegans: structural and functional features, specific expression in GABA-ergic neurons, and involvement in muscle function. J. Biol. Chem. 2005;280:2065–2077. doi: 10.1074/jbc.M408470200. [DOI] [PubMed] [Google Scholar]
- 46.Fei Y-J, et al. Expression cloning of a mammalian proton-coupled oligopeptide transporter. Nature. 1994;368:563–566. doi: 10.1038/368563a0. [DOI] [PubMed] [Google Scholar]
- 47.Beg AA, Jorgensen EM. EXP-1 is an excitatory GABA-gated cation channel. Nat. Neurosci. 2003;6:1145–1152. doi: 10.1038/nn1136. [DOI] [PubMed] [Google Scholar]
- 48.Li MZ, Elledge SJ. Harnessing homologous recombination in vitro to generate recombinant DNA via SLIC. Nat. Methods. 2007;4:251–256. doi: 10.1038/nmeth1010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.