Abstract
Previously, it was shown that ethanol dependent animals display increased sensitivity to the general opioid receptor antagonist nalmefene compared to naltrexone. It was hypothesized that the dissociable effects of the two antagonists was attributable to a κ-opioid receptor mechanism. Nucleus accumbens dynorphin is upregulated following chronic ethanol exposure and such neuroadaptations could contribute to nalmefene’s increased potency in ethanol-dependent animals. To test this hypothesis, male Wistar rats were trained to self-administer ethanol using an operant conditioning procedure. Animals were then implanted with bilateral intra-accumbens shell guide cannulae and assigned to either a chronic intermittent ethanol vapor exposure condition (to induce dependence) or an air-exposed control group. Following a one-month exposure period, nalmefene, nor-binaltorphimine (nor-BNI; selective for κ-opioid receptors) or a combination of the selective opioid receptor antagonists CTOP and naltrindole (selective for the μ- and δ-opioid receptors, respectively) were site-specifically infused into the nucleus accumbens shell prior to ethanol self-administration sessions during acute withdrawal. Nalmefene and CTOP / naltrindole dose-dependently reduced ethanol self-administration in nondependent and dependent animals, whereas nor-BNI selectively attenuated ethanol self-administration in ethanol-dependent animals without affecting the self-administration of nondependent animals. Further analysis indentified that intra-accumbens shell nalmefene was more potent in ethanol dependent animals and that the increased potency was attributable to a κ-opioid receptor mechanism. These data support the concept that dysregulation of DYN / κ-opioid receptor systems contributes to the excessive self-administration observed in dependent animals and suggest that pharmacotherapeutics for ethanol dependence that target κ-opioid receptors, in addition to μ- and δ-opioid receptors, are preferable than those that target μ- and δ-opioid receptor mechanisms alone.
1. Introduction
Alcohol use disorders, comprising alcohol abuse and dependence, are a pervasive problem, with rates in the United States for person 18 years of age and older climbing from 7.41% in 1991-1992 to 8.5% in 2004 (Grant et al. 2004). Further, factors related to alcohol consumption have been shown to be the third leading cause of preventable death (Mokdad et al. 2004). Presently there are only three FDA-approved medications for the treatment of alcohol abuse and dependence (Heilig and Egli 2006), none of which target the negative emotional states that accompany acute and protracted withdrawal from alcohol (Heilig and Koob 2007). Clearly, an impetus exists to continue studying and developing effective pharmacotherapies to treat alcohol abuse and dependence, particularly those therapies targeting symptoms not addressed by current pharmacological strategies.
Acute ethanol stimulates the release of the endogenous opioid peptides β-endorphin (END), enkephalin (ENK), and dynorphin (DYN; Gianoulakis et al. 1996; Marinelli et al. 2003; Marinelli et al. 2004; Dai et al. 2005; Marinelli et al. 2005; Marinelli et al. 2006). Nonspecific opioid receptor antagonists can effectively reduce ethanol consumption in humans (Volpicelli et al. 1992; Mason et al. 1994) and reduce ethanol consummatory and self-administration behavior in rats (Gonzales and Weiss 1998; Stromberg et al. 2001; Coonfield et al. 2002; Shoemaker et al. 2002; Walker and Koob 2008; Walker and Ehlers 2009). Subtype-selective antagonists of the μ- and δ-opioid receptor (MOR and DOR, for which the endogenous ligands are END and ENK, respectively) have been shown to reduce ethanol self-administration (Stromberg et al. 1998; Hyytia and Kiianmaa 2001). Antagonists selective for the κ-opioid receptor (KOR, for which DYN is the endogenous ligand) generally show no effect on nondependent ethanol self-administration (Williams and Woods 1998; Doyon et al. 2006; Walker and Koob 2008; Logrip et al. 2008; Walker et al. 2010b), but see Mitchell (2005). Thus, evidence suggests that the MOR and DOR are viable targets to reduce the positive reinforcing effects of ethanol in nondependent cohorts, whereas DYN / KOR systems do not appear to be involved in the positive reinforcing effects of ethanol.
Recent evidence comparing the FDA-approved naltrexone to another opioid receptor antagonist, nalmefene, showed that nalmefene and naltrexone were comparably efficacious for reducing ethanol self-administration in nondependent animals, an effect that was attributed to their similar affinity for the MOR. However, in ethanol-dependent animals, nalmefene was more effective than naltrexone for attenuating ethanol self-administration (Walker and Koob 2008). It was posited that nalmefene’s increased efficacy in dependent animals was due to KOR binding affinity differences between the two compounds. Nalmefene has equipotent binding affinity with naltrexone at the MOR, but unlike naltrexone, has a higher affinity for the KOR and DOR in rats (Michel et al. 1985) and the κ receptor in humans (Bart et al. 2005). Specifically, nalmefene had a two-fold increase in affinity at the KOR in rats compared to naltrexone (Michel et al. 1985) at low doses. Indeed, when nondependent and ethanol-dependent animals were pretreated with a selective KOR antagonist, ethanol-dependent animals showed significant reductions in self-administration, whereas nondependent animals were unaffected (Walker and Koob 2008; Walker et al. 2010b). Thus, KOR antagonists selectively impacted dependent animals which suggested that neuroadaptations in DYN / KOR systems occur during the transition to dependence and could be a viable target for pharmacotherapies to treat ethanol dependence. KORs are morphologically situated in a manner that enables them to oppose the effects of MOR agonists (Di Chiara and Imperato 1988b) and directly inhibit motivationally-relevant mesolimbic dopamine neurons with terminal regions in the nucleus accumbens shell (AcbSh; Di Chiara and Imperato 1988a). Furthermore, chronic ethanol exposure results in neuroadaptations in AcbSh DYN / KOR systems that are reflective of an upregulated state (Przewlocka et al. 1997; Lindholm et al. 2000; Lindholm et al. 2007) and increased DYN / KOR systems signaling produces behaviors indicative of a negative affective state (Todtenkopf et al. 2004; Carlezon et al. 2006).
The present experiment evaluated whether infusion of the opioid receptor antagonist nalmefene into the AcbSh could attenuate ethanol self-administration in ethanol-dependent animals as it has been shown to do previously in nondependent animals (June et al. 2004). Because of evidence suggesting ethanol dependent animals showed increased sensitivity to nalmefene, when compared to naltrexone, for reducing ethanol self-administration, in part, due to a KOR mechanism of action (Walker and Koob 2008) and evidence supporting chronic ethanol-induced neuroadaptations of the DYN / KOR receptor system in the nucleus accumbens (Przewlocka et al. 1997; Lindholm et al. 2000; Lindholm et al. 2007), it was hypothesized that nalmefene would show increased potency in dependent animals. Furthermore, to elucidate whether the effects of intra-AcbSh nalmefene could be linked to particular opioid receptor subtypes, antagonists selective for the MOR, DOR and KOR were infused into the AcbSh of nondependent and ethanol-dependent animals prior to self-administration sessions and compared for their ability to attenuate operant self-administration of ethanol.
2. Materials and Methods
2.1 Animals
Fifty-four adult male Wistar rats (bred from Charles River Laboratory stock) approximately 70 days old at the beginning of the study were communally housed (2-3 per cage) in a temperature (21 ± 2° C) controlled vivarium on a 12-hr reverse light cycle (lights off at 6 am) with ad libitum food and water available. Prior to operant training, animals were handled daily for a one-week period. This work adheres to the National Research Council Guide for the Care and Use of Laboratory Animals (National Research Council (NRC) 1996) and was approved by the Washington State University Institutional Animal Care and Use Committee. All efforts were made to minimize animal suffering, to reduce the number of animals used and to utilize alternatives to in vivo techniques.
2.2 Acquisition of Operant Ethanol Self-Administration
Animals were trained to self-administer a 10% ethanol (w/v) solution in standard operant chambers (Med Associates, St. Albans, VT) with custom drinking wells (Behavioral Pharma, La Jolla, CA) using a sweetener-fade procedure (Samson 1986) that has been described in detail elsewhere (Walker and Koob 2007). In short, acquisition of the operant response (using a fixed-ratio 1 (FR-1) schedule of reinforcement) occurred using a sweetened fluid (0.125% saccharin and 3% glucose) as the reinforcer that is preferred to other sweeteners such as sucrose (Valenstein et al. 1967) without necessitating water or food deprivation. Next, 10% ethanol (w/v) was added to the solution and then over three weeks, the sweetener was removed from the solution with 10% ethanol as the final solution. Once the operant response was acquired and when ethanol was added to the solution, a second lever was introduced that allowed for water self-administration according to a FR-1 schedule of reinforcement. Once responding for ethanol stabilized (defined as three sessions with < 10% deviation), animals were assigned to one of two groups (air- or ethanol vapor-exposed) that were matched based on ethanol self-administration and proceeded to surgical phase.
2.3 Surgical Procedures
Animals were implanted with bilateral guide cannulae (22 gauge) targeting a location 2 mm above the AcbSh (AP +2.2, ML ± 1.0, DV −5.8, from bregma according to (Paxinos and Watson 2005)). All surgeries were conducted under 2% isoflurane gas anesthesia (VetOne, Meridian, ID). The guide cannulae were implanted using standard stereotaxic techniques and were secured in placed with acrylic dental cement (Lang, Wheeling, IL) and four machine screws (0/80 × 1/8; Fastenal, Moscow, ID) that served as an anchor for the acrylic. The open ends of the guide cannulae were sealed by inserting bilateral obdurators (PlasticsOne, Roanoke, VA) to maintain patency and reduce risk of infection. Obdurators were held in place with stainless steel cap nuts (Fastenal, Moscow, ID). Following surgery, post-operative analgesics (flunixin, MWI Veterinary Supply, Meridian, ID) and antibiotics (Baytril, MWI Veterinary Supply, Meridian, ID) were administered daily for 5 days. Following one week of recovery, the animals were transferred to the vapor chamber apparatus for the dependence induction phase of the experiment.
2.4 Intermittent Ethanol Vapor Exposure
Intermittent vapor exposure (14 hours on, 10 hours off) effectively induces a wide range of behaviors indicative of an ethanol dependent-like state such as excessive ethanol self-administration in both fixed- and progressive-ratio schedules of reinforcement (O’Dell et al. 2004; Walker and Koob 2007), increased anxiety-like (Valdez et al. 2002) and depressive-like (Walker et al. 2010a) behaviors. The vapor exposure apparatus (La Jolla Alcohol Research, Inc., La Jolla, CA) allows for blood alcohol levels (BALs) to be titrated according to desired target BALs by manipulating the rate at which 95% ethanol is vaporized and adjusting the air-flow to each cage. Target BALs over the course of the experiment were 175 – 225 mg% and determined twice weekly by collecting the blood from the tail (~0.25 ml). Blood samples were centrifuged at 15000 rpm for 15 min and 5 μl of plasma was assayed using the Analox AM1 (Analox Instruments Ltd., Lunenburg, MA). Calibrations of the Analox AM1 were conducted prior to each set of BAL determinations.
2.5 Drugs
Nalmefene was provided by Lundbeck Research USA and nor-BNI, CTOP and naltrindole were purchased from Tocris Bioscience, Ellisville, MO. All compounds were soluble in aCSF (pH 7.2–7.4 was composed of 145 mM NaCl, 2.8 mM KCl, 1.2 mM MgCl2, 1.2 mM CaCl2, 5.4 mM d-Glucose and 0.25 mM ascorbic acid; Alvarez-Jaimes et al. 2009) at the concentrations listed in section 2.7.
2.6 Post-Vapor Ethanol Self-Administration and Pharmacological Challenges
Following the dependence induction period, air- and vapor-exposed animals were allowed to self-administer (FR-1) ethanol for 30 minutes twice weekly during acute withdrawal (6 hours after the ethanol vapor had terminated on a given day). The twice weekly 30 minute sessions continued until stable responding (as defined in Section 2.2) was achieved.
Once stable, there were three phases of testing: 1) sham infusions 2) artificial cerebrospinal fluid (aCSF) infusions and 3) pharmacological challenges. Sham infusions occurred to habituate the animals to the infusion process and consisted of insertion of bilateral internal cannulae (28GA, Plastics One) for three minutes; with no infusions at that time. After the three minute period, the animal was transferred to the operant testing room and given a five minute waiting period before the self-administration session began, thus infusions of aCSF and pharmacological ligands initiated eight minutes prior to operant self-administration sessions.
Following stable sham responding (in this case defined as less than 10% deviation for 2 self-administration sessions), the same procedure was followed for infusions of aCSF (0.5uL/side over two minutes). The bilateral internal cannulae were left in place for an additional minute, for a total of a 3 min infusion procedure time. The aCSF infusions occurred until stable responding was established (< than 10% deviation over 2 sessions). These critically important sham and aCSF infusions served to establish that no changes in ethanol responding occurred due to the infusion procedures and that any changes in self-administration were due to the pharmacological effects of the ligands under examination.
All pharmacological infusions occurred as described above for the aCSF infusions. After each self-administration session, the animals were returned to the vapor-chambers for their intermittent exposure regimen. Thus, each post-dependence induction self-administration session occurred under identical conditions of acute withdrawal. Aside from the evaluation of nalmefene, selective antagonists for the MOR (D-Pen-Cys-Tyr-D-Trp-Orn-Thr-Pen-Thr-NH2; CTOP), DOR (naltrindole) and KOR (nor-binaltorphimine; nor-BNI) were also evaluated. Because of the apparent dichotomy between the ability of MOR / DOR antagonists and KOR antagonists to regulate nondependent ethanol self-administration (see section 1) and the hypothesis that nalmefene’s enhanced potency in dependent animal is, in part, based on a KOR mechanism of action, the present study compared MOR / DOR antagonist combinations to the effects of a KOR antagonist.
2.7 Intra-Accumbens Pharmacology
Nalmefene (0, 0.125, 1.25 and 12.5 μg / side; n=9 for air-exposed and n=7 for vapor-exposed) and CTOP / naltrindole (0, 62.5/62.5, 125/125 and 250/250 ng / side; n=8 for air-exposed and n=7 for vapor-exposed) dose-response curves were conducted according to a within-subjects Latin square design, in which each animal was exposed to all of the drug doses in a counterbalanced fashion. Although the animals displayed stable responding under aCSF conditions, a vehicle dose was included in the Latin square dose-response curves to control for potential drug carryover effects or potential cellular trauma due to repeated injections. All drug infusions occurred in a volume of 0.5 μl / side over 2 min with aCSF as the vehicle. The nalmefene, CTOP / naltrindole combination and nor-BNI doses were based off of those utilized previously (Hyytia and Kiianmaa 2001; June et al. 2004; Walker and Koob 2008).
One characteristic of nor-BNI does not allow for the use of a within-subject dose-response curve, namely an extended duration of action. In rats, nor-BNI has been shown to produce maximal rightward shifts in the dose-response curves of KOR agonists for up to seven weeks (Picker et al. 1996). In support, under conditions comparable to those used in the present study, nor-BNI continued to attenuate alcohol self-administration in dependent animals for approximately two weeks after nor-BNI treatment ceased (Walker et al. 2011). An additional consideration when utilizing nor-BNI to selectively target KORs is previous nociception-related evidence from mice suggesting that nor-BNI has mild affinity for the MOR immediately after administration that appears to last at least 2 h (Broadbear et al. 1994). It is notable, however, that the transient MOR affinity of nor-BNI that has been observed in mice (e.g., Broadbear et al. 1994) was not replicated in rats (Picker et al. 1996). Indeed, our own studies, in rats, have confirmed that there are no observed differences in the effects of nor-BNI when administered immediately or 24 hours before ethanol self-administration sessions in nondependent and ethanol-dependent rats (Walker and Koob 2008; Walker et al. 2011).
Thus, following stable behavior in the sham and aCSF infusions, all animals were given a one-time infusion of nor-BNI immediately prior to self-administration sessions (4 μg / side; n=9 for air-exposed and n=8 for vapor-exposed). In most situations comparable to those in the present study (within subject design using a single dose), it would be appropriate to use a crossover design. However, given that nor-BNI has such long-term effects, those animals receiving nor-BNI prior to aCSF could be subjected to post-dependence self-administration sessions for extremely extended periods of time before returning to baseline so that a vehicle test could occur. Such temporal differences in the length of vapor exposure and duration of self-administration were considered to be greater confounds when planning the experiment and were avoided by using the current design.
2.8 Histology
Following the completion of the pharmacological challenges, all animals were infused with 0.5 μl / side of 0.6% cresyl violet and euthanized with intraperitoneal sodium pentobarbital injections. The brains were then removed and stored in 4% formalin at 4° C until histology was completed. On the day of histological examination, the brains were frozen to 20° C and mounted on specimen discs to be sliced using a cryostat 1850 (Leica, Bannockburn, IL). 40 μm sections were mounted on slides and the placement of injection sites were evaluated for appropriate intra-AcbSh placement. Only those animals with appropriate placement were included in the data analysis of the present study.
2.9 Statistical Analysis
To confirm that one month of intermittent alcohol vapor exposure successfully induced dependence for the nalmefene, nor-BNI and CTOP / naltrindole cohorts, a mixed-model two-way analysis of variance (ANOVA) was conducted to independently compare the baseline and post-dependence ethanol self-administration sessions for the vapor- and air-exposed animals following pharmacological challenges. The within-subject variable was session (the average of the final three self-administration sessions prior to the dependence induction period compared to the vehicle-treated post-dependence alcohol consumption) and the between-groups variable was level of vapor exposure (air- or vapor-exposure).
The nalmefene, nor-BNI and CTOP / naltrindole dose-response curves for the air- and vapor-exposed animals were analyzed using a mixed model two-way ANOVA with exposure condition as the between-groups factor and dose as the within-subject factor, with post-hoc tests conducted if a significant interaction was found. Subsequently, one-way repeated measure ANOVAs, or paired-sample t-tests for nor-BNI, were individually conducted for the air- and vapor-exposed conditions. For the nalmefene- and CTOP / naltrindole-treated animals, post-hoc least significant difference (LSD) tests were conducted if a significant main effect of dose was established.
To evaluate whether there was a shift in the potency of nalmefene that could be attributed to specific opioid receptor mechanisms of action, the percent reduction of alcohol self-administration in air- compared to vapor-exposed animals were evaluated by two-way ANOVA for the nalmefene and CTOP / naltrindole doses that were determined to be significantly different from vehicle as determined by the one-way ANOVA (see above) and for nor-BNI using an independent-samples t-test.
To address the specificity of the intracranial infusions, those animals with misplaced cannulae that resulted in removal from the statistical analyses were used as negative control animals. The percent reduction from vehicle was calculated for each animal and compared to zero using a one-sample t-test.
3. Results
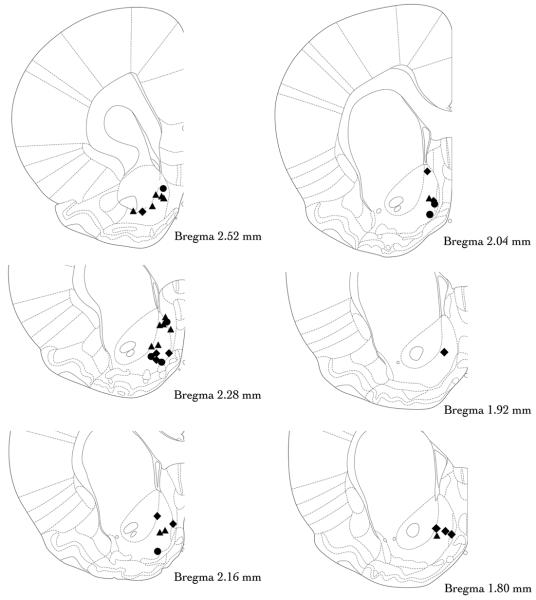
Two animals were removed because the injection sites of the internal cannulae were located outside the AcbSh (see below) with one hemisphere in the nucleus accumbens core and the other hemisphere in between the AcbSh and the midline. Three other animals were lost due to head stages that were damaged / removed and one animal was removed prior to the onset of pharmacological challenges because of an inability to display stable responding. Thus, of the 54 animals that began the study, 48 successfully completed the study, had appropriate cannulae placement (see Fig. 1) and were included in the data analysis.
Figure 1. Histological confirmation of infusion sites targeting the nucleus accumbens shell.
Animals were administered a nalmefene (denoted by filled circles), CTOP / naltrindole (filled diamonds) or nor-BNI (filled triangles) directly into the nucleus accumbens shell prior to ethanol self-administration sessions. Note that for the sake of simplicity, the cannula sites depicted here show placements in only one hemisphere but are meant to reflect the bilateral locations of each cannula tip. Coronal sections from The Rat Brain in Stereotaxic Coordinates (Paxinos and Watson 2005).
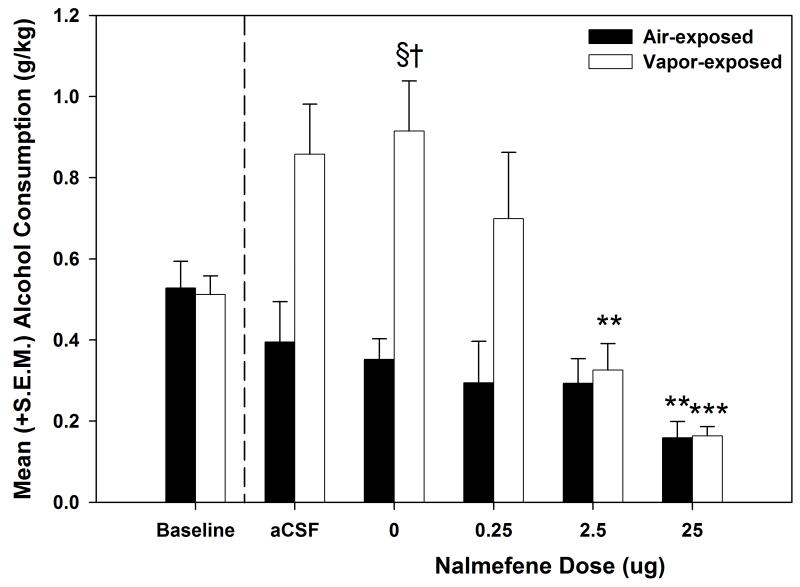
To confirm that successful dependence induction resulted from intermittent vapor exposure for the nalmefene-challenged animals, a mixed model two-way ANOVA compared baseline and vehicle-treated self-administration sessions for the air- and vapor-exposed animals. The results (see Fig. 2) identified a main effect of Exposure (F (1, 14) = 18.315, p < 0.01) and an Exposure × Session interaction (F (1, 14) = 10.876, p < 0.01). Post-hoc analyses indicated that the vapor-exposed animal significantly increased their self-administration following one month of vapor exposure (p < 0.05) and that the vapor-exposed animals had significantly elevated ethanol self-administration levels when compared to the air-exposed animals on the vehicle-treated self-administration session (p < 0.001). The mixed model two-way ANOVA conducted on the data from the nalmefene dose-response curve (see Fig. 2) indicated a main effect of Dose (F (3, 42) = 22.833, p < 0.001), a main effect of Exposure (F (1, 14) = 6.833, p < 0.05) and a Dose × Exposure interaction (F (3, 42) = 10.076, p < 0.001). Post-hoc analyses showed that the ethanol consumption following administration of 0.25 μg nalmefene significantly differed between the air- and vapor-exposed animals (p < 0.05), but that there were no differences between the two conditions from the 2.5 and 25 μg doses (p > 0.05). Individual one-way ANOVAs conducted on the effects of nalmefene for the air- and vapor-exposed animals showed main effects of dose (F (3, 24) = 3.860, p < 0.05 and F (3, 18) = 17.231, p < 0.001, respectively). Post-hoc LSD tests identified that the 25 μg dose of nalmefene significantly (p < 0.01) reduced ethanol self-administration in the air-exposed animals, whereas both the 2.5 and 25 μg doses of nalmefene significantly (p < 0.01) reduced ethanol self-administration in vapor-exposed animals.
Figure 2. Effect of nalmefene on nondependent and ethanol-dependent ethanol self-administration.
Mean (+S.E.M.) ethanol consumption (g/kg) during baseline, and following a one-month dependence induction period (represented by the dashed line), aCSF-treated and nalmefene -treated sessions for air-exposed (n=9) and alcohol vapor-exposed (n=7) male Wistar rats. A nalmefene dose-response curve (0 – 12.5 μg/side in 0.5 μl over 2 min) was conducted in the nucleus accumbens shell and nalmefene was shown to dose-dependently reduce alcohol self-administration in both air- and vapor-exposed animals († = p < 0.05 when compared to the corresponding air-exposed group, § = p < 0.05 when compared to baseline of the same exposure condition, ** = p < 0.01 and *** = p ≤ 0.001 when compared to the vehicle dose of the same exposure condition).
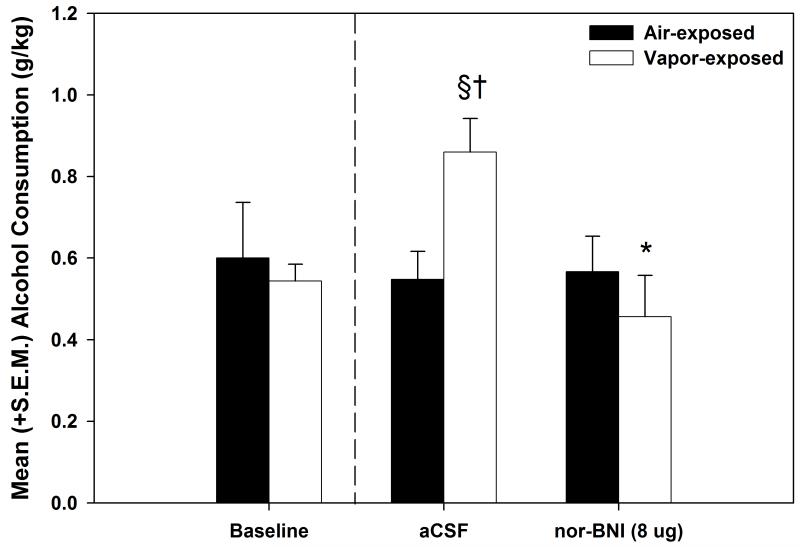
To verify dependence induction for the animals in the nor-BNI component of the experiment (see Fig. 3), a mixed model two-way ANOVA was used to compared baseline and vehicle-treated self-administration sessions for the air- and vapor-exposed animals. The results identified an Exposure × Session interaction (F (1, 15) = 4.882, p < 0.05). Post-hoc analyses showed that the increase in self-administration on the vehicle-treated session was significantly elevated when compared to baseline responding (p < 0.05) and that responding for the vapor-exposed animals was significantly elevated when compared to the air-exposed animals on the vehicle-treated self-administration session (p ≤ 0.01). When evaluating the impact of KOR antagonism in the AcbSh, the mixed model two-way ANOVA conducted on the effects of nor-BNI (see Fig. 3) indicated a main effect of Dose (F (1, 15) = 10.925, p < 0.01) and a Dose × Exposure interaction (F (1, 15) = 13.138, p < 0.01). Post-hoc analyses identified a selective effect of nor-BNI that significantly reduced (p < 0.01) ethanol self-administration in the vapor-exposed animals while leaving the air-exposed ethanol self-administration intact.
Figure 3. Effect of nor-BNI on nondependent and ethanol-dependent ethanol self-administration.
Mean (+S.E.M.) ethanol consumption (g/kg) during baseline, aCSF-treated and pharmacological challenge with nor-BNI for air-exposed (n=9) and alcohol vapor-exposed (n=8) rats. Following a one-month dependence induction period (represented by the dashed line), the animals received bilateral sham and aCSF infusions (0.5 μl/side over 2 min). After confirmation that responding was unaffected by the site-specific control infusions, norBNI (4 μg/side over 2 min) was infused prior to a final self-administration session. Nor-BNI selectively reduced operant self-administration for alcohol in the vapor-exposed dependent animals while leaving nondependent responding intact († = p < 0.05 when compared to the corresponding air-exposed group, § = p < 0.05 when compared to baseline of the same exposure condition, * = p < 0.05 when compared to the vapor-exposed aCSF-treated group).
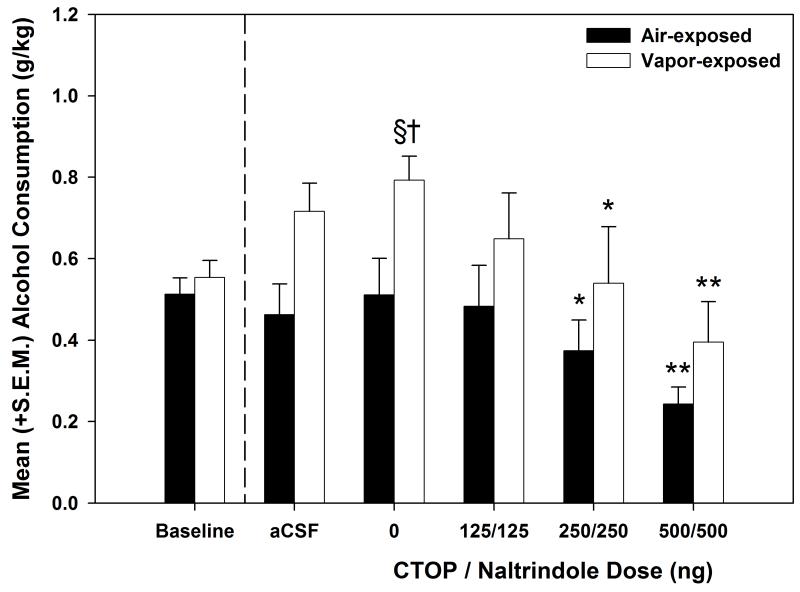
Dependence induction was substantiated in the CTOP / naltrindole cohort (see Fig. 4) using a mixed model two-way ANOVA to compared baseline and vehicle-treated self-administration sessions for the air- and vapor-exposed animals. The results identified a main effect of Exposure (F (1, 13) = 8.773, p < 0.05) an Exposure × Session interaction (F (1, 13) = 7.098, p < 0.05). Post-hoc analyses showed that ethanol self-administration significantly increased from baseline to vehicle-treated sessions (p < 0.05) and that self-administration for the vapor-exposed animals was significantly elevated when compared to the air-exposed animals on the vehicle-treated self-administration session (p ≤ 0.01). The mixed model two-way ANOVA conducted on the CTOP / naltrindole dose-response curve (see Fig. 4) indicated a main effect of Dose (F (3, 39) = 13.937, p < 0.001. Individual one-way ANOVAs conducted on the effects of CTOP / naltrindole for the air- and vapor-exposed animals showed main effects of dose (F (3, 21) = 4.719, p < 0.05 and F (3, 18) = 10.536, p < 0.001, respectively). Post-hoc LSD tests identified that the 250/250 and 500/500 ng dose of the CTOP / naltrindole combination significantly (p < 0.05, 0.01, respectively) reduced ethanol self-administration in the air- and vapor-exposed animals.
Figure 4. Effect of CTOP / naltrindole on nondependent and ethanol-dependent ethanol self-administration.
Mean (+S.E.M.) alcohol consumption (g/kg) during baseline, aCSF-treated and CTOP / naltrindole-treated sessions for air-exposed (n=8) and alcohol vapor-exposed (n=7) rats. The CTOP / naltrindole combination dose-dependently decreased alcohol self-administration for both air-exposed and vapor-exposed animals († = p < 0.05 when compared to the corresponding air-exposed group, § = p < 0.05 when compared to baseline of the same exposure condition, * = p < 0.05 and ** = p ≤ 0.01 when compared to the vehicle dose of the same exposure condition).
The mean (± S.E.M) water consumption (g/kg) following the nalmefene challenge (0.0, 0.25, 2.5 and 25 μg) for the air- and vapor-exposed animals was .62 (± .21) / .46 (± .21), .78 (± .26) / .22 (± .08), .66 (± .22) / .28 (± .11) and .60 (± .2) / .37 (± .14), respectively. For air- and vapor-exposed nor-BNI challenges (0.0 and 8μg) the values were 3.33 (± 1.11) / 4.38 (± 1.55) and 3.67 (± 1.22) / 2.38 (± .84), respectively. Lastly, following CTOP / naltrindole challenges (0, 125/125, 250/250 and 500/500 ng) in air- and vapor-exposed animals, consumption of water (g/kg) was 1.44 (± .51) / .85 (± .32), 1.07 (± .39) / .82 (± .31), 1.05 (± .37) / .70 (± .26) and .97 (± .34) / .98 (± .37), respectively. Two-way ANOVAs were used to evaluate the effects of nalmefene, nor-BNI and the CTOP / naltrindole combination on water consumption (g/kg). None of the compounds significantly altered water consumption.
The percent reduction from vehicle was analyzed for nalmefene and the CTOP / naltrindole combination (see Fig. 5) using a two-way ANOVA. Main effects of dose (F (1, 14) = 4.707, p < 0.05) and exposure (F (1, 14) = 6.711, p < 0.05) were identified for nalmefene, whereas only a main effect of dose (F (1, 13) = 5.011, p < 0.05) was shown for the CTOP / naltrindole combination. The main effect of dose identified that nalmefene and the CTOP / naltrindole combination produced dose-dependent reductions in ethanol self-administration. However, the main effect of exposure confirmed that nalmefene was more potent in dependent animals when compared to nondependent animals, a result that was consistent for both moderate and higher doses. The paired-sample t-test conducted on the percent reduction from vehicle produced by nor-BNI identified a significant difference (t (15) = −3.698, p < 0.01) between the effects of nor-BNI in dependent compared to nondependent animals. Indeed, nor-BNI selectively reduced ethanol self-administration in dependent animals while leaving nondependent self-administration intact.
Figure 5. Percent reduction from vehicle following nonselective and selective opioid receptor antagonism.

Mean (± S.E.M.) percent reduction from vehicle following nalmefene, nor-BNI or the CTOP / naltrindole combination. Nalmefene showed a significant increase in potency in dependent animals (* = p < 0.05 when comparing exposure conditions), nor-BNI showed selective efficacy in the dependent phenotype (** = p < 0.01 when compared to air-exposed animals), whereas the CTOP / naltrindole combination showed no differences between air- and vapor-exposed animals. These data suggest that the increased potency of nalmefene in dependent animals is attributable to a KOR mechanism.
The two animals removed from the experiments following histological examination due to misplaced cannulae were used to represent a negative control group. The data from these animals provided information regarding the specificity of the antagonist infusions. Both cases were vapor-exposed animals; one from the nalmefene experiment and the other from the nor-BNI experiment. The percent reduction from vehicle, for the largest dose of nalmefene and for the single nor-BNI infusion, was calculated to assess the specificity of the infusions. The mean (± S.E.M) percent reduction from vehicle for these animals was 10.63% (± 3.81). A one-sample t-test identified that such a change was not statistically significant (t (1) = 2.79, p > 0.05). Thus, as assessed in this small number of animals, infusions of the tested compounds outside of the AcbSh did not reliably alter the self-administration of ethanol in dependent animals.
4. Discussion
The primary purpose of this study was to evaluate the efficacy of intra-accumbens nalmefene infusion to attenuate ethanol self-administration in dependent rats. A secondary objective was to identify whether alterations in the potency of nalmefene that were observed in ethanol dependent animals could be attributed to a specific opioid receptor mechanism of action. After one month of intermittent vapor exposure and prior to pharmacological challenges, the vapor-exposed groups displayed the excessive ethanol self-administration that is characteristic of a dependence-like phenotype (Roberts et al. 2000; O’Dell et al. 2004; Walker and Koob 2007).
When nalmefene was infused into the AcbSh, a dose-dependent decrease in ethanol self-administration occurred for both the nondependent and ethanol-dependent groups, but water self-administration was unaffected. These data are consistent with previous research (June et al. 1998; June et al. 2004; Walker and Koob 2008) showing that both peripheral and intra-accumbens nalmefene show efficacy for reducing ethanol self-administration in nondependent animals. However, to date, only peripherally administered nalmefene had been tested in dependent animals (Walker and Koob 2008), and thus, these results confirm that blockade of opioid receptors in the AcbSh by nalmefene can attenuate ethanol self-administration in dependent animals.
Like nalmefene, when infused into the AcbSh, the CTOP / naltrindole combination dose-dependently reduced ethanol self-administration in both nondependent and ethanol-dependent animals at dose-ranges that were consistent with data published previously (Hyytia and Kiianmaa 2001) in nondependent Wistar rats. Thus, antagonism of the MOR and/or DOR in the AcbSh can significantly reduce ethanol self-administration in both nondependent and dependent animals. In contrast, a comparison of intra-accumbens shell KOR antagonism with nor-BNI in nondependent and ethanol-dependent animals identified dissociable effects of nor-BNI. Nor-BNI selectively attenuated ethanol self-administration in dependent animals, but did not affect the motivation for ethanol of nondependent animals. This dissociation is consistent with effects of systemic and intracerebroventricular nor-BNI administration (Walker and Koob 2008; Walker et al. 2010b), although this is the first example of this effect following site-specific AcbSh KOR antagonism. One question that has been be posited relates to whether increasing the dosage of nor-BNI might impact nondependent self-administration of ethanol. Because the guiding theoretical basis of this research suggests that nor-BNI is blocking neuroadaptive changes that are specific to dependence (i.e., targeting systems that are recruited during dependence), we would not anticipate any effects in nondependent animals by increasing the dosage. This is supported by the fact that we have not observed nondependent effects of nor-BNI following systemic, intracerebroventricular or site-specific infusions (Walker and Koob 2008; Walker et al. 2011; present results).
When evaluating the dose ranges of nalmefene that reduced self-administration in nondependent compared to dependent animals, a very interesting effect was observed, in that a moderate dose of nalmefene significantly attenuated responding in dependent animals, whereas the highest dose tested was needed to significantly reduce responding in nondependent animals. However, because the post-dependence baseline level of responding was higher in the dependent animals, the fact that a lower dose was needed to significantly impact responding in dependent animals could simply be a function of rate-dependent effects of the antagonist and not be reflective of altered potency. To control for such a possibility, the percent reduction from baseline was calculated for those doses that showed significant differences in at least one of the exposure conditions and compared to determine whether there was support for the concept of altered potency. Indeed, the percent reduction by nalmefene in dependent animals was significantly elevated compared to nondependent animals and thus, nalmefene was shown to be more potent in ethanol-dependent animals.
Assessment of the percent reduction in self-administration by intra-AcbSh infusion of CTOP / naltrindole or nor-BNI showed that the CTOP / naltrindole combination reduced responding in a comparable manner regardless of whether the animals were nondependent or ethanol-dependent, whereas nor-BNI selectively reduced self-administration in dependent animals. From this, it could be inferred that nalmefene’s increased potency in dependent animals is linked to a KOR mechanism as opposed to MOR / DOR mechanism. This interpretation is not stating that MOR / DOR antagonism is ineffective in dependent animals, as the MOR / DOR antagonists were able to dose-dependently reduce self-administration. Instead, the KOR mechanism is identified as an additional pharmacotherapeutic target with a theoretically distinct basis that putatively involves negative reinforcement mechanisms rather than positive reinforcement mechanisms as the MOR / DOR does. Indeed, it was the KOR mechanism that was hypothesized to have distinguished nalmefene from naltrexone in reducing ethanol self-administration in dependent animals, whereas both compounds showed comparable efficacy in nondependent animals that was proposed to be reflective of their comparable affinity for the MOR and ability to block the positive reinforcing effects of ethanol (Walker and Koob 2008). Thus, pharmacotherapeutics that incorporate the KOR as a target, in addition to the MOR and DOR, appear to be preferable than those focusing on the MOR and DOR alone.
Certain properties of the ligands used in these experiments would be prudent to include in the discussion. The first relates to the classification of nalmefene as an antagonist at the KOR. Hormonal and intracellular signaling data established with nalmefene have led one group to suggest that nalmefene could be a partial agonist at the KOR (Bart et al. 2005). In regards to the results of the present study, if nalmefene was determined to be a partial agonist at the KOR, based o the fact that DYN levels have been shown to be upregulated following chronic ethanol exposure, then nalmefene could still serve as a functional antagonist at the KOR due to competition with endogenous DYN and, if bound, would produce less of an effect than DYN itself. Thus, the net effect would be decreased signaling via the KOR.
The second relates to the appropriate pretreatment times when using nor-BNI and focuses on the specificity of nor-BNI for the KOR immediately following administration. Evidence from mice tested in nociceptive assays has suggested that nor-BNI has mild affinity for the MOR immediately after administration that appears to last at least 2 h (Broadbear et al. 1994). Because of this, some researchers posit the use of extended pretreatment durations (e.g., 24-hrs). However, the transient MOR affinity of nor-BNI that has been observed in mice (e.g., Broadbear et al. 1994) was not replicated using rats (Picker et al. 1996). Furthermore, our own studies, in rats, have confirmed that there are no observed differences in the effects of nor-BNI when administered immediately or 24 hours prior to ethanol self-administration sessions in nondependent and ethanol-dependent rats (Walker and Koob 2008; Walker et al. 2011). Most importantly, if nor-BNI has MOR affinity that is functionally relevant when assessing motivational circuitry and behaviors, then one would predict that nondependent ethanol self-administration should be impacted if nor-BNI treatment occurred immediately prior to self-administration sessions. However, as observed in our previous study using a central route of administration (Walker and Koob 2008), infusions of nor-BNI immediately prior to self-administration sessions did not impact nondependent ethanol self-administration. This effect was replicated in the present study following AcbSh infusions of nor-BNI and additionally supported by the fact that selectively targeting the MOR / DOR did reduce nondependent self-administration of ethanol. Thus, under the conditions used in the present study, there were no behavioral indications of an initial MOR mechanism of action for nor-BNI when administered immediately prior to testing.
The importance of DYN / KOR systems in ethanol abuse and dependence is becoming increasingly apparent. However, the precise nature of the changes that occur during the transition to an ethanol dependent state remain to be established. As determined by the results of the present study, antagonism of KORs in the AcbSh functionally impacts ethanol-dependent animals exclusively. The AcbSh is one terminal region of the mesolimbic DA system which has been implicated as a signaling system for biologically relevant information through which natural reinforcers (e.g., see Hull et al. 1999; Carelli 2002; Kelley et al. 2002; Kelley et al. 2005) and drugs of abuse (e.g., see Koob 2000; Maldonado 2003; Di Chiara et al. 2004; Ikegami and Duvauchelle 2004) exert their behavioral effects. Dysregulation of mesolimbic DA system functioning has been posited as a basis for disorders of affect, such as depression (Nestler and Carlezon 2006) and self-medication of disorders of affect has been hypothesized to be a major contributor to excessive alcohol and drug use and relapse (Markou et al. 1998). Although considerable work has focused on KOR modulation of DA transmission, it must be noted that KORs within the AcbSh have been shown to modulate other neurotransmitters than DA alone (Fields et al. 2007; Land et al. 2009) and therefore, the specific neurotransmitter system(s) impacted by an upregulated DYN system remain to be established.
KOR stimulation produces dysphoria, place aversions (Mucha and Herz 1985; Pfeiffer et al. 1986; Land et al. 2008) and a phenotype indicative of a depressive-like state (e.g., see Mague et al. 2003; Todtenkopf et al. 2004). Chronic ethanol exposure results in increased prodynorphin mRNA levels in the nucleus accumbens (Acb; Przewlocka et al. 1997) and increased expression of DYN B in the Acb (Lindholm et al. 2000). In an elegant study measuring dopamine release within the Acb of naive and chronic ethanol-treated animals (Lindholm et al. 2007), both naive and ethanol-treated animals had comparable reductions in dopamine release following KOR agonist treatment, however, the chronic ethanol-treated animals showed increased dopamine release following administration of a selective KOR antagonist. Collectively, the prior evidence and the current results are consistent with the concept of increased signaling through the KOR in ethanol dependence during acute withdrawal that putatively produces a negative affective state which results in excessive ethanol self-administration. It follows that by blocking KORs in the AcbSh, as was done in the present study, the need to ‘self-medicate’ was attenuated and the ethanol-dependent animals displayed a behavioral phenotype that was consistent with nondependent animals rather than dependent animals.
5. Conclusions
Intermittent ethanol vapor exposure produced excessive ethanol self-administration during acute withdrawal in dependent animals, an effect that was ameliorated by AcbSh infusions of nalmefene, CTOP / naltrindole and nor-BNI. Intra-AcbSh nalmefene was shown to be more potent in ethanol-dependent animals, an effect that was attributed to a KOR mechanism of action. These results highlight nalmefene as an effective pharmacotherapy for both nondependent and ethanol-dependent populations and the DYN / KOR as a pharmacotherapeutic target for the treatment of alcoholism.
Acknowledgements
Support for this research was provided, in part, by R01AA020394-01 from the National Institute on Alcohol Abuse and Alcoholism, Lundbeck Research USA, Inc and the WSU Alcohol and Drug Abuse Research Program according to the State of Washington Initiative Measure No. 171. The authors would like to thank Jessica Bilimoria, Allison Brown, Tricia Christensen and Hunter Hoglen for their technical assistance.
References
- Alvarez-Jaimes L, Stouffer DG, Parsons LH. Chronic ethanol treatment potentiates ethanol-induced increases in interstitial nucleus accumbens endocannabinoid levels in rats. J Neurochem. 2009;111:37–48. doi: 10.1111/j.1471-4159.2009.06301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bart G, Schluger JH, Borg L, Ho A, Bidlack JM, Kreek MJ. Nalmefene induced elevation in serum prolactin in normal human volunteers: partial kappa opioid agonist activity? Neuropsychopharmacology. 2005;30:2254–2262. doi: 10.1038/sj.npp.1300811. [DOI] [PubMed] [Google Scholar]
- Broadbear JH, Negus SS, Butelman ER, de Costa BR, Woods JH. Differential effects of systemically administered nor-binaltorphimine (nor-BNI) on kappa-opioid agonists in the mouse writhing assay. Psychopharmacology (Berl) 1994;115:311–319. doi: 10.1007/BF02245071. [DOI] [PubMed] [Google Scholar]
- Carelli RM. Nucleus accumbens cell firing during goal-directed behaviors for cocaine vs. ‘natural’ reinforcement. Physiol Behav. 2002;76:379–387. doi: 10.1016/s0031-9384(02)00760-6. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Beguin C, DiNieri JA, Baumann MH, Richards MR, Todtenkopf MS, et al. Depressive-like effects of the kappa-opioid receptor agonist salvinorin A on behavior and neurochemistry in rats. J Pharmacol Exp Ther. 2006;316:440–447. doi: 10.1124/jpet.105.092304. [DOI] [PubMed] [Google Scholar]
- Coonfield DL, Hill KG, Kaczmarek HJ, Ferraro FM, III, Kiefer SW. Low doses of naltrexone reduce palatability and consumption of ethanol in outbred rats. Alcohol. 2002;26:43–47. doi: 10.1016/s0741-8329(01)00180-x. [DOI] [PubMed] [Google Scholar]
- Dai X, Thavundayil J, Gianoulakis C. Differences in the peripheral levels of beta-endorphin in response to alcohol and stress as a function of alcohol dependence and family history of alcoholism. Alcohol Clin Exp Res. 2005;29:1965–1975. doi: 10.1097/01.alc.0000187599.17786.4a. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Bassareo V, Fenu S, De Luca MA, Spina L, Cadoni C, et al. Dopamine and drug addiction: the nucleus accumbens shell connection. Neuropharmacology. 2004;47(Suppl 1):227–241. doi: 10.1016/j.neuropharm.2004.06.032. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci U S A. 1988a;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Opposite effects of mu and kappa opiate agonists on dopamine release in the nucleus accumbens and in the dorsal caudate of freely moving rats. J Pharmacol Exp Ther. 1988b;244:1067–1080. [PubMed] [Google Scholar]
- Doyon WM, Howard EC, Shippenberg TS, Gonzales RA. Kappa-opioid receptor modulation of accumbal dopamine concentration during operant ethanol self-administration. Neuropharmacology. 2006;51:487–496. doi: 10.1016/j.neuropharm.2006.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields HL, Hjelmstad GO, Margolis EB, Nicola SM. Ventral tegmental area neurons in learned appetitive behavior and positive reinforcement. Annu Rev Neurosci. 2007;30:289–316. doi: 10.1146/annurev.neuro.30.051606.094341. [DOI] [PubMed] [Google Scholar]
- Gianoulakis C, Krishnan B, Thavundayil J. Enhanced sensitivity of pituitary beta-endorphin to ethanol in subjects at high risk of alcoholism. Arch Gen Psychiatry. 1996;53:250–257. doi: 10.1001/archpsyc.1996.01830030072011. [DOI] [PubMed] [Google Scholar]
- Gonzales RA, Weiss F. Suppression of ethanol-reinforced behavior by naltrexone is associated with attenuation of the ethanol-induced increase in dialysate dopamine levels in the nucleus accumbens. J Neurosci. 1998;18:10663–10671. doi: 10.1523/JNEUROSCI.18-24-10663.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Dawson DA, Stinson FS, Chou SP, Dufour MC, Pickering RP. The 12-month prevalence and trends in DSM-IV alcohol abuse and dependence: United States, 1991-1992 and 2001-2002. Drug Alcohol Depend. 2004;74:223–234. doi: 10.1016/j.drugalcdep.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Heilig M, Egli M. Pharmacological treatment of alcohol dependence: target symptoms and target mechanisms. Pharmacol Ther. 2006;111:855–876. doi: 10.1016/j.pharmthera.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Heilig M, Koob GF. A key role for corticotropin-releasing factor in alcohol dependence. Trends Neurosci. 2007;30:399–406. doi: 10.1016/j.tins.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull EM, Lorrain DS, Du J, Matuszewich L, Lumley LA, Putnam SK, et al. Hormone-neurotransmitter interactions in the control of sexual behavior. Behav Brain Res. 1999;105:105–116. doi: 10.1016/s0166-4328(99)00086-8. [DOI] [PubMed] [Google Scholar]
- Hyytia P, Kiianmaa K. Suppression of ethanol responding by centrally administered CTOP and naltrindole in AA and Wistar rats. Alcohol Clin Exp Res. 2001;25:25–33. doi: 10.1111/j.1530-0277.2001.tb02123.x. [DOI] [PubMed] [Google Scholar]
- Ikegami A, Duvauchelle CL. Dopamine mechanisms and cocaine reward. Int Rev Neurobiol. 2004;62:45–94. doi: 10.1016/S0074-7742(04)62002-2. [DOI] [PubMed] [Google Scholar]
- June HL, Cummings R, Eiler WJ, Foster KL, McKay PF, Seyoum R, et al. Central opioid receptors differentially regulate the nalmefene-induced suppression of ethanol- and saccharin-reinforced behaviors in alcohol-preferring (P) rats. Neuropsychopharmacology. 2004;29:285–299. doi: 10.1038/sj.npp.1300338. [DOI] [PubMed] [Google Scholar]
- June HL, Grey C, Warren-Reese C, Durr LF, Ricks-Cord A, Johnson A, et al. The opioid receptor antagonist nalmefene reduces responding maintained by ethanol presentation: preclinical studies in ethanol-preferring and outbred Wistar rats. Alcohol Clin Exp Res. 1998;22:2174–2185. [PubMed] [Google Scholar]
- Kelley AE, Bakshi VP, Haber SN, Steininger TL, Will MJ, Zhang M. Opioid modulation of taste hedonics within the ventral striatum. Physiol Behav. 2002;76:365–377. doi: 10.1016/s0031-9384(02)00751-5. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Baldo BA, Pratt WE, Will MJ. Corticostriatal-hypothalamic circuitry and food motivation: integration of energy, action and reward. Physiol Behav. 2005;86:773–795. doi: 10.1016/j.physbeh.2005.08.066. [DOI] [PubMed] [Google Scholar]
- Koob GF. Neurobiology of addiction. Toward the development of new therapies. Ann N Y Acad Sci. 2000;909:170–185. doi: 10.1111/j.1749-6632.2000.tb06682.x. [DOI] [PubMed] [Google Scholar]
- Land BB, Bruchas MR, Lemos JC, Xu M, Melief EJ, Chavkin C. The dysphoric component of stress is encoded by activation of the dynorphin kappa-opioid system. J Neurosci. 2008;28:407–414. doi: 10.1523/JNEUROSCI.4458-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land BB, Bruchas MR, Schattauer S, Giardino WJ, Aita M, Messinger D, et al. Activation of the kappa opioid receptor in the dorsal raphe nucleus mediates the aversive effects of stress and reinstates drug seeking. Proc Natl Acad Sci U S A. 2009;106:19168–19173. doi: 10.1073/pnas.0910705106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindholm S, Ploj K, Franck J, Nylander I. Repeated ethanol administration induces short- and long-term changes in enkephalin and dynorphin tissue concentrations in rat brain. Alcohol. 2000;22:165–171. doi: 10.1016/s0741-8329(00)00118-x. [DOI] [PubMed] [Google Scholar]
- Lindholm S, Rosin A, Dahlin I, Georgieva J, Franck J. Ethanol alters the effect of kappa receptor ligands on dopamine release in the nucleus accumbens. Physiol Behav. 2007;92:167–171. doi: 10.1016/j.physbeh.2007.05.039. [DOI] [PubMed] [Google Scholar]
- Logrip ML, Janak PH, Ron D. Dynorphin is a downstream effector of striatal BDNF regulation of ethanol intake. FASEB J. 2008;22:2393–2404. doi: 10.1096/fj.07-099135. [DOI] [PubMed] [Google Scholar]
- Mague SD, Pliakas AM, Todtenkopf MS, Tomasiewicz HC, Zhang Y, Stevens WC, Jr., et al. Antidepressant-like effects of kappa-opioid receptor antagonists in the forced swim test in rats. J Pharmacol Exp Ther. 2003;305:323–330. doi: 10.1124/jpet.102.046433. [DOI] [PubMed] [Google Scholar]
- Maldonado R. The neurobiology of addiction. J Neural Transm Suppl. 2003:1–14. doi: 10.1007/978-3-7091-0541-2_1. [DOI] [PubMed] [Google Scholar]
- Marinelli PW, Bai L, Quirion R, Gianoulakis C. A microdialysis profile of Met-enkephalin release in the rat nucleus accumbens following alcohol administration. Alcohol Clin Exp Res. 2005;29:1821–1828. doi: 10.1097/01.alc.0000183008.62955.2e. [DOI] [PubMed] [Google Scholar]
- Marinelli PW, Lam M, Bai L, Quirion R, Gianoulakis C. A microdialysis profile of dynorphin A(1-8) release in the rat nucleus accumbens following alcohol administration. Alcohol Clin Exp Res. 2006;30:982–990. doi: 10.1111/j.1530-0277.2006.00112.x. [DOI] [PubMed] [Google Scholar]
- Marinelli PW, Quirion R, Gianoulakis C. A microdialysis profile of beta-endorphin and catecholamines in the rat nucleus accumbens following alcohol administration. Psychopharmacology (Berl) 2003;169:60–67. doi: 10.1007/s00213-003-1490-2. [DOI] [PubMed] [Google Scholar]
- Marinelli PW, Quirion R, Gianoulakis C. An in vivo profile of beta-endorphin release in the arcuate nucleus and nucleus accumbens following exposure to stress or alcohol. Neuroscience. 2004;127:777–784. doi: 10.1016/j.neuroscience.2004.05.047. [DOI] [PubMed] [Google Scholar]
- Markou A, Kosten TR, Koob GF. Neurobiological similarities in depression and drug dependence: a self-medication hypothesis. Neuropsychopharmacology. 1998;18:135–174. doi: 10.1016/S0893-133X(97)00113-9. [DOI] [PubMed] [Google Scholar]
- Mason BJ, Ritvo EC, Morgan RO, Salvato FR, Goldberg G, Welch B, et al. A double-blind, placebo-controlled pilot study to evaluate the efficacy and safety of oral nalmefene HCl for alcohol dependence. Alcohol Clin Exp Res. 1994;18:1162–1167. doi: 10.1111/j.1530-0277.1994.tb00098.x. [DOI] [PubMed] [Google Scholar]
- Michel ME, Bolger G, Weissman BA. Binding of a new opiate antagonist, nalmefene, to rat brain membranes. Methods Find Exp Clin Pharmacol. 1985;7:175–177. [PubMed] [Google Scholar]
- Mitchell JM, Liang MT, Fields HL. A single injection of the kappa opioid antagonist norbinaltorphimine increases ethanol consumption in rats. Psychopharmacology (Berl) 2005;182:384–392. doi: 10.1007/s00213-005-0067-7. [DOI] [PubMed] [Google Scholar]
- Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual causes of death in the United States, 2000. JAMA. 2004;291:1238–1245. doi: 10.1001/jama.291.10.1238. [DOI] [PubMed] [Google Scholar]
- Mucha RF, Herz A. Motivational properties of kappa and mu opioid receptor agonists studied with place and taste preference conditioning. Psychopharmacology (Berl) 1985;86:274–280. doi: 10.1007/BF00432213. [DOI] [PubMed] [Google Scholar]
- National Research Council (NRC) Guide for the Care and Use of Laboratory Animals. National Academy Press; Washington, D.C.: 1996. [Google Scholar]
- Nestler EJ, Carlezon WA. The mesolimbic dopamine reward circuit in depression. Biol Psychiatry. 2006;59:1151–1159. doi: 10.1016/j.biopsych.2005.09.018. [DOI] [PubMed] [Google Scholar]
- O’Dell LE, Roberts AJ, Smith RT, Koob GF. Enhanced alcohol self-administration after intermittent versus continuous alcohol vapor exposure. Alcohol Clin Exp Res. 2004;28:1676–1682. doi: 10.1097/01.alc.0000145781.11923.4e. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Elsevier, Academic Press; San Diego: 2005. [Google Scholar]
- Pfeiffer A, Brantl V, Herz A, Emrich HM. Psychotomimesis mediated by kappa opiate receptors. Science. 1986;233:774–776. doi: 10.1126/science.3016896. [DOI] [PubMed] [Google Scholar]
- Picker MJ, Mathewson C, Allen RM. Opioids and rate of positively reinforced behavior: III. Antagonism by the long-lasting kappa antagonist norbinaltorphimine. Behav Pharmacol. 1996;7:495–504. [PubMed] [Google Scholar]
- Przewlocka B, Turchan J, Lason W, Przewlocki R. Ethanol withdrawal enhances the prodynorphin system activity in the rat nucleus accumbens. Neurosci Lett. 1997;238:13–16. doi: 10.1016/s0304-3940(97)00829-x. [DOI] [PubMed] [Google Scholar]
- Roberts AJ, Heyser CJ, Cole M, Griffin P, Koob GF. Excessive ethanol drinking following a history of dependence: animal model of allostasis. Neuropsychopharmacology. 2000;22:581–594. doi: 10.1016/S0893-133X(99)00167-0. [DOI] [PubMed] [Google Scholar]
- Samson HH. Initiation of ethanol reinforcement using a sucrose-substitution procedure in food- and water-sated rats. Alcohol Clin Exp Res. 1986;10:436–442. doi: 10.1111/j.1530-0277.1986.tb05120.x. [DOI] [PubMed] [Google Scholar]
- Shoemaker WJ, Vavrousek-Jakuba E, Arons CD, Kwok FC. The acquisition and maintenance of voluntary ethanol drinking in the rat: effects of dopaminergic lesions and naloxone. Behav Brain Res. 2002;137:139–148. doi: 10.1016/s0166-4328(02)00290-5. [DOI] [PubMed] [Google Scholar]
- Stromberg MF, Casale M, Volpicelli L, Volpicelli JR, O’Brien CP. A comparison of the effects of the opioid antagonists naltrexone, naltrindole, and beta-funaltrexamine on ethanol consumption in the rat. Alcohol. 1998;15:281–289. doi: 10.1016/s0741-8329(97)00131-6. [DOI] [PubMed] [Google Scholar]
- Stromberg MF, Mackler SA, Volpicelli JR, O’Brien CP. Effect of acamprosate and naltrexone, alone or in combination, on ethanol consumption. Alcohol. 2001;23:109–116. doi: 10.1016/s0741-8329(00)00137-3. [DOI] [PubMed] [Google Scholar]
- Todtenkopf MS, Marcus JF, Portoghese PS, Carlezon WA., Jr. Effects of kappa-opioid receptor ligands on intracranial self-stimulation in rats. Psychopharmacology (Berl) 2004;172:463–470. doi: 10.1007/s00213-003-1680-y. [DOI] [PubMed] [Google Scholar]
- Valdez GR, Roberts AJ, Chan K, Davis H, Brennan M, Zorrilla EP, et al. Increased ethanol self-administration and anxiety-like behavior during acute ethanol withdrawal and protracted abstinence: regulation by corticotropin-releasing factor. Alcohol Clin Exp Res. 2002;26:1494–1501. doi: 10.1097/01.ALC.0000033120.51856.F0. [DOI] [PubMed] [Google Scholar]
- Valenstein ES, Cox VC, Kakolewski JW. Polydipsia elicited by the synergistic action of a saccharin and glucose solution. Science. 1967;157:552–554. doi: 10.1126/science.157.3788.552. [DOI] [PubMed] [Google Scholar]
- Volpicelli JR, Alterman AI, Hayashida M, O’Brien CP. Naltrexone in the treatment of alcohol dependence. Arch Gen Psychiatry. 1992;49:876–880. doi: 10.1001/archpsyc.1992.01820110040006. [DOI] [PubMed] [Google Scholar]
- Walker BM, Drimmer DA, Walker JL, Liu T, Mathe AA, Ehlers CL. Effects of prolonged ethanol vapor exposure on forced swim behavior, and neuropeptide Y and corticotropin-releasing factor levels in rat brains. Alcohol. 2010a;44:487–493. doi: 10.1016/j.alcohol.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker BM, Ehlers CL. Appetitive motivational experience during adolescence results in enhanced alcohol consumption during adulthood. Behav Neurosci. 2009;123:926–935. doi: 10.1037/a0016002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker BM, Koob GF. The gamma-aminobutyric acid-B receptor agonist baclofen attenuates responding for ethanol in ethanol-dependent rats. Alcohol Clin Exp Res. 2007;31:11–18. doi: 10.1111/j.1530-0277.2006.00259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker BM, Koob GF. Pharmacological Evidence for a Motivational Role of kappa-Opioid Systems in Ethanol Dependence. Neuropsychopharmacology. 2008;33:643–652. doi: 10.1038/sj.npp.1301438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker BM, Zorrilla EP, Koob GF. Systemic kappa-opioid receptor antagonism by nor-binaltorphimine reduces dependence-induced excessive alcohol self-administration in rats. Addict Biol. 2010b doi: 10.1111/j.1369-1600.2010.00226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker BM, Zorrilla EP, Koob GF. Systemic kappa-opioid receptor antagonism by nor-binaltorphimine reduces dependence-induced excessive alcohol self-administration in rats. Addict Biol. 2011;16:116–119. doi: 10.1111/j.1369-1600.2010.00226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams KL, Woods JH. Oral ethanol-reinforced responding in rhesus monkeys: effects of opioid antagonists selective for the mu-, kappa-, or delta-receptor. Alcohol Clin Exp Res. 1998;22:1634–1639. doi: 10.1111/j.1530-0277.1998.tb03960.x. [DOI] [PubMed] [Google Scholar]