Abstract
Clearance of apoptotic cells by macrophages and other phagocytic cells, called efferocytosis, is a central process in the resolution of inflammation. Although the receptor for advanced glycation end products (RAGE) has been shown to participate in a variety of acute and chronic inflammatory processes in the lungs and other organs, a role for RAGE in efferocytosis has not been reported. In the present studies, we examined the potential involvement of RAGE in efferocytosis. Macrophages from transgenic RAGE−/− mice showed a decreased ability to engulf apoptotic neutrophils and thymocytes. Pretreatment of RAGE+/+ macrophages with advanced glycation end products, which competitively bind to RAGE, or Abs against RAGE diminished phagocytosis of apoptotic cells. Overexpression of RAGE in human embryonic kidney 293 cells resulted in an increased ability to engulf apoptotic cells. Furthermore, we found that incubation with soluble RAGE enhances phagocytosis of apoptotic cells by both RAGE+/+ and RAGE−/− macrophages. Direct binding of RAGE to phosphatidylserine (PS), an “eat me” signal highly expressed on apoptotic cells, was shown by using solid-phase ELISA. The ability of RAGE to bind to PS on apoptotic cells was confirmed in an adhesion assay. Decreased uptake of apoptotic neutrophils by macrophages was found under in vivo conditions in the lungs and peritoneal cavity of RAGE−/− mice. These results demonstrate a novel role for RAGE in which it is able to enhance efferocytosis through binding to PS on apoptotic cells.
Introduction
Clearance of apoptotic cells, a process known as efferocytosis, is a major mechanism involved in the resolution of inflammation (1). Dying cells, when they are not eliminated through engulfment by phagocytes, progress to necrosis with associated release of intracellular contents that enhance inflammation and damage to surrounding tissue. Phagocytosis of apoptotic cells not only reduces inflammation through cellular clearance but also induces macrophages to produce anti-inflammatory mediators including IL-10 (2). Alterations in efferocytosis, associated with diminished clearance of apoptotic cells, are associated with chronic and acute inflammatory diseases such as rheumatoid arthritis and acute lung injury (1, 3).
The receptor for advanced glycation end products (RAGE) is expressed at low levels on multiple cell populations, including macrophages, and is present in relatively high concentrations in specific anatomic locations such as on pulmonary epithelial cells (4). Upregulated expression of RAGE in the lungs and other organs accompanies tissue injury. Interaction of RAGE with ligands, including advanced glycation end products (AGEs) or high mobility group box 1 (HMGB1), appears to participate in potentiating inflammation associated with sepsis, liver ischemia, or diabetes (5). In addition to its role in activating macrophages, neutrophils, and other cell populations involved in inflammation, HMGB1 also can contribute to enhancing the severity of inflammatory injury through binding to phosphatidylserine (PS) on the surface of apoptotic cells and diminishing their clearance by macrophages (6). In recent studies, we found that the C-terminal domain of HMGB1, a motif required for binding to RAGE, was required to inhibit ingestion of apoptotic cells by macrophages (7). Such findings suggest that RAGE may participate in efferocytosis.
In the present experiments, we examined the potential role of RAGE in the recognition and clearance of apoptotic cells. We found that RAGE is directly involved in enhancing the ingestion of apoptotic cells by macrophages and other phagocytic cells and participates in efferocytosis through binding to PS. These results delineate a novel mechanism through which RAGE may participate in inflammatory processes.
Materials and Methods
Mice
Male C57BL/6 mice (wild-type [WT]) were purchased from the National Cancer Institute-Frederick. Transgenic RAGE−/− mice were a gift from A. Bierhaus (University of Heidelberg, Heidelberg, Germany). Mice were housed and studied at the University of Alabama (Birmingham, AL) using Institutional Animal Care and Use Committee-approved protocols. Experiments were performed using 8- to 10-wk-old mice.
Reagents
Custom mixture Abs and negative selection columns for neutrophil isolation were from StemCell Technologies. Penicillin-streptomycin and Brewer thioglycollate were from Sigma-Aldrich. Goat and rabbit anti-RAGE Abs (sc-8230 and sc-5563) and IgG were from Santa Cruz Biotechnology. AGE-modified BSA was from BioVision. Recombinant chimeric mouse RAGE-Fc was from R&D Systems. Recombinant murine His-tagged RAGE was a gift from A. Bierhaus. PS, phosphatidylcholine (PC), and phosphatidylethanolamine (PE) were from Avanti Polar Lipids. Annexin V was from BD. Annexin V binding buffer was from Novagen. PKH26 was from Sigma-Aldrich. CD16/CD32 Ab was from eBioscience. The IL-10 ELISA kit was from R&D Systems. PKH26 was from Sigma-Aldrich. FITC-conjugated anti-CD11b and allophycocyanin-conjugated anti-CD 90.2 Abs were from BD. The Chromeo 488 Ab labeling kit was from Active Motif.
Isolation and induction of apoptosis in neutrophils
Mouse neutrophils were purified from bone marrow cell suspensions essentially as described previously (8). In brief, bone marrow cells were incubated with 20 µl primary Ab mixture specific to the cell surface markers F4/80, CD4, CD45R, CD5, and TER119 for 15 min at 4°C. Anti-biotin tetrameric Ab complexes (100 µl) were then added to the cells and incubated for 15 min at 4°C, followed by incubation with 60 µl colloidal magnetic dextran iron particles for 15 min at 4°C. The entire cell suspension was then placed into a column surrounded by a magnet. The T cells, B cells, RBC, monocytes, and macrophages were captured in the column, allowing the neutrophils to pass through as a result of negative selection. The cells were then pelleted and washed. Neutrophil purity, as determined by Wright–Giemsa-stained cytospin preparations, was >97%. Cell viability, as determined by trypan blue exclusion, was consistently >98%. Apoptosis was induced by heating 6 × 106 cells/ml serum-free RPMI 1640 medium at 43°C for 60 min and followed by incubation at 37°C in 5% CO2 for 150 min. With this method, >70% of the neutrophils were apoptotic (early and late apoptotic) as demonstrated by annexin V and propidium iodide staining.
Induction of apoptosis in thymocytes
To induce apoptosis, murine thymocytes were resuspended in RPMI 1640 medium containing 5% FBS and 1 µM dexamethasone at a concentration of 6 × 106 cells/ml and incubated at 37°C in 5% CO2 for 12 h. At this time point, >90% of the thymocytes were apoptotic as demonstrated by annexin V and propidium iodide staining.
Isolation and preparation of mouse peritoneal macrophages
Peritoneal macrophages were elicited from 8- to 10-wk-old mice by i.p. injection of 1 ml 3% thioglycollate. Cells were harvested 4 d later by peritoneal lavage. Macrophages (0.5 × 106) were plated on coverslips in 24-well plates in RPMI 1640 medium containing 5% FBS (Atlanta Biologicals). After 1 h at 37°C, nonadherent cells were removed by washing with medium. Macrophages were cultured in RPMI 1640 medium containing 5% FBS at 37°C and maintained under the same conditions with changes of media every 3 d. One hour before the phagocytosis assay, the macrophages were washed three times with fresh serum-free medium.
Isolation and preparation of bone marrow-derived macrophages
Bone marrow cells were obtained from 8- to 10-wk-old mice. Cells were cultured on petri dishes in DMEM–F-12 medium containing 15% FBS and 25 ng/ml GM-CSF. After 5 d, nonadherent cells were removed by washing, and fresh medium was added. Cells were allowed to grow for 5 more days and then trypsinized and plated on coverslips for phagocytosis assays.
In vitro efferocytosis assays
Phagocytosis of apoptotic neutrophils or apoptotic thymocytes by macrophages (efferocytosis) was determined by adding 3 × 106 apoptotic neutrophils or 1 × 106 apoptotic thymocytes suspended in 600 µl RPMI 1640 medium to each well of a 24-well plate containing macrophage monolayers on coverslips, followed by incubation at 37°C for 120 min. FBS was included at a final concentration of 5% during the incubation of macrophages with apoptotic cells. Noningested cells were removed by washing three times with ice-cold PBS. Cells on coverslips were fixed in 100% methanol and then stained using the Wright–Giemsa method. Phagocytosis was evaluated by a blinded observer by counting at least 300 macrophages per slide from duplicate experiments. The phagocytosis index was calculated as the ratio of macrophages containing at least one ingested apoptotic cell.
Solid-phase ELISA
The solid-phase ELISA for recombinant chimeric RAGE binding to phospholipids was carried out as described previously (9). Phospholipid solutions of PS, PE, and PC at a concentration of 3 µg/ml in methanol were added to 96-well plates and air-dried at room temperature. The wells were treated with 10 mg/ml BSA in PBS. Recombinant chimeric RAGE was added to the wells and incubated at room temperature for 2 h. Recombinant chimeric RAGE that bound to the wells was quantified by ELISA with anti-RAGE Abs (sc-8230) and a peroxidase-conjugated secondary Ab. Washing with PBS containing 0.05% Tween 20 was performed between each step.
To assess competition between RAGE and annexin V for binding to PS, a previously described method was modified as follows: phospholipid solutions of PS, PE, and PC at a concentration of 3 µg/ml in methanol were added to 96-well plates and air-dried at room temperature. The wells were then washed with PBS containing 0.05% Tween 20 and treated with increasing doses of BSA or annexin V (0, 1.25, 2.5, 5, or 10 µg/ml). The assay was then performed as described above.
Cell adhesion assay
To determine the adhesion of apoptotic cells to recombinant RAGE, 96-well culture plates were coated overnight with 5 µg/ml recombinant RAGE or BSA and washed three times with PBS. Fluorescent-labeled apoptotic thymocytes were pretreated with BSA or annexin V (5 µg/ml) in annexin V-specific buffer for 30 min and then resuspended in PBS, and 105 cells were added to each well. After 30 min of incubation at room temperature, each well was washed three times with PBS. Fluorescence intensity was determined by a plate reader at an excitation wavelength of 488 nm.
In vivo efferocytosis assay
The in vivo efferocytosis assay was performed as previously described (10) to determine the effect of RAGE on the phagocytosis of apoptotic neutrophils by alveolar macrophages. Briefly, mice were anesthetized with isofluorane, and then, 10 × 106 apoptotic neutrophils in 50 µl sterile PBS were injected intratracheally in WT or RAGE knockout mice. Two hours later, the mice were sacrificed, and bronchoalveolar lavage (BAL) was performed by using 1 ml sterile PBS containing 5 mM EDTA. Cytospin slides were prepared using 500 µl BAL. The slides were fixed and stained using the Wright–Giemsa method, and the phagocytosis index was determined.
In vivo efferocytosis in the peritoneal cavity was determined as described previously (11). Briefly, thymocytes were labeled with PKH-26 red fluorescent dye, and apoptosis was induced. Mice were anesthetized with isoflurane, and 10 × 106 apoptotic thymocytes resuspended in 100 µl PBS were injected i.p. Two hours later, the mice were sacrificed, and peritoneal lavage was performed by using 3 ml sterile PBS. Samples were resuspended in PBS containing 1% albumin, FITC-conjugated CD11b (macrophage marker) Ab, and APC-conjugated CD 90.2 (thymocyte marker) Ab. Flow cytometry was performed. The phagocytic index was calculated as the ratio of FITC+PKH26+APC− cells to all cells gated. Engulfed thymocytes are not accessible to the APC-conjugated CD 90.2 Ab. Therefore, FITC+PKH26+APC− cells are macrophages that have engulfed PKH-labeled thymocytes, whereas the APC+PKH+FITC+ cells were macrophages to which thymocytes are adherent but not engulfed.
Construction of expression plasmids and recombinant protein expression in human embryonic kidney 293T cells
Full-length human RAGE cDNA was purchased from Open Biosystems. Full-length human RAGE with C-terminal FLAG fusion construct was generated by PCR using the following primers: 5′-GCTCTAGAATGGCAGCCGGAACAGCAGTTGG-3′ (forward) and 5′-CCCTCGAGTCACTTGTCATCGTCATCCTTGTAATCAGGCCCTCCAGTACTACT CTC-3′ (reverse). The PCR product was digested with XhoI and XbaI enzyme and cloned into the XbaI–XhoI site of an FG12 vector. Human embryonic kidney (HEK) cells (HEK 293) were maintained in RPMI 1640 medium (Sigma-Aldrich) containing 10% FBS and penicillin/streptomycin solution (1/10; Sigma-Aldrich). Lipofectamine 2000 was used to transfect the FG12 constructs in HEK 293T cells.
Soluble RAGE binding to thymocytes in vitro
Viable and apoptotic thymocytes were washed three times, resuspended in PBS, and incubated with Chromeo 488-labeled BSA (6 µg/ml) or Chromeo 488-labeled soluble RAGE (sRAGE) at increasing concentrations (10, 25, 50, and 100 nM) for 3 h on ice. After incubation, the thymocytes were washed and analyzed by flow cytometry.
Effect of efferocytosis on cytokine production
To study the effect of efferocytosis on LPS-induced cytokine production, phagocytosis was performed using apoptotic thymocytes and RAGE+/+ or RAGE−/− macrophages. The macrophages were then thoroughly washed and cultured in medium containing LPS (10 ng/ml) for 16 h. IL-10 concentrations in the medium were determined by ELISA according to the manufacturer’s instructions.
Statistical analysis
Data are presented as means ± SD for each experimental group. One-way ANOVA followed by analysis with the Student-Newman-Keuls test was performed for comparisons among multiple groups, and the Student t test was used for comparisons between two groups. A p value < 0.05 was considered significant.
Results
RAGE-deficient macrophages demonstrate decreased ability to engulf apoptotic cells
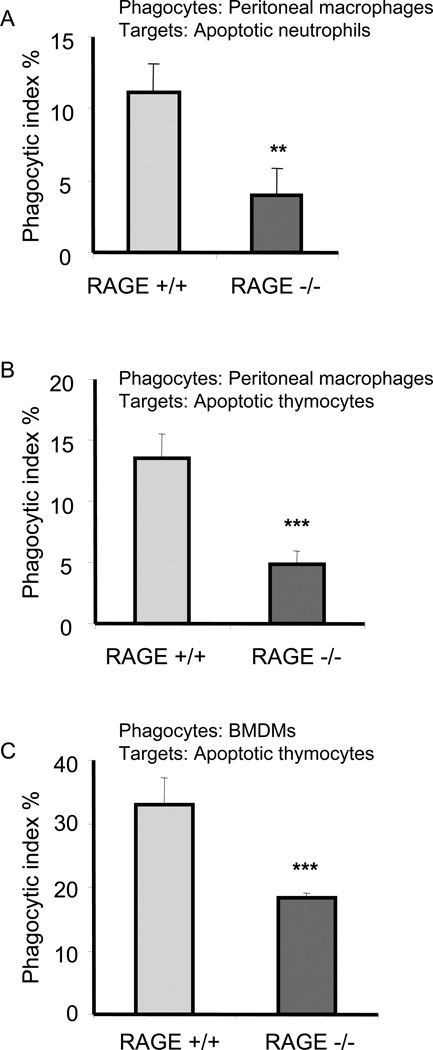
To examine the role of RAGE in efferocytosis, phagocytosis assays were performed by incubating peritoneal macrophages isolated from RAGE+/+ or RAGE−/− mice with apoptotic RAGE+/+ neutrophils. As shown in Fig. 1A, the ability of RAGE−/− macrophages to ingest apoptotic neutrophils was significantly decreased as compared with that found when apoptotic neutrophils were incubated with RAGE+/+ macrophages. To determine whether the participation of RAGE in efferocytosis was specific for apoptotic neutrophils or was generalizable to additional populations of apoptotic cells, apoptotic thymocytes were used as target cells. As shown in Fig. 1B, RAGE−/− macrophages demonstrated a diminished ability to engulf apoptotic thymocytes as compared with RAGE+/+ macrophages.
Figure 1. RAGE deficient macrophages demonstrate decreased ability to engulf apoptotic cells.
Peritoneal macrophages (A and B) or bone marrow derived macrophages (BMDMs) (C) isolated from wild type (RAGE+/+) or RAGE−/− mice were incubated with apoptotic neutrophils (A) or apoptotic thymocytes (B and C) for 120 or 60 min. Phagocytosis assays were performed as described in Materials and Methods. ** p<0.01 *** p<0.001 compared to the RAGE+/+ group.
To further characterize the role of RAGE in efferocytosis and to determine whether the ability of RAGE to enhance uptake of apoptotic cells was specific to peritoneal macrophages, we examined the ability of RAGE−/− bone marrow-derived macrophages (BMDMs) to phagocytose apoptotic cells. As shown in Fig. 1C, RAGE−/− BMDMs, like RAGE−/− peritoneal macrophages, demonstrated a decreased ability to engulf apoptotic thymocytes.
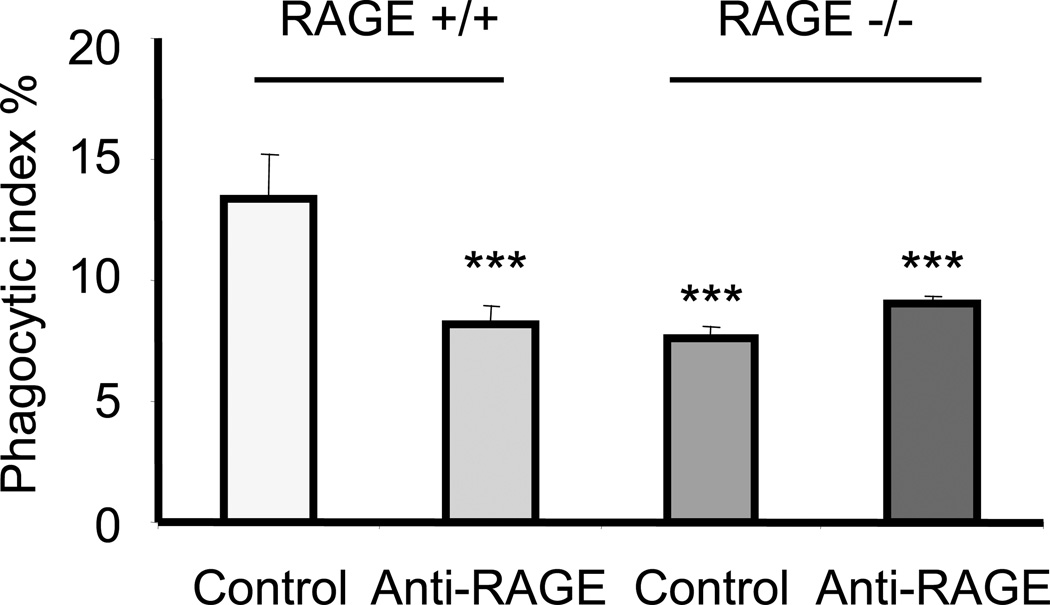
The above experiments showed that RAGE−/− macrophages have a decreased ability to engulf apoptotic cells. To determine whether such a phenomenon is a direct effect of RAGE, rather than a secondary event occurring in RAGE-deficient cells, we treated RAGE+/+ macrophages with anti-RAGE Abs and then examined their ability to engulf apoptotic cells. As shown in Fig. 2, preincubation of RAGE+/+ macrophages with anti-RAGE Abs decreased their ability to phagocytize apoptotic cells. Furthermore, we found that treatment of RAGE−/− macrophages with anti-RAGE Abs did not affect their ability to engulf apoptotic cells, indicating that the inhibitory effects of anti-RAGE Abs were specific for their interactions with RAGE.
Figure 2. Anti-RAGE antibody diminishes efferocytosis by macrophages.
RAGE+/+ or RAGE−/− macrophages were pretreated with anti-CD16/CD32 antibodies (3µg/ml) for 30 minutes to block Fc receptor on the cell surface. The cells were then washed three times with serum free medium and incubated with rabbit IgG or anti-RAGE antibodies (10µg/ml) for 1h. Cells were again washed with fresh medium and incubated with 3 × 106 apoptotic neutrophils (pre-treated with 3 µg/ml anti-CD16/CD32 antibodies for 1h). Phagocytosis assays were performed as described in Materials and Methods. *** p<0.001 compared to the phagocytic index in RAGE+/+ macrophages treated with IgG.
Expression of exogenous RAGE enhances efferocytosis
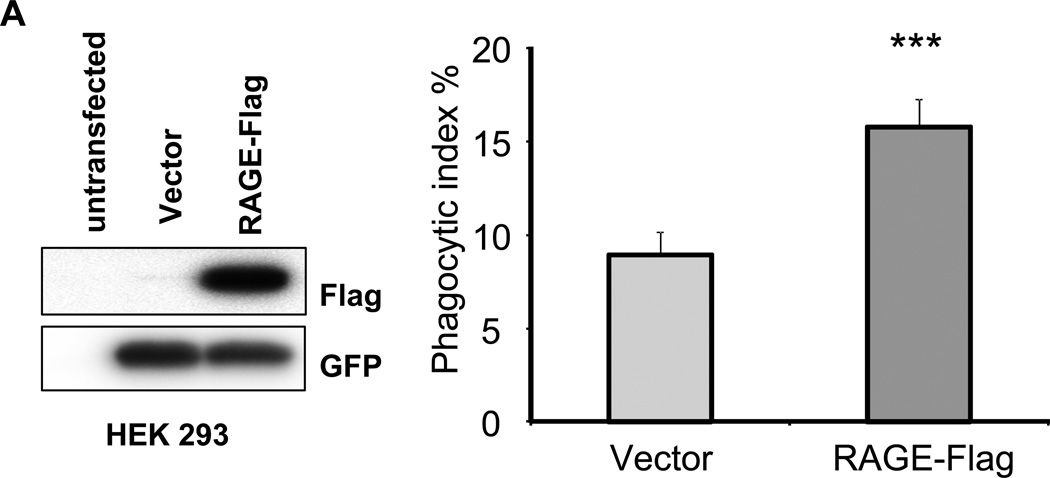
In our initial experiments (Figs. 1, 2), we showed that the absence of RAGE or blocking of RAGE by binding to AGE or anti-RAGE Abs is associated with decreased efferocytosis by macrophages. To confirm the participation of RAGE in efferocytosis, we transfected RAGE-expressing vectors into HEK 293 cells. As shown in Fig. 3A, RAGE was expressed in cells transfected with the RAGE vector but not in cells transfected with control vector. RAGE-expressing HEK 293 cells had significantly greater ability to engulf apoptotic thymocytes than did HEK 293 cells transfected with a control vector (Fig. 3B). These results directly demonstrate that expression of RAGE enhances efferocytosis.
Figure 3. Exogenously expressed RAGE in HEK 293 cells promotes efferocytosis.
(A) HEK 293 cells were transiently transfected with control vectors or RAGE expressing vectors. At 24 hours after transfection, the HEK 293 cells were collected and Western blotting performed to determine the expression of exogenous RAGE using anti-Flag antibodies. (B) HEK 293 cells were transiently transfected with control vectors or RAGE expressing vectors. At 24 hours after transfection, the HEK 293 cells were incubated with apoptotic thymocytes for 120 minutes. Phagocytosis assays were performed. Phagocytic indices were calculated as described in Materials and Methods. *** p<0.001 compared to control vector transfected cells.
AGEs inhibit efferocytosis
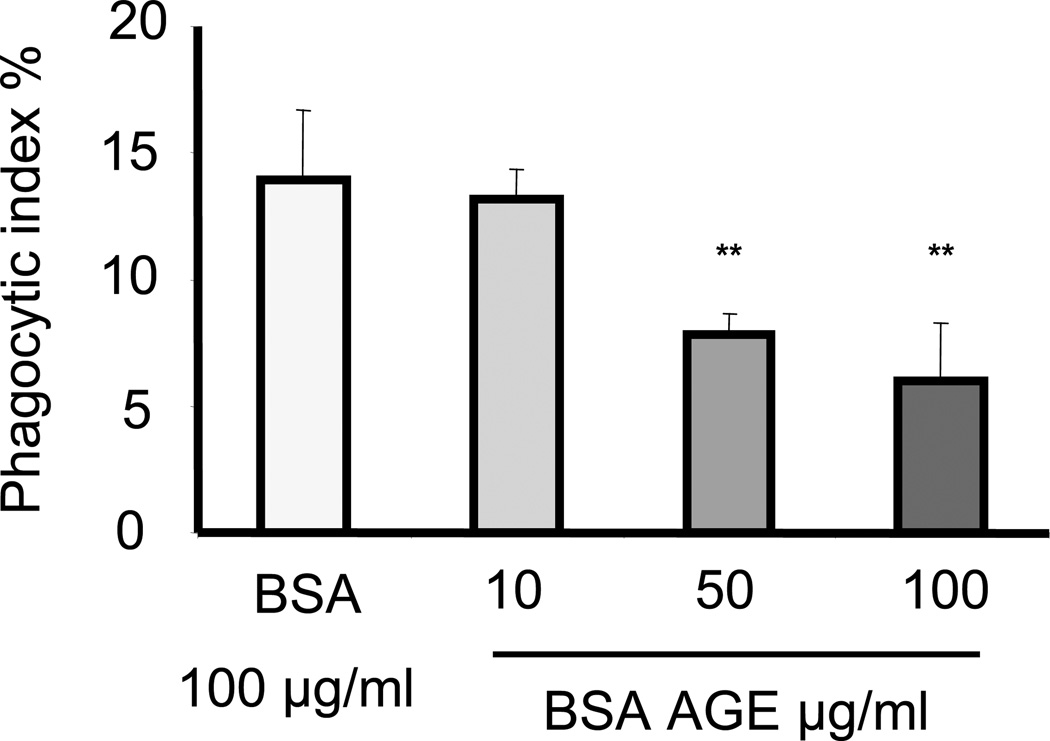
AGEs are direct ligands of RAGE (12). Because of the ability of AGEs to bind to RAGE, we hypothesized that incubation of macrophages with AGEs would reduce the ability of RAGE to interact with ligands on apoptotic target cells and would diminish RAGE-associated potentiation of efferocytosis. To examine this hypothesis, increasing concentrations of advanced glycation end-product–modified BSA (BSA-AGE) were added to cultures of peritoneal macrophages and apoptotic neutrophils during phagocytosis. As shown in Fig. 4, BSA-AGE dose-dependently inhibited the ability of macrophages to phagocytose apoptotic neutrophils.
Figure 4. BSA-AGE diminishes efferocytosis by macrophages.
Peritoneal macrophages from RAGE+/+ mice were incubated with apoptotic neutrophils in medium containing BSA or AGE modified BSA (BSA-AGE) at increasing concentrations (10, 50, or 100 µg/ml) for 120 minutes. Phagocytosis assays were then performed as described in Materials and Methods. ** p<0.01 compared to macrophages incubated with unmodified BSA.
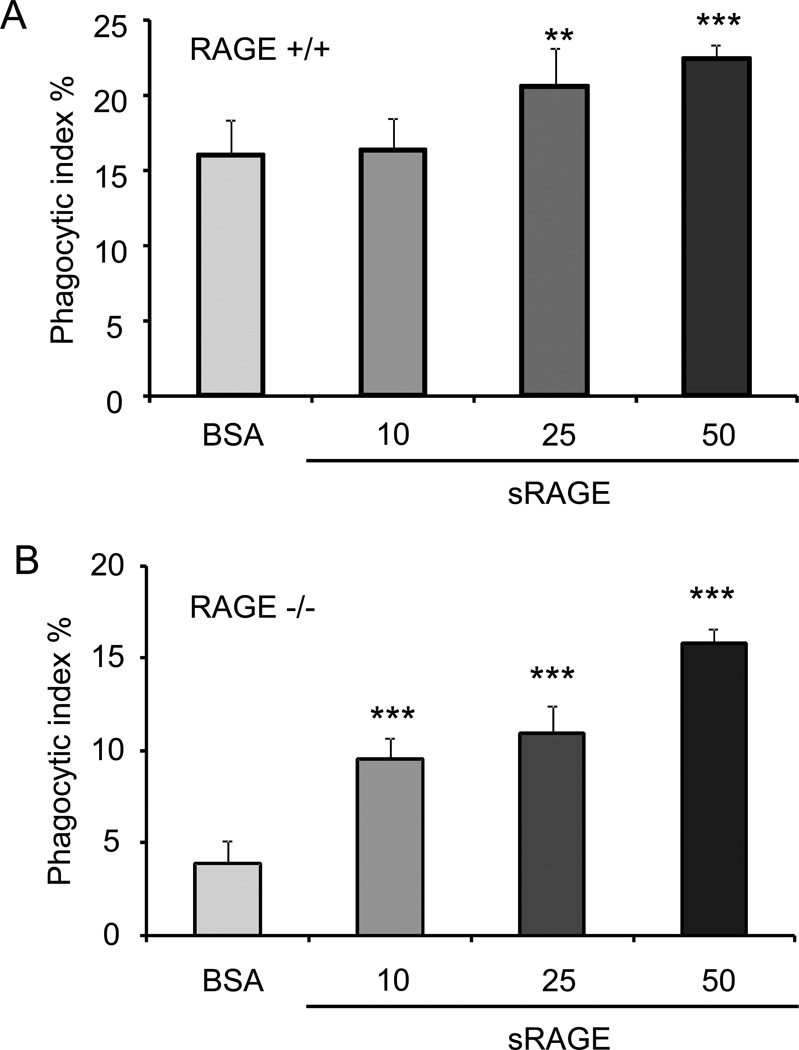
sRAGE enhances efferocytosis
sRAGE is produced by cleavage of the extracellular domain of RAGE or from splicing variants of the RAGE transcript (13, 14). sRAGE has been shown to block RAGE activities by serving as a decoy to competitively bind to RAGE ligands (15–18). Given these findings, we hypothesized that sRAGE might inhibit efferocytosis through interaction with binding partners for RAGE that are present on the apoptotic target cells. However, we found that incubation of RAGE+/+ macrophages with sRAGE enhanced phagocytosis of apoptotic cells (Fig. 5A). These data indicate that sRAGE does not block RAGE-mediated efferocytosis. To determine whether the increase in efferocytosis produced by macrophage exposure to sRAGE directly involves RAGE, we performed the same experiments using RAGE−/− macrophages and found that incubation with sRAGE also increased the phagocytosis of apoptotic cells by RAGE−/− macrophages (Fig. 5B). These data suggest that the promoting effect of sRAGE in efferocytosis is mediated through mechanisms that are independent of the presence of RAGE on the macrophage.
Figure 5. sRAGE enhances efferocytosis.
Peritoneal macrophages isolated from RAGE +/+ (A) and RAGE −/− (B) mice were incubated for 60 minutes with apoptotic thymocytes in medium containing 10 µg/ml polymyxin B and either BSA (1,2 µg/ml) or sRAGE (10, 25, 50 nM). Phagocytosis assays were performed as described in Materials and Methods. ** p<0.01 *** p<0.001 compared to the control BSA group.
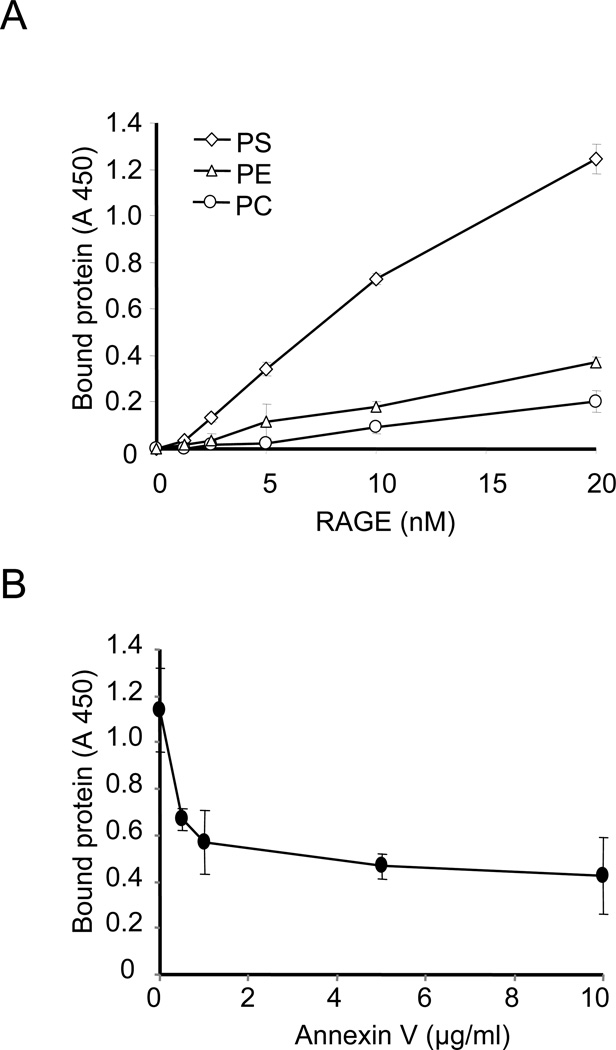
RAGE binds to PS
PS appears on the surface of cells during apoptosis and acts as an important “eat me” signal enhancing the uptake of apoptotic cells by phagocytes (19). A limited number of receptors on phagocytic cells have been shown to be capable of directly binding to PS to enhance efferocytosis. Among PS-binding receptors is the T cell/transmembrane Ig and mucin-4 (TIM-4) receptor, a member of the Ig superfamily (20). TIM-4 contains a V-type Ig terminal domain that is required for binding to PS and also for the ability of TIM-4 to enhance efferocytosis. Because RAGE also includes a V-type Ig terminal domain (21), we hypothesized that the positive effects of RAGE on efferocytosis might occur through recognition and binding to PS on the surface of apoptotic cells. To examine this hypothesis, we performed a solid-phase ELISA (10) in which wells precoated with PS, PC, or PE were exposed to increasing doses of sRAGE.
As shown in Fig. 6A, sRAGE bound to PS in a dose-dependent manner but demonstrated only minimal binding to PC and PE. These data suggest that RAGE specifically binds to PS and not to other related lipids. To examine more completely the interactions between RAGE and PS, we determined whether annexin V, which specifically binds to PS (22), would competitively inhibit the binding between sRAGE and PS. In these experiments, wells precoated with PS were incubated with increasing doses of annexin V before the addition of sRAGE. As shown in Fig. 6B, preincubation of annexin V with PS dose-dependently decreased binding of sRAGE to PS. These data suggest that RAGE may be a novel PS receptor, thereby facilitating recognition of apoptotic cells for clearance by efferocytosis. Of note, preincubation of PS with annexin V did not completely prevent the binding of PS to sRAGE. These data suggest that the regions in PS that bind to annexin V and RAGE are overlapping but not identical.
Figure 6. RAGE binds to phosphatidylserine (PS).
(A) 96-well plates were incubated with phosphatidylserine (PS), phosphatidylethanolamine (PE), or phosphatidylcholine (PC) dissolved in methanol and the methanol allowed to evaporate. The plates were then washed 3 times with PBS, followed by blocking with PBS containing 1% BSA for 1h. After removing the blocking solution, the plates were incubated with increasing doses of recombinant mouse chimeric RAGE dissolved in PBS (0, 1.25, 2.5, 5, 10, 20 nM) for 60 min, followed by washing with PBS three times. RAGE bound to the plates was quantified by ELISA as described in Materials and Methods. (B) PS pre-coated plates were incubated with increasing doses of annexin V (0, 0.5, 1, 5, 10 µg/ml) for 60 min. The plates were then washed three times with BSand RAGE dissolved in PBS (20 nM) was added to the wells. After incubation for 60 min, unbound RAGE was removed and plates washed three times. The amounts of RAGE bound to the wells were determined as in A. Assays were done in triplicate and the mean values ±standard deviation are shown. A 450, absorbance at 450 nm.
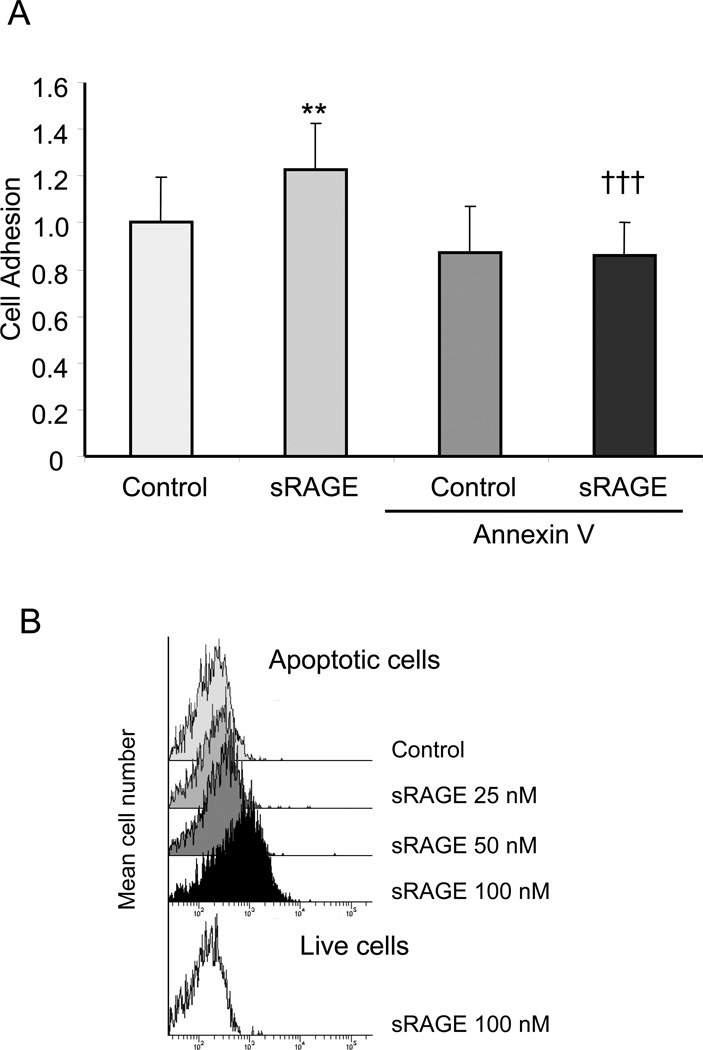
Apoptotic cells bind to RAGE through interactions with PS
To determine whether RAGE directly binds to PS on the surface of apoptotic cells, 96-well plates were precoated with BSA or sRAGE and then incubated with apoptotic thymocytes. After washing to remove nonbinding cells, the percentage of cells bound to RAGE was calculated. As shown in Fig. 7A, significantly more apoptotic cells bound to RAGE as compared with BSA.
Figure 7. Apoptotic cells bind to RAGE through interactions with phosphatidylserine.
(A) Apoptotic thymocytes labeled with PKH26 fluorescent dye were incubated with BSA or annexin V for 30 minutes, then washed three times with PBS. The cells were then added into 96 well plates pre-coated with recombinant RAGE or BSA (1 µg/ml) and incubated for 30 min. The plates were washed with PBS to remove unattached apoptotic cells and the remaining bound cells were determined by measuring the intensity of PKH26 fluorescence from each well. The intensity of PKH26 fluorescence from BSA pre-coated wells that were incubated with BSA treated apoptotic thymocytes (Control) was set to 1. ** p<0.01 compared to the Control group. ††† p<0.001 compared to the sRAGE group. (B) sRAGE binds to apoptotic cells. Apoptotic thymocytes were incubated with increasing concentrations of Chromeo-488 labeled sRAGE (25, 50, or 100 nM) or Chromeo-488 labeled BSA (6 µg/ml) for 3 hours on ice. Viable thymocytes were incubated with 100 nM of Chromeo-488 labeled sRAGE. The binding of the labeled proteins to thymocytes was determined by flow cytometry.
Given the in vitro data in Fig. 6B showing that RAGE binds to PS and that annexin V competitively inhibited interactions between RAGE and PS, we next determined whether the interactions of apoptotic cells with RAGE can also be blocked by annexin V. Consistent with the in vitro results, pretreatment of apoptotic thymocytes with annexin V decreased their binding to RAGE (Fig. 7A).
To confirm that RAGE binds directly to PS on the surface of apoptotic cells, increasing concentrations of fluorescence-labeled sRAGE or fluorescence labeled BSA were incubated with viable and apoptotic thymocytes. As shown in Fig. 7B, sRAGE was dose-dependently bound to apoptotic thymocytes, whereas BSA demonstrated a minimal basal level of binding. More importantly, sRAGE demonstrated much less binding to viable thymocytes than to apoptotic thymocytes. Given that PS is exposed on the surface of apoptotic cells, these data suggest that sRAGE likely binds to PS.
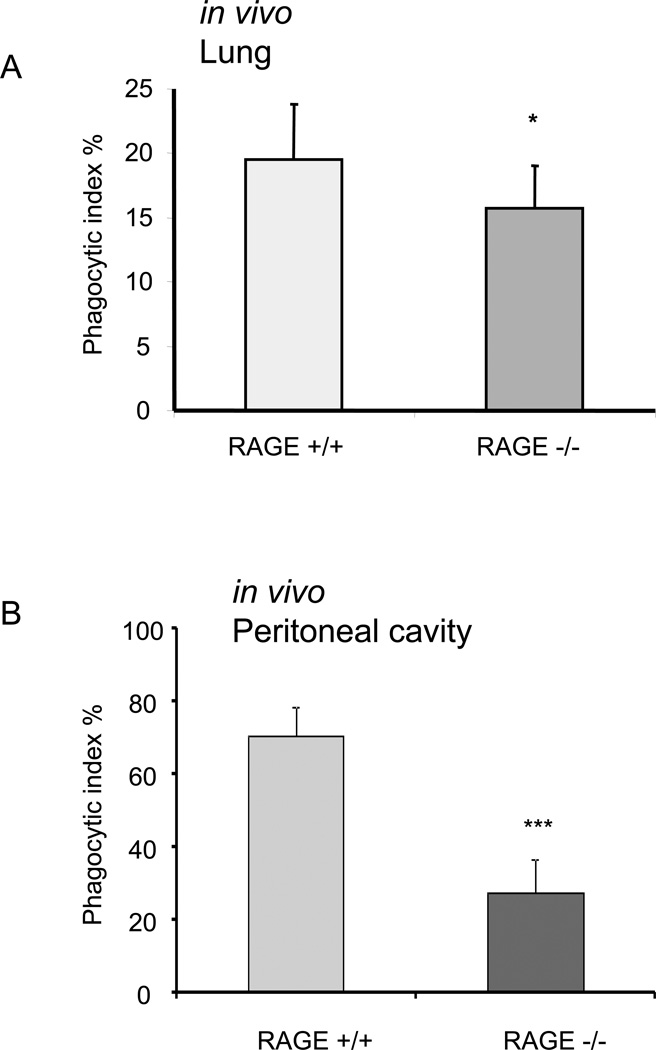
RAGE participates in efferocytosis under in vivo conditions
To determine whether RAGE is involved in efferocytosis in vivo, RAGE+/+ and RAGE−/− mice were injected intratracheally with apoptotic neutrophils. After 2 h, BALs were obtained, and the percentage of macrophages that engulfed apoptotic neutrophils was determined. As shown in Fig. 8A, phagocytosis of apoptotic neutrophils by alveolar macrophages was significantly decreased in RAGE−/− mice as compared with that found in control RAGE+/+ mice. To further characterize the role of RAGE in efferocytosis in vivo, RAGE+/+ and RAGE−/− mice were injected i.p. with PKH26-labeled apoptotic thymocytes. After 2 h, peritoneal lavages were obtained, and the percentage of peritoneal macrophages that had ingested apoptotic thymocytes was determined by flow cytometry. As shown in Fig. 8B, phagocytosis of apoptotic thymocytes by peritoneal macrophages was significantly decreased in RAGE−/− mice as compared with that found in control RAGE+/+ mice.
Figure 8. RAGE participates in efferocytosis under in vivo conditions.
RAGE+/+ or RAGE−/− mice were injected i.t. (5 mice in each group) with 10 × 106 apoptotic neutrophils (A) or i.p (3 mice in each group) with 10 × 106 PKH26 labeled apoptotic thymocytes (B). The mice were sacrificed 2 hours later and bronchoalveolar (A) or peritoneal (B) lavage performed. Phagocytic indices were determined as described in materials and methods. * p<0.05 compared to RAGE+/+ mice. *** p<0.001 compared to RAGE+/+ mice.
Enhanced IL-10 production associated with efferocytosis is comparable between RAGE+/+ and RAGE−/− macrophages
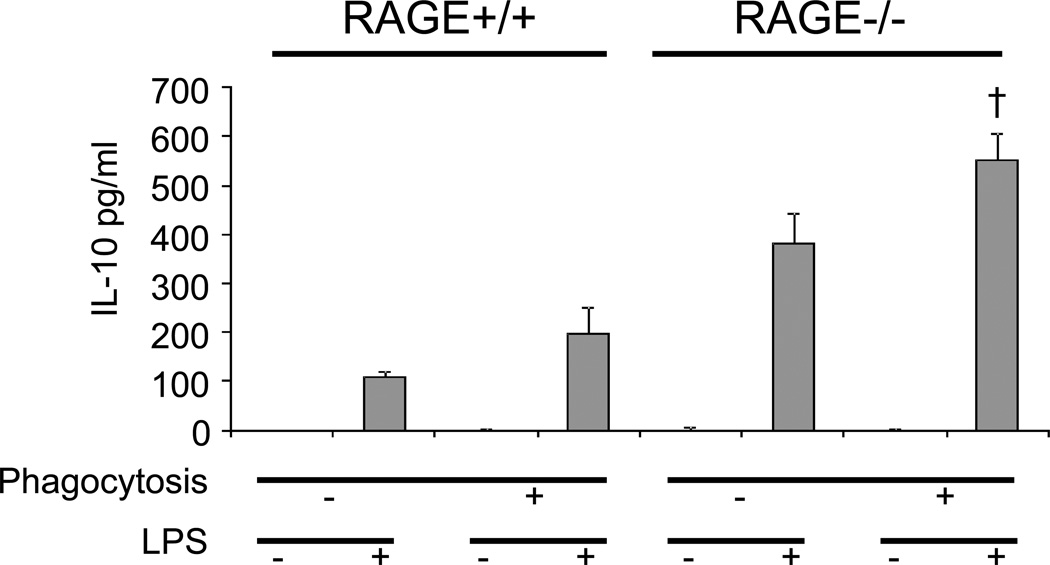
Previous studies have shown that efferocytosis enhances the production of anti-inflammatory cytokines, such as IL-10, by macrophages after endotoxin stimulation (2). Therefore, we determined whether the decrease in efferocytosis of RAGE−/− macrophages is associated with alterations in the production of IL-10. As shown in Fig. 9, phagocytosis of apoptotic cells enhanced the production the IL-10 in RAGE+/+ macrophages after LPS stimulation, consistent with previous studies (2). LPS-treated RAGE−/− macrophages produced more IL-10 than did RAGE+/+ macrophages. However, phagocytosis of apoptotic cells enhanced the production of IL-10 in RAGE−/− macrophages after LPS stimulation to an extent comparable to that found in RAGE+/+ macrophages. These data suggest that the enhanced production of anti-inflammatory cytokines after efferocytosis is not correlated to the extent of change in efferocytosis and also indicate that RAGE may have roles in modulating production of anti-inflammatory cytokines after TLR stimulation that are independent of effects of efferocytosis.
Figure 9. Efferocytosis enhanced IL-10 production in both RAGE+/+ and RAGE−/− macrophages.
RAGE+/+ and RAGE−/− macrophages were incubated without or with apoptotic thymocytes. After phagocytosis the macrophages were washed thoroughly with PBS to remove adherent thymocytes. The macrophages were then cultured with 0 or 10 ng/ml LPS. After 16h, levels of IL-10 in the culture supernatants were determined by ELISA. † p<0.05 compared with RAGE−/− macrophages after exposure to LPS (no effero
Discussion
These studies demonstrate a novel role for RAGE in enhancing efferocytosis through facilitating the recognition of apoptotic cells by macrophages. Inhibition of interactions of RAGE with apoptotic cells through treatment of macrophages with anti-RAGE Abs or AGEs, or by use of RAGE−/− macrophages, was associated with diminished phagocytosis of apoptotic target cells, including thymocytes and neutrophils. The ability of RAGE to potentiate efferocytosis was demonstrated under in vitro conditions with peritoneal macrophages or BMDMs and was also present in vivo in the lungs as shown by diminished engulfment of apoptotic neutrophils by alveolar macrophages in RAGE−/− mice. Similarly, overexpression of RAGE by transfection in HEK 293 cells, which are nonprofessional phagocytes, enhanced their ability to engulf apoptotic cells.
We found that the ability of RAGE to facilitate the recognition and uptake of apoptotic cells occurs through binding to PS, a well established eat me signal that is upregulated on the surface of apoptotic cells. Three receptors directly involved in efferocytosis have previously been shown to bind directly to PS (i.e., TIM-4, brain angiogenesis inhibitor 1, and stabilin-2 (23–25)). Like RAGE, members of the TIM family have a V-type Ig terminal domain, and in the case of TIM-4, this domain is required for binding to PS and enhancement of efferocytosis (23). Although the specific domain in RAGE that interacts with PS is presently unknown, it is likely to include the V-type Ig domain, especially as this sequence has previously been shown to participate in recognition of RAGE ligands during cellular activation (26).
In previous experiments, we found that HMGB1, a known ligand for RAGE, binds to PS on apoptotic neutrophils and diminishes their engulfment by macrophages under both in vitro and in vivo conditions (6). The C-terminal tail of HMGB1, which is required for binding to RAGE, is necessary for the inhibitory effects of HMGB1 on efferocytosis (7). Therefore, it is possible that competitive interactions between HMGB1 and RAGE for binding to PS on apoptotic cells contribute to the ability of HMGB1 to diminish efferocytosis.
Previous studies have primarily focused on the proinflammatory contributions of RAGE, including the ability of RAGE ligation to activate macrophages and other cell populations involved in inflammation, and the participation of RAGE in systemic inflammatory responses associated with conditions such as sepsis, diabetes, and atherosclerosis. Previously described RAGE ligands include AGEs, HMGB1, β-amyloid peptide, β-fibrils, and S100B/calgranulins (27–29). The present experiments demonstrate that PS is also a ligand for RAGE. These studies also delineate a novel function for RAGE in efferocytosis and suggest that RAGE may be directly involved in enhancing the resolution of inflammation through facilitating the uptake and clearance of apoptotic cell populations. Decreased clearance of apoptotic cells in tissue sites appears to potentiate inflammatory and autoimmune responses. For example, diminished clearance of apoptotic cells is associated with severe systemic lupus erythematosis (30), chronic obstructive pulmonary disease, and cystic fibrosis. Elevated expression of RAGE has been found in a number of inflammatory conditions and is associated with worse outcomes in sepsis and acute lung injury (16, 31). These findings are seemingly contradictory to our current data showing that RAGE participates in the clearance of apoptotic cells and thereby may contribute to resolution of inflammation. However, enhanced expression of RAGE is often accompanied by increased levels of its proinflammatory ligands such as HMGB1 and AGE (28). The proinflammatory effects of RAGE observed in previous studies are likely caused by elevated expression of RAGE ligands, rather than by RAGE itself. Therefore, a role for RAGE in efferocytosis may be a feedback mechanism to limit inflammation in which phagocytes can limit excessive breakdown of apoptotic cells. Furthermore, consistent with the previously described (27, 28) proinflammatory effects of RAGE ligands, our present and previous studies (6–8) demonstrated that both HMGB1 and AGE inhibit efferocytosis and, through such actions, may delay the resolution of inflammation.
Treatment with sRAGE has been shown to improve the outcome in acute inflammatory conditions such as those induced by sepsis and ischemia-reperfusion, which are accompanied by the accumulation of activated neutrophils in the lungs and other organs (15, 18, 32). The presumptive mechanism for the benefit of sRAGE in such conditions has been through binding RAGE ligands and preventing cellular activation that would occur through the interaction of proinflammatory molecules with RAGE. However, the present experiments demonstrate an alternate mechanism by which sRAGE could reduce the severity of inflammatory organ injury. In particular, sRAGE, through binding to PS on the surface of apoptotic neutrophils and other apoptotic cells that accumulate in sites of tissue damage, could facilitate their clearance by macrophages and additional phagocytic populations, thereby enhancing the resolution of inflammation. Because we also found that RAGE itself mediates efferocytosis, it is possible that sRAGE could block efferocytosis by acting as a decoy to competitively bind to PS on the surface of apoptotic cells and thereby prevent recognition of PS during phagocytosis. However, although both RAGE and sRAGE bind to PS, they may use different mechanisms in activating phagocytes to ingest apoptotic cells. In particular, although RAGE ligation activates Rac1 and Erk kinase, sRAGE has been shown to directly bind to macrophages through Mac-1, an integrin involved in the clearance of apoptotic cells, and thereby could effectively bridge PS on apoptotic cells with macrophages (33, 34).
In the present studies, RAGE not only appeared to serve in a tethering role between apoptotic and phagocytic cells but also was involved in enhancing the phagocytosis of apoptotic cells by macrophages and RAGE-expressing HEK cells. A potential mechanism by which RAGE engagement may directly participate in phagocytosis is through activation of ERK and Rac1 kinases. In particular, ERK and Rac1 kinases are activated during phagocytosis and play a central role in the ingestion of apoptotic cells during efferocytosis. Binding between RAGE and its ligands has been shown to result in activation of both Rac1 and ERK (35, 36). In the case of Rac1, interaction of the cytoplasmic domain of RAGE with diaphanous-1 appears to link cellular activation through RAGE with activation of Rac1 (35).
These studies, by demonstrating that RAGE binds to PS and participates in efferocytosis, delineate a novel role for RAGE in modulating inflammatory processes associated with tissue injury and repair. In particular, interactions between RAGE and molecules that are expressed on the cell surface and directly involved in apoptosis, such as PS, have not been described previously. The ability of RAGE to bind to PS and to facilitate the phagocytosis and clearance of apoptotic cells suggests that therapies, including the administration of sRAGE, that result in the increased expression of RAGE on the surface of macrophages and other phagocytic cells and that facilitate interaction between RAGE and PS may be beneficial in removing dying cells from sites of tissue injury and enhancing resolution of inflammation. In addition, treatments aimed at diminishing tissue levels of RAGE ligands, including AGEs and HMGB1, that interfere with the ability of RAGE to bind to PS might have dual effects in minimizing organ injury through reducing the inflammatory injury directly produced by these RAGE ligands and also by allowing RAGE to more effectively participate in efferocytosis.
Acknowledgments
This work was supported by NIH grants GM087748 and HL076206 to E.A.
References
- 1.Haslett C. Granulocyte apoptosis and its role in the resolution and control of lung inflammation. Am J Respir Crit Care Med. 1999;160:S5–S11. doi: 10.1164/ajrccm.160.supplement_1.4. [DOI] [PubMed] [Google Scholar]
- 2.Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Henson PM. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J Clin Invest. 1998;101:890–898. doi: 10.1172/JCI1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baumann I, Kolowos W, Voll RE, Manger B, Gaipl U, Neuhuber WL, Kirchner T, Kalden JR, Herrmann M. Impaired uptake of apoptotic cells into tingible body macrophages in germinal centers of patients with systemic lupus erythematosus. Arthritis Rheum. 2002;46:191–201. doi: 10.1002/1529-0131(200201)46:1<191::AID-ART10027>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 4.Schmidt AM, Yan SD, Yan SF, Stern DM. The biology of the receptor for advanced glycation end products and its ligands. Biochim Biophys Acta. 2000;1498:99–111. doi: 10.1016/s0167-4889(00)00087-2. [DOI] [PubMed] [Google Scholar]
- 5.Su X, Looney MR, Gupta N, Matthay MA. Receptor for advanced glycation end-products (RAGE) is an indicator of direct lung injury in models of experimental lung injury. Am J Physiol Lung Cell Mol Physiol. 2009;297:L1–L5. doi: 10.1152/ajplung.90546.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu G, Wang J, Park YJ, Tsuruta Y, Lorne EF, Zhao X, Abraham E. High mobility group protein-1 inhibits phagocytosis of apoptotic neutrophils through binding to phosphatidylserine. J Immunol. 2008;181:4240–4246. doi: 10.4049/jimmunol.181.6.4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Banerjee S, Friggeri A, Liu G, Abraham E. The C-terminal acidic tail is responsible for he inhibitory effects of HMGB1 on efferocytosis. J Leukoc Biol. 88:973–979. doi: 10.1189/jlb.0510262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Friggeri A, Yang Y, Banerjee S, Park YJ, Liu G, Abraham E. HMGB1 inhibits macrophage activity in efferocytosis through binding to the alphavbeta3-integrin. Am J Physiol Cell Physiol. 299:C1267–C1276. doi: 10.1152/ajpcell.00152.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.D'Mello V, Singh S, Wu Y, Birge RB. The urokinase plasminogen activator receptor promotes efferocytosis of apoptotic cells. J Biol Chem. 2009;284:17030–17038. doi: 10.1074/jbc.M109.010066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hanayama R, Tanaka M, Miwa K, Shinohara A, Iwamatsu A, Nagata S. Identification of a factor that links apoptotic cells to phagocytes. Nature. 2002;417:182–187. doi: 10.1038/417182a. [DOI] [PubMed] [Google Scholar]
- 11.Yang Y, Friggeri A, Banerjee S, Bdeir K, Cines DB, Liu G, Abraham E. Urokinase-type plasminogen activator inhibits efferocytosis of neutrophils. Am J Respir Crit Care Med. 182:1516–1523. doi: 10.1164/rccm.201003-0452OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Neeper M, Schmidt AM, Brett J, Yan SD, Wang F, Pan YC, Elliston K, Stern D, Shaw A. Cloning and expression of a cell surface receptor for advanced glycosylation end products of proteins. J Biol Chem. 1992;267:14998–15004. [PubMed] [Google Scholar]
- 13.Schlueter C, Hauke S, Flohr AM, Rogalla P, Bullerdiek J. Tissue-specific expression patterns of the RAGE receptor and its soluble forms--a result of regulated alternative splicing? Biochim Biophys Acta. 2003;1630:1–6. doi: 10.1016/j.bbaexp.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 14.Raucci A, Cugusi S, Antonelli A, Barabino SM, Monti L, Bierhaus A, Reiss K, Saftig P, Bianchi ME. A soluble form of the receptor for advanced glycation endproducts (RAGE) is produced by proteolytic cleavage of the membrane-bound form by the sheddase a disintegrin and metalloprotease 10 (ADAM10) Faseb J. 2008;22:3716–3727. doi: 10.1096/fj.08-109033. [DOI] [PubMed] [Google Scholar]
- 15.Sternberg DI, Gowda R, Mehra D, Qu W, Weinberg A, Twaddell W, Sarkar J, Wallace A, Hudson B, D'Ovidio F, Arcasoy S, Ramasamy R, D'Armiento J, Schmidt AM, Sonett JR. Blockade of receptor for advanced glycation end product attenuates pulmonary reperfusion injury in mice. J Thorac Cardiovasc Surg. 2008;136:1576–1585. doi: 10.1016/j.jtcvs.2008.05.032. [DOI] [PubMed] [Google Scholar]
- 16.Liliensiek B, Weigand MA, Bierhaus A, Nicklas W, Kasper M, Hofer S, Plachky J, Grone HJ, Kurschus FC, Schmidt AM, Yan SD, Martin E, Schleicher E, Stern DM, Hammerling GG, Nawroth PP, Arnold B. Receptor for advanced glycation end products (RAGE) regulates sepsis but not the adaptive immune response. J Clin Invest. 2004;113:1641–1650. doi: 10.1172/JCI18704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aleshin A, Ananthakrishnan R, Li Q, Rosario R, Lu Y, Qu W, Song F, Bakr S, Szabolcs M, D'Agati V, Liu R, Homma S, Schmidt AM, Yan SF, Ramasamy R. RAGE modulates myocardial injury consequent to LAD infarction via impact on JNK and STAT signaling in a murine model. Am J Physiol Heart Circ Physiol. 2008;294:H1823–H1832. doi: 10.1152/ajpheart.01210.2007. [DOI] [PubMed] [Google Scholar]
- 18.Zhang H, Tasaka S, Shiraishi Y, Fukunaga K, Yamada W, Seki H, Ogawa Y, Miyamoto K, Nakano Y, Hasegawa N, Miyasho T, Maruyama I, Ishizaka A. Role of soluble receptor for advanced glycation end products on endotoxin-induced lung injury. Am J Respir Crit Care Med. 2008;178:356–362. doi: 10.1164/rccm.200707-1069OC. [DOI] [PubMed] [Google Scholar]
- 19.Ravichandran KS. Find-me and eat-me signals in apoptotic cell clearance: progress and conundrums. J Exp Med. 207:1807–1817. doi: 10.1084/jem.20101157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miyanishi M, Tada K, Koike M, Uchiyama Y, Kitamura T, Nagata S. Identification of Tim4 as a phosphatidylserine receptor. Nature. 2007;450:435–439. doi: 10.1038/nature06307. [DOI] [PubMed] [Google Scholar]
- 21.Koch M, Chitayat S, Dattilo BM, Schiefner A, Diez J, Chazin WJ, Fritz G. Structural Basis for Ligand Recognition and Activation of RAGE. Structure. 18:1342–1352. doi: 10.1016/j.str.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koopman G, Reutelingsperger CP, Kuijten GA, Keehnen RM, Pals ST, van Oers MH. Annexin V for flow cytometric detection of phosphatidylserine expression on B cells undergoing apoptosis. Blood. 1994;84:1415–1420. [PubMed] [Google Scholar]
- 23.Freeman GJ, Casasnovas JM, Umetsu DT, DeKruyff RH. TIM genes: a family of cell surface phosphatidylserine receptors that regulate innate and adaptive immunity. Immunol Rev. 235:172–189. doi: 10.1111/j.0105-2896.2010.00903.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park D, Tosello-Trampont AC, Elliott MR, Lu M, Haney LB, Ma Z, Klibanov AL, Mandell JW, Ravichandran KS. BAI1 is an engulfment receptor for apoptotic cells upstream of the ELMO/Dock180/Rac module. Nature. 2007;450:430–434. doi: 10.1038/nature06329. [DOI] [PubMed] [Google Scholar]
- 25.Park SY, Jung MY, Kim HJ, Lee SJ, Kim SY, Lee BH, Kwon TH, Park RW, Kim IS. Rapid cell corpse clearance by stabilin-2, a membrane phosphatidylserine receptor. Cell Death Differ. 2008;15:192–201. doi: 10.1038/sj.cdd.4402242. [DOI] [PubMed] [Google Scholar]
- 26.Yan SF, Ramasamy R, Schmidt AM. Soluble RAGE: therapy and biomarker in unraveling the RAGE axis in chronic disease and aging. Biochem Pharmacol. 79:1379–1386. doi: 10.1016/j.bcp.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bopp C, Bierhaus A, Hofer S, Bouchon A, Nawroth PP, Martin E, Weigand MA. Bench-to-bedside review: The inflammation-perpetuating pattern-recognition receptor RAGE as a therapeutic target in sepsis. Crit Care. 2008;12:201. doi: 10.1186/cc6164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Creagh-Brown BC, Quinlan GJ, Evans TW, Burke-Gaffney A. The RAGE axis in systemic inflammation, acute lung injury and myocardial dysfunction: an important therapeutic target? Intensive Care Med. 36:1644–1656. doi: 10.1007/s00134-010-1952-z. [DOI] [PubMed] [Google Scholar]
- 29.Yan SF, Ramasamy R, Schmidt AM. The receptor for advanced glycation endproducts (RAGE) and cardiovascular disease. Expert Rev Mol Med. 2009;11:e9. doi: 10.1017/S146239940900101X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Munoz LE, Lauber K, Schiller M, Manfredi AA, Herrmann M. The role of defective clearance of apoptotic cells in systemic autoimmunity. Nat Rev Rheumatol. 6:280–289. doi: 10.1038/nrrheum.2010.46. [DOI] [PubMed] [Google Scholar]
- 31.Uchida T, Shirasawa M, Ware LB, Kojima K, Hata Y, Makita K, Mednick G, Matthay ZA, Matthay MA. Receptor for advanced glycation end-products is a marker of type I cell injury in acute lung injury. Am J Respir Crit Care Med. 2006;173:1008–1015. doi: 10.1164/rccm.200509-1477OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hofmann MA, Drury S, Fu C, Qu W, Taguchi A, Lu Y, Avila C, Kambham N, Bierhaus A, Nawroth P, Neurath MF, Slattery T, Beach D, McClary J, Nagashima M, Morser J, Stern D, Schmidt AM. RAGE mediates a novel proinflammatory axis: a central cell surface receptor for S100/calgranulin polypeptides. Cell. 1999;97:889–901. doi: 10.1016/s0092-8674(00)80801-6. [DOI] [PubMed] [Google Scholar]
- 33.Mevorach D, Mascarenhas JO, Gershov D, Elkon KB. Complement-dependent clearance of apoptotic cells by human macrophages. J Exp Med. 1998;188:2313–2320. doi: 10.1084/jem.188.12.2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Y, Wang H, Piper MG, McMaken S, Mo X, Opalek J, Schmidt AM, Marsh CB. sRAGE induces human monocyte survival and differentiation. J Immunol. 185:1822–1835. doi: 10.4049/jimmunol.0903398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hudson BI, Kalea AZ, Del Mar Arriero M, Harja E, Boulanger E, D'Agati V, Schmidt AM. Interaction of the RAGE cytoplasmic domain with diaphanous-1 is required for ligand-stimulated cellular migration through activation of Rac1 and Cdc42. J Biol Chem. 2008;283:34457–34468. doi: 10.1074/jbc.M801465200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ishihara K, Tsutsumi K, Kawane S, Nakajima M, Kasaoka T. The receptor for advanced glycation end-products (RAGE) directly binds to ERK by a D-domain-like docking site. FEBS Lett. 2003;550:107–113. doi: 10.1016/s0014-5793(03)00846-9. [DOI] [PubMed] [Google Scholar]