Abstract
Natural killer (NK)-cell killing of virus-infected cells is regulated in part by the engagement of activation and coactivation receptors. In this issue of Cell Host & Microbe, Shah et al. (2010) demonstrate that HIV-1 protects infected cells from NK-cell-mediated killing by hindering NK-cell degranulation through downmodulation of NTB-A coactivation receptor ligands by the Vpu accessory protein.
Natural killer (NK) cells are a key component of the innate immune system, given their ability to lyse target cells and to serve as an early source of immunoregulatory cytokines. Hence, they play an important role in immune surveillance and defense against viruses before the emergence of virus-specific adaptive immune responses (Lanier, 2008). NK-cell function is regulated by a wide range of receptors, activating or inhibitory, expressed at the surface of NK cells. Ligands for these receptors may be present on target cells. A broadly accepted view is that integration of the intracellular signals originating from these receptors during target cell recognition determines whether a NK cell will eliminate a potential target (Bryceson et al., 2006). NKG2D is a potent NK-cell activation receptor that recognizes genotoxic stress-induced molecules, such as ULBP-1 and -2, as ligands at the surface of target cells. Although activation of NK receptors is necessary for NK-cell-mediated cell lysis of target cells, they are insufficient to induce the production of lytic granules in resting NK cells—a process designated as degranulation—and, indeed, require the concomitant triggering of coactivation receptors (Bryceson et al., 2006). One such factor that regulates NK-cell cytolysis is the NK-T and B cell antigen (NTB-A) coactivator receptor. NTB-A is a member of the signaling lymphocytic activation molecule (SLAM) family of receptors. It is a type 1 transmembrane protein of the immunoglobulin superfamily that functions as a homotypic ligand-coactivation NK receptor pair.
Previous studies showed that HIV-1 infection leads to a decrease in surface expression of HLA-A and -B inhibitory ligands through the action of the Nef viral protein (Cohen et al., 1999) and an increase of NKG2D activating ligands through the action of HIV-1 Vpr (Richard et al., 2010; Ward et al., 2009). This represents a configuration that should make HIV-1-infected cells optimal targets for NK-cell-mediated destruction. However, NK-cell-mediated killing of autologous primary CD4+ T cells infected with HIV-1 was reported to be modest at best (Bonaparte and Barker, 2003). In this issue of Cell Host & Microbe, Shah et al. (2010) provide an explanation for the inefficient NK-cell-mediated killing of HIV-1-infected cells. They report that the HIV-1 Vpu accessory protein interferes with NK-cell degranulation by downmodulating NTB-A at the cell surface of infected cells.
A defining feature of primary immuno-deficiency viruses is their ability to encode a set of viral proteins—the so-called accessory proteins—which include Vif, Vpr, Vpu, and Nef in the case of HIV-1 (Kirchhoff, 2010). Overall, these proteins act as adaptors and recruit fundamental host cell machineries to create a cellular environment that is favorable for viral persistence, replication, transmission, dissemination, and immune evasion. HIV-1 Vpu is a type 1 membrane-associated protein that is unique to HIV-1 and some simian immunodeficiency viruses (SIVs) (Kirchhoff, 2010). The protein was found to contribute to HIV-1-induced downmodulation of the CD4 receptor by mediating a rapid degradation of receptor molecules in the endoplasmic reticulum via the ubiquitin-proteasome system. A key process in Vpu-mediated CD4 degradation is the recruitment of β-TrCP, a component of the SCF (β-TrCP) E3 ubiquitin ligase and the trans-ubiquitination of acceptor residues in the cytosolic tail of CD4. In addition to its effect on CD4 catabolism, Vpu promotes the release of assembled virions by antagonizing Tetherin (also called BST2), an interferon-induced antiviral host protein (also called a restriction factor) that directly crosslinks HIV-1 virions on the host cell surface (Figure 1). Tetherin antagonism by Vpu is species specific and correlates with the downregulation of Tetherin at the cell surface. The mechanism by which Vpu downregulates Tetherin and promotes efficient release of HIV-1 virions has recently attracted considerable attention (Kirchhoff, 2010). The emerging conscensus is that Vpu associates with Tetherin via transmembrane domain (TMD) interactions and sequesters the restriction factor in a peri-nuclear compartment that costains with trans-Golgi markers prior to targeting it for lysosomal degradation in a β-TrCP-dependent manner.
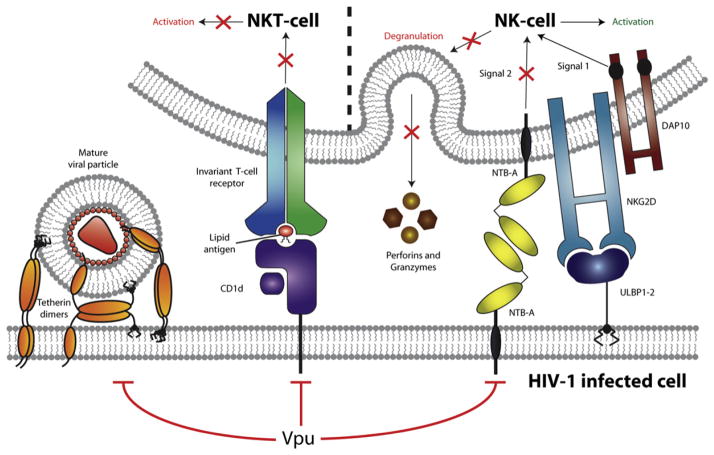
Figure 1. HIV-1 Vpu Interferes with the Activity of Several Host Cell Surface Factors Involved in Innate Immunity.
Expression of HIV-1 Vpu in infected cells leads to the cell-surface downmodulation of Tetherin, CD1d, and NTB-A. By decreasing Tetherin levels at the cell surface, Vpu prevents tethering of mature viruses to the plasma membrane of infected cells. Downmodulation of CD1d prevents presentation of lipid antigens to CD1d-restricted NKT cells, thereby avoiding their activation. Finally, Vpu protects infected cells from NK-cell-mediated killing by downmodulating NTB-A, since both NTB-A- and NKG2D-triggered signals are required for NK-cell degranulation. Overall, these Vpu activities reduce the efficiency of the innate immune response directed against HIV-1-infected cells.
The study of Barker and colleagues (Shah et al., 2010) now reveals that Vpu, alone, can target another membrane-associated protein, NTB-A, and downregulate its levels at the surface of HIV-1-infected cells (Figure 1). NTB-A downmodulation by Vpu was found to be mechanistically distinct from CD4 and Tetherin downmodulation, since Vpu did not alter the steady-state levels of NTB-A and did not rely on the recruitment of β-TrCP to reduce cell surface NTB-A levels. Although Vpu did not increase NTB-A endocytosis as a mean to deplete the coactivation NK receptor ligand from the cell surface, the viral protein was shown to interact with NTB-A through its TMD, and the Vpu TMD was found to be required for NTB-A downmodulation. Together, these results suggest that Vpu might downmodulate NTB-A through an alteration of the trafficking and/or recycling of the protein and as such sequesters NTB-A in an intracellular compartment. In that regard, it is interesting to note that downmodulation of NTB-A by Vpu shares several similarities with a recently reported activity of HIV-1 Vpu, that is, the inhibition of surface expression of CD1d, a membrane-associated protein expressed by antigen-presenting cells (APCs) such as monocytes, macrophages, and dendritic cells (DCs) (Moll et al., 2010). CD1d is involved in presenting exogenous pathogen-derived lipid antigens to CD1d-restricted natural killer T (NKT) cells expressing an invariant αβ T cell receptor, thus resulting in the mutual activation of both APC and NKT-cells and the subsequent activation of a cellular immune reponse (Figure 1). As observed with NTB-A, Vpu did not enhance constitutive CD1d endocytosis or induce rapid CD1d degradation. Instead, Vpu was found to interact with CD1d and suppress its recycling from endosomal compartments to the cell surface by retaining CD1d in intracellular compartments that included early endosomes. Importantly, this interference with the CD1d antigen presentation pathway strongly inhibited the ability of infected DC to activate CD1d-restricted NKT cells. The fact that Vpu is now reported to target three membrane-associated cell-surface proteins involved in various aspects of the innate immune response raises the possibility that Vpu may be a key factor used by HIV-1 to evade innate immunity (Figure 1).
The results presented by Barker and colleagues (Shah et al., 2010) raise many new questions about the interaction of Vpu and NTB-A. One important future goal will be to assess precisely how Vpu downmodulates NTB-A levels at the cell surface of infected cells. Furthermore, given that the Vpu TMD is involved in complex formation with Tetherin, and NTB-A, two proteins sharing little sequence homology, it remains unclear how the specific recognition of Tetherin and NTB-A by Vpu is governed and how can Vpu control both host proteins simultaneously.
Another important result from the Shah et al. (2010) study is that NTB-A engagement is required for NK cells to degranulate when the activation receptor NKG2D is triggered, thus establishing an activation/coactivation receptor pair that may be functional during HIV-1 infection. Interestingly, although HIV-1-infected cells induced vigourous NKG2D-dependent activation of NK-cells, they failed to trigger efficient degranulation (Figure 1). Indeed, Vpu-mediated downmodulation of NTB-A was found to hinder NK-cell degranulation, thus ultimately protecting infected cell from NK-cell-mediated lysis. While Vpu may in part protect HIV-1-infected cells from NK-cell-mediated lysis, it will be important to assess the implication of the sustained activation of NK cells via NKG2D on the function of NK cells. Indeed, accumulating evidence indicates that NK-cell function is compromised during HIV-1 infection through poorly understood mechanisms (Alter and Altfeld, 2006). The fact that the emergence of a novel subset of NK cells that lacks the majority of NK-cell functions directly correlates with viral load suggests that the presence of viral antigens has a role in NK-cell dysfunction. Hence, HIV-1 might employ a dual strategy to interfere with NK-cell function; early in infection, HIV-1 might interfere specifically with NK-cell degranulation through the action of Vpu as a mean to protect infected cells from NK-cell-mediated killing, whereas the sustained activation of NK cell triggered by Vpr might impede NK-cell function as a whole during the chronic phase of infection. Clearly, the study by Shah et al. (2010) marks only the beginning of a fascinating story that will shed new light on an important but still poorly understood aspect of the interaction of HIV-1 with NK cells.
References
- Alter G, Altfeld M. Curr Mol Med. 2006;6:621–629. doi: 10.2174/156652406778195035. [DOI] [PubMed] [Google Scholar]
- Bonaparte MI, Barker E. AIDS. 2003;17:487–494. doi: 10.1097/00002030-200303070-00003. [DOI] [PubMed] [Google Scholar]
- Bryceson YT, March ME, Ljunggren HG, Long EO. Immunol Rev. 2006;214:73–91. doi: 10.1111/j.1600-065X.2006.00457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen GB, Gandhi RT, Davis DM, Mandelboim O, Chen BK, Strominger JL, Baltimore D. Immunity. 1999;10:661–671. doi: 10.1016/s1074-7613(00)80065-5. [DOI] [PubMed] [Google Scholar]
- Kirchhoff F. Cell Host Microbe. 2010;8:55–67. doi: 10.1016/j.chom.2010.06.004. [DOI] [PubMed] [Google Scholar]
- Lanier LL. Nat Rev Immunol. 2008;8:259–268. doi: 10.1038/nri2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll M, Andersson SK, Smed-Sorensen A, Sandberg JK. Blood. 2010;116:1876–1884. doi: 10.1182/blood-2009-09-243667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard J, Sindhu S, Pham TN, Belzile JP, Cohen EA. Blood. 2010;115:1354–1363. doi: 10.1182/blood-2009-08-237370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah AH, Sowrirajan B, Davis ZB, Ward JP, Campbell EM, Planelles V, Barker E. Cell Host Microbe. 2010;8:397–409. doi: 10.1016/j.chom.2010.10.008. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward J, Davis Z, DeHart J, Zimmerman E, Bosque A, Brunetta E, Mavilio D, Planelles V, Barker E. PLoS Pathog. 2009;5:e1000613. doi: 10.1371/journal.ppat.1000613. [DOI] [PMC free article] [PubMed] [Google Scholar]