Abstract
Studies of mother–infant relationships in nonhuman primates have increasingly attempted to understand the neuroendocrine bases of interindividual variation in mothering styles and the mechanisms through which early exposure to variable mothering styles affects infant behavioral development. In this study of free-ranging rhesus macaques on Cayo Santiago, Puerto Rico, we aimed to: 1) compare lactating and nonlactating females to investigate whether lactation is associated with changes in plasma cortisol, prolactin and oxytocin, as well as changes in CSF levels of serotonin and dopamine metabolites (5-HIAA and HVA); 2) examine the extent to which interindividual variation in maternal physiology is associated with variation in maternal behavior; 3) examine the extent to which interindividual variation in infant physiology and behavior is accounted for by variation in maternal physiology and behavior. Lactating females had higher plasma concentrations of cortisol, prolactin, and oxytocin but lower CSF concentrations of HVA than nonlactating females. Variation in maternal rejection behavior was positively correlated with variation in maternal plasma cortisol levels and in CSF 5-HIAA levels while variation in the time spent nursing and grooming was associated with maternal plasma oxytocin levels. Infants who were protected more by their mothers had higher cortisol levels than those who were protected less, while infants who were rejected more had lower CSF 5-HIAA than infants who were rejected less. Since exposure to high levels of maternal protectiveness and rejection is known to affect the offspring’s behavior and responsiveness to the environment later in life, our results are consistent with the hypothesis that these effects are mediated by long-term changes in the activity of the offspring’s HPA axis and brain serotonergic system.
Keywords: Maternal behavior, Infant behavior, Cortisol, Monoamine neurotransmitters, Oxytocin, Prolactin, Rhesus monkeys
1. Introduction
Since the early studies of rhesus macaques conduced by Robert Hinde and colleagues in the 1960s and 1970s [1,2], research on maternal behavior and infant development in nonhuman primates has emphasized the study of individuals in complex social environments, the quantification of mother–infant interactions with ethological observational methods, and the investigation of the causes and consequences of naturally occurring interindividual variation in mothering style [3–7]. Studies of macaques, baboons and vervet monkeys have shown that most variability in mothering style occurs along the two dimensions of maternal protectiveness and rejection, although in some cases, a third dimension—maternal warmth—was also identified [8–10]. The maternal protectiveness dimension includes variation in the extent to which the mother physically restrains her infant, and initiates proximity and contact with her infant. The maternal rejection dimension includes the extent to which the mother limits the timing and duration of contact. Maternal warmth refers to the extent to which the mother allows nursing and cradles and grooms her infant. These dimensions of mothering style in monkeys share several similarities with parenting style dimensions in humans, as well as with particular patterns of mother–infant attachment [7]. In monkeys, although infant age, maternal age, and maternal experience are known to influence maternal behavior, individual differences in mothering style tend to be consistent over time and across infants [2,6].
Variation in mothering style is generally accounted for by a combination of maternal characteristics (e.g., age, parity, dominance rank, temperament), infant characteristics (e.g., sex, age, baseline activity levels), and the surrounding environment (e.g., stress and support from other group members, ecological variables) [6,7]. Individual differences in mothering style have long-term effects on the offspring’s tendency to respond to challenges or explore the environment. For example, infants reared by highly rejecting (or less responsive) mothers generally develop independence at an earlier age (e.g. spend more time out of contact with their mothers, explore the environment more, and play more with their peers) than infants reared by mothers with low rejection levels [11–14]. In contrast, infants reared by more protective mothers tend to be delayed in the acquisition of their independence and are relatively fearful and cautious when faced with challenging situations [15–18]. Effects of mothering style on offspring behavior extend into adulthood [16,17,19–24]. For example, mothering style affects the offspring’s parenting behavior in adulthood, as there are often significant similarities in maternal behavior between mothers and daughters [25–28].
Although observational studies of primate maternal behavior and infant development continue to provide valuable information, recent research has increasingly investigated the physiological mechanisms underlying variation in behavior. For example, recent studies have investigated the relation between physiological changes accompanying pregnancy and lactation and changes in maternal motivation [29–32], the physiological correlates of individual differences in mothering style [33–41], and the physiological mechanisms through which early exposure to variable mothering style affects offspring behavioral development [19,21,23,28,42]. Research on the physiological substrates of maternal motivation and mothering style has focused on hormones of the hypothalamic-pituitary-gonadal (HPG) and the hypothalamic-pituitary-adrenal (HPA) axes, such as estrogen, progesterone, prolactin, and cortisol [31–33,36,37,43], as well as on endogenous opioids [44,45] and brain monoamines [28,39,46–48]. Although studies of rodents and sheep have implicated oxytocin in maternal motivation and behavior [49–51], there is limited research on oxytocin and maternal behavior in primates [52–54]. Cortisol and the monoamine neurotransmitters have also been investigated to elucidate the mechanisms through which differences in mothering style may result in variation in the offspring’s behavioral development and emotional reactivity [19,21,23,24,42].
In this study we investigated the relation between physiological variables, maternal behavior, and infant behavior in a free-ranging population of rhesus macaques. Specifically, we aimed to: 1) compare lactating and nonlactating females to investigate whether lactation is associated with changes in plasma cortisol, prolactin and oxytocin and CSF serotonin and dopamine metabolites; 2) examine the extent to which interindividual variation in maternal physiology is associated with variation in maternal behavior; 3) examine the extent to which interindividual variation in infant physiology and infant behavior is accounted for by variation in maternal physiology and maternal behavior. We hypothesized that there may be causal relationships between physiology and behavior, and between maternal behavior/physiology and infant behavior/physiology. However, given the constraints of research with free-ranging monkeys, our study was not designed to test cause–effect relationships between variables.
2. Materials and methods
2.1. Subjects
This study was conducted with the free-ranging population of rhesus macaques on Cayo Santiago, a 15.2 ha island located 1 km off the southeastern coast of Puerto Rico. During the study period, the population included approximately 850 animals distributed among 6 naturally formed social groups. Monkeys on Cayo Santiago forage on vegetation and are provisioned with rainwater and commercial monkey chow. Rhesus macaques are seasonal breeders. In the Cayo Santiago population, there is a 6-month mating season beginning in March, followed by a 6-month birth season beginning in September. Some rhesus females give birth every year while others do so every other year. Colony records are updated with daily censuses of all animals. The Cayo Santiago database includes information on each animal’s date of birth and death, maternal relatedness and genealogy, as well as group membership, reproductive, and health history.
The data presented here were collected as part of a larger study of stress and reproduction in multiparous, experienced females. Study subjects were all adult females in the population who, when the study began, were between 15 and 25 years of age (n=53; mean age±SE: 18.3±0.3). All subjects were multiparous and belonged to 6 different social groups (F=17; R=16; S=7; V=5; HH=4; KK=4). They were classified as being high- or low-ranking depending on whether their rank fell within the top half or bottom half of the dominance hierarchy within their group. Dominance hierarchies were established on the basis of data on aggressive and submissive interactions collected by trained observers.
2.2. Procedure
All subjects were trapped between January 15 and March 15, 2007. At the time of capture, 25 females were lactating and had live infants (infant age range: 8–134 days; mean±SE=72.8±7.5 days) and 23 of them were neither pregnant nor lactating (nonlactating). The nonlactating females had offspring born in previous years but none of them had a successful conception in the mating season prior to this study. Five females who were either pregnant at the time of capture (as assessed through a palpation of the abdomen, and confirmed retrospectively from the date of birth) or whose infant had recently died were excluded from data analyses.
All females were inspected by a veterinarian at the time of sampling and found to be in general good health. Therefore, there was no hint that the nonlactating females in this study did not conceive for health problems, or that the lactating females were in poor health.
Monkeys were captured in feeding corrals, which were provisioned daily with monkey chow. Trapping generally occurred between 8:30 and 12:00. Subjects were netted or captured by hand in the corral, and placed into a standard squeeze cage, where they remained for overnight housing. The following morning, all adult females and infants were anesthetized with ketamine (approximately 10 mg/kg via IM injection) and a 5-ml (2 ml for infants) blood sample was collected from them. All blood samples were collected from the femoral vein into EDTA tubes. Samples were refrigerated for 20 min and centrifuged for 15 min before plasma was aliquoted into microcentrifuge tubes. Plasma samples were frozen on dry ice immediately after centrifugation and were kept at −80 °C until analyses occurred. With one exception, only infants older than 2 months of age (n=16) were sampled due to the very small size of younger infants. Blood samples were collected between 7:15 and 10:40 (average time of day: 8:10± 12.9 min). Samples were collected, on average, 63.9±7.4 min after the door of the laboratory was first opened (range: 7–207 min), and 28.3± 4.8 min after ketamine administration (range 0–127 min). Immediately following the blood sample collection and while the subjects were still under anesthesia, one 2-ml sample of CSF was also collected from the cisterna magna using a needle. The CSF samples were placed on dry ice immediately after collection and kept at −70 C until assay.
Lactating and nonlactating females did not differ significantly in their age (lactating: 17.8±0.3 years; nonlactating: 19.0±0.6; t=1.59, df=46, NS), dominance rank (lactating: high=10; low=15; nonlactating: high=13; low=10; chi square: 1.31, df=1; NS), or any variables related to blood sampling (time of day, time since beginning of the procedure, and time since ketamine injection). Cortisol and prolactin plasma concentrations were analyzed by radioimmunoassay using a commercially available kit (Diagnostic Systems Laboratories, Webster, TX). For cortisol, intra-assay coefficient of variation was 4.90% and inter-assay coefficients were 4.50% and 8.74%. For prolactin, intra-assay coefficient of variation was 5.64% and inter-assay coefficients were 4.12% and 6.67%. Oxytocin plasma concentrations were anayzed using an enzymeimmuno assay kit (Assay Designs, Ann Arbor, MI) previously validated in rodents [55]. Intra-assay variation was less than 3% and inter-assay variation was approximately 10%, with the level of detection at 11.7 pg/ml and cross reactivity with other peptides at less than 0.01%. Samples were diluted 4 fold prior to assay. CSF concentrations of the serotonin metabolite 5-HIAA and the dopamine metabolite HVA were analyzed using liquid chromatography with electrochemical detection [56]. Plasma cortisol concentrations and CSF monoamine metabolite levels were analyzed for both adult females and infants. Plasma prolactin and oxytocin concentrations were analyzed only for adult females. Data on some physiological variables were available for only a subset of the study subjects. The sample sizes (or degrees of freedom) for each data analysis are provided in the Results section.
Behavioral data were available for a subset of lactating females (see Results). These females and their infants were observed from the day of parturition to the day before the beginning of the trapping procedures. No behavioral data were collected after the trapping procedures began. Females and their infants were observed during 30-min observation sessions a minimum of two times per week. Behavioral data were recorded on a paper checksheet and using a tape recorder whenever necessary. Behavior durations were recorded with a stopwatch. Behavioral data analyzed here included: frequencies of contacts made and broken by mothers and infants; frequencies of approaches and leaves within 1 m; frequencies of maternal restraining and rejection; time spent in nipple contact (nursing); time spent by mothers grooming their infants [see 8,57, for behavioral definitions]. Behavioral data were analyzed as frequencies and duration per hour of observation. Hourly rates of behavior were averaged across the first 3 months of lactation. As in previous studies [23], a Maternal Protectiveness Index was calculated combining measures of contact-making, approaching, and restraining; a Maternal Rejection Index was calculated combining measures of contact-breaking, leaving, and rejection; and a Maternal Warmth Index was calculated combining measures of nursing and grooming.
Statistical tests included Student’s t tests for paired and unpaired samples, Pearson’s correlations, analysis of variance (ANOVA), simple regression analyses and chi squares. All tests were two-tailed. Probabilities<0.05 were considered statistically significant.
3. Results
3.1. Differences between lactating and nonlactating females in physiological variables and correlations between these variables
Plasma concentrations of cortisol and prolactin were significantly higher in lactating than in nonlactating females (cortisol: t=2.54; df =45; p=0.01; prolactin: t=3.68; df =46; p=0.0006; Table 1). Plasma oxytocin concentrations were higher in lactating females as well, but the difference only approached statistical significance (t=1.83, df =43; p=0.07; Table 1). CSF HVA was significantly lower in lactating than in nonlactating females (t=−2.24; df =31; p=0.03), whereas there was no significant difference in CSF 5-HIAA between lactating and nonlactating females (Table 1). There were no significant correlations between plasma concentrations of cortisol, prolactin, and oxytocin. HVA was positively correlated with 5-HIAA (r=0.35, n=33, p=0.04) and negatively correlated with cortisol (r=−0.38; n=32; p=0.03) and oxytocin (r=−0.36, n=30; p=0.05) across all females. HVA was also positively correlated with prolactin (r=0.51; n=19; p=0.02) but only among lactating females.
Table 1.
Comparison of physiological variables (mean+SE) between lactating and nonlactating females
| Lactating females | Nonlactating females | |
|---|---|---|
| Plasma cortisol (ug/dl) | 39.98±1.98 | 32.17±2.39 |
| Plasma prolactin (ng/ml) | 38.67±7.75 | 8.25±1.76 |
| Plasma oxytocin (pg/ml) | 311.41±15.32 | 267.86±18.29 |
| CSF HVA (ng/ml) | 247.84±10.61 | 292.93±18.45 |
| CFS 5-HIAA (ng/ml) | 48.88±3.06 | 45.94±2.74 |
See text for statistical results.
Female age was not significantly correlated with any of the physiological variables. Low ranking females had significantly higher plasma cortisol levels (41.92±2.18 ug/dl) than high ranking females (30.48±1.75 ug/dl; F1,43=4.37; p=0.04), regardless of whether they were lactating or nonlactating. Rank had no significant main effects on plasma oxytocin and prolactin, nor on CSF 5-HIAA and HVA, and there was no significant interaction between rank and reproductive condition for any of these physiological variables. Dominance rank did not significantly affect any of the measures of maternal behavior or of infant behavior and physiology that are examined in the next sections.
3.2. Relationships between physiology and maternal behavior in lactating females
The Maternal Rejection Index was positively correlated with both maternal plasma cortisol levels (r=0.65; n=11; p=0.02; Fig. 1a) and with maternal CSF concentrations of 5-HIAA (r=0.66; n=9; p=0.05; Fig. 1b). There were no significant correlations between the Maternal Rejection Index and HVA, oxytocin or prolactin. The Maternal Protectiveness Index was not correlated with any maternal physiological variables. The Maternal Warmth Index was significantly positively correlated with plasma oxytocin levels (r=0.84; n=9; p=0.004; Fig. 1c).
Fig. 1.
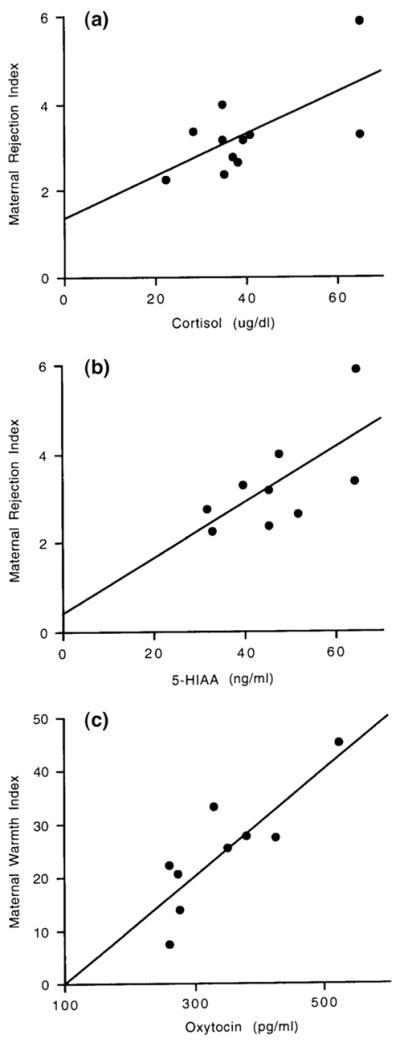
(a). Correlation between the mothers’ plasma cortisol levels and their Maternal Rejection Index. (b). Correlation between the mothers’ CSF 5-HIAA levels and their Maternal Rejection Index. (c). Correlation between the mothers’ plasma oxytocin levels and their Maternal Warmth Index.
3.3. Relation between maternal physiology/behavior and infant physiology/behavior
Plasma cortisol concentrations of infants (26.84±3.08 ug/dl) were significantly lower than those of their mothers (39.98±1.98 ug/dl; t=4.17; df =15; p =0.0008). Infants had significantly higher CSF concentrations of 5-HIAA and HVA than their mothers (5-HIAA: infants=132.07±5.55; mothers=48.88±3.06; t=13.13; df=12; p<0.0001; HVA: infants=381.36±17.42; mothers=247.84±10.61; t=4.74; df=12; p=0.0005). There was no significant correlation between infant and maternal cortisol levels, or between infant and maternal CSF levels of 5-HIAA or HVA.
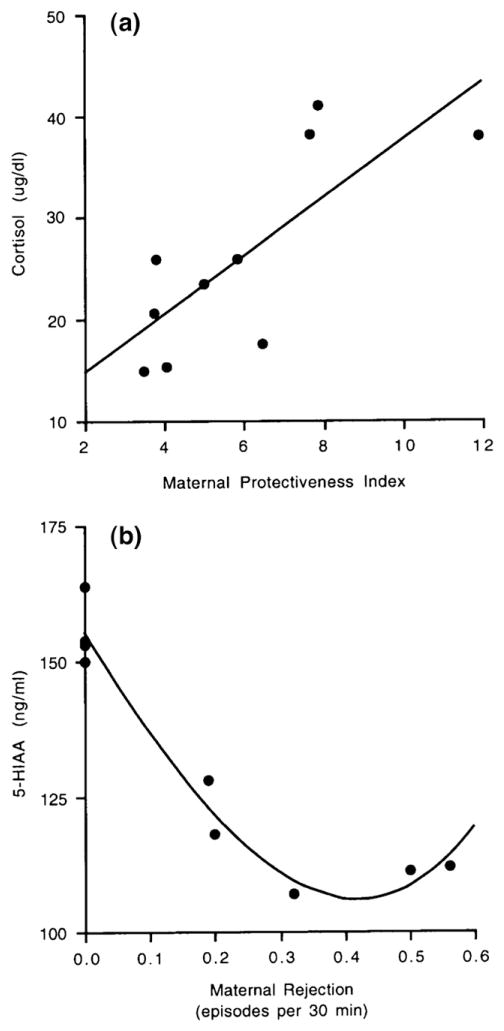
Variation in infant physiological measures was significantly predicted by maternal behavior. Specifically, variation in infant plasma cortisol levels was accounted for by the Maternal Protectiveness Index (r=0.77; n=10; p=0.009; Fig. 2a) so that infants who were protected more by their mothers had higher plasma cortisol levels. Furthermore, variation in infant CSF 5-HIAA levels was accounted for by the average maternal rejection rate in the first 3 months of life (r=−0.88, n=9; p=0.001; Fig. 2b) and more weakly, also by the Maternal Rejection Index (r=0.62; n=9; p=0.07). Thus, infants who were rejected more by their mothers had lower CSF concentrations of 5-HIAA.
Fig. 2.
(a). Correlation between the Maternal Protectiveness Index and the infants’ plasma cortisol levels. (b). Correlation between the average maternal rejection rates in the first 3 months of infant life and the infants’ CSF 5-HIAA levels.
In addition to infant physiology, infant behavior was predicted by maternal behavior as well. Differences in the Maternal Protectiveness and the Maternal Rejection Index were associated with differences in contact-breaking and contact-making behavior by infants. Specifically, infants who were protected more by their mothers broke contact with them more often (r=0.71; n=11; p=0.01). Both maternal protectiveness and infant cortisol predicted infant leaving behavior: infants who were protected more and had higher cortisol levels left their mothers more often than infants who were protected less and had lower cortisol levels (Protectiveness; r=0.74; n=11; p=0.009; cortisol: r=0.62; n=10; p=0.05). Conversely, the more infants were rejected by their mothers, the more they made contact with them (r=0.67; n=11; p=0.02). Differences in the Maternal Warmth Index were not associated with any differences in infant behavior.
4. Discussion
Our study suggests the following: 1) that lactation is accompanied by changes in concentrations of several hormones, neuropeptides, and monoamines; (2) that individual differences in some of these physiological variables among lactating females are associated with differences in mothering style; (3) and that variation in mothering style is associated with variation in both infant physiology and infant behavior.
Lactating females had higher plasma concentrations of cortisol than nonlactating females. In rhesus macaques and other primates, higher cortisol during lactation may be associated with motherhood-related psychosocial stress [58]. In our study, the lactating females had young and vulnerable infants while the nonlactating females were multiparous individuals with older offspring born in previous years. Thus, one possible interpretation of the difference in cortisol between lactating and nonlactating females is that mothers with young infants are more stressed than mothers of older offspring. Consistent with the notion that low dominance rank is associated with greater psychosocial stress [59], low ranking females had higher cortisol levels than high ranking females. The difference in cortisol between lactating and nonlactating females, however, was not affected by dominance rank. Therefore, changes in HPA axis activity associated with lactation do not appear to vary in relation to social status in free-ranging rhesus macaques. Consistent with previous findings obtained in other species of nonhuman primates [33,36], we found evidence for a relation between HPA axis activity and maternal behavior in free-ranging rhesus macaques. Specifically, the observed positive correlation between plasma cortisol levels and maternal rejection rates suggests that mothers who experienced higher levels of psychosocial stress, independent of dominance rank, rejected their infants more frequently than mothers with lower stress. In contrast, variation in the maternal protectiveness dimension of mothering style was not associated with plasma cortisol levels or any other physiological variables [23,39, but see 47]. These findings suggest that maternal rejection is a stress-sensitive component of mothering style in rhesus macaques. Consistent with this explanation, high maternal rejection rates are associated with neurochemical profiles suggestive of chronic stress [39] and are characteristic of females who were abused by their mothers in infancy and became themselves abusive mothers [27,28,57].
In addition to higher cortisol, lactating females also had higher plasma concentrations of prolactin and oxytocin than nonlactating females. Prolactin is involved in both milk production and responsiveness to stress, and prolactin levels are typically elevated in lactating females compared to nonlactating ones [50]. Prolactin levels are normally regulated by dopamine through a negative feedback mechanism. Prolactin activates dopaminergic neurons in the arcuate nucleus of the hypothalamus; these neurons project to the median eminence, releasing dopamine into the hypophyseal portal blood to reach D2 receptors on lactotrophs in the anterior pituitary and inhibit prolactin release [50]. During pregnancy and lactation, however, this feedback mechanism is suppressed and overridden by the inhibitory effect that suckling has on dopaminergic activity [60]. In a study of common marmosets (Callithrix jacchus), plasma prolactin concentrations were found to increase after infant carrying [61], suggesting that the release of this hormone can be modulated by parental behavior. Although studies of rodents have suggested that prolactin may facilitate the expression of maternal care, evidence that prolactin is important for maternal behavior in sheep and primates is equivocal [50]. In this study, we found no significant correlation between plasma prolactin levels and any measures of maternal behavior in lactating female rhesus macaques.
Oxytocin is released during labor and following nipple stimulation, thus resulting in higher plasma levels of this neuropeptide in lactating than in nonlactating females [62]. Similar to prolactin, oxytocin release is regulated by other neurotransmitters as well as by hormones such as estrogens, which may also increase the number of oxytocin receptors in specific areas of the brain [50]. A number of studies of rodents and sheep have implicated oxytocin in the regulation of maternal motivation and behavior [49,50, but see 63]. In the present study of rhesus macaques, the maternal warmth component of mothering style, which includes time spent nursing and grooming, was associated with maternal plasma oxytocin levels. This finding represents the first evidence linking oxytocin and maternal behavior in free-ranging primates. Preliminary evidence that oxytocin may be related to female responsiveness to infants was previously obtained with only 1 or 2 individuals tested in highly artificial laboratory conditions [52–54]. A previous study measuring oxytocin in free-ranging female rhesus macaques did not find any correlations between oxytocin and social behavior [64]. Moreover, Schwandt et al. [64] and Winslow et al. [65] found no correlation between plasma oxytocin and CSF oxytocin levels in rhesus monkeys. In recent human studies, however, plasma oxytocin levels were found to be positively correlated with mother–infant bonding (e.g. maternal affectionate touch, positive affect, and frequent checking of the infant) and with trait attachment scores [66–67]. Therefore, although the relation between plasma and brain oxytocin and that between brain oxytocin and primate maternal behavior remains unclear, there is now some evidence that plasma oxytocin concentrations may be related to maternal care and attachment in human and nonhuman primates.
In this study, lactating females had significantly lower CSF concentrations of HVA than nonlactating females, but similar CSF concentrations of 5-HIAA. Rodent studies of monoamines and their metabolites generally report changes in these neurotransmitter systems during pregnancy and lactation, but these changes are often contradictory and therefore poorly understood. The low CSF levels of HVA reported in lactating female rhesus macaques are likely the result of suckling’s inhibitory effect on the brain dopaminergic system and its failure to respond to stimulation from high prolactin levels [60]. The role of monoamine neurotransmitters in the regulation of maternal behavior is generally understudied in rodents [50], while studies of nonhuman primates have mostly reported correlations between the serotonin metabolite 5-HIAA and some aspects of maternal behavior [68]. In this study, maternal rejection was positively correlated with CSF 5-HIAA levels. This is consistent with the result of a previous study involving older rhesus mothers who abused their infants [39]. In another study involving young first-time mothers, however, high maternal rejection was associated with lower rather than higher CSF 5-HIAA levels [28]. Since brain serotonergic function in rhesus macaques has been shown to be influenced by early experience [23,69], it is possible that the relation between CSF levels of 5-HIAA changes as a function of reproductive and maternal experience.
In this study, we measured physiological and behavioral variables not only in mothers but also in infants. Blood and CSF samples were collected from infants who were at least 2 months old at the time of capture and were assayed only for plasma cortisol and serotonin and dopamine metabolite concentrations. Infants had lower cortisol levels but higher 5-HIAA and HVA levels than their mothers. There were no significant correlations between infant and maternal physiological variables, but variation in infant cortisol and 5-HIAA levels was predicted by variation in their mothers’ behavior. Specifically, infants who were protected more by their mothers had higher cortisol levels than those who were protected less, while infants who were rejected more had lower CSF 5-HIAA than infants who were rejected less. Previous studies have shown that infants reared by more protective mothers are delayed in the acquisition of their independence and fearful in response to challenges later in life, while highly rejected infants are more independent and more willing to explore novel objects and environments later in life (see Introduction for references). In this study, infants who were protected more by their mothers broke contact with them and left them more often, while the more infants were rejected by their mothers the more they made contact with them. The seeming contradiction between our results and those of previous studies is explained by the notion that the maternal behavior in monkeys has short- and long-term effects on infant behavior in opposite directions [70]. Maternal protectiveness has the short-term effect of increasing infant contact-breaking and leaving behavior [71,72], whereas in the long run it results in lower infant independence and clinginess to the mother [70]. Similarly, maternal rejection initially results in increased infant attempts to maintain contact with the mother [71,72], but in the long run it enhances infant independence [70].
Although the correlations between maternal and infant behavior reported here likely reflect short-term effects of mothering style on infant development and/or mother–infant conflict over the amount of time spent in contact [71,72], we suggest that the observed relationship between maternal behavior and infant physiology provides some hints as to the physiological mechanisms underlying long-term effects of early experience on infant development. In this study, infants who were protected more had higher cortisol levels than infants who were protected less. Higher cortisol levels may be one of the mechanisms through which these infants become behaviorally inhibited and hyperreactive to stress later in life when compared to the infants of less protective mothers. The negative correlation between maternal rejection rates and CSF 5-HIAA levels in the infants replicates a similar result obtained with another population of rhesus macaques [23,24]. In this previous study, this correlation was obtained with both cross-fostered and noncrossfostered infants, suggesting that it does not reflect genetic similarities between mothers and offspring. Rather, exposure to variable maternal rejection rates in infancy appears to affect the development of the offspring’s serotonergic system [58]. The brain serotonergic system is involved in the regulation of impulse control, and low CSF 5-HIAA is associated with high impulsivity and anxiety in both nonhuman primates and humans [69]. It is possible that exposure to moderate-to-high rates of maternal rejection early in life favors behavioral disinhibition through brain serotonergic mechanisms, whereas exposure to extremely high rates of rejection, such as those exhibited by rhesus abusive mothers, results in pathological impulsivity and anxiety.
Clearly, our correlational results cannot be interpreted as evidence of cause–effect relationships between maternal and infant behavior, or between physiological and behavioral variables. However, one possible interpretation of our findings, which is consistent with previous research, is that maternal behavior has short-term effects on infant behavior as well as long-lasting effects on infant physiology, including the activity of their HPA axis and brain monoaminergic systems. Long-term physiological alterations in infants, in turn, may affect their behavioral development and contribute to the previously reported association between exposure to particular mothering styles in infancy and particular profiles of behavior and responsiveness to the environment later in life [23,24]. Behavioral development is clearly also influenced by genetic characteristics, interactions between genotype and environment, and environmental situations encountered later in life. A comprehensive understanding of variability in development, therefore, requires an appreciation of the relative role of genes and experience and their interactions, experimental manipulations of both biological and environmental variables, and the longitudinal study of a much larger number of individuals than those used in this study. Nevertheless, our study contributes to the growing body of research investigating interindividual variability in maternal behavior and infant development in nonhuman primates, and to our understanding of the physiological mechanisms underlying such behavioral variability.
Acknowledgments
We thank Richelle Fulks and Misael Rivera for assistance with data collection, and Melissa Gerald and the staff of the Caribbean Primate Research Center for logistical support and assistance with animal capture and handling. This research was supported by NIH grant R21-AG029862 to D.M. This publication was made possible by grant number CM-5-P40RR003640 from the NIH National Center for Research Resources (NCRR) to the Caribbean Primate Research Center of the University of Puerto Rico.
Footnotes
Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NCRR or NIH.
References
- 1.Hinde RA, Spencer-Booth Y. The study of mother–infant interaction in captive group-living rhesus monkeys. Proc R Soc Lond B. 1968;169:177–201. [Google Scholar]
- 2.Hinde RA, Spencer-Booth Y. Towards understanding individual differences in rhesus mother–infant interaction. Anim Behav. 1971;19:165–73. [Google Scholar]
- 3.Altmann J. Baboons mothers and infants. Cambridge, MA: Harvard University Press; 1980. [Google Scholar]
- 4.Berman CM. Variation in mother–infant relationships: traditional and nontraditional factors. In: Small MF, editor. Female primates: studies by women primatologists. New York: Alan Liss; 1984. pp. 17–36. [Google Scholar]
- 5.Fairbanks LA. Individual differences in maternal styles: causes and consequences for mothers and offspring. Adv Study Behav. 1996;25:579–611. [Google Scholar]
- 6.Fairbanks LA. Parenting. In: Maestripieri D, editor. Primate psychology. Cambridge, MA: Harvard University Press; 2003. pp. 144–70. [Google Scholar]
- 7.Maestripieri D. The biology of human parenting: Insights from nonhuman primates. Neurosci Biobehav Rev. 1999;23:411–22. doi: 10.1016/s0149-7634(98)00042-6. [DOI] [PubMed] [Google Scholar]
- 8.Maestripieri D. Social and demographic influences on mothering style in pigtail macaques. Ethology. 1998;104:379–85. [Google Scholar]
- 9.Schino G, D’Amato FR, Troisi A. Mother–infant relationships in Japanese macaques: sources of interindividual variation. Anim Behav. 1995;49:151–8. [Google Scholar]
- 10.Tanaka I. Variability in the development of mother–infant relationships among free-ranging Japanese macaques. Primates. 1989;30:477–91. [Google Scholar]
- 11.Simpson MJA. Effects of early experience on the behaviour of yearling rhesus monkeys (Macaca mulatta) in the presence of a strange object: classification and correlation approaches. Primates. 1985;26:57–72. [Google Scholar]
- 12.Simpson AE, Simpson MJA. Short-term consequences of different breeding histories for captive rhesus macaque mothers and young. Behav Ecol Sociobiol. 1985;18:83–9. [Google Scholar]
- 13.Simpson MJA, Gore MA, Janus M, Rayment FDG. Prior experience of risk and individual differences in enterprise shown by rhesus monkey infants in the second half of their first year. Primates. 1989;30:493–509. [Google Scholar]
- 14.Simpson MJA, Datta SB. Predicting infant enterprise from early relationships in rhesus macaques. Behaviour. 1990;116:42–63. [Google Scholar]
- 15.Fairbanks LA, McGuire MT. Mother–infant relationships in vervet monkeys: response to new adult males. Int J Primatol. 1987;8:351–66. [Google Scholar]
- 16.Fairbanks LA, McGuire MT. Long-term effects of early mothering behavior on responsiveness to the environment in vervet monkeys. Dev Psychobiol. 1988;21:711–24. doi: 10.1002/dev.420210708. [DOI] [PubMed] [Google Scholar]
- 17.Fairbanks LA, McGuire MT. Maternal protectiveness and response to the unfamiliar in vervet monkeys. Am J Primatol. 1993;30:119–29. doi: 10.1002/ajp.1350300204. [DOI] [PubMed] [Google Scholar]
- 18.Vochteloo JD, Timmermans PJA, Duijghuisen JAH, Vossen JMH. Effects of reducing the mother’s radius of action on the development of mother–infant relationships in longtailed macaques. Anim Behav. 1993;45:603–12. [Google Scholar]
- 19.Bardi M, Bode AE, Ramirez SM, Brent LY. Maternal care and the development of the stress response. Am J Primatol. 2005;66:263–78. doi: 10.1002/ajp.20143. [DOI] [PubMed] [Google Scholar]
- 20.Bardi M, Huffman MA. Effects of maternal style on infant behavior in Japanese macaques (Macaca fuscata) Dev Psychobiol. 2002;41:364–72. doi: 10.1002/dev.10065. [DOI] [PubMed] [Google Scholar]
- 21.Bardi M, Huffman MA. Maternal behavior and maternal stress are associated with infant behavioral development in macaques. Dev Psychobiol. 2006;48:1–9. doi: 10.1002/dev.20111. [DOI] [PubMed] [Google Scholar]
- 22.Schino G, Speranza L, Troisi A. Early maternal rejection and later social anxiety in juvenile and adult Japanese macaques. Dev Psychobiol. 2001;38:186–90. doi: 10.1002/dev.1012. [DOI] [PubMed] [Google Scholar]
- 23.Maestripieri D, Higley JD, Lindell SG, Newman TK, McCormack KM, Sanchez MM. Early maternal rejection affects the development of monoaminergic systems and adult abusive parenting in rhesus macaques. Behav Neurosci. 2006;120:1017–24. doi: 10.1037/0735-7044.120.5.1017. [DOI] [PubMed] [Google Scholar]
- 24.Maestripieri D, McCormack KM, Lindell SG, Higley JD, Sanchez MM. Influence of parenting style on the offspring’s behavior and CSF monoamine metabolite levels in crossfostered and noncrossfostered female rhesus macaques. Behav Brain Res. 2006;175:90–5. doi: 10.1016/j.bbr.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 25.Fairbanks LA. Early experience and cross-generational continuity of mother–infant contact in vervet monkeys. Dev Psychobiol. 1989;22:669–81. doi: 10.1002/dev.420220703. [DOI] [PubMed] [Google Scholar]
- 26.Berman CM. Intergenerational transmission of maternal rejection rates among free-ranging rhesus monkeys. Anim Behav. 1990;39:329–37. [Google Scholar]
- 27.Maestripieri D. Early experience affects the intergenerational transmission of infant abuse in rhesus monkeys. Proc Natl Acad Sci U SA. 2005;102:9726–9. doi: 10.1073/pnas.0504122102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maestripieri D, Lindell SG, Higley JD. Intergenerational transmission of maternal behavior in rhesus monkeys and its underlying mechanisms. Dev Psychobiol. 2007;49:165–71. doi: 10.1002/dev.20200. [DOI] [PubMed] [Google Scholar]
- 29.Bardi M, Shimizu K, Barrett GM, Huffman MA, Borgognini-Tarli SM. Differences in the endocrine and behavioral profiles during the peripartum period in macaques. Physiol Behav. 2003;80:185–94. doi: 10.1016/j.physbeh.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 30.Maestripieri D, Wallen K. Interest in infants varies with reproductive condition in group-living female pigtail macaques (Macaca nemestrina) Physiol Behav. 1995;57:353–8. doi: 10.1016/0031-9384(94)00222-q. [DOI] [PubMed] [Google Scholar]
- 31.Maestripieri D, Zehr JL. Maternal responsiveness increases during pregnancy and after estrogen treatment in macaques. Horm Behav. 1998;34:223–30. doi: 10.1006/hbeh.1998.1470. [DOI] [PubMed] [Google Scholar]
- 32.Pryce CR, Dobeli M, Martin RD. Effects of sex steroids on maternal motivation in the common marmoset (Callithrix jacchus): development and application of an operant system with maternal reinforcement. J Comp Psychol. 1993;107:99–115. doi: 10.1037/0735-7036.107.1.99. [DOI] [PubMed] [Google Scholar]
- 33.Bahr NI, Pryce CR, Dobeli M, Martin RD. Evidence from urinary cortisol that maternal behavior is related to stress in gorillas. Physiol Behav. 1998;64:429–37. doi: 10.1016/s0031-9384(98)00057-2. [DOI] [PubMed] [Google Scholar]
- 34.Bardi M, French JA, Ramirez SM, Brent L. The role of the endocrine system in baboon maternal behavior. Biol Psychiatry. 2004;55:724–32. doi: 10.1016/j.biopsych.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 35.Bardi M, Shimizu K, Barrett GM, Borgognini-Tarli SM, Huffman MA. Peripartum cortisol levels and mother–infant interactions in Japanese macaques. Am J Phys Anthropol. 2003;120:298–304. doi: 10.1002/ajpa.10150. [DOI] [PubMed] [Google Scholar]
- 36.Bardi M, Shimizu K, Barrett GM, Borgognini-Tarli SM, Huffman MA. Peripartum sex steroids and maternal style in rhesus and Japanese macaques. Gen Comp Endocrinol. 2003;133:323–31. doi: 10.1016/s0016-6480(03)00193-x. [DOI] [PubMed] [Google Scholar]
- 37.Bardi M, Shimizu K, Borgognini-Tarli S. Mother–infant relationships and maternal estrogen metabolite changes in macaques (Macaca fuscata, M. mulatta) Primates. 2003;44:91–8. doi: 10.1007/s10329-002-0019-3. [DOI] [PubMed] [Google Scholar]
- 38.Bardi M, Shimizu K, Fujita S, Borgognini-Tarli S, Huffman MA. Hormonal correlates of maternal style in captive macaques (Macaca fuscata and M. mulatta) Int J Primatol. 2001;22:647–62. [Google Scholar]
- 39.Maestripieri D, Lindell SG, Ayala A, Gold PW, Higley JD. Neurobiological characteristics of rhesus macaque abusive mothers and their relation to social and maternal behavior. Neurosci Biobehav Rev. 2005;29:51–7. doi: 10.1016/j.neubiorev.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 40.Maestripieri D, Megna NL. Hormones and behavior in abusive and nonabusive rhesus macaque mothers. 2: Mother–infant interactions. Physiol Behav. 2000;71:43–9. doi: 10.1016/s0031-9384(00)00338-3. [DOI] [PubMed] [Google Scholar]
- 41.Nguyen N, Gesquiere L, Alberts SC, Altmann J. Endocrine and social sources of variation in the mother–infant relationship in wild baboons in Amboseli, Kenya. Am J Primatol. 2006;68:139–40. [Google Scholar]
- 42.Maestripieri D. Effects of early experience on female behavioural and reproductive development in rhesus macaques. Proc R Soc Lond B. 2005;272:1243–8. doi: 10.1098/rspb.2005.3059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ziegler TE, Wegner FH, Carlson AA, Lazaro-Perea C, Snowdon CT. Prolactin levels during the periparturitional period in the biparental cotton-top tamarin (Saguinus oedipus): interactions with gender, androgen levels, and parenting. Horm Behav. 2000;38:111–22. doi: 10.1006/hbeh.2000.1606. [DOI] [PubMed] [Google Scholar]
- 44.Graves FC, Wallen K, Maestripieri D. Opioids and attachment in rhesus macaque abusive mothers. Behav Neurosci. 2002;116:489–93. doi: 10.1037//0735-7044.116.3.489. [DOI] [PubMed] [Google Scholar]
- 45.Martel FL, Nevison CM, Rayment FD, Simpson MJA, Keverne EB. Opioid receptor blockade reduces maternal affect and social grooming in rhesus monkeys. Psychoneuroendocrinology. 1993;18:307–21. doi: 10.1016/0306-4530(93)90027-i. [DOI] [PubMed] [Google Scholar]
- 46.Cleveland A, Westergaard GC, Trenkle MK, Higley JD. Physiological predictors of reproductive outcome and mother–infant behaviors in captive rhesus macaque females (Macaca mulatta) Neuropsychopharmacology. 2004;29:901–10. doi: 10.1038/sj.npp.1300361. [DOI] [PubMed] [Google Scholar]
- 47.Fairbanks LA, Melega WP, McGuire MT. CSF 5-HIAA is associated with individual differences in maternal protectiveness in vervet monkeys. Am J Primatol. 1998;45:179–80. [Google Scholar]
- 48.Lindell SG, Higley JD, Shannon C, Linnoila M. Low levels of CSF 5-HIAA in female rhesus macaques predict mother–infant interaction patterns and mother’s CSF 5-HIAA correlates with infant’s CSF 5-HIAA. Am J Primatol. 1997;42:129. [Google Scholar]
- 49.Carter CS. Neuroendocrine perspectives on social attachment and love. Psychoneurondocrinology. 1998;223:779–818. doi: 10.1016/s0306-4530(98)00055-9. [DOI] [PubMed] [Google Scholar]
- 50.Numan M, Insel TR. The neurobiology of parental behavior. New York: Springer; 2003. [Google Scholar]
- 51.Young LJ, Winslow JT, Wang Z, Gingrich B, Guo Q, Matzuk MM, et al. Gene targeting approaches to neuroendocrinology: oxytocin, maternal behavior, and affiliation. Horm Behav. 1997;31:221–31. doi: 10.1006/hbeh.1997.1377. [DOI] [PubMed] [Google Scholar]
- 52.Boccia ML, Goursaud AS, Bachevalier J, Anderson KD, Pedersen CA. Peripherally administered non-peptide oxytocin antagonist, L368,899, accumulates in limbic brain areas: a new pharmacological tool for the study of social motivation in non-human primates. Horm Behav. 2007;52:344–51. doi: 10.1016/j.yhbeh.2007.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cooke B, Higley JD, Shannon C, Lindell SG, Higley HM, Suomi SJ, et al. Rearing history and CSF oxytocin as predictors of maternal competency in rhesus macaques. Am J Primatol. 1997;42:102. [Google Scholar]
- 54.Holman SD, Goy RW. Experiential and hormonal correlates of care-giving in rhesus macaques. Motherhood in human and nonhuman primates. In: Pryce CR, Martin RD, Skuse D, editors. Biosocial determinants. Basel: Karger; 1995. pp. 87–93. [Google Scholar]
- 55.Kramer KM, Cushing BS, Carter CS, Wu J, Ottinger MA. Sex and species differences in plasma oxytocin using an enzyme immunoassay. Can J Zool. 2004;82:1194–200. [Google Scholar]
- 56.Seppala T, Scheinin M, Capone A, Linnoila M. Liquid chromatographic assay for CSF catecholamines using electrochemical detection. Acta Pharmacol Toxicol. 1984;55:81–7. doi: 10.1111/j.1600-0773.1984.tb01966.x. [DOI] [PubMed] [Google Scholar]
- 57.Maestripieri D. Parenting styles of abusive mothers in group-living rhesus macaques. Anim Behav. 1998;55:1–11. doi: 10.1006/anbe.1997.0578. [DOI] [PubMed] [Google Scholar]
- 58.Maestripieri D, Hoffman CL, Fulks R, Gerald MS. Plasma cortisol responses to stress in lactating and nonlactating female rhesus macaques. Horm Behav. 2008;53:170–6. doi: 10.1016/j.yhbeh.2007.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sapolsky RM. The influence of social hierarchy on primate health. Science. 2005;308:648–52. doi: 10.1126/science.1106477. [DOI] [PubMed] [Google Scholar]
- 60.Voogt JL, Lee Y, Yang S, Arbogast L. Regulation of prolactin secretion during pregnancy and lactation. Progr Brain Res. 2001;133:173–85. doi: 10.1016/s0079-6123(01)33013-3. [DOI] [PubMed] [Google Scholar]
- 61.Roberts RL, Jenkins T, Lawler T, Wegner FH, Norcross JL, Bernhards DE, et al. Prolactin levels are elevated after infant carrying in parentally inexperienced common marmosets. Physiol Behav. 2001;72:713–20. doi: 10.1016/s0031-9384(01)00430-9. [DOI] [PubMed] [Google Scholar]
- 62.Russell JA, Leng G. Sex, parturition, and motherhood without oxytocin? J Endocrinol. 1998;157:343–59. doi: 10.1677/joe.0.1570343. [DOI] [PubMed] [Google Scholar]
- 63.Insel TR, Gingrich B, Young LJ. Oxytocin: who needs it? Progr Brain Res. 2001;133:59–66. doi: 10.1016/s0079-6123(01)33005-4. [DOI] [PubMed] [Google Scholar]
- 64.Schwandt ML, Howell S, Bales K, Jaffe BD, Westergaard GC, Higley JD. Associations between the neuropeptides oxytocin and vasopressin and the behavior of free-ranging female rhesus macaques (Macaca mulatta) Am J Phys Anthropol Suppl. 2007;44:210–1. [Google Scholar]
- 65.Winslow JT, Noble PL, Lyons CK, Sterk SM, Insel TR. Rearing effects on cerebrospinal fluid oxytocin concentration on social buffering in rhesus monkeys. Neuropsychopharmacology. 2003;28:910–8. doi: 10.1038/sj.npp.1300128. [DOI] [PubMed] [Google Scholar]
- 66.Feldman R, Weller A, Zagoory-Sharon O, Levine A. Evidence for a neuroendocrinological foundation of human affiliation: plasma oxytocin levels across pregnancy and the postpartum period predict mother–infant bonding. Psych Sci. 2007;18:965–70. doi: 10.1111/j.1467-9280.2007.02010.x. [DOI] [PubMed] [Google Scholar]
- 67.Tops M, Van Peer JM, Korf J, Wijers AA, Tucker DM. Anxiety, cortisol, and attachment predict plasma oxytocin. Psychophysiology. 2007;44:444–9. doi: 10.1111/j.1469-8986.2007.00510.x. [DOI] [PubMed] [Google Scholar]
- 68.Maestripieri D. The role of the brain serotonergic system in the origin and transmission of adaptive and maladaptive variations in maternal behavior in rhesus macaques. In: Bridges R, editor. Neurobiology of the parental mind. Amsterdam: Elsevier; 2008. pp. 163–73. [Google Scholar]
- 69.Higley JD. Aggression. In: Maestripieri D, editor. Primate psychology. Cambridge: Harvard University Press; 2003. pp. 17–40. [Google Scholar]
- 70.Hinde RA. Mother–infant relations in rhesus monkeys. In: White NF, editor. Ethology and psychiatry. Toronto: University of Toronto Press; 1974. pp. 29–46. [Google Scholar]
- 71.Maestripieri D. Parent–offspring conflict in primates. Int J Primatol. 2002;23:923–51. [Google Scholar]
- 72.Maestripieri D. Genetic aspects of mother–offspring conflict in rhesus macaques. Behav Ecol Sociobiol. 2004;55:381–7. [Google Scholar]