Abstract
Purpose
Pain in response to physical activity is common in people with chronic musculoskeletal pain and is likely a barrier to regular exercise, which would lead to a sedentary lifestyle. We recently developed a model of exercise-induced pain that is associated with increased activation of neurons in the medullary raphe nuclei, i.e., the nucleus raphe obscurus (NRO) and nucleus raphe pallidus (NRP). Because the NRO and NRP not only modulate motor output but also respond to noxious stimuli, we hypothesized that the NRO and NRP were key nuclei in the interaction between pain and exercise. We tested whether exercise enhances hyperalgesia through activation of N-methyl D-aspartate (NMDA) receptors in the NRO/NRP.
Methods
Muscle insult was induced by two injections of pH 5.0 saline 5 d apart into one gastrocnemius muscle. We initially tested whether hyperalgesia developed in mice injected with acidic saline (pH 5.0) into the gastrocnemius muscle immediately after a 30-min or 2-h exercise task or 2 h after a 2-h exercise task. Next, we tested whether blockade of NMDA receptors in the NRO/NRP during the exercise task prevented the development of exercise-induced hyperalgesia. Finally, we evaluated changes in phosphorylation of the NR1 subunit of the NMDA receptor (pNR1) after the exercise task at times in which muscle insult was given in behavioral experiments, i.e., immediately after a 30-min or 2-h exercise task or 2 h after the 2-h exercise task.
Results
All exercise conditions enhanced nociception (hyperalgesia) after combining with two injections of pH 5.0 saline. Microinjection of AP5 (1.0–0.1 nmol; 2-amino-5-phophonopenanoate) dose-dependently prevented the development of exercise-induced hyperalgesia. All exercise conditions increased pNR1 in the NRO and NRP.
Conclusions
Thus, exercise-induced pain in sedentary mice is associated with increased phosphorylation and activation of NMDA receptors in the NRO/NRP, suggesting that changes in central excitability mediate an interaction between unaccustomed exercise and pain.
Keywords: RAPHE PALLIDUS, RAPHE OBSCURUS, RAPHE MAGNUS, GLUTAMATE, PAIN, FATIGUE
In people with chronic musculoskeletal pain, a single bout of exercise enhances pain (8,29). Pain in response to physical activity could be a barrier to regular exercise and participation in rehabilitation and could consequently lead to patients choosing a sedentary lifestyle (8). We recently developed an animal model to investigate the underlying mechanisms of exercise-induced pain (28,36). In this exercise-induced pain model, we show increases in activation of neurons in the medullary raphe nuclei (28).
It is now generally accepted that brainstem pathways from the rostral ventromedial medulla (RVM) facilitate nociceptive responses as measured behaviorally or by recording from dorsal horn neurons (26). The nucleus raphe magnus (NRM) is a key nucleus within the RVM that mediates descending facilitation, contains neurons that express serotonin, and microinjection of low doses of glutamate into the NRM facilitates pain (26). Interestingly, the caudal raphe nuclei, i.e., the nucleus raphe obscurus (NRO) and nucleus raphe pallidus (NRP), also respond to noxious stimuli, express serotonin, and microinjection of low doses of glutamate into the NRO and NRP facilitates nociception (38). Although the NRO and NRP are well known to modulate motor output with descending projections to motor neurons, these neurons also project to the deep dorsal horn of the spinal cord and respond to noxious input (14,23). Descending facilitatory influences from the NRM not only mediate hyperalgesia associated with inflammation (see Porreca et al. [26]) and that associated with repeated intramuscular injection of pH 4.0 saline (6) but also respond to motor input (11). Thus, the medullary raphe nuclei may be an important link between exercise and pain.
N-methyl D-aspartate (NMDA) receptors in the RVM seem to play a significant role in the development of hyperalgesia. For example, blockade of glutamate receptors in the NRM reverses the hyperalgesia produced by colon inflammation, mustard oil inflammation of the hind limb, and repeated intramuscular acid injection (5,6,35). Further, there are alterations in the NMDA receptor subunits in the NRM after inflammation (22). Prior work from our laboratory shows that increasing expression of the NR1 subunit of the NMDA receptor in the RVM enhances nociception and decreasing expression prevents development of chronic muscle pain (7), showing the importance of NR1 in the development of pain behaviors. Others have shown that activation of cyclic AMP (cAMP) in nociceptive neurons enhances NMDA currents, phosphorylation of NR1 enhances channel conductance, and phosphorylation of NR1 increases trafficking of the NMDA receptor complex to the cell membrane (4,10,19). These data show the importance of NR1 phosphorylation in neuronal excitability.
Therefore, we tested the hypothesis that a whole-body exercise task results in an enhanced secondary hyperalgesia to repeated intramuscular acid injections through activation of NMDA receptors in the NRO and NRP.
METHODS
Mice
All experiments were approved by the University of Iowa Animal Care and Use Committee and were conducted in accordance with National Institutes of Health guidelines and adhered to the American College of Sports Medicine animal care standards. Mice used were on a C57Bl/ 6J background and were bred at the University of Iowa Animal Care Facility or purchased from Jackson Laboratories (Bar Harbor, ME). Both male (n = 43) and female mice (n = 35), 4–8 months of age, were used in these studies.
Behavior testing
Mice were trained to the running wheel, the grip force, and the restraining devices (cubicle and glove) two times per day for 2 d. Mice were placed in small clear cubicles on an elevated wire mesh table for 20 min to acclimate to the testing for paw withdrawal thresholds with von Frey filaments. Mice were placed in a glove for 5 min to acclimate for the testing of the mechanical muscle withdrawal thresholds with tweezers. Mice were placed in a running wheel for 10 min to acclimate to the wheel. For grip force, mice were familiarized to the apparatus by performing the grip force task three times at each training session.
Mechanical sensitivity of the paw was tested using von Frey filaments. A series of five von Frey filaments (0.07, 0.16, 0.33, 0.54, and 1.4 mN) was applied to the hind paws 10 times per trial, and this was repeated twice as previously described (28,36). The two trials were averaged to generate one number per testing period. There were three testing periods: before the first injection, 5 d after the first injection, and 24 h after the second injection. To measure muscle sensitivity, a pair of forceps was applied to the gastrocnemius muscle until the animal withdrew from the stimulus. Three trials on each side were given at each testing period and averaged. Grip force was used to measure fatigue immediately after the fatigue task by comparing with the grip force before the fatigue task for both the hind limbs and the forelimbs. Mice are pulled by the tail until they withdraw from a metal grip plate. Grip force was measured before and immediately after the run for both the forelimb and hind limb, and an average of five trials was recorded. A decrease in grip force after running was interpreted as muscle fatigue.
Implantation of guide cannulae
Intracerebral guide cannulae were placed in the NRO 7 d before the first intramuscular injection of either pH 5.0. The mice were anesthetized with ketamine/xylazine (dose) and positioned in a stereotaxic head holder. The skulls were exposed, and a small hole was drilled for placement of guide cannulae. The cannula was placed 6.8 mm caudal from the bregma (intra-aural = −6.8 mm, mediolateral = 0.0 mm, dorsoventral = −4.8 mm from the surface of the skull). The cannulae were secured to the skull with dental cement, and mice were allowed to recover before testing. To examine placement of the cannula, an equivalent volume of methylene blue dye was injected through the cannula at the end of the experiment. Mice were then euthanized; the brain was removed and postfixed in 10% formalin. The day before cutting, brains were transferred to 30% sucrose. Then, the brain was cross-sectioned into 35- to 40-μm sections in a cryostat and examined under a light microscope for placement of the cannula.
On day 5 of testing, the mice were injected, 15 min before a 2-h fatigue task, with 0.2 μL of AP5 (1 nmol) or vehicle (saline) into the cannula. Two hours after the fatigue task, mice were injected with the second injection of pH 5.0 into the left gastrocnemius muscle (4 h after AP5 injection).
Exercise task
Mice were placed in a running wheel and ran for 30 min or 2 h depending on the protocol. Mice generally ran continuously, but when they stopped running, they were encouraged to start again by gently tapping the wheel. This way, all mice ran at approximately the same speed, and mice within a group ran approximately the same distance. The distance each mouse ran was recorded for each mouse (m). Previous experiments show that the 2-h exercise task alone does not produce tissue damage and does not result in hyperalgesia 24 h later (28,36).
Intramuscular injection of nociceptive stimuli
To induce muscle insult, two injections of 20 μL of pH 5.0 sterile saline solution (corrected with 0.1N HCl) were given. Mice were briefly anesthetized with isoflurane (3%), and the injections were made into the left gastrocnemius muscle. Five days after the first injection, the gastrocnemius muscle was injected again with 20 μL of pH 5.0 saline. Injections were given immediately after the fatigue task (2 h or 30 min) or 2 h after the fatigue task or in a group of nonfatigued mice.
Immunohistochemistry for phosphorylation of the NR1 subunit of the NMDA receptor
Mice were transcardially perfused with heparinized saline followed by 4% paraformaldehyde. The brain was removed, stored in 30% sucrose overnight, and cut into 20-μm slides. All sections were immunohistochemically stained simultaneously for the phosphorylation of the NR1 subunit of the NMDA receptor (pNR1) (1:1000, Ser897; Millipore, Billerica, MA; catalog no. ABN99) using standard immunofluorescent techniques. Specifically, sections were blocked in 3% normal goat serum, avidin, and biotin before incubation in the primary antibody overnight at room temperature. The next day, sections were rinsed, blocked in 3% normal goat serum, and then incubated for 1 h at room temperature in biotinylated immunoglobulin G (1:1000; Invitrogen, Eugene, OR). These sections were then reacted with streptavidin conjugated to Alexa Fluor 568 (1:1000; Invitrogen) for 1 h at room temperature. Sections were coverslipped with Vectashield (Vector Labs, Inc., Burlingame, CA) and stored until analysis.
Images of the stained sections were taken in the Central Microscopy Facility at the University of Iowa. We used an Olympus BX-51 light microscope equipped with a SPOT camera (RT Slider; Diagnostic Instruments, Inc.). For a resolution sufficient for counting pNR1-positive cells, images were taken with a 20× objective lens. Five sections of the NRO and of the NRP were digitally imaged and stored for later analysis. Cells were quantified by manually counting total numbers in a given area using Image J software (National Institutes of Health). Specifically, a standard size (area of 10,430 μm2 (70 × 149 μm) for NRO and 5320 μm2 (70 × 76 μm) for NRP) was applied to each section. Cells were counted if they contained a nucleus and were positively stained for pNR1. As a control within animals, pNR1 was counted in the right and left facial nucleus for each group using the same procedures outlined above.
Protocol
The first series of experiments tested whether the muscle insult had to be near in time to the exercise task and whether it was necessary to perform the exercise task for 2 h to a level of fatigue. We therefore tested for differences in the development of hyperalgesia between 1) mice injected immediately after the 2-h exercise task and those injected 2 h after the 2-h exercise task and 2) mice injected immediately after a 2-h exercise task and mice injected immediately after a 30-min exercise task. Mice were therefore separated into groups for behavior experiments as follows: 1) non-exercised control mice (n = 22), 2) 2-h exercise task with acid injected immediately after the run (n = 16), 3) 30-min exercise task and injected immediately after the run (n = 12), and 4) 2-h exercise task and injected 2 h after the run (n = 16). A separate group of animals tested grip force after either the 2-h exercise task (n = 6) or the 30-min exercise task (n = 8).
The second experiment tested whether activation of NMDA receptors in the caudal raphe during the exercise task mediated the development of hyperalgesia 24 h later. We used a separate group of animals that performed a 2-h exercise task and were injected with acid 2 h after the exercise task to separate the drug effects between the exercise task and the intramuscular injection. In this group, we microinjected 1 nmol (n = 8), 0.3 nmol (n = 5), or 0.1 nmol (n = 5) of AP5 or vehicle (n = 6) 15 min before the exercise task.
In the third experiment, we tested whether the fatigue task increased NMDA receptor activity examining whether there were increases in pNR1 (Ser897). In a separate group of animals, we examined the number of cells stained for pNR1 immediately after the 30-min exercise task (n = 4), immediately after the 2-h exercise task (n = 4), or 2 h after the 2-h exercise task (n = 4) and compared with controls that did not exercise (n = 4). These were all times in which we injected the muscle with acidic saline in relation to the exercise task.
Statistical analysis
For the first series of experiments, a repeated-measures ANOVA tested for differences across time (dependent, paw mechanical sensitivity, three periods) and between groups (independent) for paw mechanical sensitivity. We also tested for differences between sex (independent) and fatigue condition (independent). For muscle mechanical sensitivity, we tested for differences between groups (independent) with a one-way ANOVA. For the second experiment, we tested for differences in mechanical sensitivity between drug dose and saline (independent) with a one-way ANOVA. For the third experiment, we tested for differences between groups (independent) with a one-way ANOVA. If there were significant effects with the ANOVA, appropriate post hoc testing was performed with a Tukey test for individual differences between groups. Values are presented as the mean ± SEM. P < 0.05 was considered significant.
RESULTS
Exercise task
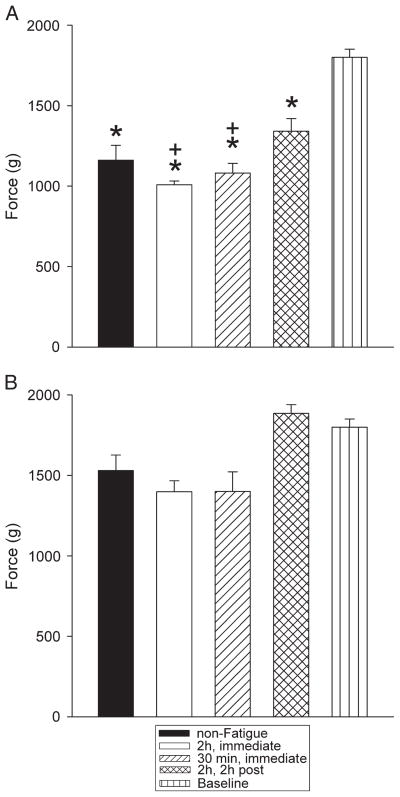
Animals ran 815 ± 43 and 727 ± 38 m for the groups injected immediately and 2 h after the 2-h exercise task, respectively. In the group that performed the 30-min exercise task, they ran 176 ± 29 m. This resulted in a similar speed of running for all groups: 2 h, immediate = 407 ± 29 m·h−1; 2 h, 2 h after = 364 ± 19 m·h−1; 30 min, immediate = 351 ± 59 m·h−1. There was no difference between males and females in running speed. Grip force significantly decreased (P < 0.05) immediately after the 2-h exercise task for both the hind paw and the forepaw to values averaging 92% ± 2.5% of baseline and 92% ± 1.3% of baseline, respectively. However, there was no significant change (P > 0.05) in grip force after the 30-min exercise task for the hind paw or the forepaw with values averaging 97% ± 3.7% of baseline and 100% ± 2.5% of baseline, respectively. The decrease in grip force was significantly greater for the group that performed the longer duration exercise (2 h) when compared with the group that performed the shorter duration exercise (30 min) (P < 0.05).
Mechanical sensitivity
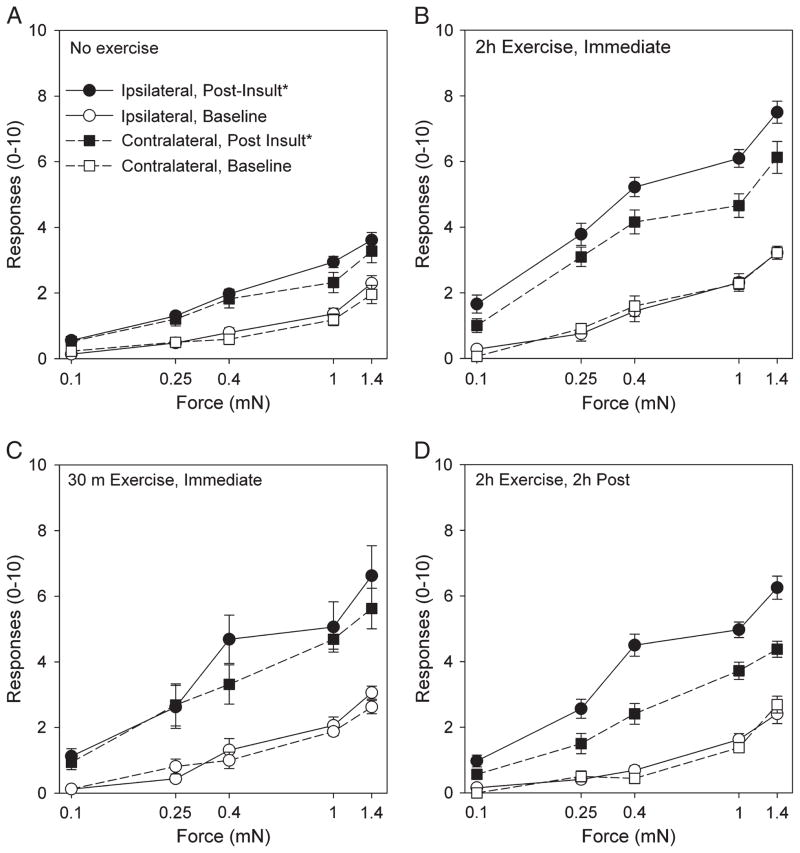
There were a significantly increased number of withdrawals to repeated application of von Frey filaments applied to the hind paw in the animals that performed the exercise task before injection of pH 5.0 saline when compared with those that did not perform the exercise task (P < 0.05). Repeated injections of pH 5.0 saline without the exercise task had no significant changes in responses to repeated stimuli when compared with baseline values (Fig. 1A). The enhanced effect occurred in animals injected immediately after the 2-h exercise task (Fig. 1B), immediately after the 30-min exercise task (Fig. 1C), and 2 h after the 2-h exercise task, both ipsilaterally and contralaterally (Fig. 1D).
FIGURE 1.
Graphs show the mechanical sensitivity of the paw in response to an exercise task in combination with muscle insult. The values before muscle insult are shown with open symbols, and those after the muscle insult, with or without exercise, are shown with closed symbols. The ipsilateral side is shown with circles, and the contralateral side is shown with squares. The number of withdrawals to repeated application of von Frey filaments of increasing bending forces applied to the paw in the group that did not receive the exercise task (A). *P < 0.05. The no-exercise group was significantly different from the group that performed the 2-h exercise task and with the second acid injection given immediately after the exercise task (B), the group that performed the 30-min exercise task and with the second acid injection given immediately after the exercise task (C), and the group that performed the 2-h exercise task and with the second acid injection given 2 h after the exercise task (D).
Muscle withdrawal thresholds were decreased 24 h after two pH 5.0 intramuscular injections similarly in all groups injected with pH 5.0 regardless of whether or not the animals performed the exercise task, when compared with a group not injected with acidic saline (P < 0.05). However, those that were injected immediately after the exercise task (2-h or 30-min task) showed a greater decrease than the group injected 2 h after the exercise task and the nonexercised group (P < 0.05) (Fig. 2).
FIGURE 2.
Withdrawal threshold of the muscle for the ipsilateral (A) and the contralateral (B) sides is shown for each group. *Significantly different from uninjected controls (P < 0.05); +significantly different from pH 5.0 injections without the exercise task (P < 0.05).
Because we have previously shown a difference between males and females after exercise-enhanced carrageenan injection (28), we analyzed both male and female mice. There were no differences between males and females with both showing an equivalent increase in responses to repeated application of von Frey filaments and an equivalent decrease in withdrawal thresholds of the muscle.
Effect of blockade of NMDA receptors
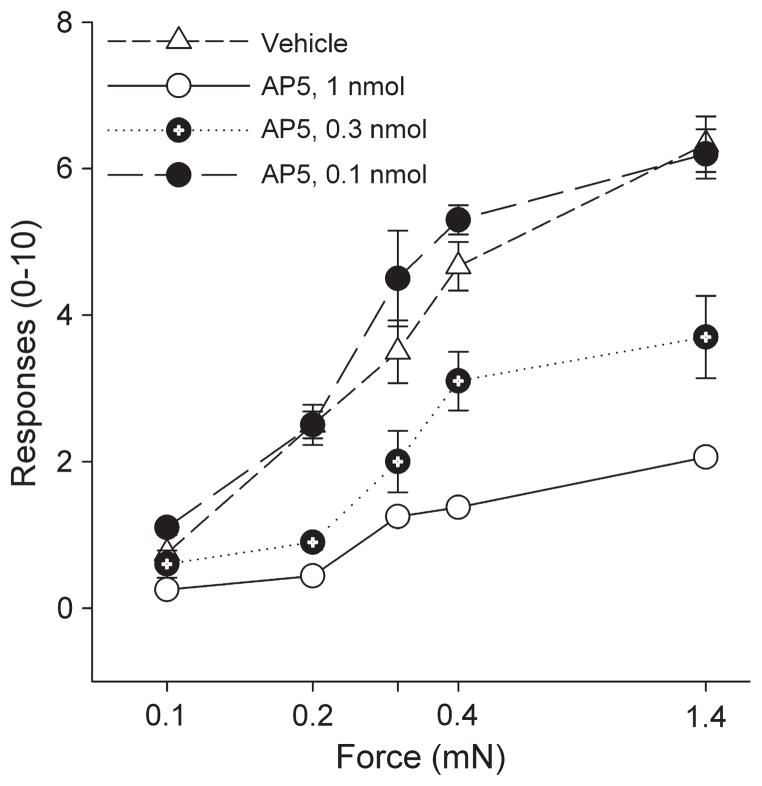
Because NMDA receptors in the brainstem are critical for the development of hyperalgesia after muscle insult (6) and the NRO and NRP are activated by the exercise task, we examined whether blockade of NMDA receptors in the NRO/ NRP during the fatigue task would prevent the development of hyperalgesia 24 h later. AP5 (0.1, 0.3, and 1 nmol) microinjected into the NRO/NRP just before the exercise task prevented the development of hyperalgesia 24 h later when compared with vehicle-injected controls in a dose-dependent manner (Fig. 3). The highest dose (1-nmol dose) significantly prevented the exercise-induced hyperalgesia when compared with the 0.3 and 0.1 nmol and vehicle control group (P < 0.05).
FIGURE 3.
Line graphs represent the number of withdrawal to repeated application of von Frey filaments for different bending forces in animals that were pretreated in the NRO/NRP with different doses of AP5 or vehicle before the exercise task. The withdrawal thresholds were significantly lower in the animals treated with 1 and 0.3 nmol of AP5. *Significantly different from vehicle, P < 0.05.
Increased expression of pNR1 in response to the exercise task
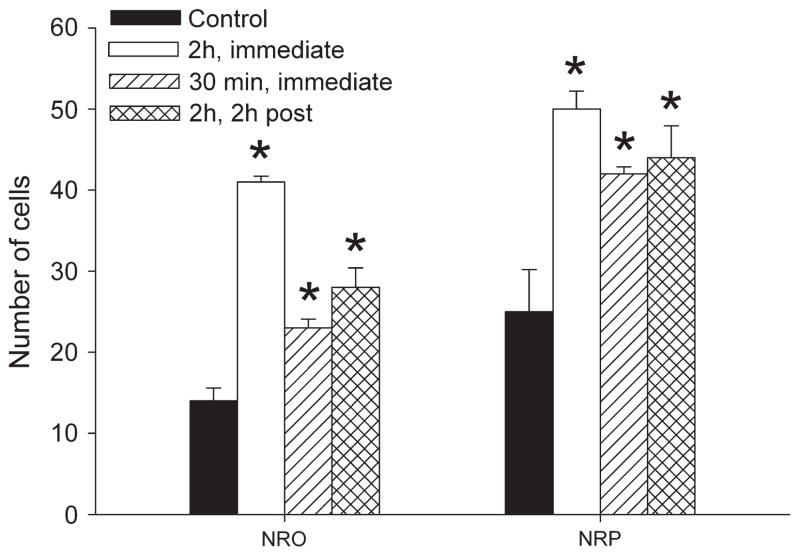
To test whether NMDA receptors are activated in response to the exercise task, we examined the expression of pNR1 immediately and 2 h after the 2-h exercise task and immediately after the 30-min exercise task, when the second intramuscular acid injection was performed. Cell bodies immunostained for pNR1 in both the NRO and the NRP after all exercise conditions and representative sections are shown in Figure 4. Quantification of the number of cells stained for pNR1 showed there was a significant increase in the number of immunostained cells for pNR1 immediately after the 2-h exercise task, immediately after the 30-min exercise task, and 2 h after the 2-h exercise task when compared with controls that did not exercise (Fig. 5) (P < 0.05). To control for potential staining differences among animals, we counted the number of pNR1-positive cells in the facial nucleus of the medulla for each group bilaterally. There was no difference between controls (left = 138 ± 5, right = 147 ± 4) and immediately after the 30-min exercise task (left = 139 ± 2, right = 138 ± 4), immediately after the 2-h exercise task (left = 143 ± 4, right = 142 ± 1), and 2 h after the 2-h exercise task (left = 139 ± 2, right = 142 ± 2).
FIGURE 4.
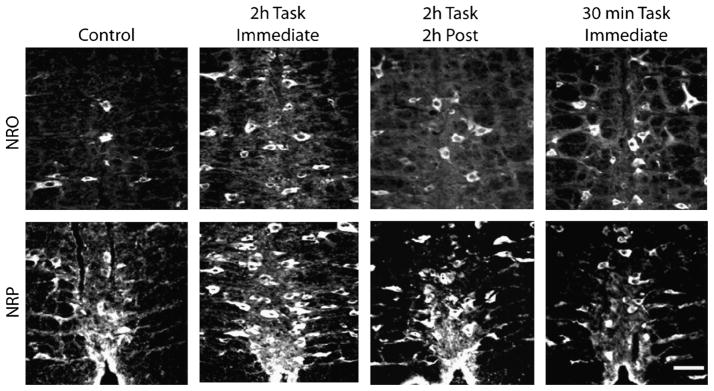
Immunohistochemical staining for pNR1 in the NRO and the NRP in each group: unexercised control, 2-h exercise immediately after task, 2-h exercise 2 h after task, and 30-min exercise immediately after task. Notice the increased number of positively labeled cells after the fatigue task compared with controls.
FIGURE 5.
Bar graph showing the number of cells in five sections counted from the NRO and the NRP in each group: unexercised control, 2-h exercise immediately after task, 2-h exercise 2 h after task, and 30-min exercise immediately after task. Significant increases occurred for all groups with the greatest increase immediately after the 2-h exercise task. *Significantly less than P < 0.05.
DISCUSSION
The current study shows that unaccustomed exercise in sedentary mice enhances the nociceptive response to low-dose muscle insult. The exercise task increases pNR1 in the NRO and NRP, and blockade of NMDA receptors in these same nuclei during the exercise task prevents the enhanced nociceptive response to muscle insult. We propose that there is an interaction between pain and exercise at the level of the brainstem that is modulated by physical activity.
These data are consistent with our prior studies showing that unaccustomed physical activity enhances the response to low-dose muscle insult with acidic saline or carrageenan (28,36). We extend these findings by showing that a shorter duration exercise task, i.e., 30 min, also enhanced the response to a low-dose muscle insult. This 30-min task was not associated with measurable muscle fatigue immediately after the task suggesting that muscle fatigue is not a necessary component to the enhancement of pain. It suggests, rather, that unaccustomed exercise activates pathways, peripherally or centrally, that enhance the response to muscle insult.
Interestingly, we show that the exercise task increases the number of raphe neurons that are positive for phosphorylated NR1 in the caudal medullary raphe nuclei, i.e., NRO and NRP. We interpret this as an increased pNR1. Classically, the NRO and the NRP are thought to modulate motor responses. We previously showed that the 2-h exercise task increases c-fos expression in these nuclei (28), and prior studies showed increased neuronal activity in these nuclei during fatiguing exercise tasks and spontaneous treadmill running (12,16,32). NRO and NRP neurons project to motor nuclei in the brainstem and the ventral horn of the spinal cord (13,20). The NRO and the NRP are the caudal extension of the NRM located in the rostral medulla. The NRM is well known for its role in pain modulation including facilitation of nociceptive responses (for a review, see Porreca et al. [26]). However, the role of the NRO and NRP in processing of nociceptive information is less clear. Noxious stimulation evokes an increase in c-fos expression in the NRO (25) and directly activates neurons in the NRO and NRP (9). Electrical or chemical stimulation of neurons in the NRO and the NRP, similar to the NRM, enhances both tail flick withdrawal and dorsal horn neuron responses to noxious stimuli (38). In contrast, electrical or chemical stimulation of the NRO and NRP at higher intensities, similar to the NRM, inhibits the tail flick and dorsal horn neuron responses to noxious stimuli (38). Together, these data support that the NRO and the NRP can modulate noxious stimulation in addition to motor responses. We propose that these nuclei are key sites to the interaction between exercise and pain.
The potential ability of the NRO and the NRP to modulate nociceptive activity may lie at sites outside the spinal cord. As stated above, the NRO and the NRP primarily send projections to the ventral horn of the spinal cord; however, nociceptive information is processed in the dorsal horn. It is possible that the NRO and NRP modulate the NRM, which has been shown to modulate nociceptive transmission (26). Indeed, there are projections from the NRO and, to a lesser extent, the NRP to the NRM as well as other nuclei in the RVM involved in nociceptive modulation (37). On the other hand, the projections from NRO and NRP to the spinal cord include the deep dorsal horn and lamina VII (17). In fact, Jones and Light (17) show that NRM and NRP have similar spinal projections in the rat innervating all laminae of the dorsal and ventral horn with strongest projections to the deep dorsal horn (30%) and the ventral horn (33%). Deep tissue input from muscle sends input to the deep dorsal horn, and noxious stimulation of muscle activates neurons in the deep dorsal horn and laminae VII and X (15,30). Thus, the NRO and the NRP could produce their effects by modulating nociceptive information at the level of the spinal cord and/or through the NRM. Further, NRO/NRP could be an interface between nociceptive and motor responses to such stimulus.
We propose that NMDA receptors in the NRO and the NRP are sensitized by unaccustomed exercise, which then sets them up to respond in an exaggerated way to a minor muscle insult. The current study shows that there is enhanced pNR1 (Ser897, protein kinase A site) in the NRO and NRP immediately and 2 h after the exercise task—times when muscle insult is given. In the NRM, activation of the cAMP pathway increases activity of cells containing μ-opioid receptors; these cells are thought to facilitate nociception (2). Further, in conditions of increased nociceptive activity, induced by chronic morphine exposure, there are increases in glutamatergic transmission in the RVM that is prevented by blockade of the cAMP pathway (2). Although it is not known whether the NRO and NRP also contain similar cell types as the NRM, such as ON cells (facilitation cells), in other conditions, they respond similarly to noxious stimuli as NRM cells (38). Works in other brain areas also show enhanced neuronal activity in response to activation of the cAMP pathway (3,31,39). More specifically, activation of the cAMP pathway enhances NMDA receptor activity (3,39) and increases surface expression of NMDA channels (4). These data together support the notion that the increased pNR1 results in enhanced neuronal activity and sensitivity of raphe neurons.
We and others previously show a role for glutamate in the medullary raphe in the facilitation of pain. Specifically, there is an increased release of glutamate in the NRM during muscle insult with acidic saline (27), low doses of glutamate microinjected into the medullary raphe (NRO, NRP, NRM) facilitate nociception (38), and blockade of NMDA receptors in the NRM prevents or reverses hyperalgesia in several animal models of pain (6,34). We suggest that the NRO and NRP also involve activation of NMDA receptors that result in pain in response to a combination of exercise in a sedentary animal with a muscle insult. In particular, the current study shows that blockade of NMDA receptors in the NRO and NRP during the exercise task prevented the onset of hyperalgesia 24 h after a subsequent injection of a low-dose muscle insult. We gave the NMDA microinjection before the 2-h exercise task and injected the muscle insult 2 h after the completion of the exercise task, 4 h after the NMDA microinjection. We therefore expect that the muscle insult was given after the NMDA antagonist was no longer effective. This would suggest that there is increased glutamate during the exercise task that activates NMDA receptors to sensitize these neurons to subsequent stimuli. This may occur through increases in activation of the cAMP pathway.
In individuals with chronic musculoskeletal pain, increased physical activity can exacerbate pain (8,29). This increase in pain not only occurs during the increased activity but also outlasts the task, sometimes for days. Pain in response to physical activity could provide a barrier to regular exercise and participation in rehabilitation and could consequently lead to a sedentary lifestyle. Quantitative sensory testing shows enhanced temporal summation in patients with fibromyalgia after a single exercise bout, suggesting that this effect is due to enhanced central excitability (18). People with osteoarthritis, fibromyalgia, and chronic fatigue syndrome also present with physical fatigue manifested as decreased strength, decreased endurance, general feelings of muscle weakness and fatigue, and an increased sense of effort during exercise (21,24,33). In contrast, a regular exercise program reduces pain in a variety of chronic musculoskeletal conditions (1). Understanding the mechanisms that produce pain in response to unaccustomed exercise could lead to strategies to reduce the enhanced pain in response to unaccustomed exercise, allowing people with chronic pain to engage in regular physical activity and rehabilitation.
Acknowledgments
This work was supported by grants AR053509 and AR052316 from the National Institutes of Health.
The authors thank the Central Microscopy Facility at the University of Iowa for technical support with imaging.
Footnotes
There is no conflict of interest for Kathleen A. Sluka, Jessica Danielson, Lynn Rasmussen, or Luis Felipe DaSilva. The authors have declared that no competing interests exist.
The results of the present study do not constitute endorsement by the American College of Sports Medicine.
References
- 1.Bement MK. Exercise-induced hypoalgesia: an evidence-based review. In: Sluka KA, editor. Mechanisms and Management of Pain for the Physical Therapist. Seattle (WA): IASP Press; 2009. pp. 143–66. [Google Scholar]
- 2.Bie B, Pan ZZ. Increased glutamate synaptic transmission in the nucleus raphe magnus neurons from morphine-tolerant rats. Mol Pain. 2005;1:7. doi: 10.1186/1744-8069-1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cerne R, Jiang M, Randic M. Cyclic adenosine 3′5′-monophosphate potentiates excitatory amino acid and synaptic responses of rat spinal dorsal horn neurons. Brain Res. 1992;596:111–23. doi: 10.1016/0006-8993(92)91538-p. [DOI] [PubMed] [Google Scholar]
- 4.Chen BS, Roche KW. Regulation of NMDA receptors by phosphorylation. Neuropharmacology. 2007;53:362–8. doi: 10.1016/j.neuropharm.2007.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coutinho SV, Urban MO, Gebhart GF. Role of glutamate receptors and nitric oxide in the rostral ventromedial medulla in visceral hyperalgesia. Pain. 1998;78:59–69. doi: 10.1016/S0304-3959(98)00137-7. [DOI] [PubMed] [Google Scholar]
- 6.Da Silva LFS, Desantana JM, Sluka KA. Activation of NMDA receptors in the brainstem, rostral ventromedial medulla, and nucleus reticularis gigantocellularis mediates mechanical hyper-algesia produced by repeated intramuscular injections of acidic saline in rats. J Pain. 2010;11:378–87. doi: 10.1016/j.jpain.2009.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Da Silva LFS, Walder RY, Davidson BL, Wilson SP, Sluka KA. Changes in expression of NMDA-NR1 receptor subunits in the rostral ventromedial medulla modulates pain behaviors. Pain. 2010;151(1):155–61. doi: 10.1016/j.pain.2010.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Damsgard E, Thrane G, Anke A, Fors T, Roe C. Activity-related pain in patients with chronic musculoskeletal disorders. Disabil Rehabil. 2010;32:1428–37. doi: 10.3109/09638280903567877. [DOI] [PubMed] [Google Scholar]
- 9.Dantas MA, Co W, Futuro-Neto HA. Responses of neurons of the nucleus raphe obscurus to noxious stimuli. Braz J Med Biol Res. 1990;23:923–6. [PubMed] [Google Scholar]
- 10.Ehlers MD, Tingley WG, Huganir RL. Regulated subcellular distribution of the NR1 subunit of the NMDA receptor. Science. 1995;269:1734–7. doi: 10.1126/science.7569904. [DOI] [PubMed] [Google Scholar]
- 11.Fields HL, Malick A, Burstein R. Dorsal horn projection targets of ON and OFF cells in the rostral ventromedial medulla. J Neurophysiol. 1995;74:1742–59. doi: 10.1152/jn.1995.74.4.1742. [DOI] [PubMed] [Google Scholar]
- 12.Fornal CA, Martin-Cora FJ, Jacobs BL. “Fatigue” of medullary but not mesencephalic raphe serotonergic neurons during locomotion in cats. Brain Res. 2006;1072:55–61. doi: 10.1016/j.brainres.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 13.Fort P, Luppi PH, Sakai K, Salvert D, Jouvet M. Nuclei of origin of monoaminergic, peptidergic, and cholinergic afferents to the cat trigeminal motor nucleus: a double-labeling study with cholera-toxin as a retrograde tracer. J Comp Neurol. 1990;301:262–75. doi: 10.1002/cne.903010209. [DOI] [PubMed] [Google Scholar]
- 14.Holstege JC, Kuypers HG. Brainstem projections to spinal moto-neurons: an update. Neuroscience. 1987;23:809–21. doi: 10.1016/0306-4522(87)90160-6. [DOI] [PubMed] [Google Scholar]
- 15.Hu JY, Zhao ZQ. Differential contributions of NMDA and non-NMDA receptors to spinal Fos expression evoked by superficial tissue and muscle inflammation in the rat. Neuroscience. 2001;106:823–31. doi: 10.1016/s0306-4522(01)00299-8. [DOI] [PubMed] [Google Scholar]
- 16.Jacobs BL, Martin-Cora FJ, Fornal CA. Activity of medullary serotonergic neurons in freely moving animals. Brain Res Brain Res Rev. 2002;40:45–52. doi: 10.1016/s0165-0173(02)00187-x. [DOI] [PubMed] [Google Scholar]
- 17.Jones SL, Light AR. Termination patterns of serotoninergic medullary raphespinal fibers in the rat lumbar spinal cord: an anterograde immunohistochemical study. J Comp Neurol. 1990;297:267–82. doi: 10.1002/cne.902970209. [DOI] [PubMed] [Google Scholar]
- 18.Kadetoff D, Kosek E. The effects of static muscular contraction on blood pressure, heart rate, pain ratings and pressure pain thresholds in healthy individuals and patients with fibromyalgia. Eur J Pain. 2007;11:39–47. doi: 10.1016/j.ejpain.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 19.Lin Q, Wu J, Willis WD. Effects of protein kinase A activation on the responses of primate spinothalamic tract neurons to mechanical stimuli. J Neurophysiol. 2002;88:214–21. doi: 10.1152/jn.2002.88.1.214. [DOI] [PubMed] [Google Scholar]
- 20.Manaker S, Tischler LJ, Morrison AR. Raphespinal and reticulospinal axon collaterals to the hypoglossal nucleus in the rat. J Comp Neurol. 1992;322:68–78. doi: 10.1002/cne.903220106. [DOI] [PubMed] [Google Scholar]
- 21.Mengshoel AM, Vollestad NK, Forre O. Pain and fatigue induced by exercise in fibromyalgia patients and sedentary healthy subjects. Clin Exp Rheumatol. 1995;13:477–82. [PubMed] [Google Scholar]
- 22.Miki K, Zhou QQ, Guo W, et al. Changes in gene expression and neuronal phenotype in brain stem pain modulatory circuitry after inflammation. J Neurophysiol. 2002;87:750–60. doi: 10.1152/jn.00534.2001. [DOI] [PubMed] [Google Scholar]
- 23.Millhorn DE, Hokfelt T, Verhofstad AA, Terenius L. Individual cells in the raphe nuclei of the medulla oblongata in rat that contain immunoreactivities for both serotonin and enkephalin project to the spinal cord. Exp Brain Res. 1989;75:536–42. doi: 10.1007/BF00249904. [DOI] [PubMed] [Google Scholar]
- 24.Nijs J, De Meirleir K, Wolfs S, Duquet W. Disability evaluation in chronic fatigue syndrome: associations between exercise capacity and activity limitations/participation restrictions. Clin Rehabil. 2004;18:139–48. doi: 10.1191/0269215504cr708oa. [DOI] [PubMed] [Google Scholar]
- 25.Pinto M, Lima D, Castro-Lopes J, Tavares I. Noxious-evoked c-fos expression in brainstem neurons immunoreactive for GABAB, mu-opioid and NK-1 receptors. Eur J Neurosci. 2003;17:1393–402. doi: 10.1046/j.1460-9568.2003.02586.x. [DOI] [PubMed] [Google Scholar]
- 26.Porreca F, Ossipov MH, Gebhart GF. Chronic pain and medullary descending facilitation. Trends Neurosci. 2002;25:319–25. doi: 10.1016/s0166-2236(02)02157-4. [DOI] [PubMed] [Google Scholar]
- 27.Radhakrishnan R, Sluka KA. Increased glutamate and decreased glycine release in the rostral ventromedial medulla during induction of a pre-clinical model of chronic widespread muscle pain. Neurosci Lett. 2009;457:141–5. doi: 10.1016/j.neulet.2009.03.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sluka KA, Rasmussen LA. Fatiguing exercise enhances hyper-algesia to muscle inflammation. Pain. 2010;148(2):188–97. doi: 10.1016/j.pain.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Staud R, Robinson ME, Price DD. Isometric exercise has opposite effects on central pain mechanisms in fibromyalgia patients compared to normal controls. Pain. 2005;118:176–84. doi: 10.1016/j.pain.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 30.Taguchi T, John V, Hoheisel U, Mense S. Neuroanatomical pathway of nociception originating in a low back muscle (multifidus) in the rat. Neurosci Lett. 2007;427:22–7. doi: 10.1016/j.neulet.2007.08.021. [DOI] [PubMed] [Google Scholar]
- 31.Vadakkan KI, Wang H, Ko SW, et al. Genetic reduction of chronic muscle pain in mice lacking calcium/calmodulin-stimulated ade-nylyl cyclases. Mol Pain. 2006;2:7. doi: 10.1186/1744-8069-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Veasey SC, Fornal CA, Metzler CW, Jacobs BL. Response of serotonergic caudal raphe neurons in relation to specific motor activities in freely moving cats. J Neurosci. 1995;15:5346–59. doi: 10.1523/JNEUROSCI.15-07-05346.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wallman KE, Sacco P. Sense of effort during a fatiguing exercise protocol in chronic fatigue syndrome. Res Sports Med. 2007;15:47–59. doi: 10.1080/15438620601184331. [DOI] [PubMed] [Google Scholar]
- 34.Wei H, Pertovaara A. MK-801, an NMDA receptor antagonist, in the rostroventromedial medulla attenuates development of neuropathic symptoms in the rat. Neuroreport. 1999;10:2933–7. doi: 10.1097/00001756-199909290-00011. [DOI] [PubMed] [Google Scholar]
- 35.Xu M, Kim CJ, Neubert MJ, Heinricher MM. NMDA receptor-mediated activation of medullary pro-nociceptive neurons is required for secondary thermal hyperalgesia. Pain. 2007;127:253–62. doi: 10.1016/j.pain.2006.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yokoyama T, Lisi TL, Moore SA, Sluka KA. Muscle fatigue increases the probability of developing hyperalgesia in mice. J Pain. 2007;8:692–9. doi: 10.1016/j.jpain.2007.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zagon A. Internal connections in the rostral ventromedial medulla of the rat. J Auton Nerv Syst. 1995;53:43–56. doi: 10.1016/0165-1838(94)00164-f. [DOI] [PubMed] [Google Scholar]
- 38.Zhuo M, Gebhart GF. Biphasic modulation of spinal nociceptive transmission from the medullary raphe nuclei in the rat. J Neurophysiol. 1997;78:746–58. doi: 10.1152/jn.1997.78.2.746. [DOI] [PubMed] [Google Scholar]
- 39.Zou X, Lin Q, Willis WD. Role of protein kinase A in phosphorylation of NMDA receptor 1 subunits in dorsal horn and spino-thalamic tract neurons after intradermal injection of capsaicin in rats. Neuroscience. 2002;115:775–86. doi: 10.1016/s0306-4522(02)00490-6. [DOI] [PubMed] [Google Scholar]