Summary
Background
We assessed effectiveness, safety, and tolerability of paclitaxel or fluorouracil when added to radiation plus cisplatin followed by adjuvant chemotherapy in a programme of selected bladder preservation for patients with muscle invasive bladder cancer.
Methods
In our randomised phase 2 trial, we enrolled patients with T2–4a transitional cell carcinoma of the bladder at 24 medical centres in the USA. We randomly allocated patients to receive paclitaxel plus cisplatin (paclitaxel group) or fluorouracil plus cisplatin (fluorouracil group) with twice-daily radiation in random block sizes per site on the basis of clinical T-stage (T2 vs T3–4). Patients and physicians were aware of treatment assignment. All patients had transurethral resection of bladder tumour and twice-daily radiotherapy to 40·3 Gy, along with allocated chemotherapy, followed by cystoscopic and biopsy assessment of response. Patients who had a tumour response with downstaging to T0, Tcis, or Ta received consolidation chemoradiotherapy to 64·3 Gy, with the same chemotherapy regimen as in the induction phase. Patients received adjuvant cisplatin-gemcitabine-paclitaxel after the end of chemoradiotherapy. If, after induction, persistent disease was graded as T1 or worse, we recommended patients undergo cystectomy and adjuvant chemotherapy. We assessed the primary endpoints of rates of treatment completion and toxic effects in all randomly allocated patients. This study is registered with ClinicalTrials.gov, number NCT00055601.
Findings
Between Dec 13, 2002, and Jan 11, 2008, we enrolled 97 patients, of whom 93 were eligible for analysis. Median follow-up was 5·0 years (IQR 5·0–6·2). Of 46 patients in the paclitaxel group, 45 (98%) completed induction (16 [35%] with grade 3–4 toxicity), 39 (85%) completed induction and consolidation (11 [24%] with grade 3–4 toxicity due to consolidation), and 31 (67%) completed the entire protocol with adjuvant chemotherapy. 34 (85%) of 40 assessable patients in the paclitaxel group had grade 3–4 toxicity during adjuvant chemotherapy. Of 47 patients in the fluorouracil group, 45 (96%) completed induction (nine [19%] with grade 3–4 toxicity), 39 (83%) completed induction and consolidation (12 [26%] had grade 3–4 toxicity due to consolidation), and 25 (53%) completed the entire protocol with adjuvant chemotherapy. 31 (76%) of 41 assessable patients in the fluorouracil group had grade 3–4 toxicity during adjuvant chemotherapy. Five (11%) patients treated with the paclitaxel regimen and three (6%) patients treated with the fluorouracil regimen developed late grade 3–4 radiotherapy toxicities. 11 (24%) patients treated with the paclitaxel regimen and 16 (34%) patients treated with the fluorouracil regimen developed late grade 3–4 toxicities unrelated to radiotherapy. One patient (in the fluorouracil group) died during follow-up. Six (13%) patients in the paclitaxel group and in three (6%) patients in the fluorouracil group discontinued due to treatment-related toxicity.
Interpretation
In the absence of phase 3 data, our findings could inform selection of a bladder-sparing trimodality chemotherapy regimen for patients with muscle invasive bladder cancer.
Funding
US National Cancer Institute.
Introduction
Definitive treatments for localised muscle invasive bladder cancer can be broadly divided into two groups—those that seek to remove the bladder and those that try to spare the bladder. In the past 50 years, oncologists have embraced organ-preserving therapies in many cancers. The best outcomes are usually achieved when each patient is assessed in a multidisciplinary setting and the team of physicians, including surgeons, medical and radiation oncologists, and other specialists, jointly assess whether surgical extirpation can be avoided or reserved as a salvage option without compromising survival of the patient.
Bladder-sparing strategies combine maximum transurethral resection of bladder tumour (TURBT) followed by a course of concurrent radiotherapy and sensitising chemotherapy. An early assessment of treatment response is often done with cystoscopy and rebiopsy sampling either during chemoradiotherapy or soon afterwards. Incomplete responders are advised to undergo immediate cystectomy. Patients with conserved bladders are followed up with close cystoscopic surveillance, with prompt salvage cystectomy at the first sign of invasive recurrence.
When based on clinical rather than pathological staging, contemporary cystectomy series1–5 report 5-year overall survival of 40–60% for clinical T2 or greater muscle invasive bladder cancer. In the absence of data from randomised trials, caution should be used when comparisons are made between bladder-preservation treatments and upfront cystectomy, because selection of patients and differences between clinical and pathological staging influences these outcomes. Improved radiotherapy techniques combined with better concurrent chemotherapeutic regimens and prompt salvage of recurrences have made native bladder preservation possible for many patients, while not compromising the chance for cure.6 Studies with long-term follow-up7 have allayed fears of substantial rates of late tumour recurrences and of poor bladder function. European Association of Urology consensus guidelines accept bladder preservation for selected patients with muscle invasive bladder cancer.8 The National Comprehensive Cancer Network updated their 2013 guidelines9 in view of the growing evidence supporting use of bladder-preserving approaches in selected patients with clinical T2 and T3 bladder cancer.
Radiation combined with cisplatin as a sensitiser is the most studied strategy for bladder preservation in the USA, although several other chemotherapy drugs have been assessed. The optimum combination of chemotherapeutic drugs needs to be identified not only to improve efficacy but also to minimise toxic effects related to treatment. We aimed to assess addition of paclitaxel or fluorouracil to cisplatin and radiotherapy to improve treatment options for patients with muscle invasive bladder cancer.
Methods
Study design and participants
In our multicentre, randomised phase 2 study, we recruited patients with primary carcinomas of the bladder and histological evidence of muscularis propria invasion, AJCC10 clinical stages T2–4a, Nx or N0, and M0, without hydronephrosis. We enrolled patients at 24 centres in the USA (appendix). Patients were regarded as potential surgical candidates by participating urologists. Patients with involvement of the prostatic urethra with transitional-cell cancer that was visibly completely resected and without evidence of stromal invasion of the prostate remained eligible. If radiological assessment of a lymph node was interpreted as positive, taking into consideration the location and size criteria of greater than 1 cm in any dimension, the lymph node had to be further assessed by lymphadenectomy or percutaneous needle biopsy sampling. Patients with confirmed node metastases on histology or cytology were not eligible for inclusion. Patients were regarded as having an adequately functioning bladder by urologist and radiation oncologist and had undergone a maximal transurethral resection (maximum defined as safe as possible) of the bladder tumour. Study participants were regarded as capable of tolerating systemic chemotherapy combined with pelvic radiotherapy and a radical cystectomy by the joint agreement of the participating urologist, radiation oncologist, and medical oncologist. Eligible patients had to have a Zubrod performance status of 1 or lower and a haemoglobin concentration of at least 0·1 g/L, a white blood cell count of at least 4000 cells per mL, an absolute neutrophil count of at least 1800 cells per mL, a platelet count of at least 100 000 platelets per μL, a serum creatinine of 132·6 μmol/L or less, a serum bilirubin of 34·2 μmol/L or less, and a creatinine clearance of at least 1 mL/s; if a patient's creatinine clearance was more than 1 mL/s, then a serum creatinine of up to 159·1 mmol/L was allowed at the discretion of the study chair.
Previous systemic chemotherapy, pelvic radiotherapy, and concurrent drugs that had potential nephrotoxicity or ototoxicity were not permitted. Previous or concurrent malignancy were permitted if the patient has been disease-free for at least 5 years (patients with stage T1a prostate cancer, carcinoma in situ of the uterine cervix, or non-melanoma skin cancer within the previous 5 years could also be enrolled). The protocol was approved by institutional review boards at each study site and patients signed a study-specific informed consent form before study entry.
Randomisation and masking
We randomly allocated patients to receive paclitaxel plus cisplatin with twice-daily radiation (paclitaxel group) or fluorouracil plus cisplatin with twice-daily radiation (fluorouracil group). Randomisation was done with a central interactive voice response system in random block sizes per site, stratified by clinical T stage (T2 vs T3–4). To balance treatment groups, we used an allocation scheme as described by Zelen.11 The allocation sequence was generated and trial group assignment was done at the Radiation Therapy Oncology Group (RTOG) headquarters. Patients and physicians were aware of treatment assignment.
Procedures
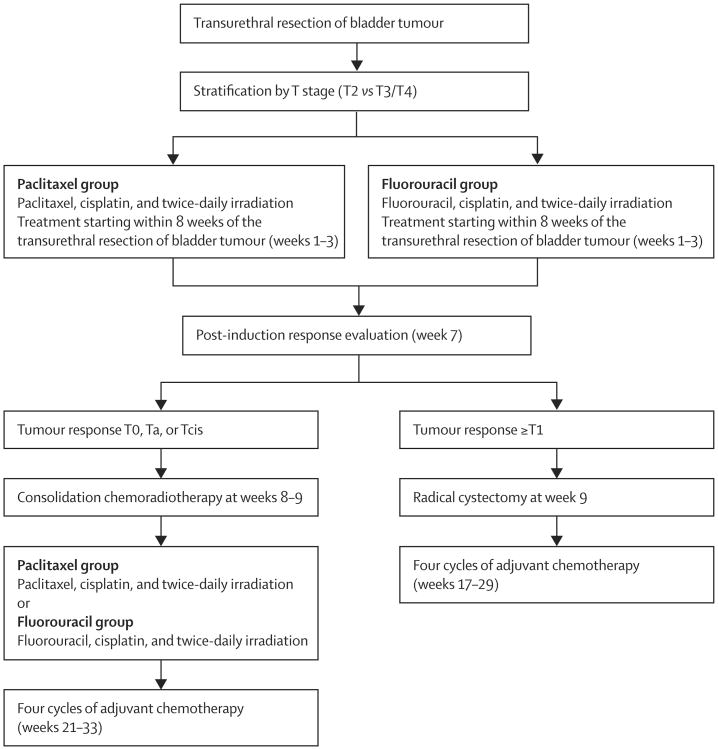
We screened and enrolled patients before protocol-defined treatment. Protocol-defined treatment began within 8 weeks after transurethral resection and endoscopic assessment. After TURBT, we stratified patients by T stage and randomly allocated them to treatment groups (figure 1). Induction therapy was 13 days of concomitant-boost radiotherapy. Patients were treated with 1·6 Gy radiotherapy to the pelvis in the morning for all 13 days, and in the evening they received 1·5 Gy to the bladder for the first five sessions and 1·5 Gy to the tumour for the last eight sessions (20·8 Gy to the pelvis, 28·3 Gy to whole bladder, and 40·3 Gy to the bladder tumour). The minimal interval between treatments was 4 h.
Figure 1. Treatment scheme.
Patients in the paclitaxel group received paclitaxel 50 mg/m2 on days 1, 8, and 15, and cisplatin 15 mg/m2 on days 1–3, 8–10, and 15–17. Patients in the fluorouracil group received fluorouracil 400 mg/m2 and cisplatin 15 mg/m2 on days 1–3, 8–10, and 15–17. Patients who achieved a complete response (CR; defined as T0) or near CR (defined as Ta or Tcis) received consolidation chemoradiation consisting of 1·5 Gy pelvic radiotherapy delivered twice per day for 8 days to 24 Gy (total dose: 64·3 Gy to the tumour and 44·8 Gy to the pelvic lymph nodes) with the following chemotherapy regimens: paclitaxel 50 mg/m2 on days 1 and 8 and cisplatin 15 mg/m2 on days 1, 2, 8, and 9 in the paclitaxel group and fluorouracil 400 mg/m2 on days 1–3 and 8–10 with cisplatin 15 mg/m2 on days 1,2, 8, and 9 in the fluorouracil group. Patients who had tumours staged as T1 or greater after the induction phase were treated with cystectomy. Adjuvant chemotherapy began 12 weeks after consolidation chemoradiotherapy or 8 weeks after cystectomy. Adjuvant chemotherapy was gemcitabine 1000 mg/m2, paclitaxel 50 mg/m2, and cisplatin 35 mg/m2 given on days 1 and 8 and repeated every 21 days for four cycles, with appropriate dose changes on the basis of toxic effects.
We followed-up patients with conserved bladders with cystoscopy, tumour-site rebiopsy sampling, bimanual examination under anaesthesia, urine cytology, and abdominal/pelvic CT scans, and chest radiographs every 3 months in the first year, every 4 months in the second year, every 6 months for the following 3 years, and once per year thereafter. After 1–2 years, in patients with negative assessments by office endoscopic evaluation and cytology, the biopsy sampling and examination under anaesthesia were usually omitted. Patients were considered for intravesical therapy for non-invasive recurrences and salvage radical cystectomy for invasive recurrences.
The primary endpoints of this phase 2 trial were completion and safety of the two regimens. We regarded a patient as having completed a radiotherapy and chemotherapy regimen if they had been treated per protocol or with acceptable variation guidelines specified based on review. We did not collect data for treatment delays and dose reductions. We graded adverse events according to the Common Terminology Criteria for Adverse Events, version 2.0. Secondary endpoints included complete response after induction therapy, defined as a negative bimanual examination under anaesthesia and all biopsy samples negative for any tumour at the sites of the pretreatment tumour or tumours, bladder-intact survival, bladder function, and the value of biomarkers as prognostic factors. Bladder function and biomarker analyses will be reported separately. We measured time to bladder-intact survival from the date of randomisation to occurrence of cystectomy or death; in the case of censoring, we used the last follow-up date. Overall survival from the date of randomisation was not included in the secondary endpoints during the trial design. Cause of death was defined by the investigators and reported in follow-up case report forms.
Statistical analysis
We designed this study to detect a 90% completion rate versus a 70% protocol completion rate in previous RTOG studies for each chemotherapy regimen. We used a two-sided binomial test with a null hypothesis of 70% completion rate against the alternative 90% completion rate. On the basis of this binomial distribution, we required 39 patients per group to test the hypothesis with a significance level of 0·05 and a power of 87%. From the RTOG 95-06 trial, we expected about 10% of eligible patients to stop per-protocol treatment before reaching the adjuvant chemotherapy phase. To allow for this dropout, and another 10% for ineligible cases, we planned to enrol 48 patients per treatment group. For analysis of completion, toxicity and complete response rate after induction, we assumed the treatment groups had a binomial distribution and calculated percentage completion with exact confidence intervals.12 We calculated overall survival and bladder-intact survival with the Kaplan-Meier method,13 with table estimates and graphs. We included unknown T stages in the denominator at each treatment phase (induction, consolidation, and adjuvant). After the induction phase, we treated patients with unknown T stages as having not completed either consolidation or adjuvant treatment. We did all analyses for this paper with SAS software, version 9.2.
This study is registered with ClinicalTrials.gov, number NCT00055601.
Results
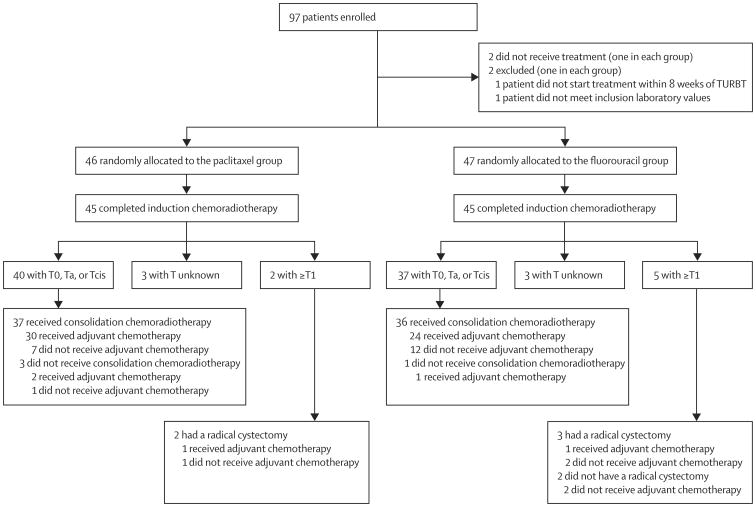
Between Dec 13, 2002, and Jan 11, 2008, we enrolled 97 patients, two of whom did not receive treatment and two did not meet inclusion criteria (figure 2). Median follow-up was 5·0 years (IQR 5·0–6·2) for the 93 eligible participants who were randomly allocated to treatment groups (table 1).
Figure 2. Trial profile.
TURBT=transurethral resection of bladder tumour.
Table 1. Baseline characteristics.
| Paclitaxel group (n=46) | Fluorouracil group (n=47) | |
|---|---|---|
| Age, years | 65 (48–82; 60–73) | 67 (44–83; 61–74) |
|
| ||
| Sex | ||
| Male | 38 (83%) | 40 (85%) |
| Female | 8 (17%) | 7 (15%) |
|
| ||
| Zubrod performance status | ||
| 0 | 42 (91%) | 42 (89%) |
| 1 | 4 (9%) | 5 (11%) |
|
| ||
| Tumour stage | ||
| T2 | 44 (96%) | 44 (94%) |
| T3–4 | 2 (4%) | 3 (6%) |
Data are median (range; IQR) or n (%).
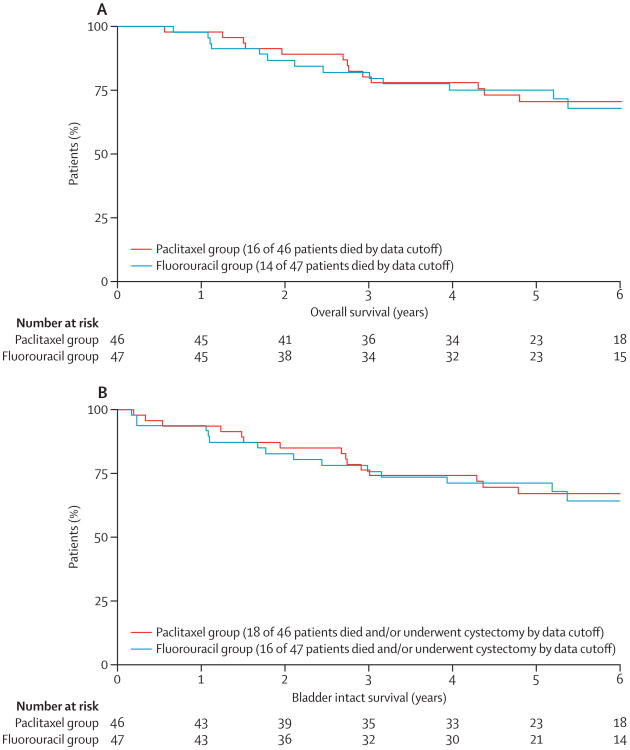
By data cutoff on Oct 16, 2012, 16 patients in the paclitaxel group and 14 patients in the fluorouracil group had died. 5-year overall survival was 71% (95% CI 57–84) in the paclitaxel group and 75% (62–88) in the fluorouracil group, whereas median overall survival in each group was not reached (figure 3). Ten of 16 patients in the paclitaxel group died from bladder cancer, two died from secondary primary cancers, and four died from other causes. In the fluorouracil group, six (43%) of 14 patients died from bladder cancer, one patient died from secondary primary cancer, one died from treatment complications, and six died from other causes. 5-year bladder-intact survival was 67% (95% CI 53–81) in the paclitaxel group and 71% (57–84) in the fluorouracil group (figure 3).
Figure 3. Kaplan-Meier curves for overall survival (A) and bladder-intact survival (B), by treatment group.
45 (98%, 95% CI 89–100) of 46 patients completed induction with the paclitaxel regimen; 40 (89%) of these 45 patients had T0, Ta, or Tcis. Two patients in the paclitaxel group had bladder disease staged as T1 or worse and both patients underwent early salvage cystectomy. 45 (96%, 95% CI 86–99) of 47 patients completed induction with the fluorouracil regimen; 37 (82%) of these 45 patients achieved a response of T0, Ta, or Tcis. Five patients in the fluorouracil group had bladder disease staged as T1 or worse, but only three of these patients underwent early salvage cystectomy (figure 2). After the induction phase, 33 (72%, 95% CI 57–84) patients had a CR in the paclitaxel group as did 30 (62%, 46–76) in the fluorouracil group.
16 patients (35%) in the paclitaxel group and nine patients (19%) in the fluorouracil group developed grade 3–4 toxic effects after induction chemoradiotherapy (table 2). Most toxic effects were haematological, metabolic, or genitourinary.
Table 2. Toxic effects associated with induction chemotherapy and radiotherapy.
| Paclitaxel group (n=46) | Fluorouracil group (n=47) | |||||||
|---|---|---|---|---|---|---|---|---|
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 1 | Grade 2 | Grade 3 | Grade 4 | |
| Allergy or immunology | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Auditory or hearing | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Blood or bone marrow | 21 | 7 | 5 | 1 | 18 | 7 | 3 | 1 |
| Cardiovascular (arrhythmia) | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 |
| Cardiovascular (general) | 5 | 0 | 0 | 0 | 2 | 0 | 0 | 0 |
| Constitutional symptoms | 23 | 4 | 0 | 0 | 19 | 8 | 1 | 0 |
| Dermatological or skin | 7 | 2 | 0 | 0 | 4 | 2 | 0 | 0 |
| Gastrointestinal | 25 | 15 | 0 | 1 | 27 | 15 | 0 | 0 |
| Haemorrhage | 2 | 2 | 1 | 0 | 2 | 4 | 0 | 0 |
| Hepatic | 10 | 3 | 1 | 0 | 9 | 0 | 1 | 0 |
| Infection febrile neutropenia | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Metabolic or laboratory | 13 | 6 | 5 | 3 | 11 | 8 | 3 | 0 |
| Musculoskeletal | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Neurology | 6 | 6 | 1 | 0 | 8 | 1 | 0 | 0 |
| Other | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Pain | 7 | 2 | 2 | 0 | 7 | 3 | 0 | 0 |
| Pulmonary | 1 | 5 | 0 | 0 | 2 | 1 | 0 | 0 |
| Renal or genitourinary | 15 | 8 | 4 | 0 | 18 | 8 | 2 | 0 |
| Sexual or reproductive function | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 |
No grade 5 toxic effects were reported. Patients could report >1 adverse event (graded according to Common Terminology Criteria for Adverse Events, version 2.0).
39 (85%, 95% CI 71–94) patients in the paclitaxel group and 39 (83%, 69–92) in the fluorouracil group completed induction and consolidation regimens. 11 (24%) patients in the paclitaxel group and 12 (26%) in the fluorouracil group developed grade 3–4 toxic effects after the consolidation phase (table 3). Nine patients in the paclitaxel group and two patients in the fluorouracil group had grade 3 or 4 metabolic toxicities. Fluorouracil consolidation resulted in five genitourinary and three gastrointestinal grade 3–4 toxic effects, compared with no patients treated with paclitaxel consolidation.
Table 3. Toxic effects associated with consolidation chemotherapy and radiotherapy.
| Paclitaxel group (n=46) | Fluorouracil group (n=47) | |||||||
|---|---|---|---|---|---|---|---|---|
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 1 | Grade 2 | Grade 3 | Grade 4 | |
| Blood or bone marrow | 19 | 13 | 3 | 0 | 24 | 6 | 3 | 0 |
| Cardiovascular (general) | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Constitutional symptoms | 21 | 9 | 0 | 0 | 23 | 3 | 2 | 0 |
| Dermatological or skin | 11 | 1 | 1 | 0 | 3 | 0 | 1 | 0 |
| Endocrine | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Gastrointestinal | 13 | 17 | 0 | 0 | 21 | 5 | 3 | 0 |
| Haemorrhage | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hepatic | 10 | 2 | 0 | 0 | 3 | 0 | 0 | 0 |
| Infection febrile neutropenia | 1 | 1 | 0 | 0 | 0 | 2 | 0 | 0 |
| Lymphatics | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Metabolic or laboratory | 9 | 7 | 8 | 1 | 12 | 6 | 2 | 0 |
| Musculoskeletal | 1 | 0 | 0 | 0 | 2 | 1 | 0 | 0 |
| Neurology | 9 | 4 | 0 | 0 | 3 | 2 | 2 | 0 |
| Other | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 |
| Pain | 7 | 2 | 0 | 0 | 4 | 1 | 0 | 0 |
| Pulmonary | 3 | 3 | 0 | 0 | 1 | 0 | 0 | 0 |
| Renal or genitourinary | 20 | 8 | 0 | 0 | 15 | 6 | 4 | 1 |
| Sexual or reproductive function | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
No grade 5 toxic effects were reported. Patients could report >1 adverse event (graded according to Common Terminology Criteria for Adverse Events, version 2.0).
31 of 39 patients in the paclitaxel group who completed consolidation phase were able to complete adjuvant chemotherapy, yielding an overall treatment completion rate of 67% (95% CI 51–80). 25 of 39 patients in the fluorouracil group who completed consolidation phase were able to complete adjuvant chemotherapy, yielding an overall treatment completion rate of 53% (38–68). Of 42 patients in the paclitaxel group who were assessable after induction chemoradiotherapy, 40 started adjuvant chemotherapy and were analysed for adjuvant chemotherapy toxicity. Of 42 patients in the fluorouracil group who were assessable after induction chemoradiotherapy, 41 started adjuvant chemotherapy and were analysed for adjuvant chemotherapy toxicity. 34 (85%) of these patients in the paclitaxel group and 31 (76%) patients in the fluorouracil group developed grade 3–4 toxic effects as a result of undergoing adjuvant chemotherapy (table 4), with most of these patients having haematological adverse events.
Table 4. Toxic effects associated with adjuvant chemotherapy.
| Paclitaxel group (n=40) | Fluorouracil group (n=41) | |||||||
|---|---|---|---|---|---|---|---|---|
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 1 | Grade 2 | Grade 3 | Grade 4 | |
| Auditory or hearing | 1 | 2 | 1 | 0 | 1 | 0 | 0 | 0 |
| Blood or bone marrow | 5 | 3 | 20 | 9 | 2 | 5 | 15 | 12 |
| Cardiovascular (arrhythmia) | 1 | 1 | 1 | 0 | 2 | 1 | 0 | 0 |
| Cardiovascular (general) | 3 | 1 | 1 | 1 | 5 | 3 | 1 | 0 |
| Constitutional symptoms | 18 | 13 | 1 | 0 | 9 | 13 | 8 | 0 |
| Dermatological or skin | 11 | 5 | 0 | 0 | 5 | 5 | 1 | 0 |
| Endocrine | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Gastrointestinal | 16 | 8 | 1 | 0 | 13 | 10 | 1 | 0 |
| Haemorrhage | 3 | 0 | 3 | 0 | 2 | 1 | 1 | 0 |
| Hepatic | 9 | 3 | 2 | 0 | 13 | 6 | 0 | 0 |
| Infection febrile neutropenia | 0 | 2 | 3 | 1 | 1 | 2 | 4 | 2 |
| Lymphatics | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Metabolic or laboratory | 3 | 9 | 10 | 1 | 8 | 8 | 9 | 2 |
| Musculoskeletal | 1 | 1 | 0 | 0 | 3 | 3 | 0 | 0 |
| Neurology | 15 | 1 | 2 | 1 | 10 | 5 | 2 | 0 |
| Ocular or visual | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Other | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pain | 9 | 3 | 0 | 0 | 8 | 5 | 0 | 0 |
| Pulmonary | 10 | 4 | 1 | 0 | 7 | 4 | 2 | 0 |
| Renal or genitourinary | 17 | 4 | 2 | 0 | 14 | 7 | 0 | 0 |
| Sexual or reproductive function | 2 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
No grade 5 toxic effects were reported. Patients could report >1 adverse event (graded according to Common Terminology Criteria for Adverse Events, version 2.0).
During the follow-up period after completion of all active treatments, 11 (24%) of 46 patients in the paclitaxel group and 16 (34%) of 47 patients in the fluorouracil group had grade 3–4 toxic effects regarded as unrelated to radiotherapy, with half of these toxicities related to bone marrow suppression (table 5). One patient in the fluorouracil group died from febrile neutropenia complicated with infection. Late radiotherapy toxicity, graded 3–4, developed in five (11%) patients treated with paclitaxel and three (6%) patients treated with fluorouracil; most of these toxicities were related to contracted bladder or haematuria (table 6). In the paclitaxel group, six (13%) patients discontinued therapy because of treatment-related toxicity compared with three (6%) patients in the fluorouracil group.
Table 5. Late toxic effects not related to radiotherapy.
| Paclitaxel group (n=46) | Fluorouracil group (n=47) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 | |
| Auditory or hearing | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Blood or bone marrow | 8 | 5 | 8 | 1 | 0 | 8 | 6 | 7 | 4 | 0 |
| Cardiovascular (arrhythmia) | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 |
| Cardiovascular (general) | 4 | 0 | 0 | 0 | 0 | 0 | 3 | 2 | 1 | 0 |
| Constitutional symptoms | 10 | 5 | 0 | 0 | 0 | 11 | 3 | 1 | 1 | 0 |
| Dermatological or skin | 3 | 1 | 0 | 0 | 0 | 4 | 5 | 0 | 0 | 0 |
| Endocrine | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Gastrointestinal | 8 | 5 | 0 | 0 | 0 | 10 | 3 | 1 | 0 | 0 |
| Haemorrhage | 2 | 0 | 1 | 0 | 0 | 2 | 0 | 2 | 0 | 0 |
| Hepatic | 3 | 0 | 0 | 0 | 0 | 2 | 2 | 0 | 0 | 0 |
| Infection febrile neutropenia | 0 | 1 | 2 | 0 | 0 | 1 | 1 | 3 | 1 | 1 |
| Lymphatics | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Metabolic or laboratory | 5 | 4 | 1 | 0 | 0 | 8 | 4 | 3 | 0 | 0 |
| Musculoskeletal | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Neurology | 6 | 5 | 0 | 1 | 0 | 2 | 5 | 2 | 0 | 0 |
| Pain | 5 | 1 | 0 | 0 | 0 | 3 | 2 | 1 | 0 | 0 |
| Pulmonary | 3 | 3 | 0 | 0 | 0 | 1 | 2 | 1 | 2 | 0 |
| Renal or genitourinary | 9 | 2 | 3 | 0 | 0 | 11 | 2 | 2 | 2 | 0 |
| Sexual or reproductive function | 0 | 1 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 |
Patients could report >1 adverse event (graded according to Common Terminology Criteria for Adverse Events, version 2.0).
Table 6. Late toxic effects related to radiotherapy.
| Paclitaxel group (n=46) | Fluorouracil group (n=47) | |||||||
|---|---|---|---|---|---|---|---|---|
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 1 | Grade 2 | Grade 3 | Grade 4 | |
| Bladder | 9 | 5 | 2 | 1 | 12 | 3 | 2 | 0 |
| Bowel | 2 | 3 | 1 | 0 | 2 | 2 | 0 | 0 |
| Kidney | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 |
| Other | 9 | 2 | 1 | 0 | 4 | 2 | 1 | 0 |
| Skin | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
No grade 5 toxic effects were reported. Patients could report >1 adverse event (graded according to Common Terminology Criteria for Adverse Events, version 2.0).
54 (58%) of the 93 enrolled patients completed induction, consolidation chemoradiotherapy, and adjuvant chemotherapy, whereas 19 patients did not complete adjuvant chemotherapy course after completion of consolidation chemoradiotherapy. 5-year overall survival in patients who completed the entire course was 81% (44 of 54 patients) compared with 49% (nine of 19 patients) for those who did not receive adjuvant chemotherapy (p=0·002).
Discussion
Cisplatin-based chemoradiotherapy, supplemented by either paclitaxel or fluorouracil, can lead to high proportions of patients achieving complete responses to the induction phase, with good bladder-intact survival and overall survival. However, treatment with either regimen has notable adverse effects, to the extent that only about half of patients were able to complete the treatment programme, including an adjuvant course of chemotherapy. In the past 25 years, single institutions in North America and Europe and multicentre cooperative groups have enrolled more than 1200 patients with muscle invasive bladder cancer in bladder-preserving protocols7,14–18 and our data adds to the growing prospective evidence for chemoradiation in this setting (panel). Because our study was designed as a phase 2 trial, the conclusions cannot be regarded as definitive, but clinicians can draw lessons in regard to cancer control and treatment morbidity.
Panel. Research in context.
Systematic review
We searched PubMed for articles published between 1980 and 2013 in English with the search terms “muscle invasive bladder cancer”, “radiation therapy”, and “bladder-preservation”. Several previous studies have shown good rates of bladder preservation in treatment of patients with muscle invasive bladder cancer.7,14–18 Such bladder-sparing strategies have not reduced overall survival, because 5-year survival with combined modality therapy and cystectomy reserved for salvage for patients who had an incomplete response or who recurred locally do not differ from those reported in studies that used immediate cystectomy. Preoperative radiation, when combined with cisplatin alone, cisplatin and fluorouracil, or cisplatin and paclitaxel, results in the downstaging to pT0 of a large proportion of patients. When transurethral resection of a bladder tumour (TURBT), radiation, and concurrent chemotherapy are combined, 70% or more of patients have achieved complete responses.7 The radiation sensitising effects of cisplatin are well established, and the opportunity to safely enhance this effect by the simultaneous administration of a second radiation sensitiser such as fluorouracil or paclitaxel has been a goal of Radiation Therapy Oncology Group (RTOG) protocols since 1995. Our phase 2 randomised trial was based on the RTOG experience in bladder preservation (RTOG 95-06 and 99-06),17,19 in which aggressive TURBT has been combined with twice-daily irradiation sensitised with cisplatin and either fluorouracil or paclitaxel. These two studies have already established dosing schedules of the three drugs.
Interpretation
Cisplatin with either paclitaxel or fluorouracil in combination with radiotherapy achieves good results in terms of cancer control and bladder preservation. These data add to the growing prospective literature on chemoradiation in muscle invasive bladder cancer and further supports trimodality bladder-preservation therapy as a viable alternative to upfront cystectomy. Although the trial was not designed to compare the two regimens directly, the paclitaxel regimen seemed to cause more acute side-effects, primarily metabolic, whereas the fluorouracil regimen seemed to cause more genitourinary and gastrointestinal toxic effects during the consolidation phase. Moreover, the fluorouracil regimen might cause profound bone marrow suppression in the long term. In the absence of phase 3 data, clinicians could use this information for selection of chemotherapy regimen when offering patients with muscle invasive bladder cancer a bladder-sparing trimodal therapy.
Complete responses were achieved in 72% (95% CI 57–84) of patients in the paclitaxel group and 62% (46–76) of patients in the fluorouracil group. Notably, the confidence intervals overlap, and because the study was not designed to compare these outcomes, the only conclusion that can be drawn is that similar proportions of patients might achieve complete responses with both regimens. These numbers are similar to those from a study of 348 patients treated on successive prospective cisplatin-based trimodality protocols between 1986 and 2002, in which 72% of patients had a complete response.7 Other groups have achieved higher and lower rates, dependent on selection factors as much as treatment programmes. The Tsukuba University Hospital study20 of 111 patients treated with intra-arterial cisplatin and methotrexate as part of their trimodality therapy resulted in 82% of patients achieving a complete response.
The regimen that achieves the highest proportion of complete responses, translating into improved overall survival or bladder-intact survival, while minimising side-effects, will eventually become the standard component of trimodality therapy, but further phase 3 randomised studies are necessary to reach that conclusion. Although we cannot compare toxicity between the groups in our study, we noted a potentially increased proportion of acute side-effects (primarily metabolic) with the paclitaxel regimen and a potentially increased proportion of genitourinary and gastrointestinal toxicity during the consolidation phase with the fluorouracil regimen. Concurrently with cisplatin and radiation, the fluorouracil regimen might cause a greater level of bone marrow suppression than paclitaxel in the long term. Patients in both treatment groups had problems with the toxicity of the adjuvant chemotherapy portion of their treatment, but patients in the fluorouracil group might have had a lower rate of adjuvant therapy completion than in the paclitaxel group.
Chemoradiotherapy not based on cisplatin for bladder cancer has recently been highlighted by excellent results of the UK multicenter Bladder Cancer 2001 (BC2001) phase 3 study.21 The trial randomly allocated 360 patients with muscle invasive bladder cancer with a median age of 72 years (IQR 64–76) to undergo radiotherapy alone or with synchronous chemotherapy, consisting of fluorouracil (500 mg/m2 on days 1–5 and 16–20) and mitomycin (12 mg/m2 on day 1). With a median follow-up of 5·8 years, the 2-year locoregional-free survival was 67%, and 5-year overall survival was 48% in the chemoradiotherapy arm. 36% of patients had acute grade 3–4 toxicities and 8·3% had late grade 3–4 toxicities. These data established an alternative effective and tolerable radiosensitisation regimen22 and future studies are needed to compare fluorouracil-mitomycin with cisplatin-based chemoradiotherapy in the trimodality therapy for muscle invasive bladder cancer.
An important technical difference between trials led by RTOG in trimodality therapy for muscle invasive bladder cancer and large European trials, such as BC2001,21 is the design of the radiation fields. Whereas many European institutions use whole-bladder or partial-bladder radiation, RTOG trials always include pelvic fields to cover pelvic lymph nodes. The importance of lymphadenectomy during radical cystectomy in muscle invasive bladder cancer is well accepted,23 with lymph node involvement noted in about 20% of patients with pT2 and 40% of patients with pT3 bladder cancer.24,25 Whether pelvic irradiation adds to improvement in outcomes or an increase in adverse events compared with bladder-only irradiation needs to be addressed.
Bladder cancer frequently affects elderly patients and toxic effects of treatment are a concern that probably prevents many of these patients from receipt of aggressive treatment—either radical surgery or bladder preservation trimodality therapy. Treatment patterns for almost 30 000 patients with muscle invasive bladder cancer have been characterised from data collected at facilities accredited by the American College of Surgeons Commission on Cancer.26 Aggressive therapy, defined as any open surgery or radiotherapy with a total radiation dose of 50 Gy or more, was delivered to only 52·5% of patients with non-metastatic muscle invasive bladder cancer. Fewer old patients received aggressive treatment—45% of individuals older than 70 years and only 33% of those older than 80 years. Safety and tolerability thus need to be critically examined for trimodality therapy as such therapy has the potential to fill a clear need gap in the elderly population. We analysed outcomes and toxic effects in our study by age (data not shown) and noted much the same overall survival and bladder-intact survival in patients aged 70 years and younger and those who were older than 70 years. Notably, we reported better outcomes with fluorouracil and cisplatin in younger patients (≤70 years) and paclitaxel and cisplatin in older (>70 years) patients. Because this trial was not designed to compare outcomes between two treatment groups, any conclusion from this analysis can only be hypothesis generating and future studies should investigate whether this age-based approach might improve overall results in patients with muscle invasive bladder cancer.
Because most patients in our study were men, the application of these results in women should be done with caution and, indeed, previous investigations have reported differences between the sexes.27 The main limitation of this study was the small sample size, which did not allow formal statistical comparison between the two treatment groups. Patients and clinicians were not masked to the treatment-group assignment, which might also be a limitation of our trial design. We did not prospectively record or analyse protocol adherence, including treatment delays or dose reductions. Data for quality of life with a bladder function analysis is forthcoming and will be published as a separate report, after increased follow-up of patients in this trial to capture late events. Clinicians should use their judgment and select the cisplatin-based regimen that would be least likely to produce long-term adverse events based on a patient's clinical characteristics. For elderly patients or those with poor renal function, the best policy might be to avoid cisplatin regimens and use fluorouracil plus mitomycin instead.21,28 Future studies are needed to compare cisplatin-based radiosensitisation with fluorouracil-mitomycin radiosensitisation in patients with muscle invasive bladder cancer.
This trial allowed patients downstaged to Ta and Tcis in addition to T0 at the time of biopsy sampling after induction therapy to continue with the bladder-preservation protocol. We plan to assess the long-term bladder tumour control outcomes of the 59 (63%) of 93 patients with completed pathological response (T0) and the 18 (19%) individuals with residual Ta or Tcis after 40 Gy.
Renewed interest exists in treatment of some patients with muscle invasive bladder cancer with TURBT alone. Pathological T0 disease has been reported in up to 15% of cystectomy specimens, suggesting that TURBT alone offers sufficient tumour control in a few patients.29 Historically, recurrence-free survival and long-term survival of patients with T2–3 bladder cancer achieved by single modality therapy were disappointing compared with a radical surgical approach. Whitmore and colleagues30 did not note any complete remissions after TURBT alone for patients with clinical T3 bladder cancer. Barnes and colleagues31 reported a 5-year overall survival of 27% for 85 patients with T2 bladder cancer after TURBT alone. The most promising experience with TURBT alone at the Memorial Sloan-Kettering Cancer Center (New York, NY, USA) showed a 10-year disease-specific survival of 76% with a 57% rate of bladder preservation.32 Similarly, a retrospective review of TURBT alone in patients with muscle invasive bladder cancer treated at MD Anderson Cancer Center (Houston, TX, USA) showed that only 35 (11%) of 327 patients were eligible for the single modality transurethral resection bladder-preservation protocol, which required T0 at the time of second TURBT.33 The investigators reported a bladder-intact survival of 59%; however, the median follow-up was only 2·5 years. TURBT alone might be a good option for carefully selected patients with complete resection of the whole tumour. However, this restrictive inclusion criterion would include very few patients presenting with muscle invasive bladder cancer. A trimodality bladder preservation protocol is more inclusive, and the addition of chemoradiation allows for microscopic disease to be eradicated, allowing for good rates of bladder preservation.
Much of the progress in the selective bladder preservation treatment modality can be attributed to successive clinical trials that incorporated and built on the findings from preceding publications. Our phase 1–2 study (RTOG protocol 99-06)17 assessed the safety and effectiveness of a combination of paclitaxel and cisplatin chemotherapy with twice-daily radiation. That protocol started out with six cycles of adjuvant gemcitabine and cisplatin, but was amended. For 50 patients who were treated with four cycles, 23 (46%) had grade 3 toxic effects (vs 42% in the present study with four cycles of adjuvant cisplatin-gemcitabine-paclitaxel), 26% had grade 4 toxicity (vs 28%) and 2% died (vs 1%). Comparison of results between different studies is difficult, but four cycles of adjuvant cisplatin-gemcitabine-paclitaxel seem to be equivalent to four cycles of adjuvant cisplatin-gemcitabine in terms of rates of acute toxicity.
The next key (rather than incremental) step to improve treatment of bladder cancer will undoubtedly come from the translation of biomedical research into clinical practice. Biological markers in the future will probably guide selection of best treatment modalities for patients with muscle invasive bladder cancer, both in terms of cystectomy versus bladder preservation approaches and with personalisation of chemotherapeutic drugs for patients selected for bladder preservation trimodality therapy.
Overall, this multicentre, phase 2, randomised study in patients with muscle invasive bladder cancer showed that cisplatin with either paclitaxel or fluorouracil in combination with radiotherapy achieves good results in terms of cancer control and bladder preservation but completion of four cycles of systemic adjuvant chemotherapy is difficult. Frequency of toxic effects seemed similar for paclitaxel or fluorouracil regimens, and clinical judgment with physician experience should guide the selection of chemotherapy regimen.
Acknowledgments
This trial was undertaken by the RTOG, and was supported by RTOG grant U10 CA21661 and Community Clinical Oncology Program grant U10 CA37422 from the US National Cancer Institute (NCI).
Role of the funding source: This trial was designed by the members of RTOG, all data were collected and analysed by RTOG. Interpretation of the data and contents of the report are solely the responsibility of the authors and do not necessarily represent the official views of the US National Cancer Institute. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Footnotes
Contributors: WS, DK, RU, C-LW, MB, HS, and AZ participated in study design, accrual and treatment of patients, data collection, analysis, and interpretation, and edited the report. DH participated in data collection and analysis and edited the report. TM participated in data analysis and wrote and edited the initial and final reports.
Conflicts of interest: We declare that we have no conflicts of interest.
References
- 1.Stein JP, Lieskovsky G, Cote R, et al. Radical cystectomy in the treatment of invasive bladder cancer: long-term results in 1,054 patients. J Clin Oncol. 2001;19:666–75. doi: 10.1200/JCO.2001.19.3.666. [DOI] [PubMed] [Google Scholar]
- 2.Dalbagni G, Genega E, Hashibe M, et al. Cystectomy for bladder cancer: a contemporary series. J Urol. 2001;165:1111–16. [PubMed] [Google Scholar]
- 3.Grossman HB, Natale RB, Tangen CM, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med. 2003;349:859–66. doi: 10.1056/NEJMoa022148. [DOI] [PubMed] [Google Scholar]
- 4.Madersbacher S, Hochreiter W, Burkhard F, et al. Radical cystectomy for bladder cancer today—a homogeneous series without neoadjuvant therapy. J Clin Oncol. 2003;21:690–96. doi: 10.1200/JCO.2003.05.101. [DOI] [PubMed] [Google Scholar]
- 5.Hautmann RE, Gschwend JE, de Petriconi RC, Kron M, Volkmer BG. Cystectomy for transitional cell carcinoma of the bladder: results of a surgery only series in the neobladder era. J Urol. 2006;176:486–92. doi: 10.1016/j.juro.2006.03.038. [DOI] [PubMed] [Google Scholar]
- 6.Efstathiou JA, Quinn DI, Stenzl A, et al. Radiation-based bladder preserving strategies: Radiation alone or combined with other modalities. In: Soloway MS, Khoury S, editors. Bladder cancer: international consultation on bladder cancer—Vienna. 2nd. Vol. 21. Paris, France: Editions; 2012. pp. 316–26. [Google Scholar]
- 7.Efstathiou JA, Spiegel DY, Shipley WU, et al. Long-term outcomes of selective bladder preservation by combined-modality therapy for invasive bladder cancer: the MGH experience. Eur Urol. 2012;61:705–11. doi: 10.1016/j.eururo.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 8.Gakis G, Efstathiou J, Lerner SP, et al. ICUD-EAU International Consultation on Bladder Cancer 2012: radical cystectomy and bladder preservation for muscle-invasive urothelial carcinoma of the bladder. Eur Urol. 2013;63:45–57. doi: 10.1016/j.eururo.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 9.National Comprehensive Cancer Network. Bladder cancer. [accessed May 18, 2013]; http://www.nccn.org/professionals/physician_gls/pdf/bladder.pdf.
- 10.Edge S, Byrd D, Compton C, Fritz A, Greene F, Trotti A. AJCC cancer staging manual. 7th. New York, NY: Springer; 2010. [Google Scholar]
- 11.Zelen M. The randomization and stratification of patients to clinical trials. J Chronic Dis. 1974;27:365–75. doi: 10.1016/0021-9681(74)90015-0. [DOI] [PubMed] [Google Scholar]
- 12.Clopper CJ, Pearson ES. The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika. 1934;26:404–13. [Google Scholar]
- 13.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:447–57. [Google Scholar]
- 14.Tester W, Caplan R, Heaney J, et al. Neoadjuvant combined modality program with selective organ preservation for invasive bladder cancer: results of Radiation Therapy Oncology Group phase II trial 8802. J Clin Oncol. 1996;14:119–26. doi: 10.1200/JCO.1996.14.1.119. [DOI] [PubMed] [Google Scholar]
- 15.Housset M, Maulard C, Chretien Y, et al. Combined radiation and chemotherapy for invasive transitional-cell carcinoma of the bladder: a prospective study. J Clin Oncol. 1993;11:2150–57. doi: 10.1200/JCO.1993.11.11.2150. [DOI] [PubMed] [Google Scholar]
- 16.Rodel C, Grabenbauer GG, Kuhn R, et al. Combined-modality treatment and selective organ preservation in invasive bladder cancer: long-term results. J Clin Oncol. 2002;20:3061–71. doi: 10.1200/JCO.2002.11.027. [DOI] [PubMed] [Google Scholar]
- 17.Kaufman DS, Winter KA, Shipley WU, et al. Phase I-II RTOG study (99–06) of patients with muscle-invasive bladder cancer undergoing transurethral surgery, paclitaxel, cisplatin, and twice-daily radiotherapy followed by selective bladder preservation or radical cystectomy and adjuvant chemotherapy. Urology. 2009;73:833–37. doi: 10.1016/j.urology.2008.09.036. [DOI] [PubMed] [Google Scholar]
- 18.Mak R, Hunt D, Shipley WU, et al. Long-term outcomes in patients with muscle-invasive bladder cancer after bladder-preserving combined-modality therapy: a pooled analysis of RTOG 8802, 8903, 9506, 9706, 9906, and 0233. Proc Am Soc Clin Oncol. 2012;30(suppl 5):abstr 264. doi: 10.1200/JCO.2014.57.5548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaufman DS, Winter KA, Shipley WU, et al. The initial results in muscle-invading bladder cancer of RTOG 95-06: phase I/II trial of transurethral surgery plus radiation therapy with concurrent cisplatin and 5-fluorouracil followed by selective bladder preservation or cystectomy depending on the initial response. Oncologist. 2000;5:471–76. doi: 10.1634/theoncologist.5-6-471. [DOI] [PubMed] [Google Scholar]
- 20.Onozawa M, Miyanaga N, Hinotsu S, et al. Analysis of intravesical recurrence after bladder-preserving therapy for muscle-invasive bladder cancer. Jpn J Clin Oncol. 2012;42:825–30. doi: 10.1093/jjco/hys105. [DOI] [PubMed] [Google Scholar]
- 21.James ND, Hussain SA, Hall E, et al. Radiotherapy with or without chemotherapy in muscle-invasive bladder cancer. N Engl J Med. 2012;366:1477–88. doi: 10.1056/NEJMoa1106106. [DOI] [PubMed] [Google Scholar]
- 22.Efstathiou JA, Shipley WU. Words of wisdom. Re: Radiotherapy with or without chemotherapy in muscle-invasive bladder cancer. Eur Urol. 2013;63:181–82. doi: 10.1016/j.eururo.2012.10.032. [DOI] [PubMed] [Google Scholar]
- 23.Tilki D, Brausi M, Colombo R, et al. Lymphadenectomy for bladder cancer at the time of radical cystectomy. Eur Urol. 2013 doi: 10.1016/j.eururo.2013.04.036. published online April 29. http://dx.doi.org/10.1016/j.eururo.2013.04.036. [DOI] [PubMed]
- 24.Vazina A, Dugi D, Shariat SF, Evans J, Link R, Lerner SP. Stage specific lymph node metastasis mapping in radical cystectomy specimens. J Urol. 2004;171:1830–34. doi: 10.1097/01.ju.0000121604.58067.95. [DOI] [PubMed] [Google Scholar]
- 25.Tarin TV, Power NE, Ehdaie B, et al. Lymph node-positive bladder cancer treated with radical cystectomy and lymphadenectomy: effect of the level of node positivity. Eur Urol. 2012;61:1025–30. doi: 10.1016/j.eururo.2012.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gray PJ, Fedewa SA, Shipley WU, Efstathiou JA, Virgo KS, Zietman AL. Receipt of aggressive therapies for muscle-invasive bladder cancer: results from the national cancer data base. Journal of Clinical Oncology Proc Am Soc Clin Oncol. 2012;30(suppl 5):abstr 272. [Google Scholar]
- 27.Zietman AL, Sacco D, Skowronski U, et al. Organ conservation in invasive bladder cancer by ransurethral resection, chemotherapy and radiation: results of a urodynamic and quality of life study on long-term survivors. J Urol. 2003;170:1772–76. doi: 10.1097/01.ju.0000093721.23249.c3. [DOI] [PubMed] [Google Scholar]
- 28.Shipley WU, Zietman AL. Old drugs, new purpose—bladder cancer turning a corner. N Engl J Med. 2012;366:1540–41. doi: 10.1056/NEJMe1201531. [DOI] [PubMed] [Google Scholar]
- 29.Thrasher JB, Frazier HA, Robertson JE, Paulson DF. Does stage of pT0 cystectomy specimen confer a survival advantage in patients with minimally invasive bladder cancer? J Urol. 1994;152:393–96. doi: 10.1016/s0022-5347(17)32746-5. [DOI] [PubMed] [Google Scholar]
- 30.Whitmore WF, Jr, Batata MA, Ghoneim MA, Grabstald H, Unal A. Radical cystectomy with or without prior irradiation in the treatment of bladder cancer. J Urol. 1977;118:184–87. doi: 10.1016/s0022-5347(17)57942-2. [DOI] [PubMed] [Google Scholar]
- 31.Barnes RW, Dick AL, Hadley HL, Johnston OL. Survival following transurethral resection of bladder carcinoma. Cancer Res. 1977;37:2895–97. [PubMed] [Google Scholar]
- 32.Herr HW. Transurethral resection of muscle-invasive bladder cancer: 10-year outcome. J Clin Oncol. 2001;19:89–93. doi: 10.1200/JCO.2001.19.1.89. [DOI] [PubMed] [Google Scholar]
- 33.Leibovici D, Kassouf W, Pisters LL, et al. Organ preservation for muscle-invasive bladder cancer by transurethral resection. Urology. 2007;70:473–76. doi: 10.1016/j.urology.2007.05.007. [DOI] [PubMed] [Google Scholar]