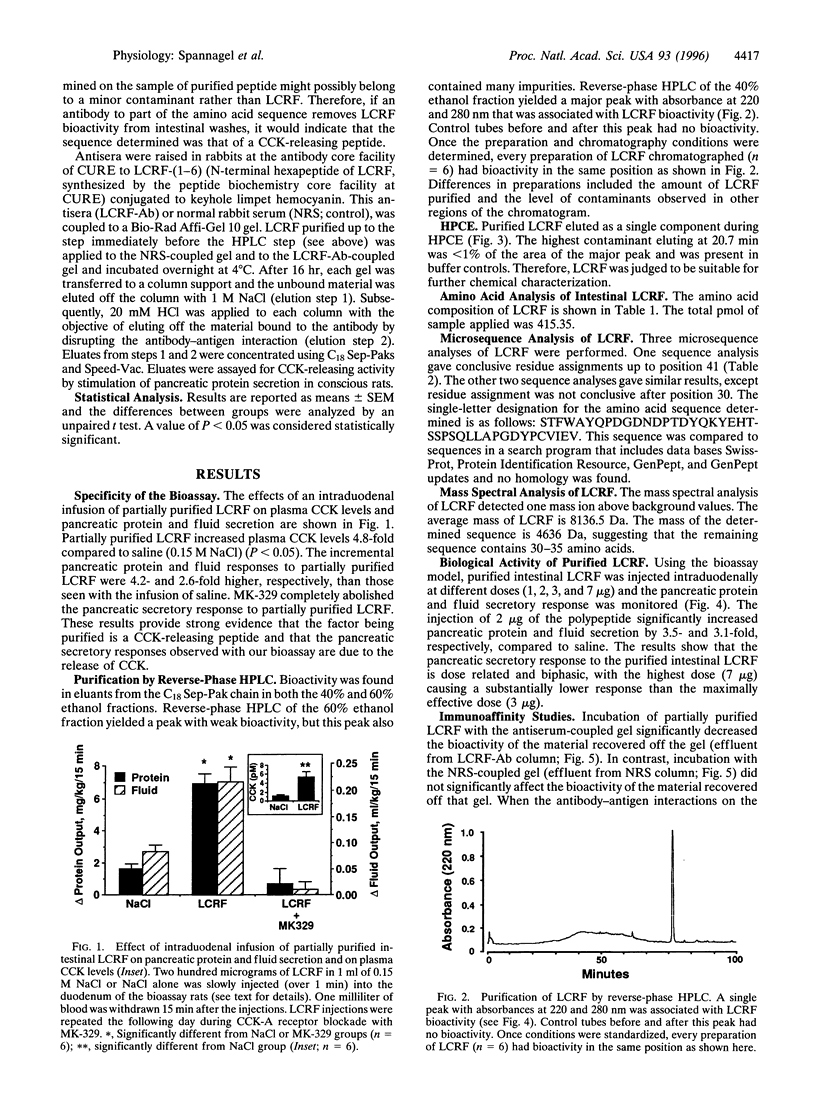
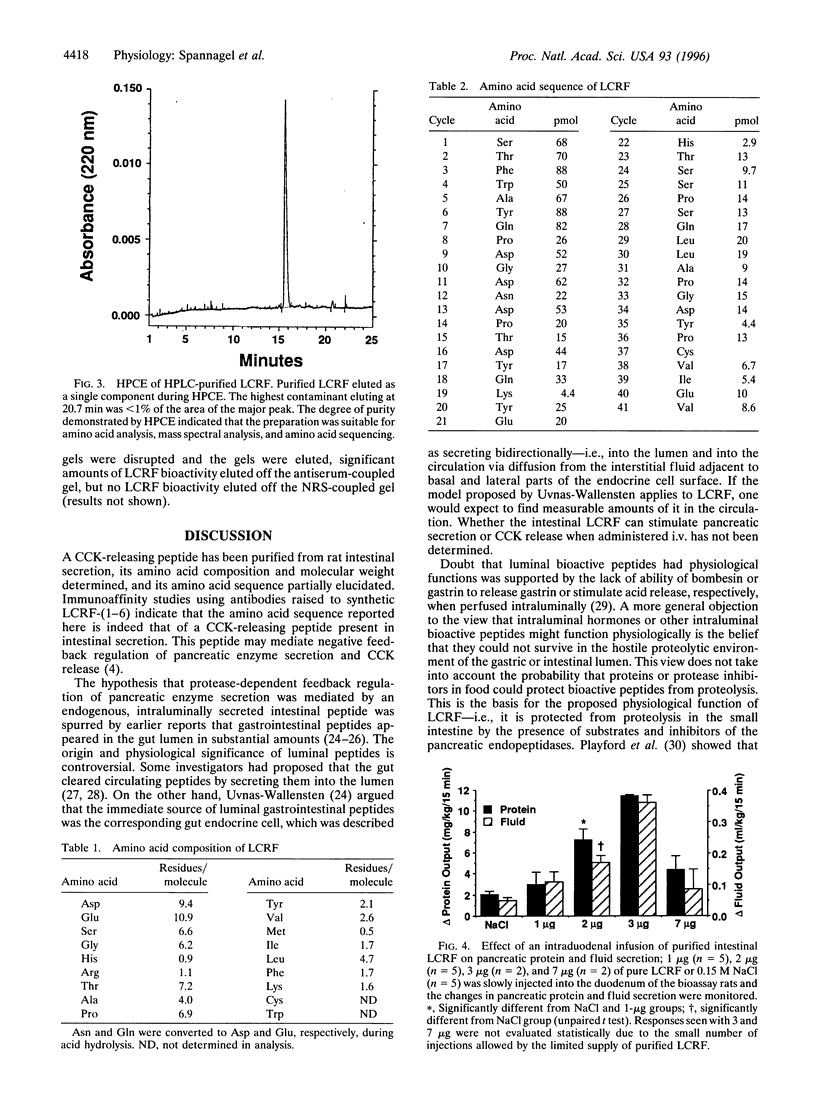
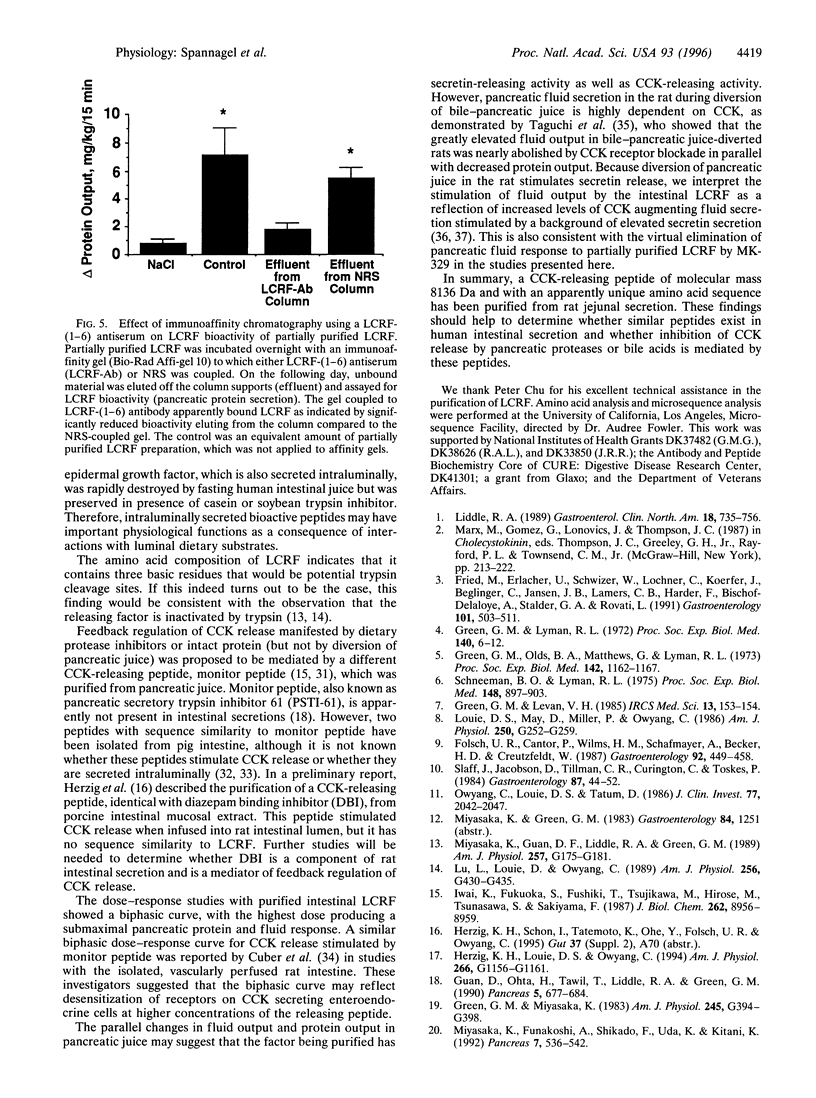
Abstract
Cholecystokinin (CCK) secretion in rats and humans is inhibited by pancreatic proteases and bile acids in the intestine. It has been hypothesized that the inhibition of CCK release caused by pancreatic proteases is due to proteolytic inactivation of a CCK-releasing peptide present in intestinal secretion. To purify the putative luminal CCK-releasing factor (LCRF), intestinal secretions were collected by perfusing a modified Thiry-Vella fistula of jejunum in conscious rats. From these secretions, the peptide was concentrated by ultrafiltration followed by low-pressure reverse-phase chromatography and purified by reverse-phase high-pressure liquid chromatography. Purity was confirmed by high-performance capillary electrophoresis. Fractions were assayed for CCK-releasing activity by their ability to stimulate pancreatic protein secretion when infused into the proximal small intestine of conscious rats. Partially purified fractions strongly stimulated both pancreatic secretion and CCK release while CCK receptor blockade abolished the pancreatic response. Amino acid analysis and mass spectral analysis showed that the purified peptide is composed of 70-75 amino acid residues and has a mass of 8136 Da. Microsequence analysis of LCRF yielded an amino acid sequence for 41 residues as follows: STFWAYQPDGDNDPTDYQKYEHTSSPSQLLAPGDYPCVIEV. When infused intraduodenally, the purified peptide stimulated pancreatic protein and fluid secretion in a dose-related manner in conscious rats and significantly elevated plasma CCK levels. Immunoaffinity chromatography using antisera raised to synthetic LCRF-(1-6) abolished the CCK releasing activity of intestinal secretions. These studies demonstrate, to our knowledge, the first chemical characterization of a luminally secreted enteric peptide functioning as an intraluminal regulator of intestinal hormone release.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agerberth B., Ostenson C. G., Efendic S., Jörnvall H. Pancreatic secretory trypsin inhibitor (PSTI) isolated from pig intestine. Influence on insulin and somatostatin release. FEBS Lett. 1991 Apr 9;281(1-2):227–230. doi: 10.1016/0014-5793(91)80399-n. [DOI] [PubMed] [Google Scholar]
- Agerberth B., Söderling-Barros J., Jörnvall H., Chen Z. W., Ostenson C. G., Efendić S., Mutt V. Isolation and characterization of a 60-residue intestinal peptide structurally related to the pancreatic secretory type of trypsin inhibitor: influence on insulin secretion. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8590–8594. doi: 10.1073/pnas.86.21.8590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang T. M., Chey W. Y., Kim M. S., Lee K. Y. The release of biologically active secretin-like immunoreactivity into duodenal lumen of dogs. J Physiol. 1981 Nov;320:393–401. doi: 10.1113/jphysiol.1981.sp013957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chey W. Y., Lee K. Y., Chang T. M., Chen Y. F., Millikan L. Potentiating effect of secretin on cholecystokinin-stimulated pancreatic secretion in dogs. Am J Physiol. 1984 Mar;246(3 Pt 1):G248–G252. doi: 10.1152/ajpgi.1984.246.3.G248. [DOI] [PubMed] [Google Scholar]
- Cuber J. C., Bernard G., Fushiki T., Bernard C., Yamanishi R., Sugimoto E., Chayvialle J. A. Luminal CCK-releasing factors in the isolated vascularly perfused rat duodenojejunum. Am J Physiol. 1990 Aug;259(2 Pt 1):G191–G197. doi: 10.1152/ajpgi.1990.259.2.G191. [DOI] [PubMed] [Google Scholar]
- Fried M., Erlacher U., Schwizer W., Löchner C., Koerfer J., Beglinger C., Jansen J. B., Lamers C. B., Harder F., Bischof-Delaloye A. Role of cholecystokinin in the regulation of gastric emptying and pancreatic enzyme secretion in humans. Studies with the cholecystokinin-receptor antagonist loxiglumide. Gastroenterology. 1991 Aug;101(2):503–511. doi: 10.1016/0016-5085(91)90031-f. [DOI] [PubMed] [Google Scholar]
- Fushiki T., Iwai K. Two hypotheses on the feedback regulation of pancreatic enzyme secretion. FASEB J. 1989 Feb;3(2):121–126. doi: 10.1096/fasebj.3.2.2644146. [DOI] [PubMed] [Google Scholar]
- Fölsch U. R., Cantor P., Wilms H. M., Schafmayer A., Becker H. D., Creutzfeldt W. Role of cholecystokinin in the negative feedback control of pancreatic enzyme secretion in conscious rats. Gastroenterology. 1987 Feb;92(2):449–458. doi: 10.1016/0016-5085(87)90141-7. [DOI] [PubMed] [Google Scholar]
- Green G. M., Lyman R. L. Feedback regulation of pancreatic enzyme secretion as a mechanism for trypsin inhibitor-induced hypersecretion in rats. Proc Soc Exp Biol Med. 1972 May;140(1):6–12. doi: 10.3181/00379727-140-36384. [DOI] [PubMed] [Google Scholar]
- Green G. M., Miyasaka K. Rat pancreatic response to intestinal infusion of intact and hydrolyzed protein. Am J Physiol. 1983 Sep;245(3):G394–G398. doi: 10.1152/ajpgi.1983.245.3.G394. [DOI] [PubMed] [Google Scholar]
- Green G. M., Olds B. A., Matthews G., Lyman R. L. Protein, as a regulator of pancreatic enzyme secretion in the rat. Proc Soc Exp Biol Med. 1973 Apr;142(4):1162–1167. doi: 10.3181/00379727-142-37199. [DOI] [PubMed] [Google Scholar]
- Guan D., Ohta H., Tawil T., Liddle R. A., Green G. M. CCK-releasing activity of rat intestinal secretion: effect of atropine and comparison with monitor peptide. Pancreas. 1990 Nov;5(6):677–684. doi: 10.1097/00006676-199011000-00007. [DOI] [PubMed] [Google Scholar]
- Herzig K. H., Louie D. S., Owyang C. Somatostatin inhibits CCK release by inhibiting secretion and action of CCK-releasing peptide. Am J Physiol. 1994 Jun;266(6 Pt 1):G1156–G1161. doi: 10.1152/ajpgi.1994.266.6.G1156. [DOI] [PubMed] [Google Scholar]
- Inoue K., Ayalon A., Yazigi R., Watson L. C., Rayford P. L., Thompson J. C. Removal of circulating gastrin and cholecystokinin into the lumen of the small intestine. Digestion. 1982;24(2):118–125. doi: 10.1159/000198786. [DOI] [PubMed] [Google Scholar]
- Iwai K., Fukuoka S., Fushiki T., Tsujikawa M., Hirose M., Tsunasawa S., Sakiyama F. Purification and sequencing of a trypsin-sensitive cholecystokinin-releasing peptide from rat pancreatic juice. Its homology with pancreatic secretory trypsin inhibitor. J Biol Chem. 1987 Jul 5;262(19):8956–8959. [PubMed] [Google Scholar]
- Jordan P. H., Jr, Yip B. S. Origin of gastrin in gastric juice. Am J Surg. 1974 Sep;128(3):336–339. doi: 10.1016/0002-9610(74)90168-8. [DOI] [PubMed] [Google Scholar]
- Lake-Bakaar G., Beidas S. Route of access of secretin into rabbit duodenal lumen during acid perfusion. Pancreas. 1991 Nov;6(6):673–678. doi: 10.1097/00006676-199111000-00009. [DOI] [PubMed] [Google Scholar]
- Lake-Bakaar G., Tovoli S., Li H., Straus E., Yalow R. S. Recovery of secretin in acid small intestinal lumen perfusates in the rabbit. Horm Metab Res. 1981 Dec;13(12):682–685. doi: 10.1055/s-2007-1019375. [DOI] [PubMed] [Google Scholar]
- Li P., Lee K. Y., Chang T. M., Chey W. Y. Mechanism of acid-induced release of secretin in rats. Presence of a secretin-releasing peptide. J Clin Invest. 1990 Nov;86(5):1474–1479. doi: 10.1172/JCI114864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddle R. A., Goldfine I. D., Williams J. A. Bioassay of plasma cholecystokinin in rats: effects of food, trypsin inhibitor, and alcohol. Gastroenterology. 1984 Sep;87(3):542–549. [PubMed] [Google Scholar]
- Liddle R. A. Integrated actions of cholecystokinin on the gastrointestinal tract: use of the cholecystokinin bioassay. Gastroenterol Clin North Am. 1989 Dec;18(4):735–756. [PubMed] [Google Scholar]
- Louie D. S., May D., Miller P., Owyang C. Cholecystokinin mediates feedback regulation of pancreatic enzyme secretion in rats. Am J Physiol. 1986 Feb;250(2 Pt 1):G252–G259. doi: 10.1152/ajpgi.1986.250.2.G252. [DOI] [PubMed] [Google Scholar]
- Lu L., Louie D., Owyang C. A cholecystokinin releasing peptide mediates feedback regulation of pancreatic secretion. Am J Physiol. 1989 Feb;256(2 Pt 1):G430–G435. doi: 10.1152/ajpgi.1989.256.2.G430. [DOI] [PubMed] [Google Scholar]
- Miyasaka K., Funakoshi A., Shikado F., Uda K., Kitani K. Effect of taurocholate on CCK release and pancreatic secretion produced by two CCK-releasing peptides in conscious rats. Pancreas. 1992;7(5):536–542. doi: 10.1097/00006676-199209000-00005. [DOI] [PubMed] [Google Scholar]
- Miyasaka K., Guan D. F., Liddle R. A., Green G. M. Feedback regulation by trypsin: evidence for intraluminal CCK-releasing peptide. Am J Physiol. 1989 Aug;257(2 Pt 1):G175–G181. doi: 10.1152/ajpgi.1989.257.2.G175. [DOI] [PubMed] [Google Scholar]
- Owyang C., Louie D. S., Tatum D. Feedback regulation of pancreatic enzyme secretion. Suppression of cholecystokinin release by trypsin. J Clin Invest. 1986 Jun;77(6):2042–2047. doi: 10.1172/JCI112534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Playford R. J., Woodman A. C., Clark P., Watanapa P., Vesey D., Deprez P. H., Williamson R. C., Calam J. Effect of luminal growth factor preservation on intestinal growth. Lancet. 1993 Apr 3;341(8849):843–848. doi: 10.1016/0140-6736(93)93057-8. [DOI] [PubMed] [Google Scholar]
- Sarfati P., Green G. M., Morisset J. Secretion of protein, fluid, and immunoreactive somatostatin in rat pure pancreatic juice: adaptation to chronic cerulein and secretin treatment. Pancreas. 1988;3(4):375–382. doi: 10.1097/00006676-198808000-00002. [DOI] [PubMed] [Google Scholar]
- Schneeman B. O., Lyman R. L. Factors involved in the intestinal feedback regulation of pancreatic enzyme secretion in the rat. Proc Soc Exp Biol Med. 1975 Mar;148(3):897–903. doi: 10.3181/00379727-148-38656. [DOI] [PubMed] [Google Scholar]
- Slaff J., Jacobson D., Tillman C. R., Curington C., Toskes P. Protease-specific suppression of pancreatic exocrine secretion. Gastroenterology. 1984 Jul;87(1):44–52. [PubMed] [Google Scholar]
- Sun G., Lee K. Y., Chang T. M., Chey W. Y. Effect of pancreatic juice diversion on secretin release in rats. Gastroenterology. 1989 Apr;96(4):1173–1179. doi: 10.1016/0016-5085(89)91638-7. [DOI] [PubMed] [Google Scholar]
- Taguchi S., Green G. M., Nakano I., Hatta Y. Inhibitory effects of the cholecystokinin antagonist loxiglumide on pancreatic exocrine secretion and pancreatic growth in conscious rats. Int J Pancreatol. 1992 Apr;11(2):67–73. doi: 10.1007/BF02925977. [DOI] [PubMed] [Google Scholar]
- Uvnäs-Wallensten K. Luminal secretion of gut peptides. Clin Gastroenterol. 1980 Sep;9(3):545–553. [PubMed] [Google Scholar]



