SUMMARY
Tumor-specific pyruvate kinase M2 (PKM2) is instrumental in both aerobic glycolysis and gene transcription. PKM2 regulates G1-S phase transition by controlling cyclin D1 expression. However, it is not known whether PKM2 directly controls cell cycle progression. We show here that PKM2, but not PKM1, binds to the spindle checkpoint protein Bub3 during mitosis and phosphorylates Bub3 at Y207. This phosphorylation is required for Bub3-Bub1 complex recruitment to kinetochores, where it interacts with Blinkin and is essential for correct kinetochore-microtubule attachment, mitotic/spindle-assembly checkpoint, accurate chromosome segregation, cell survival and proliferation, and active EGF receptor-induced brain tumorigenesis. In addition, the level of Bub3 Y207 phosphorylation correlated with histone H3-S10 phosphorylation in human glioblastoma specimens and with glioblastoma prognosis. These findings highlight the role of PKM2 as a protein kinase controlling the fidelity of chromosome segregation, cell cycle progression, and tumorigenesis.
Keywords: PKM2, Bub3, EGFR, phosphorylation, kinetochore, spindle-assembly checkpoint, tumorigenesis
INTRODUCTION
Pyruvate kinase regulates the final rate-limiting step of glycolysis, which catalyzes the transfer of a phosphate group from phosphoenolpyruvate (PEP) to adenosine diphosphate (ADP) for production of pyruvate and adenosine triphosphate (ATP) (Lu, 2012). Four pyruvate kinase isoforms (M1, M2, L, and R) exist in mammals and are expressed in different types of cells and tissues. PKM2 is generated from alternate splicing of PKM pre-mRNA by the inclusion of exon 10 (PKM2) and the exclusion of exon 9 (PKM1) (Yang and Lu, 2013). In nude mouse xenografts, PKM2 was shown to be instrumental in the Warburg effect and tumor formation (Christofk et al., 2008). In addition to its important roles in glycolysis (Koppenol et al., 2011; Vander Heiden et al., 2009), PKM2 has essential non-metabolic functions in tumorigenesis (Yang et al., 2011). PKM2 was shown to bind to c-Src-phosphorylated Y333 of β-catenin in the nucleus, which is essential for β-catenin transactivation (Lu, 2012; Yang et al., 2011). In addition, PKM2 directly phosphorylates histone H3 at T11, leading to H3-K9 acetylation and transcription of genes, including CCND1 (encoding for cyclin D1) and MYC (Yang et al., 2012b; Yang et al., 2012c). These findings clearly demonstrate that PKM2 regulates G1-S phase transition by controlling cyclin D1 expression (Yang et al., 2012b). However, whether PKM2 plays a role in regulating mitosis is unknown.
Before cell division, the replicated genome must be accurately segregated to ensure the continued growth and development of the daughter cells (Holland and Cleveland, 2009; Tanaka et al., 2005). Errors in chromosomal segregation can lead to the loss or gain of chromosomes in daughter cells. This condition is called aneuploidy (Holland and Cleveland, 2009). To maintain the fidelity of chromosome segregation, eukaryotes have evolved a control mechanism, often referred to as the cell cycle checkpoint or the mitotic or spindle assembly checkpoint (SAC), which monitors the status of kinetochore-microtubule (K-MT) attachments and delays anaphase onset until all the chromosomes are correctly aligned on the metaphase plate (Cheeseman and Desai, 2008; Musacchio and Salmon, 2007). SAC component proteins include the evolutionarily conserved Bub1, Bub3, Mad1, Mad2, BubR1 (Mad3 in yeast), Mps1, centromere-associated protein (CENP)-E, and Aurora B proteins (Musacchio and Salmon, 2007). SAC proteins inhibit the ubiquitin ligase activity of the anaphase-promoting complex/cyclosome (APC/C) and the proteasome-mediated destruction of securin and mitotic cyclin B, which blocks separase-dependent cohesion cleavage, the separation of sister chromatids, and cyclin B degradation-dependent mitotic exit (Musacchio and Salmon, 2007). Bub3, Bub1, and BubR1 form cell-cycle-constitutive complexes and are interdependent for kinetochore localization during prometaphase by binding to Blinkin (also known as KNL1, Spc7, Spc105, AF15q14, D40, and CASC5), a member of the conserved KMN (KNL1/Mis12 complex/Ndc80 complex) network of kinetochore proteins (Bolanos-Garcia and Blundell, 2011; Kiyomitsu et al., 2007). Mps1 phosphorylates Blinkin or its homologs to recruit SAC components (London et al., 2012; Shepperd et al., 2012; Yamagishi et al., 2012). Depletion of Bub3 and Bub1 results in misaligned chromosomes in which kinetochores fail to achieve end-on binding to microtubules (Logarinho et al., 2008; Meraldi and Sorger, 2005). These results indicate that the Bub3-Bub1 complex, in addition to its role in the SAC-regulated delay of anaphase, is essential for the establishment of correct K-MT attachments and required for proper chromosome segregation (Logarinho and Bousbaa, 2008).
In this report, we show that PKM2 binds to Bub3 during mitosis and phosphorylates Bub3 at Y207, which is required for recruitment of the Bub3-Bub1 complex to Blinkin and kinetochores and the subsequent regulation of chromosome segregation, cell proliferation, and tumorigenesis.
RESULTS
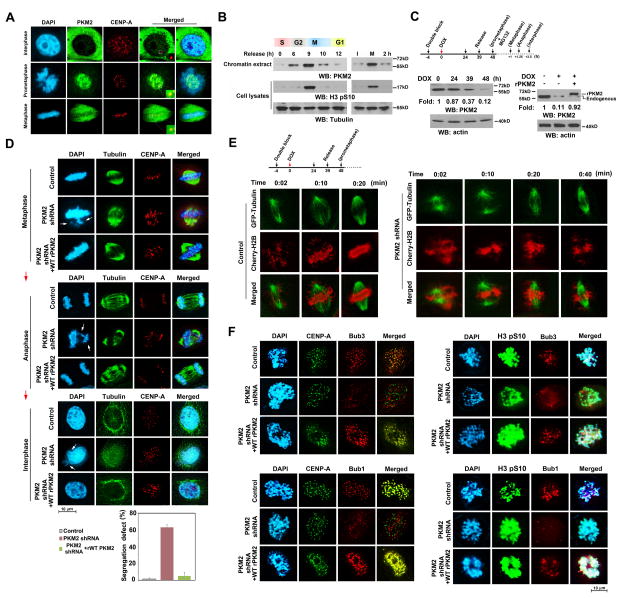
PKM2 is required for the fidelity of chromosome segregation and kinetochore localization of Bub3 and Bub1
To examine whether PKM2 plays a role in mitosis, we synchronized HeLa human cervical cancer cells in the G1 phase with a double-thymidine block and then released the block by removing thymidine for 12 hours. Immunofluorescence analyses showed that PKM2 co-localized with chromatin and CENP-A, a centromere-specific histone H3 variant and a marker of kinetochore localization (Cheeseman and Desai, 2008), primarily in prometaphase (and to a lesser extent in metaphase), but not in interphase (Figure 1A). The observed co-localization was abrogated by expression of PKM2 shRNA (Figure S1A). In line with this finding, immunoblotting studies revealed that PKM2 was enriched in chromatin extracts of mitotic cells that were indicated by the mitosis marker phospho-histone H3-S10 (Cheung et al., 2000) (Figure 1B, left panel). PKM2 association with chromatin was also observed in cells treated with nocodazole after a double-thymidine block that arrested the cells at mitosis (Figure 1B, right panel). The amount of chromatin-associated PKM2 was reduced after mitotic exit prompted by removal of nocodazole for 2 hours. These results suggest a role for PKM2 in mitosis progression.
Figure 1. PKM2 is required for the fidelity of chromosome segregation and kinetochore localization of Bub3 and Bub1.
(A) HeLa cells in different phases of the cell cycle were immunostained with the indicated antibodies. Nuclei were stained with DAPI (blue).
(B) HeLa cells synchronized by thymidine double block (2 mM) were released for the indicated periods of time (left panel) or for 6 h, followed by nocodazole (20 ng/ml) treatment for 12 h (right panel) with or without removal of nocodazole for 2 h thereafter. Chromatin extracts or cell lysates were prepared.
(C, D) U87/EGFRvIII cells synchronized by thymidine double block (2 mM) were released for the indicated periods of time. Doxycycline (500 ng/ml) was added at the indicated time point to induce PKM2 shRNA or scrambled shRNA as a control expression. U87/EGFRvIII cells with expression of PKM2 shRNA were reconstituted with or without WT rPKM2 expression. Immunoblotting analyses were performed with the indicated antibodies (C). MG132 (25 μM) was added at the indicated time point and incubated with the cells for 1 h to sustain the cells in metaphase. MG132 were then removed for 15 min or 2.5 h to release the cells into anaphase or interphase, respectively. These cells were immunostained with the indicated antibodies. One hundred metaphase cells in each indicated phase of mitosis were analyzed. Data represent the mean ± SD of three independent experiments. The white arrows point to the fragmented chromatin, lagging chromosomes, or micronuclei (D).
(E) U87/EGFRvIII cells expressing GFP-tubulin and mCherry-Histone H2B (for chromosome staining) synchronized by thymidine double block (2 mM) were released for 9 h. Doxycycline (500 ng/ml) was added with thymidine to induce PKM2 shRNA expression. Live-cell confocal time-lapse images were taken at the indicated time points.
(F) U87/EGFRvIII cells synchronized by thymidine double block (2 mM) were released for 9 h. Doxycycline (500 ng/ml) was added at the indicated time point in Figure 1C to induce PKM2 shRNA expression. The cells were stained with the indicated antibodies.
See also Figure S1.
To avoid the effect of PKM2 depletion on G1-S transition and investigate whether PKM2 plays a role in mitosis regulation, we expressed doxycycline-inducible PKM2 shRNA and depleted PKM2 in the mitotic HeLa cells (data not shown) and U87 human glioblastoma (GBM) cells expressing an active EGFRvIII mutant (Figure 1C, left panel), which were released from a double-thymidine block. Flow cytometric analysis showed that about 70% cells were in mitosis and only small potion of cells were in G0/G1 phase that may result from unsynchronized PKM2 depletion in heterogeneous GBM cells (some tumor cells having less PKM2 may have earlier depletion than others were arrested at G0/G1 phases) (Figure S1B). PKM2 depletion during mitosis prevented microtubule spindle attachment to kinetochores in metaphase (Figure 1D, top panel) and resulted in abnormal chromosome segregation and lagging chromatids in anaphase (Figure 1D, middle panel) and higher levels of micronuclei in the cells that had exited mitosis (Figure 1D, bottom panel). These PKM2 depletion-induced mitotic defects were rescued by the reconstituted expression of RNAi-resistant wild-type (WT) rPKM2 (Figures 1C right panel and 1D). In addition, live-cell time-lapse image analyses showed that PKM2 depletion, which did not significantly affect spindle assembly, still impaired spindle attachment to chromosomes (Figures 1E, S1C). These results indicated that PKM2 is required for correct K-MT attachment and the fidelity of chromosome segregation.
PKM2 phosphorylates histone H3-T11, leading to H3-K9 acetylation (Yang et al., 2012b), which could preclude histone H3-K9 methylation for the recruitment of chromosome passenger complex (CPC) proteins, such as Aurora B, to centromeric DNA for regulating chromosome segregation (Verdaasdonk and Bloom, 2011; Zhang et al., 2010). To test this possibility, we performed ChIP assays with antibodies for H3-pT11, acetylated H3-K9, methylated H3-K4 and H3-K9, and CENP-A and primers specific for centromeric DNA. We found that PKM2 depletion did not affect the levels of H3-T11 phosphorylation, acetylation or methylation of H3-K4 and H3-K9, or CENP-A expression in centromeric DNA regions (Figure S1D), suggesting that PKM2 does not phosphorylate histone H3 across kinetochore regions or affect CENP-A centromere localization. Immunofluorescence analyses showed that PKM2 depletion, which had no effect on the localization of CENP-A, did not alter the localization of other kinetochore proteins, such as CENP-C, -T, and -U, in the interphase and prometaphase of HeLa cells (Figure S1E). The recruitment of CPC proteins, such as Aurora B, to kinetochore regions was also not largely affected (Figure S1F). In contrast, PKM2 depletion, which abrogated the co-localization between PKM2 and the Bub3 and Bub1 complex (Figure S1G), blocked the recruitment of Bub3 and Bub1 to kinetochores during the prometaphase of U87/EGFRvIII (Figure 1F) and HeLa (data not shown) cells, without affecting the expression levels of Bub3 and Bub1 as well as CENP-A, -C, -T, and -U and Aurora-B (Figure S1H). The PKM2 depletion-induced defect was rescued by the reconstituted expression of rPKM2 (Figures 1F, S1G). These results indicated that PKM2 is essential for the translocation of Bub3 and Bub1 to kinetochores.
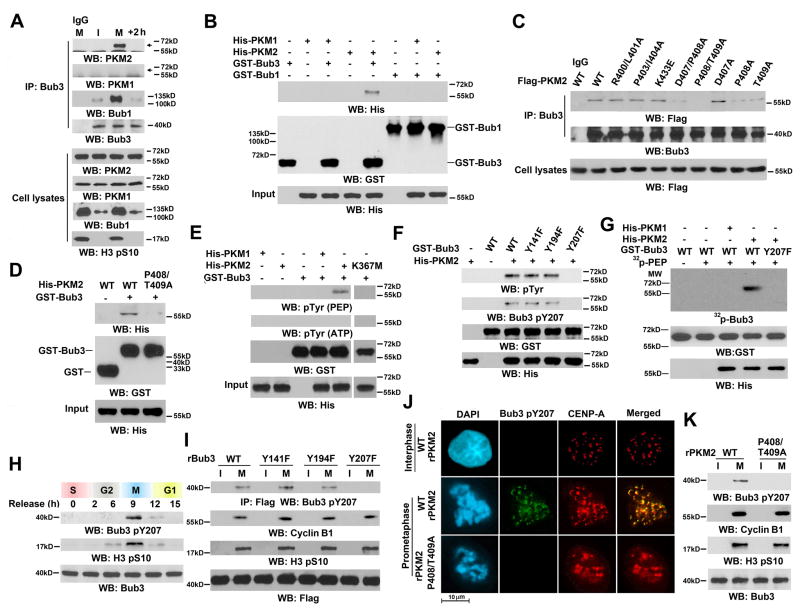
PKM2, but not PKM1, interacts with and phosphorylates Bub3 at Y207
To determine the relationship between PKM2 and the Bub3-Bub1 complex, we synchronized HeLa cells with a double-thymidine block followed with or without nocodazole treatment. Immunoblotting of immunoprecipitated Bub3 with an anti-PKM2 or an anti-PKM1 antibody showed that, in contrast to the constant association between Bub3 and Bub1 (Figure 2A, the third panel), Bub3 interacts with PKM2, but not with endogenous (Figure 2A, top two panels) or overexpressed PKM1 (Figure S2A), during mitosis, but not in interphase or after mitosis exit. To examine whether PKM2 directly binds to Bub3 or Bub1, we incubated bacterially purified recombinant GST-Bub3 or GST-Bub1 with purified recombinant His-PKM2 or His-PKM1. We found that PKM2, but not PKM1, binds to Bub3, but not Bub1 (Figure 2B). In line with this finding, co-immunoprecipitation analyses showed that Bub1 depletion did not affect the interaction between PKM2 and Bub3 (Figure S2B, left panel). In contrast, Bub3 depletion disrupted the binding of PKM2 to Bub1 (Figure S2B, right panel), further supporting that PKM2 directly binds to Bub3.
Figure 2. PKM2, but not PKM1, interacts with and phosphorylates Bub3 at Y207.
Immunoblotting, immunoprecipitation (A–I, K), or immunofluorescence (J) analyses were performed with the indicated antibodies. Nuclei were stained with DAPI (blue).
(A) HeLa cells synchronized by thymidine double block (2 mM) with or without release for 6 h were treated with nocodazole (20 ng/ml) for 12 h with or without release for 2 h. I, interphase; M, mitosis.
(B) Purified recombinant GST-Bub1 or GST-Bub3 was mixed with purified His-PKM2 or His-PKM1. A GST pull-down assay was performed.
(C) HeLa cells with PKM2 depletion and reconstituted expression of Flag-tagged PKM2 proteins were synchronized in mitosis by thymidine double block (2 mM) with release for 9 h.
(D) Purified recombinant GST or GST-Bub3 was mixed with indicated purified His-PKM2 protein. A GST pull-down assay was performed.
(E) In vitro phosphorylation analyses were performed by mixing purified WT His-PKM2, His-PKM2 K367M, or His-PKM1 with purified GST-Bub3 in the presence of PEP or ATP.
(F) In vitro phosphorylation analyses were performed by mixing purified His-PKM2 with the indicated recombinant GST-Bub3 proteins in the presence of PEP.
(G) In vitro phosphorylation assays were performed by mixing purified WT His-PKM2 or His-PKM1 with the indicated recombinant GST-Bub3 proteins in the presence of 32P-labeled PEP.
(H) HeLa cells synchronized by thymidine double block (2 mM) were released for the indicated periods of time.
(I) HeLa cells with depleted Bub3 and reconstituted expression of the indicated Bub3 protein were synchronized by thymidine double block (2 mM) with or without release for 9 h. I, interphase; M, mitosis.
(J) HeLa cells with PKM2 depletion and reconstituted expression of WT rPKM2 or rPKM2 P408/T409A were immunostained with the indicated antibodies. The cells in interphase and prometaphase were examined.
(K) HeLa cells with depleted PKM2 and reconstituted expression of WT rPKM2 or rPKM2 P408T409A were synchronized by thymidine double block (2 mM) with or without release for 9 h. I, interphase; M, mitosis.
See also Figures S2 and S3.
Mutations of the polar or hydrophobic surface residues of PKM2 R400, L401, P403, L404, D407, P408, T409, and K433 coded by the PKM2-specific exon 10 and expression of these mutants showed that the P408A and T409A mutants reduced binding ability to Bub3, and a combined mutation of these residues more significantly abrogated the interaction between PKM2 and Bub3 in mitotic HeLa (Figure 2C) and U87/EGFRvIII (Figure S2C) cells. These findings were further supported by a GST pulldown assay showing that His-PKM2 P408/T409A mutant largely lost its interaction with purified recombinant GST-Bub3 (Figure 2D).
Because PKM2 can function as a protein kinase (Gao et al., 2012; Yang et al., 2012b), we tested whether Bub3 is a substrate of PKM2 kinase activity. An in vitro protein kinase assay of recombinant PKM2 or PKM1 mixed with recombinant Bub3 showed that PKM2, but not inactive PKM2 K367M mutant or PKM1, phosphorylated Bub3; Bub3 phosphorylation was detected by anti-phospho-Tyr (Figure 2E), but not anti-phospho-Ser or anti-phospho-Thr (data not shown) antibodies. Notably, this phosphorylation occurred in the presence of PEP, the physiological phosphate group donor of PKM2, but not in the presence of ATP. Liquid chromatography-coupled ion trap mass spectrometry (LC-MS/MS) analyses displayed that PKM2 phosphorylated Bub3 at Y207 (Figure S2D). Mutations of Bub3 Y141, Y194, and Y207 into phenylalanines showed that Bub3 Y207F, but not WT Bub3, Bub3 Y141F, or Bub3 Y194F, was resistant to phosphorylation by PKM2, as detected by a general anti-phospho-Tyr antibody, an antibody that specifically recognizes phosphorylated Bub3 Y207 (Figures 2F, S2E), and autoradiography using 32P-labeled PEP (Figure 2G). In addition, Bub3 phosphorylation identified by 32P-labeled PEP was alleviated by cold PEP, whereas ATP did not affect PEP-dependent Bub3 Y207 phosphorylation (Figure S2F). Furthermore, Bub3 Y207 phosphorylation was enhanced by increasing the amount of PKM2 in the reactions (Figure S2G). These results indicated that PKM2 interacts with and phosphorylates Bub3 at Y207 in vitro. The recombinant WT PKM2 in the presence of fructose 1,6-bisphosphate (FBP), which forms a tetramer (Gao et al., 2012), sufficiently phosphorylated Bub3, as does dimerized PKM2 R399E mutant (Gao et al., 2012) (Figure S2H), suggesting that both dimers and tetramers of PKM2 can phosphorylate Bub3 in vitro.
To test whether PKM2 phosphorylates Bub3 in cells, we synchronized U87 overexpressign EGFR cells with nocodazole treatment or a double-thymidine block and showed that PKM2 interacted with Bub3 (Figure S2I) and Bub3 Y207 was phosphorylated during mitosis (Figure 2H). In contrast, PKM2 co-immunoprecipitated with β-catenin only in the cells in G1 phase that were serum-starved and EGF-treated for 6 hours, but not in mitotic cells (Figure S2I), suggesting that PKM2 regulates the functions of β-catenin and Bub3 in different phases of cell cycle for cell cycle progression. The cell cycle phase-dependent regulation of Bub3 by PKM2 was further supported by treatment with cyclin-dependent kinase 1 inhibitor RO-3306, which blocked histone H3 S10 phosphorylation, arrested the cells at G2-M border (Vassilev, 2006), and inhibited the association between PKM2 and Bub3 (Figure S2J). In line with the finding that PKM2 did not interact with Bub1, purified PKM2 did not phosphorylate purified GST-Bub1 (Figure S2K). In addition, 32P-phosphate-metabolic labeling of U87/EGFRvIII cells revealed that PKM2 depletion largely blocked phosphorylation of Bub3, but not Bub1 (Figure S2M).
To further support that PKM2 phosphorylates Bub3 Y207 during mitosis, we depleted endogenous Bub3 and reconstituted the expression of RNAi-resistant WT rBub3, rBub3 Y141F, rBub3 Y194F, or rBub3 Y207F in HeLa cells (Figure S3A) or in U87/EGFRvIII cells (Figure S3B). We demonstrated that only rBub3 Y207F was resistant to phosphorylation during mitosis (Figures 2I, S3C; accumulation of cyclin B1 as a marker of mitosis). These results were further supported by immunofluorescence analyses showing that phosphorylated Bub3 Y207 co-localized with CENP-A and was detected in prometaphase, but not in interphase (Figures 2J, S3D). In addition, depletion of endogenous PKM2 and reconstituted expression of WT rPKM2 or rPKM2 P408/T409A in HeLa cells (Figure S3E) revealed that expression of rPKM2 P408/T409A mutant, but not its WT counterpart, blocked Bub3 Y207 phosphorylation during mitosis (Figures 2J, S3D). These results were further supported by evidences showing that expression of rPKM2 P408/T409A mutant, which in contrast to its WT counterpart did not affect its glycolytic enzyme activity (Figure S3F), nuclear translocation (Figure S3G), or protein kinase ability to induce cyclin D1 expression (Yang et al., 2012b) (Figure S3H), failed to phosphorylate Bub3 Y207 during mitosis of HeLa (Figure 2K) and U87/EGFRvIII (Figure S3I) cells. In contrast, expression of rPKM2 P408/T409A, which phosphorylated histone H3 T11 and STAT3 Y705 but not Bub3 in vitro (Figure S3J), did not affect PKM2-mediated phosphorylation of histone H3 T11 upon EGF treatment and STAT3 Y705 phosphorylation (Gao et al., 2012; Yang et al., 2012b) (Figure S3K). These results indicated that PKM2 specifically phosphorylates Bub3 Y207 during mitosis.
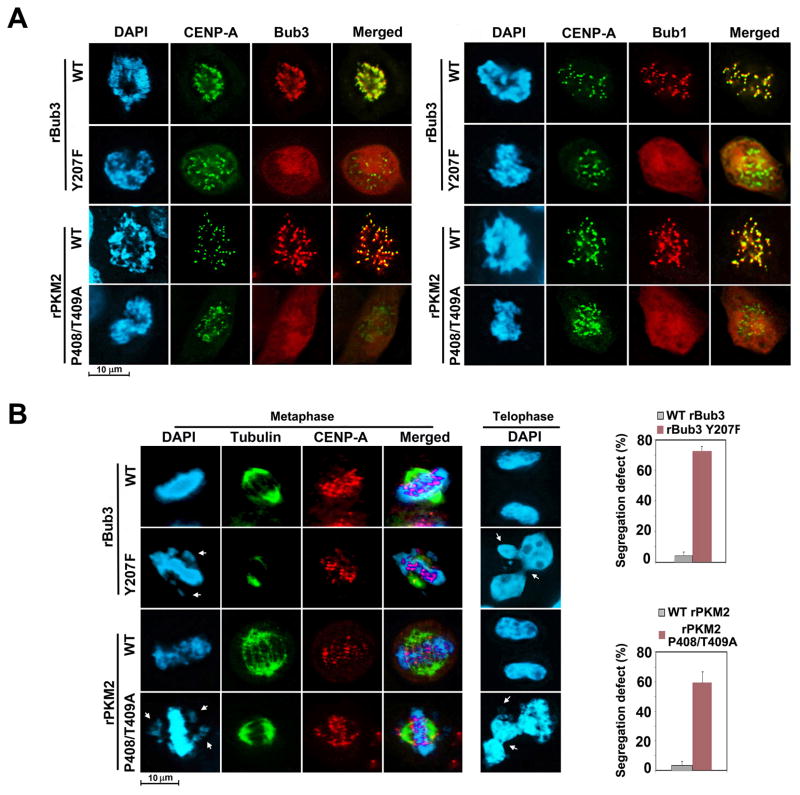
PKM2-dependent Bub3 Y207 phosphorylation is required for recruitment of Bub3 and Bub1 to kinetochores and accurate chromosome segregation
Purified recombinant GST-Bub3 Y207F, like its WT counterpart, bound to purified recombinant His-Bub1, indicating that Bub3 Y207 phosphorylation is not required for the association between Bub3 and Bub1 (Figure S4A). In agreement with these findings, a co-immunoprecipitation assay showed that Bub3 Y207F and its WT counterpart bound similarly to PKM2 and Bub1 (Figure S4B), indicating that Bub3 Y207 phosphorylation does not alter formation of the Bub3, Bub1, and PKM2 complex.
To determine whether Bub3 Y207 phosphorylation regulates Bub3 kinetochore localization, we immunostained HeLa cells with depleted endogenous Bub3 and reconstituted expression of WT rBub3 or rBub3 Y207F (Figure S3A). We showed that rBub3 Y207F, unlike its WT counterpart, failed to co-localize with CENP-A during prometaphase (Figures 3A and S4C, left panel; histone H3 pS10 staining as a marker of mitosis), indicating that PKM2-dependent Bub3 Y207 phosphorylation is required for kinetochore recruitment of Bub3. In addition, rBub3 Y207F expression blocked co-localization of Bub1 with CENP-A (Figures 3A and S4C, right panel). Similarly, reconstituted expression of rPKM2 P408/T409A blocked kinetochore recruitment of both Bub3 and Bub1 (Figures 3A and S4C, bottom panels). Furthermore, HeLa (Figure 3B), U87/EGFRvIII (Figure S4D), and U251 GBM (Figure S4E) cells with depletion of Bub3 or PKM2 and reconstituted expression of rBub3 Y207F or rPKM2 P408/T409A exhibited increased incidences of mitotic defects, as reflected by the misalignment of chromosomes in the metaphase plate and defective chromosome segregation represented by the increased incidence of lagging chromosomes and micronuclei in telophase. These results indicated that PKM2-dependent Bub3 Y207 phosphorylation is required for kinetochore recruitment of Bub3 and Bub1, correct K-MT attachments, and proper chromosome segregation.
Figure 3. PKM2-dependent Bub3 Y207 phosphorylation is required for recruitment of Bub3 and Bub1 to kinetochores and accurate chromosome segregation.
Immunofluorescence analyses were performed with the indicated antibodies. Nuclei were stained with DAPI (blue).
(A) HeLa cells with depleted Bub3 and reconstituted expression of WT rBub3 or rBub3 Y207F (top two panels) or with depleted PKM2 and reconstituted expression of WT rPKM2 or rPKM2 P408T409A (bottom two panels) were immunostained with the indicated antibodies. The cells in prometaphase were examined.
(B) HeLa cells with depleted Bub3 and reconstituted expression of WT rBub3 or rBub3 Y207F or with depleted PKM2 and reconstituted expression of WT rPKM2 or rPKM2 P408T409A were synchronized by thymidine double block (2 mM) and released for 9 h followed by MG132 (25 μM) treatment for 1 h (for staining the cells in metaphase). MG132 was then removed for 30 min (for staining the cells in telophase). One hundred cells in mitosis were analyzed. Data represent the mean ± SD of three independent experiments (right panel).
See also Figure S4.
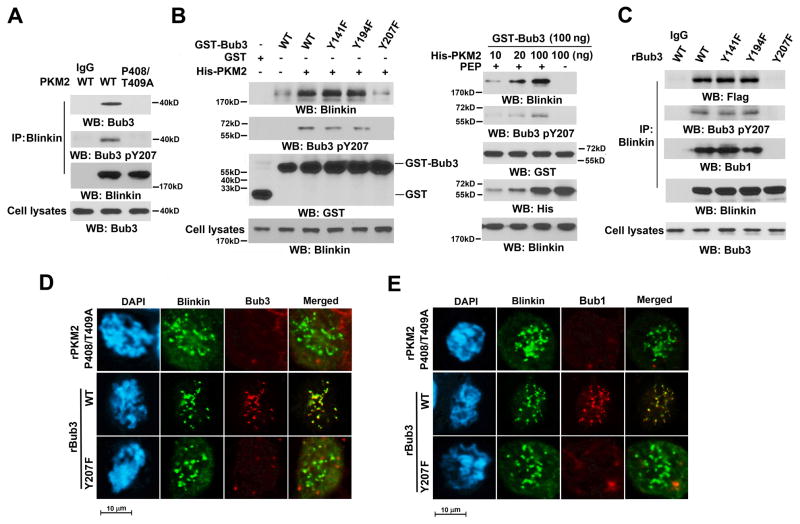
PKM2-dependent Bub3 Y207 phosphorylation is required for recruitment of Bub3 and Bub1 to Blinkin
Blinkin interaction with Bub1 is required for recruitment of Bub1 to kinetochores (Kiyomitsu et al., 2007). To examine whether Bub3 Y207 phosphorylation regulates the binding of the Bub3-Bub1 complex to Blinkin, we performed a double-thymidine block followed by co-immunoprecipitation analyses with an anti-Blinkin antibody. We showed that Blinkin interacted with phosphorylated Bub3 Y207 during mitosis in HeLa (Figure 4A) and U87/EGFRvIII (Figure S5A) cells with reconstituted expression of WT rPKM2, but not rPKM2 P408/T409A. Co-immunoprecipitation analyses showed that PKM2 associated with Bub3 and Bub1, but not Blinkin, during mitosis (Figure S5B), suggesting that PKM2 does not complex with Blinkin at kinetochores.
Figure 4. PKM2-dependent Bub3 Y207 phosphorylation is required for recruitment of Bub3 and Bub1 to Blinkin.
(A) HeLa cells with PKM2 depletion and reconstituted expression of WT rPKM2 or rPKM2 P408T409A were synchronized by thymidine double block (2 mM) and released for 9 h. Immunoprecipitation of endogenous Blinkin was performed.
(B) Purified recombinant GST or GST-Bub3 proteins were mixed with or without purified recombinant His-PKM2 (left panel) in different dosages (right panel) for an in vitro phosphorylation reaction in the presence (left panel) or absence (last lane of right panel) of PEP. The reaction mixtures were then incubated with the lysates of endogenous PKM2-depleted HeLa cells synchronized by thymidine double block (2 mM) and arrested at mitosis by nocodazole (20 ng/ml) treatment for 9 h. GST pull-down assays were performed.
(C) HeLa cells with Bub3 depletion and reconstituted expression of Flag-tagged Bub3 proteins were synchronized by thymidine double block (2 mM) and arrested at mitosis by nocodazole (20 ng/ml) treatment for 9 h. Immunoprecipitation analyses were performed with an anti-Blinkin antibody.
(D, E) HeLa cells with depleted PKM2 and reconstituted expression rPKM2 P408T409A or depleted Bub3 and reconstituted expression of WT rBub3 or rBub3 Y207F were synchronized by thymidine double block (2 mM) and arrested at mitosis by nocodazole (20 ng/ml) treatment for 9 h. The cells were then immunostained with the indicated antibodies. The cells in prometaphase were examined.
See also Figure S5.
The essential role of Bub3 Y207 phosphorylation in the regulation of the interaction between Bub3 and Blinkin was further supported by GST pull-down assays, which showed that purified GST-Bub3 interacted with a limited amount of Blinkin from mitotic HeLa (Figure 4B, left panel) and U87/EGFRvIII (Figure S5C) cells with endogenous PKM2 depletion (Figures S3E, S3G). However, incubation of purified WT Bub3, Bub3 Y141F, or Bub3 Y194F, but not Bub3 Y207F, with purified His-PKM2, which phosphorylated Bub3 Y207, significantly enriched the association between Bub3 and Blinkin in Hela (Figure 4B, left panel) and U87/EGFRvIII (Figure S5C) cells in a manner dependent on the amount of PKM2 and the presence of PEP (Figure 4B, right panel). In addition, co-immunoprecipitation with an anti-Blinkin antibody showed that Blinkin interacted with Bub1, WT rBub3, rBub3 Y141F, and rBub3 Y194F, but not with rBub3 Y207F, in HeLa (Figure 4C) and U87/EGFRvIII (Figure S5D) cells with reconstituted expression of these Bub3 proteins (Figures S3A and S3B).
These observations were further supported by immunofluorescence analyses showing that reconstituted expression of rPKM2 P408/T409A blocked the co-localization of Blinkin with Bub3 (Figure 4D, top panel) or Bub1 (Figure 4E, top panel). In addition, Bub3 Y207F, unlike its WT counterpart, failed to co-localize with Blinkin in kinetochores during prometaphase (Figure 4D, middle and bottom panels), and Bub3 Y207F expression blocked recruitment of Bub1 to Blinkin at kinetochores (Figure 4E, middle and bottom panels). In contrast, reconstituted expression of rBub3 Y207F or rPKM2 P408/T409A did not affect the co-localization of Blinkin with CENP-A at the centromere (Figure S5E). These results indicated that PKM2-dependent Bub3 Y207 phosphorylation is required for recruitment of Bub3 and Bub1 to kinetochores to interact with Blinkin.
Mps1 phosphorylates Blinkin or its homologs to recruit Bub1 to Blinkin in a Bub3-dependent manner (London et al., 2012; Shepperd et al., 2012; Yamagishi et al., 2012). The treatment of U87/EGFRvIII cells with an Mps1 inhibitor (reversine), which inhibited Bub3/Bub1 kinetochore translocation (data not shown), did not affect the interaction between Bub3 and PKM2 or affect PKM2-dependent Bub3 Y207 phosphorylation in mitosis (Figure S5F, left panel). In addition, PKM2 depletion did not affect the interaction between Blinkin and Mps1 (Figure S5F, middle panel), and the interaction between Blinkin and Mps1 was not enhanced in U87/EGFRvIII cells compared with that in U87 cells (Figure S5F, right panel). These results suggest that PKM2-dependent Bub3 phosphorylation and Mps1-mediated Blinkin phosphorylation are separate regulatory mechanisms that coordinately and precisely mediate the translocation of the Bub3/Bub1 complex to kinetochores.
PKM2-dependent Bub3 Y207 phosphorylation is required for spindle assembly checkpoint, cell survival, and cell proliferation
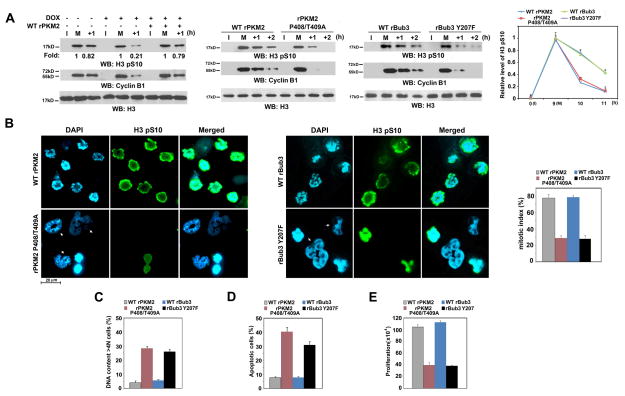
Bub3 and Bub1 are required for SAC and delay the onset of anaphase; failure of SAC leads to an accelerated mitosis exit (Bolanos-Garcia and Blundell, 2011). A double-thymidine block and release of U87/EGFRvIII (Figure 5A) and HeLa (Figure S5G) cells with depleted PKM2 or Bub3 and reconstituted expression of WT rPKM2, rPKM2 P408/T409A, WT rBub3, or rBub3 Y207F showed that PKM2 depletion resulted in rapid downregulation of histone H3-S10 phosphorylation and cyclin B1 expression (Figure 5A, left panel), suggesting an accelerated mitosis exit. This SAC defect was rescued by the reconstituted expression of WT rPKM2 (Figure 5A, left panel), indicating a specific SAC regulation by PKM2. In addition, expression of rPKM2 P408/T409A or rBub3 Y207F, in contrast to expression of their WT counterparts, also led to an accelerated mitosis exit (Figure 5A middle and right panels; Figure S5G), indicating an instrumental role of PKM2-dependent Bub3 phosphorylation in SAC regulation. These observations were supported by immunofluorescence analyses showing that about 80% of the HeLa cells with reconstituted expression of WT rPKM2 or WT rBub3 were arrested in mitosis in the presence of nocodazole treatment for 24 hours (Figure 5B). In contrast, only about 30% of the cells with reconstituted expression of rPKM2 P408/T409A or rBub3 Y207F were arrested in mitosis, and a significant fraction of these cells failed to undergo mitotic arrest (Figure 5B).
Figure 5. PKM2-dependent Bub3 Y207 phosphorylation is required for spindle assembly checkpoint, cell survival, and cell proliferation.
(A) U87/EGFRvIII cells with PKM2 depletion and reconstituted expression of WT rPKM2 (left panel) or rPKM2 P408T409A (middle panel) or with Bub3 depletion and reconstituted expression of WT rBub3 or rBub3 Y207F (right panel) were synchronized by thymidine double block (2 mM) and released for the indicated periods of time. Immunoblotting analyses were performed with the indicated antibodies. The intensity of H3 pS10 was quantified (right panel). Data represent the mean ± SD of three independent experiments. I, interphase; M, mitosis.
(B) HeLa cells with PKM2 depletion and reconstituted expression of WT rPKM2 or rPKM2 P408T409A or with Bub3 depletion and reconstituted expression of WT rBub3 or rBub3 Y207F were synchronized by thymidine double block (2 mM) and released for 6 h, followed by nocodazole (20 ng/ml) treatment for 24 h. Immunofluorescence analyses were performed with an anti-histone H3-pS10 antibody. One hundred cells were analyzed. Data represent the mean ± SD of five independent experiments.
(C, D) HeLa cells with PKM2 depletion and reconstituted expression of WT rPKM2 or rPKM2 P408T409A or with Bub3 depletion and reconstituted expression of WT rBub3 or rBub3 Y207F were synchronized by thymidine double block (2 mM) and released for 6 h, followed by nocodazole (20 ng/ml) treatment for 36 h. Flow cytometric analyses of mitotic (C) and apoptotic cells (D) was performed. Data represent the mean ± SD of five independent experiments.
(E) HeLa cells (2 × 104) with PKM2 depletion and reconstituted expression of WT rPKM2 or rPKM2 P408T409A or with Bub3 depletion and reconstituted expression of WT rBub3 or rBub3 Y207F were plated and counted 7 days after seeding in DMEM with 2% bovine calf serum. Data represent the mean ± SD of three independent experiments.
See also Figures S5 and S6.
These findings were further supported by flow cytometric analyses. With exposure to nocodazole, about 27–30% of the cells with reconstituted expression of rPKM2 P408/T409A or Bub3 Y207F underwent another round of DNA replication in the absence of cell division and had DNA content greater than 4N (Figures 5C, S5H), indicating a defective SAC. Notably, a significantly higher fraction of these cells underwent cell death than their WT counterparts (Figures 5D, S5H). We also found a higher percentage of apoptosis among the cells with reconstituted expression of rPKM2 P408/T409A or rBub3 Y207F than among their WT counterparts in the absence of nocodazole treatment (data not shown). These results indicated that PKM2 kinase activity and PKM2-dependent Bub3 Y207 phosphorylation are required for SAC to prevent abnormal mitosis exit and apoptosis. In line with this mitosis defect, cells with reconstituted expression of rPKM2 P408/T409A and rBub3 Y207F exhibited inhibited cell growth, in contrast to their WT counterpart cells (Figure 5E).
To determine whether out finding has general applications, we depleted expression of PKM2 in MDA-MB-231 human breast cancer cells, DU145 human prostate cancer cells, A549 human lung adenocarcinoma cells, PANC-1 human pancreatic cancer cells, and SW480 human colon adenocarcinoma cells. As shown in Figure S5I, Bub3 Y207 was phosphorylated during mitosis, and this phosphorylation was significantly blocked by PKM2 depletion, which was accompanied by an increase in the percentage of aneuploid cells.
PKM2 is highly expressed during embryonic development (Vander Heiden et al., 2009). To determine whether the function of PKM2 in mitosis is also shared by rapidly proliferating embryonic cells, we depleted PKM2 or Bub3 from immortalized mouse embryonic fibroblasts (MEFs) and reconstituted their expression with WT rPKM2, rPKM2 P408/T409A, WT rBub3, or rBub3 Y207F (Figure S6A). Figure S6B shows Bub3 Y207 was phosphorylated in mitosis, and this phosphorylation was blocked by rPKM2 P408/T409A expression (Figure S6C). In addition, expression of rPKM2 P408/T409A and rBub3 Y207F blocked Bub3 recruitment to the kinetochores (Figure S6D). In addition, a significantly higher fraction of the cells expressing these mutants had DNA content greater than 4N (left panel), underwent apoptosis (middle panel), and had inhibited cell proliferation (right panel) compared with their WT counterparts (Figure S6E). Expression of EGFRvIII in MEFs, which increased PKM2 expression (Figure S6F), the binding of PKM2 to Bub3, Bub3 Y207 phosphorylation (Figure S6G), and cell proliferation (Figure S6E, right panel), resulted in more dramatic defects in cell mitosis and cell proliferation upon reconstituted expression of rPKM2 P408/T409A and rBub3 Y207F than did expression of these mutants in MEFs without EGFRvIII expression (Figures S6A, S6E). These results indicate that PKM2 is instrumental in controlling the mitosis progression of tumor cells and rapidly proliferating MEFs and strongly suggest that tumor cells with enhanced PKM2 expression have increased dependence on PKM2’s regulation on mitosis.
In agreement with the finding that rPKM2 P408/T409A mutant did not affect glycolytic enzyme activity in vitro, we did not detect a significant difference in glucose uptake (Figure S6H) or lactate production (Figure S6I) from the cells with reconstituted expression of rPKM2 P408/T409A and rBub3 Y207F before they entered mitosis. However, expression of both rPKM2 P408/T409A and rBub3 Y207F resulted in decreased glucose uptake and lactate production in the cells after exit from cell mitosis and division, indicating that aneuploid cells with altered gene expression can have inhibited aerobic glycolysis.
To determine whether the role of PKM2 in the regulation of Bub3 is evolutionarily conserved, we examined whether the FBP-regulated isoform of pyruvate kinase CDC19 (Pyk1) in Saccharomyces cerevisiae (Boles et al., 1997) has a similar function to that of human PKM2. Pyk1, which does not contain P408/T409, failed to phosphorylate recombinant human Bub3 in vitro (Figure S6J). In addition, reconstituted expression of Pyk1 and human rPKM2 P408/T409A in PKM2-depleted U87/EGFRvIII cells resulted in a similar rate of segregation defect (Figure S6K). These results strongly suggest that Bub3 regulation by PKM2 is a late-evolving phenomenon for controlling cell mitosis.
Bub3 Y207 phosphorylation is required for tumorigenesis
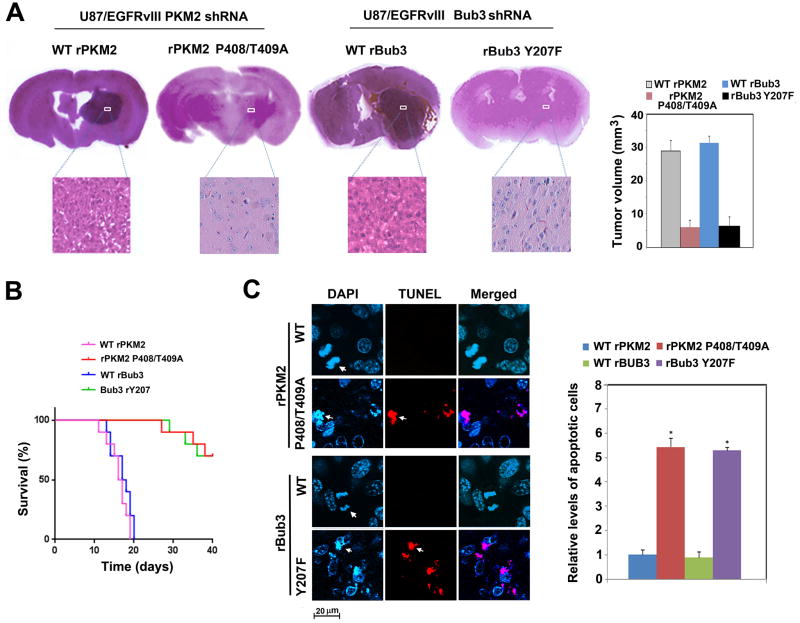
To determine the role of PKM2-dependent Bub3 Y207F phosphorylation in brain tumor development, we intracranially injected endogenous PKM2- or Bub3-depleted U87/EGFRvIII cells with reconstituted expression of WT rPKM2, rPKM2 P408/T409A, WT rBub3, or rBub3 Y207F into athymic nude mice. U87/EGFRvIII cells expressing WT rPKM2 or WT rBub3 elicited rapid tumorigenesis (Figure 6A). In contrast, rPKM2 P408/T409A and rBub3 Y207F expression significantly inhibited EGFRvIII-driven tumor growth, prolonged mouse survival (Figures 6A and 6B), and promoted tumor cell apoptosis (Figure 6C). Similar tumorigenesis results were obtained by using GSC11 human primary GBM cells with endogenous Bub3 depletion and reconstituted expression of WT rBub3 or rBub3 Y207F (Figures S7A, S7B). These results indicated that PKM2-dependent Bub3 Y207 phosphorylation is instrumental in EGFR-promoted tumor development.
Figure 6. Bub3 Y207 phosphorylation is required for tumorigenesis.
(A) A total of 5 × 105 U87/EGFRvIII cells with PKM2 depletion and reconstituted expression of WT rPKM2 or rPKM2 P408/T409A (left panel) or with Bub3 depletion and reconstituted expression of WT rBub3 or rBub3 Y207F (middle panel) were intracranially injected into athymic nude mice. The mice were sacrificed and examined for tumor growth. H&E-stained coronal brain sections show representative tumor xenografts. Tumor volumes were measured by using length (a) and width (b) and calculated using the equation: V = ab2/2. Data represent the means ± SD of 7 mice (right panel).
(B, C) A total of 5 × 105 U87/EGFRvIII cells with PKM2 depletion and reconstituted expression of WT rPKM2 or rPKM2 P408/T409A or with Bub3 depletion and reconstituted expression of WT rBub3 or rBub3 Y207F were intracranially injected into athymic nude mice. The mouse survival times were recorded. Data represent the means ± SD of 10 mice (B). The number of TUNEL-positive cells per mm2 tumor tissue area was counted. Data represent the mean ± SD of 5 mice from each group. *P<0.01: statistically significant value in relation with tumor cells expressing WT rPKM2 or WT rBub3 (C).
See also Figure S7.
Bub3 Y207 phosphorylation positively correlates with the level of H3-S10 phosphorylation in human tumor specimens
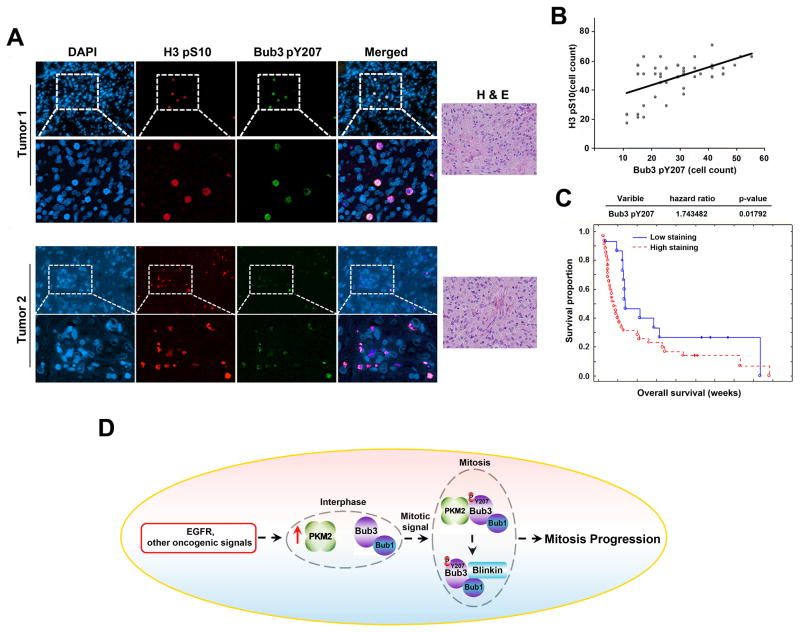
Bub3 Y207 phosphorylation correlates with H3-S10 phosphorylation during mitosis (Figure 2H). To further define the clinical relevance of our finding that PKM2 phosphorylates Bub3 Y207, we used immunofluorescent analyses to examine the levels of Bub3 Y207 phosphorylation and H3-S10 phosphorylation in serial sections of 50 human primary GBM specimens (World Health Organization [WHO] grade IV). The antibody specificities were validated by using specific blocking peptides (Figure S2E). As shown in Figure 7A, Bub3 Y207 phosphorylation co-localized with H3-S10 phosphorylation. In addition, the levels of Bub3 Y207 and H3-S10 phosphorylation were correlated. Quantification of the staining showed that these correlations were significant (Figure 7B; r = 0.78, p < 0.0001).
Figure 7. Bub3 Y207 phosphorylation positively correlates with the level of H3-S10 phosphorylation in human GBM specimens.
(A, B) H&E staining and immunofluorescent staining with anti–phospho-Bub3 Y207 and anti–phospho-H3-S10 antibodies were performed on 50 GBM specimens. Representative photos of two tumors are shown (A). We quantitatively scored the tissue sections by counting positively stained cells in 10 microscopic fields. (Pearson product moment correlation test; r = 0.78, p < 0.0001) (B). Note that some of the dots on the graphs represent more than one specimen (some scores overlapped).
(C) The survival times for 50 GBM patients, who received standard adjuvant radiotherapy after surgery, followed by treatment with an alkylating agent (temozolomide in most cases), with low (0–4 staining scores, blue curve) versus high (4.1–8 staining scores, red curve) Bub3 Y207 phosphorylation (low, 15 patients; high, 35 patients) were compared. The table (top) shows the multivariate analysis, indicating the significance level of the association of Bub3 Y207 phosphorylation (p = 0.01792) with patient survival. Empty circles represent deceased patients, and filled circles represent censored (alive at last clinical follow-up) patients.
(D) PKM2, which is upregulated in its expression by activation of EGFR or other oncogenic signalings, binds to and phosphorylates Bub3 at Y207 and regulates the binding of Bub3-Bub1 complex to Blinkin at kinetochores, spindle assembly, and mitotic checkpoint in tumor cells.
See also Figure S7.
In addition, co-localization of Bub3 Y207 phosphorylation with H3-S10 phosphorylation and correlation of the levels of Bub3 Y207 and H3-S10 phosphorylation were also observed in 50 human lung adenocarcinoma specimens (Figure S7C, S7D; r = 0.71, p < 0.001). Furthermore, brain tumor patients whose tumors had low Bub3 Y207 phosphorylation (15 cases) had a median survival duration of 69.8 weeks; those GBM patients whose tumors had high levels of Bub3 Y207 phosphorylation (35 cases) had a significantly lower median survival duration of 40.5 weeks (Figure 7C). These results support a role for PKM2-dependent Bub3 Y207 phosphorylation in the clinical behavior of human GBM and lung adenocarcinoma and reveal a relationship between Bub3 Y207 phosphorylation and the mitotic progression of tumor cells.
DISCUSSION
The mitotic checkpoint is a major cell cycle control mechanism that guards against chromosome missegregation and the subsequent production of aneuploid daughter cells (Holland and Cleveland, 2009). PKM2 plays a key role as a cytosolic glycolytic enzyme in the regulation of cancer cell glycolysis. PKM2 also processes important nuclear functions and plays a critical role in regulating gene transcription by phosphorylating histone H3 (Yang et al., 2012b; Yang et al., 2011) and functioning as a transcriptional co-activator (Gao et al., 2012; Luo et al., 2011; Yang et al., 2011). However, whether PKM2 directly regulates cell cycle progression by mediating the mitosis process is not known. In this report, we demonstrate that PKM2 interacts with Bub3 and phosphorylates Bub3 Y207, which leads to the recruitment of the Bub3-Bub1 complex to Blinkin in kinetochores, precise control of kinetochore-spindle microtubule attachment and SAC, and subsequently, accurate chromosome segregation and proliferation of tumor cells.
Aneuploidy is associated with cancer and tumorigenesis; however, it also adversely affects cell proliferation and the growth of organisms owing to the gain or loss of hundreds or thousands of genes and the disruption of a large array of cellular activities. Thus, aneuploidy can either promote or suppress tumor formation, depending on the genetic and cellular context, including the specific genes on the abnormal chromosome, the extent of the aneuploidy, the already-accumulated genetic errors, and specific features unique to the cell type (Holland and Cleveland, 2009). In mammals, complete inactivation of the mitotic checkpoint leads to massive chromosome missegregation, cell death, and early embryonic lethality (Dobles et al., 2000; Michel et al., 2001; Williams et al., 2008). Depleting the SAC proteins BubR1 or Mad2 or inhibiting BubR1 kinase activity causes apoptotic cell death in human cancer cells (Kops et al., 2004). Depletion of Bub1, Bub3, or Blinkin all leads to chromosome missegregation and mitosis defects (Kiyomitsu et al., 2007; Logarinho and Bousbaa, 2008). Consistent with the critical role of SAC proteins in mitosis, Bub1-null mice are embryonically lethal (Jeganathan et al., 2007). Similarly, Bub3-null embryos accumulate mitotic errors in the form of micronuclei, chromatin bridging, lagging chromosomes, and irregular nuclear morphology that result in failure to survive. Bub3-null embryos treated with a spindle-depolymerizing agent fail to arrest in metaphase and show an increase in mitotic defects (Kalitsis et al., 2000). In line with these evidences of the essential roles of Bub1, Bub3, and Blinkin in kinetochore-spindle microtubule attachment and mitotic checkpoint, we found that reconstituted expression of the Bub3-binding defect mutant of PKM2 in endogenous PKM2-depleted cancer cells displayed a similar mitotic defect, aneuoploid formation, and cell apoptosis. Our findings support that PKM2-dependent Bub3 Y207 phosphorylation regulates the mitotic functions of the Bub3-Bub1-Blinkin complex and governs the integrity of chromosome segregation and cell survival and proliferation.
Pyk1, a yeast homolog of human PKM2, does not contain P408/T409 for interacting Bub3 and failed to phosphorylate human Bub3 and rescue the defect induced by PKM2 depletion in tumor cells. In addition, Bub3 in budding yeast does not conserve Bub3 Y207. These findings strongly suggest that Bub3 regulation by PKM2 is a late-evolving phenomenon for controlling the mitosis of tumor cells and rapidly dividing normal mammalian cells. It was recently shown that the β-propeller of yeast Bub3 is important for the binding of Bub3 to the phosphorylated MELT peptides of Blinkin (Primorac et al., 2013). In addition, mutation of R217 and R239 in the β-propeller largely reduced the interaction between Bub3 and Blinkin, suggesting that the β-propeller is responsible for Bub3’s interaction with Blinkin. In line with this finding, Y207 of mammalian Bub3, which aligns with the F222 of the Bub3 yeast homolog, is in the interface involved in the interaction with Blinkin. We demonstrated that the basic binding of Bub3 to Blinkin in tumor cells is substantially enhanced after Bub3 Y207 is phosphorylated by PKM2. These results strongly suggest that oncogenic signaling in mammalian cells regulates the mitosis through the same Bub3-Blinkin interface with a posttranslational modification to increase the binding of Bub3 to Blinkin.
The tumor cells with expression of the active EGFRvIII mutant, which resulted in an increased PKM2 expression (Yang et al., 2012a), had increased dependence on PKM2 during mitotic progression. This strongly suggests that the tumor cells developed their own specific regulatory mechanism for mitosis by regulating the expression of PKM2, which in turn regulated cell-cycle progression via a feedback mechanism. In line with these results, the MEFs, which have lower expression of PKM2 than that in tumor cells, had low rates of defects during mitotic progression under the condition of PKM2 depletion. However, expression of EGFRvIII oncoprotein in these fibroblasts increased PKM2 expression and enhanced the interaction between PKM2 and Bub3 and Bub3 Y207 phosphorylation, which led to increased dependence of the cells on PKM2-regulated mitotic progression. These findings suggest that normal cells, especially adult normal cells with low levels of PKM2 expression, primarily depend on other mechanisms to regulate cell mitosis, which can be abnormally regulated by aberrantly expressed PKM2 upon activation of oncogenes (Figure 7D).
Abnormally high expression of SAC proteins such as MAD2, kinetochore component HEC1, and PKM2 is common in human cancers, and elevated levels of these proteins are often associated with a poor prognosis (Holland and Cleveland, 2009; Mazurek, 2007; Yang et al., 2011). In contrast, reduced expression of SAC proteins such as CENP-E and BubR1, resulting from CENP-E haploinsufficiency and BubR1 heterozygosity, respectively, lowered the tumor incidence in mice (Holland and Cleveland, 2009; Rao et al., 2005). The findings that interruption of Bub3 Y207 phosphorylation results in increased cell apoptosis and inhibition of tumor cell proliferation and EGFR-promoted tumorigenesis and that Bub3 Y207 phosphorylation correlates with the mitotic progression of tumor cells in GBM and lung cancer specimens highlight the nonmetabolic function of PKM2 as a protein kinase controlling the mitotic process and may provide a molecular basis for improving the diagnosis and treatment of tumors with upregulated PKM2.
EXPERIMENTAL PROCEDURES
Materials
Rabbit polyclonal antibodies recognizing Bub3, phospho-Bub3 Y207, PKM1, PKM2, and phospho-histone H3-S10 were obtained from Signalway Antibody (College Park, MD). A polyclonal antibody for cyclin D1 was purchased from Santa Cruz Biotechnology (Santa Cruz, CA).
Transfection
Cells were plated at a density of 4 × 105/60-mm dish 18 h prior to transfection. Transfection was performed using HyFect reagents (Denville Scientific) according to the vendor’s instructions. Transfected cultures were selected with puromycin (5 μg/ml), hygromycin (200 μg/ml), or G418 (400 μg/ml) for 10–14 days at 37°C. At that time, antibiotic-resistant colonies were picked, pooled, and expanded for further analysis under selective conditions.
Cell proliferation assay
Cells (2 × 104) were plated and counted 7 days after seeding in DMEM with 0.5% bovine calf serum. Data represent the mean ± standard deviation (S.D.) of three independent experiments.
Flow cytometric analysis
Cells (1 × 106) were fixed in 70% ethanol on ice for 3 h, spun down, and incubated for 1 h at 37°C in PBS with DNase-free RNase A (100 μg/ml) and propidium iodide (50 μg/ml). Cells were then analyzed by fluorescence-activated cell sorting (FACS).
Supplementary Material
HIGHLIGHTS.
PKM2 binds to and phosphorylates Bub3 at Y207.
Bub3 Y207 phosphorylation is required for Bub3-Bub1 complex to interact with Blinkin.
Bub3 Y207 phosphorylation is required for correct kinetochore-microtubule attachment.
Bub3 Y207 phosphorylation is required for SAC and EGFR-induced brain tumorigenesis.
Acknowledgments
We thank Don Cleveland (Ludwig Institute for Cancer Research, UCSD) for LAP-CENP-T, and CENP-U plasmids, Iain M. Cheeseman (Whitehead Institute for Biomedical Research, MIT) for mCherry-H2B plasmid, and Katsumi Kitagawa (St. Jude Children’s Research Hospital) for His-Bub3 and Bub1 plasmids. We thank Lewis Cantley and Costas Lyssiotis for their insightful suggestions and Dawn Chalaire for her critical reading of this manuscript.
This work was supported by National Cancer Institute grants 2R01CA109035 (Z.L.), 1R0CA169603 (Z.L.), and CA16672 (Cancer Center Support Grant), research grants (RP110252 and RP130389; Z.L.) from the Cancer Prevention and Research Institute of Texas (CPRIT), an American Cancer Society Research Scholar Award (RSG-09-277-01-CSM; Z.L.), the James S. McDonnell Foundation 21st Century Science Initiative in Brain Cancer Research Award (220020318; Z.L.), and the Odyssey Fellowship from The University of Texas MD Anderson Cancer Center (J.Y.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bolanos-Garcia VM, Blundell TL. BUB1 and BUBR1: multifaceted kinases of the cell cycle. Trends in biochemical sciences. 2011;36:141–150. doi: 10.1016/j.tibs.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boles E, Schulte F, Miosga T, Freidel K, Schluter E, Zimmermann FK, Hollenberg CP, Heinisch JJ. Characterization of a glucose-repressed pyruvate kinase (Pyk2p) in Saccharomyces cerevisiae that is catalytically insensitive to fructose-1,6-bisphosphate. Journal of bacteriology. 1997;179:2987–2993. doi: 10.1128/jb.179.9.2987-2993.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheeseman IM, Desai A. Molecular architecture of the kinetochore-microtubule interface. Nature reviews Molecular cell biology. 2008;9:33–46. doi: 10.1038/nrm2310. [DOI] [PubMed] [Google Scholar]
- Cheung P, Allis CD, Sassone-Corsi P. Signaling to chromatin through histone modifications. Cell. 2000;103:263–271. doi: 10.1016/s0092-8674(00)00118-5. [DOI] [PubMed] [Google Scholar]
- Christofk HR, Vander Heiden MG, Harris MH, Ramanathan A, Gerszten RE, Wei R, Fleming MD, Schreiber SL, Cantley LC. The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature. 2008;452:230–233. doi: 10.1038/nature06734. [DOI] [PubMed] [Google Scholar]
- Dobles M, Liberal V, Scott ML, Benezra R, Sorger PK. Chromosome missegregation and apoptosis in mice lacking the mitotic checkpoint protein Mad2. Cell. 2000;101:635–645. doi: 10.1016/s0092-8674(00)80875-2. [DOI] [PubMed] [Google Scholar]
- Gao X, Wang H, Yang JJ, Liu X, Liu ZR. Pyruvate kinase M2 regulates gene transcription by acting as a protein kinase. Molecular cell. 2012;45:598–609. doi: 10.1016/j.molcel.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland AJ, Cleveland DW. Boveri revisited: chromosomal instability, aneuploidy and tumorigenesis. Nature reviews Molecular cell biology. 2009;10:478–487. doi: 10.1038/nrm2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeganathan K, Malureanu L, Baker DJ, Abraham SC, van Deursen JM. Bub1 mediates cell death in response to chromosome missegregation and acts to suppress spontaneous tumorigenesis. The Journal of cell biology. 2007;179:255–267. doi: 10.1083/jcb.200706015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalitsis P, Earle E, Fowler KJ, Choo KH. Bub3 gene disruption in mice reveals essential mitotic spindle checkpoint function during early embryogenesis. Genes & development. 2000;14:2277–2282. doi: 10.1101/gad.827500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyomitsu T, Obuse C, Yanagida M. Human Blinkin/AF15q14 is required for chromosome alignment and the mitotic checkpoint through direct interaction with Bub1 and BubR1. Dev Cell. 2007;13:663–676. doi: 10.1016/j.devcel.2007.09.005. [DOI] [PubMed] [Google Scholar]
- Koppenol WH, Bounds PL, Dang CV. Otto Warburg’s contributions to current concepts of cancer metabolism. Nature reviews Cancer. 2011;11:325–337. doi: 10.1038/nrc3038. [DOI] [PubMed] [Google Scholar]
- Kops GJ, Foltz DR, Cleveland DW. Lethality to human cancer cells through massive chromosome loss by inhibition of the mitotic checkpoint. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:8699–8704. doi: 10.1073/pnas.0401142101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logarinho E, Bousbaa H. Kinetochore-microtubule interactions “in check” by Bub1, Bub3 and BubR1: The dual task of attaching and signalling. Cell Cycle. 2008;7:1763–1768. doi: 10.4161/cc.7.12.6180. [DOI] [PubMed] [Google Scholar]
- Logarinho E, Resende T, Torres C, Bousbaa H. The human spindle assembly checkpoint protein Bub3 is required for the establishment of efficient kinetochore-microtubule attachments. Mol Biol Cell. 2008;19:1798–1813. doi: 10.1091/mbc.E07-07-0633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London N, Ceto S, Ranish JA, Biggins S. Phosphoregulation of Spc105 by Mps1 and PP1 regulates Bub1 localization to kinetochores. Current biology : CB. 2012;22:900–906. doi: 10.1016/j.cub.2012.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Z. Nonmetabolic functions of pyruvate kinase isoform M2 in controlling cell cycle progression and tumorigenesis. Chinese journal of cancer. 2012;31:5–7. doi: 10.5732/cjc.011.10446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo W, Hu H, Chang R, Zhong J, Knabel M, O’Meally R, Cole RN, Pandey A, Semenza GL. Pyruvate kinase M2 is a PHD3-stimulated coactivator for hypoxia-inducible factor 1. Cell. 2011;145:732–744. doi: 10.1016/j.cell.2011.03.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazurek S. Pyruvate kinase type M2: a key regulator within the tumour metabolome and a tool for metabolic profiling of tumours. Ernst Schering Found Symp Proc. 2007:99–124. doi: 10.1007/2789_2008_091. [DOI] [PubMed] [Google Scholar]
- Meraldi P, Sorger PK. A dual role for Bub1 in the spindle checkpoint and chromosome congression. The EMBO journal. 2005;24:1621–1633. doi: 10.1038/sj.emboj.7600641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel LS, Liberal V, Chatterjee A, Kirchwegger R, Pasche B, Gerald W, Dobles M, Sorger PK, Murty VV, Benezra R. MAD2 haplo-insufficiency causes premature anaphase and chromosome instability in mammalian cells. Nature. 2001;409:355–359. doi: 10.1038/35053094. [DOI] [PubMed] [Google Scholar]
- Musacchio A, Salmon ED. The spindle-assembly checkpoint in space and time. Nature reviews Molecular cell biology. 2007;8:379–393. doi: 10.1038/nrm2163. [DOI] [PubMed] [Google Scholar]
- Primorac I, Weir JR, Chiroli E, Gross F, Hoffmann I, van Gerwen S, Ciliberto A, Musacchio A. Bub3 reads phosphorylated MELT repeats to promote spindle assembly checkpoint signaling. eLife. 2013;2:e01030. doi: 10.7554/eLife.01030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao CV, Yang YM, Swamy MV, Liu T, Fang Y, Mahmood R, Jhanwar-Uniyal M, Dai W. Colonic tumorigenesis in BubR1+/-ApcMin/+ compound mutant mice is linked to premature separation of sister chromatids and enhanced genomic instability. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:4365–4370. doi: 10.1073/pnas.0407822102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepperd LA, Meadows JC, Sochaj AM, Lancaster TC, Zou J, Buttrick GJ, Rappsilber J, Hardwick KG, Millar JB. Phosphodependent recruitment of Bub1 and Bub3 to Spc7/KNL1 by Mph1 kinase maintains the spindle checkpoint. Current biology : CB. 2012;22:891–899. doi: 10.1016/j.cub.2012.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka TU, Stark MJ, Tanaka K. Kinetochore capture and bi-orientation on the mitotic spindle. Nature reviews Molecular cell biology. 2005;6:929–942. doi: 10.1038/nrm1764. [DOI] [PubMed] [Google Scholar]
- Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassilev LT. Cell cycle synchronization at the G2/M phase border by reversible inhibition of CDK1. Cell Cycle. 2006;5:2555–2556. doi: 10.4161/cc.5.22.3463. [DOI] [PubMed] [Google Scholar]
- Verdaasdonk JS, Bloom K. Centromeres: unique chromatin structures that drive chromosome segregation. Nature reviews Molecular cell biology. 2011;12:320–332. doi: 10.1038/nrm3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams BR, Prabhu VR, Hunter KE, Glazier CM, Whittaker CA, Housman DE, Amon A. Aneuploidy affects proliferation and spontaneous immortalization in mammalian cells. Science. 2008;322:703–709. doi: 10.1126/science.1160058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagishi Y, Yang CH, Tanno Y, Watanabe Y. MPS1/Mph1 phosphorylates the kinetochore protein KNL1/Spc7 to recruit SAC components. Nature cell biology. 2012;14:746–752. doi: 10.1038/ncb2515. [DOI] [PubMed] [Google Scholar]
- Yang W, Lu Z. Regulation and function of pyruvate kinase M2 in cancer. Cancer letters. 2013;339:153–158. doi: 10.1016/j.canlet.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W, Xia Y, Cao Y, Zheng Y, Bu W, Zhang L, You MJ, Koh MY, Cote G, Aldape K, et al. EGFR-induced and PKCepsilon monoubiquitylation-dependent NF-kappaB activation upregulates PKM2 expression and promotes tumorigenesis. Molecular cell. 2012a;48:771–784. doi: 10.1016/j.molcel.2012.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W, Xia Y, Hawke D, Li X, Liang J, Xing D, Aldape K, Hunter T, Alfred Yung WK, Lu Z. PKM2 phosphorylates histone H3 and promotes gene transcription and tumorigenesis. Cell. 2012b;150:685–696. doi: 10.1016/j.cell.2012.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W, Xia Y, Ji H, Zheng Y, Liang J, Huang W, Gao X, Aldape K, Lu Z. Nuclear PKM2 regulates beta-catenin transactivation upon EGFR activation. Nature. 2011;480:118–122. doi: 10.1038/nature10598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W, Zheng Y, Xia Y, Ji H, Chen X, Guo F, Lyssiotis CA, Aldape K, Cantley LC, Lu Z. ERK1/2-dependent phosphorylation and nuclear translocation of PKM2 promotes the Warburg effect. Nature cell biology. 2012c;14:1295–1304. doi: 10.1038/ncb2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Zhang Z, Chen G, Zhao M, Wang D, Zhang X, Du Z, Xu Y, Yu X. FK228 induces mitotic catastrophe in A549 cells by mistargeting chromosomal passenger complex localization through changing centromeric H3K9 hypoacetylation. Acta biochimica et biophysica Sinica. 2010;42:677–687. doi: 10.1093/abbs/gmq077. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.