Abstract
Pituitary adenylate cyclase activating peptide (PACAP), a potent neuropeptide which crosses the blood–brain barrier, is known to provide neuroprotection in rat stroke models of middle cerebral artery occlusion (MCAO) by mechanism(s) which deserve clarification. We confirmed that following i.v. injection of 30 ng/kg of PACAP38 in rats exposed to 2 h of MCAO focal cerebral ischemia and 48 h reoxygenation, 50 % neuroprotection was measured by reduced caspase-3 activity and volume of cerebral infarction. Similar neuroprotective effects were measured upon PACAP38 treatment of oxygen–glucose deprivation and reoxygenation of brain cortical neurons. The neuroprotection was temporally associated with increased expression of brain-derived neurotrophic factor, phosphorylation of its receptor—tropomyosin-related kinase receptor type B (trkB), activation of phosphoinositide 3-kinase and Akt, and reduction of extracellular signal-regulated kinases 1/2 phosphorylation. PACAP38 increased expression of neuronal markers beta-tubulin III, microtubule-associated protein-2, and growth-associated protein-43. PACAP38 induced stimulation of Rac and suppression of Rho GTPase activities. PACAP38 down-regulated the nerve growth factor receptor (p75NTR) and associated Nogo-(Neurite outgrowth-A) receptor. Collectively, these in vitro and in vivo results propose that PACAP exhibits neuroprotective effects in cerebral ischemia by three mechanisms: a direct one, mediated by PACAP receptors, and two indirect, induced by neurotrophin release, activation of the trkB receptors and attenuation of neuronal growth inhibitory signaling molecules p75NTR and Nogo receptor.
Keywords: Stroke, Apoptosis, Neuroprotection, PACAP, BDNF, trkB, p75, NgR, Akt, Erk1/2
Introduction
Pituitary adenylate cyclase activating peptide (PACAP) was originally isolated from ovine hypothalamic extracts (Miyata et al. 1989), sharing the highest homology with vasoactive intestinal polypeptide of the secretin/glucagon family. PACAP exists in two forms: PACAP38, which is the predominant form in the nervous system, and PACAP27, which consists of N-terminal 27 amino acids of PACAP38 (Arimura 1998). While both PACAP isoforms activate adenylate cyclase with equal potency, PACAP38 is 200-fold more potent than PACAP27 in promoting activation of phospholipase C (Deutsch and Sun 1992). PACAP is widely distributed in the brain and peripheral organs, notably in the endocrine pancreas, gonads, and respiratory and urogenital tracts (Sherwood et al. 2000). In the nervous system, PACAP is widely expressed in the embryonic brain at the onset of neurogenesis and it has also been shown to be upregulated after brain injury (Skoglosa et al. 1999). PACAP is involved in various processes such as the regulation of hormonal secretion, energy metabolism, and neuronal development, proliferation, regeneration, and survival (Magistretti et al. 1998; Vaudry et al. 1998a). PACAP has three distinct receptor subtypes, the PACAP-specific PAC1 receptor, and the two PACAP/vasoactive intestinal polypeptide-indifferent VPAC1 and VPAC2 receptors (Harmar and Lutz 1994). Pituitary adenylate cyclase receptor type 1 (PAC1) is highly abundant in the brain, pituitary, and adrenal glands (Vaudry et al. 2000b). PACAP has both in vitro and in vivo antiapoptotic effects, can increase the survival of the in vitro neuronal cultures exposed to ischemia-like conditions, and has neuroprotective effects in the in vivo animal models of focal and transient global ischemia (Uchida et al. 1996; Reglodi et al. 2000b, 2004).
Brain-derived neurotrophic factor (BDNF) is a prototype member of neurotrophin family of growth factors which has pleiotropic effects on neuronal development and synaptic plasticity as well neuronal survival (Cowansage et al. 2010). BDNF induces its biological effects in neurons by binding to the tyrosine kinase trk family of receptors, which in turn activate the typical signal transduction molecules extracellular signal-regulated kinases 1/2 (Erk1/2), protein kinase B (Akt), and phosphoinositide phospholipase C γ (Miller and Kaplan 2001). A few studies provided evidences that BDNF mediates the neuroprotective effects of PACAP38 on rat cortical neurons exposed to N-methyl-d-aspartic acid (NMDA) excitatory insults (Frechilla et al. 2001). PACAP can also exert trophic effects in neurons through crosstalk mechanism involving trk receptors and utilization of their tyrosine kinase signaling (Lee et al. 2002b), a process defined as transactivation (Shi et al. 2010). PACAP has been reported to increase the tyrosine kinase activity of nerve growth factor (NGF) receptor trkA in pheochromocytoma (PC12) cells (Lazarovici and Fink 1999) and BDNF tropomyosin-related kinase receptor type B (trkB) in hippo-campal neurons, as well as promote the survival of hippo-campal neurons after trophic factor withdrawal (Lee et al. 2002a). PACAP can activate phosphoinositide 3-kinase (PI3-K) and Erk1/2 (Botia et al. 2007), which are two of the critical signaling pathways involved in neuronal survival under various apoptotic paradigms. One of the downstream targets of PI3-K, PKB/Akt, when activated, can prevent apoptosis through phosphorylation and activation of multiple downstream signaling proteins caspase-9, glycogen synthase kinase 3, nuclear factor-kappa B, and the fork head family of transcription factors and changing the activation ratio Bax (member of the pro-apoptotic Bcl-2 protein family) to BAD (Bcl-2-associated death promoter) (White et al. 2000). PACAP induced neuronal outgrowth (neuritogenic activity) in rat chromaffin cells (Wolf and Krieglstein 1995), human neuroblastoma cells (Deutsch et al. 1993), and PC12 cells (Lazarovici et al. 1998; Vaudry et al. 2002). PACAP can delay or prevent apoptosis in sympathetic neurons after NGF deprivation (Chang and Korolev 1997), in PC12 cells after serum deprivation or ceramide treatment (Tanaka et al. 1997; Hartfield et al. 1998), or in cerebellar granule cells after serum and potassium withdrawal (Cavallaro et al. 1996; Kienlen Campard et al. 1997; Villalba et al. 1997; Vaudry et al. 1998a). The neuroprotective effect of PACAP is likely to be mediated through the inhibition of caspase-3 activity (Vaudry et al. 2000a).
The first study on PACAP effect on stroke indicated that delayed administration of PACAP induced, by virtue of its ability to cross the blood–brain barrier(Somogyvari-Vigh et al. 2000), protective effects in transient middle cerebral artery occlusion (MCAO) in the rat model, expressed by reduction of the volume of brain infarct (Reglodi et al. 2000a). This finding was also confirmed in rats habituated to moderate hyperthermic conditions (Reglodi et al. 2000b) and also in a two-vessel occlusion model in mice (Doan et al. 2011). An additional important study correlated the neuroprotection by endogenous and exogenous PACAP following stroke to the differential modulation of the cerebrocortical transcriptome (Dejda et al. 2011b). Later studies further indicated that endogenous PACAP is involved in suppression of edema in the ischemic brain (Nakamachi et al. 2010). The neuroprotective effect of PACAP after MCAO is not only due to its ability to inhibit apoptosis, but also to moderate reduction of the inflammatory response (Dejda et al. 2011b) via a mechanism involving interleukin-6 signaling (Ohtaki et al. 2006). PACAP protective effects in MCAO and in a variety of models of neurodegenerative diseases in vitro and in vivo (Reglodi et al. 2011) offer a novel therapeutic approach. However, PACAP cannot be used in the clinic as a drug because of its poor metabolic stability. Stable analogues may provide lead compounds towards development of novel anti-stroke drugs (Bourgault et al. 2011). This strategy is promising and requires a better understanding of the cellular and molecular mechanisms of PACAP-induced neuroprotection.
Our working hypothesis considered that PACAP provides neuroprotection by several potential mechanisms: a direct one involving activation of PAC1 receptors. Other indirect mechanisms may involve PACAP-induced autocrine release of neurotrophins such as BDNF, which by activation of its respective trkB receptor provide neuroprotective signals and induce neurite outgrowth by modulation of small GTPases and attenuation of neuronal growth inhibitory molecules. In the present study, we mechanistically evaluated the effect of PACAP38 in a rat model of transient MCAO and rat cortical neurons exposed to oxygen–glucose deprivation (OGD), focusing on the level and activity of BDNF receptors and signaling. We propose that PACAP induced neuroprotection by mobilizing BDNF–trkB receptor signaling, activating PI3-K/Akt pathways, inhibiting the hyperphosphorylation of Erk1/2 and induction of neurite outgrowth by modulation of the activity balance between the Ras homolog gene family, member A (RhoA) to Ras-related C3 botulinum toxin substrate 1 (Rac1), and down-regulating nerve growth factor receptor (p75NTR) and Nogo receptors (NgR). These findings propose that PACAP38 induces neuroprotection in stroke models both directly and indirectly by involving neurotrophin signaling.
Material and Methods
Primary Cortical Neuronal Cell Culture
Cultured cortical neurons were prepared from embryonic day 17 Wistar rats (Kuperstein and Yavin 2002). Cells were mechanically dissociated from tissue pieces by trituration following enzymatic incubation for 20 min in calcium- and magnesium-free Hank’s balanced saline buffer containing 2 mg/ml of trypsin. Dissociated cells were plated in poly-d-lysine-coated dishes and grown in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10 % fetal bovine serum, 100 µg/ml streptomycin, and 100 U/ml penicillin (Invitrogen, Carlsbad, CA, USA) overnight at 37 °C with 5 % CO2. The medium was replaced the next day with Neurobasal medium supplemented with 2 % B27 supplement and maintained in vitro for 12–14 days before the start of the experiments.
In Vitro Model of Ischemia
Neuronal cultures were maintained under normal growth conditions before switching to ischemia-like conditions (OGD). Cell culture medium was replaced and incubated in glucose-free DMEM under hypoxia for 1 h (Lecht et al. 2010). Then, the medium was replaced with Neurobasal medium containing normal amounts of glucose and grown under regular culture conditions for 48 h of reperfusion. Control cultures were maintained under regular culture conditions (normoxia).
RT-PCR Analysis
For RT-PCR analysis, samples were collected and total RNA was isolated using Trizol reagent (Invitrogen). PCR reactions were carried out for 40 cycles (94 °C for 1 min, 65 °C for 1 min, and 72 °C for 2 min) and PCR products were separated on 2 % agarose gel (Arien-Zakay et al. 2009). RT-PCR was performed using the following PCR primer pairs for trkB, p75, NgR, and β-actin:
- BDNF (141 bp):
- Sense: 5′-GCGGCAGATAAAAAGACTGC-3′
- Antisense: 5′-GCAGCCTTCCTTCGTGTAAC-3′
- trkB (664 bp):
- Sense: 5′-CCGCTAGGATTTGGTGTACTGAGCCTTCT-3′
- Antisense: 5′-CCACTGTCATCAGATGAAATGTTCGTTATCCT-3′
- p75 (663 bp):
- Sense: 5′-AGCCAACCAGACCGTGTGTG-3′
- Antisense: 5′-TTGCAGCTGTTCCACCTCTT-3′
- NgR (113 bp):
- Sense: 5′-TGCACAGTCTTGACCGTCTC-3′
- Antisense: 5′-GAGGTTGTTGGCAAACAGGT-3′
- β-Actin (285 bp, internal control):
- Sense: 5′-CATGAAGTGTGACGTTGACATTCGT-3′
- Antisense: 5′-TTAGAAGCATTTGCGGTGCACGATG-3′
Enzyme-Linked Immunosorbent Assay (ELISA)
Samples were lysed in radioimmunoprecipitation assay buffer (RIPA) (20 mM Tris–HCl pH 7.4, 150 mM NaCl, 1 % NP-40, 1 % Na-deoxycholate, 1 mM EDTA, 1 mM EGTA, 1 mM Na3VO4, 50 mM NaF, 1 mM phenylmethyl-sulfonyl fluoride, and protease inhibitor cocktail set I (Calbiochem, Darmstadt, Germany)). Equal amounts of lysates were used for the assay. To measure the BDNF expression and secretion, an ELISA kit (Promega, Madison, WI, USA) was employed as previously described (Arien-Zakay et al. 2009).
Rac1 and RhoA Activity Assays
Samples were lysed in RIPA buffer. RhoA and Rac1 activity was measured using assay kits from Upstate (Millipore, Billerica, MA, USA). The protocols were performed according to the manufacturer’s instructions. Equal amounts of lysates were incubated with GST-PBD (p21-binding domain of human PAK-1) or GST-RBD (Rho binding domain of rhotekin) to precipitate GTP-bound Rac1 and GTP-bound RhoA, respectively. Precipitated GTP-bound Rac1 or RhoA was analyzed on a 14 % SDS-PAGE and immunoblotted using antibodies specific for Rac1 and RhoA. The total amounts of Rac1 or RhoA were also measured by immunoblotting using the same set of lysates and antibodies.
Immunoprecipitation and Western Blotting Analysis
Western blotting analyses were performed as previously reported (Jiang et al. 1997). Cells were lysed in RIPA buffer for 30 min on ice and soluble proteins were obtained after centrifugation. For immunoblotting, equal amounts of lysate were subjected to SDS gel electrophoresis. Separated proteins were then electro-transferred to Immobilon membranes (Millipore). After exposure to the desired antibodies, proteins were visualized by an enhanced chemiluminescence protein detection kit (Pierce, Rockford, IL, USA). For immunoprecipitation, equal amounts of lysate were precleared with protein A-agarose (Pierce) for 1 h at 4 °C. Supernatants were then incubated overnight at 4 °C with the appropriate antibody. Protein A-agarose was added and the samples were further incubated for 1 h at 4 °C. The protein A-agarose beads were collected, washed three times with lysis buffer, once with 25 mM Tris, pH 6.8, and then resuspended in 4× SDS sample buffer. The supernatants were boiled and subjected to SDS-PAGE. The immunoblotting was performed with the appropriate antibody (Jiang et al. 1997).
Immunocytochemistry
Cells grown on chamber slides were fixed with 4 % paraformaldehyde and then incubated in 20 % normal serum for 30 min. Cells were then treated with a pre-determined dilution of each of the primary antibody for 1 h at 4 °C. Following incubation with primary antibody, cells were washed three to four times in cold PBS and then reacted with fluorescein or Texas red-labeled secondary antibody (Vector, Burlingame, CA, USA). The visualization of neural markers was performed by immunofluorescence technique and laser scanning confocal microscopy (Chen et al. 2005). The following antibodies were used: anti-phospho-Akt (Cell Signaling, Danvers, MA, USA); anti-Bax (B-9), anti-phospho-Erk, anti-phospho-trkB (H-181), anti-phospho-p85, and anti-phospho-tyrosine (PY99) (Santa Cruz, Santa Cruz, CA, USA); anti-BDNF and anti-p75NTR (Promega); and anti-neuron-specific class III beta-tubulin (TuJ1) (Covance, Princeton, NJ, USA).
In Vivo Model of Ischemia
The procedures were performed according to published protocols (Cai et al. 2001). Male Wistar rats (275–300 g) were purchased from Charles River (Wilmington, MA, USA). After 5 days in quarantine, each rat was anesthetized with 3.5 % halothane and spontaneously respired with 0.5 % halothane in a 2:1 N2O/O2 mixture using a face mask. A normal range of blood gases and pH was maintained during ischemia and reperfusion in animals with face mask inhalation. Body temperature was maintained constant (37 °C) with a recirculating pad and K module and monitored via an intrarectal type T thermocouple. PE-50 catheters were placed into the femoral artery and vein to obtain blood for analysis of systemic arterial pressure, blood gases, glucose, and hemoglobin concentration and for drug administration, respectively. Supplemental fluids (15–25 ml lactated ringer) were given subcutaneously after the reperfusion onset.
In order to generate a highly reproducible lesion in this model of MCAO, it is essential that MCAO is induced in animals with a narrow range of body weight (275–300 g). MCAO was induced by advancing a 4–0 surgical nylon monofilament with an expanded (heated) tip into the internal carotid artery (ICA) to block the origin of the MCAO; reperfusion was performed by withdrawal of the filament. A 2-cm incision was made at the center of the animal neck, and the right common carotid artery (CCA), external carotid artery (ECA), and ICA were exposed under a Zeiss operating microscope. Care was taken to avoid injury to the vagus nerve. The ICA was further dissected to identify the pterygopalatine artery branch and the intracranial ICA branch. The CCA and ICA were temporarily clamped using micro-surgical clips (Codman & Shurtleff, Inc., Warsaw, IN, USA). A 5–0 silk suture was tied loosely at the origin of the ECA and ligated to the distal end of the ECA. A 4–0 surgical nylon suture, with its tip rounded by heating near a flame, was introduced into the ECA lumen through a small puncture. The silk suture around the ECA origin was tightened around the intraluminal nylon suture to prevent bleeding, and the microsurgical clips were removed. A length of 18.5–19.5 mm of nylon suture, determined according to the animal’s weight, was gently advanced from the ECA into the lumen of the ICA until the suture blocks the origin of the MCAO closing the incision (Chen et al. 2005). In all experiments, rats were subjected to 2 h of right MCAO followed by i.v. injection with saline or 30 ng/kg of PACAP38 into the tail vein. The rats were allowed to survive for 2 days to allow about 48 h of reoxygenation. Thereafter, the brains were removed for the different ELISA, western blotting, RT-PCR, and immunohistochemistry evaluations. All experiments were conducted in strict accordance with the Henry Ford Health System’s Institutional Animal Care and Use Committee.
Brain Fixation and Sectioning
Two days after MCAO, rats were anesthetized by pentobarbital (40 mg/kg, i.p.) and the cardiovascular system was transcardially perfused first, with 0.1 M phosphate buffer, and then with 4 % paraformaldehyde in 0.1 M phosphate buffer. These procedures were carried out under controlled pressure of 100 mm Hg, which is equal to the mean arterial blood pressure of the rat. Brains were removed and immersed in the same fixative overnight at 4 °C. Sectioning the brain was completed in a rat brain matrix (Activational Systems, Warren, MI, USA), which allows to cut the entire rat brain into seven coronal slices (labeled A to G from front to back) each measuring 2.0 mm in thickness. A series of adjacent 6 µm-thick sections were cut for the 2-mm block D (bregma level +1.0 to +3.0) in the coronal plain and stained with hematoxylin and eosin for evaluation of infarct volume (Shehadah et al. 2010). In some experiments, the sections were stained with 2,3,5-triphenyltetrazolium chloride (TTC): slices were immersed for 20 min in a 2 % solution of TTC in normal saline at 37 °C, then fixed in 10 % phosphate-buffered formalin at 4 °C. TTC reacts with intact mitochondrial respiratory enzymes to generate a bright red color that contrasts with the pale color of the infarction. The caudal side of each slice was photographed. The area of the infarction (unstained) was traced and measured using Image J (http://rsb.info.nih.gov/ij/download/), and the infarction volume per brain (cubic millimeter) was calculated from the measured infarction area (Koumura et al. 2008).
Immunohistochemistry
Sections (6 µm) were deparaffinized and hydrated through a series of graded ethanol solutions. Antigen retrieval methodology was used to enhance the immunoreactivity. Each section was placed in boiled citrate buffer (pH 6) within a microwave oven (650–720 W) and was blocked and pretreated with a normal serum, depending upon the specific primary antibodies that were used. Sections were incubated with an antibody, treated with peroxidase–avidin–biotin complex (ABC kit, Vector) peroxidase-conjugated reagents, developed with 3,3′-diaminobenzidine (DAB), and counterstained with hematoxylin. Controls were treated with non-immune serum for the primary antibody or omission of the primary antibodies. The following primary antibodies were used: anti-caspase-3 and anti-phospho-Akt (Cell Signaling); anti-microtubule-associated protein-2 (MAP-2) (Sigma-Aldrich, St. Louis, MO, USA); anti-TuJ1 (Covance); anti-GAP-43, anti-actin, anti-phospho-Erk, anti-phospho-trkB (H-181), anti-phospho-p85, anti-phospho-tyrosine (PY99), and anti-NgR (Santa Cruz, Princeton, NJ, USA); and anti-p75NTR (Promega). For double immunostaining, the sections were processed with DAB staining and then pretreated with a normal serum and fluorescein antibody binding which was detected using fluorescein isothiocyanate-conjugated secondary antibodies (Vector).
Measurement of Infarct Volume
The volume of cerebral tissue infarction was measured by a light microscope using a Global Lab Image Analysis Program (Data Translation, Marlboro, MA, USA). The area of infarction and the area of both hemispheres (square millimeter) were calculated on hematoxylin and eosin-stained sections, by tracing the areas on the computer screen, and the volume (cubic millimeter) was determined by integrating the appropriate areas with the section interval thickness. To reduce errors associated with processing of tissue for histological analysis, an indirect volume calculation was also performed (Chen et al. 2005).
Statistics
Each experimental group contained eight to ten rats (n=–10). All in vitro experiments were performed with four to nine different cultures (n=24–54). Analysis of variance followed by t tests with Bonferroni correction was used to compare differences between the control and the PACAP-treated groups both in vitro and in vivo. All data are presented as means±SE and a p<0.05 was considered statistically significant.
Results
Neuroprotective Effect of PACAP in MCAO Rat Model
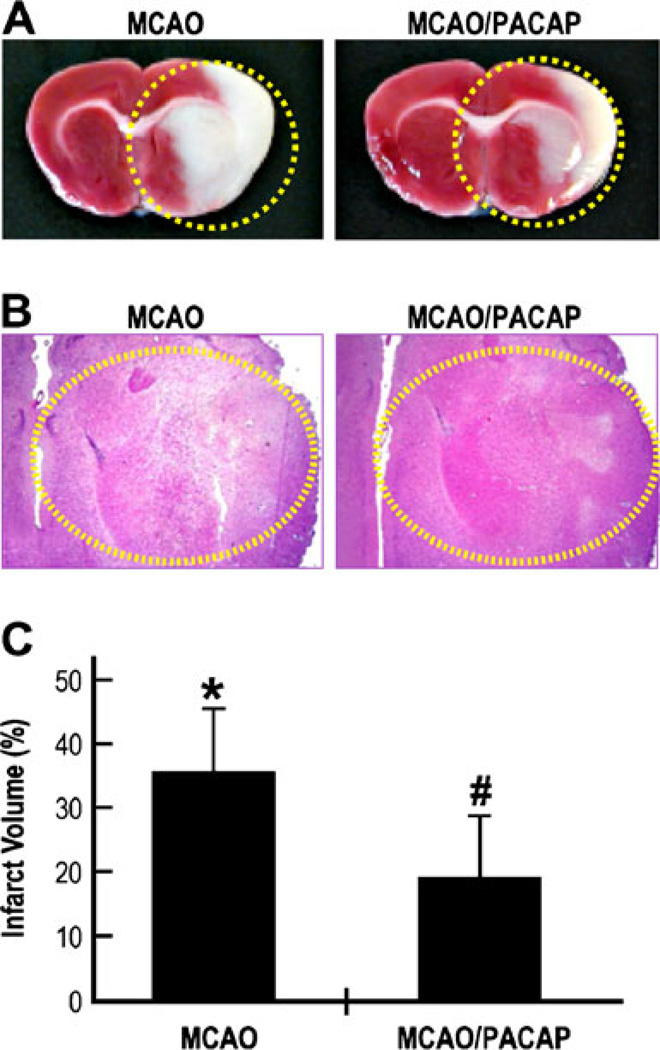
To examine the neuroprotective effects of PACAP in vivo, the rats were subjected to 2 h of unilateral MCAO followed by reperfusion for 48 h. A large area of edema was observed in the saline-treated animals, but was markedly reduced upon PACAP treatment (Fig. 1a, b, circled area). To examine the extent of PACAP-mediated protection, the infarct volume (Fig. 1a, circled area) was measured (Fig. 1c). The infarct volume was approximately 36 % in MCAO animals injected with saline, but was significantly reduced (p<0.01, n=8) to approximately 18.8 % after PACAP treatment. These results suggest that PACAP has about 50 % protective effect against ischemic brain damage in vivo, confirming previous studies.
Fig. 1.
Neuroprotective effects of PACAP after focal cerebral ischemia measured by reduced infarct volume. a Rats were subjected to 2 h of right MCAO followed by 48 h of reoxygenation in the presence or absence of PACAP38 injected i.v. at a dose of 30 ng/kg into the tail vein. The rats were allowed to survive for 2 days. Brains were dissected, perfused, fixed, and cut into sections of 2 mm. A series of adjacent 6-µm-thick sections were cut from the 2-mm block (bregma level +1.0 to +3.0) in the coronal plane and stained with a TTC and b hematoxylin and eosin; MCAO: saline-treated; MCAO/PACAP: PACAP38-treated. c The infarct volume represents the percentage of the area of lesion in the ipsilateral hemisphere vs. the corresponding area in the contralateral hemisphere. *p<0.01 compared to healthy brains; #p< 0.01 compared to MCAO brains
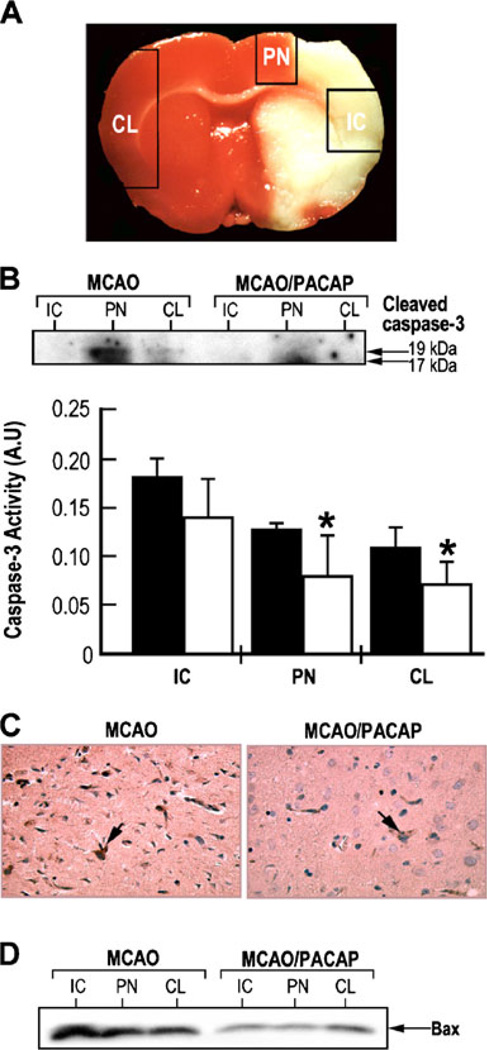
Caspase-3 is one of the important mediators of apoptotic cell death and can be activated after transient focal ischemia (Davoli et al. 2002). To examine the expression and activation of caspase-3, in the ischemic core (IC) and penumbra regions (PN) in rat brains subjected to focal cerebral ischemia, tissue samples were dissected from these areas of the ipsilateral hemisphere as well as from the corresponding region of the contralateral hemisphere (CL) as shown in Fig. 2a. Western blotting analysis showed the appearance of cleaved caspase-3 products (molecular weight of 17 and 19 kDa), the cleavage product of pro-caspase-3, in the PN of the MCAO brains but very little in the PN of corresponding tissue of the PACAP38-treated MCAO brains (Fig 2b, insert). No apparent cleavage of caspase-3 was observed in other brain regions. Caspase-3 activity was greatly increased in the ischemic core and penumbra region of the ipsilateral side of the brain after MCAO. PACAP significantly reduced the caspase-3 activity in these brain regions, back to the levels of those in the contralateral hemisphere (Fig. 2b). Due to the extensive cell death in the ischemic core, the variability in caspase-3 activity compromised the caspase-3 measurements. Similar observations of reduction in caspase-3 staining were also found with coronal brain sections immunostained with anti-active caspase-3 antibody (Fig 2c). These results suggest that PACAP may prevent neuronal apoptotic cell death through the suppression of caspase-3 activity and are consistent with studies demonstrating that PACAP-mediated neuronal survival is involved in the suppression of caspase-3 activity in cerebellar granule cells (Vaudry et al. 2000a). The changes in expression of pro-apoptotic member Bax were examined using the same set of tissue samples as shown in Fig. 2d. Bax expression was increased in the IC and PN tissue in saline-injected rats while its expression was greatly reduced in the corresponding areas in PACAP-treated rats (Fig. 2d). These results suggest that Bax protein is also involved in PACAP-mediated neuroprotection.
Fig. 2.
Neuroprotective effects of PACAP after focal cerebral ischemia measured by reduced caspase-3 and Bax (a member of the pro-apoptotic Bcl-2 protein family) expression. a Representative coronal brain sections (4 mm) stained with 2,3,5-triphenyltetrazolium chloride to demonstrate the regions of tissue dissection. Ipsilateral hemisphere: ischemic core (IC) and penumbra (PN) boundary zone to the ischemic core; contralateral hemisphere (CL). b PACAP reduces caspase-3 activity under ischemic conditions. Insert, western blotting analysis using the antibody against active caspase-3 and caspase-3 activity measured by a colorimetric kit. Black bars: saline; white bars: PACAP38; *p<0.05 compared to saline. c Immunostaining of coronal brain sections (6 µM) using anti-active caspase-3 antibody. d Western blotting analysis of Bax using the tissue samples from ischemic core (IC), penumbra (PN), and contralateral (CL) hemispheres. MCAO rats injected with either saline (MCAO) or PACAP38 (MCAO/PACAP)
PACAP Preserves the Level of Neuronal Markers in MCAO Rat Model
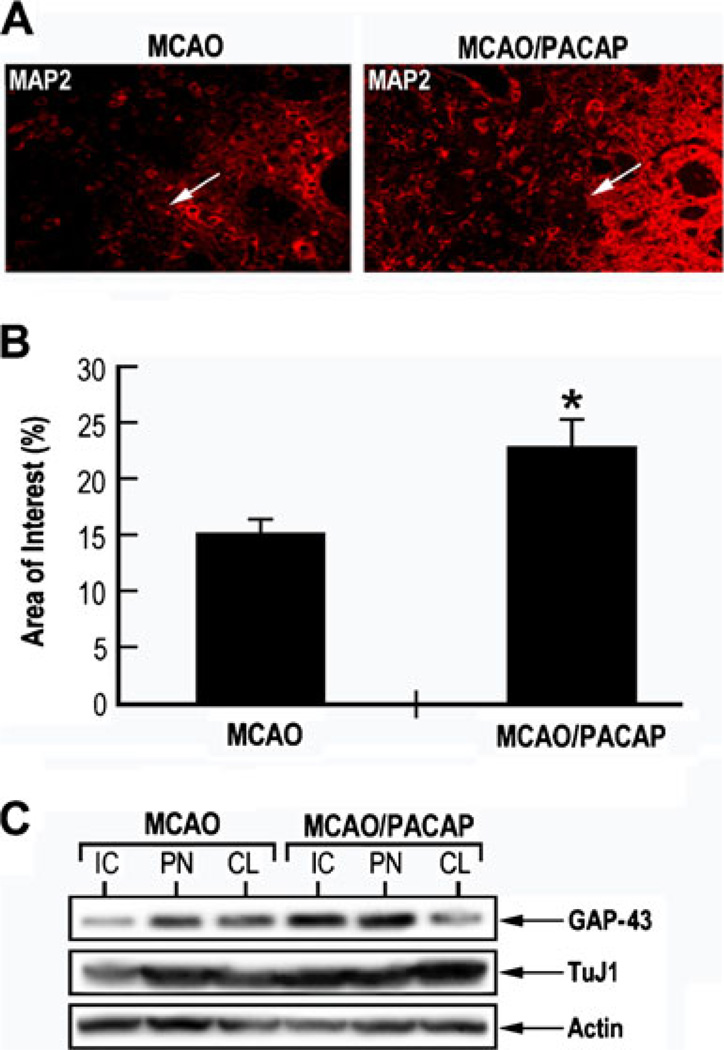
The change of expression of neuronal specific marker MAP-2, in the animal model of focal cerebral ischemia was examined by immunohistochemistry, before and after PACAP treatment. Higher MAP-2 immunostaining was observed after PACAP treatment in the ipsilateral subventricular zone and ischemic boundary regions, respectively, as compared to corresponding untreated regions (Fig 3a, b). Western blotting analysis was performed using dissected tissue samples from IC and PN of ipsilateral and contralateral hemispheres. Growth-associated protein-43 (GAP-43) is a neuronal specific protein expressed during axonal out-growth and regeneration and localized at the growth cone (Benowitz et al. 1990). TuJ1 antibody can recognize class III beta-tubulin expressed exclusively in neurons and stains the neuronal cell bodies, dendrites, axons, and axonal terminations (Geisert and Frankfurter 1989). Both TuJ1 and GAP-43 expressions were reduced in the IC of saline-injected MCAO animal, but were higher expressed in the IC of PACAP-treated MCAO animal (Fig. 3c). These results of PACAP38 preservation of the expression of the selected neuronal markers MAP-2, TuJ1, and GAP-43 associated with neuroprotection and regeneration (Li et al. 1998) provide another experimental evidence of PACAP38-induced neuroprotection.
Fig. 3.
PACAP induced MAP-2 TuJ1 and Gap-43 immunoreactivity in MCAO model. a Immunostaining of MAP-2 was performed on coronal brain sections from MCAO rats injected with either saline (MCAO) or PACAP38 (MCAO/PACAP), using a monoclonal antibody against MAP-2. b Quantitative analysis of MAP-2 immunopositive staining in the ischemic penumbra (PN) area. *p<0.05 compared to MCAO. c Western blotting analysis of different neuronal cytoskeletal markers. Brain tissue samples from MCAO rats injected with either saline (MCAO) or PACAP38 (MCAO/PACAP) were dissected and cell lysates were prepared. Equal amounts of lysates were immunoblotted using specific monoclonal antibodies against TuJ1, GAP-43, and actin. IC ischemic core, PN penumbra, CL contralateral
PACAP Induced Expression of BDNF and Phosphorylation of trkB/PI3-K–Akt in MCAO Rat Model
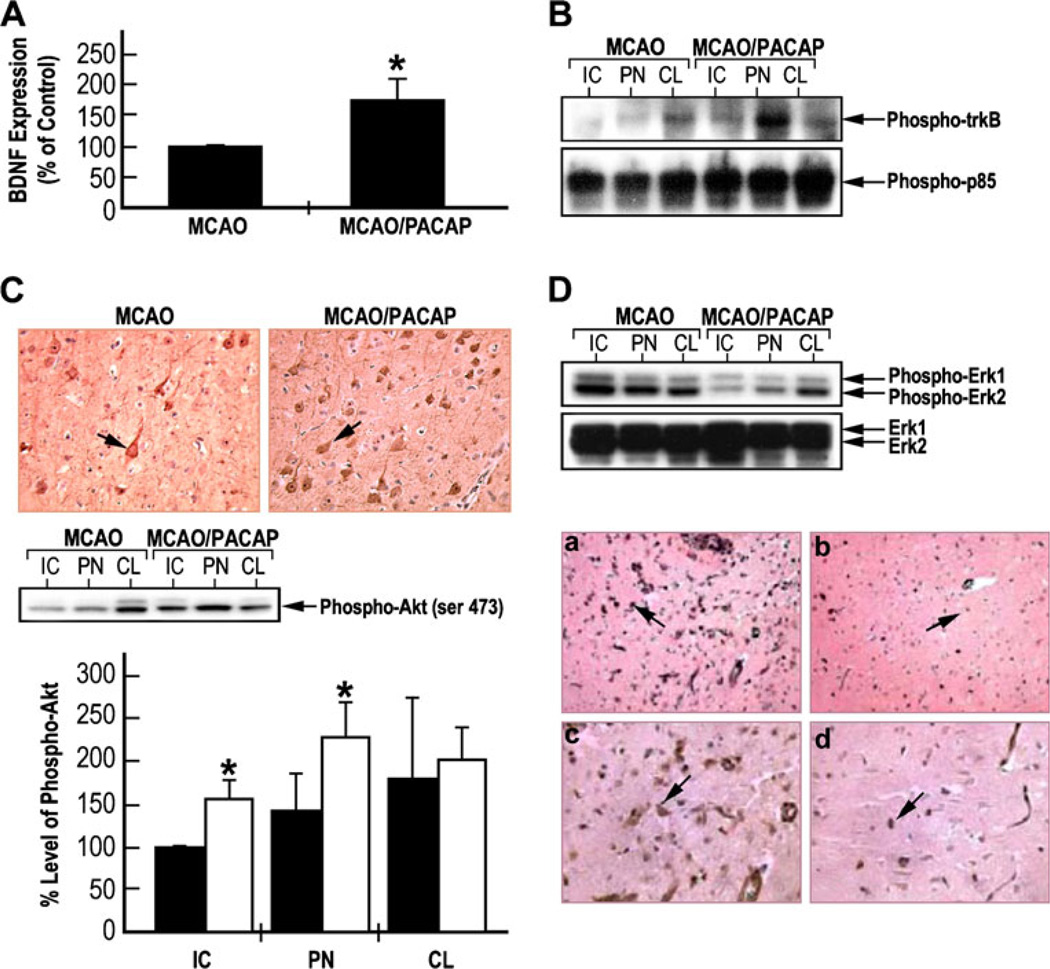
To measure changes in BDNF levels, ELISA was used with dissected tissue homogenates of the ipsilateral and contralateral hemispheres of the brain of MCAO rats compared to PACAP-treated MCAO animals (Fig. 4a). PACAP treatment increased by twofold the level of BDNF. In parallel experiments, the levels of phospho-trkB and PI3-K–Akt signaling pathways, PI3-K regulatory subunit p85, and its down-stream signaling protein Akt were measured (Fig. 4b, c). Equal amounts of lysates from the IC, PN, and CL areas were immunoprecipitated with anti-phosphotyrosine anti-body and immunoblotted with anti-trkB or anti-PI3-K p85 antibody (Fig. 4b). Increased tyrosine phosphorylation of trkB and p85 was observed in the tissue from MCAO/PACAP-treated rats. In addition, the same tissue samples were subjected to western blotting analyses using phospho-Akt antibodies (Fig. 4c). Increased phosphorylation of Akt was observed in the ischemic core and penumbra regions after PACAP treatment of MCAO rats. These results suggest that PACAP may activate trkB-mediated signaling pathways most probably due to the increased expression of BDNF.
Fig. 4.
PACAP induced expression of BDNF and phosphorylation of trkB/PI3-K–Akt in MCAO rat model. a ELISA evaluation of BDNF expression in lysates from tissue samples from penumbra of ipsilateral hemisphere of the MCAO rats injected with either saline or PACAP38; *p<0.01 compared to MCAO. b PACAP-induced tyrosine phosphorylation of trkB and PI3-K p85. Equal amounts of tissue samples of the MCAO rats injected with either saline or PACAP38 were immunoprecipitated with anti-phosphotyrosine antibody and then immunoblotted with anti-trkB or anti-PI3-K p85 antibodies. c PACAP-induced Akt phosphorylation: upper part, immunostaining of coronal brain sections with anti-phospho-Akt antibody; middle part, western blotting analysis of phospho-Akt levels; lower part, the levels of phospho-Akt were measured from three western blotting and represented as mean±SD; black bars—saline; white bars—PACAP; *p<0.05 compared to saline in the same group. d PACAP reduced Erk1/2 phosphorylation. Upper part western blotting analysis was performed using antibodies against phospho-Erk1/2 and basal Erk1. Lower part immunostaining of the coronal brain sections using anti-phospho-Erk1/2. a, b Low magnification (×20); c, d high magnification (×40); a, c—MCAO; b, d— MCAO/PACAP. IC ischemic core, PN penumbra, CL contralateral
In order to examine the extent of phosphorylation of Erk1/2 in the animal MCAO rat model, tissue samples were obtained as described and subjected to western blotting analysis. As expected, MCAO induced Erk1/2 phosphorylation, and after PACAP treatment, the levels of phospho-Erk1/2 were significantly reduced in IC and PN areas of the ipsilateral hemisphere, while the levels were not changed in the CL hemisphere, as also evident in the immunohisto-chemical experiments (Fig. 4d). These findings suggest that activation of Erk1/2 may be involved in the cell death process under ischemic insult, and attenuation of Erk1/2 phosphorylation by PACAP may contribute to its neuroprotective effects.
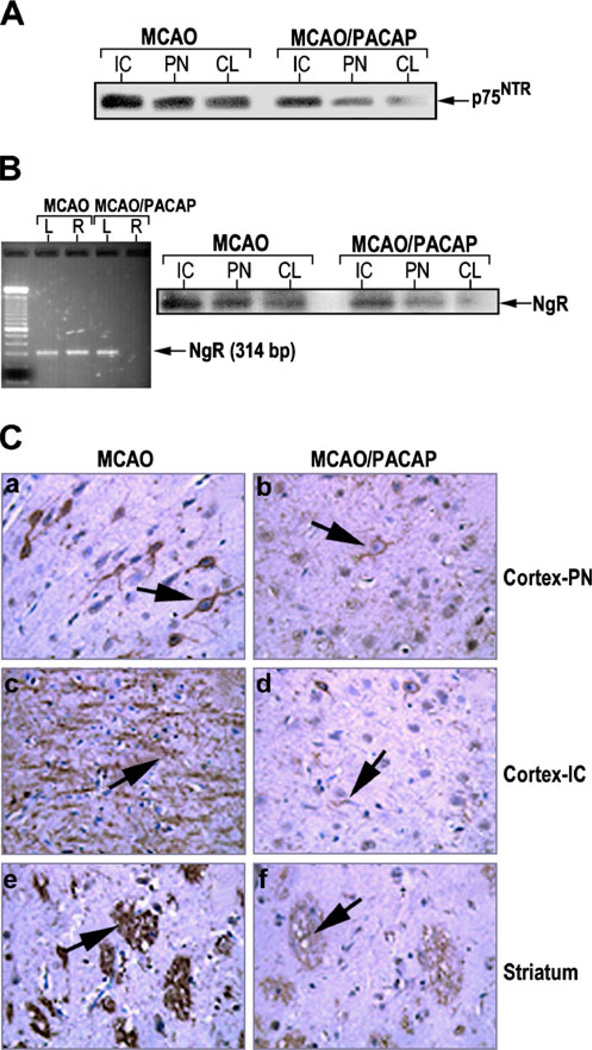
To examine the expression of p75NTR receptor, and NgR-associated protein Nogo, known as a neuronal axon growth inhibitory molecule receptor, brain lysates or sections from the MCAO animals treated with either saline or PACAP were subjected to western blotting, RT-PCR, or immunohistochemistry (Fig. 5). Figure 5a clearly indicates a decrease in the expression of p75NTR after PACAP treatment in the tissue samples from IC and PN of ipsilateral hemispheres and nonsignificantly from the CL hemisphere of MCAO rats compared to samples from animals injected with saline. Western blotting analysis was performed to examine the expression of NgR in the tissues dissected from different brain regions (Fig. 5b). In brains of MCAO rats, NgR expression was observed in ischemic and penumbra tissues, but less NgR expression was observed in the contralateral hemisphere. In addition, NgR expression was greatly reduced in the IC, PN, or CL tissues in PACAP-treated MCAO rats. RT-PCR analysis using total RNA derived from the tissue samples dissected from ipsilateral or contralateral hemispheres showed that PACAP suppressed the expression of NgR in the ipsilateral side but showed no apparent difference in the contralateral side (Fig. 5b). The NgR immunoreactivity in the sections of saline-treated MCAO animals was greatly increased in the cortex and striatum. However, the immunoreactivity was rarely detected in the brain of PACAP-treated MCAO rats (Fig. 5c). These findings suggest that PACAP38 suppresses NgR expression, which in turn may reduce the interaction between NgR and neuronal growth inhibitory proteins, and prevent the induction of intrinsic neuronal outgrowth inhibitory signaling mechanisms as previously noted with other neuroprotective drugs (Shehadah et al. 2010).
Fig. 5.
PACAP reduction of p75NTR/NgR expression in MCAO rat model. a PACAP mediated downregulation of p75NTR and b down-regulation of Nogo receptor (NgR). The tissues were dissected from ischemic core (IC) and penumbra (PN) areas of the ipsilateral hemisphere, as well as the corresponding region of the contralateral (CL) hemisphere, from the rats subjected to MCAO or MCAO followed by PACAP38 treatment. Equal amounts of lysates were subjected to western blotting analysis using anti-p75NTR or anti-NgR receptor antibodies. b Left—PACAP-mediated downregulation of Nogo receptors measured by RT-PCR. L: tissue sample from contralateral hemisphere; R: tissue sample from ipsilateral hemisphere. Total RNA was isolated and RT-PCR was performed at 40 cycles at 94 °C for 1 min, 62 °C for 1 min, and 72 °C for 1 min. Samples were separated on 2 % agarose gel. c Immunostaining of coronal brain sections with anti-Nogo receptor antibody indicating PACAP downregulation of Nogo receptor expression. The 6-µm-thick sections were derived from the 2-mm brain block D (bregma level +1.0 to +3.0) stained with anti-Nogo receptor antibody. a, b Cortex at penumbra; c, d cortex at ischemic core; e, f striatum. Arrows indicate NgR positive cells
PACAP Induced Neuroprotection, BDNF Release, and trkB and Akt Phosphorylation in Cultured Cortical Neurons Subjected to OGD
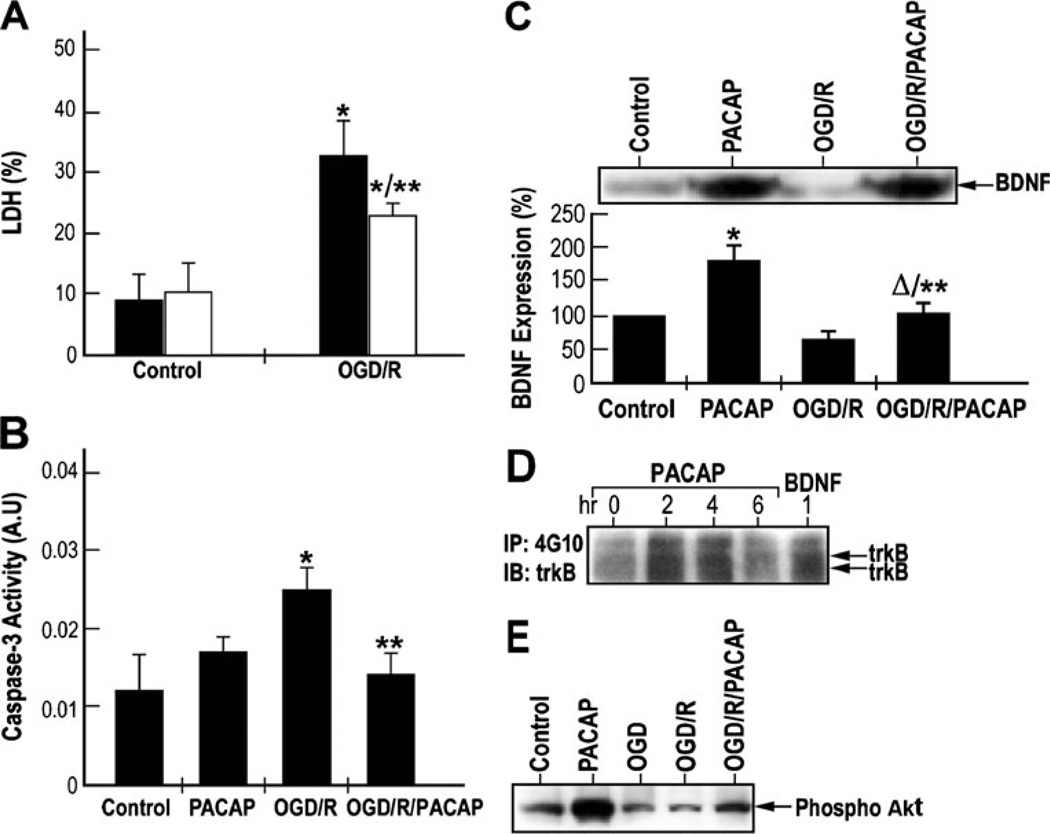
To measure the protective effects of PACAP under ischemia-like conditions (oxygen and glucose deprivation–reperfusion), primary cortical neurons were subjected to 1 h of OGD (Lobner and Choi 1996) followed by 48 h of reperfusion (OGD/R), in the absence or presence of 100 nM of PACAP38. The extent of cell death was measured by lactate dehydrogenase (LDH) release assay. LDH release increased over threefold after OGD–reperfusion as compared to control, thereby reflecting an increase in necrotic cell death (Fig. 6a). PACAP treatment under normoxic conditions did not change the LDH release, but under OGD reduced by 35 % the increase of LDH release, conferring neuroprotection. Caspase-3 activity measurements indicate that OGD–reperfusion induced approximately 2.7-fold increase in caspase-3 activity while PACAP treatment suppressed this induction by about 25 % (Fig. 6b). These results are similar to those obtained from LDH release assay and clearly indicate the neuroprotective effect of PACAP on cultured cortical neurons subjected to OGD.
Fig. 6.
PACAP induced neuroprotection, BDNF release, and trkB and Akt phosphorylation in cultured cortical neurons subjected to oxygen–glucose deprivation (OGD). a Neuroprotective effects of PACAP on OGD-induced neuronal cell death in vitro. Cultured cortical neurons were subjected to OGD for 1 h followed by reperfusion for 48 h, in the absence (OGD/R) or presence (OGD/R/PACAP) of 100 nM of PACAP38. The levels of released and total lactate dehydrogenase (LDH) were measured by using a CytoTox 96 cytotoxicity assay kit. Results were presented as mean±SD, *p<0.05 compared to control; **p<0.05 compared to the saline group exposed to oxygen–glucose deprivation followed by reoxygenation (OGD/R) (black—saline; white —PACAP38). b Inhibitory effects of PACAP on caspase-3 activity under OGD followed by reperfusion. Cultured cortical neurons were subjected to OGD for 1 h followed by reperfusion as in a. Caspase-3 activity was measured using caspase-3 assay kit. Results were presented as mean±SD. *p <0.05 compared to control; **p <0.05 compared to OGD/R. c Upper—western blotting analysis of BDNF expression. Lower—quantization of BDNF levels using ELISA. *p< 0.001 compared to control; **p<0.001 compared to OGD/R; Δp<0.05 compared to PACAP. d Effects of PACAP on the activation of trkB-mediated signaling pathways in cultured cortical neurons. Cultured cortical neurons were treated with 100 nM of PACAP38 for 2, 4, and 6 h. Cells were also treated with 50 ng/ml of BDNF for 1 h. Equal amounts of lysates were immunoprecipitated with anti-phosphotyrosine antibody and immunoblotted with anti-trkB or anti-PI3-K p85 antibodies. e PACAP induces Akt phosphorylation under ischemic conditions. Cultured cortical neurons were subjected to OGD for 1 h followed by reperfusion for 48 h, in the absence or presence of 100 nM of PACAP38. Equal amounts of lysates were subjected to western blotting analysis. Phosphorylation of Akt was detected by using an anti-phospho-Akt antibody (ser 43)
PACAP treatment of the neurons under normoxic conditions significantly induced the increase of BDNF expression after 24 h (Fig. 6c) in line with a previous report (Frechilla et al. 2001). OGD–reperfusion significantly reduced BDNF expression, which could be partially reversed after PACAP treatment (Fig. 6c). These results were further confirmed by western blotting analysis of BDNF protein (Fig. 6c, insert). These data suggest that PACAP can induce BDNF expression under normal or ischemic conditions, which may contribute to its neuroprotective effect.
To examine PACAP activation of trkB receptor phosphorylation, cultured cortical neuronal cells were treated with 100 nM of PACAP38 for 0, 2, 4, and 6 h, and tyrosine-phosphorylated trkB protein was immunoprecipitated. Increased trkB immunoreactivity was observed after 2 and 4 h of PACAP treatment, indicative of tyrosine phosphorylation of trkB receptor (Fig. 6d). BDNF was used as a positive control to show that it could induce trkB phosphorylation. The levels of phospho-Akt were also examined in these experiments (Fig. 6e). PACAP induced Akt phosphorylation under control conditions. OGD insult did not cause changes in Akt phosphorylation. However, OGD–reperfusion reduced Akt phosphorylation and PACAP could prevent this effect. These results suggest that PACAP may prevent ischemia-induced apoptotic cell death through the suppression of caspase-3 (Fig. 6b), as well as by the induction of pro-survival Akt activation (Fig. 6e), both critical for PACAP-mediated neuroprotection.
PACAP Induced Neurite Outgrowth Under Ischemic Conditions in Cultured Cortical Neurons Subjected to OGD
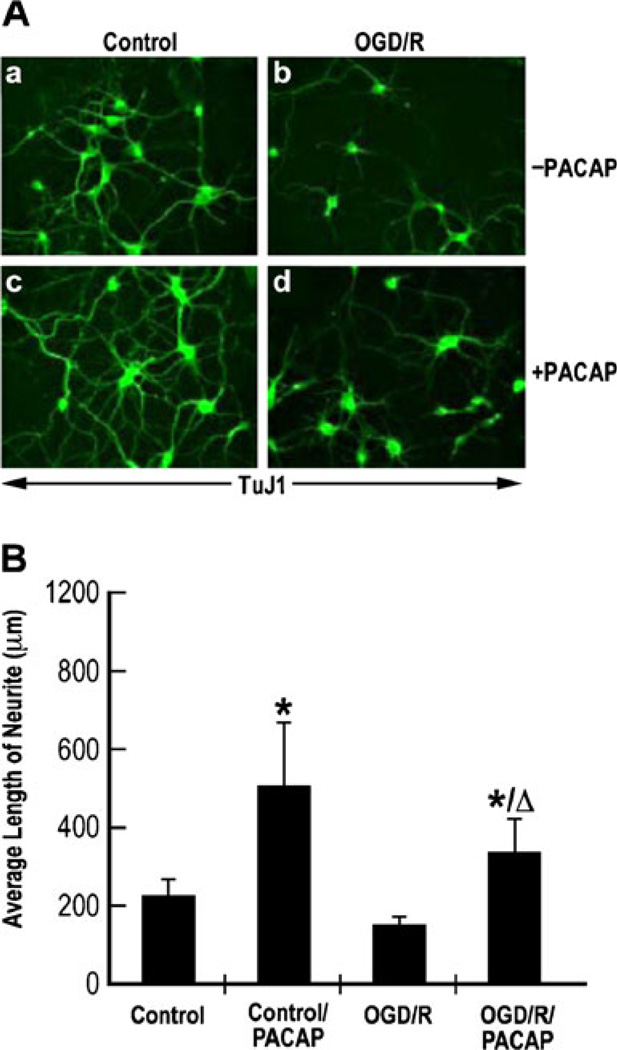
Immunostaining was performed in fixed cultured cortical neurons under normoxia or OGD-reperfusion conditions, before or after PACAP treatment using a monoclonal anti-body anti-TuJ1. PACAP induced an increase in immunore-activity of TuJ1 expression under control or OGD conditions (Fig. 7a). The average length of neurite per cell was 275±45 µm under the control conditions, while PACAP increased it to about 610 ± 157 µm. OGD–reperfusion reduced the average length of neurite to 180 ± 17 µm from 275 µm, but PACAP restored the neurite length to 410 ± 105 µm (Fig. 7b), suggesting that PACAP can induce neurite outgrowth under OGD–reperfusion conditions.
Fig. 7.
PACAP induced neurite outgrowth under ischemic conditions in cultured cortical neurons subjected to OGD. a Immunostaining of cultured cortical neurons with TuJ1. Cultured cortical neurons subjected to OGD followed by reoxygenation (OGD/R) or exposed to normoxic conditions (control) were left untreated (−PACAP; a, b) or treated with 100 nM of PACAP38 for 48 h (+PACAP; c, d). Cells were fixed and subjected to immunostaining using TuJ1 antibody. b Quantization of the average length of neurite from different conditions shown in a. Photomicrographs were taken at × 40 magnification with a digital camera. The axons were then traced and measured using SigmaScan analysis system. Results were presented as mean±SD. *p<0.05 as compared to control; Δp<0.05 as compared to oxygen–glucose deprivation followed by reoxygenation (OGD/R)
PACAP Induced Rac Activity and Suppression of Rho Activity in MCAO and OGD Stroke Models
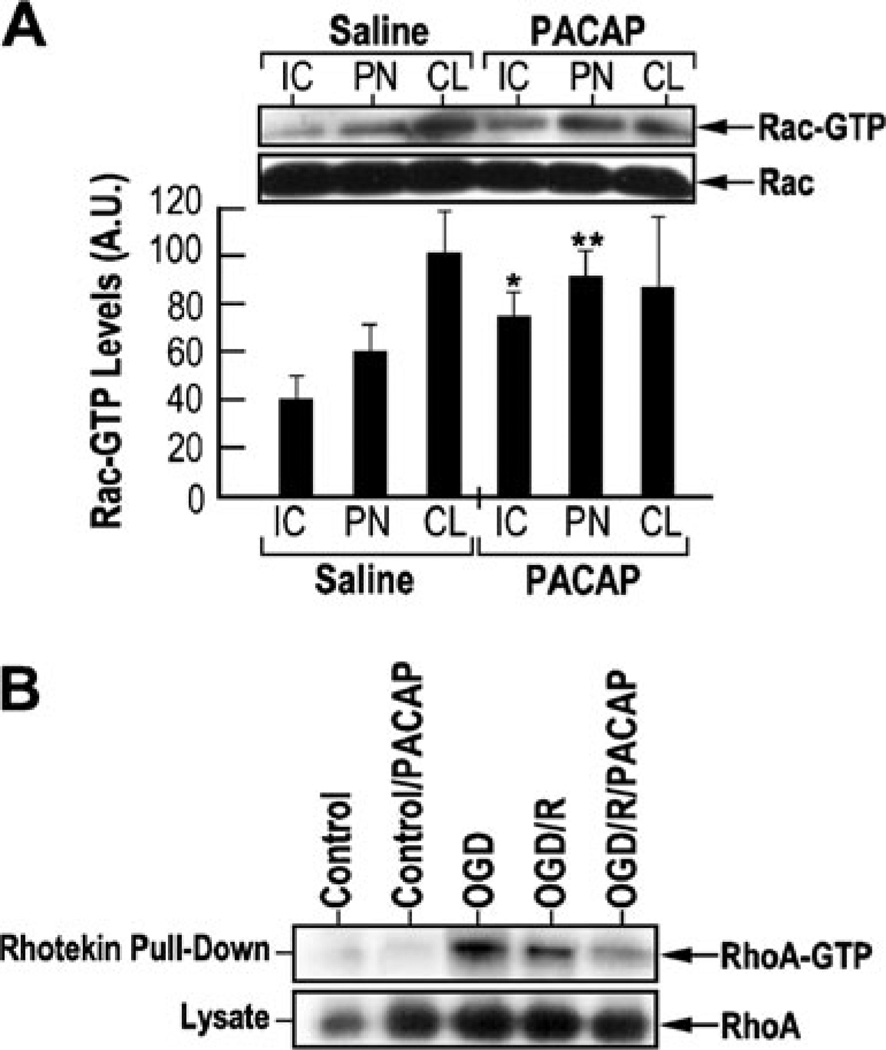
Rho GTPases, such as Rac1 and RhoA, have been implicated in the process of neurite outgrowth (Kao et al. 2002). Rho family of GTPases can signal to the actin cytoskeleton through various downstream effector proteins, which require the binding of active GTP-bound forms of Rho GTPases (Hall and Nobes 2000). The binding domains in these effector proteins have been used to pull down and quantify the GTP-bound Rho GTPases. To examine the effects of PACAP on the activities of Rho GTPases, Rac-GTP levels were examined in the IC, PN, and CL tissue samples derived from saline-injected and PACAP-treated rats Fig. 8a). PACAP induced approximately 50 and 30 % increase of Rac-GTP levels in the IC and PN regions, respectively. To examine the effects of PACAP on RhoA activity, cultured cortical neurons were subjected to OGD–reperfusion conditions and RhoA-GTP levels were measured (Fig. 8b). OGD or OGD–reperfusion increased the levels of RhoA-GTP, while the levels were significantly suppressed by PACAP treatment. These results indicate that PACAP can induce Rac activity and suppress Rho activity under ischemic conditions in vivo and in vitro. The balance of Rac and Rho activities in PACAP-mediated neural damage and plasticity under ischemic conditions remains to be further characterized.
Fig. 8.
PACAP induction of Rac1 (Ras-related C3 botulinum toxin substrate 1) and suppression of RhoA (Ras homolog gene family, member A) activity. a The effect of PACAP on Rac activity. Insert— equal amounts of lysates from the brain areas of rats subjected to MCAO or MCAO followed by PACAP38 treatment were incubated with PAK-1-PBD (p21-binding domain of human PAK-1). Precipitated Rac-GTP proteins were separated on 14 % SDS polyacrylamide gel and immunoblotted with anti-Rac1 antibody. Equal amounts of lysates were run separately to measure the total Rac levels. Quantization of Rac-GTP levels in various brain regions with or without PACAP treatment, using the data obtained from western blotting experiments. IC ischemic core, PN penumbra, CL contralateral. b The effect of PACAP RhoA activity in cultured cortical neurons subjected to 1 h of OGD or 1 h of OGD followed by 1 h of reperfusion, in the absence or presence of 100 nM PACAP38. RhoA-GTP activity was measured using a Rhotekin pull-down assay kit. Equal amounts of lysates were subjected to immunoblotting to measure the total RhoA levels among different samples. Control—normoxia; control/PACAP—normoxia and 100 nM PACAP treatment; oxygen–glucose deprivation—OGD alone; OGD/R—OGD followed by 48 h of reoxygenation; OGD/R/PACAP—OGD/R with 100 nM PACAP treatment
Discussion
In the present study, using a rat model of MCAO transient focal cerebral stroke and cultured cortical neurons exposed to OGD—reperfusion ischemic insult, it has been found that (1) PACAP38 reduced the infarct volume, confirming pre-vious studies; (2) PACAP exerted neuroprotective effects in the MCAO animal model and cultured cortical neurons likely through the increased expression and release of BDNF, increased phosphorylation of BDNF receptor trkB and PI3-K/Akt and suppression of caspase-3 activity; (3) PACAP increased the expression of the neuronal structural protein MAP-2, TuJ1, and GAP-43 in neurons in the boundary zone to the ischemic core suggestive of neuroprotective mechanisms; (4) PACAP reduced neuronal growth inhibitory molecules p75NTR and NgR under ischemic conditions in vivo most probably contributing to the neuroprotection and functional recovery after the insult; and (5) PACAP induces Rac while suppressing RhoA activity, a critical balance in the promotion of neurite outgrowth and regeneration after stroke.
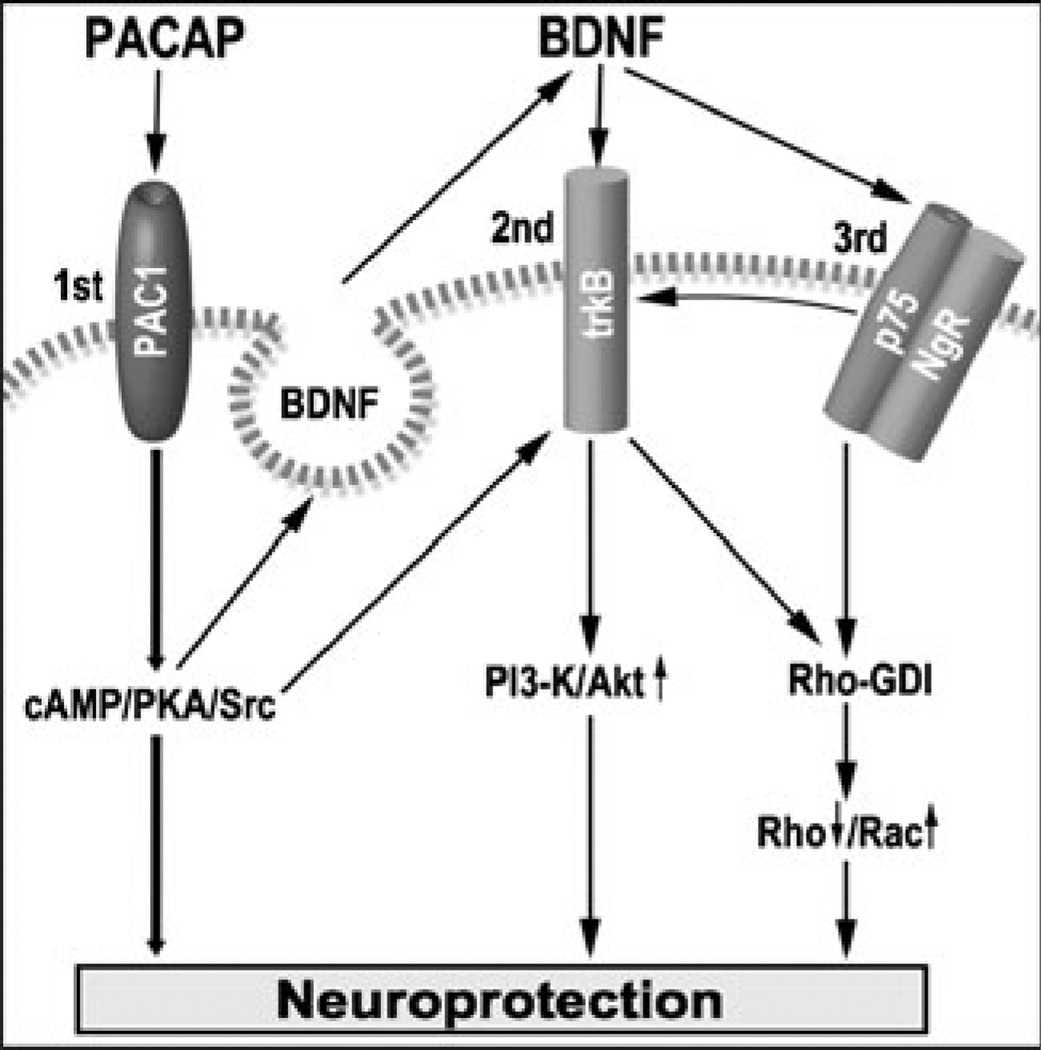
These data are summarized in a mechanistic working model presented in Fig. 9, proposing multiple pathways by which PACAP provides neuroprotection against ischemic brain damage and promotes neurite outgrowth after injury. These beneficial activities can be induced from one hand by activation of PAC1 receptors and stimulation of cAMP-dependent PKA, Src, and other signaling molecules (Onaka et al. 2003). PACAP can increase intracellular cAMP and induce the phosphorylation of the cAMP response element binding protein (CREB) through the activation of PKA (Schomerus et al. 1996; Vaudry et al. 1998b), which is an important transcription factor in many physiological and pathological conditions including synaptic plasticity and neuronal survival (Bito and Takemoto-Kimura 2003). Persistent activation of CREB is associated with neuronal survival in focal and global cerebral ischemia (Tanaka et al. 2000; Jin et al. 2001), therefore providing a strong neuroprotective signal.
Fig. 9.
Schematic diagram of potential crosstalk signaling pathway between PACAP and BDNF leading to neuroprotection; 1st, 2nd, and 3rd represent the three mechanisms leading to neuroprotection; arrow up—activation; arrow down—inhibition; striped line—plasma membrane
PACAP reduced the phosphorylation of Erk1/2 in the MCAO rat model. Apparently, this finding contradicts earlier studies which indicated a direct correlation between PACAP-induced neuroprotection and PACAP activation of Erk1/2 in a glutamate-induced retina lesion model (Racz et al. 2008) and in C2-ceramide-induced apoptosis of cerebellar granule cells (Vaudry et al. 2003). Extracellular signal-regulated protein kinases 1 and 2 (ERK1/2), which are members of the mitogen-activated protein kinase superfamily, have been well characterized and are known to be involved in cell survival; however, recent evidence suggests that the activation of ERK1/2 also contributes to cell death in some cell types and organs under certain conditions. For example, ERK1/2 is activated in neuronal and renal epithelial cells upon exposure to oxidative stress and toxicants and deprivation of growth factors, and inhibition of the ERK pathway blocks apoptosis. ERK activation also occurs in animal models of ischemia- and trauma-induced brain injury and cisplatin-induced renal injury, and inactivation of ERK reduces the extent of tissue damage. In some studies, ERK has been implicated in apoptotic events upstream of mitochondrial cytochrome c release, whereas other studies have suggested the converse that ERK acts downstream of mitochondrial events and upstream of caspase-3 activation. ERK also can contribute to cell death through the suppression of the antiapoptotic signaling molecule Akt (Zhuang and Schnellmann 2006). Administration of the MEK inhibitor, U0126, can attenuate ischemia-induced brain damage after either forebrain or focal ischemia (Namura et al. 2001), suggesting that Erk1/2 activation may actively participate in the ischemic cell death process. Although transient Erk1/2 activation is considered as a survival signaling event, prolonged stimulation of Erk1/2 appears to be related to neuronal cell death, consistent with the concept of the paradox of dual survival/death role of Erk1/2 in neurons (Zhuang and Schnellmann 2006). Therefore, in the MCAO model, the effect of PACAP on Erk1/2 phosphorylation may depend on the degree of insult, and definitely, the reduced Erk1/2 phosphorylation observed in our study may be interpreted as a neuroprotective effect.
On another hand, PACAP increased BDNF expression and most probably its autocrine/exocrine release (Fig. 9). In theory, this release can activate BDNF trkB receptors which in turn can activate PI3-K/Akt signaling pathway therefore conferring neuroprotection by a second mechanism. A similar possibility was proposed for PACAP-induced release of BDNF and activation of NMDA receptors (Yaka et al. 2003), and these data are also reminiscent of PACAP-induced phosphorylation of Akt in cardiomyocytes exposed to ischemia (Racz et al. 2008). Activation of trkB can also result from PACAP-induced transactivation of trkB using Src signaling (Lee et al. 2002b; Shi et al. 2010). Another effect of PACAP was its ability to reduce neuronal inhibitory molecules p75NTR and NgR under ischemic conditions. Since p75NTR level was decreased and p75NTR level regulates the activity of trkB (Zaccaro et al. 2001), it is tempting to propose that this third mechanism is also responsible for the increase phosphorylation activity of trkB receptor.
Rho GTPases, RhoA, and Rac1 are expressed throughout the nervous system. Inhibition of RhoA can induce neurite outgrowth and CNS axonal regeneration (Kubo and Yamashita 2007), while activation of Rac1 may promote neurite outgrowth (Kozma et al. 1997). Direct interaction of the Rho GDP dissociation inhibitor (Rho-GDI) with p75NTR has been observed, and this interaction leads to the activation of RhoA, which can be further strengthened by Nogo (Yamashita and Tohyama 2003). Neurotrophin-dependent tyrosine phosphorylation of Ras guanine releasing factor 1 and associated neurite outgrowth is dependent on trk receptor (Robinson et al. 2005), suggesting that trkB can induce neurite outgrowth by modulating the Rho–Rac balance. Therefore, it is tempting to propose that PACAP induced decreased of p75NTR/NgR as well as activation of trkB, contributed to the reduced RhoA-GTP observed in vitro with cortical neurons exposed to OGD–reperfusion, and increased Rac1 activity observed in the MCAO stroke model. We believe that during the first 48 h, the neurotrophin effect is neuroprotective and increased labeling of neurites is a reflection of reduced cell death. We cannot exclude the possibility that the increased presence of neurites at 48 h post-stroke is also indicative of an early onset of a neurorestorative effect, including plasticity and neurite remodeling. The in-creased neurites are clearly indicative of sparing and neuroprotection, but may also indicate neurite outgrowth and the onset of neurorestoration. This third mechanism may explain PACAP reduction of neurite damage and increased neurite plasticity under ischemic conditions. These findings are in line with the concept that neurotrophic factors can influence axonal outgrowth through the modulation of downstream signaling proteins, such as PKA, PI3-K/Akt, and Rho GTPase (Ozdinler and Erzurumlu 2001; Kao et al. 2002).
In conclusion, the neuroprotection and neuroplasticity effects of PACAP38 in the above stroke models indicate cross-talk effects of PAC1 and trkB receptors, which can amplify the molecular signals attempting to cope with the ischemic insult. cAMP/PKA has been implicated in neurite outgrowth in vitro and in vivo, and it may be one of the potential therapeutic targets for stimulating the regeneration after neuronal injury or neurodegeneration (Pazyra-Murphy et al. 2009). PI3-K–Akt is involved in the modulation of axonal regeneration, such as preventing the injury-induced motoneuron death and promoting axon regeneration (Fiore et al. 2009). PACAP by mobilizing BDNF–trkB signaling may induce a stronger activation of PI3K/Akt signaling pathway generating a significant neuroprotective effect. This study extends the observation that PACAP is simply neuroprotective after stroke by acting simultaneously on several mechanisms (Dejda et al. 2011a) including attenuation of mitochondrial apoptotic pathway, reduction of inflammatory response (Ohtaki et al. 2006), and probably mobilization of neurotrophin signaling. Therefore, development of PACAP-based therapeutic agents (Doan et al. 2011; Reglodi et al. 2011) may benefit stroke patients by inducing neurotrophin expression and signaling (Jeanneteau and Chao 2006). Clarifying the mechanisms of PACAP-induced, neurotrophin-mediated neuroprotection and neurite outgrowth under ischemic conditions will further contribute towards this goal.
Acknowledgments
PL holds the Jacob Gitlin Chair in Physiology and is affiliated and supported by David R. Bloom Center for Pharmacy and Dr. Adolf and Klara Brettler Center for Research in Molecular Pharmacology and Therapeutics at the Hebrew University of Jerusalem, Israel. This work was also supported in part by NIH grants PO1 NS023393 (MC) and RO1 AG037506 (MC). The authors wish to thank Ms. Zehava Cohen for graphic work.
Abbreviations
- Akt
PKB protein kinase B
- Bax
Member of the pro-apoptotic Bcl-2 protein family
- BDNF
Brain-derived neurotrophic factor
- Erk1/2
Extracellular signal-regulated kinases 1/2
- GAP-43
Growth-associated protein-43
- LDH
Lactate dehydrogenase
- MAP-2
Microtubule-associated protein-2
- MCAO
Middle cerebral artery occlusion
- NgR
Nogo receptor (inhibitor of neurite outgrowth Nogo 66)
- OGD
Oxygen–glucose deprivation
- p75NTR
Nerve growth factor receptor
- PAC1
Pituitary adenylate cyclase receptor type 1
- PACAP
Pituitary adenylate cyclase activating peptide
- PI3-K
Phosphoinositide 3-kinase
- Rac1
Ras-related C3 botulinum toxin substrate 1
- RhoA
Ras homolog gene family member A
- Rho-GDI
Rho GDP dissociation inhibitor
- trkB
Tropomyosin-related kinase receptor type B
- TuJ1
Neuron-specific class III beta-tubulin
Contributor Information
Philip Lazarovici, Email: philipl@ekmd.huji.ac.il, School of Pharmacy Institute for Drug Research, Faculty of Medicine, The Hebrew University of Jerusalem, POB 12065, Jerusalem 91120, Israel.
Gadi Cohen, School of Pharmacy Institute for Drug Research, Faculty of Medicine, The Hebrew University of Jerusalem, POB 12065, Jerusalem 91120, Israel.
Hadar Arien-Zakay, School of Pharmacy Institute for Drug Research, Faculty of Medicine, The Hebrew University of Jerusalem, POB 12065, Jerusalem 91120, Israel.
Jieli Chen, Department of Neurology, Henry Ford Health Sciences Center, Detroit, MI 48202, USA.
Chunling Zhang, Department of Neurology, Henry Ford Health Sciences Center, Detroit, MI 48202, USA.
Michael Chopp, Department of Neurology, Henry Ford Health Sciences Center, Detroit, MI 48202, USA.
Hao Jiang, Department of Neurology, Henry Ford Health Sciences Center, Detroit, MI 48202, USA.
References
- Arien-Zakay H, Lecht S, Bercu MM, et al. Neuroprotection by cord blood neural progenitors involves antioxidants, neurotrophic and angiogenic factors. Exp Neurol. 2009;216:83–94. doi: 10.1016/j.expneurol.2008.11.006. [DOI] [PubMed] [Google Scholar]
- Arimura A. Perspectives on pituitary adenylate cyclase activating polypeptide (PACAP) in the neuroendocrine, endocrine, and nervous systems. Jpn J Physiol. 1998;48:301–331. doi: 10.2170/jjphysiol.48.301. [DOI] [PubMed] [Google Scholar]
- Benowitz LI, Perrone-Bizzozero NI, Neve RL, Rodriguez W. GAP-43 as a marker for structural plasticity in the mature CNS. Prog Brain Res. 1990;86:309–320. doi: 10.1016/s0079-6123(08)63187-8. [DOI] [PubMed] [Google Scholar]
- Bito H, Takemoto-Kimura S. Ca(2+)/CREB/CBP-dependent gene regulation: a shared mechanism critical in long-term synaptic plasticity and neuronal survival. Cell Calcium. 2003;34:425–430. doi: 10.1016/s0143-4160(03)00140-4. [DOI] [PubMed] [Google Scholar]
- Botia B, Basille M, Allais A, et al. Neurotrophic effects of PACAP in the cerebellar cortex. Peptides. 2007;28:1746–1752. doi: 10.1016/j.peptides.2007.04.013. [DOI] [PubMed] [Google Scholar]
- Bourgault S, Chatenet D, Wurtz O, et al. Strategies to convert PACAP from a hypophysiotropic neurohormone into a neuroprotective drug. Curr Pharm Des. 2011;17:1002–1024. doi: 10.2174/138161211795589337. [DOI] [PubMed] [Google Scholar]
- Cai D, Qiu J, Cao Z, McAtee M, Bregman BS, Filbin MT. Neuronal cyclic AMP controls the developmental loss in ability of axons to regenerate. J Neurosci. 2001;21:4731–4739. doi: 10.1523/JNEUROSCI.21-13-04731.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavallaro S, Copani A, D'Agata V, et al. Pituitary adenylate cyclase activating polypeptide prevents apoptosis in cultured cerebellar granule neurons. Mol Pharmacol. 1996;50:60–66. [PubMed] [Google Scholar]
- Chang JY, Korolev VV. Cyclic AMP and sympathetic neuronal programmed cell death. Neurochem Int. 1997;31:161–167. doi: 10.1016/s0197-0186(96)00145-3. [DOI] [PubMed] [Google Scholar]
- Chen J, Zhang C, Jiang H, et al. Atorvastatin induction of VEGF and BDNF promotes brain plasticity after stroke in mice. J Cereb Blood Flow Metab. 2005;25:281–290. doi: 10.1038/sj.jcbfm.9600034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowansage KK, LeDoux JE, Monfils MH. Brain-derived neurotrophic factor: a dynamic gatekeeper of neural plasticity. Curr Mol Pharmacol. 2010;3:12–29. doi: 10.2174/1874467211003010012. [DOI] [PubMed] [Google Scholar]
- Davoli MA, Fourtounis J, Tam J, et al. Immunohistochemical and biochemical assessment of caspase-3 activation and DNA fragmentation following transient focal ischemia in the rat. Neuroscience. 2002;115:125–136. doi: 10.1016/s0306-4522(02)00376-7. [DOI] [PubMed] [Google Scholar]
- Dejda A, Bourgault S, Doan ND, et al. Identification by photo-affinity labeling of the extracellular N-terminal domain of PAC1 receptor as the major binding site for PACAP. Biochimie. 2011a;93:669–677. doi: 10.1016/j.biochi.2010.12.010. [DOI] [PubMed] [Google Scholar]
- Dejda A, Seaborn T, Bourgault S, et al. PACAP and a novel stable analog protect rat brain from ischemia: insight into the mechanisms of action. Peptides. 2011b;32:1207–1216. doi: 10.1016/j.peptides.2011.04.003. [DOI] [PubMed] [Google Scholar]
- Deutsch PJ, Sun Y. The 38-amino acid form of pituitary adenylate cyclase-activating polypeptide stimulates dual signaling cascades in PC12 cells and promotes neurite outgrowth. J Biol Chem. 1992;267:5108–5113. [PubMed] [Google Scholar]
- Deutsch PJ, Schadlow VC, Barzilai N. 38-Amino acid form of pituitary adenylate cyclase activating peptide induces process outgrowth in human neuroblastoma cells. J Neurosci Res. 1993;35:312–320. doi: 10.1002/jnr.490350311. [DOI] [PubMed] [Google Scholar]
- Doan ND, Bourgault S, Dejda A, et al. Design and in vitro characterization of PAC1/VPAC1-selective agonists with potent neuroprotective effects. Biochem Pharmacol. 2011;81:552–561. doi: 10.1016/j.bcp.2010.11.015. [DOI] [PubMed] [Google Scholar]
- Fiore R, Khudayberdiev S, Christensen M, et al. Mef2-mediated transcription of the miR379–410 cluster regulates activity-dependent dendritogenesis by fine-tuning Pumilio2 protein levels. EMBO J. 2009;28:697–710. doi: 10.1038/emboj.2009.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frechilla D, Garcia-Osta A, Palacios S, Cenarruzabeitia E, Del Rio J. BDNF mediates the neuroprotective effect of PACAP-38 on rat cortical neurons. Neuroreport. 2001;12:919–923. doi: 10.1097/00001756-200104170-00011. [DOI] [PubMed] [Google Scholar]
- Geisert EE, Jr, Frankfurter A. The neuronal response to injury as visualized by immunostaining of class III beta-tubulin in the rat. Neurosci Lett. 1989;102:137–141. doi: 10.1016/0304-3940(89)90068-2. [DOI] [PubMed] [Google Scholar]
- Hall A, Nobes CD. Rho GTPases: molecular switches that control the organization and dynamics of the actin cytoskeleton. Philos Trans R Soc Lond B Biol Sci. 2000;355:965–970. doi: 10.1098/rstb.2000.0632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmar T, Lutz E. Multiple receptors for PACAP and VIP. Trends Pharmacol Sci. 1994;15:97–99. doi: 10.1016/0165-6147(94)90042-6. [DOI] [PubMed] [Google Scholar]
- Hartfield PJ, Bilney AJ, Murray AW. Neurotrophic factors prevent ceramide-induced apoptosis downstream of c-Jun N-terminal kinase activation in PC12 cells. J Neurochem. 1998;71:161–169. doi: 10.1046/j.1471-4159.1998.71010161.x. [DOI] [PubMed] [Google Scholar]
- Jeanneteau F, Chao MV. Promoting neurotrophic effects by GPCR ligands. Novartis Found Symp. 2006;276:181–189. [PubMed] [Google Scholar]
- Jiang H, Movsesyan V, Fink DW, Jr, et al. Expression of human p140trk receptors in p140trk-deficient, PC12/endothelial cells results in nerve growth factor-induced signal transduction and DNA synthesis. J Cell Biochem. 1997;66:229–244. [PubMed] [Google Scholar]
- Jin K, Mao XO, Simon RP, Greenberg DA. Cyclic AMP response element binding protein (CREB) and CREB binding protein (CBP) in global cerebral ischemia. J Mol Neurosci. 2001;16:49–56. doi: 10.1385/JMN:16:1:49. [DOI] [PubMed] [Google Scholar]
- Kao HT, Song HJ, Porton B, et al. A protein kinase A-dependent molecular switch in synapsins regulates neurite outgrowth. Nat Neurosci. 2002;5:431–437. doi: 10.1038/nn840. [DOI] [PubMed] [Google Scholar]
- Kienlen Campard P, Crochemore C, Rene F, Monnier D, Koch B, Loeffler JP. PACAP type I receptor activation promotes cerebellar neuron survival through the cAMP/PKA signaling pathway. DNA Cell Biol. 1997;16:323–333. doi: 10.1089/dna.1997.16.323. [DOI] [PubMed] [Google Scholar]
- Koumura A, Nonaka Y, Hyakkoku K, et al. A novel calpain inhibitor, ((1 S)-1((((1 S)-1-benzyl-3-cyclopropylamino-2,3-dioxopropyl) amino)carbonyl)-3-met hylbutyl) carbamic acid 5-methoxy-3-oxapentyl ester, protects neuronal cells from cerebral ischemia-induced damage in mice. Neuroscience. 2008;157:309–318. doi: 10.1016/j.neuroscience.2008.09.007. [DOI] [PubMed] [Google Scholar]
- Kozma R, Sarner S, Ahmed S, Lim L. Rho family GTPases and neuronal growth cone remodelling: relationship between increased complexity induced by Cdc42Hs, Rac1, and acetylcholine and collapse induced by RhoA and lysophosphatidic acid. Mol Cell Biol. 1997;17:1201–1211. doi: 10.1128/mcb.17.3.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo T, Yamashita T. Rho-ROCK inhibitors for the treatment of CNS injury. Recent Pat CNS Drug Discov. 2007;2:173–179. doi: 10.2174/157488907782411738. [DOI] [PubMed] [Google Scholar]
- Kuperstein F, Yavin E. ERK activation and nuclear translocation in amyloid-beta peptide- and iron-stressed neuronal cell cultures. Eur J Neurosci. 2002;16:44–54. doi: 10.1046/j.1460-9568.2002.02056.x. [DOI] [PubMed] [Google Scholar]
- Lazarovici P, Fink D., Jr Heterologous upregulation of nerve growth factor-TrkA receptors in PC12 cells by pituitary adenylate cyclase-activating polypeptide (PACAP) Mol Cell Biol Res Commun. 1999;2:97–102. doi: 10.1006/mcbr.1999.0158. [DOI] [PubMed] [Google Scholar]
- Lazarovici P, Jiang H, Fink D., Jr The 38-amino-acid form of pituitary adenylate cyclase-activating polypeptide induces neurite outgrowth in PC12 cells that is dependent on protein kinase C and extracellular signal-regulated kinase but not on protein kinase A, nerve growth factor receptor tyrosine kinase, p21(ras) G protein, and pp60(c-src) cytoplasmic tyrosine kinase. Mol Pharmacol. 1998;54:547–558. doi: 10.1124/mol.54.3.547. [DOI] [PubMed] [Google Scholar]
- Lecht S, Arien-Zakay H, Marcinkiewicz C, Lelkes PI, Lazarovici P. Nerve growth factor-induced protection of brain capillary endothelial cells exposed to oxygen-glucose deprivation involves attenuation of Erk phosphorylation. J Mol Neurosci. 2010;41:183–192. doi: 10.1007/s12031-009-9318-0. [DOI] [PubMed] [Google Scholar]
- Lee FS, Rajagopal R, Chao MV. Distinctive features of Trk neurotrophin receptor transactivation by G protein-coupled receptors. Cytokine Growth Factor Rev. 2002a;13:11–17. doi: 10.1016/s1359-6101(01)00024-7. [DOI] [PubMed] [Google Scholar]
- Lee FS, Rajagopal R, Kim AH, Chang PC, Chao MV. Activation of Trk neurotrophin receptor signaling by pituitary adenylate cyclase-activating polypeptides. J Biol Chem. 2002b;277:9096–9102. doi: 10.1074/jbc.M107421200. [DOI] [PubMed] [Google Scholar]
- Li Y, Jiang N, Powers C, Chopp M. Neuronal damage and plasticity identified by microtubule-associated protein 2, growth-associated protein 43, and cyclin D1 immunoreactivity after focal cerebral ischemia in rats. Stroke. 1998;29:1972–1981. doi: 10.1161/01.str.29.9.1972. [DOI] [PubMed] [Google Scholar]
- Lobner D, Choi DW. Preincubation with protein synthesis inhibitors protects cortical neurons against oxygen-glucose deprivation-induced death. Neuroscience. 1996;72:335–341. doi: 10.1016/0306-4522(95)00561-7. [DOI] [PubMed] [Google Scholar]
- Magistretti PJ, Cardinaux JR, Martin JL. VIP and PACAP in the CNS: regulators of glial energy metabolism and modulators of glutamatergic signaling. Ann N Y Acad Sci. 1998;865:213–225. doi: 10.1111/j.1749-6632.1998.tb11181.x. [DOI] [PubMed] [Google Scholar]
- Miller FD, Kaplan DR. Neurotrophin signalling pathways regulating neuronal apoptosis. Cell Mol Life Sci. 2001;58:1045–1053. doi: 10.1007/PL00000919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata A, Arimura A, Dahl RR, et al. Isolation of a novel 38 residue-hypothalamic polypeptide which stimulates adenylate cyclase in pituitary cells. Biochem Biophys Res Comm. 1989;164:567–574. doi: 10.1016/0006-291x(89)91757-9. [DOI] [PubMed] [Google Scholar]
- Nakamachi T, Ohtaki H, Yofu S, et al. Endogenous pituitary adenylate cyclase activating polypeptide is involved in suppression of edema in the ischemic brain. Acta Neurochir Suppl. 2010;106:43–46. doi: 10.1007/978-3-211-98811-4_6. [DOI] [PubMed] [Google Scholar]
- Namura S, Iihara K, Takami S, et al. Intravenous administration of MEK inhibitor U0126 affords brain protection against fore-brain ischemia and focal cerebral ischemia. Proc Natl Acad Sci USA. 2001;98:11569–11574. doi: 10.1073/pnas.181213498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtaki H, Nakamachi T, Dohi K, et al. Pituitary adenylate cyclase-activating polypeptide (PACAP) decreases ischemic neuronal cell death in association with IL-6. Proc Natl Acad Sci USA. 2006;103:7488–7493. doi: 10.1073/pnas.0600375103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onaka T, Ikeda K, Yamashita T, Honda K. Facilitative role of endogenous oxytocin in noradrenaline release in the rat supraoptic nucleus. Eur J Neurosci. 2003;18:3018–3026. doi: 10.1046/j.1460-9568.2003.03037.x. [DOI] [PubMed] [Google Scholar]
- Ozdinler PH, Erzurumlu RS. Regulation of neurotrophin-induced axonal responses via Rho GTPases. J Comp Neurol. 2001;438:377–387. doi: 10.1002/cne.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazyra-Murphy MF, Hans A, Courchesne SL, et al. A retrograde neuronal survival response: target-derived neurotrophins regulate MEF2D and bcl-w. J Neurosci. 2009;29:6700–6709. doi: 10.1523/JNEUROSCI.0233-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racz B, Gasz B, Gallyas F, Jr, et al. PKA-Bad-14-3-3 and Akt-Bad-14-3-3 signaling pathways are involved in the protective effects of PACAP against ischemia/reperfusion-induced cardiomyocyte apoptosis. Regul Pept. 2008;145:105–115. doi: 10.1016/j.regpep.2007.09.015. [DOI] [PubMed] [Google Scholar]
- Reglodi D, Somogyvari-Vigh A, Vigh S, Kozicz T, Arimura A. Delayed systemic administration of PACAP38 is neuroprotective in transient middle cerebral artery occlusion in the rat. Stroke. 2000a;31:1411–1417. doi: 10.1161/01.str.31.6.1411. [DOI] [PubMed] [Google Scholar]
- Reglodi D, Somogyvari-Vigh A, Vigh S, Maderdrut JL, Arimura A. Neuroprotective effects of PACAP38 in a rat model of transient focal ischemia under various experimental conditions. Ann NY Acad Sci. 2000b;921:119–128. doi: 10.1111/j.1749-6632.2000.tb06958.x. [DOI] [PubMed] [Google Scholar]
- Reglodi D, Fabian Z, Tamas A, et al. Effects of PACAP on in vitro and in vivo neuronal cell death, platelet aggregation, and production of reactive oxygen radicals. Regul Pept. 2004;123:51–59. doi: 10.1016/j.regpep.2004.05.012. [DOI] [PubMed] [Google Scholar]
- Reglodi D, Kiss P, Lubics A, Tamas A. Review on the protective effects of PACAP in models of neurodegenerative diseases in vitro and in vivo. Curr Pharm Des. 2011;17:962–972. doi: 10.2174/138161211795589355. [DOI] [PubMed] [Google Scholar]
- Robinson KN, Manto K, Buchsbaum RJ, MacDonald JI, Meakin SO. Neurotrophin-dependent tyrosine phosphorylation of Ras guanine-releasing factor 1 and associated neurite outgrowth is dependent on the HIKE domain of TrkA. J Biol Chem. 2005;280:225–235. doi: 10.1074/jbc.M410454200. [DOI] [PubMed] [Google Scholar]
- Schomerus C, Maronde E, Laedtke E, Korf HW. Vasoactive intestinal peptide (VIP) and pituitary adenylate cyclase-activating polypeptide (PACAP) induce phosphorylation of the transcription factor CREB in subpopulations of rat pinealocytes: immunocytochemical and immunochemical evidence. Cell Tissue Res. 1996;286:305–313. doi: 10.1007/s004410050700. [DOI] [PubMed] [Google Scholar]
- Shehadah A, Chen J, Cui X, Roberts C, Lu M, Chopp M. Combination treatment of experimental stroke with Niaspan and Simvastatin, reduces axonal damage and improves functional outcome. J Neurol Sci. 2010;294:107–111. doi: 10.1016/j.jns.2010.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwood NM, Krueckl SL, McRory JE. The origin and function of the pituitary adenylate cyclase-activating polypeptide (PACAP)/glucagon superfamily. Endocr Rev. 2000;21:619–670. doi: 10.1210/edrv.21.6.0414. [DOI] [PubMed] [Google Scholar]
- Shi GX, Jin L, Andres DA. Src-dependent TrkA transactivation is required for pituitary adenylate cyclase-activating polypeptide 38-mediated Rit activation and neuronal differentiation. Mol Biol Cell. 2010;21:1597–1608. doi: 10.1091/mbc.E09-12-1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoglosa Y, Lewen A, Takei N, Hillered L, Lindholm D. Regulation of pituitary adenylate cyclase activating polypeptide and its receptor type 1 after traumatic brain injury: comparison with brain-derived neurotrophic factor and the induction of neuronal cell death. Neuroscience. 1999;90:235–247. doi: 10.1016/s0306-4522(98)00414-x. [DOI] [PubMed] [Google Scholar]
- Somogyvari-Vigh A, Pan W, Reglodi D, Kastin AJ, Arimura A. Effect of middle cerebral artery occlusion on the passage of pituitary adenylate cyclase activating polypeptide across the blood–brain barrier in the rat. Regul Pept. 2000;91:89–95. doi: 10.1016/s0167-0115(00)00123-3. [DOI] [PubMed] [Google Scholar]
- Tanaka J, Koshimura K, Murakami Y, Sohmiya M, Yanaihara N, Kato Y. Neuronal protection from apoptosis by pituitary adenylate cyclase-activating polypeptide. Regul Pept. 1997;72:1–8. doi: 10.1016/s0167-0115(97)01038-0. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Nogawa S, Nagata E, et al. Erratum to: effects of blockade of voltage-sensitive Ca(2+)/Na(+) channels by a novel phenylpyrimidine derivative, NS-7, on CREB phosphorylation in focal cerebral ischemia in the rat. Brain Res. 2000;881:248. doi: 10.1016/s0006-8993(00)02866-3. [DOI] [PubMed] [Google Scholar]
- Uchida D, Arimura A, Somogyvari-Vigh A, Shioda S, Banks WA. Prevention of ischemia-induced death of hippocampal neurons by pituitary adenylate cyclase activating polypeptide. Brain Res. 1996;736:280–286. doi: 10.1016/0006-8993(96)00716-0. [DOI] [PubMed] [Google Scholar]
- Vaudry D, Basille M, Anouar Y, Fournier A, Vaudry H, Gonzalez BJ. The neurotrophic activity of PACAP on rat cerebellar granule cells is associated with activation of the protein kinase A pathway and c-fos gene expression. Ann NY Acad Sci. 1998a;865:92–99. doi: 10.1111/j.1749-6632.1998.tb11167.x. [DOI] [PubMed] [Google Scholar]
- Vaudry D, Gonzalez BJ, Basille M, Anouar Y, Fournier A, Vaudry H. Pituitary adenylate cyclase-activating polypeptide stimulates both c-fos gene expression and cell survival in rat cerebellar granule neurons through activation of the protein kinase A pathway. Neuroscience. 1998b;84:801–812. doi: 10.1016/s0306-4522(97)00545-9. [DOI] [PubMed] [Google Scholar]
- Vaudry D, Gonzalez BJ, Basille M, et al. The neuroprotective effect of pituitary adenylate cyclase-activating polypeptide on cerebellar granule cells is mediated through inhibition of the CED3-related cysteine protease caspase-3/CPP32. Proc Natl Acad Sci USA. 2000a;97:13390–13395. doi: 10.1073/pnas.97.24.13390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaudry D, Gonzalez BJ, Basille M, Pamantung TF, Fournier A, Vaudry H. PACAP acts as a neurotrophic factor during histogenesis of the rat cerebellar cortex. Ann NY Acad Sci. 2000b;921:293–299. doi: 10.1111/j.1749-6632.2000.tb06980.x. [DOI] [PubMed] [Google Scholar]
- Vaudry D, Pamantung TF, Basille M, et al. PACAP protects cerebellar granule neurons against oxidative stress-induced apoptosis. Eur J Neurosci. 2002;15:1451–1460. doi: 10.1046/j.1460-9568.2002.01981.x. [DOI] [PubMed] [Google Scholar]
- Vaudry D, Falluel-Morel A, Basille M, et al. Pituitary adenylate cyclase-activating polypeptide prevents C2-ceramide-induced apoptosis of cerebellar granule cells. J Neurosci Res. 2003;72:303–316. doi: 10.1002/jnr.10530. [DOI] [PubMed] [Google Scholar]
- Villalba M, Bockaert J, Journot L. Pituitary adenylate cyclase-activating polypeptide (PACAP-38) protects cerebellar granule neurons from apoptosis by activating the mitogen-activated protein kinase (MAP kinase) pathway. J Neurosci. 1997;17:83–90. doi: 10.1523/JNEUROSCI.17-01-00083.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White BC, Sullivan JM, DeGracia DJ, et al. Brain ischemia and reperfusion: molecular mechanisms of neuronal injury. J Neurol Sci. 2000;179:1–33. doi: 10.1016/s0022-510x(00)00386-5. [DOI] [PubMed] [Google Scholar]
- Wolf N, Krieglstein K. Phenotypic development of neonatal rat chromaffin cells in response to adrenal growth factors and glucocorticoids: focus on pituitary adenylate cyclase activating polypeptide. Neurosci Lett. 1995;200:207–210. doi: 10.1016/0304-3940(95)12116-l. [DOI] [PubMed] [Google Scholar]
- Yaka R, He DY, Phamluong K, Ron D. Pituitary adenylate cyclase-activating polypeptide (PACAP(1–38)) enhances N-methyl-D-aspartate receptor function and brain-derived neurotrophic factor expression via RACK1. J Biol Chem. 2003;278:9630–9638. doi: 10.1074/jbc.M209141200. [DOI] [PubMed] [Google Scholar]
- Yamashita T, Tohyama M. The p75 receptor acts as a displacement factor that releases Rho from Rho-GDI. Nat Neurosci. 2003;6:461–467. doi: 10.1038/nn1045. [DOI] [PubMed] [Google Scholar]
- Zaccaro MC, Ivanisevic L, Perez P, Meakin SO, Saragovi HU. p75 Co-receptors regulate ligand-dependent and ligand-independent Trk receptor activation, in part by altering Trk docking subdomains. J Biol Chem. 2001;276:31023–31029. doi: 10.1074/jbc.M104630200. [DOI] [PubMed] [Google Scholar]
- Zhuang S, Schnellmann RG. A death-promoting role for extracellular signal-regulated kinase. J Pharmacol Exp Ther. 2006;319:991–997. doi: 10.1124/jpet.106.107367. [DOI] [PubMed] [Google Scholar]