Abstract
Introduction
Stroke remains the leading cause of adult disability. Thus, it is imperative to develop restorative therapies for ischemic stroke designed specifically to treat the intact brain tissue to stimulate functional benefit. Therapies targeting amplification of brain repair processes with nitric oxide (NO) donors and phosphodiesterase type 5 (PDE5) inhibitors in preclinical studies are emerging and showing improvement of functional recovery after stroke.
Area covered
This review will mainly cover the effect of NO donors, which produce NO, and PDE5 inhibitors, which elevate cyclic guanosine 3′,5′-monophosphate (cGMP), on neural restorative events in ischemic brain and highlight mechanisms underlying their restorative therapeutic activity .
Expert opinion
During stroke recovery, interwoven restorative events occur in ischemic brain, which include angiogenesis, neurogenesis, oligodendrogenesis, astrogliosis, and neurite outgrowth. Emerging preclinical data indicate that restorative therapies targeting multiple parenchymal cells including neural stem cells, cerebral endothelial cells, astrocytes, oligodendrocytes, neurons would be more effective than agents with a single cell target. Preclinical data suggest that elevated cGMP levels induced by NO donors and PDE5 inhibitors act on cerebral endothelial cells, neural stem cells, and oligodendrocyte progenitor cells to enhance stroke-induced angiogenesis, neurogenesis and oligodendrogenesis, respectively. These interacting remodeling events collectively improve neurological function after stroke.
Keywords: Nitric oxide, cGMP, PDE5 inhibitor, stroke, angiogenesis, neurogenesis
1. Introduction
Ischemic stroke triggered by blockage of blood flow within an artery in the brain by a clot constitutes more than 80% of stroke that is third leading cause of death and remains the leading cause of adult disability [1–6]. There have been substantial advances in understanding ischemic neuronal injury. However, although neuroprotection has been validated in experimental stroke, clinical trials show that none of neuroprotective drugs achieve clinical benefit for treatment of acute stroke [1–6]. The only Food and Drug Administration (FDA) approved treatment for acute stroke (within 4.5h) is thrombolysis with tissue plasminogen activator (tPA) that is applied to approximately 3% to 8.5% of stroke patients [1, 2, 7]. Thus, it is imperative to develop therapies for ischemic stroke designed specifically to reduce neurological deficits, which can be employed to treat the vast majority of patients.
Studies in animal models of stroke and patients with stroke show that brain repair processes take place, which are associated with limited neurological improvement. Brain repair includes cerebral angiogenesis, axonal and dendritic sprouting, oligodendrogenesis and neurogenesis. Emerging data from preclinical experiments indicate that pharmacological and cell-based therapies targeting amplification of brain repair processes after stroke substantially improve functional recovery [8–16].
Nitric oxide (NO) is an activator of soluble guanylate cyclase that causes cyclic guanosine 3′,5′-monophosphate (cGMP) formation in target cells [17, 18]. Stimulation of natriuretic peptide also generates cGMP [17, 18]. Phosphodiesterase type 5 (PDE5) enzyme is highly specific for hydrolysis of cGMP [19, 20]. The effect of NO and NO synthase (NOS) on acute stroke has been extensively investigated (please see review [21]). NO reacts with excessive superoxide generated immediately after stroke and forms peroxynitrite, which leads to cell death [22, 23]. Additional neurotoxicity of NO is mediated by protein S-nitrosylation in which NO reacts with specific cysteine thiols to regulate protein activities [24, 25]. In general, preclinical studies demonstrate that NO generated by the neuronal and inducible NOS (nNOS and iNOS) after stroke is detrimental to neuronal survival, whereas endothelial NOS (eNOS) and endothelial NO are neuroprotective [21]. A clinical trial is currently under way to determine if an NO donor is effective for treatment of stroke (Efficacy of Nitric Oxide in Stroke study)[26]. Readers can find comprehensive information of the effects of NO and reactive oxygen species on acute stroke in recently published excellent review articles [21, 23, 27]. In this review, we will mainly cover the effect of NO donors, which produce NO, and PDE5 inhibitors, which elevate cGMP, on neural restorative events in ischemic brain and highlight mechanisms underlying their restorative therapeutic effects.
2. The effect of NO donors and PDE5 inhibitors on stroke-induced angiogenesis
Stroke induces angiogenesis in the adult human and rodent brains [28, 29]. Angiogenesis, the sprouting of new capillaries from pre-existing vessels, is initiated at peri-infarct areas during the first few weeks and persists for several months after the onset of stroke [30–32]. Experimental studies indicate that the NO/cGMP signaling pathway amplifies angiogenesis in the ischemic brain. Systemic administration of the NO donor, DETANONOate, to rats 24 h after stroke substantially increases angiogenesis in the peri-infarct region [8]. The effect of an NO donor on cerebral angiogenesis likely occurs through the NO/cGMP signaling pathway, because blockage of soluble guanylate cyclase by a pharmacological inhibitor abolishes the NO donor induced angiogenesis [8]. Furthermore, treatment with PDE5 inhibitors, sildenafil and tadalafil, initiated 24h after stroke elevates brain cGMP levels and markedly increases angiogenesis [8, 33]. Elevation of cGMP levels in the ischemic brain by PDE5 inhibitors is likely specific, because administration of tadalafil to ischemic rats does not substantially change brain cAMP levels [33]. Advanced age is a major risk factor and a leading cause of severe disability for stroke patients [1, 2, 7, 34–36]. The PDE5 enzyme is present in the brain and aging decreases the basal brain levels of cGMP [37]. Aging also reduces angiogenesis [38, 39]. However, treatment of stroke with DETANONOate and sildenafil in animals at the age of 18 months considerably augments brain cGMP levels and angiogenesis to a similar level, respectively, observed in young adult ischemic rats treated with these compounds[40]. These data indicate that even in aged animals, angiogenic potential is present in cerebral endothelial cells in response to these treatments. In addition to cerebral endothelial cells, circulating endothelial progenitor cells also contribute to the formation of new vessels in the ischemic brain [41]. In a mouse model of hindlimb ischemia, vardenafil, another PDE5 inhibitor, promotes angiogenesis through enhancement of mobilization of endothelial progenitor cells [42]. However, whether endothelial progenitor cells contribute to ischemia-induced cerebral angiogenesis by the NO/cGMP pathway remains to be determined.
Newly generated vessels are permeable, which could exacerbate ischemic damage [43]. Thus, it is critical to examine whether angiogenesis observed in the peri-infarct region generates functional vessels. Longitudinal MRI measurements of ischemic brain show that the angiogenic peri-infarct region exhibits a transient increase in vascular permeability 2 to 3 weeks after stroke. After that, vascular leakage subsides, whereas CBF is elevated 6 weeks after stroke [44]. However, treatment of stroke with sildenafil leads to early (3 to 4 weeks after stroke) augmentation of CBF in the angiogenic peri-infarct region and elevated CBF persists for at least 6 weeks after stroke [40, 45, 46]. These data suggest that angiogenic vessels increased by sildenafil have biological function. More importantly, the enhanced angiogenesis is correlated with improvement of neurological outcome during stroke recovery in ischemic animals treated with DETANONOate, sildenafil and tadalafil [8, 33]. Patients with stroke who have higher vascular density exhibit better neurological outcomes [29, 47, 48]. Ablation of stroke-induced angiogenesis with endostatin exacerbates neurological outcome in experimental stroke [49]. Collectively, these data suggest that angiogenesis improves neurological outcome. However, the effect of angiogenesis on improved neurological function could be either direct or indirect, as detailed further below.
3. The effect of NO donors and PDE5 inhibitors on stroke-induced neurogenesis
During brain development, neural stem cells in the ventricular zone (VZ) generate neuroblasts that migrate to the cortex to populate cortical neurons [50]. Several studies have suggested that the NO/cGMP pathway is involved in cortical neurogenesis [51–53]. Treatment of pregnant rats with nitroarginine-methylester (L-NAME), an inhibitor of NOS, reduces cGMP levels by approximately 50% and cortical neurons by approximately 40% in fetal brain [52]. However, administration of sildenafil along with L-NAME completely reverses the effect of L-NAME on cGMP and cortical neurogenesis [52]. In addition, incubation of neural stem cells harvested from the subventricular zone (SVZ) of postnatal mice with an NO donor (NOC-18) for 24h substantially promotes proliferation of these cells in a cGMP-dependent manner [51]. More importantly, incubation of human neural progenitor cells with the NOC-18 considerably increases neuronal cell motility by elevating cGMP levels [53]. Together, these studies suggest that the NO/cGMP pathway acts on neural stem cell proliferation and/or neuroblast migration to mediate the cortical neurogenesis. In the adult rodent, the VZ is replaced by an ependymal layer, while the SVZ of the lateral ventricle shrinks and persists [54]. There are at least two regions of adult rodent brain containing neural stem cells, one is the SVZ of the lateral ventricle and the other is the subgranular zone of the dentate gyrus [50]. Under physiological conditions, neural stem cells in the SVZ generate neuroblasts that migrate to the olfactory bulb where they differentiate into interneurons [50]. After stroke, neuroblasts generated in the SVZ migrate to peri-infarct regions and some of them express phenotypes of mature neurons in young and aged animals [55]. In addition, stroke induced neurogenesis has been detected in patients [56–59]. These data provide a compelling argument for further research to develop therapies by amplifying endogenous neurogenesis in the injured brain [55, 60]. Compounds that target the NO/cGMP pathway in neural progenitor cells have been investigated in non-ischemic and ischemic brains of the adult rodent [8, 61, 62]. Under non-ischemic conditions, inhibition of nNOS by either pharmacological inhibitors including L-NAME and 7-nitroindazole(7NI), or genetic ablation of nNOS promotes proliferation of neural progenitor cells in the SVZ and the subgranular zone of the dentate gyrus [63, 64]. Immunohistochemistry analysis reveals that SVZ neural progenitor cells do not express nNOS [63]. In vitro, co-culture of SVZ neural progenitor cells with neurons derived from wild-type mice suppresses progenitor cell proliferation, while this effect is abolished when the progenitor cells are co-cultured with neurons harvested from nNOS−/− mice [65]. Together, these data indicate that under physiological condition endogenous NO from nNOS exerts negative control of neural progenitor cell proliferation. The negative effect of nNOS on ischemia-induced neurogenesis has also been reported [66, 67] (Table 1). Adult nNOS−/− mice subjected to stroke exhibit substantial enhancement of neurogenesis compared to wild-type mice [66]. Blockage of nNOS activity by 7NI also further increases stroke-induced neurogenesis [66]. In contrast to nNOS, ablation of eNOS and iNOS genes or inhibition of iNOS by aminoguanidine blocks stroke-induced neurogenesis [67–69] (Table 1). Further studies indicate that augmentation of stroke-induced neurogenesis by blockage of nNOS is mediated by upregulation of iNOS, because knockdown of iNOS suppresses 7NI-enhanced neurogenesis in ischemic brain [67]. Under physiological conditions, nNOS and eNOS primarily contribute to NO production in the brain [21]. However, in ischemic brain, iNOS upregulated by stroke also forms NO [21, 67, 69]. Together, these studies suggest that elevation of NO after stroke regardless of its source could enhance neurogenesis. Indeed, administration of NO donors initiated 24 h after stroke significantly increases neurogenesis in the ischemic brain [8, 61, 62]. Although NO exerts neurotoxicity by formation of peroxynitrite and S-nitrosylation, NO donors given 24h after stroke do not exacerbate ischemic cell damage, suggesting that elevation of cGMP through activation of soluble guanylate cyclase by NO donors could contribute to augmentation of neurogenesis in ischemic brain. Several studies have investigated this possibility. Adult SVZ neural progenitor cells express PDE5 [37]. Inhibition of neural progenitor cell PDE5 by sildenafil considerably augments cGMP levels and increases progenitor cell proliferation and neuronal differentiation [37]. Later treatments (either 1 or 7 days after stroke) of ischemic animals with sildenafil substantially increase newly generated neurons in the peri-infarct regions and these new neurons exhibit phenotypes of mature neuronal markers [9, 37, 70] (Table 1). Stroke-induced neurogenesis enhanced by DETANONOate, sildenafil and tadalafil is observed not only in young adult, but also in aged rats, although the absolute numbers of newly generated neurons are less in aged rats than that in young adult rats [8, 33, 61, 62] (Table 1). In vitro data indicate that sildenafil can directly act on neural stem cells to enhance neurogenesis by increasing stem cell proliferation and differentiation [37]. This is further supported by a recent in vivo study [71]. By tracking progeny of SVZ nestin lineage neural stem cells after stroke, administration of sildenafil to ischemic mice at age of 12 months robustly increases new neurons in peri-infarct striatum and new oligodendrocytes in peri-infarct corpus callosum [71]. Ablation of newly generated neurons in the ischemic exacerbates neurological function [72, 73]. Oligodendrocytes myelinate axons and stroke induces axonal outgrowth in the peri-infarct areas [74–76]. Thus, in addition to angiogenesis, amplification of endogenous neurogenesis and oligodendrogenesis in the ischemic brain by the NO donors and PDE5 inhibitors could contribute to improvement of neurological outcome after stroke.
Table 1.
The effect of NO and PDE5 inhibitors on neurogenesis in ischemic brain.
| Species | Ischemic lesion | neurogenesis | references | |
|---|---|---|---|---|
| NO donors | ||||
| DETANONOate | rat | No change* | increase | 61, 62 |
| NOS inhibitors | ||||
| 7NI | mouse | reduction | increase | 66, 67 |
| aminoguanidine | mouse | reduction | reduction | 67, 69 |
| Transgenic mice | ||||
| eNOS−/− | mouse | increase | reduction | 68 |
| iNOS−/− | mouse | reduction | reduction | 67, 69 |
| nNOS−/− | mouse | reduction | increase | 66, 67 |
| PDE5 inhibitors | ||||
| sildenafil | rat, mouse | No change* | increase | 61, 62, 71 |
| tadalafil | rat | No change* | increase | 33 |
compounds were given 24h after the onset of stroke. Data of ischemic lesion and neurogenesis presented in the cited studies are statistically significant (p<0.05).
4. The effect the NO/cGMP pathway on coupling of neurogenesis and angiogenesis in the ischemic brain
Under physiological conditions, neurogenesis in the SVZ of the lateral ventricle and the subgranular zone of the dentate gyrus occurs within an angiogenic niche [77–79]. Angiogenesis and neurogenesis induced by stroke are coupled [28, 31, 32, 43, 49, 80–84]. Cerebral endothelial cells harvested from the ischemic boundary augment generation of neurons from neural progenitor cells derived from the non-ischemic SVZ, whereas ischemic neural progenitor cells promote naïve cerebral endothelial cells to enhance in vitro angiogenesis [84, 85]. Transplantation of neural progenitor cells into ischemic brain promotes angiogenesis [86]. Migration of neuroblasts generated in the SVZ to the ischemic boundary is closely associated with cerebral vessels [32, 44, 49]. Suppressing angiogenesis substantially attenuates migration of neuroblasts in the SVZ to the ischemic region and exacerbates neurological deficits [49]. Thus, enhancement of angiogenesis and neurogenesis in ischemic brain by the NO/cGMP pathway are likely coupled.
Vascular endothelial growth factor (VEGF) and its receptor, VEGF receptor 2 (VEGFR2), appear to play an important role in mediating coupling of angiogenesis and neurogenesis. DETANONOate and sildenafil upregulate VEGF expression in cerebral endothelial cells through elevation of cGMP and blockage of VEGFR2 abolishes cGMP-enhanced angiogenesis [8]. In addition to angiogenesis, VEGF secreted by cerebral endothelial cells can further amplify neurogenesis [84] (Fig. 1). On the other hand, SVZ neural progenitor cells express an array of angiogenic factors, including angiopoietin 2, VEGFR2 and fibroblast growth factor [87]. VEGF generated by SVZ neural progenitor cells facilities angiogenesis [84]. Accordingly, neurogenesis enhanced by NO donors and PDE5 inhibitors could likely magnify angiogenesis in ischemic brain.
Figure 1.
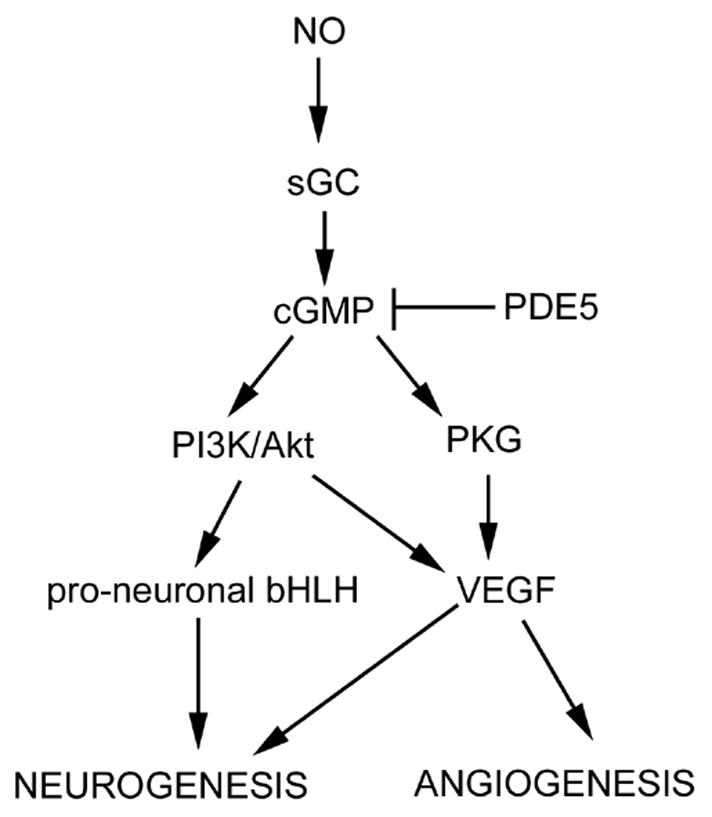
Schematic representation of signaling pathways that may be involved in NO donors and PDE5 inhibitors-induced angiogenesis and neurogenesis in the ischemic brain. sGC = soluble guanylate cyclase, PKG = cGMP-dependent protein kinases.
The phosphatidylinositol 3 kinase (PI3K)/Akt pathway affects multiple cellular functions such as cell survival, proliferation, differentiation and migration [88, 89]. NO/cGMP activates the PI3K/Akt signaling pathway in neural progenitor cells and endothelial cells [10, 13, 90] (Fig. 1). Activation of the PI3K/Akt pathway is required for VEGF induced angiogenesis [90]. Elevation of cGMP by sildenafil increases Akt activity in SVZ neural stem cells, whereas blockage of the PI3K/Akt by the PI3K inhibitor, LY 294002, suppresses cGMP-induced Akt activity [37]. Pro-neuronal basic helix-loop-helix (bHLH) transcription factors, such as mammalian achaete-scute homolog 1 (Mash1) and neurogenin 1 (Ngn1) mediate neural stem cell differentiation into neurons [91, 92]. Akt activity regulates the assembly and activity of bHLH–coactivator complexes to promote neural stem cell differentiation into neurons [89]. Inhibition of the PI3K/Akt pathway in neural stem cells suppresses Mash1 and Ngn1 expression [89]. Therefore, NO/cGMP increases Akt activity, which consequently upregulates pro-neuronal transcription factors, leading to amplification of neurogenesis in ischemic brain (Fig. 1). Collectively, these data suggest that the PI3K/Akt signaling pathway plays an important role in coupling of angiogenesis and neurogenesis boosted by NO/cGMP and that neurogenesis and angiogenesis in ischemic brain act in concert to promote improvement of neurological function after treatment of stroke with NO donors and PDE5 inhibitors.
There are two types of cGMP-dependent protein kinases (PKGs), type I (PKG-1) and II (PKG-II)[93, 94]. Sildenafil suppresses cardiac hypertrophy after heart failure and regulates angiogenesis through PKG1 [95, 96] (Fig. 1). However, the role of PKG-1 and PKG-2 in mediating therapeutic effects of NO donors and PDE5 inhibitors remain poorly documented in ischemic brain.
5. Expert opinion and conclusion
Mechanisms underlying neurotoxicity of NO include formation of peroxynitrite, the reaction product from NO and excessive superoxide, and protein S-nitrosylation [22–25]. Based on these mechanisms, inhibition of nNOS and protein S-nitrosylation and antioxidant therapies by inhibition of reactive oxygen species formation have been demonstrated to reduce ischemic neuronal damage in experimental stroke [23, 24, 66, 67]. However, a clinical trial of the free radical trapping agent NXY-059 failed to achieve clinical benefit for treatment of patients with acute stroke [27, 97], whereas the clinical application of agents targeting inhibition of nNOS and protein S-nitrosylation is unknown. Analysis of preclinical studies and failed clinical trials suggests that neuroprotection alone without restoration of tissue perfusion and vascular integrity may not be effective for the treatment of acute stroke [2, 3, 5, 6]. Without adequate tissue perfusion, neuroprotective agents may not reach or will be present at a suboptimal concentration in the ischemic penumbra where damaged neurons still can be rescued within limited time period. NO increases CBF by dilating cerebral blood vessels and inhibiting platelet aggregation [98]. Preclinical studies in acute stroke show that inhalation of NO improves CBF and reduces ischemic cell damage, suggesting that prompt restoration of tissue perfusion by NO diminishes the neurotoxicity of NO in ischemic brain. A recent review of patients with ischemia/reperfusion injury induced by cardiopulmonary bypass, organ transplant, or myocardial infarction indicates that administration of NO donors is safe and reduces ischemia/reperfusion injury [99]. A clinical trial, Efficacy of Nitric Oxide in Stroke study, is currently under way to determine safety and efficacy of transdermal glyceryl trinitrate, an NO donor, in patients with acute stroke [26].
In contrast to acute stroke, during stroke recovery, interwoven restorative events are present in ischemic brain, which include angiogenesis, neurogenesis, oligodendrogenesis, astrogliosis, and neurite outgrowth. Agents used for restorative therapies given days after the onset of stroke have accesses to the target tissues via CBF. Emerging preclinical data indicate that restorative therapies targeting multiple parenchymal cells including neural stem cells, cerebral endothelial cells, astrocytes, oligodendrocytes, neurons would be more effective than agents with a single pharmacological target. Preclinical data reviewed above suggest that elevated cGMP levels by NO donors and PDE5 inhibitors act on cerebral endothelial cells and neural stem cells to enhance stroke-induced angiogenesis and neurogenesis, respectively. These interacting remodeling events collectively improve neurological function after stroke.
A case report shows that in a compassionate use application, sildenafil has evoked remarkable recovery in a locked-in patient [100]. A safety study of sildenafil (25 mg daily for 2 weeks) shortly after ischemic stroke onset has been conducted in 12 patients [101]. The study shows that sildenafil at this dose given days 2 to 9 after symptom onset is safe in patients with mild to moderately severe stroke [101]. However, due to patient recruitment issues, a dose-tiered of sildenafil clinical Phase I safety trial in stroke patients was terminated (www.clinicaltrials.gov). Based on the experimental studies showing promising restorative therapies of NO donors and PDE5 inhibitors after stroke, additional clinical trails are warranted.
Acknowledgments
This work was supported by NIH grant AG037506.
Bibliography
- 1.Zivin JA. Acute stroke therapy with tissue plasminogen activator (tPA) since it was approved by the U.S. Food and Drug Administration (FDA) Ann Neurol. 2009;66(1):6–10. doi: 10.1002/ana.21750. [DOI] [PubMed] [Google Scholar]
- 2.del Zoppo GJ. Inflammation and the neurovascular unit in the setting of focal cerebral ischemia. Neuroscience. 2009;158(3):972–82. doi: 10.1016/j.neuroscience.2008.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Armstead WM, et al. Signaling, delivery and age as emerging issues in the benefit/risk ratio outcome of tPA For treatment of CNS ischemic disorders. J Neurochem. 2010;113(2):303–12. doi: 10.1111/j.1471-4159.2010.06613.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fisher M, Bastan B. Treating acute ischemic stroke. Curr Opin Drug Discov Devel. 2008;11(5):626–32. [PubMed] [Google Scholar]
- 5.Chavez JC, et al. Pharmacologic interventions for stroke: looking beyond the thrombolysis time window into the penumbra with biomarkers, not a stopwatch. Stroke. 2009;40(10):e558–63. doi: 10.1161/STROKEAHA.109.559914. [DOI] [PubMed] [Google Scholar]
- 6.Ginsberg MD. Current status of neuroprotection for cerebral ischemia: synoptic overview. Stroke. 2009;40(3 Suppl):S111–4. doi: 10.1161/STROKEAHA.108.528877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lo EH, Dalkara T, Moskowitz MA. Mechanisms, challenges and opportunities in stroke. Nat Rev Neurosci. 2003;4(5):399–415. doi: 10.1038/nrn1106. [DOI] [PubMed] [Google Scholar]
- 8.Zhang R, et al. Nitric oxide enhances angiogenesis via the synthesis of vascular endothelial growth factor and cGMP after stroke in the rat. Circ Res. 2003;92(3):308–13. doi: 10.1161/01.res.0000056757.93432.8c. [DOI] [PubMed] [Google Scholar]
- 9.Zhang R, et al. Sildenafil (Viagra) induces neurogenesis and promotes functional recovery after stroke in rats. Stroke. 2002;33(11):2675–80. doi: 10.1161/01.str.0000034399.95249.59. [DOI] [PubMed] [Google Scholar]
- 10.Chen J, et al. Statins induce angiogenesis, neurogenesis, and synaptogenesis after stroke. Ann Neurol. 2003;53(6):743–51. doi: 10.1002/ana.10555. [DOI] [PubMed] [Google Scholar]
- 11.Lu D, et al. Atorvastatin reduces neurological deficit and increases synaptogenesis, angiogenesis, and neuronal survival in rats subjected to traumatic brain injury. J Neurotrauma. 2004;21(1):21–32. doi: 10.1089/089771504772695913. [DOI] [PubMed] [Google Scholar]
- 12.Shyu WC, et al. Functional recovery of stroke rats induced by granulocyte colony-stimulating factor-stimulated stem cells. Circulation. 2004;110(13):1847–54. doi: 10.1161/01.CIR.0000142616.07367.66. [DOI] [PubMed] [Google Scholar]
- 13.Wang L, et al. Treatment of stroke with erythropoietin enhances neurogenesis and angiogenesis and improves neurological function in rats. Stroke. 2004;35(7):1732–7. doi: 10.1161/01.STR.0000132196.49028.a4. [DOI] [PubMed] [Google Scholar]
- 14.Jin K, et al. Post-ischemic administration of heparin-binding epidermal growth factor-like growth factor (HB-EGF) reduces infarct size and modifies neurogenesis after focal cerebral ischemia in the rat. J Cereb Blood Flow Metab. 2004;24(4):399–408. doi: 10.1097/00004647-200404000-00005. [DOI] [PubMed] [Google Scholar]
- 15.Taguchi A, et al. Administration of CD34+ cells after stroke enhances neurogenesis via angiogenesis in a mouse model. J Clin Invest. 2004;114(3):330–8. doi: 10.1172/JCI20622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Willing AE, et al. Mobilized peripheral blood cells administered intravenously produce functional recovery in stroke. Cell Transplant. 2003;12(4):449–54. doi: 10.3727/000000003108746885. [DOI] [PubMed] [Google Scholar]
- 17.Garthwaite J, et al. Potent and selective inhibition of nitric oxide-sensitive guanylyl cyclase by 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one. Mol Pharmacol. 1995;48(2):184–8. [PubMed] [Google Scholar]
- 18.Dirnagl U, Iadecola C, Moskowitz MA. Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci. 1999;22(9):391–7. doi: 10.1016/s0166-2236(99)01401-0. [DOI] [PubMed] [Google Scholar]
- 19.Corbin JD, Francis SH. Cyclic GMP phosphodiesterase-5: target of sildenafil. J Biol Chem. 1999;274(20):13729–32. doi: 10.1074/jbc.274.20.13729. [DOI] [PubMed] [Google Scholar]
- 20.Bednar MM. The role of sildenafil in the treatment of stroke. Curr Opin Investig Drugs. 2008;9(7):754–9. [PubMed] [Google Scholar]
- 21.Terpolilli NA, Moskowitz MA, Plesnila N. Nitric oxide: considerations for the treatment of ischemic stroke. J Cereb Blood Flow Metab. 2012;32(7):1332–46. doi: 10.1038/jcbfm.2012.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eliasson MJ, et al. Neuronal nitric oxide synthase activation and peroxynitrite formation in ischemic stroke linked to neural damage. J Neurosci. 1999;19(14):5910–8. doi: 10.1523/JNEUROSCI.19-14-05910.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Olmez I, Ozyurt H. Reactive oxygen species and ischemic cerebrovascular disease. Neurochem Int. 2012;60(2):208–12. doi: 10.1016/j.neuint.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 24.Shi ZQ, et al. S-nitrosylated SHP-2 contributes to NMDA receptor-mediated excitotoxicity in acute ischemic stroke. Proc Natl Acad Sci U S A. 2013;110(8):3137–42. doi: 10.1073/pnas.1215501110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stamler JS. Redox signaling: nitrosylation and related target interactions of nitric oxide. Cell. 1994;78(6):931–6. doi: 10.1016/0092-8674(94)90269-0. [DOI] [PubMed] [Google Scholar]
- 26.Glyceryl trinitrate vs. control, and continuing vs. stopping temporarily prior antihypertensive therapy, in acute stroke: rationale and design of the Efficacy of Nitric Oxide in Stroke (ENOS) trial (ISRCTN99414122) Int J Stroke. 2006;1(4):245–9. doi: 10.1111/j.1747-4949.2006.00059.x. [DOI] [PubMed] [Google Scholar]
- 27.Amaro S, Chamorro A. Translational stroke research of the combination of thrombolysis and antioxidant therapy. Stroke. 2011;42(5):1495–9. doi: 10.1161/STROKEAHA.111.615039. [DOI] [PubMed] [Google Scholar]
- 28.Zhang ZG, et al. VEGF enhances angiogenesis and promotes blood-brain barrier leakage in the ischemic brain. J Clin Invest. 2000;106(7):829–838. doi: 10.1172/JCI9369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krupinski J, et al. Role of angiogenesis in patients with cerebral ischemic stroke. Stroke. 1994;25(9):1794–8. doi: 10.1161/01.str.25.9.1794. [DOI] [PubMed] [Google Scholar]
- 30.Garcia J, Cox J, Hudgins W. Ultrastructure of the microvasculature in experimental cerebral infarction. Acta Neuropathol. 1971;18(4):273–285. doi: 10.1007/BF00688441. [DOI] [PubMed] [Google Scholar]
- 31.Zhang ZG, et al. Correlation of VEGF and Angiopoietin Expression With Disruption of Blood-Brain Barrier and Angiogenesis After Focal Cerebral Ischemia. J Cereb Blood Flow Metab. 2002;22(4):379–392. doi: 10.1097/00004647-200204000-00002. [DOI] [PubMed] [Google Scholar]
- 32.Thored P, et al. Long-term neuroblast migration along blood vessels in an area with transient angiogenesis and increased vascularization after stroke. Stroke. 2007;38(11):3032–9. doi: 10.1161/STROKEAHA.107.488445. [DOI] [PubMed] [Google Scholar]
- 33.Zhang L, et al. Tadalafil, a long-acting type 5 phosphodiesterase isoenzyme inhibitor, improves neurological functional recovery in a rat model of embolic stroke. Brain Res. 2006 doi: 10.1016/j.brainres.2006.08.028. [DOI] [PubMed] [Google Scholar]
- 34.DiNapoli VA, et al. Early disruptions of the blood-brain barrier may contribute to exacerbated neuronal damage and prolonged functional recovery following stroke in aged rats. Neurobiol Aging. 2008;29(5):753–64. doi: 10.1016/j.neurobiolaging.2006.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tanne D, et al. Intravenous tissue plasminogen activator for acute ischemic stroke in patients aged 80 years and older : the tPA stroke survey experience. Stroke. 2000;31(2):370–5. doi: 10.1161/01.str.31.2.370. [DOI] [PubMed] [Google Scholar]
- 36.Manolio TA, et al. Short-term predictors of incident stroke in older adults. The Cardiovascular Health Study. Stroke. 1996;27(9):1479–86. doi: 10.1161/01.str.27.9.1479. [DOI] [PubMed] [Google Scholar]
- 37.Wang L, et al. Activation of the PI3-K/Akt pathway mediates cGMP enhanced-neurogenesis in the adult progenitor cells derived from the subventricular zone. J Cereb Blood Flow Metab. 2005;25(9):1150–8. doi: 10.1038/sj.jcbfm.9600112. [DOI] [PubMed] [Google Scholar]
- 38.Beck H, Plate KH. Angiogenesis after cerebral ischemia. Acta Neuropathol. 2009;117(5):481–96. doi: 10.1007/s00401-009-0483-6. [DOI] [PubMed] [Google Scholar]
- 39.Petcu EB, et al. Angiogenesis in old-aged subjects after ischemic stroke: a cautionary note for investigators. J Angiogenes Res. 2010;2:26. doi: 10.1186/2040-2384-2-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ding G, et al. Longitudinal magnetic resonance imaging of sildenafil treatment of embolic stroke in aged rats. Stroke. 2011;42(12):3537–41. doi: 10.1161/STROKEAHA.111.622092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang ZG, et al. Bone marrow-derived endothelial progenitor cells participate in cerebral neovascularization after focal cerebral ischemia in the adult mouse. Circ Res. 2002;90(3):284–8. doi: 10.1161/hh0302.104460. [DOI] [PubMed] [Google Scholar]
- 42.Sahara M, et al. A phosphodiesterase-5 inhibitor vardenafil enhances angiogenesis through a protein kinase G-dependent hypoxia-inducible factor-1/vascular endothelial growth factor pathway. Arterioscler Thromb Vasc Biol. 2010;30(7):1315–24. doi: 10.1161/ATVBAHA.109.201327. [DOI] [PubMed] [Google Scholar]
- 43.Zhang Z, Chopp M. Vascular endothelial growth factor and angiopoietins in focal cerebral ischemia. Trends Cardiovasc Med. 2002;12(2):62–6. doi: 10.1016/s1050-1738(01)00149-9. [DOI] [PubMed] [Google Scholar]
- 44.Jiang Q, et al. Investigation of neural progenitor cell induced angiogenesis after embolic stroke in rat using MRI. Neuroimage. 2005;28(3):698–707. doi: 10.1016/j.neuroimage.2005.06.063. [DOI] [PubMed] [Google Scholar]
- 45.Ding G, et al. Angiogenesis detected after embolic stroke in rat brain using magnetic resonance T2*WI. Stroke. 2008;39(5):1563–8. doi: 10.1161/STROKEAHA.107.502146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li L, et al. Angiogenesis and improved cerebral blood flow in the ischemic boundary area detected by MRI after administration of sildenafil to rats with embolic stroke. Brain Res. 2007;1132(1):185–92. doi: 10.1016/j.brainres.2006.10.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Slevin M, et al. Serial measurement of vascular endothelial growth factor and transforming growth factor-beta1 in serum of patients with acute ischemic stroke. Stroke. 2000;31(8):1863–70. doi: 10.1161/01.str.31.8.1863. [DOI] [PubMed] [Google Scholar]
- 48.Krupinski J, et al. Prognostic value of blood vessel density in ischaemic stroke [letter; comment] Lancet. 1993;342(8873):742. doi: 10.1016/0140-6736(93)91734-4. [DOI] [PubMed] [Google Scholar]
- 49.Ohab JJ, et al. A neurovascular niche for neurogenesis after stroke. J Neurosci. 2006;26(50):13007–16. doi: 10.1523/JNEUROSCI.4323-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Alvarez-Buylla A, Lim DA. For the long run: maintaining germinal niches in the adult brain. Neuron. 2004;41(5):683–6. doi: 10.1016/s0896-6273(04)00111-4. [DOI] [PubMed] [Google Scholar]
- 51.Carreira BP, et al. Nitric oxide stimulates the proliferation of neural stem cells bypassing the epidermal growth factor receptor. Stem Cells. 2010;28(7):1219–30. doi: 10.1002/stem.444. [DOI] [PubMed] [Google Scholar]
- 52.Gomez-Pinedo U, et al. cGMP modulates stem cells differentiation to neurons in brain in vivo. Neuroscience. 2010;165(4):1275–83. doi: 10.1016/j.neuroscience.2009.11.046. [DOI] [PubMed] [Google Scholar]
- 53.Tegenge MA, et al. Nitric oxide stimulates human neural progenitor cell migration via cGMP-mediated signal transduction. Cell Mol Life Sci. 2011;68(12):2089–99. doi: 10.1007/s00018-010-0554-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Morshead CM, Craig CG, van der Kooy D. In vivo clonal analyses reveal the properties of endogenous neural stem cell proliferation in the adult mammalian forebrain. Development. 1998;125(12):2251–61. doi: 10.1242/dev.125.12.2251. [DOI] [PubMed] [Google Scholar]
- 55.Zhang RL, Zhang ZG, Chopp M. Ischemic stroke and neurogenesis in the subventricular zone. Neuropharmacology. 2008;55(3):345–52. doi: 10.1016/j.neuropharm.2008.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jin K, et al. Evidence for stroke-induced neurogenesis in the human brain. Proc Natl Acad Sci U S A. 2006;103(35):13198–202. doi: 10.1073/pnas.0603512103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Macas J, et al. Increased generation of neuronal progenitors after ischemic injury in the aged adult human forebrain. J Neurosci. 2006;26(50):13114–9. doi: 10.1523/JNEUROSCI.4667-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Minger SL, et al. Endogenous neurogenesis in the human brain following cerebral infarction. Regen Med. 2007;2(1):69–74. doi: 10.2217/17460751.2.1.69. [DOI] [PubMed] [Google Scholar]
- 59.Curtis MA, Kam M, Faull RL. Neurogenesis in humans. Eur J Neurosci. 2011;33(6):1170–4. doi: 10.1111/j.1460-9568.2011.07616.x. [DOI] [PubMed] [Google Scholar]
- 60.Alvarez-Buylla A, et al. The heterogeneity of adult neural stem cells and the emerging complexity of their niche. Cold Spring Harb Symp Quant Biol. 2008;73:357–65. doi: 10.1101/sqb.2008.73.019. [DOI] [PubMed] [Google Scholar]
- 61.Zhang R, et al. A nitric oxide donor induces neurogenesis and reduces functional deficits after stroke in rats. Ann Neurol. 2001;50(5):602–11. doi: 10.1002/ana.1249. [DOI] [PubMed] [Google Scholar]
- 62.Chen J, et al. Combination therapy of stroke in rats with a nitric oxide donor and human bone marrow stromal cells enhances angiogenesis and neurogenesis. Brain Res. 2004;1005(1–2):21–8. doi: 10.1016/j.brainres.2003.11.080. [DOI] [PubMed] [Google Scholar]
- 63.Packer MA, et al. Nitric oxide negatively regulates mammalian adult neurogenesis. Proc Natl Acad Sci U S A. 2003;100(16):9566–71. doi: 10.1073/pnas.1633579100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Moreno-Lopez B, et al. Nitric oxide is a physiological inhibitor of neurogenesis in the adult mouse subventricular zone and olfactory bulb. J Neurosci. 2004;24(1):85–95. doi: 10.1523/JNEUROSCI.1574-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Luo CX, et al. Bidirectional regulation of neurogenesis by neuronal nitric oxide synthase derived from neurons and neural stem cells. Stem Cells. 2010;28(11):2041–52. doi: 10.1002/stem.522. [DOI] [PubMed] [Google Scholar]
- 66.Sun Y, et al. Neuronal nitric oxide synthase and ischemia-induced neurogenesis. J Cereb Blood Flow Metab. 2005;25(4):485–92. doi: 10.1038/sj.jcbfm.9600049. [DOI] [PubMed] [Google Scholar]
- 67.Luo CX, et al. Reduced neuronal nitric oxide synthase is involved in ischemia-induced hippocampal neurogenesis by up-regulating inducible nitric oxide synthase expression. J Neurochem. 2007;103(5):1872–82. doi: 10.1111/j.1471-4159.2007.04915.x. [DOI] [PubMed] [Google Scholar]
- 68.Chen J, et al. Endothelial nitric oxide synthase regulates brain-derived neurotrophic factor expression and neurogenesis after stroke in mice. J Neurosci. 2005;25(9):2366–75. doi: 10.1523/JNEUROSCI.5071-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhu DY, et al. Expression of inducible nitric oxide synthase after focal cerebral ischemia stimulates neurogenesis in the adult rodent dentate gyrus. J Neurosci. 2003;23(1):223–9. doi: 10.1523/JNEUROSCI.23-01-00223.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang RL, et al. Delayed treatment with sildenafil enhances neurogenesis and improves functional recovery in aged rats after focal cerebral ischemia. J Neurosci Res. 2006;83(7):1213–9. doi: 10.1002/jnr.20813. [DOI] [PubMed] [Google Scholar]
- 71.Zhang RL, et al. Sildenafil enhances neurogenesis and oligodendrogenesis in ischemic brain of middle-aged mouse. PLoS ONE. 2012;7(10):e48141. doi: 10.1371/journal.pone.0048141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sun F, et al. Ablation of neurogenesis attenuates recovery of motor function after focal cerebral ischemia in middle-aged mice. PLoS ONE. 2012;7(10):e46326. doi: 10.1371/journal.pone.0046326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang X, et al. Conditional depletion of neurogenesis inhibits long-term recovery after experimental stroke in mice. PLoS ONE. 2012;7(6):e38932. doi: 10.1371/journal.pone.0038932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ueno Y, et al. Axonal outgrowth and dendritic plasticity in the cortical peri-infarct area after experimental stroke. Stroke. 2012;43(8):2221–8. doi: 10.1161/STROKEAHA.111.646224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang L, et al. Erythropoietin amplifies stroke-induced oligodendrogenesis in the rat. PLoS ONE. 2010;5(6):e11016. doi: 10.1371/journal.pone.0011016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Carmichael ST. Cellular and molecular mechanisms of neural repair after stroke: making waves. Ann Neurol. 2006;59(5):735–42. doi: 10.1002/ana.20845. [DOI] [PubMed] [Google Scholar]
- 77.Shen Q, et al. Adult SVZ stem cells lie in a vascular niche: a quantitative analysis of niche cell-cell interactions. Cell Stem Cell. 2008;3(3):289–300. doi: 10.1016/j.stem.2008.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tavazoie M, et al. A specialized vascular niche for adult neural stem cells. Cell Stem Cell. 2008;3(3):279–88. doi: 10.1016/j.stem.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Palmer TD, Willhoite AR, Gage FH. Vascular niche for adult hippocampal neurogenesis. J Comp Neurol. 2000;425(4):479–94. doi: 10.1002/1096-9861(20001002)425:4<479::aid-cne2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 80.Morris DC, et al. Measurement of cerebral microvessel diameters after embolic stroke in rat using quantitative laser scanning confocal microscopy. Brain Res. 2000;876(1–2):31–6. doi: 10.1016/s0006-8993(00)02543-9. [DOI] [PubMed] [Google Scholar]
- 81.Zhang ZG, et al. Up-regulation of neuropilin-1 in neovasculature after focal cerebral ischemia in the adult rat. J Cereb Blood Flow Metab. 2001;21(5):541–9. doi: 10.1097/00004647-200105000-00008. [DOI] [PubMed] [Google Scholar]
- 82.Lin TN, et al. Induction of angiopoietin and Tie receptor mRNA expression after cerebral ischemia-reperfusion. J Cereb Blood Flow Metab. 2000;20(2):387–95. doi: 10.1097/00004647-200002000-00021. [DOI] [PubMed] [Google Scholar]
- 83.Jin K, et al. Vascular endothelial growth factor (VEGF) stimulates neurogenesis in vitro and in vivo. Proc Natl Acad Sci U S A. 2002;99(18):11946–50. doi: 10.1073/pnas.182296499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Teng H, et al. Coupling of angiogenesis and neurogenesis in cultured endothelial cells and neural progenitor cells after stroke. J Cereb Blood Flow Metab. 2008;28(4):764–71. doi: 10.1038/sj.jcbfm.9600573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang L, et al. Neural progenitor cells treated with EPO induce angiogenesis through the production of VEGF. J Cereb Blood Flow Metab. 2008;28(7):1361–8. doi: 10.1038/jcbfm.2008.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Roitbak T, Li L, Cunningham LA. Neural stem/progenitor cells promote endothelial cell morphogenesis and protect endothelial cells against ischemia via HIF-1alpha-regulated VEGF signaling. J Cereb Blood Flow Metab. 2008;28(9):1530–42. doi: 10.1038/jcbfm.2008.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Liu XS, et al. Stroke induces gene profile changes associated with neurogenesis and angiogenesis in adult subventricular zone progenitor cells. J Cereb Blood Flow Metab. 2007;27(3):564–74. doi: 10.1038/sj.jcbfm.9600371. [DOI] [PubMed] [Google Scholar]
- 88.Katakowski M, et al. Phosphoinositide 3-kinase promotes adult subventricular neuroblast migration after stroke. J Neurosci Res. 2003;74(4):494–501. doi: 10.1002/jnr.10775. [DOI] [PubMed] [Google Scholar]
- 89.Vojtek AB, et al. Akt regulates basic helix-loop-helix transcription factor-coactivator complex formation and activity during neuronal differentiation. Mol Cell Biol. 2003;23(13):4417–27. doi: 10.1128/MCB.23.13.4417-4427.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kawasaki K, et al. Activation of the phosphatidylinositol 3-kinase/protein kinase Akt pathway mediates nitric oxide-induced endothelial cell migration and angiogenesis. Mol Cell Biol. 2003;23(16):5726–37. doi: 10.1128/MCB.23.16.5726-5737.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kageyama R, et al. Roles of bHLH genes in neural stem cell differentiation. Exp Cell Res. 2005;306(2):343–8. doi: 10.1016/j.yexcr.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 92.Parras CM, et al. Mash1 specifies neurons and oligodendrocytes in the postnatal brain. Embo J. 2004;23(22):4495–505. doi: 10.1038/sj.emboj.7600447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Schlossmann J, Feil R, Hofmann F. Signaling through NO and cGMP-dependent protein kinases. Ann Med. 2003;35(1):21–7. doi: 10.1080/07853890310004093. [DOI] [PubMed] [Google Scholar]
- 94.Feil R, et al. Cyclic GMP-dependent protein kinases and the cardiovascular system: insights from genetically modified mice. Circ Res. 2003;93(10):907–16. doi: 10.1161/01.RES.0000100390.68771.CC. [DOI] [PubMed] [Google Scholar]
- 95.Takimoto E, et al. Chronic inhibition of cyclic GMP phosphodiesterase 5A prevents and reverses cardiac hypertrophy. Nat Med. 2005;11(2):214–22. doi: 10.1038/nm1175. [DOI] [PubMed] [Google Scholar]
- 96.Pyriochou A, et al. The phosphodiesterase 5 inhibitor sildenafil stimulates angiogenesis through a protein kinase G/MAPK pathway. J Cell Physiol. 2007;211(1):197–204. doi: 10.1002/jcp.20929. [DOI] [PubMed] [Google Scholar]
- 97.Feuerstein GZ, et al. Missing steps in the STAIR case: a Translational Medicine perspective on the development of NXY-059 for treatment of acute ischemic stroke. J Cereb Blood Flow Metab. 2008;28(1):217–9. doi: 10.1038/sj.jcbfm.9600516. [DOI] [PubMed] [Google Scholar]
- 98.Madden JA. Role of the vascular endothelium and plaque in acute ischemic stroke. Neurology. 2012;79(13 Suppl 1):S58–62. doi: 10.1212/WNL.0b013e3182695836. [DOI] [PubMed] [Google Scholar]
- 99.Roberts BW, et al. Nitric oxide donor agents for the treatment of ischemia/reperfusion injury in human subjects: a systematic review. Shock. 2013;39(3):229–39. doi: 10.1097/SHK.0b013e31827f565b. [DOI] [PubMed] [Google Scholar]
- 100.Silver B, et al. Recovery in a patient with locked-in syndrome. Can J Neurol Sci. 2006;33(2):246–9. doi: 10.1017/s0317167100005084. [DOI] [PubMed] [Google Scholar]
- 101.Silver B, et al. Sildenafil treatment of subacute ischemic stroke: a safety study at 25-mg daily for 2 weeks. J Stroke Cerebrovasc Dis. 2009;18(5):381–3. doi: 10.1016/j.jstrokecerebrovasdis.2009.01.007. [DOI] [PubMed] [Google Scholar]


