Abstract
Objective
In vitro data and pilot data suggest that green tea catechins may possess chemopreventive activity for cervical cancer and precursor lesions. We conducted a randomized, double-blind, placebo controlled trial of Polyphenon E (decaffeinated and enriched green tea catechin extract) in women with persistent human papillomavirus (HPV) infection and low grade cervical intraepithelial neoplasia (CIN1) to evaluate the potential of Polyphenon E for cervical cancer prevention.
Methods
Ninety-eight eligible women were randomized to receive either Polyphenon E (containing 800 mg epigallocatechin gallate) or placebo once daily for 4 months. The primary study outcome was oncogenic HPV clearance and clearance of CIN1.
Results
Polyphenon E was shown to be acceptable, safe and well tolerated. There was no difference in the response rate by treatment allocation. Complete response, defined as negative for high risk HPV and normal histopathology, was noted in 7 (17.1%) and 6 (14.6%) women in the Polyphenon E and placebo arms, respectively. Progression, defined as persistent oncogenic HPV with histopathologic evidence of progression, was more common in the Polyphenon E group than in the placebo group [6 (14.6%) vs. 3 (7.7%)].
Conclusion
Based on the largest randomized placebo-controlled trial of a green tea extract for HPV related cervical disease, we conclude that four months of Polyphenon E intervention did not promote the clearance of persistent high risk HPVand related CIN 1. Further studies may be necessary to better delineate the risk factors for persistent HPV infection and biology of the disease to facilitate the evaluation of chemopreventive strategies.
Keywords: Polyphenon E, cervical intraepithelial neoplasia, HPV infection
Introduction
Cervical cancer is the most common gynecologic malignancy in women worldwide with an estimated annual incidence of 470,000 cases [1]. Persistent high-risk human papillomavirus (HPV) infection is a requisite precursor to the development of nearly all (>99%) of pre-malignant (high grade cervical intraepithelial neoplasia) and invasive carcinomas of the cervix. The recent introduction of virus-like particle prophylactic HPV vaccination provides an effective primary prevention strategy for squamous and adenomatous malignancies of the cervix. Prophylactic vaccines however are type-specific (16/18 and 16/18/6/11, respectively) and relatively expensive making them inaccessible to many domestic and developing world underserved populations. Though recent encouraging results from the National Health and Nutrition Examination Survey (NHANES) indicate that within four years of HPV vaccination introduction there has been a 56% decrease in vaccine-type HPV prevalence among females age 14–19 years[2], there has not been a decrease in HPV prevalence among older age groups, and current bivalent and quadrivalent prophylactic type restricted vaccines leave nearly a third of high-risk oncogenic strains without a viable primary prevention strategy. A range of non-surgical treatments have been evaluated for the treatment of cervical cancer precursor lesions and prevention of cervical cancer, but the results of these studies have been clinically inconclusive and/or have been associated with safety concerns.
In vitro studies have suggested that green tea catechins may exert chemopreventive activity for cervical cancer. We have shown that epigallocatechin gallate (EGCG, a major green tea catechin) and Polyphenon E (a decaffeinated, enriched, and defined mixture of green tea catechins) inhibited the growth of HPV-positive cervical cancer cells and HPV-immortalized cervical epithelial cells in a dose dependent fashion [3]. Induction of apoptosis and cell cycle arrest was observed in EGCG and Polyphenon E treated cells. Apoptosis-related proteins, p53 and p21 showed dose dependent increase while p27 was not affected. HPV-E7 protein expression was decreased with green tea catechin treatment. In addition, green tea catechins or EGCG has been shown to inhibit epidermal growth factor receptor activation [4], telomerase activity [5], phosphatidylinosital 3-kinase/Akt and extracellular signal-regulated kinase 1/2 signaling pathways [6] in HR HPV immortalized cervical epithelial cells or cervical cancer cells which lead to growth cession.
Preliminary clinical efficacy of green tea extracts (Polyphenon E or EGCG) in patients with HPV infected cervical lesions has been reported [7]. Fifty-one patients with various degrees of cervical dysplasia (mild, moderate, and severe) were divided to receive oral Polyphenon E capsules (containing 200 mg EGCG), EGCG capsules (200 mg), vaginal Polyphenon E ointment, or a combination of oral and vaginal Polyphenon E. Following 8 to 12 weeks of intervention, 23 of 33 subjects receiving either oral or vaginal Polyphenon E demonstrated regression of cervical dysplasia, with a trend toward clearance of HPV. Clinical efficacy of topical sinecatechins, a defined green tea extract, in the treatment of external genital and perianal warts has been documented and topical green tea therapy is now commercially available for treatment of condyloma.[8]
Based on these preliminary findings, we conducted a randomized, double-blind, placebo controlled clinical trial of oral Polyphenon E in women with persistent high risk HPV (HR HPV) infection and concomitant low grade cervical intraepithelial neoplasia (CIN1) to evaluate the potentials of Polyphenon E for cervical cancer prevention. We hypothesized that oral Polyphenon E intervention would induce the histologic regression of CIN1 by promoting the clearance on oncogenic HPV.
Materials and Methods
Study Drugs
Polyphenon E contains 85–95% total catechins, with 56–72% as EGCG. The agent was provided to National Cancer Institute (IND number 58,367), Division of Cancer Prevention (NCI, DCP) by Mitsui Norin. This study used Polyphenon E oral capsules, standardized to contain 200 mg EGCG per capsule, and matched placebo capsules, supplied by NCI, DCP. Study capsules were stored at room temperature and protected from environmental extremes.
Study Population
The study was conducted primarily at the University of Arizona (Tucson, Arizona), with additional accrual at Maricopa Integrated Health System (Phoenix, Arizona), and Southern Pines Women’s Health Center (Southern Pines, North Carolina). All potential participants were identified by participating community clinicians, who provided documentation of HPV, and/or cytology. In cases where oncogenic HPV status was unknown, specimens were collected to establish baseline HPV status during the pre-screening phase of the trial. Participants were required to be at least 18 years of age, had normal liver and kidney function, and good performance status. To be eligible subjects were required to be positive for HR HPV by DNA hybrid capture and have at least one biopsy with histologically documented low-grade cervical intraepithelial neoplasia (CIN) at the time of enrollment. To document persistence prior to enrollment, eligibility also required (6 to 12 months prior) a positive HR HPV on DNA hybrid capture and either LSIL cytology or histologically documented CIN1 on cervical biopsy.
Participants were excluded if they were pregnant or breast feeding, consumed tea regularly within 1 month of enrollment, had a history of allergic reaction to tea or related dietary projects, had been treated for genital condyloma within 30 days of enrollment, were receiving other investigational agents, had prior pelvic irradiation, were HIV positive, had uncontrolled intercurrent illness, had invasive or high grade intraepithelial neoplasia, or had a history of cancer except non-melanoma skin cancer. The study was approved by the Institutional Review Boards of the University of Arizona as well as the collaborating institutions. Written informed consent in English or Spanish was obtained from all participants.
Study Procedures
During the initial visit, participants underwent eligibility evaluation. Baseline HPV, cytology and histopathology data were reviewed as part of eligibility determination. Each participant underwent an interview and brief physical exam to document medical history, performance status, height, weight, blood pressure, pulse, and temperature measurements. Blood samples were collected for complete blood count with differentials and blood chemistry panel. A urine sample was collected for urine pregnancy test. Participants also underwent baseline colposcopy to confirm the presence of an evaluable low grade CIN lesion occupying at least 1 quadrant and to exclude the presence of a concurrent high grade CIN. Colposcopic biopsy with endocervical curettage was obtained if not performed within 90 days prior to enrollment. Specimens were collected for cervical cytology and HR HPV testing.
Upon determination of eligibility, participants were randomized to receive Polyphenon E or placebo. An adaptive allocation randomization procedure was implemented [9] to balance the two groups on the basis of age. Participants were instructed to take 4 study capsules each morning with food for 4 months. Participants returned to the clinic every month for the following procedures: urine collection for pregnancy test, assessment of adverse events, compliance verification with review of drug diary and pill count, and blood collection for hepatic panel.
At the end of the 4-month intervention, blood samples were collected for complete blood count with differentials and blood chemistry panel. A urine sample was collected for urine pregnancy test. All participants underwent a standardized post-intervention colposcopic evaluation with collection of endocervical curettage and collection of specimens for cervical cytology and HR HPV testing. This included biopsy of all clinically visible lesions as well as random biopsies of three of four quadrants when no lesions were visible. All histopathology specimens were reviewed in a blinded fashion by an experienced gynecologic pathologist and were subjected to a second quality control review.
The primary study outcome was HR HPV clearance and resolution of the low grade CIN at month 4. HR HPV status was determined using DNA hybrid capture testing using the high risk oncogenic probe (HC2, Hologic, Lexington Massachusetts) and performed by a commercial laboratory. Resolution of low grade CIN was based on evaluation of cervical cytology and biopsy/endocervical curettage obtained at month 4. This time frame was selected due to safety considerations and the desire to avoid disease progression.
The following criteria were established a priori for the assessment of the study outcomes: Complete Response (CR) -- clearance of HR HPV and complete colposcopic, histologic and cytologic resolution of disease; Partial Response (PR) -- clearance of HR HPV with evidence of CIN1 on cervical biopsy, endocervical curettage, or ASCUS/LSIL cytology; No Response (NR) -- persistent HR HPV positivity, with or without evidence of CIN1; Progression (PG) -- persistent HR HPV positivity, with histopathologic evidence of progression to CIN2, 3, or invasive cancer.
The study also documented the toxicity of Polyphenon E compared to placebo in this cohort. The NCI Common Terminology Criteria for Adverse Events (CTCAE) version 3.0 was used for adverse event description and grading.
Statistical Analysis
The statistical analyses were performed for this trial used the intention-to-treat principle. The sample size estimate for this trial was based on a two-sided Fisher’s exact test at a significance level of 0.05. The spontaneous regression rate for HPV at 4 months in the placebo group was estimated to be approximately 10% (based on our own work in the same study population) [10]. It was estimated that 68 participants per arm would allow for detection of a Polyphenon E related increase in the HPV clearance rate from 10% to 35%, with a power of 0.93. Based on these considerations and taking into account a potential loss to follow-up rate of 15%, we proposed enrolling 78 subjects in each arm.
Two-sample test with unequal variances was performed to compare each continuous baseline variable between groups. Fisher’s exact test was performed to compare each categorical baseline variable between groups. Fisher’s exact test was also performed to compare each of the primary outcomes, HR HPV clearance and histologic resolution, between groups. In addition, for each primary outcome, logistic regression was performed to adjust for participant’s baseline characteristics, such as age, smoking status, current sexual activity, lifetime partners, age at first intercourse and race/ethnicity.
Finally, a scheduled interim futility analysis was conducted at the midpoint of enrollment for the trial which led to early closure of the trial. Pre-specified stopping rules were that the study would be discontinued if the complete regression rate in the treatment group was equal to or less than the rate observed in the placebo group. Based on the expected rates of 10% regression in placebo and 35% regression in the treatment group and interim sample sizes of 39 subjects per group, the probability of committing type II error (i.e., halting the trial when in fact the true placebo and treatment rates are as expected) was approximately 0.004. Thus, the interim analysis has a negligible effect on overall statistical power.
Results
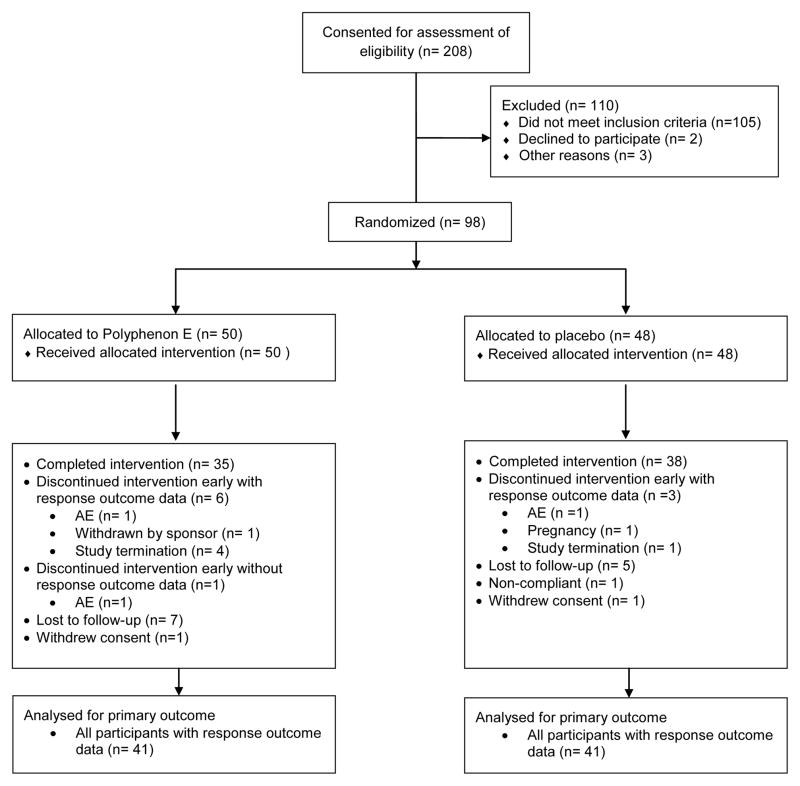
The study was closed to accrual based on the results of the protocol-specified interim futility analysis. In total 208 women were screened for eligibility at the three study centers. The screen fail rate was higher than anticipated with HPV negativity, absence of CIN and high-grade CIN as the top three screen fail reasons. Ninety-eight women met all eligibility criteria and were randomized to one of two study arms. The Consort flow diagram is shown in Figure 1.
Figure 1.
Consort flow diagram.
Of 50 participants randomized to receive Polyphenon E, 35 completed the intervention, six discontinued intervention early with response outcome data, one discontinued intervention early without response outcome data, seven were lost to follow-up, and one withdrew consent. Of the 48 participants randomized to receive placebo, 38 completed the intervention, three discontinued but had evaluable response outcome data, five were lost to follow-up, one was non-compliant with study procedures, and one withdrew consent.
Subject characteristics for the entire study population are summarized in Table 1. The age range was balanced between the two treatment groups with the mean age of 28.48 in the Polyphenon E group and 28.27 in the placebo group. The treatment groups are also well balanced in terms of smoking status, sexual history and race/ethnicity. Of note, our trial included a high proportion of Hispanic participants (45% overall).
Table 1.
Characteristics of the study population
| Variable | All Subjects (98) | Poly-E (50) | Placebo (48) | p-valuea |
|---|---|---|---|---|
| Age | 0.9020 | |||
| Range | 18–58 | 19–58 | 18–56 | |
| Mean | 28.38 | 28.48 | 28.27 | |
| Standard Deviation | 8.39 | 8.78 | 8.05 | |
| Weight(pounds) | 0.9034 | |||
| Range | 89–307 | 89–268 | 92–307 | |
| Mean | 149.29 | 149.78 | 148.77 | |
| Standard Deviation | 40.83 | 39.48 | 42.63 | |
| Height (inches) | 0.7077 | |||
| Range | 53–72 | 56–72 | 53–70 | |
| Mean | 63.69 | 63.58 | 63.81 | |
| Standard Deviation | 3.01 | 2.85 | 3.20 | |
| Race | 0.6470 | |||
| American Indian | 2 (2%) | 1 (2%) | 1 (2%) | |
| Asian | 3 (3%) | 0 | 3 (6%) | |
| African American | 5 (5%) | 3 (6%) | 2 (4%) | |
| White | 78 (80%) | 41 (82%) | 37 (77%) | |
| Other (incl. mixed) | 3 (3%) | 1 (2%) | 2 (4%) | |
| Unknown | 7 (7%) | 4 (8%) | 3 (6%) | |
| Ethnicity | 1.0000 | |||
| Hispanic | 44 (45%) | 22 (44%) | 22 (46%) | |
| Non-Hispanic | 54 (55%) | 28 (56%) | 26 (54%) | |
| Smoking status | 1.0000 | |||
| Never smoked | 79 (81%) | 40 (80%) | 39 (81%) | |
| Past smoker | 5 (5%) | 3 (6%) | 2 (4%) | |
| Current smoker | 14 (14%) | 7 (14%) | 7 (15%) | |
| Current sexual activity | 0.6317 | |||
| No | 22 (22%) | 10 (20%) | 12 (25%) | |
| Yes, one partner | 75 (77%) | 39 (78%) | 36 (75%) | |
| Yes, > one partner | 1 (1%) | 1 (2%) | 0 | |
| Age at 1st intercourse | 0.8283 | |||
| Range | 12–23 | 14–23 | 12–23 | |
| Mean | 16.7 | 16.8 | 16.7 | |
| Standard deviation | 2.26 | 2.20 | 2.34 | |
| Total lifetime partners | 0.0890 | |||
| Range | 1–50 | 1–50 | 1–20 | |
| Mean | 7.5 | 8.7 | 6.3 | |
| Standard Deviation | 7.01 | 8.61 | 4.71 |
based on a two-sample t-test with unequal variances for continuous variables and a Fisher’s exact test for categorical variables.
Table 2 summarizes the response outcome data by treatment group. In keeping with an intent-to-treat analysis, all participants with response outcome data (n= 41 in each arm) were included in the analysis. There was no difference in response rate by treatment allocation. Complete response, that is a negative HR HPV and normal histopathology, was noted in 7 (17.1%) and 6 (14.6%) for the treatment and placebo arms respectively. Partial response occurred more frequently in the placebo group and progression was more common in the treatment group, although neither of these findings were statistically significant. A logistic regression analysis which accounted for treatment group, age, smoking status, current sexual activity, lifetime partners, age at first intercourse and race/ethnicity failed to reveal any treatment effect when these variables were considered (data not shown). In addition, analyses to assess differences between treatment and placebo arms if grouped by ages under and over 30 showed no significant differences (data not shown).
Table 2.
Summary of response outcomes by treatment group designation
| Group | Progression (P) | No Response (NR) | Partial Response (PR) | Complete Response (CR) | Total |
|---|---|---|---|---|---|
| Poly-E | 6 (14.6%) | 27 (65.9%) | 1 (2.4%) | 7 (17.1%) | 41 |
| Placebo | 3 (7.7%) | 26 (63.4%) | 6 (14.6%) | 6 (14.6%) | 41 |
The p-value is 0.21 based on a Fisher’s exact test.
HPV outcome data by treatment group included all participants with HPV outcome data (n= 41 in each arm) in the analysis (Table 3). Likewise, end of study HR HPV positive status was found to be similar between the two groups with 31 (75.6%) in the Polyphenon E group and 29 (70.7%) in the placebo arm (p=0.80), and was not modified by logistic regression analysis that accounted for co-variables (data not shown).
Table 3.
HPV outcomes by treatment group
| Group | Negative | Positive | Total |
|---|---|---|---|
| Poly-E | 10 (24.4%) | 31 (75.6%) | 41 |
| Placebo | 12 (29.3%) | 29 (70.7%) | 41 |
The p-value is 0.80 based on a Fisher’s exact test.
All participants with histological outcome data (n= 41 in the Polyphenon E arm; n= 39 in the placebo arm) were included in the analysis of histological outcomes by treatment group (Table 4). The histological outcomes were similar for the Polyphenon E and placebo groups. More than half of all subjects had normal histology at the conclusion of the trial regardless of treatment group allocation. There was a statistically non-significant histological progression to CIN 2 in the Polyphenon E group with 7 cases (17.1%) compared to 3 cases (7.7%) in the placebo.
Table 4.
Histological outcomes by treatment group allocation
| Group | Normal | CIN1 | CIN2 | Total |
|---|---|---|---|---|
| Poly-E | 21 (51.2%) | 13 (31.7%) | 7 (17.1%) | 41 |
| Placebo | 21 (53.9%) | 16 (38.5%) | 3 (7.7%) | 39 |
p-value= 0.47 based on a Fisher’s exact test.
In general, the study agent was well tolerated by study participants. Medication adherence, as measured by pill count, was found to be excellent across the two groups, and was further substantiated by urinary catechin metabolite testing, with the average urinary EGC concentration measured at 652 ng/ml in the Polyphenon E arm compared to 59 ng/ml in the placebo arm. There were a total of 163 and 136 adverse events in the placebo and Polyphenon E groups, respectively. Two serious adverse events occurred in one participant in the placebo arm with both events attributed to changes in seizure and anxiety medications. Two participants in the Polyphenon E group discontinued the study early due to adverse events (one for gastrointestinal related events and the other for ALT and AST elevation). One participant in the placebo group discontinued intervention early due to adverse events (for persistent ALT and AST elevation).
Adverse events occurring in greater than 5 percent of subjects treated with Polyphenon E or placebo, regardless of attribution, were documented. These were all Grade 1 or Grade 2 events, except one Grade 3 ALT elevation and one Grade 3 back pain in the Polyphenon E arm and one Grade 3 ALT and AST elevation in the placebo arm. Nausea was reported more frequently by subjects receiving Polyphenon E compared to those receiving placebo, 32.0% vs. 18.8%, respectively. Other AEs that occurred more frequently in the Polyphenon E arm than that in the placebo arm include vomiting (6.0% vs. 2.1%), cervicitis (12.0% vs. 6.3%), sinus infection (6.0% vs. 0%), dizziness (14.0% vs. 6.3%), back pain (6.0% vs. 4.2%), stomach ache (12.0% vs. 8.3%), upper respiratory conditions related to cold symptoms (8.0% vs. 6.3%), and irregular menses (6.0% vs. 4.2%). In addition, elevated ALT was noted in 10.0% of subjects in the Polyphenon E group compared to 2.1% in the placebo group. Elevated AST was noted in 8.0% of subjects in the Polyphenon E group compared to 2.1% in the placebo group. It should be noted that in all cases the liver function tests returned to baseline after discontinuation of study agent, although in one case this did not occur until approximately 6 months post discontinuation. A summary of adverse events is provided in Supplemental Table 2.
Discussion
The search for chemopreventive agents for use in cervical cancer prevention is logical given the well characterized, protracted preinvasive character of CIN, encouraging preclinical laboratory data, and epidemiologic findings that suggest a protective role for a variety of nutritional agents. These chemoprevention studies, however, have been largely disappointing and plagued by a variety of methodological challenges. Problems include a lack of consensus as to the appropriate grade of disease to be studied (CIN 1, 2 or 3), the appropriate definition of response (e.g. histologic regression, viral clearance) given significant rates of spontaneous regression, the selection of appropriate endpoint biomarkers, as well as ethical and safety considerations (withholding treatment for potentially preinvasive disease).
Despite these challenges, a significant number of trials have been conducted in this area over the past 30 years. Investigators have long shown great interest in looking at the role of micronutrients and pharmaceutical interventions in cervical cancer prevention. Although some success has been found in animal models, most have shown mixed results in human trials. In our review of 22 combined clinical trials (Supplemental Table 1) that assessed a variety of interventions including antioxidants, suicide inhibitors of ornithine decarboxylase, retinoids, and micronutrients. There was no consistent clinical effect for any of these interventions. Chemoprevention trials conducted among women with high grade cervical intraepithelial neoplasia using retinoids [11], β-carotene [12], difluoromethylornithine [13], indole-3-carbinol [14], amilogene[15], and cyclooxygenase inhibitors have been largely inconclusive and were associated with patient acceptability concerns.
Green tea, green tea extract, green tea catechins, and EGCG have been shown to inhibit carcinogenesis induced by a wide variety of carcinogens in rodent cancer models. The principal components in green tea are catechins (30–42%) and flavonols (5–10%). The major catechins are (-)-epicatechin (EC), (-)-epicatechin gallate (ECG), (-)-epigallocatechin (EGC), and (-)-epigallocatechin gallate (EGCG), with EGCG being the most abundant. Tea is one of the most widely consumed beverages worldwide, although utilization varies greatly in different populations. Tea is manufactured in three basic forms: black, green, or oolong, which differ mostly in the degree of oxidation during production. Additionally, green teas relative low cost makes it an attractive agent for chemoprevention in low resource settings.
In this study, we sought to investigate the effect of Polyphenon E in women with persistent HR HPV infection and concomitant CIN1 to evaluate the potential of Polyphenon E for cervical cancer prevention. This was based on promising pre-clinical data showing that green tea, green tea extract, green tea catechins, and EGCG inhibit carcinogens; and on preliminary clinical data showing regression of cervical dysplasia with Polyphenon E. In addition, potential advantages for Polyphenon E as a chemopreventive agent are a presumed high patient acceptability and safety profile.
We have now conducted the largest randomized placebo-controlled trial of a green tea extract for HPV related cervical disease. Definitive results show that Polyphenon E is acceptable, safe and well tolerated. However, despite the promising in vitro data and the pilot clinical data, in our study, Polyphenon E was not shown to promote the resolution of persistent HR HPV and related CIN 1. However, perhaps intervention at different points in the development of SIL/cancer may yield different results. Since primary HPV screening is done now over age 30, intervening early with HR HPV positive cases with no SIL, or HPV genotyping may show that HPV 16/18 have different response to these kinds of treatments than other HR HPV. In addition, intervening as an adjuvant to established treatment of HSIL may have improved outcomes and decreased progression. The development of other molecular markers of progression may also identify specific populations that would be responders or non-responders to these novel chemopreventive treatments.
There are several limitations and strengths to our study that should be noted. One limitation is the length of follow-up of 4 months for our study, which was selected due to concern about a small but real risk for disease progression. Even though all participants had documented persistent HPV infections, the duration of the persistence could potentially be incidental. However, this should have been accounted for by the randomization procedure to balance known and unknown factors between groups. Another potential limitation one could argue is that the catechins may not distribute extensively to the target tissue. Use of a vaginal agent rather than an oral agent could have been more effective. A final potential limitation is that it could be argued that the 10% spontaneous regression rate was too low and would under-power the study. This threshold was selected based on the regression rate of high grade disease in the setting of HR HPV infection. This was used as a best proxy for the regression rate of persistent HR HPV infection.
A strength of this study was that it was a randomized, double-blind placebo-controlled study, which is the gold standard to reduce spurious causality and bias. Other strengths of this study include the assessment of clinically meaningful histologic and viral endpoints, a high degree of adherence across patients, and verification of agent intake using urine catechin metabolites.
Trials conducted to date in cervical chemoprevention have been mostly disappointing. There remains a tremendous need to find acceptable, safe, and inexpensive non-surgical treatments to prevent cervical cancer. These clinical studies are methodologically difficult to conduct due to very high rates of spontaneous regression of high-risk HPV infection, even in the setting of persistence. In addition, there is significant variation in the performance and yield of colposcopy that is operator dependent and that impacts the determination of study endpoints. Finally, there is a lack of consensus as to a reference standard that would be less invasive than a LEEP/conization that would be acceptable in this population of young reproductive age women. Further studies will continue to be necessary in order to develop better methodologies to facilitate evaluation of chemopreventive strategies in this organ site.
Supplementary Material
Highlights.
We conducted the largest randomized trial of a green tea extract for HPV cervical disease.
Results show that Polyphenon E is acceptable, safe and well tolerated.
Polyphenon E was not shown to promote the resolution of persistent high risk HPV.
Acknowledgments
This article was partially written using funding provided by the National Cancer Institute of the National Institutes of Health under Award Number R25CA078447.
This work was supported by a contract (N01-CN35158) from the National Cancer Institute and the Arizona Cancer Center Support Grant (P30CA023074). The authors would like to acknowledge Bonita Weible, Steven Rodney, Donna Vining, Roberta Kline, Wendy Thomas, Susan Vanzzini, Sarah Owen, Bonnie Monaco, Blanca-Flor Jimenez, Nicole Trimarche for their excellent assistance in the conduct of the clinical study and Catherine Cordova for her efforts in tea catechin analysis. Finally we are deeply indebted to the many women who were screened for or took part in this clinical trial.
The abbreviations used are
- HPV
human papillomavirus
- CIN1
low grade cervical intraepithelial neoplasia
- EGCG
epigallocatechin gallate
Footnotes
Conflict of Interest Statement
The authors declare no financial conflicts of interest.
This trial is registered under ClinicalTrials.gov. The ClinicalTrials.gov Identifier is NCT00303823.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bosch X. Human papillomavirus and cervical cancer-burden and assessment causality. J Natl Cancer Inst Monogr. 2003;31:3–13. doi: 10.1093/oxfordjournals.jncimonographs.a003479. [DOI] [PubMed] [Google Scholar]
- 2.Markowitz LE, et al. Reduction in Human Papillomavirus (HPV) Prevalence Among Young Women Following HPV Vaccine Introduction in the United States, National Health and Nutrition Examination Surveys, 2003–2010. J Infect Dis. 2013;208(3):385–93. doi: 10.1093/infdis/jit192. [DOI] [PubMed] [Google Scholar]
- 3.Zou C, et al. Green tea compound in chemoprevention of cervical cancer. Int’l J Gyn Cancer. 2010;20:617–24. doi: 10.1111/IGC.0b013e3181c7ca5c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sah JF, et al. Epigallocatechin-3-gallate inhibits epidermal growth factor receptor signaling pathway. Evidence for direct inhibition of ERK1/2 and AKT kinases. Journal of Biological Chemistry. 2004;279(13):12755–62. doi: 10.1074/jbc.M312333200. [DOI] [PubMed] [Google Scholar]
- 5.Yokoyama M, et al. The tea polyphenol, (-)-epigallocatechin gallate effects on growth, apoptosis, and telomerase activity in cervical cell lines. Gynecologic Oncology. 2004;92(1):197–204. doi: 10.1016/j.ygyno.2003.09.023. [DOI] [PubMed] [Google Scholar]
- 6.Zhang Q, et al. Green tea extract and (-)-epigallocatechin-3-gallate inhibit hypoxia- and serum-induced HIF-1alpha protein accumulation and VEGF expression in human cervical carcinoma and hepatoma cells. Mol Cancer Ther. 2006;5(5):1227–38. doi: 10.1158/1535-7163.MCT-05-0490. [DOI] [PubMed] [Google Scholar]
- 7.Ahn WS, et al. Protective effects of green tea extracts (polyphenon E and EGCG) on human cervical lesions. European Journal of Cancer Prevention. 2003;12(5):383–90. doi: 10.1097/00008469-200310000-00007. [DOI] [PubMed] [Google Scholar]
- 8.Tatti S, et al. Sinecatechins, a defined green tea extract, in the treatment of external anogenital warts: a randomized controlled trial. Obstet Gynecol. 2008;111(6):1371–9. doi: 10.1097/AOG.0b013e3181719b60. [DOI] [PubMed] [Google Scholar]
- 9.Aickin M. Randomization, balance, and the validity and efficiency of design-adaptive allocation methods. J Stat Plan Infer. 2001;(94):97–119. [Google Scholar]
- 10.Barker B, et al. The correlation between colposcopically directed cervical biopsy and loop electrosurgical excision procedure pathology and the effect of time on that agreement. Gynecol Oncol. 2001;82(1):22–6. doi: 10.1006/gyno.2001.6245. [DOI] [PubMed] [Google Scholar]
- 11.Meyskens FL, et al. Enhancement of regression of CIN II with topically applied all-trans-retinoic acid: a randomzied trial. J Natl Cancer Inst. 1994;86:539–543. doi: 10.1093/jnci/86.7.539. [DOI] [PubMed] [Google Scholar]
- 12.Romney SL, et al. Effects of beta-carotene and other factors on outcome of cervical dysplasia and human papillomavirus infection. Gynecol Oncol. 65:483–492. doi: 10.1006/gyno.1997.4697. [DOI] [PubMed] [Google Scholar]
- 13.Vlastos AT, et al. Results of a phase II double-blinded randomized clinical trial of difluoromethylornithine for cervical intraepithelial neoplasia grades 2 to 3. Clinical Cancer Research. 2005;11:390–396. [PubMed] [Google Scholar]
- 14.Bell MC, et al. Placebo-controlled trial of indole-3-carbinol in the treatment of CIN. Gynecologic Oncology. 2000;78(2):123–9. doi: 10.1006/gyno.2000.5847. [DOI] [PubMed] [Google Scholar]
- 15.Garcia FAR, et al. ZYC101a for treatment of high grade intraepithelial neoplasia: A randomized controlled trial. Obstet Gynecol. 2004;103(2):317–26. doi: 10.1097/01.AOG.0000110246.93627.17. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.