Abstract
Background
The effect of opioids on inflammation and immune responses is an important subject of investigation because immunoregulatory cytokines are produced in the central nervous system and opioid receptors are widespread in these cells.
Objectives
The aim of this study was to evaluate the immunomodulatory effect of morphine on the C3 expression (both constitutive and proinflammatory cytokine-induced C3 expression) in primary rat astrocytes.
Methods
Primary rat astrocytes were untreated or treated with morphine in different concentrations (10–6 to 10–2 M) before incubation without or with 5 U/mL tumor necrosis factor-α (TNF-α), and C3 protein and mRNA expressions were measured. Similarly, astrocytes were treated with 10–3 M morphine and stimulated with other proinflammatory cytokines, including 10 ng/mL interleukin-8 (IL-8) and 5 U/mL IL-1β. Astrocytes were exposed to 10–5 M naloxone for 2 hours before adding morphine, and TNF-α and C3 protein was measured. Tumor growth factor-β (TGF-β) was measured from the supernatants of each proinflammatory cytokine.
Results
All results are expressed as mean percentages of C3 production by normalizing C3 without morphine or any cytokine treatment as 100%. Constitutive C3 protein production was decreased at morphine 10–3 M (57.2%) and 10–2 M (30.1%). Pretreatment with morphine suppressed induction of C3 expression at both the protein and mRNA levels in astrocytes stimulated with TNF-α, IL-8, and IL-1β (P < 0.05) in a dose-dependent manner. The inhibition of C3 protein production by morphine (10–3 M; 33%) was partially attenuated by naloxone (52.0%) (P < 0.05). The pretreatment of astrocytes with morphine (10–3 M) before stimulation with TNF-α, IL-8, and IL-1β increased by 33% (P < 0.05), decreased by 15.2% (P < 0.05), and did not change the production of TGF-β protein, respectively.
Conclusions
Morphine downregulated both constitutive and proinflammatory cytokine-induced C3 expression of astrocytes at the transcriptional level, but not in a cytokine-specific manner.
Key words: astrocytes, complement 3, morphine, proinflammatory cytokines
Introduction
The number of recent reports on the broad effects of opioids on the inflammatory response is growing.1–4 In particular, findings that opioid receptors are present on the cells of the immune system and that exogenous opioids can suppress immunological activities suggest potential immunoregulatory and/or anti-inflammatory activities of opioids.5–8 Furthermore, the modulation of immune function by opioids and the implications for susceptibility to infection have been studied extensively because these effects may have significant therapeutic consequences.1–4 The effect of morphine, a classic μ-receptor agonist, on immune function has been studied closely because of its widespread clinical use compared with other opioids. Evidence supporting the ability of morphine (in both acute and long-term treatment) to suppress a variety of immunological end points has been uncovered.1–4,9–11 The reported inhibitory effects of morphine on innate and adapted immune responses include alterations in antibody responses, macrophage and natural killer cell activity, cytokine expression, phagocytic activity, and more.1,4,9 In addition, there is growing support for the role of morphine on immune parameters of the central nervous system (CNS), and many investigations indicate that cells within the brain (neurons and glial cells) express opioid receptors.10,12–15 Although numerous studies have shown that opioid-induced immunosuppression can be mediated indirectly through opioid receptors located within the CNS12–15 or through direct interactions with immunocytes,2 the precise cellular mechanisms underlying the immunomodulatory effects of opioids on the CNS require additional investigation.
Considerable evidence supports the potential for glial cells to serve as important modulatory neurotropic and neuroimmune elements in the CNS.16–19 Among the glial cells, astrocytes are immunologically competent cells known to participate in inflammatory events in the CNS by secreting molecules, including cytokines and complements.17,20 Attempts to elucidate the role of complement in the physiological and pathological function of the immune system have been reported previously.21–25 The third component of complement (C3), the most abundant complement protein in human plasma, is an acute-phase protein that plays a key initial role in the activation of the inflammatory cascade of the complement system.21 However, in the host, uncontrolled activation and synthesis of complement may result in inflammation and tissue damage.25 In the CNS, there is ample evidence that complement plays a role in demyelination and inflammation.22,26 For example, the detrimental effects of complement activation have been implicated in multiple neuropathological diseases, including Alzheimer's disease, multiple sclerosis, and stroke.22 Recent studies have revealed that both human and rat astrocytes produce mRNA and protein for many components of the complement system, and proinflammatory cytokines such as tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), and interferon-γ (IFN-γ) are known to upregulate production of complement factors, including C3 in astrocytes.27–29 In line with these findings, it is of interest to determine the role of morphine in the modulation of immune functions on C3 production by astrocytes under inflammatory conditions.
The aim of this study was to evaluate the immunomodulatory effect of morphine on both constitutive and proinflammatory cytokine-induced C3 expression in primary rat astrocytes.
Methods
This study was approved by the Samsung Bio Research Institute Ethical Committee on animal research.
Astrocyte Cultures
Primary rat astrocyte cultures were established as described previously.30 Briefly, the brains of 1- to 3-day-old neonatal rats (Sprague-Dawley, Charles River, Wilmington, Massachusetts) were removed aseptically and placed in ice cold isolation solution medium (Dulbecco's modified Eagle's medium with sodium pyruvate and 4.5 mg/mL of glucose, mixed 1/1 (vol/vol) with Ham F-12 medium containing 15 mM HEPES 100 U/mL of penicillin and 100 μg/mL of streptomycin). After removal of the meninges under the dissecting microscope, the brain tissue was mechanically dissociated by pipetting and passed through 2 sequential sievings of nylon mesh (210- and 130-μm pore size, respectively). The cells were pelleted by centrifugation and re-suspended in the culture medium made from the isolation medium with the addition of 10% fetal bovine serum and 50 U/mL of penicillin and 50 μg/mL of streptomycin. Twenty millimeters of cell suspension at a concentration of 7.5 × 105 cells/mL was placed in each 75 cm2 tissue culture flask and incubated at 37°C in a humidified atmosphere with 5% carbon dioxide. The medium was refreshed every 3 days. On day 8, the flasks were filled with 15 mL of fresh medium, capped tightly, and fastened securely to an orbital shaker. The flasks were shaken at 250 rpm in a 37°C incubator for 24 hours before the oligodendrocyte-enriched supernatant was removed. The original flasks received 15 mL of fresh medium and were returned to shake for an additional 48 hours at 100 rpm to obtain highly purified astrocytes. Astrocytes isolated using this method are known to be 95% to 97% pure when examined by immunofluorescent staining for glial fibrillary acidic protein, which is characteristically produced by these cells. Fluorescence-activated cell-sorting analysis of these cultures showed that <10% of the cells stain positively with fluorescence isothiocyanate isomer-conjugated anti-rat CD 11b, a monoclonal antibody that recognizes the iC3b receptor, which is a cell surface marker of microglia.
C3 Protein Determination by ELISA Technique
C3 protein was identified in the astrocyte culture supernatant using a sandwich ELISA method.29 The 96-well polystyrene plates (Nunc Immunoplate, MaxiSorp, Thomas Scientific, Swedesboro, New York) were coated with 10 μg/mL of goat immunoglobulin-G (IgG) to rat C3 (Cappel Lab, Durham, North Carolina) for 18 hours at 4°C. All the washings and dilutions were carried out with a solution containing phosphate buffer, pH 7.4, 0.5 M sodium chloride, 1% bovine serum albumin, and 0.1% Triton X-100. The plates were incubated with 1:1000, 1:10,000, 1:50,000, and 1:100,000 diluted normal rat serum or 1:1, 1:2, and 1:4 diluted supernatant for 1 hour at 37°C . After 3 washings, the plates were incubated with a peroxidase-conjugated goat IgG to rat C3 (1:10,000 dilution) for 1 hour at 37°C . The reaction was developed with orthophenylenediamine, and the absorbance was read at 490 nm in an ELISA reader (Dynatech Lab. Inc., Alexandria, Virginia). Each plate had controls where the culture supernatant was replaced with washing buffer. The net absorbance was obtained by subtracting the average of triplicate controls from the average of triplicate sample values. The standard curve was generated in each assay with normal rat serum. The detection limit using this method was 8 ng/mL.
RNA Preparation and Northern Analysis
Astrocyte monolayers were lysed in buffer containing guanidine isothiocyanate and β-mercaptoethanol, and total cellular RNA in lysate was purified by ultracentrifugation through a cesium chloride cushion for 18 hours at 35,000 rpm using a SW 60 Beckman rotor (Beckman Instruments Inc., Palo Alto, California).31 The RNA samples underwent electrophoresis in a 0.8% agarose-formaldehyde gel, transferred to nitrocellulose paper, and baked for 2 hours at 80°C . Hybridization with specific cDNA probes was carried out in a solution containing 50% formamide, 45% hybridization buffer (5× somatic stem cell (SSC), 1× Denhardt solution, 0.1% SDS [sodium dodecyl sulfate]),32,33 and 5% denatured herring sperm DNA by incubating the nitrocellulose paper with phosphorus 32 (32P)-labeled cDNA probes for 48 hours at 37°C. Radiolabeled probes were prepared by incorporating 32P into the cDNA fragments using an oligolabeling kit (Pharmacia Fine Chemicals, Piscataway, New Jersey) according to the manufacturer's instructions with (α)dCTP deoxy-cytidine-triphosphate-32P (NEN, Boston, Massachusetts). Nitrocellulose papers were washed sequentially with 2× sodium chloride citrate (SCC), 0.1% SDS, and 0.5× SSC, 0.1% SDS, then exposed to X-OMAT AR5 Kodak film (Rochester, NY) at –70°C for varying periods. The densitometric analysis was performed on a LKB Ultrascan (LKB Instruments, Bromma, Sweden) using autoradiographs obtained using various exposures. The density ratios of C3 mRNA and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA were used to normalize the C3 mRNA expression.
Effect of Morphine on C3 Protein Expression in Primary Rat Astrocyte Cells
In preliminary experiments, we examined whether morphine alone had an effect on C3 protein production by primary rat astrocytes. Astrocytes were untreated or treated with morphine on a 10-fold scale from 10–6 to 10–2 M for 6 hours. Culture supernatants were then collected and assayed for C3 protein.
Effect of TNF-α on C3 Protein Expression in Primary Rat Astrocyte Cells
Primary rat astrocytes cells were incubated with 5 U/mL of human recombinant TNF-α (R&D System, Minneapolis, Minnesota) at 37°C for 24 hours, and the level of C3 protein expression was measured. TNF-α (5 U/mL) stimulation for 24 hours resulted in a 41% increase in C3 protein compared with untreated cells (data not shown).
Effect of Morphine on C3 Protein and mRNA Expression in Astrocyte Cells Treated with TNF-α, IL-8, AND IL-1β
To determine whether pretreating primary rat astrocyte cells with morphine would suppress the ability of TNF-α to enhance C3 protein expression, cells were either untreated or treated with morphine on a 10-fold scale from 10–6 to 10–2 M for 6 hours before incubation with TNF-α 5 U/mL for 24 hours. C3 protein and mRNA levels were measured. Similar experiments were performed on astrocytes using IL-8 (10 ng/mL), IL-1β (5 U/mL), and morphine 10–3 M.
Effect of Naloxone on Inhibition of C3 Production by Morphine
Primary rat astrocytes cells were treated with naloxone 10–5 M for 2 hours before adding morphine 10–3 M. Next, 5 U/mL TNF-α was added to cells for an additional 24 hours. The amount of C3 protein production was measured. The same experiments were performed 4 times, and mean data were analyzed.
Transforming Growth Factor-β (TGF-β) Determination by ELISA Technique
To determine whether the suppressive effect of morphine on inflammatory cytokine-enhanced C3 production was mediated by the anti-inflammatory cytokine TGF-β, astrocytes were pretreated with 10–3 M of morphine for 6 hours and incubated with TNF-α, IL-8, or IL-lβ for 30 hours. The levels of TGF-β proteins in the corresponding supernatants were assayed by ELISA technique. Identical experiments were performed 3 times, and mean data were used for analysis.
The primary objective of our study was to determine the immunomodulatory effect of morphine on both constitutive and proinflammatory cytokine-induced C3 production. The secondary objective was to determine the role of anti-inflammatory cytokine, TGF-β, as the main mechanism of suppressive effect of morphine on proinflammatory cytokine-induced C3 production.
Statistical Analysis
The data presented are expressed as mean (SD). The effect of morphine was analyzed using the Mann-Whitney U test and Dunnett's multiple comparison test for comparison to reference parameter only. In all comparisons, P < 0.05 was considered significant.
Results
Effect of Morphine on C3 Protein Expression in Primary Rat Astrocyte Cells
Results are expressed as mean percentage of C3 production by normalizing constitutive C3 as 100%. The addition of morphine to the cell culture for 6 hours decreased C3 production (decrease of 56.6% at a morphine concentration of 10–6, 57.2% at 10–3 , and 30.1% at 10–2) (Table I).
Table I.
Comparison of C3 production in rat astrocytes treated with incremental dose of morphine (10–6 to 10–2 M).
| Constitutive C3 Level (untreated with morphine, baseline) | Morphine Concentration (M. –log) | 10–6 | 10–5 | 10–4 | 10–3 | 10–2 |
|---|---|---|---|---|---|---|
| 100% | C3 production (%) | 56.6⁎ | 95.2 | 90.7 | 57.2⁎ | 30.1⁎ |
Values are expressed as mean percent C3 production.
P < 0.05 compared with baseline.
Effect of Morphine on C3 Protein and mRNA Expression in Astrocytes Cells Treated with TNF-α, IL-8, and IL-1β
Results are expressed as mean percentages of C3 production by normalizing C3 induced by TNF-α (5 U/mL) as the reference. At morphine concentrations from 10–6 to 10–4 M, C3 protein levels decreased by approximately 60% of the baseline level, compared with C3 protein levels measured in astrocytes treated with TNF-α alone. At morphine concentrations 10–3 and 10–2, C3 protein levels decreased by 33.1% and 23%, respectively (Table II). At morphine concentrations 10–3, C3 mRNA level decreased to 11.0% (Table III).
Table II.
Comparison of C3 production in rat astrocytes treated with tumor necrosis factor-α (TNF-α) (5 U/mL) and incremental dose of morphine (10–6 to 10–2 M).
| C3 Induced by TNF-α Alone as 100% (baseline) | TNF-α (5 U/mL) + Morphine Concentration (M. –log) | 10–6 | 10–5 | 10–4 | 10–3 | 10–2 |
|---|---|---|---|---|---|---|
| 100 | C3 production (%) | 64⁎ | 65⁎ | 63⁎ | 33⁎ | 23⁎ |
Values are expressed as mean percent C3 production in rat astrocytes.
P < 0.05 compared with baseline.
Table III.
Comparison of mRNA density ratio of C3 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) in rat astrocytes treated with tumor necrosis factor-α (TNF-α) and incremental dose of morphine (10–6 to 10–3 M).
| TNF-α (5 U/mL) Alone (baseline) | TNF-α (5 U/mL) + Morphine Concentration (M. –log) | 10–6 | 10–5 | 10–3 |
|---|---|---|---|---|
| 100 | mRNA density ratio of C3 and GAPDH (%) | 70⁎ | 53⁎ | 11⁎ |
Values are expressed as mean percent mRNA density ratio of C3 and GAPDH.
P < 0.05 compared with baseline.
Similar results were observed in IL-8 and IL-1β stimulated astrocytes. Results are expressed as mean percentages of the baseline C3 production by normalizing C3 induced by IL-8 (10 ng/mL) and IL-1β (5 U/mL) by 100%. Pretreatment with morphine (10–3 M) before the addition of IL-8 (10 ng/mL) and IL-1β (5 U/mL) also affected the amounts of C3 produced, significantly reducing cytokine-mediated enhancement in C3 protein levels by 43% and 25%, respectively (P < 0.05). The suppressive effect of morphine pretreatment was also evident on C3-specific mRNA expression. C3-specific mRNA density ratios were significantly decreased, with IL-8–mediated enhancement reduced by 20% and IL-1β–mediated enhancement reduced by 22% (Table IV).
Table IV.
The percent C3 production and mRNA density ratio of C3 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) in rat astrocytes treated with proinflammatory cytokines (interleukin-8 [IL-8] or IL-1β) and morphine.
| IL-8 (10 ng/mL) | IL-1β (5 U/mL) | |
|---|---|---|
| C3 production (%) | ||
| Proinflammatory cytokine alone | 100 | 100 |
| Treated with morphine | 43⁎ | 25⁎ |
| mRNA density ratio of C3 and GAPDH (%) | ||
| Proinflammatory cytokine alone | 100 | 100 |
| Treated with morphine | 20⁎ | 22⁎ |
Values are mean percent C3 production and mRNA density ratio of C3 and GAPDH level, respectively.
P < 0.05 compared with baseline.
Effect of Naloxone on Inhibition of C3 Production by Morphine
The inhibition of C3 protein production by 10–3 M of morphine (33%) was partially attenuated by 10–5 M of naloxone (52.0%) compared with C3 levels induced by TNF-α (5 U/mL) alone (considered as reference) (P < 0.05) (Table V).
Table V.
The effect of naloxone (10–5 M) on inhibition of C3 production by morphine (10–3 M) in rat astrocytes.
| TNF-α (5 U/ml) Only (baseline) | TNF-α (5 U/mL) + Morphine (10–3 M) | Naloxone + TNF-α (5 U/mL) and Morphine (10–3 M) | |
|---|---|---|---|
| 100 | C3 production (%) | 33⁎ | 52† |
TNF-α = tumor necrosis factor-α.
Values are mean percent C3 production.
P < 0.05 compared with cells stimulated with TNF-α (5 U/ml) only.
P < 0.05 compared with cells incubated with TNF-α (5 U/mL) and morphine (10–3 M).
Transforming Growth Factor-β Determination
The pretreatment of astrocytes with 10–3 M of morphine before stimulation with TNF-α, IL-8 ,and IL-1β increased by 33.3% (P < 0.05), decreased by 15.2% (P < 0.05), and did not change the production of TGF-β protein, respectively (Table VI).
Table VI.
The changes in tumor growth factor-β (TGF-β) levels (picogram per milliliter) in supernatants of cytokine-stimulated rat astrocytes culture untreated and treated with morphine (10–3 M).
| TNF-α | IL-8 | IL-1β | |
|---|---|---|---|
| TGF-β levels,pg/mL | |||
| Not treated with morphine | 180.1 (40.3) | 252.2 (10.3) | 141.2 (11.1) |
| Treated with morphine | 241.1 (88.0)⁎ | 213.8 (I8.5)† | 146.9 (29.9) |
IL = interleukin.
Values are mean (SD).
P < 0.05 compared with cells stimulated with TNF-α (5 U/mL) only.
P < 0.05 compared with cells stimulated with IL-8 (10 ng/mL) only.
Discussion
The major emphasis of our study was to investigate the role of morphine on C3 production in astrocytes and to elucidate the molecular mechanisms by which morphine regulates C3 levels when stimulated by proinflammatory cytokines (TNF-α, IL-8, and IL-1β). We observed that morphine downregulated both constitutive C3 and increased expression of the complement component C3 induced by proinflammatory cytokines TNF-α, IL-8, and IL-1β in rat astrocytes.
The role of opioid receptors in morphine-induced changes in the immune system has been suggested and several studies have identified opioid receptors on immune cells.5–8 Moreover, astrocytes have been reported to express μ-, δ-, and κ-opioid receptor mRNA at different levels,6 and μ-receptor has been ascribed to play a central role in mediating the immunomodulatory effects of opioids on astrocytes of the CNS.10 In our study, the treatment of rat astrocytes with only morphine, a primary μ-agonist, and in different concentrations for 6 hours showed variable suppressive effects on C3 protein levels. These effects were observed up to morphine concentrations of 10–4, but definite dose-dependent suppression was observed at higher morphine concentrations (10–3 and 10–2 M). These results demonstrated the potential for direct suppressive effect of morphine on constitutive expression of C3 in astrocytes, especially at higher concentrations.
Generally, inflammatory cytokines such as IL-1, INF-γ, and TNF-α have been demonstrated to induce the expression of C3 in astrocytes,27–29, but recent studies have suggested that cytokines can also induce μ-receptor expressions in both immune cells and neuronal cells.5,34,35 Ruzicka et al5 investigated the effects of IL-1β on the levels of opioid receptor mRNAs in rat astrocyte enriched cultures and found that the level of μ-receptor mRNA was increased. Activation of astrocytes by proinflammatory cytokines may lead to increased synthesis of opioid receptors, and the subsequently increased morphine binding may provide a molecular basis for greater morphine modulation of immune function in the inflammatory setting. Our results support this finding, in that the degree of suppression of C3 protein production was greater when astrocytes were pretreated with morphine (10–3 M) and proinflammatory cytokines (TNF-α; 33%, IL-8; 42%, and IL-1β; 25%) compared with the suppression of constitutive C3 productions with morphine alone (57%). This finding suggests that the suppressive effect of morphine might be greater under conditions of inflammation. In addition, experiments with morphine and TNF-α suggest that transcriptional regulation may play a more important role in C3 suppression as there is a correlation between the reduction of C3 protein and mRNA levels seen in dose-response studies. Also, similar results were observed with other proinflammatory cytokines, IL-8 and IL-1β, suggesting that the mechanism of morphine-mediated C3 suppression for all 3 cytokines occurs primarily through the impairment of transcription of C3 mRNA. In addition, the extent of suppression was not cytokine specific, in that C3 protein and mRNA levels were reduced by similar degrees for all 3 proinflammatory cytokines.
In our study, naloxone, a specific μ-receptor antagonist, was used to determine the role of the μ-receptor on the inhibition of C3 production by morphine. This experiment demonstrated that the inhibition of C3 protein production by morphine (33%) was partially attenuated by 10–5 M naloxone compared with the level of C3 induced by TNF-α alone as (Figure). Although μ-receptor is known as the major binding site for morphine, morphine also has good affinity for all 3 types of opioid receptors.9 Thus, it does not appear that morphine acts solely on the μ-receptor. In addition, previous studies have suggested that morphine modulates the immune system in both naloxone sensitive and insensitive manners.36 Our results also suggest that the effects of morphine on the suppression of C3 expression was only partially mediated through the μ-receptor. Additional studies are warranted to elucidate the role of other opioid receptors on morphine-mediated immunosuppression.
Figure.
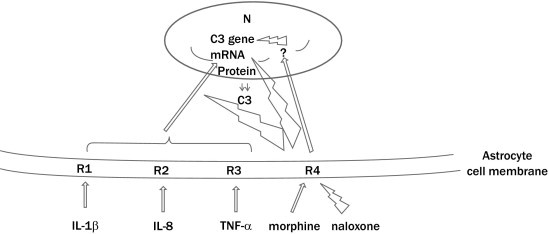
Schematic diagram of modulation of C3 gene expression by morphine and proinflammatory cytokines in primary rat astrocytes. N = nucleus; R1 = interleukin-1β (IL-1β) receptor; R2 = IL-8 receptor; R3 = TNF-α receptor; R4 = μ-receptor;  = excitatory,
= excitatory,  = inhibitory, ? = some genes that would inhibit C3 gene expression.
= inhibitory, ? = some genes that would inhibit C3 gene expression.
Morphine has been demonstrated to induce TGF-β release from peripheral blood mononuclear cell cultures.37 TGF-β has a potent immunosuppressive effect and has been shown to inhibit the signaling pathways of inflammatory cytokines, as well as to reduce C3 production by astroglioma cell lines induced by INF-γ, IL-β, and TNF-α.16,27,28 In addition, the suppression of C3 expression by TGF-β occurs in cytokine-specific patterns, with TGF-β appearing to play an important role in regulating C3 production in astrocytes, especially under inflammatory conditions.16 Our study also measured the level of TGF-β to determine whether morphine stimulated TGF-β production, which subsequently led to the suppression of C3 expression. When astrocytes were pretreated with morphine, the level of TGF-β in supernatants was decreased with exposure to IL-8, increased in the presence of TNF-α, and did not change with IL-1β-stimulated astrocytes. In addition, the maximum level of TGF-β did not exceed 250 pg/mL, which may not have been sufficient to suppress the proinflammatory cytokine-induced C3 production.19 The marked suppression of C3 production by morphine is unlikely to be completely dependent on morphine-potentiated release of TGF-β.
These results suggest that suppression of both constitutive and proinflammatory cytokine-induced C3 protein and mRNA expression is mediated at least in part by opioid receptors on the astrocytes. The complex interaction between opioids and the immune system makes it difficult to weigh the impact of each one of these factors in intricate pathways of immunosuppression. The clinical implications of these findings are yet to be determined but it is possible that the use of morphine may potentially impact susceptibility to infection.
There are several limitations to this study. First, limitations in study design include using a single concentration of morphine (10–3 M) to determine its suppressive effects on other proinflammatory cytokines (IL-8, and IL-1β). Additional data on the effects of morphine on IL-8 and IL-1β might have been revealed by using incremental doses of morphine (10–6 to 10–2 M); however, 10–3 M morphine was chosen based on our result from the TNF-α experiment in which definite suppression of C3 protein and C3 mRNA was observed, and similarly, a suppressive effect of morphine pretreatment on IL-8 and IL-1β was observed in both C3 protein and C3 mRNA expression. Second, different concentrations of both morphine and naloxone might have revealed more detailed information on the role of μ-receptor antagonists on the inhibition of C3 production by morphine. Third, morphine has good affinity for all 3 types of opioid receptors. Therefore, additional study with pure opioid receptor antagonists is needed to elucidate the role of other opioid receptors on morphine-mediated immunosuppression.
Conclusions
The results of our study suggest that morphine may downregulate both constitutive and proinflammatory cytokine-induced C3 expression of astrocytes at a transcriptional level and this downregulation may not occur in a cytokine-specific manner.
Acknowledgments
All authors meet internationally accepted criteria for authorship and participated in study design, implementation, analysis, and/or manuscript preparation. All authors have read and approved the manuscript. The work raises no ethical issues, and the authors have no conflicts of interest to disclose. This work was supported by Samsung Bio Research Institute, which approved the study conception. Dr. Sangmin M. Lee received the Samsung Bio Research Institute Grant (C-99-035-1). Dr. Chung Su Kim participated in study design and implementation of experimental work. Dr. Ko participated in data collection and manuscript preparation. Drs. Lee and Shin participated in data analysis. Dr. Choi participated in statistical analysis. Drs. Lee and Kim participated in implementation of experimental work. Dr. Lee participated in study design, implementation, and manuscript preparation.
References
- 1.Roy S., Wang J., Kelschenbach J. Modulation of immune function by morphine: Implications for susceptibility to infection. J Neuroimmune Pharmacol. 2006;1:77–89. doi: 10.1007/s11481-005-9009-8. [DOI] [PubMed] [Google Scholar]
- 2.Shavit Y., Lewis J.W., Terman G.W. Opioid peptides mediate the suppressive effect of stress on natural killer cell cytotoxicity. Science. 1984;223:188–190. doi: 10.1126/science.6691146. [DOI] [PubMed] [Google Scholar]
- 3.Stein C., Schäfer M., Cabot P.J. Opioids and inflammation. In: Boorsook, editor. Molecular Biology of Pain. International Association for the Study of Pain; Seattle, WA: 1997. pp. 25–43. [Google Scholar]
- 4.Vallejo R., de Leon-Casasola O., Benyamin R. Opioid therapy and immunosuppression: A review. Am J Ther. 2004;11:354–365. doi: 10.1097/01.mjt.0000132250.95650.85. [DOI] [PubMed] [Google Scholar]
- 5.Ruzicka B.B., Akil H. The interleukin-1beta-mediated regulation of proenkephalin and opioid receptor messenger RNA in primary astrocyte-enriched cultures. Neuroscience. 1997;79:517–524. doi: 10.1016/s0306-4522(96)00669-0. [DOI] [PubMed] [Google Scholar]
- 6.Ruzicka B.B., Fox C.A., Thompson R.C. Primary astroglial cultures derived from several rat brain regions differentially express mu, delta and kappa opioid receptor mRNA. Brain Res Mol Brain Res. 1995;34:209–220. doi: 10.1016/0169-328x(95)00165-o. [DOI] [PubMed] [Google Scholar]
- 7.Beagles K., Wellstein A., Bayer B. Systemic morphine administration suppresses genes involved in antigen presentation. Mol Pharmacol. 2004;65:437–442. doi: 10.1124/mol.65.2.437. [DOI] [PubMed] [Google Scholar]
- 8.Chuang T.K., Killam K.F., Jr, Chuang L.F. Mu opioid receptor gene expression in immune cells. Biochem Biophys Res Commun. 1995;216:922–930. doi: 10.1006/bbrc.1995.2709. [DOI] [PubMed] [Google Scholar]
- 9.Bidlack J.M., Khimich M., Parkhill A.L. Opioid receptors and signaling on cells from the immune system. J Neuroimmune Pharmacol. 2006;1:260–269. doi: 10.1007/s11481-006-9026-2. [DOI] [PubMed] [Google Scholar]
- 10.Mahajan S.D., Schwartz S.A., Shanahan T.C. Morphine regulates gene expression of alpha- and beta-chemokines and their receptors on astroglial cells via the opioid mu receptor. J Immunol. 2002;169:3589–3599. doi: 10.4049/jimmunol.169.7.3589. [DOI] [PubMed] [Google Scholar]
- 11.Peterson P.K., Molitor T.W., Chao C.C. The opioid-cytokine connection. J Neuroimmunol. 1998;83:63–69. doi: 10.1016/s0165-5728(97)00222-1. [DOI] [PubMed] [Google Scholar]
- 12.Dobrenis K., Makman M.H., Stefano G.B. Occurrence of the opiate alkaloid-selective mu3 receptor in mammalian microglia, astrocytes and Kupffer cells. Brain Res. 1995;686:239–248. doi: 10.1016/0006-8993(95)00452-v. [DOI] [PubMed] [Google Scholar]
- 13.Alonzo N.C., Bayer B.M. Opioids, immunology, and host defenses of intravenous drug abusers. Infect Dis Clin North Am. 2002;16:553–569. doi: 10.1016/s0891-5520(02)00018-1. [DOI] [PubMed] [Google Scholar]
- 14.Bryant H.U., Bernton E.W., Kenner J.R., Holaday J.W. Role of adrenal cortical activation in the immunosuppressive effects of chronic morphine treatment. Endocrinology. 1991;128:3253–3258. doi: 10.1210/endo-128-6-3253. [DOI] [PubMed] [Google Scholar]
- 15.Eriksson P.S., Hansson E., Ronnback L. Delta and kappa opiate receptors in primary astroglial cultures from rat cerebral cortex. Neurochem Res. 1990;15:1123–1126. doi: 10.1007/BF01101714. [DOI] [PubMed] [Google Scholar]
- 16.Barnum S.R., Jones J.L. Transforming growth factor-beta 1 inhibits inflammatory cytokine-induced C3 gene expression in astrocytes. J Immunol. 1994;152:765–773. [PubMed] [Google Scholar]
- 17.Shin M.L., Lieberman A.P., Fisher S.N. Methodological evaluation of tumor necrosis factor production in central nervous system glial cells. Meth Neurosci. 1993;17:16–34. [Google Scholar]
- 18.Maranto J., Rappaport J., Datta P.K. Regulation of complement component C3 in astrocytes by IL-1beta and morphine. J Neuroimmune Pharmacol. 2008;3:43–51. doi: 10.1007/s11481-007-9096-9. [DOI] [PubMed] [Google Scholar]
- 19.Morganti-Kossmann M.C., Kossmann T., Brandes M.E. Autocrine and paracrine regulation of astrocyte function by transforming growth factor-beta. J Neuroimmunol. 1992;39:163–173. doi: 10.1016/0165-5728(92)90185-n. [DOI] [PubMed] [Google Scholar]
- 20.Brosnan C.F., Selmaj K., Raine C.S. Hypothesis: A role for tumor necrosis factor in immune-mediated demyelination and its relevance to multiple sclerosis. J Neuroimmunol. 1988;18:87–94. doi: 10.1016/0165-5728(88)90137-3. [DOI] [PubMed] [Google Scholar]
- 21.Frank M.M., Fries L.F. The role of complement in inflammation and phagocytosis. Immunol Today. 1991;12:322–326. doi: 10.1016/0167-5699(91)90009-I. [DOI] [PubMed] [Google Scholar]
- 22.Morgan B.P., Gasque P., Singhrao S., Piddlesden S.J. The role of complement in disorders of the nervous system. Immunopharmacology. 1997;38:43–50. doi: 10.1016/s0162-3109(97)00059-3. [DOI] [PubMed] [Google Scholar]
- 23.Quezado Z.M., Hoffman W.D., Winkelstein J.A. The third component of complement protects against Escherichia coli endotoxin-induced shock and multiple organ failure. J Exp Med. 1994;179:569–578. doi: 10.1084/jem.179.2.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roumen R.M., Redl H., Schlag G. Inflammatory mediators in relation to the development of multiple organ failure in patients after severe blunt trauma. Crit Care Med. 1995;23:474–480. doi: 10.1097/00003246-199503000-00010. [DOI] [PubMed] [Google Scholar]
- 25.Volanakis J.E. The role of complement in innate and adaptive immunity. Curr Top Microbiol Immunol. 2002;266:41–56. doi: 10.1007/978-3-662-04700-2_4. [DOI] [PubMed] [Google Scholar]
- 26.Shin M.L., Koski C.L. The complement system in demyelination. In: Martenson R.E., editor. Myelin: Biology and Chemistry. CRC Press; Boca Raton, FL: 1992. pp. 801–831. [Google Scholar]
- 27.Barnum S.R., Jones J.L., Benveniste E.N. Interferon-gamma regulation of C3 gene expression in human astroglioma cells. J Neuroimmunol. 1992;38:275–282. doi: 10.1016/0165-5728(92)90020-l. [DOI] [PubMed] [Google Scholar]
- 28.Barnum S.R., Jones J.L., Benveniste E.N. Interleukin-1 and tumor necrosis factor-mediated regulation of C3 gene expression in human astroglioma cells. Glia. 1993;7:225–236. doi: 10.1002/glia.440070306. [DOI] [PubMed] [Google Scholar]
- 29.Rus H.G., Kim L.M., Niculescu F.I., Shin M.L. Induction of C3 expression in astrocytes is regulated by cytokines and Newcastle disease virus. J Immunol. 1992;148:928–933. [PubMed] [Google Scholar]
- 30.McCarthy K.D., de Vellis J. Preparation of separate astroglial and oligodendroglial cell cultures from rat cerebral tissue. J Cell Biol. 1980;85:890–902. doi: 10.1083/jcb.85.3.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chirgwin J.M., Przybyla A.E., MacDonald R.J., Rutter W.J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979;18:5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- 32.Thomas P. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci USA. 1980;77:5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Raj N.B., Pitha P.M. Analysis of interferon mRNA in human fibroblast cells induced to produce interferon. Proc Natl Acad Sci USA. 1981;78:7426–7430. doi: 10.1073/pnas.78.12.7426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Börner C., Kraus J., Schröder H. Transcriptional regulation of the human mu-opioid receptor gene by interleukin-6. Mol Pharmacol. 2004;66:1719–1726. doi: 10.1124/mol.104.003806. [DOI] [PubMed] [Google Scholar]
- 35.Kraus J., Borner C., Giannini E. Regulation of mu-opioid receptor gene transcription by interleukin-4 and influence of an allelic variation within a STAT6 transcription factor binding site. J Biol Chem. 2001;276:43901–43908. doi: 10.1074/jbc.M107543200. [DOI] [PubMed] [Google Scholar]
- 36.Bryant H.U., Holaday J.W. Opioids in Immunologic Processes. Springer; Berlin: 1993. pp. 361–385. [Google Scholar]
- 37.Chao C.C., Hu S., Molitor T.W. Morphine potentiates transforming growth factor-beta release from human peripheral blood mononuclear cell cultures. J Pharmacol Exp Ther. 1992;262:19–24. [PubMed] [Google Scholar]


