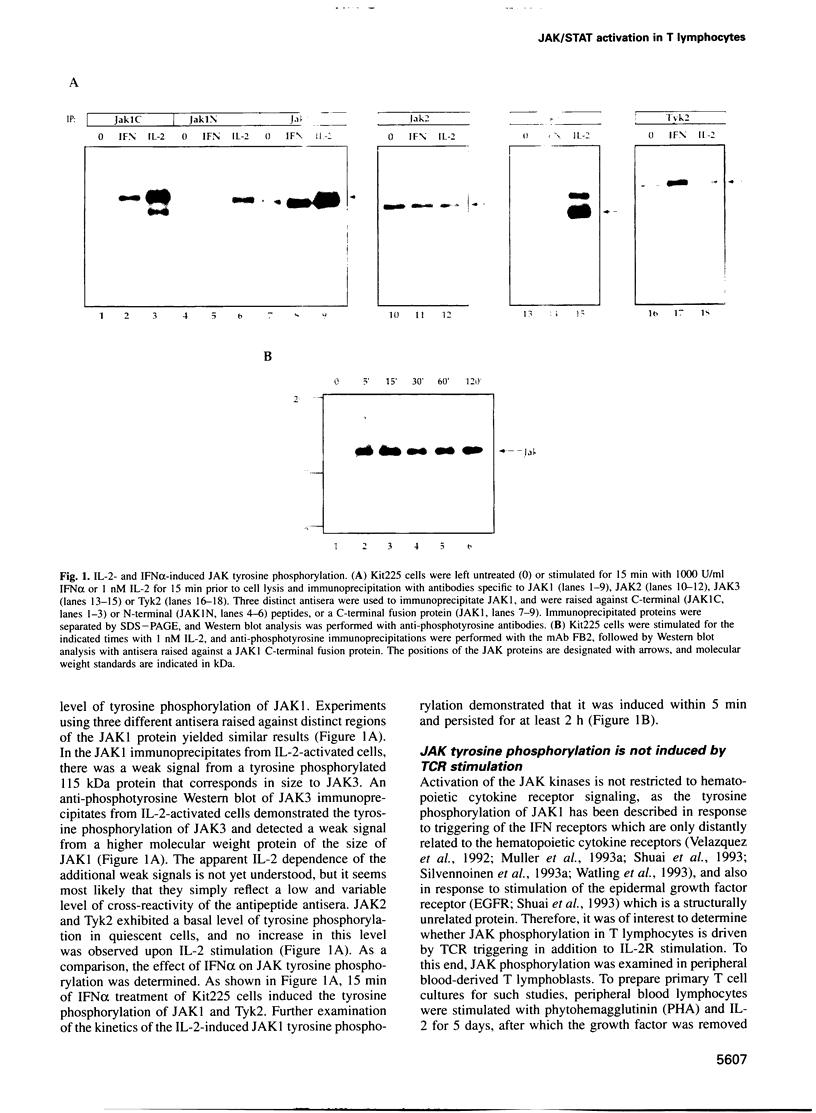
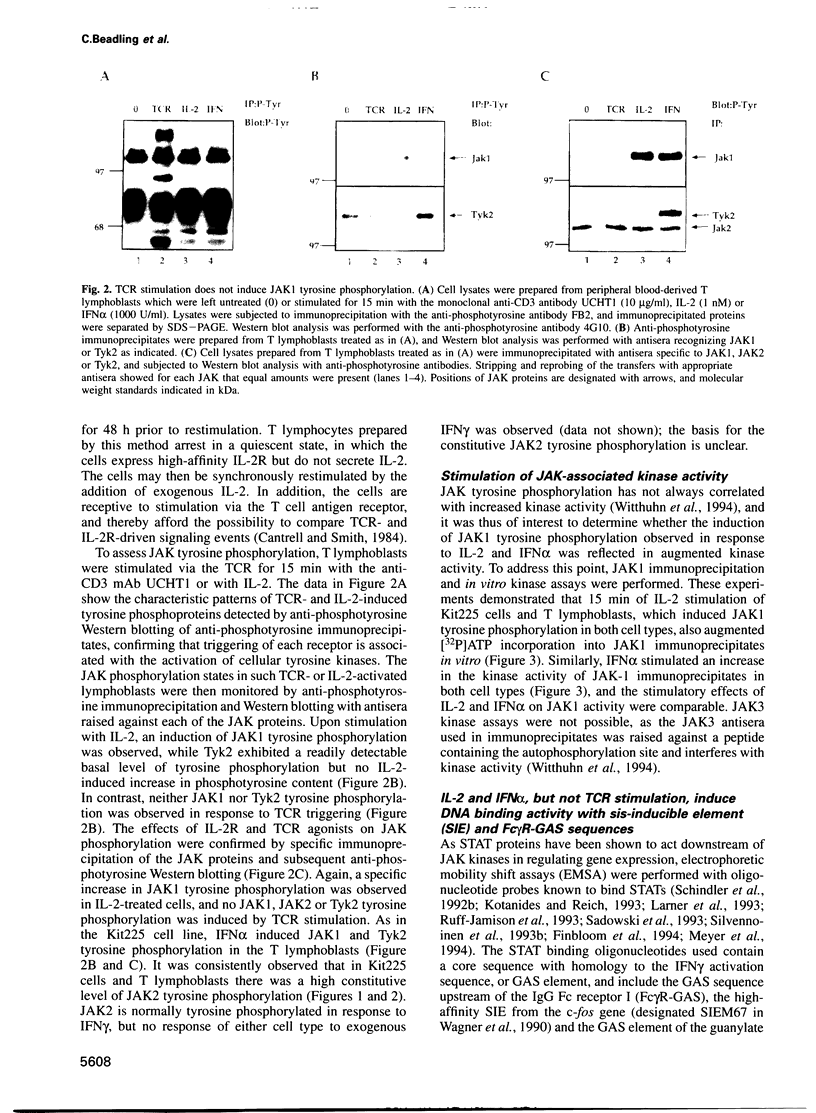
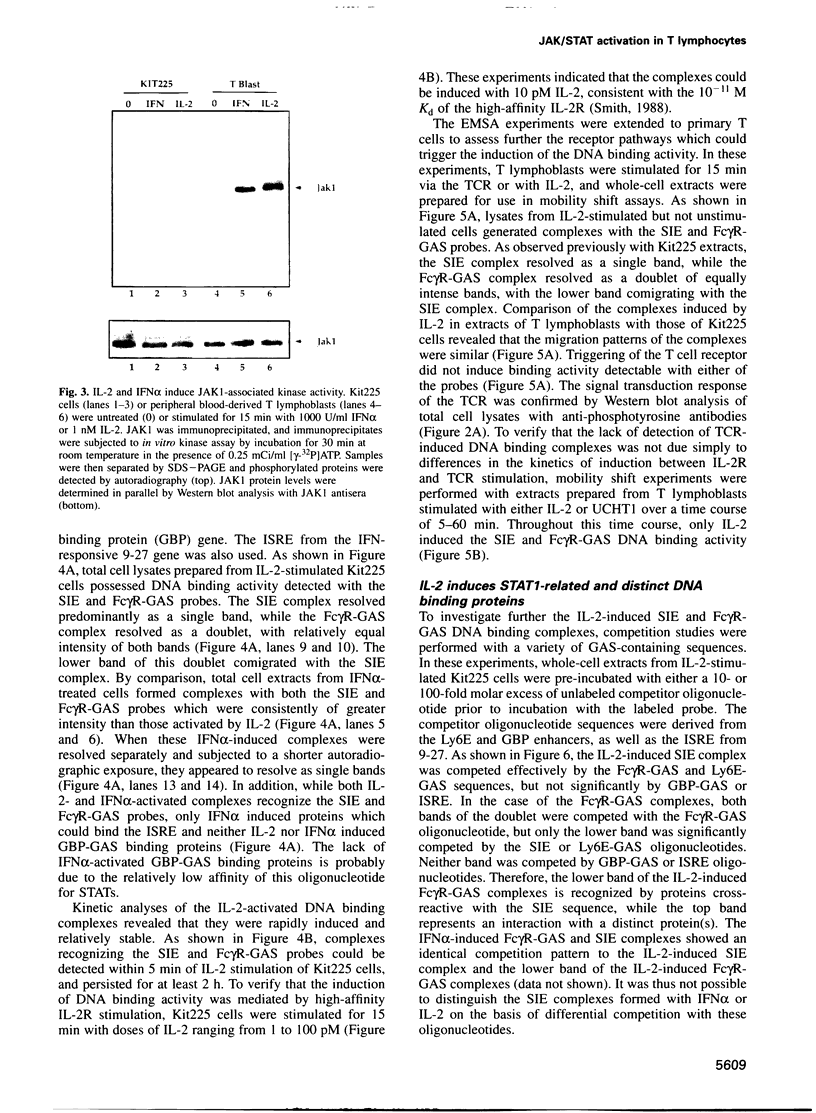
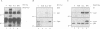Abstract
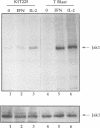
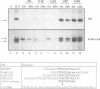
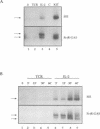
The activation of Janus protein tyrosine kinases (JAKs) and signal transducer and activator of transcription (STAT) proteins by interleukin (IL)-2, the T cell antigen receptor (TCR) and interferon (IFN) alpha was explored in human peripheral blood-derived T cells and the leukemic T cell line Kit225. An IL-2-induced increase in JAK1 and JAK3, but not JAK2 or Tyk2, tyrosine phosphorylation was observed. In contrast, no induction of tyrosine phosphorylation of JAKs was detected upon stimulation of the TCR. IFN alpha induced the tyrosine phosphorylation of JAK1 and Tyk2, but not JAK2 or JAK3. IFN alpha activated STAT1, STAT2 and STAT3 in T cells, but no detectable activation of these STATs was induced by IL-2. However, IL-2 regulates the DNA binding and tyrosine phosphorylation of two STAT-like protein complexes which do not include STAT1, STAT2 or STAT3. STAT4 is not activated by IL-2. The activation of STAT5 cannot be excluded, so the IL-2-activated complexes most probably include at least one novel STAT. No STAT activity was detected in TCR-stimulated lymphocytes, indicating that the JAK/STAT pathway defined in this study constitutes an IL-2R-mediated signaling event which is not shared by the TCR. Finally, in other cell types the correlation between JAK1 activation and the induction of STAT1 has suggested that JAK1 may activate STAT1. The observation that IL-2 and IFN alpha activate JAK1 to a comparable degree, but only IFN alpha activates STAT1, indicates that JAK1 activation is not the only determining factor for STAT1 activation. Moreover, the data show that JAK1 stimulation is also not sufficient for STAT3 activation.
Full text
PDF

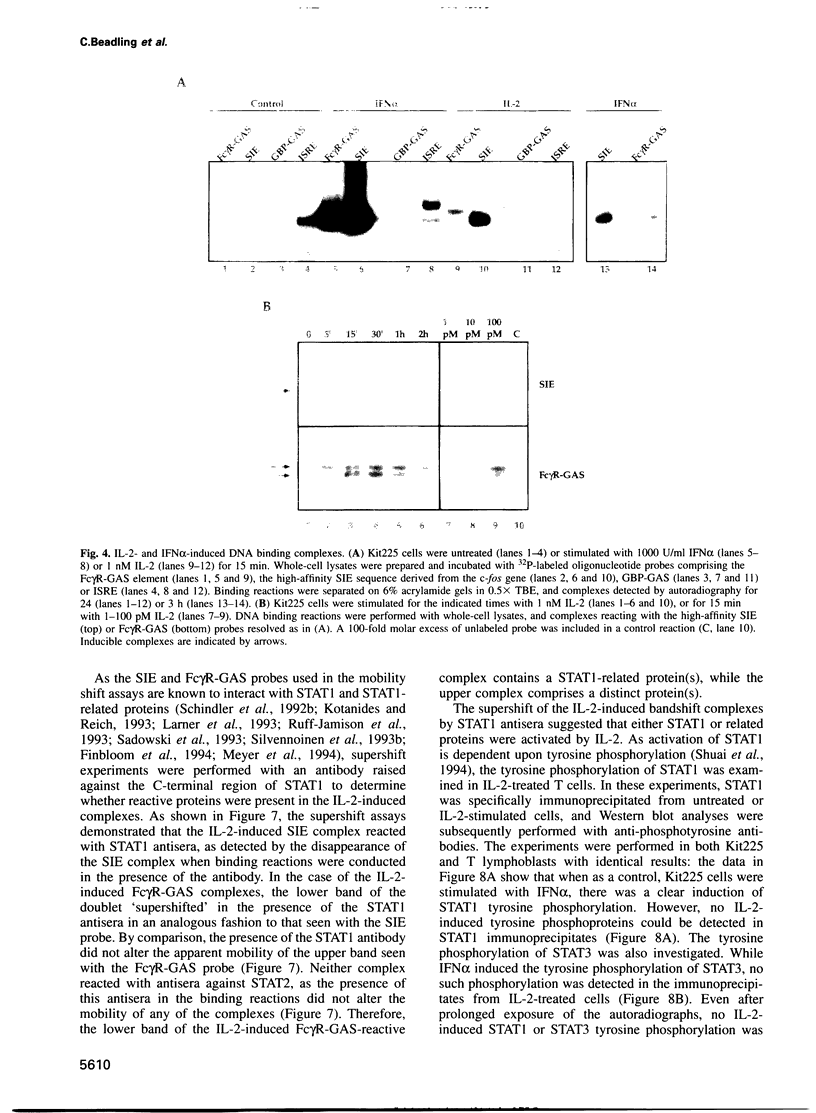
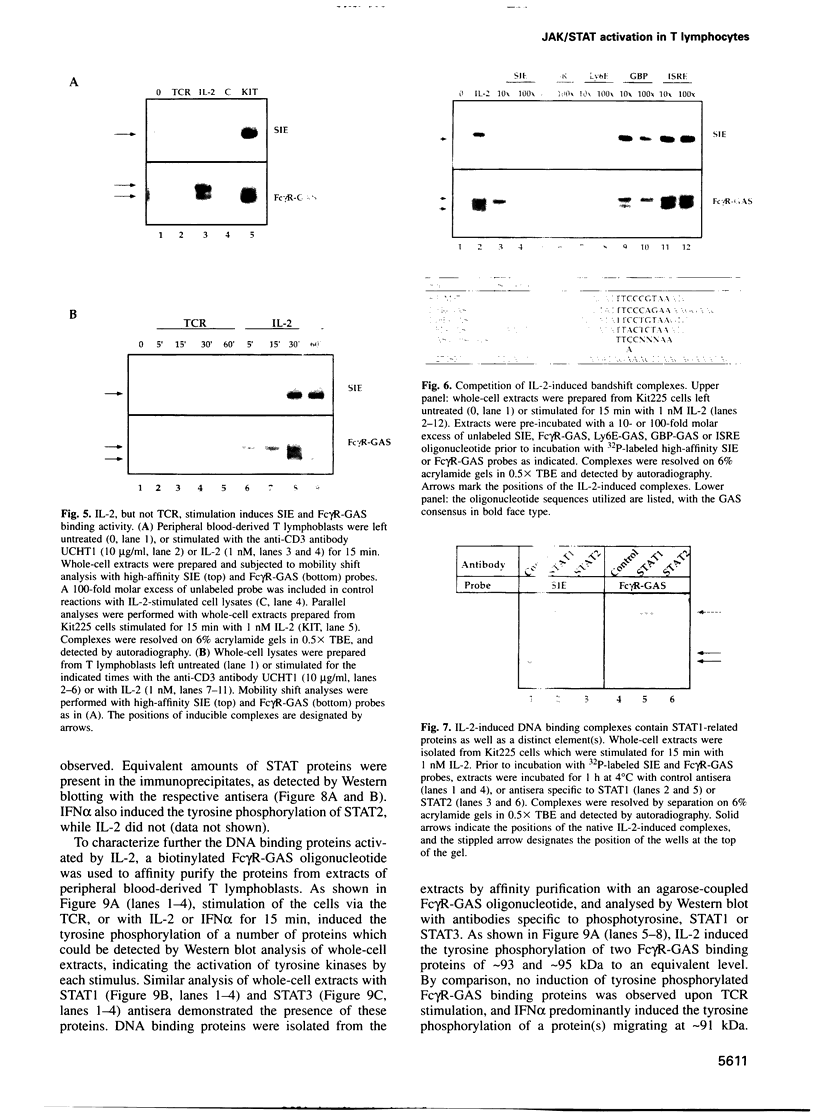
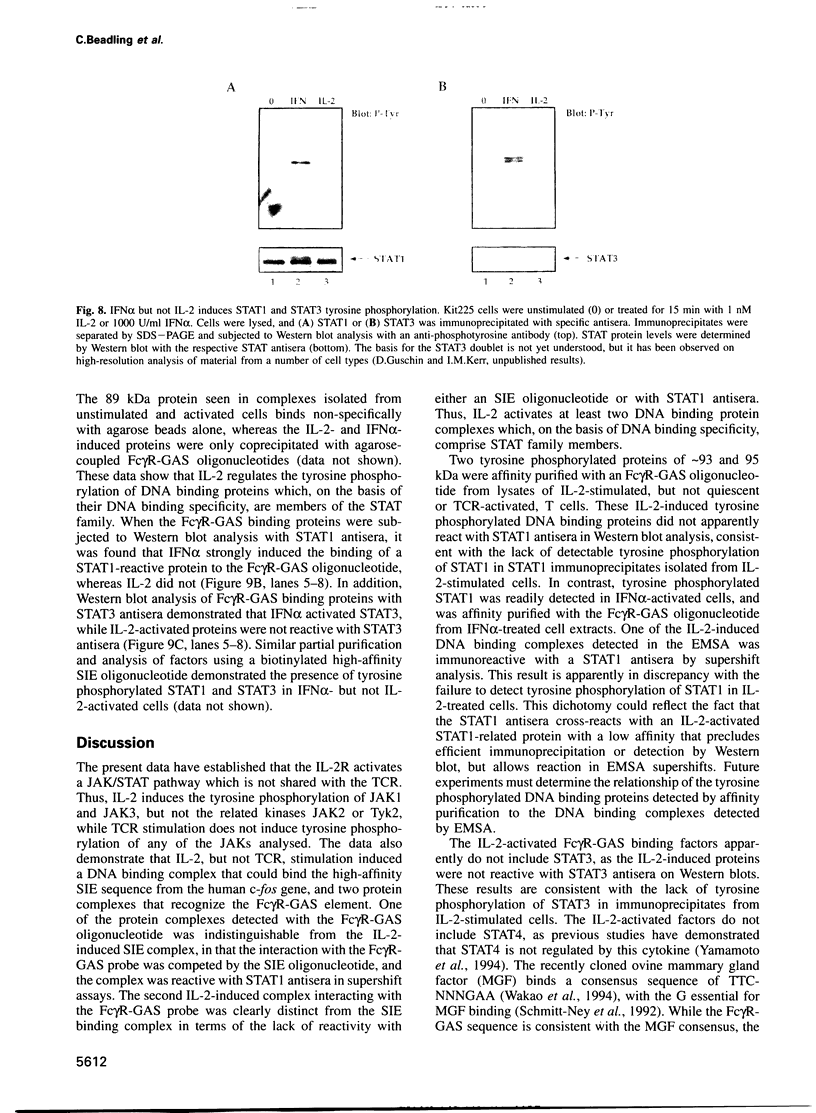
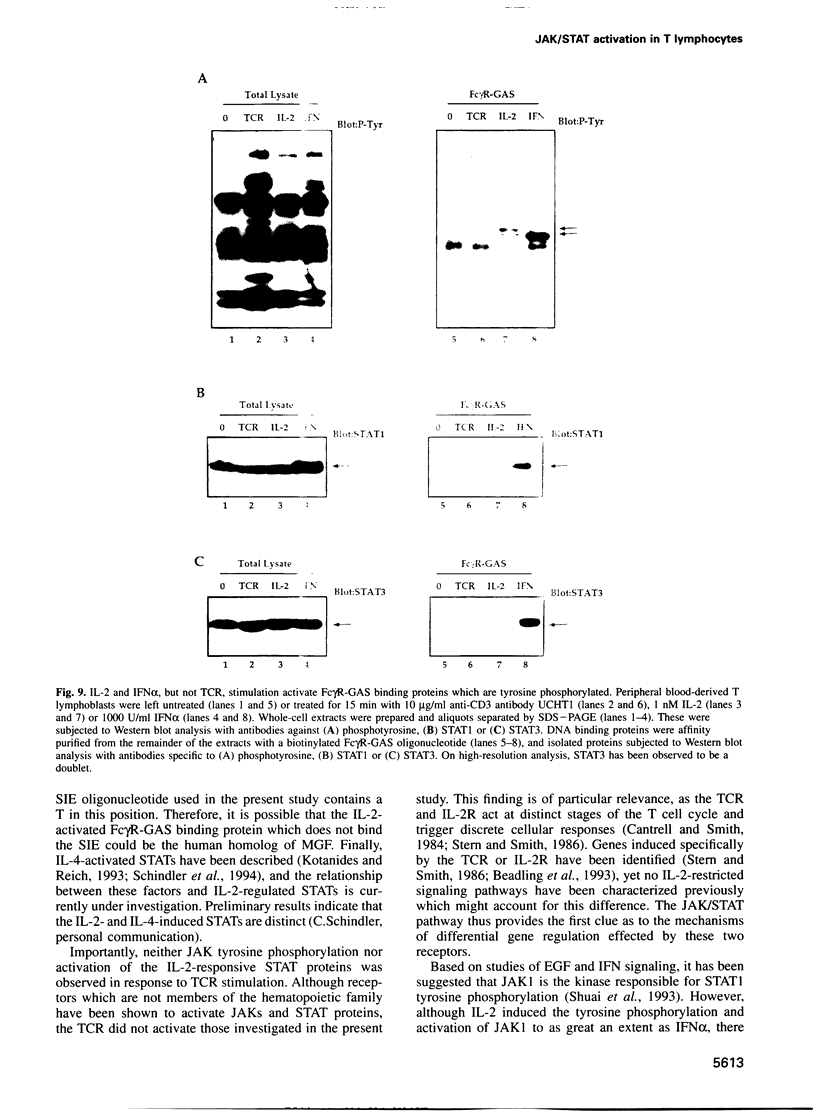


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akira S., Nishio Y., Inoue M., Wang X. J., Wei S., Matsusaka T., Yoshida K., Sudo T., Naruto M., Kishimoto T. Molecular cloning of APRF, a novel IFN-stimulated gene factor 3 p91-related transcription factor involved in the gp130-mediated signaling pathway. Cell. 1994 Apr 8;77(1):63–71. doi: 10.1016/0092-8674(94)90235-6. [DOI] [PubMed] [Google Scholar]
- Argetsinger L. S., Campbell G. S., Yang X., Witthuhn B. A., Silvennoinen O., Ihle J. N., Carter-Su C. Identification of JAK2 as a growth hormone receptor-associated tyrosine kinase. Cell. 1993 Jul 30;74(2):237–244. doi: 10.1016/0092-8674(93)90415-m. [DOI] [PubMed] [Google Scholar]
- Asao H., Takeshita T., Ishii N., Kumaki S., Nakamura M., Sugamura K. Reconstitution of functional interleukin 2 receptor complexes on fibroblastoid cells: involvement of the cytoplasmic domain of the gamma chain in two distinct signaling pathways. Proc Natl Acad Sci U S A. 1993 May 1;90(9):4127–4131. doi: 10.1073/pnas.90.9.4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustine J. A., Sutor S. L., Abraham R. T. Interleukin 2- and polyomavirus middle T antigen-induced modification of phosphatidylinositol 3-kinase activity in activated T lymphocytes. Mol Cell Biol. 1991 Sep;11(9):4431–4440. doi: 10.1128/mcb.11.9.4431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamford R. N., Grant A. J., Burton J. D., Peters C., Kurys G., Goldman C. K., Brennan J., Roessler E., Waldmann T. A. The interleukin (IL) 2 receptor beta chain is shared by IL-2 and a cytokine, provisionally designated IL-T, that stimulates T-cell proliferation and the induction of lymphokine-activated killer cells. Proc Natl Acad Sci U S A. 1994 May 24;91(11):4940–4944. doi: 10.1073/pnas.91.11.4940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazan J. F. Structural design and molecular evolution of a cytokine receptor superfamily. Proc Natl Acad Sci U S A. 1990 Sep;87(18):6934–6938. doi: 10.1073/pnas.87.18.6934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beadling C., Johnson K. W., Smith K. A. Isolation of interleukin 2-induced immediate-early genes. Proc Natl Acad Sci U S A. 1993 Apr 1;90(7):2719–2723. doi: 10.1073/pnas.90.7.2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonni A., Frank D. A., Schindler C., Greenberg M. E. Characterization of a pathway for ciliary neurotrophic factor signaling to the nucleus. Science. 1993 Dec 3;262(5139):1575–1579. doi: 10.1126/science.7504325. [DOI] [PubMed] [Google Scholar]
- Cantrell D. A., Smith K. A. The interleukin-2 T-cell system: a new cell growth model. Science. 1984 Jun 22;224(4655):1312–1316. doi: 10.1126/science.6427923. [DOI] [PubMed] [Google Scholar]
- Cosman D., Lyman S. D., Idzerda R. L., Beckmann M. P., Park L. S., Goodwin R. G., March C. J. A new cytokine receptor superfamily. Trends Biochem Sci. 1990 Jul;15(7):265–270. doi: 10.1016/0968-0004(90)90051-c. [DOI] [PubMed] [Google Scholar]
- Darnell J. E., Jr, Kerr I. M., Stark G. R. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994 Jun 3;264(5164):1415–1421. doi: 10.1126/science.8197455. [DOI] [PubMed] [Google Scholar]
- Feldman G. M., Petricoin E. F., 3rd, David M., Larner A. C., Finbloom D. S. Cytokines that associate with the signal transducer gp130 activate the interferon-induced transcription factor p91 by tyrosine phosphorylation. J Biol Chem. 1994 Apr 8;269(14):10747–10752. [PubMed] [Google Scholar]
- Finbloom D. S., Petricoin E. F., 3rd, Hackett R. H., David M., Feldman G. M., Igarashi K., Fibach E., Weber M. J., Thorner M. O., Silva C. M. Growth hormone and erythropoietin differentially activate DNA-binding proteins by tyrosine phosphorylation. Mol Cell Biol. 1994 Mar;14(3):2113–2118. doi: 10.1128/mcb.14.3.2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu X. Y., Kessler D. S., Veals S. A., Levy D. E., Darnell J. E., Jr ISGF3, the transcriptional activator induced by interferon alpha, consists of multiple interacting polypeptide chains. Proc Natl Acad Sci U S A. 1990 Nov;87(21):8555–8559. doi: 10.1073/pnas.87.21.8555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu X. Y., Schindler C., Improta T., Aebersold R., Darnell J. E., Jr The proteins of ISGF-3, the interferon alpha-induced transcriptional activator, define a gene family involved in signal transduction. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7840–7843. doi: 10.1073/pnas.89.16.7840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu X. Y., Zhang J. J. Transcription factor p91 interacts with the epidermal growth factor receptor and mediates activation of the c-fos gene promoter. Cell. 1993 Sep 24;74(6):1135–1145. doi: 10.1016/0092-8674(93)90734-8. [DOI] [PubMed] [Google Scholar]
- Giri J. G., Ahdieh M., Eisenman J., Shanebeck K., Grabstein K., Kumaki S., Namen A., Park L. S., Cosman D., Anderson D. Utilization of the beta and gamma chains of the IL-2 receptor by the novel cytokine IL-15. EMBO J. 1994 Jun 15;13(12):2822–2830. doi: 10.1002/j.1460-2075.1994.tb06576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabstein K. H., Eisenman J., Shanebeck K., Rauch C., Srinivasan S., Fung V., Beers C., Richardson J., Schoenborn M. A., Ahdieh M. Cloning of a T cell growth factor that interacts with the beta chain of the interleukin-2 receptor. Science. 1994 May 13;264(5161):965–968. doi: 10.1126/science.8178155. [DOI] [PubMed] [Google Scholar]
- Graves J. D., Downward J., Izquierdo-Pastor M., Rayter S., Warne P. H., Cantrell D. A. The growth factor IL-2 activates p21ras proteins in normal human T lymphocytes. J Immunol. 1992 Apr 15;148(8):2417–2422. [PubMed] [Google Scholar]
- Greenlund A. C., Farrar M. A., Viviano B. L., Schreiber R. D. Ligand-induced IFN gamma receptor tyrosine phosphorylation couples the receptor to its signal transduction system (p91). EMBO J. 1994 Apr 1;13(7):1591–1600. doi: 10.1002/j.1460-2075.1994.tb06422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatakeyama M., Kono T., Kobayashi N., Kawahara A., Levin S. D., Perlmutter R. M., Taniguchi T. Interaction of the IL-2 receptor with the src-family kinase p56lck: identification of novel intermolecular association. Science. 1991 Jun 14;252(5012):1523–1528. doi: 10.1126/science.2047859. [DOI] [PubMed] [Google Scholar]
- Hatakeyama M., Mori H., Doi T., Taniguchi T. A restricted cytoplasmic region of IL-2 receptor beta chain is essential for growth signal transduction but not for ligand binding and internalization. Cell. 1989 Dec 1;59(5):837–845. doi: 10.1016/0092-8674(89)90607-7. [DOI] [PubMed] [Google Scholar]
- Horak I. D., Gress R. E., Lucas P. J., Horak E. M., Waldmann T. A., Bolen J. B. T-lymphocyte interleukin 2-dependent tyrosine protein kinase signal transduction involves the activation of p56lck. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):1996–2000. doi: 10.1073/pnas.88.5.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori T., Uchiyama T., Tsudo M., Umadome H., Ohno H., Fukuhara S., Kita K., Uchino H. Establishment of an interleukin 2-dependent human T cell line from a patient with T cell chronic lymphocytic leukemia who is not infected with human T cell leukemia/lymphoma virus. Blood. 1987 Oct;70(4):1069–1072. [PubMed] [Google Scholar]
- Ihle J. N., Witthuhn B. A., Quelle F. W., Yamamoto K., Thierfelder W. E., Kreider B., Silvennoinen O. Signaling by the cytokine receptor superfamily: JAKs and STATs. Trends Biochem Sci. 1994 May;19(5):222–227. doi: 10.1016/0968-0004(94)90026-4. [DOI] [PubMed] [Google Scholar]
- Johnston J. A., Kawamura M., Kirken R. A., Chen Y. Q., Blake T. B., Shibuya K., Ortaldo J. R., McVicar D. W., O'Shea J. J. Phosphorylation and activation of the Jak-3 Janus kinase in response to interleukin-2. Nature. 1994 Jul 14;370(6485):151–153. doi: 10.1038/370151a0. [DOI] [PubMed] [Google Scholar]
- Kawamura M., McVicar D. W., Johnston J. A., Blake T. B., Chen Y. Q., Lal B. K., Lloyd A. R., Kelvin D. J., Staples J. E., Ortaldo J. R. Molecular cloning of L-JAK, a Janus family protein-tyrosine kinase expressed in natural killer cells and activated leukocytes. Proc Natl Acad Sci U S A. 1994 Jul 5;91(14):6374–6378. doi: 10.1073/pnas.91.14.6374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler D. S., Veals S. A., Fu X. Y., Levy D. E. Interferon-alpha regulates nuclear translocation and DNA-binding affinity of ISGF3, a multimeric transcriptional activator. Genes Dev. 1990 Oct;4(10):1753–1765. doi: 10.1101/gad.4.10.1753. [DOI] [PubMed] [Google Scholar]
- Kishimoto T., Taga T., Akira S. Cytokine signal transduction. Cell. 1994 Jan 28;76(2):253–262. doi: 10.1016/0092-8674(94)90333-6. [DOI] [PubMed] [Google Scholar]
- Kondo M., Takeshita T., Ishii N., Nakamura M., Watanabe S., Arai K., Sugamura K. Sharing of the interleukin-2 (IL-2) receptor gamma chain between receptors for IL-2 and IL-4. Science. 1993 Dec 17;262(5141):1874–1877. doi: 10.1126/science.8266076. [DOI] [PubMed] [Google Scholar]
- Kotanides H., Reich N. C. Requirement of tyrosine phosphorylation for rapid activation of a DNA binding factor by IL-4. Science. 1993 Nov 19;262(5137):1265–1267. doi: 10.1126/science.7694370. [DOI] [PubMed] [Google Scholar]
- Krolewski J. J., Lee R., Eddy R., Shows T. B., Dalla-Favera R. Identification and chromosomal mapping of new human tyrosine kinase genes. Oncogene. 1990 Mar;5(3):277–282. [PubMed] [Google Scholar]
- Lamb P., Kessler L. V., Suto C., Levy D. E., Seidel H. M., Stein R. B., Rosen J. Rapid activation of proteins that interact with the interferon gamma activation site in response to multiple cytokines. Blood. 1994 Apr 15;83(8):2063–2071. [PubMed] [Google Scholar]
- Larner A. C., David M., Feldman G. M., Igarashi K., Hackett R. H., Webb D. S., Sweitzer S. M., Petricoin E. F., 3rd, Finbloom D. S. Tyrosine phosphorylation of DNA binding proteins by multiple cytokines. Science. 1993 Sep 24;261(5129):1730–1733. doi: 10.1126/science.8378773. [DOI] [PubMed] [Google Scholar]
- Lütticken C., Wegenka U. M., Yuan J., Buschmann J., Schindler C., Ziemiecki A., Harpur A. G., Wilks A. F., Yasukawa K., Taga T. Association of transcription factor APRF and protein kinase Jak1 with the interleukin-6 signal transducer gp130. Science. 1994 Jan 7;263(5143):89–92. doi: 10.1126/science.8272872. [DOI] [PubMed] [Google Scholar]
- Merida I., Diez E., Gaulton G. N. IL-2 binding activates a tyrosine-phosphorylated phosphatidylinositol-3-kinase. J Immunol. 1991 Oct 1;147(7):2202–2207. [PubMed] [Google Scholar]
- Meyer D. J., Campbell G. S., Cochran B. H., Argetsinger L. S., Larner A. C., Finbloom D. S., Carter-Su C., Schwartz J. Growth hormone induces a DNA binding factor related to the interferon-stimulated 91-kDa transcription factor. J Biol Chem. 1994 Feb 18;269(7):4701–4704. [PubMed] [Google Scholar]
- Mills G. B., Zhang N., Schmandt R., Fung M., Greene W., Mellors A., Hogg D. Transmembrane signalling by interleukin 2. Biochem Soc Trans. 1991 Apr;19(2):277–287. doi: 10.1042/bst0190277. [DOI] [PubMed] [Google Scholar]
- Miyajima A., Mui A. L., Ogorochi T., Sakamaki K. Receptors for granulocyte-macrophage colony-stimulating factor, interleukin-3, and interleukin-5. Blood. 1993 Oct 1;82(7):1960–1974. [PubMed] [Google Scholar]
- Müller M., Briscoe J., Laxton C., Guschin D., Ziemiecki A., Silvennoinen O., Harpur A. G., Barbieri G., Witthuhn B. A., Schindler C. The protein tyrosine kinase JAK1 complements defects in interferon-alpha/beta and -gamma signal transduction. Nature. 1993 Nov 11;366(6451):129–135. doi: 10.1038/366129a0. [DOI] [PubMed] [Google Scholar]
- Müller M., Laxton C., Briscoe J., Schindler C., Improta T., Darnell J. E., Jr, Stark G. R., Kerr I. M. Complementation of a mutant cell line: central role of the 91 kDa polypeptide of ISGF3 in the interferon-alpha and -gamma signal transduction pathways. EMBO J. 1993 Nov;12(11):4221–4228. doi: 10.1002/j.1460-2075.1993.tb06106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson S. E., Oates A. C., Harpur A. G., Ziemiecki A., Wilks A. F., Layton J. E. Tyrosine kinase JAK1 is associated with the granulocyte-colony-stimulating factor receptor and both become tyrosine-phosphorylated after receptor activation. Proc Natl Acad Sci U S A. 1994 Apr 12;91(8):2985–2988. doi: 10.1073/pnas.91.8.2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi M., Nakamura Y., Russell S. M., Ziegler S. F., Tsang M., Cao X., Leonard W. J. Interleukin-2 receptor gamma chain: a functional component of the interleukin-7 receptor. Science. 1993 Dec 17;262(5141):1877–1880. doi: 10.1126/science.8266077. [DOI] [PubMed] [Google Scholar]
- Pellegrini S., Schindler C. Early events in signalling by interferons. Trends Biochem Sci. 1993 Sep;18(9):338–342. doi: 10.1016/0968-0004(93)90070-4. [DOI] [PubMed] [Google Scholar]
- Remillard B., Petrillo R., Maslinski W., Tsudo M., Strom T. B., Cantley L., Varticovski L. Interleukin-2 receptor regulates activation of phosphatidylinositol 3-kinase. J Biol Chem. 1991 Aug 5;266(22):14167–14170. [PubMed] [Google Scholar]
- Ruff-Jamison S., Chen K., Cohen S. Induction by EGF and interferon-gamma of tyrosine phosphorylated DNA binding proteins in mouse liver nuclei. Science. 1993 Sep 24;261(5129):1733–1736. doi: 10.1126/science.8378774. [DOI] [PubMed] [Google Scholar]
- Russell S. M., Keegan A. D., Harada N., Nakamura Y., Noguchi M., Leland P., Friedmann M. C., Miyajima A., Puri R. K., Paul W. E. Interleukin-2 receptor gamma chain: a functional component of the interleukin-4 receptor. Science. 1993 Dec 17;262(5141):1880–1883. doi: 10.1126/science.8266078. [DOI] [PubMed] [Google Scholar]
- Sadowski H. B., Shuai K., Darnell J. E., Jr, Gilman M. Z. A common nuclear signal transduction pathway activated by growth factor and cytokine receptors. Science. 1993 Sep 24;261(5129):1739–1744. doi: 10.1126/science.8397445. [DOI] [PubMed] [Google Scholar]
- Satoh T., Nakafuku M., Miyajima A., Kaziro Y. Involvement of ras p21 protein in signal-transduction pathways from interleukin 2, interleukin 3, and granulocyte/macrophage colony-stimulating factor, but not from interleukin 4. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3314–3318. doi: 10.1073/pnas.88.8.3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler C., Fu X. Y., Improta T., Aebersold R., Darnell J. E., Jr Proteins of transcription factor ISGF-3: one gene encodes the 91-and 84-kDa ISGF-3 proteins that are activated by interferon alpha. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7836–7839. doi: 10.1073/pnas.89.16.7836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler C., Kashleva H., Pernis A., Pine R., Rothman P. STF-IL-4: a novel IL-4-induced signal transducing factor. EMBO J. 1994 Mar 15;13(6):1350–1356. doi: 10.1002/j.1460-2075.1994.tb06388.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler C., Shuai K., Prezioso V. R., Darnell J. E., Jr Interferon-dependent tyrosine phosphorylation of a latent cytoplasmic transcription factor. Science. 1992 Aug 7;257(5071):809–813. doi: 10.1126/science.1496401. [DOI] [PubMed] [Google Scholar]
- Schmitt-Ney M., Happ B., Ball R. K., Groner B. Developmental and environmental regulation of a mammary gland-specific nuclear factor essential for transcription of the gene encoding beta-casein. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):3130–3134. doi: 10.1073/pnas.89.7.3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuai K., Horvath C. M., Huang L. H., Qureshi S. A., Cowburn D., Darnell J. E., Jr Interferon activation of the transcription factor Stat91 involves dimerization through SH2-phosphotyrosyl peptide interactions. Cell. 1994 Mar 11;76(5):821–828. doi: 10.1016/0092-8674(94)90357-3. [DOI] [PubMed] [Google Scholar]
- Shuai K. Interferon-activated signal transduction to the nucleus. Curr Opin Cell Biol. 1994 Apr;6(2):253–259. doi: 10.1016/0955-0674(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Shuai K., Schindler C., Prezioso V. R., Darnell J. E., Jr Activation of transcription by IFN-gamma: tyrosine phosphorylation of a 91-kD DNA binding protein. Science. 1992 Dec 11;258(5089):1808–1812. doi: 10.1126/science.1281555. [DOI] [PubMed] [Google Scholar]
- Shuai K., Ziemiecki A., Wilks A. F., Harpur A. G., Sadowski H. B., Gilman M. Z., Darnell J. E. Polypeptide signalling to the nucleus through tyrosine phosphorylation of Jak and Stat proteins. Nature. 1993 Dec 9;366(6455):580–583. doi: 10.1038/366580a0. [DOI] [PubMed] [Google Scholar]
- Silvennoinen O., Ihle J. N., Schlessinger J., Levy D. E. Interferon-induced nuclear signalling by Jak protein tyrosine kinases. Nature. 1993 Dec 9;366(6455):583–585. doi: 10.1038/366583a0. [DOI] [PubMed] [Google Scholar]
- Silvennoinen O., Schindler C., Schlessinger J., Levy D. E. Ras-independent growth factor signaling by transcription factor tyrosine phosphorylation. Science. 1993 Sep 24;261(5129):1736–1739. doi: 10.1126/science.8378775. [DOI] [PubMed] [Google Scholar]
- Silvennoinen O., Witthuhn B. A., Quelle F. W., Cleveland J. L., Yi T., Ihle J. N. Structure of the murine Jak2 protein-tyrosine kinase and its role in interleukin 3 signal transduction. Proc Natl Acad Sci U S A. 1993 Sep 15;90(18):8429–8433. doi: 10.1073/pnas.90.18.8429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K. A. Interleukin-2: inception, impact, and implications. Science. 1988 May 27;240(4856):1169–1176. doi: 10.1126/science.3131876. [DOI] [PubMed] [Google Scholar]
- Stahl N., Boulton T. G., Farruggella T., Ip N. Y., Davis S., Witthuhn B. A., Quelle F. W., Silvennoinen O., Barbieri G., Pellegrini S. Association and activation of Jak-Tyk kinases by CNTF-LIF-OSM-IL-6 beta receptor components. Science. 1994 Jan 7;263(5143):92–95. doi: 10.1126/science.8272873. [DOI] [PubMed] [Google Scholar]
- Stern J. B., Smith K. A. Interleukin-2 induction of T-cell G1 progression and c-myb expression. Science. 1986 Jul 11;233(4760):203–206. doi: 10.1126/science.3523754. [DOI] [PubMed] [Google Scholar]
- Takeshita T., Asao H., Ohtani K., Ishii N., Kumaki S., Tanaka N., Munakata H., Nakamura M., Sugamura K. Cloning of the gamma chain of the human IL-2 receptor. Science. 1992 Jul 17;257(5068):379–382. doi: 10.1126/science.1631559. [DOI] [PubMed] [Google Scholar]
- Turner B., Rapp U., App H., Greene M., Dobashi K., Reed J. Interleukin 2 induces tyrosine phosphorylation and activation of p72-74 Raf-1 kinase in a T-cell line. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1227–1231. doi: 10.1073/pnas.88.4.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velazquez L., Fellous M., Stark G. R., Pellegrini S. A protein tyrosine kinase in the interferon alpha/beta signaling pathway. Cell. 1992 Jul 24;70(2):313–322. doi: 10.1016/0092-8674(92)90105-l. [DOI] [PubMed] [Google Scholar]
- Wagner B. J., Hayes T. E., Hoban C. J., Cochran B. H. The SIF binding element confers sis/PDGF inducibility onto the c-fos promoter. EMBO J. 1990 Dec;9(13):4477–4484. doi: 10.1002/j.1460-2075.1990.tb07898.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakao H., Gouilleux F., Groner B. Mammary gland factor (MGF) is a novel member of the cytokine regulated transcription factor gene family and confers the prolactin response. EMBO J. 1994 May 1;13(9):2182–2191. doi: 10.1002/j.1460-2075.1994.tb06495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watling D., Guschin D., Müller M., Silvennoinen O., Witthuhn B. A., Quelle F. W., Rogers N. C., Schindler C., Stark G. R., Ihle J. N. Complementation by the protein tyrosine kinase JAK2 of a mutant cell line defective in the interferon-gamma signal transduction pathway. Nature. 1993 Nov 11;366(6451):166–170. doi: 10.1038/366166a0. [DOI] [PubMed] [Google Scholar]
- Weiss A., Littman D. R. Signal transduction by lymphocyte antigen receptors. Cell. 1994 Jan 28;76(2):263–274. doi: 10.1016/0092-8674(94)90334-4. [DOI] [PubMed] [Google Scholar]
- Wilks A. F., Harpur A. G., Kurban R. R., Ralph S. J., Zürcher G., Ziemiecki A. Two novel protein-tyrosine kinases, each with a second phosphotransferase-related catalytic domain, define a new class of protein kinase. Mol Cell Biol. 1991 Apr;11(4):2057–2065. doi: 10.1128/mcb.11.4.2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witthuhn B. A., Quelle F. W., Silvennoinen O., Yi T., Tang B., Miura O., Ihle J. N. JAK2 associates with the erythropoietin receptor and is tyrosine phosphorylated and activated following stimulation with erythropoietin. Cell. 1993 Jul 30;74(2):227–236. doi: 10.1016/0092-8674(93)90414-l. [DOI] [PubMed] [Google Scholar]
- Witthuhn B. A., Silvennoinen O., Miura O., Lai K. S., Cwik C., Liu E. T., Ihle J. N. Involvement of the Jak-3 Janus kinase in signalling by interleukins 2 and 4 in lymphoid and myeloid cells. Nature. 1994 Jul 14;370(6485):153–157. doi: 10.1038/370153a0. [DOI] [PubMed] [Google Scholar]
- Yamamoto K., Quelle F. W., Thierfelder W. E., Kreider B. L., Gilbert D. J., Jenkins N. A., Copeland N. G., Silvennoinen O., Ihle J. N. Stat4, a novel gamma interferon activation site-binding protein expressed in early myeloid differentiation. Mol Cell Biol. 1994 Jul;14(7):4342–4349. doi: 10.1128/mcb.14.7.4342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Z., Wen Z., Darnell J. E., Jr Stat3 and Stat4: members of the family of signal transducers and activators of transcription. Proc Natl Acad Sci U S A. 1994 May 24;91(11):4806–4810. doi: 10.1073/pnas.91.11.4806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Z., Wen Z., Darnell J. E., Jr Stat3: a STAT family member activated by tyrosine phosphorylation in response to epidermal growth factor and interleukin-6. Science. 1994 Apr 1;264(5155):95–98. doi: 10.1126/science.8140422. [DOI] [PubMed] [Google Scholar]
- Zmuidzinas A., Mamon H. J., Roberts T. M., Smith K. A. Interleukin-2-triggered Raf-1 expression, phosphorylation, and associated kinase activity increase through G1 and S in CD3-stimulated primary human T cells. Mol Cell Biol. 1991 May;11(5):2794–2803. doi: 10.1128/mcb.11.5.2794. [DOI] [PMC free article] [PubMed] [Google Scholar]