Abstract
Context:
Polycystic ovary syndrome (PCOS) is a triad of anovulation, insulin resistance, and hyperandrogenism. Androgen excess may correlate with metabolic risk and PCOS consensus criteria define androgen excess on the basis of serum T. Here we studied the utility of the androgen precursor serum androstenedione (A) in conjunction with serum T for predicting metabolic dysfunction in PCOS.
Patients and Methods:
Eighty-six PCOS patients fulfilling Rotterdam diagnostic consensus criteria and 43 age- and body mass index-matched controls underwent measurement of serum androgens by tandem mass spectrometry and an oral glucose tolerance test with homeostatic model assessment of insulin resistance and insulin sensitivity index calculation. We analyzed 24-hour urine androgen excretion by gas chromatography/mass spectrometry.
Results:
PCOS patients had higher levels of serum androgens and urinary androgen metabolites than controls (all P < .001). Within the PCOS cohort, both serum A and T were positively correlated with the free androgen index (T × 100/SHBG) and total androgen metabolite excretion (all P < .001). All subjects with T above the normal reference range [high T (HT)] also had high A (HA/HT group, n = 56). However, the remaining 30 patients had normal T levels, either in the presence of HA (HA/NT; n = 20) or normal A (NA/NT; n = 10). The groups did not differ in age or BMI. The HA/HT and HA/NT groups had higher total androgen excretion than NA/NT (P < .01 and P < .05, respectively). Multiple linear regression showed a strong negative association between serum androstenedione and insulin sensitivity. The incidence of dysglycemia according to an oral glucose tolerance test increased with the severity of androgen phenotype (NA/NT, 0%; HA/NT, 14%; HA/HT, 25%, P = .03).
Conclusion:
Simultaneous measurement of serum T and A represents a useful tool for predicting metabolic risk in PCOS women. HA levels are a sensitive indicator of PCOS-related androgen excess.
Polycystic ovary syndrome (PCOS) is defined by a clinical triad of anovulation, insulin resistance, and androgen excess (1). It has an estimated prevalence of 5%–10% in women of reproductive age and consumes most assisted conception health care budgets (2, 3). Diagnostic criteria for PCOS have changed significantly in recent years, acknowledging the heterogeneity of clinical phenotype (4, 5). The long-term health consequences of PCOS are increasingly recognized, ranging from metabolic dysfunction to increased cardiovascular events (6–8). Androgen excess remains the cardinal clinical feature that defines the condition and may contribute at least in part to the adverse metabolic phenotype.
However, a lack of clarity persists on which androgens should be measured, what constitutes normal reference ranges, and which analytical technique should be used (9). Equally we have little insight into whether different patterns of biochemical hyperandrogenemia define distinct PCOS subgroups in terms of metabolic risk. The most accurate strategy for quantification of biochemical androgen excess in PCOS is the measurement of total 24-hour urinary androgen metabolite excretion (10); however, urinary steroid profiling by gas chromatography/mass spectrometry (GC/MS) is time consuming and expensive. In routine clinical practice, serum total T remains the most commonly measured and widely available marker for the estimation of biochemical androgen excess (2). However, the measurement of T is severely limited by a number of factors including the following: 1) T circulates bound to SHBG and other proteins such as albumin, and only the unbound or free fraction enters into target tissues (11); 2) the measurement of free T by direct RIA is highly inaccurate (12); 3) direct immunoassays for total T are highly variable (13); and 4) T circulates in low nanomolar range concentrations, with the lower end of the normal female reference range below the limit of quantitation in all assays available.
Androgen excess in PCOS originates from the ovaries and adrenals (14); moreover, increased peripheral conversion of T to the most potent androgen 5α-dihydrotestosterone by systemically up-regulated 5α-reductase activity has been well documented (15, 16). Androstenedione (A) is the immediate precursor of T. In addition to direct secretion by ovaries and adrenals, A can be generated in peripheral tissues from its precursor dehydroepiandrosterone (DHEA) by the enzyme 3β-hydroxysteroid dehydrogenase type 2 (17). In addition to mainly ovarian production, about 25% of plasma T derives from the conversion from A by 17β-hydroxysteroid dehydrogenase type 5 (17β-HSD5 or AKR1C3) in adipose and other peripheral tissues (18). Serum A is inconsistently measured in routine clinical investigation of suspected PCOS because clinicians remain uncertain about its diagnostic value. Limited previous work suggests that 10% of PCOS patients may be misclassified as normoandrogenemic if A is not measured (19).
The independent health risks of androgen excess in women are increasingly apparent (20). We therefore hypothesized that patients with the coelevation of A and T and therefore increased circulating androgen burden represent a distinct subgroup with adverse metabolic parameters compared with those with normal androgens or solitary elevation of either A or T. We thus also hypothesized that A represents a more sensitive marker for the biochemical detection of androgen excess. We therefore carried out a detailed analysis of androgen synthesis and metabolism to test these hypotheses in a large cohort of women with PCOS according to Rotterdam criteria in comparison with a group of body mass index (BMI)-matched healthy controls.
Materials and Methods
Subjects
Women with PCOS were recruited from outpatient clinics at the Queen Elizabeth Hospital Birmingham and Birmingham Women's Hospital. This observational, cross-sectional study was approved by the South Birmingham Research Ethics Committee, and all participants gave their written informed consent. PCOS was diagnosed according to the Rotterdam European Society of Human Reproduction and Embryology 2004 criteria, with the presence of two or more of the following: oligo-/anovulation, clinical signs of hyperandrogenism, and polycystic ovaries on ultrasound (4). Other causes of oligomenorrhea and/or androgen excess were excluded by history, physical examination, and biochemical assessment. Healthy controls were recruited via local advertisement, with a diagnosis of PCOS excluded by clinical and biochemical parameters. Exclusion criteria for both groups were current or recent treatment with glucocorticoids, congenital adrenal hyperplasia, hyperprolactinemia, thyroid dysfunction, pregnancy, age younger than 18 or older than 45 years, oral contraceptive use within 3 months prior to recruitment, and diabetes mellitus.
Clinical protocol
Participants attended the National Institute of Health Research/Wellcome Trust Clinical Research Facility at the Queen Elizabeth Hospital Birmingham after an overnight fast. Each patient provided a 24-hour urine sample for urinary steroid metabolite analysis by GC/MS as previously reported (21). Baseline anthropometric assessment included height, weight, BMI (kilograms per square meter), and blood pressure measurement. A modified Ferriman-Gallwey scoring system for hirsutism was used as described previously (22). Dual-energy x-ray absorptiometry (DXA) was used for body composition assessment using a Hologic Discovery/W DXA (software version Apex 3.0; Hologic Inc). Specific fat phenotype was measured using android, gynecoid, peripheral (arms and legs), and trunk regions of interest, with subsequent calculation of android to gynecoid and trunk to peripheral fat ratios (23).
Blood samples were drawn for fasting plasma glucose and insulin and serum T, A, DHEA, DHEA sulfate (DHEAS), SHBG, FSH and LH, total cholesterol, high-density lipoprotein, and triglycerides. Each participant underwent a 75-g oral glucose tolerance test (OGTT) with blood sampling at 30-minute intervals for 2 hours. Glucose and insulin were measured using methods described previously (24). Subjects were categorized as having normal glucose tolerance or dysglycemia on the basis of 2-hour glucose values (normal glucose tolerance <7.8 mmol/L, dysglycemia ≥7.8 mmol/L). Homeostasis model assessment of insulin resistance (HOMA-IR) was calculated using the following formula: [fasting glucose (millimoles per liter) * fasting insulin (milliinternational units per liter)/22.5]. Insulin sensitivity index (ISI) was calculated using the following formula: ISI = [75 000 + (glucose0 − glucose120) × 1.15 × 180 × 0.19 × body weight (kg)]/120 × log (insulinmean) × glucosemean] =(mg·l2·mmol−1·mIU−1.min−1) (25, 26).
Serum and urine steroid measurements
Serum steroids were measured by liquid chromatography/tandem mass spectrometry (LC/MS-MS) using a Waters Xevo mass spectrometer with Acquity uPLC system. LC/MS-MS conditions were an electrospray ionization source with capillary voltage 4.0 kV, a source temperature of 150°C, and a desolvation temperature of 500°C. Serum steroid oxime analysis, which facilitates enhanced detection by formation of oxime derivatives of the steroid oxogroups (27), was used for the measurement of T, A, and DHEA and carried out in positive mode, whereas the measurement of serum DHEAS was performed in negative mode. T, A, and DHEA were extracted from 200 μL serum via liquid-liquid extraction using 1 mL tert-butyl-methyl-ether followed by derivatization into steroid oximes using 100 μL derivatization mixture (0.16 g hydroxylamine in 8 mL pyridine). For protein precipitation and extraction of DHEAS, 20 μL ZnSO4, 0.1 mM, and 100 μL acetonitrile were added to 20 μL serum before evaporation under constant nitrogen flow (28). All steroids were separated using an optimized gradient system consisting of methanol with 0.1% formic acid and quantified referring to a linear calibration series with appropriate internal standards. Each steroid was identified by matching retention times and two mass transitions in comparison with a deuterated reference compound.
Serum A and T were categorized as normal based on locally derived LC/MS-MS reference values for premenopausal women (T, 0.3–1.9 nmol/L; A, 1.4–7.4 nmol/L; percentiles of 2.5th to 97.5th of an unselected healthy female control cohort). Serum androgens were used to categorize PCOS patients into four subgroups: normal A/normal T (NA/NT), high A/normal T (HA/NT), normal A/high T (NA/HT), and high A/high T (HA/HT). The free androgen index (FAI) was calculated using the formula (T * 100)/SHBG. We also subdivided the PCOS patients into the four phenotypic subgroups arising from the Rotterdam criteria (29) as recommended at a recent National Institutes of Health meeting (3). These subgroups are defined by the presence of the following: 1) androgen excess, anovulation, and polcystic ovaries on ultrasound (AE+Anov+PCO), 2) Anov+PCO, 3) AE+PCO and 4) AE+Anov.
Urinary steroid metabolite excretion analysis was carried out by GC/MS as described previously (21). The sum of androsterone (An) and etiocholanolone (Et) was considered representative of active androgen metabolite excretion (10). Net systemic 5α-reductase activity was assessed by the ratios of An to Et and 5α-tetrahydrocortisol to tetrahydrocortisol (5α-THF/THF). 11β-hydroxysteroid dehydrogenase type 1 (11β-HSD1) activity was estimated using the ratio of the tetrahydrometabolites of cortisol (5α-THF + THF) to tetrahydrocortisone (THE) (30), and 11β-hydroxysteroid dehydrogenase type 2 (11β-HSD2) activity by the ratio of free urinary cortisol to cortisone. Total glucocorticoid metabolite excretion was assessed as the sum of 5α-THF + THF + THE + cortolones + cortols + cortisol + cortisone.
Statistical analysis
The Statistical Package for the Social Sciences (SPSS) version 21 was used for data analysis. All data are expressed as mean ± SD unless otherwise stated. Independent-samples t tests or Mann-Whitney U tests were used as appropriate for comparison between two groups. One-way ANOVA with post hoc Tukey testing was used for multiple comparisons between different groups. Correlation testing was performed using Pearson's correlation coefficient or Spearman's test as appropriate. Multiple linear regression was used to adjust for the confounding effects of different variables. Differences were considered statistically significant at P < .05.
Results
Baseline clinical and metabolic characteristics of PCOS and age- and BMI-matched healthy controls are shown in Table 1. Complete clinical, biochemical, and radiological data were available in 86 women with PCOS. Results were compared with those obtained from 43 age- and BMI-matched controls. Hirsutism, oligomenorrhea, and polycystic ovaries were present in 87%, 92%, and 73% of PCOS patients, respectively. Polycystic ovary subgroups arising from the Rotterdam criteria were matched for age and BMI and categorized as follows: 1) AE+Anov+PCO (n = 51, 59%); 2) AE+PCO (n = 6, 7%); 3) Anov+PCO (n = 9, 11%); and 4) AE+Anov (n = 20, 23%).
Table 1.
Clinical, Biochemical, and Metabolic Characteristics in the Overall PCOS Cohort (n = 86), PCOS Androgen Phenotype Subgroups, and Age- and BMI-Matched Controls (n = 43)
| Variable | Controls (n = 43) | All PCOS (n = 86) | NA/NT (n = 10) | HA/NT (n = 20) | HA/HT (n = 56) |
|---|---|---|---|---|---|
| Age, y | 32.4 ± 9.8 | 30.0 ± 7.2 | 32.9 ± 2.9 | 30.8 ± 7.9 | 28.8 ± 7.0 |
| BMI, kg/m2 | 30.3 ± 6.4 | 31.9 ± 7.1 | 30.2 ± 6.5 | 30.0 ± 5.9 | 32.1 ± 6.5 |
| Ferriman-Gallwey score | 2.1 ± 1.6 | 12.4 ± 6.7a | 9.9 ± 5.3a | 10.1 ± 6.4a | 13.9 ± 7.2a,b |
| T, nmol/L | 1.3 ± 0.4 | 2.2 ± 0.8a | 1.2 ± 0.3 | 1.6 ± 0.4 | 2.6 ± 0.7a,b |
| FAI (T × 100/SHBG) | 3.2 ± 1.8 | 9.1 ± 6.1a | 5.5 ± 3.9d | 7.3 ± 4.6d | 10.5 ± 6.5a,b |
| SHBG, nmol/L | 49.8 ± 26.6 | 32.3 ± 18.4a | 28.8 ± 11.8a | 30.8 ± 21.2a | 31.8 ± 16.5a |
| A, nmol/L | 3.5 ± 1.4 | 13.4 ± 5.6a | 5.1 ± 1.7 | 11.4 ± 4.2a,c | 14.2 ± 5.1a,c |
| DHEA, nmol/L | 20.5 ± 7.9 | 57.5 ± 30.0a | 24.4 ± 8.6 | 59.8 ± 34.8a,e | 58.0 ± 26.8a,e |
| DHEAS, μmol/L | 3.9 ± 2.0 | 6.2 ± 3.0a | 2.4 ± 1.2 | 6.3 ± 2.6c | 6.2 ± 2.7a,c |
| DHEA to DHEAS ratio | 7.8 ± 3.7 | 12.9 ± 6.8d | 12.6 ± 8.5d | 14.0 ± 10.0d | 15.4 ± 5.4d |
| LH, IU/L | 5.2 ± 2.4 | 9.9 ± 5.8d | 4.7 ± 3.2 | 9.0 ± 6.3d | 11.4 ± 9.2b,d |
| LH to FSH ratio | 0.8 ± 0.5 | 2.0 ± 1.5d | 0.9 ± 0.7 | 2.1 ± 1.9d | 2.3 ± 1.4b,d |
| Fasting glucose, mmol/L | 4.6 ± 0.5 | 4.8 ± 0.7 | 5.0 ± 0.5 | 4.7 ± 0.5 | 4.8 ± 0.8 |
| Two-hour OGTT glucose, mmol/L | 6.7 ± 1.5 | 6.5 ± 2.2 | 5.5 ± 1.2 | 6.7 ± 1.9 | 6.7 ± 2.3 |
| Fasting insulin, mIU/L | 2.0 ± 1.5 | 10.4 ± 8.6f | 8.2 ± 5.5d | 11.7 ± 8.3d | 12.3 ± 6.6a |
| HOMA-IR | 0.9 ± 0.8 | 2.3 ± 0.2f | 1.9 ± 1.1d | 2.4 ± 1.5d | 2.8 ± 1.0b,f |
| ISI (Cederholm), mg/L2/mmol · mU · min) (25) | 78.7 ± 38.0 | 54.6 ± 24.1a | 73.4 ± 54.6 | 51.0 ± 17.1a,f | 54.4 ± 25.2a,f |
| Dysglycemia, n, % | 3 (6.9%) | 17 (19.7%)d | 0 | 3 (15%) | 14 (25%)b,d |
Abbreviation: HOMA-IS, homeostatic model assessment of insulin sensitivity. PCOS subgroup classification according to high (H) or normal (N) serum A and serum T concentrations as measured by LC/MS-MS [normal T (NT) ≤1.9 nmol/L; normal A (NA) ≤7.4 nmol/L]. Data are expressed as mean ± SD unless otherwise stated. Normal glucose tolerance was a 2-hour glucose OGTT 7.7 mmol/L or less. Dysglycemia was a 2-h glucose OGTT 7.8 mmol/L or greater.
P < .001 as compared with the BMI-matched healthy controls
P < .05 as compared with the NA/NT group.
P < .001 as compared with the NA/NT group.
P < .05 as compared with the BMI-matched healthy controls.
P < .01 as compared with the NA/NT group.
P < .01 as compared with the BMI-matched healthy controls.
Circulating androgens and androgen excretion
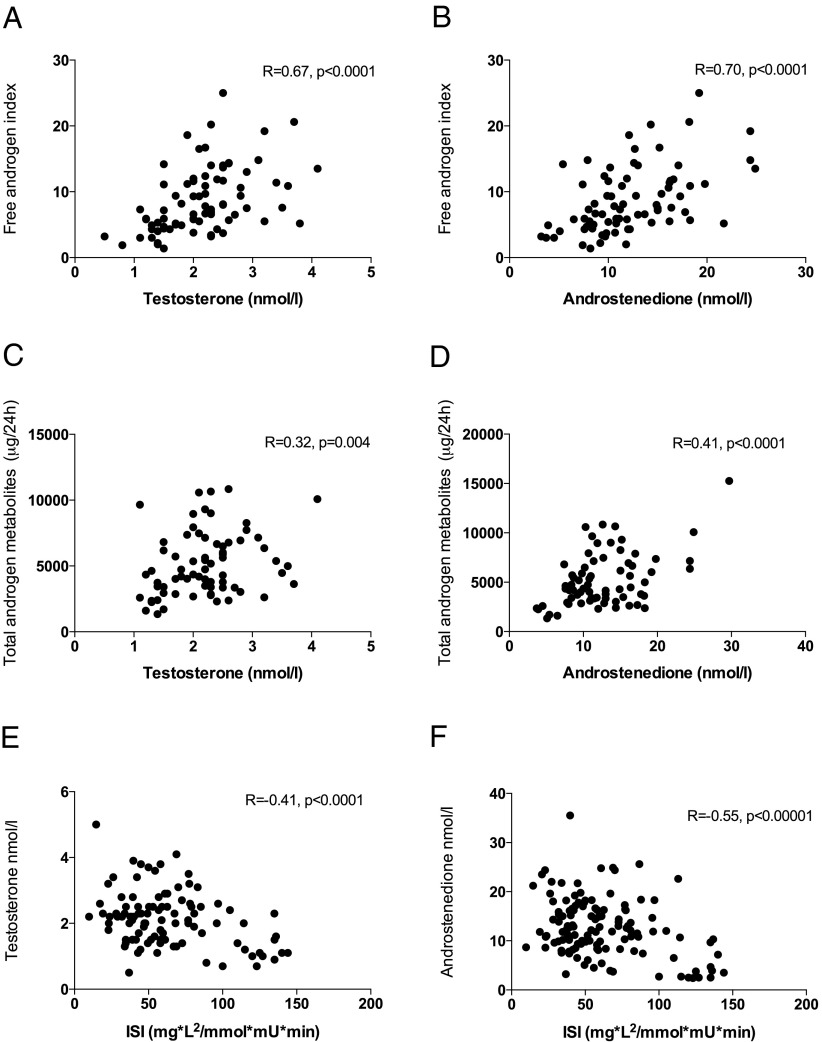
Both serum A and T were significantly higher in PCOS than in healthy controls (P < .0001 for both, Table 1). PCOS patients also had significantly higher FAI, DHEA, and DHEAS (all P < .0001) than controls. Total 24-hour urinary androgen excretion was also significantly higher in PCOS (P < .0001, Table 2), with higher excretion of An (P < .001), Et (P < .05), and DHEA (P < .05). T and A correlated strongly with FAI and total androgen metabolite excretion (Figure 1, A–D).
Table 2.
Twenty-Four-Hour Urinary Steroid Excretion in PCOS Patients, Controls, and PCOS Androgen Phenotype Subgroups
| Variable | Controls (n = 43) | All PCOS (n = 86) | NA/NT (n = 10) | HA/NT (n = 20) | HA/HT (n = 56) |
|---|---|---|---|---|---|
| Total androgen metabolites, μg per 24 h | 2882 ± 2018 | 5493 ± 2781a | 2751 ± 1760 | 4886 ± 1968b,c | 5727 ± 2530a,c |
| An, μg per 24 h | 1413 ± 906 | 2824 ± 1651a | 1452 ± 1178 | 2594 ± 1207b | 3016 ± 1553a,c |
| Et, μg per 24 h | 1468 ± 1208 | 2457 ± 725a | 1299 ± 733 | 2292 ± 1211 | 2683 ± 1469b,d |
| An/Et ratio (5α-reductase activity) | 1.1 ± 0.4 | 1.3 ± 0.6b | 1.2 ± 0.6 | 1.3 ± 0.6 | 1.3 ± 0.6 |
| Urinary DHEA, μg per 24 h | 457 ± 721 | 1740 ± 954b | 477 ± 400 | 1586 ± 960 | 1960 ± 854b,c |
| Total glucocorticoid metabolites, μg per 24 h | 6821 ± 3572 | 9624 ± 4214e | 8449 ± 4181 | 8935 ± 3404 | 9803 ± 4390b |
| 5α-THF, μg per 24 h | 940 ± 608 | 1313 ± 814b | 1250 ± 1135 | 1259 ± 759 | 1359 ± 842e |
| THF, μg per 24 h | 1206 ± 607 | 1520 ± 549b | 1489 ± 924 | 1477 ± 475 | 1634 ± 822b |
| 5α-THF/THF (5α-reductase activity) | 0.8 ± 0.3 | 0.9 ± 0.5 | 0.8 ± 0.4 | 0.8 ± 0.5 | 0.9 ± 0.5 |
| (5α-THF+THF)/THE (11β-HSD1 activity) | 0.9 ± 0.3 | 0.9 ± 0.3 | 0.9 ± 0.3 | 0.9 ± 0.2 | 0.9 ± 0.4 |
| Urinary cortisol/urinary cortisone (11β-HSD2 activity) | 0.6 ± 0.5 | 0.6 ± 0.2 | 0.5 ± 0.1 | 0.6 ± 0.2 | 0.7 ± 0.2 |
Abbreviations: total androgen metabolites, sum of An+Et; total glucocorticoid metabolites, sum of THF+5α-THF+THE+cortols+cortolones+free cortisol/cortisone. Androgen and glucocorticoid metabolites were measured by GC/MS. The PCOS subgroup classification was according to high (H) or normal (N) A and T concentrations as measured by LC/MS-MS [normal T (NT) ≤1.9 nmol/L; normal A (NA) ≤7.4 nmol/L]. Data are expressed as mean ± SD unless otherwise stated.
P < .001 as compared with the BMI-matched healthy controls
P < .05 as compared with the BMI-matched healthy controls.
P < .05 as compared with the NA/NT group.
P < .01 as compared with the NA/NT group.
P < .01 as compared with the BMI-matched healthy controls.
Figure 1.
A–F, Relationship of serum T and A with FAI, total urinary androgen metabolites, and ISI. There is a significant positive relationship between both T and A with total androgen excretion and the FAI; a negative association with ISI is observed.
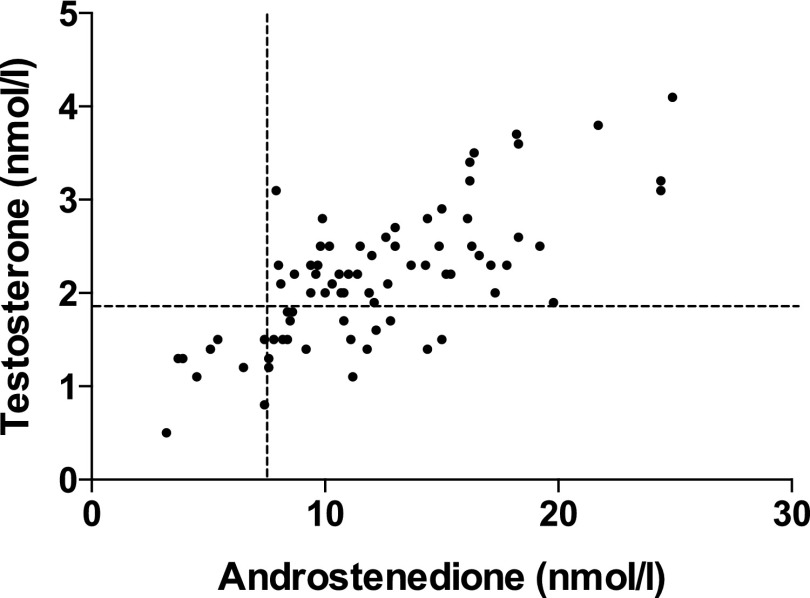
Serum A was elevated in 76 of 86 PCOS patients (88.3%); T was elevated in 56 (65.1%). A and T were elevated in 1 of 43 (2.3%) and 5 of 43 (11.6%) healthy controls, respectively. Serum levels of A and T were in the normal range in 10 and 30 PCOS patients, respectively. Patients with PCOS were subsequently divided into four androgen phenotype groups dependent on circulating androgens; NA/NT, NA/HT, HA/NT, and the most severe degree of androgen excess, HA/HT. Most patients were categorized as HA/HT (n = 56), with 20 and 10 patients classified as HA/NT and NA/NT, respectively; no patient had high T but normal A (Figure 2).
Figure 2.
Relationship of serum T and A with stratification of androgenemic subgroups: NA/NT (n = 10); HA/NT (n = 20); and HA/HT (n = 56). No individuals were identified with NA/HT (n = 0).
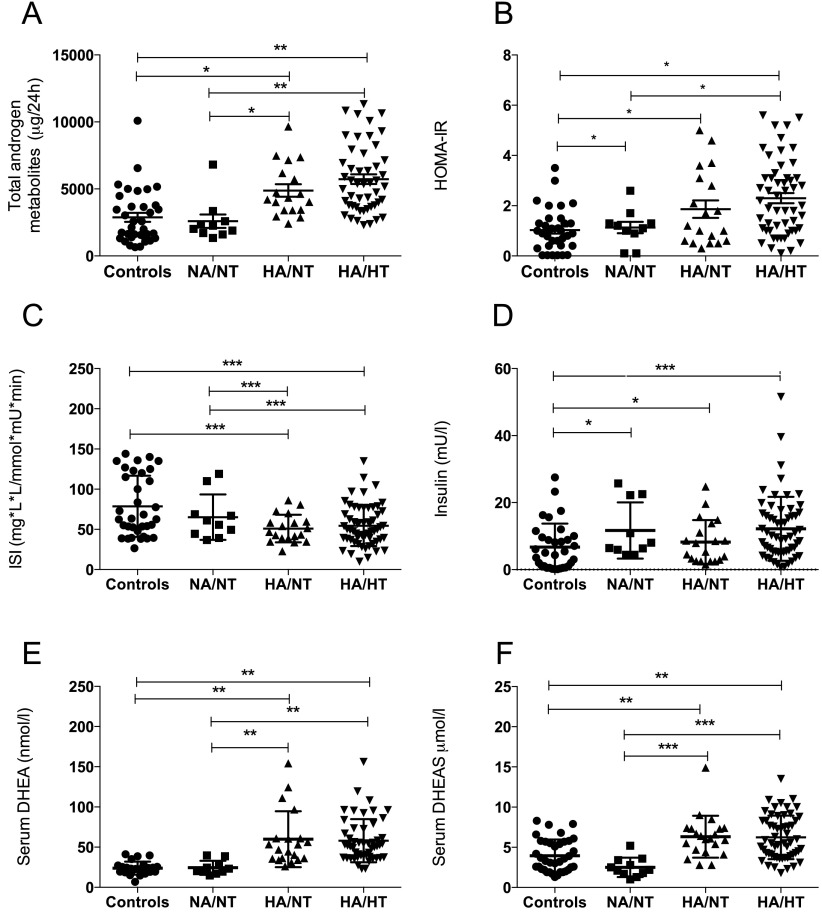
Serum androgen levels in the PCOS women with the mildest degree of biochemical androgen excess (NA/NT) were similar to those in healthy BMI-matched controls (Table 1). However, they differed significantly from controls with regard to SHBG and FAI. NA/NT women had androgen excretion rates similar to controls, whereas androgen excretion in the two other PCOS groups was significantly higher (Table 2 and Figure 3A). Similarly, serum DHEA and DHEAS were significantly higher in HA/HT and HA/NT patients as compared with the NA/NT group and the controls (Figure 3, E and F). The Ferriman-Gallwey score was significantly higher in HA/HT women compared with NA/NT (P = .04) (Table 1).
Figure 3.
Total androgen metabolites (A), HOMA-IR (B), ISI (C), fasting insulin (D), serum DHEA (E), and DHEAS (F) in the three androgen phenotype PCOS subgroups and healthy controls. *, P < .05; **, P < .01; ***, P < .001.
Metabolic characteristics
Overall, PCOS patients had higher fasting insulin and HOMA-IR values than BMI-matched controls, but fasting glucose and 2-hour values did not differ significantly (Table 1). The incidence of dyglycemia was 19.7% in the PCOS group compared with 6.9% in controls (P = .03) (Table 1). BMI was positively correlated with FAI (R = 0.4, P < .0001) and systemic 5α-reductase activity (An/Et, R = 0.21, P = .01; 5α-THF + THF, R = 0.20, P = .02). ISI values were lower in PCOS patients compared with controls (P < .001, Table 1). The ISI had a strong negative correlation with serum T and A (R = −0.41, P < .001, and R = −0.55, P < .00001, respectively, Figure 1, E and F). When PCOS and control patients were considered separately, a negative association between A and ISI was observed in both groups (R = −0.53, P < .00001, and R = −0.41, P < .001, respectively). In obese control patients, this negative association between A and ISI persists (R = −0.25, P = .04). On multiple linear regression analysis, a significant negative association was confirmed only between A and ISI [coefficient −2.69, 95% confidence interval (CI) −1.57 to −3.81, P < .0001, Supplemental Table 1, published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org]; no such association was observed between ISI and serum T, DHEAS, or DHEA.
HOMA-IR in all three PCOS subgroups was significantly higher than in controls and HA/HT women had significantly higher values than NA/NT women (P < .05 for both) (Figure 3B). However, only HA/NT and HA/HT had lower ISI values (Table 1). PCOS women in the NA/NT group had significantly higher fasting insulin and HOMA-IR than BMI-matched controls (Table 1). The rate of dysglycemia in the HA/NT and HA/HT groups was 15% and 25%, respectively; no NA/NT women were dysglycemic. No significant differences were observed in systolic blood pressure or lipid profiles between each group. No difference in insulin sensitivity was observed between the four phenotypic groups defined by the Rotterdam criteria (Supplemental Figure 1).
Body composition
No significant differences in fat distribution or total lean mass on DXA were observed between PCOS and control women or between the three androgen phenotype or Rotterdam phenotype groups (see Supplemental Table 2). The android to gynecoid ratio was strongly correlated with HOMA-IR (R = 0.53, P < .0001), FAI (R = 0.37, P < .0001), An/Et (R = 0.38, P < .0001), 5α-THF/THF (R = 0.31, P < .0001) and total glucocorticoid metabolites (R = 0.26, P = .01). There was a strong negative correlation between the android to gynecoid ratio and ISI (R = −0.41, P < .01).
Cortisol metabolism
PCOS patients and controls did not differ significantly in indices of 11β-HSD1 or 11β-HSD2 activity (Table 2). However, total glucocorticoid excretion was higher in the PCOS group (9624 ± 4214 vs 6821 ± 3572 μg per 24 h, P = .01). Urinary glucocorticoid excretion appeared to gradually increase with the degree of androgen excess, with significantly higher levels in the HA/HT group than in controls (Table 2). On multiple linear regression (Supplemental Table 1), total glucocorticoid excretion was a negative predictor of ISI (coefficient −0.003, 95% CI −0.001 to −0.005, P = .048).
Discussion
We have performed a detailed assessment of the clinical, metabolic and androgen phenotype of a cohort of PCOS, revealing that PCOS patients with coelevation of A and T have impaired indices of insulin sensitivity compared with those with normal androgens or milder hyperandrogenemia. Using multiple linear regression, we found a strong negative relationship between A and insulin sensitivity as measured by the ISI, independent of age and BMI; such a relationship was not observed for T. All our patients were diagnosed according to consensus criteria, fulfilling at least two of three criteria. Until relatively recently, it has been unclear whether women with hyperandrogenic PCOS are at increased cardiometabolic risk compared with those without androgen excess. However, recent data suggest that biochemical androgen excess poses a higher risk of liver disease, insulin resistance, and subclinical atherosclerosis compared with those with anovulation and polycystic ovaries alone (20, 31, 32). These studies suggest that hyperandrogenic PCOS represents a distinct metabolic phenotype, whereas patients without androgen excess may have a phenotype similar to healthy controls (33). However, in our cohort we also found higher fasting insulin values in normoandrogenemic PCOS compared with healthy controls, suggesting that women without androgen excess are not entirely protected from metabolic dysfunction.
Women with hyperandrogenemic PCOS in our cohort had a significantly reduced ISI; those with normal serum androgens had ISI values comparable with healthy controls. It is interesting that HOMA-IR and homeostatic model assessment of insulin sensitivity values were still unfavorable in the NA/NT group, suggestive perhaps of impaired fasting indices but preserved ability to handle an oral glucose load. Further studies are needed to corroborate and understand the associations observed here. Serum androgens, and in particular A, had a strong negative association with the ISI; it is interesting that this association was also observed in control patients with circulating A levels within the normal range. These data lend support to the hypothesis that metabolic disease in PCOS should be considered as a continuous variable, with metabolic dysfunction observed across a worsening spectrum of severity of androgen excess. It is important to remember that normal reference ranges are relatively arbitrarily defined and that androgen exposure across all patients may contribute to their metabolic phenotype. Women with normal circulating androgens may still exhibit subtle hyperandrogenemia after LH stimulation (34), which could explain the higher indices of insulin resistance, lower SHBG, and higher FAI levels observed. The long-term implications of this are unclear without longitudinal, prospective studies, but these findings have also been observed by Barber et al (31). When we subdivided our PCOS cohort into the four phenotypic groups arising from the Rotterdam consensus (Supplemental Figure 1), no significant differences were observed in insulin sensitivity or body composition between each group. It seems reasonable to argue that this strategy of subphenotyping of PCOS patients may not accurately predict those at highest metabolic risk. Admittedly, the AE+Anov+ PCO group comprised most patients, but a similar distribution of PCOS patients was also noted by Barber et al (31). Interestingly, Barber et al found that patients in their AE+Anov+PCO subgroup were more insulin resistant; however, the groups were not BMI matched, unlike in our study. For this reason we believe that comeasurement of A and T might offer a better alternative in terms of metabolic risk prediction than the previously suggested categorization by phenotype.
It is crucial that PCOS patients are correctly and accurately categorized as normoandrogenemic or hyperandrogenemic if they are to be stratified into low- and high-risk metabolic groups on this basis. Our data show that the measurement of serum T alone fails to adequately make this differentiation. Elaborate investigative tools such as LH stimulation testing or 24-hour urinary androgen metabolite excretion are not universally available in routine clinical practice. By contrast, concurrent measurement of serum T and A provides a suitable alternative. In our view, only those with normal serum levels of both androgens should be categorized as normoandrogenemic; as discussed above, even this cohort may still exhibit occult hyperandrogenism, and without prospective studies it is impossible to be certain whether they are protected from more severe metabolic disease over time. To date, there are only very limited data on the effects of androgen-lowering therapies on metabolic parameters in PCOS (35, 36), and clearly larger prospective trials are needed.
Only 65% of our patients had elevated serum T, whereas 88% had serum A concentrations above the reference range. In addition, more than two thirds of the women initially categorized as normoandrogenemic on the basis of a normal T had androgen excess as documented by increased serum A levels, revealing that the proportion of patients with biochemical androgen excess is significantly larger than previously reported (19). Based on the supposition that none of our controls have occult PCOS, this translates into a sensitivity of 88.3% (95% CI 79.7–94.3%) and a specificity of 97.7% (95% CI 87.7–99.6%) for A to detect androgen excess. Sensitivity and specificity values for T of 65.1% (95% CI 54.1–75.1%) and 88.3% (95% CI 74.9–96.1%) are less impressive. If we consider the age-related normative reference values for A in 985 women described by Haring et al (37), the demarcation between the two groups would improve the sensitivity even further, with minimum reduction of specificity.
Although serum LH values increased across each subgroup, no significant difference was observed between NA/NT women and healthy controls. We would not recommend the use of LH or the LH to FSH ratio as investigative tools in a routine PCOS workup. LH values fluctuate widely across the menstrual cycle and accurate blood sampling is inconsistently timed in the early follicular phase. It is also very unreliable in women with oligo-ovulation (29). Similarly, fasting insulin levels and HOMA-IR values were noted to increase across each androgenemic subgroup; however, these surrogate markers in isolation clearly cannot be used as a diagnostic marker for PCOS due to poor specificity, failing to accurately differentiate between PCOS-driven and multifactorial insulin resistance (7).
There have been many attempts over the last 25 years to define PCOS (1, 4, 5). Each definition focuses on hyperandrogenism and hyperandrogenemia, yet few attempts have been made to precisely define either term, and biochemical definitions remain particularly elusive. Previous work has advocated measurement of a single morning serum T sample during the early follicular phase as the diagnostic criterion for hyperandrogenemia (9). The limitations of total T as a solitary diagnostic tool for hyperandrogenemia have been described above. The clearance and bioavailability of T are affected by serum SHBG levels, which are lowered by hyperinsulinemia, but A levels are not affected by this phenomenon (38), and this may explain some of the observed discrepancies between T and A measurements. Appropriateness and reliability of analytical methods has equally been a major issue in this debate, and The Endocrine Society has appealed to international laboratories to refine techniques for androgen measurement in women (39). However, the advent of LC/MS-MS has dramatically improved the rapid detection and reliable quantification of serum steroids in both clinical and research practice (40). Accurate A measurement could now emerge from these developments as a sensitive diagnostic test for hyperandrogenemia in PCOS.
Patterns of body fat distribution on DXA did not differ between PCOS and control patients, supporting previously published work (41), nor were there differences in fat distribution between the androgenemic subgroups. These and other studies point toward a qualitative rather than quantitative defect in adipose biology in PCOS (42). 17β-HSD5 is expressed in adipose tissue, and significant extraovarian conversion to T may occur in obesity (18). 17β-HSD5 is also highly expressed in the ovary, and activating variants result in a PCOS phenotype with severe insulin resistance (43). This suggests a possible primary role for androgen excess in insulin resistance. However, the association of insulin resistance and hyperandrogenism could equally point toward an insulin-mediated effect on ovarian thecal steroidogenesis. The observation that insulin plays a key role in thecal androgen generation in vivo and in vitro supports this hypothesis (44, 45). Insulin-stimulated A generation from the ovarian theca may create an enlarged circulating pool for conversion to T, and elevated levels of the former may therefore be a surrogate marker for metabolic dysfunction.
A is also produced in significant quantities by the adrenal gland, but so far, studies on the impact of hyperinsulinism on adrenal androgen production are lacking. Impaired DHEA sulfation due to 3′-phosphoadenosine 5′-phosphosulfate synthase-2 deficiency has been shown to result in a PCOS phenotype (46). A recent paper described two sulfotransferase-2A1 genetic variants that were associated with an increase in the DHEA to DHEAS ratio in a large PCOS cohort (47); in our cohort, 10% of the PCOS patients were found to have a highly elevated DHEA level but low normal serum DHEAS, indicative of reduced DHEA sulfation capacity.
One limitation of our study is its cross-sectional nature. Whether the concurrent presence of elevated A and T corresponds to an increased risk of cardiovascular disease or a worse outcome in terms of hard clinical end points can be ascertained only by prospective studies. Longitudinal studies will reveal whether initial increases in androgen precursors later followed by additional increases in T may represent a sequence over time in individual patients. Although HOMA-IR measurement is a limited marker of insulin resistance, the ISI devised by Cederholm and Wibell (25) calculated in our cohort has been shown to perform with comparable accuracy to a hyperinsulinemic euglycemic clamp (∼R = 0.8). Similarly, it was not possible to measure serum androgens in the early follicular phase of all patients, largely due to the fact that most PCOS patients were oligo- or amenorrhoeic. Finally, the debate over what constitutes normal androgen values continues, and quoted reference ranges refer to normative values in 95% of an unselected presumptively healthy female population, implying that abnormal levels will be observed in 5% of women with no evidence of endocrinopathy.
In conclusion, these data endorse the hypothesis that increasing androgen burden is associated with an adverse metabolic phenotype and that concurrent measurement of both A and T highlights a PCOS cohort that appears to be at increased metabolic risk. We also believe on the basis of these data that serum A is a more sensitive indicator of PCOS-related androgen excess than serum total T concentrations. The utility of this novel strategy for predicting metabolic risk in PCOS women warrants investigation in prospective and longitudinal studies and these may ultimately afford a personalized approach to the management of PCOS.
Acknowledgments
We thank the nurses of the National Institute of Health Research/ Wellcome Trust Clinical Research Facility, University Hospital Birmingham National Health Service Foundation Trust (Birmingham, United Kingdom), for their help with patient recruitment and running of the study. We are also indebted to all the patients and healthy volunteers who participated in this study.
This work was supported by Wellcome Trust Clinical Research Training Fellowship 099909 (to M.W.O.), Wellcome Trust Program Grant 082809 (to P.M.S.), and Wellcome Trust Project Grant 092283 (to W.A.).
Disclosure Summary: The authors have nothing to declare.
For editorial see page 777
- A
- androstenedione
- AE+Anov
- androgen excess and anovulation
- AE+Anov+PCO
- androgen excess, anovulation, and polcystic ovaries on ultrasound
- An
- androsterone
- Anov+PCO
- anovulation and polcystic ovaries on ultrasound
- BMI
- body mass index
- CI
- confidence interval
- DHEA
- dehydroepiandrosterone
- DHEAS
- DHEA sulfate
- DXA
- dual-energy x-ray absorptiometry
- Et
- etiocholanolone
- FAI
- free androgen index
- GC/MS
- gas chromatography/mass spectrometry
- HA/HT
- high A and also T above the normal reference range
- HA/NT
- high A and normal T levels
- HOMA-IR
- homeostasis model assessment of insulin resistance
- 11β-HSD1
- 11β-hydroxysteroid dehydrogenase type 1
- 17β-HSD5
- 17β-hydroxysteroid dehydrogenase type 5
- ISI
- insulin sensitivity index
- LC/MS-MS
- liquid chromatography/tandem mass spectrometry
- NA/HT
- normal A and T above the normal reference range
- NA/NT
- normal A and normal T levels
- OGTT
- oral glucose tolerance test
- PCOS
- polycystic ovary syndrome
- THE
- tetrahydrocortisone
- 5α-THF/THF
- 5α-tetrahydrocortisol to tetrahydrocortisol
- 5α-THF + THF
- tetrahydrometabolites of cortisol.
References
- 1. Franks S. Polycystic ovary syndrome. N Engl J Med. 1995;333:853–861 [DOI] [PubMed] [Google Scholar]
- 2. Azziz R, Woods KS, Reyna R, Key TJ, Knochenhauer ES, Yildiz BO. The prevalence and features of the polycystic ovary syndrome in an unselected population. J Clin Endocrinol Metab. 2004;89:2745–2749 [DOI] [PubMed] [Google Scholar]
- 3. Fauser BC, Tarlatzis BC, Rebar RW, et al. Consensus on women's health aspects of polycystic ovary syndrome (PCOS): The Amsterdam ESHRE/ASRM-sponsored 3rd PCOS Consensus Workshop Group. Fertil Steril. 2012;97:28–38.e25 [DOI] [PubMed] [Google Scholar]
- 4. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod. 2004;19:41–47 [DOI] [PubMed] [Google Scholar]
- 5. Azziz R, Carmina E, Dewailly D, et al. The Androgen Excess and PCOS Society criteria for the polycystic ovary syndrome: the complete task force report. Fertil Steril. 2009;91:456–488 [DOI] [PubMed] [Google Scholar]
- 6. Mani H, Levy MJ, Davies MJ, et al. Diabetes and cardiovascular events in women with polycystic ovary syndrome: a 20-year retrospective cohort study. Clin Endocrinol (Oxf). 2013;78:926–934 [DOI] [PubMed] [Google Scholar]
- 7. Randeva HS, Tan BK, Weickert MO, et al. Cardiometabolic aspects of the polycystic ovary syndrome. Endocr Rev. 2012;33:812–841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Franks S. The investigation and management of hirsutism. J Fam Plann Reprod Health Care. 2012;38:182–186 [DOI] [PubMed] [Google Scholar]
- 9. Barth JH, Yasmin E, Balen AH. The diagnosis of polycystic ovary syndrome: the criteria are insufficiently robust for clinical research. Clin Endocrinol (Oxf). 2007;67:811–815 [DOI] [PubMed] [Google Scholar]
- 10. Silfen ME, Shackleton CH, Manibo AM, et al. 5α-Reductase and 11β-hydroxysteroid dehydrogenase activity in prepubertal Hispanic girls with premature adrenarche. J Clin Endocrinol Metab. 2002;87:4647–4651 [DOI] [PubMed] [Google Scholar]
- 11. Pardridge WM. Serum bioavailability of sex steroid hormones. Clin Endocrinol Metab. 1986;15:259–278 [DOI] [PubMed] [Google Scholar]
- 12. Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab. 1999;84:3666–3672 [DOI] [PubMed] [Google Scholar]
- 13. Boots LR, Potter S, Potter D, Azziz R. Measurement of total serum testosterone levels using commercially available kits: high degree of between-kit variability. Fertil Steril. 1998;69:286–292 [DOI] [PubMed] [Google Scholar]
- 14. Loughlin T, Cunningham S, Moore A, Culliton M, Smyth PP, McKenna TJ. Adrenal abnormalities in polycystic ovary syndrome. J Clin Endocrinol Metab. 1986;62:142–147 [DOI] [PubMed] [Google Scholar]
- 15. Stewart PM, Shackleton CH, Beastall GH, Edwards CR. 5 α-Reductase activity in polycystic ovary syndrome. Lancet. 1990;335:431–433 [DOI] [PubMed] [Google Scholar]
- 16. Fassnacht M, Schlenz N, Schneider SB, Wudy SA, Allolio B, Arlt W. Beyond adrenal and ovarian androgen generation: Increased peripheral 5α-reductase activity in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2003;88:2760–2766 [DOI] [PubMed] [Google Scholar]
- 17. Ehrmann DA, Barnes RB, Rosenfield RL. Polycystic ovary syndrome as a form of functional ovarian hyperandrogenism due to dysregulation of androgen secretion. Endocr Rev. 1995;16:322–353 [DOI] [PubMed] [Google Scholar]
- 18. Quinkler M, Sinha B, Tomlinson JW, Bujalska IJ, Stewart PM, Arlt W. Androgen generation in adipose tissue in women with simple obesity—a site-specific role for 17β-hydroxysteroid dehydrogenase type 5. J Endocrinol. 2004;183:331–342 [DOI] [PubMed] [Google Scholar]
- 19. Knochenhauer ES, Key TJ, Kahsar-Miller M, Waggoner W, Boots LR, Azziz R. Prevalence of the polycystic ovary syndrome in unselected black and white women of the southeastern United States: a prospective study. J Clin Endocrinol Metab. 1998;83:3078–3082 [DOI] [PubMed] [Google Scholar]
- 20. Jones H, Sprung VS, Pugh CJ, et al. Polycystic ovary syndrome with hyperandrogenism is characterized by an increased risk of hepatic steatosis compared to nonhyperandrogenic pcos phenotypes and healthy controls, independent of obesity and insulin resistance. J Clin Endocrinol Metab. 2012;97:3709–3716 [DOI] [PubMed] [Google Scholar]
- 21. Arlt W, Walker EA, Draper N, et al. Congenital adrenal hyperplasia caused by mutant p450 oxidoreductase and human androgen synthesis: analytical study. Lancet. 2004;363:2128–2135 [DOI] [PubMed] [Google Scholar]
- 22. Yildiz BO, Bolour S, Woods K, Moore A, Azziz R. Visually scoring hirsutism. Hum Reprod Update. 2010;16:51–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gregson CL, Paggiosi MA, Crabtree N, et al. Analysis of body composition in individuals with high bone mass reveals a marked increase in fat mass in women but not men. J Clin Endocrinol Metab. 2013;98:818–828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vassiliadi DA, Barber TM, Hughes BA, et al. Increased 5α-Reductase activity and adrenocortical drive in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2009;94:3558–3566 [DOI] [PubMed] [Google Scholar]
- 25. Cederholm J, Wibell L. Insulin release and peripheral sensitivity at the oral glucose tolerance test. Diabetes Res Clin Pract. 1990;10:167–175 [DOI] [PubMed] [Google Scholar]
- 26. Stumvoll M, Mitrakou A, Pimenta W, et al. Use of the oral glucose tolerance test to assess insulin release and insulin sensitivity. Diabetes Care. 2000;23:295–301 [DOI] [PubMed] [Google Scholar]
- 27. Kushnir MM, Blamires T, Rockwood AL, et al. Liquid chromatography-tandem mass spectrometry assay for androstenedione, dehydroepiandrosterone, and testosterone with pediatric and adult reference intervals. Clin Chem. 2010;56:1138–1147 [DOI] [PubMed] [Google Scholar]
- 28. Chadwick CA, Owen LJ, Keevil BG. Development of a method for the measurement of dehydroepiandrosterone sulphate by liquid chromatography-tandem mass spectrometry. Ann Clin Biochem. 2005;42:468–474 [DOI] [PubMed] [Google Scholar]
- 29. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81:19–25 [DOI] [PubMed] [Google Scholar]
- 30. Stewart PM, Boulton A, Kumar S, Clark PM, Shackleton CH. Cortisol metabolism in human obesity: impaired cortisone–>cortisol conversion in subjects with central adiposity. J Clin Endocrinol Metab. 1999;84:1022–1027 [DOI] [PubMed] [Google Scholar]
- 31. Barber TM, Wass JA, McCarthy MI, Franks S. Metabolic characteristics of women with polycystic ovaries and oligo-amenorrhoea but normal androgen levels: implications for the management of polycystic ovary syndrome. Clin Endocrinol (Oxf). 2007;66:513–517 [DOI] [PubMed] [Google Scholar]
- 32. Cakir E, Dogan M, Topaloglu O, et al. Subclinical atherosclerosis and hyperandrogenemia are independent risk factors for increased epicardial fat thickness in patients with PCOS and idiopathic hirsutism. Atherosclerosis. 2013;226:291–295 [DOI] [PubMed] [Google Scholar]
- 33. Dewailly D, Catteau-Jonard S, Reyss AC, Leroy M, Pigny P. Oligoanovulation with polycystic ovaries but not overt hyperandrogenism. J Clin Endocrinol Metab. 2006;91:3922–3927 [DOI] [PubMed] [Google Scholar]
- 34. Gilling-Smith C, Story H, Rogers V, Franks S. Evidence for a primary abnormality of thecal cell steroidogenesis in the polycystic ovary syndrome. Clin Endocrinol (Oxf). 1997;47:93–99 [DOI] [PubMed] [Google Scholar]
- 35. Fruzzetti F, Perini D, Lazzarini V, Parrini D, Gambacciani M, Genazzani AR. Comparison of effects of 3 mg drospirenone plus 20 mug ethinyl estradiol alone or combined with metformin or cyproterone acetate on classic metabolic cardiovascular risk factors in nonobese women with polycystic ovary syndrome. Fertil Steril. 2010;94:1793–1798 [DOI] [PubMed] [Google Scholar]
- 36. Studen KB, Sebestjen M, Pfeifer M, Prezelj J. Influence of spironolactone treatment on endothelial function in non-obese women with polycystic ovary syndrome. Eur J Endocrinol. 2011;164:389–395 [DOI] [PubMed] [Google Scholar]
- 37. Haring R, Hannemann A, John U, et al. Age-specific reference ranges for serum testosterone and androstenedione concentrations in women measured by liquid chromatography-tandem mass spectrometry. J Clin Endocrinol Metab. 2012;97:408–415 [DOI] [PubMed] [Google Scholar]
- 38. Anderson DC. Sex-hormone-binding globulin. Clin Endocrinol (Oxf). 1974;3:69–96 [DOI] [PubMed] [Google Scholar]
- 39. Rosner W, Auchus RJ, Azziz R, Sluss PM, Raff H. Position statement: utility, limitations, and pitfalls in measuring testosterone: an Endocrine Society position statement. J Clin Endocrinol Metab. 2007;92:405–413 [DOI] [PubMed] [Google Scholar]
- 40. Cawood ML, Field HP, Ford CG, et al. Testosterone measurement by isotope-dilution liquid chromatography-tandem mass spectrometry: validation of a method for routine clinical practice. Clin Chem. 2005;51:1472–1479 [DOI] [PubMed] [Google Scholar]
- 41. Barber TM, Golding SJ, Alvey C, et al. Global adiposity rather than abnormal regional fat distribution characterizes women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2008;93:999–1004 [DOI] [PubMed] [Google Scholar]
- 42. Barber TM, Franks S. Adipocyte biology in polycystic ovary syndrome. Mol Cell Endocrinol. 2013;373:68–76 [DOI] [PubMed] [Google Scholar]
- 43. Qin K, Ehrmann DA, Cox N, Refetoff S, Rosenfield RL. Identification of a functional polymorphism of the human type 5 17β-hydroxysteroid dehydrogenase gene associated with polycystic ovary syndrome. J Clin Endocrinol Metab. 2006;91:270–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cara JF, Rosenfield RL. Insulin-like growth factor I and insulin potentiate luteinizing hormone-induced androgen synthesis by rat ovarian thecal-interstitial cells. Endocrinology. 1988;123:733–739 [DOI] [PubMed] [Google Scholar]
- 45. Tosi F, Negri C, Perrone F, et al. Hyperinsulinemia amplifies GnRH agonist stimulated ovarian steroid secretion in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2012;97:1712–1719 [DOI] [PubMed] [Google Scholar]
- 46. Noordam C, Dhir V, McNelis JC, et al. Inactivating PAPSS2 mutations in a patient with premature pubarche. N Engl J Med. 2009;360:2310–2318 [DOI] [PubMed] [Google Scholar]
- 47. Louwers YV, de Jong FH, van Herwaarden NA, et al. Variants in SULT2A1 affect the DHEA sulphate to DHEA ratio in patients with polycystic ovary syndrome but not the hyperandrogenic phenotype. J Clin Endocrinol Metab 2013;98:3848–3855 [DOI] [PubMed] [Google Scholar]