Abstract
Context:
The impact of adolescent nonalcoholic fatty liver disease (NAFLD) on health, independent of fat mass, is unclear.
Objective:
The objective of the study was to determine the independent (of total body fat) association of ultrasound scan (USS)-determined NAFLD with liver fibrosis, insulin resistance, and dyslipidemia among healthy adolescents.
Design:
This was a cross-sectional analysis in participants from a UK birth cohort.
Participants:
One thousand eight hundred seventy-four (1059 female) individuals of a mean age of 17.9 years participated in the study.
Main Outcomes:
USS assessed liver stiffness (shear velocity, an indicator of fibrosis) and volume, fasting glucose, insulin, triglycerides, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, alanine amino transferase, aspartate amino transferase, γ-glutamyltransferase, and haptoglobin.
Results:
The prevalence of NAFLD was 2.5% [95% confidence interval (CI) 1.8–3.3] and was the same in females and males. Dual-energy X-ray absorptiometry determined total body fat mass was strongly associated with USS NAFLD: odds ratio 3.15 (95% CI 2.44–4.07) per 1 SD (∼10 kg) fat mass. Those with NAFLD had larger liver volumes and greater shear velocity. They also had higher fasting glucose, insulin, triglycerides, low-density lipoprotein cholesterol, alanine amino transferase, aspartate amino transferase, γ-glutamyltransferase, and haptoglobin and lower high-density lipoprotein cholesterol. Most associations were independent of total body fat. For example, after adjustment for fat mass and other confounders, hepatic shear velocity [mean difference 22.8% (95% CI 15.6–30.5)], triglyceride levels [23.6% (95% CI 6.0–44.2)], and insulin [39.4% (95% CI 10.7–75.5)] were greater in those with NAFLD compared with those without NAFLD.
Conclusion:
In healthy European adolescents, 2.5% have USS-defined NAFLD. Even after accounting for total body fat, those with NAFLD have more adverse levels of liver fibrosis and cardiometabolic risk factors.
Nonalcoholic fatty liver disease (NAFLD) is the most common form of liver disease in adolescents (1–4). Some adolescents with NAFLD progress to more severe forms of fibrosis and cirrhosis (5). However, the lack of studies in general populations of adolescents, as opposed to those done in small numbers of patients with established disease or clinical obesity (3–10), means it is difficult to determine the population effect of NAFLD on fibrosis/cirrhosis. Adolescents with NAFLD are also more likely to be insulin resistant and dyslipidemic, but the extent to which these associations are independent of total body fatness is unclear (1–4).
Accurately diagnosing liver pathology in large numbers of healthy young people is difficult. The gold standard of a liver biopsy is unfeasible and unethical in healthy young volunteers; in clinical practice it is recommended only in instances in which there is clear evidence that the benefits outweigh the potential harms (3). Noninvasive methods of assessment of liver fat and fibrosis using ultrasound scans (USS), magnetic resonance imaging, computed tomography scans, and biomarkers have been explored, but the studies using these methods have largely been done on small (rarely with > 100 participants) clinical cohorts with known liver disease, obesity, or diabetes (3–10). The results cannot be assumed to be the same in the general, as opposed to the clinical, population, and the potential population burden and public importance can be determined only by examining general population cohorts.
In this study we have used data from 1874 healthy adolescents to determine the independent (of fat mass) association of USS fatty liver with cardiometabolic risk factors (fasting glucose, insulin, and lipids) and markers of liver pathology [liver stiffness (a marker of fibrosis) and liver volume and fasting alanine amino transferase (ALT), aspartate amino transferase (AST), γ-glutamyltransferase (GGT), and haptoglobin].
Materials and Methods
The Avon Longitudinal Study of Parents and Children is a prospective UK population-based study that recruited a cohort of 14 541 pregnancies with expected dates of delivery April 1, 1991, to December 31, 1992 (http://www.alspac.bris.ac.uk) (11, 12). A total of 13 678 singleton live-born infants resulted from these pregnancies. The cohort has been followed up since birth, including repeat clinical assessment from age 7 years.
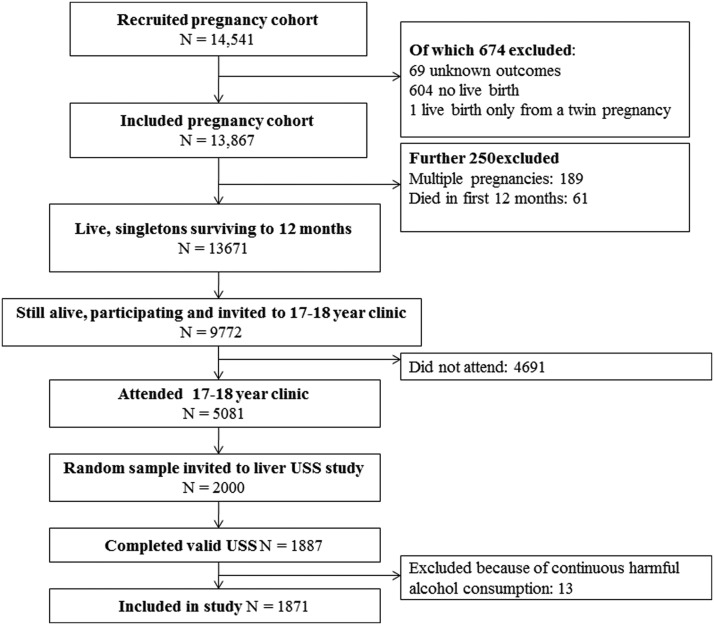
At the 17- to 18-year clinic follow-up, a random sample of 1887 participants completed a USS examination by a trained sonographer (Figure 1). These participants had been followed up since birth with completion (parental or young person) of up to 25 questionnaires and attendance at up to eight clinic assessments. Based on data from questionnaires and the clinic assessments, there was no evidence that any participants included in this study had ever experienced jaundice, nor had they ever received a doctors diagnosis of hepatitis or any other liver disease. At the time of the USS assessment, none of them were taking medications or receiving treatment that would indicate they had hepatic disease and none were taking medication known to influence liver function. We removed from the analyses 13 participants who reported continuous harmful drinking over the year prior to USS. Information on alcohol consumption was obtained by questionnaires administered 12 months before and at the same time as the USS, using the Alcohol Use Disorders Identification Tests (AUDIT) questionnaire (13). After removing 13 participants who scored greater than 16 [the threshold that defines harmful drinking (13)] at both ages, our study sample includes 1874 adolescents. Of these, 1711 (91%), 1716 (92%) and 1871 (100%) had valid liver fat, stiffness, and volume data, respectively.
Figure 1.
Participant flow from original birth cohort to the adolescent USS substudy used in this paper.
Ethical approval was obtained from the Avon Longitudinal Study of Parents and Children Law and Ethics Committee and the local National Health Service Research Ethics Committee, and all participants provided written informed consent.
Participants fasted overnight or for a minimum of 6 hours. Upper abdominal USS was completed by one of four trained sonographers using a Siemens Acuson S2000 USS system, with the participant at rest, in the dorsal decubitus position, and with both arms abducted and rested above their head. Echogenicity (our marker of liver fat) was assessed during deep inspiration and recorded as present, absent, or uncertain according to established protocols (3). This protocol has high levels of sensitivity and specificity for moderate-severe NAFLD (14, 15). Levels of agreement in identifying echogenicity between the four sonographers was high, both immediately after training and at 6-month intervals during data collection (absolute agreement of 98% or greater).
Acoustic radiation force impulse imaging (ARFI) of the right lobe of the liver was used to measure liver stiffness [shear velocity in meters/s (meters per second)] using standard protocols (7, 8), and this was used as our main indicator of liver fibrosis. The right lobe of the liver was viewed through the intercostal space such that the pulse wave was traversing an area of at least 6 cm and was not traversing any major vascular structures and the right lobe was clearly viewed. Shear velocity was assessed six times with a gap of at least 1 minute between each measurement. The highest and lowest of these measurements were excluded and the Siemens Acuson S2000 system produced a mean of the remaining four. If this mean was greater than 4 m/s, a further six measurements were taken from the left lobe. In the analyses we have used the mean of four measurements; when both right and left lobe values were available, the lowest mean of the two has been used. The coefficient of variation for the four measures was 3.6%.
In a study of 32 children (aged up to 16 y) with known chronic liver disease, the ability of the ARFI to identify liver fibrosis accurately (compared with the gold standard of histopathology) was assessed and shear velocity values for defining different levels of fibrosis determined (area under the receiver operator characteristic curves all > 0.8) (8). We used these threshold values to categorize the extent of fibrosis as follows: none, less than 1.31 m/s; mild, 1.31 or greater to less than 1.39 m/s; significant, 1.39 m/s or greater to less than 2.25 m/s; severe, advanced, 2.25 m/s or greater.
Liver volume was assessed by three sweeps through the liver. The sweeps were taken transversely (lateral to medial), from diaphragm down to the inferior pole of the liver (craniocaudal), and back to front (posterior to anterior). These produced distances, which were used to calculate liver volume with the Siemens Acuson S2000 system software.
Fasting blood samples were immediately spun and frozen at −80°C. Measurements were assayed shortly (3–9 mo) after samples were taken with no previous freeze-thaw cycles. All assays were completed in the same laboratory at the University of Glasgow. ALT, GGT, and AST were measured by automated analyzer with enzymatic methods and haptoglobin was measured by immunoturbidimetry. We defined those with an ALT of a value of 30 U/L or greater as having abnormally high ALT levels, using the most common biomarker and threshold for that biomarker used previously in adolescent populations (16).
Plasma lipids [total cholesterol, triglycerides, and high density lipoprotein cholesterol (HDLc)] were performed by modification of the standard Lipid Research Clinics Protocol using enzymatic reagents for lipid determination. Low-density lipoprotein cholesterol (LDLc) was calculated using the Friedewald equation: [LDLc = total cholesterol − (HDLc + [triglycerides × 0.45])] (17). Insulin was measured by an ELISA (Mercodia) that does not cross-react with proinsulin and plasma glucose by the automated enzymatic (hexokinase) method. All inter- and intracoefficients of variation for all of these blood based assays were less than 5%. We used fasting insulin as an indicator of insulin resistance (18–20). In our cohort, as in others (18–20), correlations of fasting insulin with homeostasis model assessment index of insulin resistance and quantitative insulin sensitivity check index were 0.97 or greater in both sexes, and the results were the same if either of these assessments were used instead of fasting insulin. We defined those with fasting insulin 90th sex-specific percentile or greater of this cohort as being insulin resistant (21).
Parental occupation was used to derive family occupational social class, with the highest of parental occupation used in all analyses. Ethnicity was based on mothers' self-report at the time of recruitment during her pregnancy. Weight and height were measured in light clothing and without shoes. A Lunar Prodigy narrow fan beam densitometer was used to perform a whole-body dual-energy X-ray absorptiometry (DXA) scan from which total body lean and fat mass and trunkal (area between the pelvis and lower end of the rib cage) fat are measured. For our main analyses, we used total-body DXA fat mass to adjust for confounding by adiposity a priori. We judged this to be the best measure of total body fat. In additional analyses, we also examined associations adjusting for DXA trunkal (central) fat and also for body mass index (BMI). We generated sex- and age-specific (in 6 mo categories) SD scores (z-scores) of DXA to determine total and trunkal fat mass and BMI to take account of differences in age and sex.
All analyses were conducted in Stata MP version 12.
The associations of sex, age, ethnicity, social class, total and trunkal fat mass, and BMI with USS fatty liver were assessed using logistic regression. In these analyses total and trunkal fat mass was adjusted for height and height squared. Multivariable linear regression was used to examine the association of USS fatty liver with continuously measured markers of cardiometabolic and liver outcomes, and a multivariable logistic regression was used to examine associations with binary outcomes. Natural logged values of all continuous outcomes were used in the linear regression analyses. The resulting regression coefficients were transformed by multiplying by 100 to give a difference in means (between those with and without fatty liver) expressed as a percentage. In model 1 we adjusted for age, sex, ethnicity, and social class. In the second model, we additionally adjusted for DXA fat mass (and height and height squared) to determine how much of any associations were driven by fatness.
In additional analyses we substituted DXA total fat mass with DXA trunk fat mass or BMI to make sure that neither of these measurements of adiposity were stronger confounders. We also adjusted for the AUDIT score results. This was a further test (in addition to removing those with persistent harmful drinking) that associations with USS fatty liver reflected nonalcoholic, as opposed to alcoholic, fatty liver.
Of the 1874 eligible participants, a small proportion had missing liver fat or shear velocity measures, and some also had missing data on potential confounding factors or blood-based outcomes (Supplemental Table 1, published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org). To increase efficiency and minimize selection bias, we used multivariate multiple imputation to impute missing data for any of the 1874 with missing data. We included all exposures, covariables, outcomes, and potential predictors of missing data in the imputation equations and imputed separately by sex. Distributions of all variables were similar in the multivariate imputation databases and when observed (Supplemental Table 2). The main adjusted associations were similar when conducted on those with complete data (Supplemental Tables 3) compared with those using the imputation databases (presented here as the main results).
Results
Of the 1711 participants with valid USS data on liver fat, 43 (2.5%) had fat in their liver [95% confidence interval (CI) 1.8–3.3]. The prevalence of liver fat was similar in females [26 of 1009 (2.6%)] and males [17 of 702 (2.4%), P = .8]. Supplemental Table 1 shows these and the distributions of other characteristics of the participants. Distributions of characteristics were similar for the subsample included in this USS study compared with those of all participants invited to the 17- to 18-year clinic (Supplemental Table 4). Compared with those who were invited to the 17- to 18-year clinic, those who attended (including those participating in the USS study) were less likely to be from manual social class families and more likely to be female; nonwhite ethnicity did not differ between the groups (Supplemental Table 4). Associations were similar in females and males (P for interaction with sex all > .1), with the exception of the association with shear velocity. All association analyses are therefore presented with both sexes combined, with sex-specific analyses in the Supplemental Material.
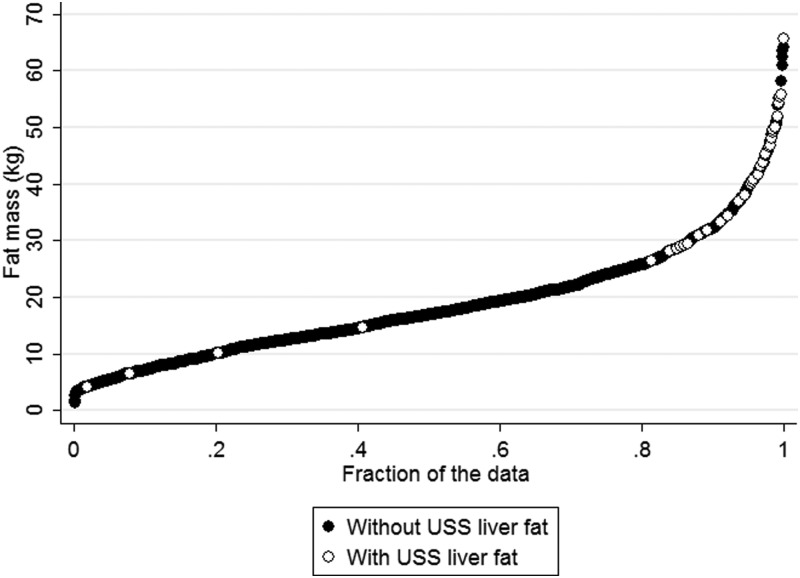
Table 1 shows the association of age, sex, social class, ethnicity, DXA total and trunkal fat mass, and BMI with USS liver fat. There was no evidence of associations of sex, age, or ethnicity with liver fat. Odds of liver fat were greater in those from lower compared with higher socioeconomic position, but the CI was wide and included the null value [odds ratio (OR) 1.91 (95% CI 0.91–4.03)]. All measures of fatness were strongly positively associated with liver fat, which increased approximately 3-fold for every 1 SD greater total or trunkal fat mass or BMI. When we mutually adjusted social class and total fat mass for each other the OR for the association of social class with liver fat attenuated to 1.55 (95% CI 0.68–3.52), whereas that with fat mass remained similar at 3.13 (2.42–4.04). Figure 2 illustrates the strong association of total fat mass with liver fat, showing that the vast majority of participants with USS liver fat are in the upper end of the fat mass distribution. This remains the case when we look at this with fat mass adjusted for height and height squared (Supplemental Figure 1). Using standard BMI definitions, the prevalence of USS liver fat were 0.4% (5 of 1226), 4.3% (12 of 279), and 22.2% (26 of 117) for those who were normal, overweight, and obese, respectively.
Table 1.
Associations With USS Fatty Liver (n = 1874)
| Risk Factor | Unit/Category | USS Fatty Liver, % | OR (95% CI) per Unit/Category | P Value |
|---|---|---|---|---|
| Age, y | Per 6 months | N/A | 1.24 (0.92–1.68) | .2 |
| Sex | Female | 3.0 | Referent | .9 |
| Male | 2.9 | 0.96 (0.54–1.73) | ||
| Social class | Nonmanual | 2.6 | Referent | .1 |
| Manual | 5.0 | 1.91 (0.91–4.03) | ||
| Ethnicity | White | 2.9 | Referent | .6 |
| Nonwhite | 4.3 | 1.42 (0.39–5.11) | ||
| Fat mass, total | Per 1 SDa,b | N/A | 3.15 (2.44–4.07) | <.001 |
| Fat mass, trunkal | Per 1 SDa,b | N/A | 3.20 (2.47–4.15) | <.001 |
| BMI | Per 1 SDa | N/A | 3.05 (2.36–3.93) | <.001 |
| Height | Per 1 SDa | N/A | 0.96 (0.71–1.29) | .8 |
Abbreviation: N/A, not applicable.
Standardized for sex and age.
Adjusted for height and height squared.
Figure 2.
Distribution of fat mass for those with and without USS liver fat.
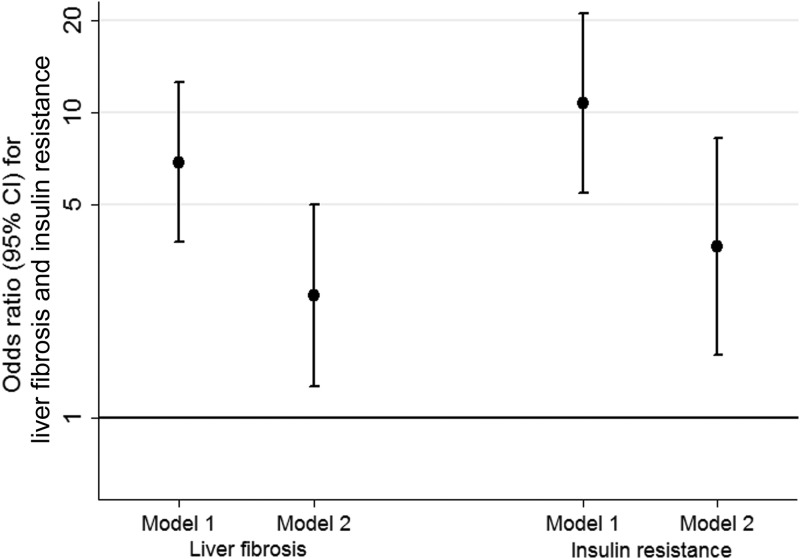
Adolescents with USS detectable liver fat had higher mean levels of insulin, glucose, triglycerides, and LDLc and lower levels of HDLc, after adjustment for age, sex, social class, and ethnicity (Table 2). With additional adjustment for total fat mass, these associations attenuated but for most associations remained. The inverse association with HDLc had confidence intervals that just included the null value after adjustment for fat mass. The positive association with LDLc was clearly attenuated to the null with adjustment for fat mass. Even after adjustment for DXA determined fat mass, adolescents with USS fatty liver had insulin levels that were approximately 40% higher than those without liver fat. Adolescents with USS fatty liver had increased risk of insulin resistance both before and after adjustment for fat mass (Figure 3).
Table 2.
Multivariable Associations of USS Fatty Liver With Cardiometabolic Risk Factors (n = 1874)
| Outcome | Mean Difference (95% CI) Comparing Those With With Those Without USS Fatty Liver, % |
|
|---|---|---|
| Model 1 | Model 2 | |
| Fasting triglycerides, % | 49.1 (29.0–72.5) | 23.6 (6.0–44.2) |
| Fasting HDLc, % | − 15.1 ( − 21.8 to − 7.8) | −8.1 (−16.0 to 0.6) |
| Fasting LDLc, % | 12.6 (1.9–24.4) | 1.5 (−9.1 to 13.4) |
| Fasting glucose, % | 7.4 (3.9–11.1) | 4.6 (1.0–8.3) |
| Fasting insulin, % | 111.0 (70.0–161.9) | 39.4 (10.7–75.5) |
All results are mean percentage differences, and the null value (of no difference between those with and without USS fatty liver) is 0. Model 1 was adjusted for age, sex, social class, and ethnicity. Model 2 was as model 1 but with additional adjustment for DXA fat mass, height, and height squared.
Figure 3.
Multivariable associations of ultrasound scan liver fat with insulin resistance and liver fibrosis. The figure shows ORs (dots) and their CIs (vertical lines) for the association of USS fatty liver with liver fibrosis (the two results on the left) and insulin resistance (the two resuluts on the right), both without (model 1) and with (model 2) adjustment for fat mass. Model 1 was adjusted for age, sex, social class, and ethnicity. Model 2 was as model 1 but with additional adjustment for DXA fat mass, height, and height squared liver fibrosis defined as 1.39 m/s or greater shear velocity. Insulin resistance was defined as 90th percentile or greater of sex-specific fasting insulin (90th percentile: females, 86.44 pmol/L; males, 87.80 pmol/L).
Adolescents with USS fatty liver had greater liver stiffness (shear velocity), a larger liver volume, and higher ALT, AST, GGT, and haptoglobin compared with those without USS fatty liver, after adjustment for age, sex, social class, and ethnicity (Table 3). With additional adjustment for fat mass, associations attenuated but positive associations remained for all. Adolescents with USS fatty liver had increased risk of significant liver fibrosis, both before and after adjustment for fat mass (Figure 3).
Table 3.
Multivariable Associations of USS Fatty Liver With Markers of Liver Damage in Adolescents (n = 1874)
| Outcome | Mean Difference (95% CI) Comparing Those With USS Fatty Liver With Those Without USS Fatty Liver, % |
|
|---|---|---|
| Model 1 | Model 2 | |
| Shear velocity, %a | 31.6 (23.9–39.7) | 22.8 (15.6–30.5) |
| Liver volume, % | 24.6 (15.6–34.2) | 9.3 (1.3–17.9) |
| ALT, % | 60.6 (36.5–88.9) | 30.9 (10.5–55.0) |
| AST, % | 19.8 (9.1–31.6) | 14.4 (3.6–26.3) |
| GGT, % | 54.4 (35.1–76.4) | 29.0 (11.9–48.6) |
| Haptoglobin, % | 48.1 (22.7–78.6) | 22.7 (0.8–49.4) |
All outcomes are mean percentage differences, and the null value (of no difference between those with and without USS fatty liver) is 0. Model 1 was adjusted for age, sex, social class, and ethnicity. Model 2 was as model 1 but with additional adjustment for DXA determined fat mass, height, and height squared.
Shear velocity is measured by ARFI.
If trunkal fat mass or BMI was used in these analyses in place of total body fat mass, the results were essentially the same as those shown (results available from authors).
Both before and after adjustment for fat mass, the positive association of USS fatty liver with shear velocity was greater in females compared with males (mean difference after adjustment for all confounders including fat mass 30.6% (95% CI 20.3–41.7) in females and 12.7% (95% CI 4.1–22.0) in males (P for sex interaction < .001). For all other outcomes, associations were similar in females and males (Supplemental Tables 5 and 6). The associations were essentially unchanged with adjustment for the AUDIT alcohol consumption score (Supplemental Tables 7 and 8).
Discussion
We have shown that among a contemporary general population of adolescents, 2.5% have USS detected fatty liver. This cohort has no known liver pathology and prolonged heavy drinking of the amount likely to cause detectable liver fat, and pathology is rare at age 17.8 years. Furthermore, we removed the small number with reported continuous harmful drinking for the 12 months prior to the study. Thus, our results suggest that the prevalence of USS detected NAFLD in healthy European adolescents is 2.5%. The prevalence was similar in females and males.
Accurately assessed total fat mass is a strong risk factor, with the odds of NAFLD increasing 3-fold for every increase in 1 SD (∼10 kg in both females and males) of total fat mass. Notably, almost all participants with NAFLD were in the upper end of the fat mass distribution (Figure 2). DXA-determined total body fat mass, trunkal fat mass, and BMI all had similar magnitudes of association with NAFLD. These results suggest that general greater fatness (rather than centrally located fatness) is a key risk factor for NAFLD in adolescence, and that using BMI to predict NAFLD is likely to be as useful as DXA fat mass, which will be more difficult to obtain in clinical practice, particularly in primary care in which risk is likely to be first assessed.
Mean levels of hepatic shear velocity, liver volume, fasting triglycerides, glucose, insulin, ALT, AST, and GGT remained higher, and those of HDLc lower, in adolescents with NAFLD compared with those without NAFLD, even after adjustment for fat mass.
Previous studies in children and adolescents have mostly used high ALT as a marker of NAFLD and in general pediatric populations have reported prevalences between 3% and 10% (1–4, 22, 23), with our results being consistent with the lower end of this range. In an autopsy study, 9.6% of children and adolescents had clinically important levels of liver fat, with the prevalence increasing with increasing age to a level of 17.3% in those aged 15–19 years (24). Our results suggest a markedly lower prevalence than that, but adolescents who die as a result of accidents tend to be from more deprived backgrounds and be more likely to consume high levels of alcohol (24). That study defined NAFLD as 5% or greater of hepatocytes containing macrovesicular fat in one liver section, which would include the whole range from mild steatosis to moderate to severe NAFLD. Our NALFD cases are likely to have largely been those with moderate to severe steatosis.
A recent USS study of an Australian adolescent cohort (n = 1170; mean age 17 y) reported a prevalence of 12.8%, with a greater prevalence in females (16.3%) compared with males (10.1%) (25). The most likely reason for the difference between that and our study is the inclusion of mild steatosis. The prevalence of moderate to severe steatosis in that study was similar in females (2.2%) and in males (3.1%), and both were similar to the prevalence in our study. More of the Australian cohort were excluded on the basis of continuous harmful drinking than in our cohort (2.7% vs 0.7%), so it is possible that greater alcohol consumption among those not excluded also explains some of the difference. Other characteristics between the two studies are almost identical.
To our knowledge this Australian study is the only previous study to assess NAFLD prevalence and correlates in a healthy population of adolescents. Consistent with our study, NAFLD was more common in those with a greater adiposity and was associated with higher levels of ALT and more adverse levels of cardiometabolic outcomes. However, whether NAFLD was associated with adverse outcomes independently of fatness was not assessed (25).
The differences in associations of USS assessed NAFLD with shear velocity (liver stiffness) between females and males in our study may be a chance finding, particularly because no other sex differences were found.
The key strength of this study is the fact that it is one of the largest studies to date to examine the association of USS fatty liver with markers of adverse liver and cardiometabolic outcomes in a healthy contemporary cohort of adolescents. We were able to adjust for DXA body fat and demonstrate that NAFLD was associated with increased levels of adverse outcomes independent of this, suggesting that NAFLD per se, and not simply as a reflection of general greater fatness, is an important risk factor for these outcomes.
USS-assessed fatty liver is not the gold standard liver biopsy; however, a liver biopsy could not be undertaken ethically in a large cohort of healthy young people. In one study of 208 children aged on average 10 years, USS fatty liver (defined with a similar protocol to that used in our study) was shown to accurately identify moderate to severe steatosis determined through liver biopsy (area under the receiver operator curve 0.87), (14), which demonstrates a similar level of accuracy to that reported in studies of adults that have compared USS with biopsy (15). USS in general has a sensitivity of 85%–90% and specificity of 70%–85% for detecting liver fat of at least 10% but lower sensitivity and specificity for lower levels of fat (26). Thus, our estimate of the prevalence of NAFLD in healthy adolescents most likely reflects the prevalence for the more moderate to severe end of the spectrum of this condition.
Our study is cross-sectional, so we cannot determine causality. This cohort consists largely (97%) of white European origin individuals, and our results do not necessarily generalize to other ethnicities.
In summary, our study, together with other published studies, suggests that among healthy contemporary European-origin adolescents between 2% and 3% have moderate to severe NAFLD. We have further shown that those with this condition have evidence of more adverse levels of insulin, glucose, and markers of dyslipidemia together with more adverse levels of liver pathology, including liver fibrosis as assessed by shear velocity, even after taking account of the strong association of total body fat with liver fat. Longer-term follow-up of these young people is important to establish the longer-term outcomes, the natural history of NAFLD and the extent to which new cases of USS detected NAFLD emerge with increasing age. The similarity of association between total fat mass, trunkal fat mass, and BMI in relation to NAFLD, and the fact that most cases occurred in those meeting criteria for obesity (BMI ≥ 30 kg/m2) illustrate that risk for this outcome can be identified by clinicians using BMI. If longer-term follow-up indicates a lasting problem in these adolescents, testing liver function/pathology in adolescents who are obese and providing advice on how to reduce liver damage in this group would be sensible.
Acknowledgments
We are extremely grateful to all of the families who took part in this study, the midwives for recruiting them, and the whole Avon Longitudinal Study of Parents and Children team, which includes interviewers, computer and laboratory technicians, clerical workers, research scientists, volunteers, managers, receptionists, and nurses.
Author contributions included the following: D.A.L. obtained the funds for the study, developed the study aims and objectives, wrote the analysis plan, wrote the first draft of the paper, and coordinated the revisions and comments from all other authors. M.C. obtained the funds for the study, contributed to developing the study aims, wrote and oversaw the ultrasound scan protocol, and made critical comments on the paper drafts. C.M.-W. undertook the statistical analyses and made critical comments on paper drafts. E.A. helped with the background literature review and made critical comments on paper drafts. A.F. obtained the funds for the study, contributed to developing the study aims and made critical comments on paper drafts. L.D.H. contributed to developing the study aims and the analysis plan and made critical comments on the paper drafts. C.D. obtained the funds for the study, contributed to developing the study aims, and made critical comments on the paper drafts. N.S. obtained the funds for the study, contributed to developing the study aims, managed the assays on the blood samples and made critical comments on the paper drafts. D.A.L. and C.M.-W. had complete access to all the data used in this study.
The comments made in this paper are those of the authors and not necessarily those of any funders.
The research leading to these results has received funding from the UK Medical Research Council (Grant G0801456), which funded the salary of C.M.-W., and the European Union's Seventh Framework Programme (FP7/2007-2013) under Grant Agreement HEALTH-F2-2009-241762 for the project FLIP. The UK Medical Research Council and Wellcome Trust (Grant 092731), together with the University of Bristol, provide core support for the Avon Longitudinal Study of Parents and Children study. D.A.L., C.M.-W., A.F., and L.D.H. work in a research unit that receives funding from the UK Medical Research Council (Grant MC_UU_12013/1-9). C.M.-W., A.F., and L.D.H. are supported by a UK Medical Research Council Postdoctoral Research Fellowship (Grants MR/J011932/1, 0701594, and G1002375, respectively), and E.A. is supported by a Medical Research Council PhD studentship. None of the funders influenced the data analysis or the interpretation of the results.
Disclosure Summary: The authors have nothing to declare.
For editorial see page 774
- ALT
- alanine amino transferase
- ARFI
- acoustic radiation force impulse imaging
- AST
- aspartate amino transferase
- AUDIT
- Alcohol Use Disorders Identification Tests
- BMI
- body mass index
- CI
- confidence interval
- DXA
- dual-energy X-ray absorptiometry
- GGT
- γ-glutamyltransferase
- HDLc
- high density lipoprotein cholesterol
- LDLc
- low density lipoprotein cholesterol
- NAFLD
- nonalcoholic fatty liver disease
- OR
- odds ratio
- USS
- ultrasound scan.
References
- 1. Cheung CR, Kelly DA. Non-alcoholic fatty liver disease in children. BMJ. 2011;343:d4460. [DOI] [PubMed] [Google Scholar]
- 2. Alisi A, Feldstein AE, Villani A, Raponi M, Nobili V. Pediatric nonalcoholic fatty liver disease: a multidisciplinary approach. Nat Rev Gastroenterol Hepatol. 2012;9:152–161 [DOI] [PubMed] [Google Scholar]
- 3. Vajro P, Lenta S, Socha P, et al. Diagnosis of nonalcoholic fatty liver disease in children and adolescents: position paper of the ESPGHAN Hepatology Committee. J Pediatr Gastroenterol Nutr. 2012;54:700–713 [DOI] [PubMed] [Google Scholar]
- 4. Della CC, Alisi A, Saccari A, De Vito R, Vania A, Nobili V. Nonalcoholic fatty liver in children and adolescents: an overview. J Adolesc Health. 2012;51:305–312 [DOI] [PubMed] [Google Scholar]
- 5. Feldstein AE, Charatcharoenwitthaya P, Treeprasertsuk S, Benson JT, Enders FB, Angulo P. The natural history of non-alcoholic fatty liver disease in children: a follow-up study for up to 20 years. Gut. 2009;58:1538–1544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nobili V, Vizzutti F, Arena U, et al. Accuracy and reproducibility of transient elastography for the diagnosis of fibrosis in pediatric nonalcoholic steatohepatitis. Hepatology. 2008;48:442–448 [DOI] [PubMed] [Google Scholar]
- 7. Marginean CO, Marginean C. Elastographic assessment of liver fibrosis in children: a prospective single center experience. Eur J Radiol. 2012;81:e870–e874 [DOI] [PubMed] [Google Scholar]
- 8. Noruegas MJ, Matos H, Goncalves I, Cipriano MA, Sanches C. Acoustic radiation force impulse-imaging in the assessment of liver fibrosis in children. Pediatr Radiol. 2012;42:201–204 [DOI] [PubMed] [Google Scholar]
- 9. Alkhouri N, Carter-Kent C, Lopez R, et al. A combination of the pediatric NAFLD fibrosis index and enhanced liver fibrosis test identifies children with fibrosis. Clin Gastroenterol Hepatol. 2011;9:150–155 [DOI] [PubMed] [Google Scholar]
- 10. Nobili V, Alisi A, Vania A, Tiribelli C, Pietrobattista A, Bedogni G. The pediatric NAFLD fibrosis index: a predictor of liver fibrosis in children with non-alcoholic fatty liver disease. BMC Med. 2009;7:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fraser A, Macdonald-Wallis C, Tilling K, et al. Cohort profile: the Avon Longitudinal Study of Parents and Children: ALSPAC mothers cohort. Int J Epidemiol. 2013;42:97–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Boyd A, Golding J, Macleod J, et al. Cohort profile: the 'Children of the 90s'; the index offspring of the Avon Longitudinal Study of Parents and Children (ALSPAC). Int J Epidemiol. 2013;42:111–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption–II. Addiction. 1993;88:791–804 [DOI] [PubMed] [Google Scholar]
- 14. Shannon A, Alkhouri N, Carter-Kent C, et al. Ultrasonographic quantitative estimation of hepatic steatosis in children with NAFLD. J Pediatr Gastroenterol Nutr. 2011;53:190–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dasarathy S, Dasarathy J, Khiyami A, Joseph R, Lopez R, McCullough AJ. Validity of real time ultrasound in the diagnosis of hepatic steatosis: a prospective study. J Hepatol. 2009;51:1061–1067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Strauss RS, Barlow SE, Dietz WH. Prevalence of abnormal serum aminotransferase values in overweight and obese adolescents. J Pediatr. 2000;136:727–733 [PubMed] [Google Scholar]
- 17. Friedewald WT, Levy RI, Frederickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without the use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502 [PubMed] [Google Scholar]
- 18. Gungor N, Saad R, Janosky J, Arslanian S. Validation of surrogate estimates of insulin sensitivity and insulin secretion in children and adolescents. J Pediatr. 2004;144:47–55 [DOI] [PubMed] [Google Scholar]
- 19. Schwartz B, Jacobs DR, Jr, Moran A, Steinberger J, Hong CP, Sinaiko AR. Measurement of insulin sensitivity in children: comparison between the euglycemic-hyperinsulinemic clamp and surrogate measures. Diabetes Care. 2008;31:783–788 [DOI] [PubMed] [Google Scholar]
- 20. Levy-Marchal C, Arslanian S, Cutfield W, et al. ESPE-LWPES-ISPAD-APPES-APEG-SLEP-JSPE, Insulin Resistance in Children Consensus Conference Group. Insulin resistance in children: consensus, perspective, and future directions. J Clin Endocrinol Metab 2010;95:5189–5198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lawlor DA, Benfield L, Logue J, et al. Association between general and central adiposity in childhood, and change in these, with cardiovascular risk factors in adolescence: prospective cohort study. BMJ 2010;341:c6224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schwimmer JB, McGreal N, Deutsch R, Finegold MJ, Lavine JE. Influence of gender, race, and ethnicity on suspected fatty liver in obese adolescents. Pediatrics. 2005;115:e561–e565 [DOI] [PubMed] [Google Scholar]
- 23. Fraser A, Longnecker MP, Lawlor DA. Prevalence of elevated alanine-aminotransferase (ALT) among US adolescents and associated factors: NHANES 1999–2004. Gastroenterology. 2007;133:1814–1820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schwimmer JB, Deutsch R, Kahen T, Lavine JE, Stanley C, Behling C. Prevalence of fatty liver in children and adolescents. Pediatrics. 2006;118:1388–1393 [DOI] [PubMed] [Google Scholar]
- 25. Ayonrinde OT, Olynyk JK, Beilin LJ, et al. Gender-specific differences in adipose distribution and adipocytokines influence adolescent nonalcoholic fatty liver disease. Hepatology. 2011;53:800–809 [DOI] [PubMed] [Google Scholar]
- 26. Bohte AE, van Werven JR, Bipat A, Stoker J. The diagnostic accuracy of US, CT, MRI and 1H-MRS for the evaluation of hepatic steatosis compared with liver biopsy: a meta-analysis. Eur Radiol. 2011;21:87–97 [DOI] [PMC free article] [PubMed] [Google Scholar]