Abstract
Context:
The focus of care in disorders of sex development (DSD) is often directed to issues related to sex and gender development. In addition, the molecular etiology remains unclear in the majority of cases.
Objective:
To report the range of associated conditions identified in the international DSD (I-DSD) Registry.
Design, Setting, and Patients:
Anonymized data were extracted from the I-DSD Registry for diagnosis, karyotype, sex of rearing, genetic investigations, and associated anomalies. If necessary, clarification was sought from the reporting clinician.
Results:
Of 649 accessible cases, associated conditions occurred in 168 (26%); 103 (61%) cases had one condition, 31 (18%) had two conditions, 20 (12%) had three conditions, and 14 (8%) had four or more conditions. Karyotypes with most frequently reported associations included 45,X with 6 of 8 affected cases (75%), 45,X/46,XY with 19 of 42 cases (45%), 46,XY with 112 of 460 cases (24%), and 46,XX with 27 of 121 cases (22%). In the 112 cases of 46,XY DSD, the commonest conditions included small for gestational age in 26 (23%), cardiac anomalies in 22 (20%), and central nervous system disorders in 22 (20%), whereas in the 27 cases of 46,XX DSD, skeletal and renal anomalies were commonest at 12 (44%) and 8 (30%), respectively. Of 170 cases of suspected androgen insensitivity syndrome, 19 (11%) had reported anomalies and 9 of these had confirmed androgen receptor mutations.
Conclusions:
Over a quarter of the cases in the I-DSD Registry have an additional condition. These associations can direct investigators toward novel genetic etiology and also highlight the need for more holistic care of the affected person.
Disorders of sex development (DSD) are a group of rare conditions that usually present in early infancy with an abnormality of the external and/or internal reproductive organs and commonly arise because of a disorder of gonadal, adrenal, or hormonal function. Although the overall birth prevalence of conditions associated with DSD may be as high as 1 in 300 (1), individual pathophysiological conditions are much rarer, limiting the study of their etiology and prognosis.
Although enormous advances in our knowledge have been achieved over the last decade, the genetic etiology in most cases of DSD remains unclear (2, 3). Furthermore, our understanding of the basis of the genetic modifiers of monogenic DSD remains in its infancy. Mammalian sex development, which is already known to be closely linked to the development of the urological system (4), occurs at critical stages of embryonic and fetal development. These windows may also be important for the development of other organs as illustrated by a number of known conditions associated with DSD (5–7). High-throughput gene sequencing technology has the potential to identify a number of attributable genetic variations but understanding the link between these variations and the clinical condition may prove to be a challenge. An improved knowledge of the range of associated conditions that exist in people with DSD not only will help with interpreting the results of the genetic analyses but also will aid with the management of the affected patient.
Although a link between DSD and associated anomalies is recognized (1), it is generally thought that conditions such as hypospadias are rarely associated with abnormalities beyond the genitourinary system (8). Given the rarity of DSD, it has been difficult to ascertain the frequency and nature of associated anomalies in these cases. The Consensus Guidelines on DSD published in 2006 stressed the need for the creation and maintenance of a database in centers of expertise (9). Such databases do exist in many regional and national centers and have provided valuable insights into many aspects of DSD, including epidemiology (1), etiology (10, 11), variation of disease expression (12), initial adjustment of parents to their affected child's condition (13), and long-term outcome (14). However, as these databases and registries lacked uniformity, an international DSD (I-DSD) registry that collects information on cases with a range of conditions has now been in operation to overcome these issues (15). We have used this model of international collaboration to report on the largest cohort of cases of DSD that has been studied for associated anomalies.
Subjects and Methods
Details of the I-DSD Registry, including its development and current operation, have been previously reported (15, 16) and are also available from its web site (www.i-dsd.org). Briefly, all clinicians belonging to recognized professional medical and scientific societies are eligible to register and report cases. This information is required when the clinician first registers as a user of the Registry. Users are then approved by the steering committee of the Registry before they can submit any cases. There are no restrictions on the age requirement of the case, with the Registry serving both adult and pediatric populations, and there is no time limit between initial presentation or diagnosis and entry into the Registry. Patient and/or parental consent must be obtained before case registration, with the level of consent tiered according to the extent to which the information may be shared (own center, own country, European Union member states, international). The I-DSD Registry is approved by the National Research Ethics Service of the United Kingdom.
The terminology used within the Registry is based on the nomenclature initially developed at the Chicago consensus meeting and which has continued to evolve subsequently (9, 15). In addition to details of diagnosis, the I-DSD Registry collects simple information on physical conditions that affect systems other than the reproductive system, as well as the occurrence of conditions such as small for gestational age (SGA), and short stature. See Supplemental Tables 1–4, published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org, for details of the data fields in the Registry.
At the time of the study in September 2012, there were 1050 cases submitted by registered clinicians from 20 centers in 14 different countries. Reporting clinicians for cases represented a range of specialties, including pediatric endocrinology, adult endocrinology, clinical genetics and biochemistry, and their countries of origin, are outlined in Supplemental Table 5. On analyzing the extracted data, 649 (62%) cases had a sufficient level of consent to allow sharing of suitable information. Anonymized data were obtained from the Registry regarding diagnosis, karyotype, sex of rearing, clinical center, genetic investigations, and any associated anomalies. Until September 2012, the field that captures “associated conditions” was labeled as “associated malformations.” The field does not seek any further information on whether the associated condition is early onset, late onset, or acquired. Short stature in congenital adrenal hyperplasia was excluded from the analysis because this is a condition that is acquired as a consequence of the management of the disorder. Furthermore, the data were subanalyzed with the exclusion of cases where the condition was known to be associated with specific anomalies, namely P450 oxidoreductase deficiency (PORD), Müllerian duct aplasia, renal dysplasia, and cervical somite anomalies (MURCS), Mayer-Rokitansky-Küster-Hauser syndrome (MRKH), Turner syndrome, and 45,X/46,XY. Where information was unclear or incomplete, the reporting clinician was contacted to obtain further information.
Results
Karyotype
Of the 649 cases analyzed, 460 (71%), were 46,XY, 121 (19%) were 46,XX, 42 (6%) were 45,X/46,XY, 8 (1%) were 45,X, 6 (1%) were 46,XX/46,XY, 2 (0.3%) were 47,XXY, with other atypical karyotypes (such as translocations) making up the remaining 10 cases (1%). Of these cases with the respective karyotypes, associated conditions were reported in 6 cases of 45,X (75%), 19 cases of 45,X/46,XY (45%), 112 cases of 46,XY DSD (24%), 27 cases of 46,XX DSD (22%), and 4 cases with the atypical karyotypes (22%). Disorders of gonadal development occur in patients with a variety of karyotypes, and in the current cohort, of the 63 cases of a disorder of gonadal development with an associated condition, 33 (52%) were 46,XY, 3 (5%) were 46,XX, 5 (8%) were 45,X, 19 (30%) were 45,X/46,XY, 1 (2%) was 47,XXY, and 2 (2%) had an atypical karyotype.
Multiple conditions
Of the 649 cases, associated conditions occurred in 168 (26%). Of these 168 cases, 103 (61%) cases had one condition each, 31 (18%) had two conditions, 20 (12%) had three conditions, and 14 (8%) had four or more conditions, with the maximum number being eight. Multiple conditions (more than one per case) were reported in 3 of 6 (50%) cases of 46,XX disorders of Müllerian development, 22 of 44 (50%) cases of nonspecific 46,XY DSD, 25 of 63 (40%) cases of disorders of gonadal development, 7 of 19 (37%) cases of disorders of androgen action, 3 of 11 (27%) cases of disorders of androgen excess, and 2 of 18 (11%) cases of disorders of androgen synthesis. Further details of the conditions encountered are outlined in Table 1.
Table 1.
Reported Anomalies According to Disorder Classification
| Disorder Type (number of accessible cases in Registry) | Number of Cases With Anomaly | Number of Cases With Each Type of Anomaly |
Number of Cases With Anomaly by Karyotype | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adrenal | Blood and Lymph | CNS | Craniofacial | ENT | Eyes | GI Tract | Heart | Renal | Respiratory | SGA | Short Stature | Skeletal | Skin | Undefined Syndrome | Other | |||
| Gonadal development (153) | 63 | 1 | 3 | 9 | 9 | 7 | 4 | 4 | 14 | 17 | 4 | 5 | 19 | 7 | - | 3 | 6 | 46,XY-–33 |
| 46,XX-–3 | ||||||||||||||||||
| 45,X-–5 | ||||||||||||||||||
| 45,X/46, XY-–19 | ||||||||||||||||||
| 47,XXY-–1 | ||||||||||||||||||
| Other-–2 | ||||||||||||||||||
| Androgen synthesis (100) | 18 | - | - | 1 | 1 | - | - | - | - | - | - | 1 | - | 13 | 1 | - | 3 | 46,XY-–9 |
| 46,XX-–9 | ||||||||||||||||||
| Androgen action (172) | 19 | - | - | 3 | 1 | - | 2 | 2 | 3 | 3 | 2 | 5 | 4 | 3 | 1 | 1 | 7 | 46,XY-–19 |
| Androgen excess (75) | 9 | - | - | 1 | 1 | - | - | 1 | 1 | 2 | - | - | - | 1 | - | 1 | 5 | 46,XY-–1 |
| 46,XX-–8 | ||||||||||||||||||
| Nonspecific XY DSD (90) | 44 | 1 | 1 | 8 | 4 | 5 | 3 | 6 | 11 | 7 | 3 | 17 | 4 | 5 | 3 | 1 | 10 | 46,XY-–44 |
| XX Müllerian devpt (12) | 6 | - | - | 1 | - | - | 1 | - | 1 | 6 | - | - | - | 2 | - | 1 | - | 46,XX-–6 |
| PMDS (5) | 1 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 1 | 46,XY-–1 |
| Leydig cell defects (17) | 1 | - | - | 1 | 1 | - | 1 | - | - | - | - | - | - | 1 | 1 | - | - | 46,XY-–1 |
| Other (25) | 7 | 1 | - | 3 | - | - | - | - | 2 | - | - | - | 1 | - | - | 1 | 1 | 46,XY-–5 |
| 46,XX-–1 | ||||||||||||||||||
| 45,X-–1 | ||||||||||||||||||
| Total (649) | 168 | 3 | 4 | 27 | 17 | 12 | 11 | 13 | 32 | 35 | 9 | 28 | 28 | 32 | 6 | 8 | 33 | |
Abbreviation: PMDS, persistent Müllerian duct syndrome. Dashes indicate no cases recorded.
Range of conditions
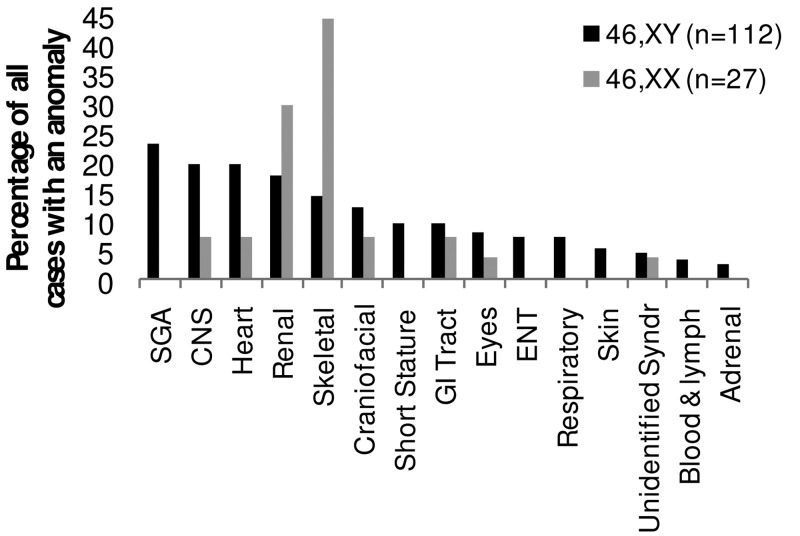
The range and distribution of associated conditions differed substantially between cases of 46,XY and 46,XX DSD (Figure 1). SGA was reported in 26 of 112 cases (23%) of 46,XY DSD with associated conditions; however, there were no cases of SGA in the 46,XX DSD group. Central nervous system (CNS) and cardiac conditions were next most frequent in 46,XY DSD, each reported in 22 of the 112 cases (20%) of 46,XY DSD with associated conditions.
Figure 1.
Range of anomalies reported in 46,XX and 46,XY DSD.
In the 90 cases of nonspecific XY DSD without any clear diagnosis on the registry, 17 (19%) were reported to be SGA, 11 (12%) had cardiac abnormalities, and 8 (9%) had involvement of the CNS (Table 1). In the 153 cases of disorders of gonadal development on the registry, 19 (12%) had short stature (15 of which occurred in cases with 45,X or 45,X/46,XY karyotype, 3 in cases of 46,XY DSD, and 1 case with an atypical karyotype), 17 (11%) had renal anomalies (7 of which occurred in cases with 45,X or 45,X/46,XY karyotype), and 14 (9%) had cardiac abnormalities (6 of which occurred in cases with 45,X or 45,X/46,XY karyotype) (Table 1).
Conditions affecting the skeleton were present in 12 of 27 (44%) cases of 46,XX DSD with an associated condition and these cases included two cases of MURCS, diagnosed clinically, seven cases of PORD all with genetic confirmation of diagnosis, two unconfirmed nonclassical 3β-hydroxysteroid dehydrogenase deficiency, and one case of 11β-hydroxylase deficiency, with genetic confirmation. Conditions involving renal development were encountered in 8 of the 27 cases (30%) and were commonest in those with a 46,XX disorder of Müllerian development, occurring in 6 of 12 cases in the Registry (50%), and present in all such cases that had an anomaly recorded.
As associated conditions are already known to occur in some syndromes associated with DSD, 8 cases of Turner syndrome, 42 cases of 45,X/46,XY, 19 cases of PORD, and 12 cases of Müllerian development anomalies were excluded from the overall 649 cases. Of the remaining 568 cases, 127 (22%) had an associated condition that did not form a recognized part of the disorder. Of these 127, 108 (85%) were 46,XY, 15 (12%) were 46,XX, 2 (2%) had a nonsex chromosome rearrangement, and there was 1 case each (1%) of 46,XX/46,XY and 47,XXY, respectively. Of these 127 cases, 75 (60%) had one condition, 22 (17%) had two conditions, 18 (14%) had three conditions, and 12 (9%) had four or more conditions, with the maximum number of conditions encountered in one patient being eight. Among these 127 cases there were 44 cases of nonspecific 46,XY DSD, 39 cases of disorders of gonadal development, 19 cases of disorders of androgen action, 10 case of disorders of androgen synthesis, 9 cases of disorders of androgen excess, 1 case each of Leydig cell defects and persistent Müllerian duct syndrome, and 6 cases classified as “other” disorders. Of the 39 cases of disorders of gonadal development, 10 (26%) had a renal condition, 8 (21%) had a cardiac condition, 8 (21%) had a CNS condition, 8 (21%) had a craniofacial disorder, 4 each (10%) had short stature, ear, nose, and throat, gastrointestinal (GI) tract, respiratory or skeletal conditions, 3 (8%) had blood and lymph conditions, eye conditions, SGA, or an unidentified syndrome, and 1 (3%) had an adrenal condition.
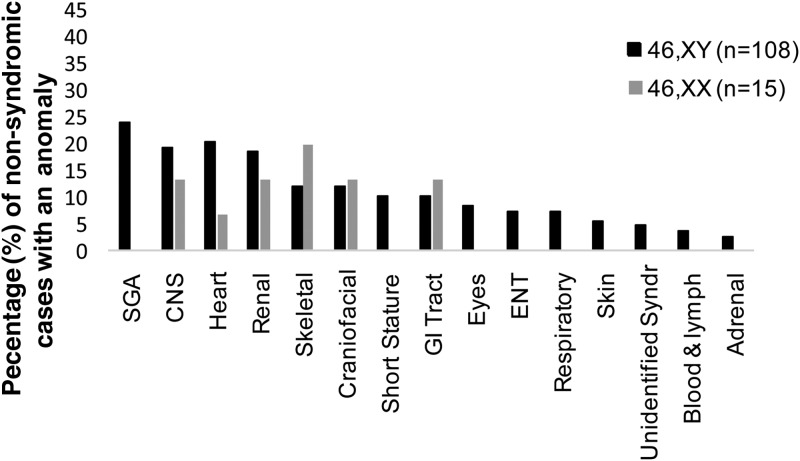
The pattern and frequency of anomalies also varied between 46,XY and 46,XX DSD after exclusion of the syndromic DSD conditions (Figure 2). Of the 451 cases of 46,XY DSD, an associated condition was reported in 108 (24%) and in 15 of 98 cases (15%) of 46,XX DSD. In 46,XY DSD, SGA was the most frequent condition in 26 cases (24%), with cardiac anomalies in 22 cases (20%) and CNS conditions in 21 cases (19%). In 46,XX DSD, there was an even spread of conditions with the most frequent condition being skeletal occurring in 3 of 15 cases (20%).
Figure 2.
Range of anomalies reported after exclusion of known DSD syndromic associations in 46,XX and 46,XY DSD.
Monogenic DSD
Of 170 cases reported as androgen insensitivity syndrome (AIS), 19 (11%) were recorded as having an associated condition. Of these 19 cases, 9 had a confirmed androgen receptor mutation, occurring in six cases of Complete AIS and three cases of Partial AIS. Associated conditions were reported to affect the renal system in two cases, skeleton in one case, skin in one case, CNS in one case, GI tract in one case, heart in one case, and an unspecified condition in five cases (Table 2). Associated conditions were also reported in 8 of 72 (11%) cases of 21α-hydroxylase deficiency (1 of which had confirmed CYP21A2 mutation, while the remainder were diagnosed clinically), 4 of 26 (15%) cases of 17β-hydroxysteroid dehydrogenase type 3 deficiency (all with confirmed HSD17B3 mutation), and 2 of 19 cases (10%) of 5α-reductase type 2 deficiency. In the 8 cases of 21α-hydroxylase deficiency, two had renal conditions. In the remainder, there was one case of a CNS condition, one affecting the GI tract, and one case each detailed as severe autism, spastic paraplegia, polyarthritis, learning disability, and one not specified.
Table 2.
Reported Anomalies in Monogenic Conditions
| Disorder | Total Number | Number Cases With Anomaly | CNS | Number of Cases With Each Anomaly |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Craniofacial | Eyes | GI Tract | Heart | Renal | Respiratory | SGA | Short Stature | Skeletal | Skin | Undefined Syndrome | Other | ||||
| Disorders of androgen synthesis | |||||||||||||||
| 17βhydroxysteroid dehydrogenase type 3 deficiency | 26 | 4 | 1 | - | - | - | - | - | - | - | - | 1 | - | - | 3 |
| 5α reductase type 2 deficiency | 19 | 2 | - | - | - | - | - | - | - | 1 | - | - | 1 | - | - |
| P450 oxidoreductase deficiency | 19 | 10 | - | 1 | - | - | - | - | - | - | - | 10 | - | - | - |
| Disorder of androgen action | |||||||||||||||
| Complete androgen insensitivity syndrome (AR mutation +ve) | 77 | 6 | 1 | - | - | 1 | 1 | 1 | - | - | - | - | 1 | - | 3 |
| Complete androgen insensitivity syndrome (AR mutation −ve/unknown) | 16 | 3 | - | - | - | - | - | 1 | - | - | - | 1 | - | - | 1 |
| Partial androgen insensitivity syndrome (AR mutation +ve) | 39 | 3 | - | - | - | - | - | 1 | - | - | - | 1 | - | - | 2 |
| Partial androgen insensitivity syndrome (AR mutation −ve) | 38 | 7 | 2 | 1 | 2 | 1 | 2 | - | 2 | 5 | 4 | 1 | - | 1 | 1 |
| Disorder of androgen excess | |||||||||||||||
| 21αHydroxylase deficiency | 72 | 8 | 1 | - | - | 1 | 1 | 2 | - | - | - | - | - | - | 5 |
| 11βHydroxysteroid dehydrogenase deficiency | 1 | 1 | - | 1 | - | - | - | - | - | - | - | 1 | - | 1 | - |
Abbreviation: AR, androgen receptor. Two cases reported as possible nonclassic 3β-hydroxysteroid dehydrogenase have been included in Table 1 as androgen synthesis disorders, but due to diagnostic uncertainty have not been included in the monogenic conditions in Table 2. Two cases described as “disorders of androgen excess” have the diagnosis “other” with no confirmed diagnosis and are therefore not included in Table 2. Dashes indicate no cases recorded.
Discussion
The results from this analysis of the I-DSD Registry reveal that associated conditions are frequent in DSD, with a rate of 27%, which is over 10 times the birth prevalence of congenital anomalies (17). This is not unexpected, given that the presence of one congenital condition is known to be associated with the presence of further anomalies because disrupting factors, whether environmental or genetic, are likely to affect multiple developmental processes. Furthermore, approximately 10% of the cases of DSD had more than one associated condition, highlighting the need for input from multiple specialists. When cases with DSD syndromes and chromosomal anomalies were excluded, the overall frequency of associated conditions was similar to the overall cohort. These figures are also in keeping with the findings of an epidemiological study carried out in Germany that identified a higher rate of 37.5% for associated malformations in infants with ambiguous genitalia (18).
Although the ranges of anomalies that are encountered are different, it is of interest that in the current cohort of cases in the I-DSD Registry, the reported prevalence of associated conditions in 46,XY and 46,XX DSD at 24% and 22% was similar. With the exclusion of the cases that are well known to be associated with anomalies, the prevalence of associated conditions in the other cases of 46,XY and 46,XX DSD was 24% and 15%, respectively. The type of conditions encountered in 45,X/46,XY cases bears a striking resemblance to the known phenotype in Turner syndrome, with partial or complete gonadal dysgenesis. This is reflected in the high frequency of associated conditions in all cases with gonadal dysgenesis. Our findings echo the description of the 45,X/46,XY phenotype by Tosson et al and Telvi et al (19, 20) and the cardiac phenotype refined by De Groote et al (21). These findings highlight the importance of a thorough screening for associated conditions in both male and female individuals with this karyotype.
It has long been recognized that there is a strong association between SGA and DSD (1, 22), and this was reflected in our findings. It was notable that there were no reported cases of SGA in 46,XX DSD. It has been speculated that altered prenatal androgen exposure may alter in utero growth but this is debatable (23, 24). An alternative hypothesis for the origins of low birth weight in 46,XY DSD is that both are the result of early placental insufficiency (25, 26) and this requires further investigation.
In 46,XX DSD, an association with skeletal and renal anomalies is recognized in conditions such as MURCS, MRKH, and PORD (7, 27). This was borne out by our results, in which renal conditions were common in women with 46,XX DSD and disorders of Müllerian development. Similarly, skeletal involvement was also observed in women with 46,XX DSD and PORD or MURCS. Renal disorders are also a recognized feature of 46,XY DSD due to the Denys Drash and WAGR (Wilms, Aniridia, Genitourinary anomalies, mental Retardation) syndromes. Furthermore, bone and kidney abnormalities combined with genital anomalies are a component of Smith-Lemli-Opitz syndrome (28). Renal tract anomalies have previously been reported as occurring in 14 of 66 females (21%) with congenital adrenal hyperplasia (29) and were also confirmed to be present in the current cohort occurring in two cases of 21α-hydroxylase deficiency. When recognized associations were excluded from the analysis, renal and skeletal disorders were still encountered in up to 4% of cases of 46,XX DSD and 46,XY DSD.
The unexpected presence of renal abnormalities in AIS cases with a confirmed androgen receptor mutation raises a question about the developmental role of the androgen receptor in the kidney. Previously, an association between Complete AIS and renal anomalies has been described in one case (30). In mice, the kidney is one of the most androgen sensitive, nonreproductive organs, with a close correlation between kidney mass and androgen levels in the male (31). In addition, several developmental genes are known to be androgen-regulated in the mouse (32). Our finding of two cases with renal anomalies in congenital adrenal hyperplasia is also worthy of emphasis, as it highlights the importance of thorough investigation of the whole renal tract in such patients. Ten cases reported as AIS without a confirmed androgen receptor mutation had an associated condition. Of these, five cases had SGA and four had short stature. Given that these associations are often encountered in cases of gonadal dysgenesis, these cases of presumed AIS need a thorough investigation of gonadal function.
It is possible that some of the associations revealed by our analysis of the Registry may represent co-occurrence of anomalies by chance. Renal tract anomalies have been reported as occurring with a frequency of 25 per 1000 in the general European population (17). This compares to a frequency in our analysis of 35 of 649, equivalent to 54 per 1000. The frequency of cardiac anomalies, the second most common anomaly in our population, is reported as 8 per 1000 in the European population (33). This compares to the occurrence of cardiac anomalies in our analysis of 32 of 649, equivalent to 49 per 1000.
The I-DSD Registry is not an epidemiological registry and, as it does not collect information on all cases of DSD, it may suffer from a reporting and selection bias. It is recognized that there may be a bias toward the reporting of more clinically unusual cases, which could increase the reported prevalence of anomalies. Registry users can also choose the extent of data sharing with other users for each individual case and this sharing may depend on the patient's or clinician's preference. Based on the access level of the investigators, only 60% of the cases in the Registry could be accessed for this study and this may have also introduced some selection bias. However, the similarities between our results and the findings of a national study suggest that the prevalence figure of 25% for associated conditions probably is representative of the true prevalence (18). In particular, cases of congenital adrenal hyperplasia may be underrepresented in the Registry due to reporting methods, given that the incidence in the general population is estimated at around 1 in 15 000 live births (34). The incidence of AIS was described in a Danish population registry as 1 in 20 400 (35), whereas MRKH is estimated as occurring in 1 in 4500 females (36). However, the Registry does not necessarily represent the population prevalence of these disorders because it relies on clinician reporting rather that population screening.
All data are self-recorded by the clinician, with little recourse to source verification of entered data, and it can be difficult in such a registry to be clear that all users have the same understanding of an associated condition that is congenital. It is also possible that some congenital conditions may not be manifested until a later age. Until recently, the data collection form asked investigators to enter data on “associated malformations,” which implies congenital conditions. More recently, the term “malformations” has been replaced by the term “conditions.” It would be beneficial in future revisions of the Registry to provide some explanatory data to ensure that conditions that are considered to be of congenital and those considered to be acquired are captured separately and there may be additional value in assessing the age of onset of the manifestation of the condition.
However, the use of an international registry of such rare conditions in such a large group of cases has, nevertheless, pointed to some new associations in DSD and the study highlights the strength of a global effort to collect data in rare diseases, which will encourage reporting of more cases of DSD with associated conditions. In conclusion, we have found that, in this cohort of cases, associated anomalies are reported in around a quarter of cases of DSD. The prevalence of associated conditions is much greater in disorders of gonadal development and nonspecific 46,XY DSD. In 46,XY DSD, the largest group of cases in the Registry, the commonest associated conditions included SGA, cardiac, and CNS. In many cases of DSD the etiology remains obscure and the current findings may lead to new research targets as well as improved care of those affected by these disorders.
Acknowledgments
K.C. and M.R. are supported by the University of Glasgow and the Yorkhill Children's Foundation. S.F.A., J.B., J.J., M.R., and R.O.S. are also supported by Medical Research Council Partnership Grant Ref. G1100236. The Registry was initiated and built under a grant from the seventh European Union framework program (Grant No. 201444).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AIS
- androgen insensitivity syndrome
- DSD
- disorders of sex development
- GI
- gastrointestinal
- I-DSD
- international DSD
- MRKH
- Mayer-Rokitansky-Küster-Hauser syndrome
- MURCS
- Müllerian duct aplasia, renal dysplasia, and cervical somite anomalies
- PORD
- P450 oxidoreductase deficiency
- SGA
- small for gestational age.
References
- 1. Ahmed SF, Dobbie R, Finlayson AR, et al. Prevalence of hypospadias and other genital anomalies among singleton births, 1988–1997, in Scotland. Arch Dis Child Fetal Neonatal Ed. 2004;89:F149–F151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ono M, Harley VR. Disorders of sex development: new genes, new concepts. Nat Rev Endocrinol. 2013;9:79–91 [DOI] [PubMed] [Google Scholar]
- 3. Ahmed SF, Bashamboo A, Lucas-Herald A, McElreavey K. Understanding the genetic aetiology in patients with XY DSD. Br Med Bull. 2013;106:67–89 [DOI] [PubMed] [Google Scholar]
- 4. Hughes IA. Minireview: sex differentiation. Endocrinology. 2001;142:3281–3287 [DOI] [PubMed] [Google Scholar]
- 5. Kreidberg JA, Sariola H, Loring JM, et al. WT-1 is required for early kidney development. Cell. 1993;74:679–691 [DOI] [PubMed] [Google Scholar]
- 6. Porter FD. Smith-Lemli-Opitz syndrome: pathogenesis, diagnosis and management. Eur J Human Genet. 2008;16:535–541 [DOI] [PubMed] [Google Scholar]
- 7. Flück CE, Tajima T, Pandey AV, et al. Mutant P450 oxidoreductase causes disordered steroidogenesis with and without Antley-Bixler syndrome. Nat Genet. 2004;36:228–230 [DOI] [PubMed] [Google Scholar]
- 8. Yang W, Carmichael SL, Shaw GM. Congenital malformations co-occurring with hypospadias in California, 1983–1997. Am J Med Genet A. 2007;143A:2627–2634 [DOI] [PubMed] [Google Scholar]
- 9. Hughes IA, Houk C, Ahmed SF, Lee PA. Consensus statement on management of intersex disorders. J Pediatr Urol. 2006;2:148–162 [DOI] [PubMed] [Google Scholar]
- 10. Ahmed SF, Cheng A, Dovey L, et al. Phenotypic features, androgen receptor binding, and mutational analysis in 278 clinical cases reported as androgen insensitivity syndrome. J Clin Endocrinol Metab. 2000;85:658–665 [DOI] [PubMed] [Google Scholar]
- 11. Gottlieb B, Beitel LK, Nadarajah A, Paliouras M, Trifiro M. The androgen receptor gene mutations database: 2012 update. Hum Mutat. 2012;33:887–894 [DOI] [PubMed] [Google Scholar]
- 12. Bebermeier JH, Brooks JD, DePrimo SE, et al. Cell-line and tissue-specific signatures of androgen receptor-coregulator transcription. J Mol Med (Berl). 2006;84:919–931 [DOI] [PubMed] [Google Scholar]
- 13. Duguid A, Morrison S, Robertson A, Chalmers J, Youngson G, Ahmed SF. The psychological impact of genital anomalies on the parents of affected children. Acta Paediatr. 2007;96:348–352 [DOI] [PubMed] [Google Scholar]
- 14. Köhler B, Kleinemeier E, Lux A, Hiort O, Gruters A, Thyen U, DSD Network Working Group Satisfaction with genital surgery and sexual life of adults with XY disorders of sex development: results from the German clinical evaluation study. J Clin Endocrinol Metab. 2012;97:577–588 [DOI] [PubMed] [Google Scholar]
- 15. Ahmed SF, Rodie M, Jiang J, Sinnott RO. The European disorder of sex development registry: a virtual research environment. Sex Dev. 2010;4:192–198 [DOI] [PubMed] [Google Scholar]
- 16. Hiort O, Wünsch L, Cools M, Looijenga L, Cuckow P. Requirements for a multicentric multidisciplinary registry on patients with disorders of sex development. J Pediatr Urol. 2012;8:624–628 [DOI] [PubMed] [Google Scholar]
- 17. EUROCAT EUROCAT Prevalence Tables (2006–2010). In: http://www.eurocat-network.eu/accessprevalencedata/prevalencetables Accessed June 3, 2013
- 18. Thyen U, Lanz K, Holterhus PM, Hiort O. Epidemiology and initial management of ambiguous genitalia at birth in Germany. Horm Res. 2006;66:195–203 [DOI] [PubMed] [Google Scholar]
- 19. Tosson H, Rose SR, Gartner LA. Description of children with 45,X/46,XY karyotype. Eur J Pediatr. 2012;171:521–529 [DOI] [PubMed] [Google Scholar]
- 20. Telvi L, Lebbar A, Del Pino O, Barbet JP, Chaussain JL. 45,X/46,XY mosaicism: report of 27 cases. Pediatrics. 1999;104:304–308 [DOI] [PubMed] [Google Scholar]
- 21. De Groote K, Cools M, De Schepper J, et al. Cardiovascular pathology in males and females with 45,X/46,XY mosaicism. PLoS One. 2013;8:e54977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Akre O, Lipworth L, Cnattingius S, Sparén P, Ekbom A. Risk factor patterns for cryptorchidism and hypospadias. Epidemiology. 1999;10:364–369 [PubMed] [Google Scholar]
- 23. Miles HL, Gidlöf S, Nordenström A, Ong KK, Hughes IA. The role of androgens in fetal growth: observational study in two genetic models of disordered androgen signalling. Arch Dis Child Fetal Neonatal Ed. 2010;95:F435–F438 [DOI] [PubMed] [Google Scholar]
- 24. Hughes IA, Northstone K, Golding J. Reduced birth weight in boys with hypospadias: an index of androgen dysfunction? Arch Dis Child Fetal Neonatal Ed. 2002;87:F150–F151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yinon Y, Kingdom JC, Proctor LK, et al. Hypospadias in males with intrauterine growth restriction due to placental insufficiency: the placental role in the embryogenesis of male external genitalia. Am J Med Genet A. 2010;152A:75–83 [DOI] [PubMed] [Google Scholar]
- 26. Sun M, Maliqueo M, Benrick A, et al. Maternal androgen excess reduces placental and fetal weights, increases placental steroidogenesis, and leads to long-term health effects in their female offspring. Am J Physiol Endocrinol Metab. 2012;303:E1373–E1385 [DOI] [PubMed] [Google Scholar]
- 27. Morcel K, Guerrier D, Watrin T, Pellerin I, Leveque J. The Mayer-Rokitansky-Kuster-Hauser (MRKH) syndrome: clinical description and genetics. J Gynecol Obstet Biol Reprod (Paris). 2008;37:539–546 [DOI] [PubMed] [Google Scholar]
- 28. Nowaczyk MJ, Irons MB. Smith-Lemli-Opitz syndrome: phenotype, natural history, and epidemiology. Am J Med Genet C Semin Med Genet. 2012;160C:250–262 [DOI] [PubMed] [Google Scholar]
- 29. Nabhan ZM, Eugster EA. Upper-tract genitourinary malformations in girls with congenital adrenal hyperplasia. Pediatrics. 2007;120:e304–307 [DOI] [PubMed] [Google Scholar]
- 30. Tokgoz H, Turksoy O, Boyacigil S, Sakman B, Yuksel E. Complete androgen insensitivity syndrome: report of a case with solitary pelvic kidney. Acta Radiol. 2006;47:222–225 [DOI] [PubMed] [Google Scholar]
- 31. Berger FG, Watson G. Androgen-regulated gene expression. Annu Rev Physiol. 1989;51:51–65 [DOI] [PubMed] [Google Scholar]
- 32. Melià MJ, Bofill N, Hubank M, Meseguer A. Identification of androgen-regulated genes in mouse kidney by representational difference analysis and random arbitrarily primed polymerase chain reaction. Endocrinology. 1998;139:688–695 [DOI] [PubMed] [Google Scholar]
- 33. Dolk H, Loane M, Garne E. Congenital heart defects in Europe: prevalence and perinatal mortality, 2000 to 2005. Circulation. 2011;123:841–849 [DOI] [PubMed] [Google Scholar]
- 34. Pang S, Shook MK. Current status of neonatal screening for congenital adrenal hyperplasia. Curr Opin Pediatr. 1997;9:419–423 [DOI] [PubMed] [Google Scholar]
- 35. Bangsbøll S, Qvist I, Lebech PE, Lewinsky M. Testicular feminization syndrome and associated gonadal tumors in Denmark. Acta Obstet Gynecol Scand. 1992;71:63–66 [DOI] [PubMed] [Google Scholar]
- 36. Guerrier D, Mouchel T, Pasquier L, Pellerin I. The Mayer-Rokitansky-Kuster-Hauser syndrome (congenital absence of uterus and vagina)—phenotypic manifestations and genetic approaches. J Negat Results Biomed. 2006;5:1. [DOI] [PMC free article] [PubMed] [Google Scholar]