Abstract
TRIC represents a novel class of trimeric intracellular cation channels. Two TRIC isoforms have been identified in both the human and mouse genomes: TRIC-A - a subtype predominantly expressed in the sarcoplasmic reticulum (SR) of muscle cells, and TRIC-B - a ubiquitous subtype expressed in the endoplasmic reticulum (ER) of all tissues. Genetic ablation of either TRIC-A or TRIC-B leads to compromised K+ permeation and Ca2+ release across the SR/ER membrane, supporting the hypothesis that TRIC channels provide a counter balancing K+ flux that reduces SR/ER membrane depolarization for maintenance of the electrochemical gradient that drives SR/ER Ca2+ release. TRIC-A and TRIC-B appear to have differential functions in Ca2+ signaling in excitable and non-excitable cells. Tric-a−/− mice display defective Ca2+ sparks and spontaneous transient outward currents in arterial smooth muscle and develop hypertension, in addition to skeletal muscle dysfunction. Knockout of TRIC-B results in abnormal IP3 receptor-mediated Ca2+ release in airway epithelial cells, respiratory defects and neonatal lethality. Double-knockout mice lacking both TRIC-A and TRIC-B show embryonic lethality due to cardiac arrest. Such an aggravated lethality indicates that TRIC-A and TRIC-B share complementary physiological functions in Ca2+ signaling in embryonic cardiomyocytes. Tric-a−/−Tric-b+/− mice are viable and susceptible to stress-induced heart failure. Recent evidence suggests that TRIC-A directly modulates the function of the cardiac ryanodine receptor (RyR2) Ca2+ release channel, which in turn controls store-overload induced Ca2+ release from the SR. Thus, the TRIC channels, in addition to providing a counter-current for SR/ER Ca2+ release, may also function as accessory proteins that directly modulate the RyR/IP3 receptor channel functions.
Keywords: RYR, IP3R, counter-ion, Ca 2+ signaling
INTRODUCTION
Ca2+ ions are important second messengers in many cellular signal transduction pathways. Compromised Ca2+ homeostasis and signaling have been linked to many human diseases, including muscle dysfunction and heart failure1–5. Two principal sources provide Ca2+ to the cell: channels in the plasma membrane (PM) that allow external Ca2+ to enter the cell, and internal stores sequestered in the endoplasmic reticulum (ER) or sarcoplasmic reticulum (SR) that release Ca2+. Junctional membrane complexes between PM and ER/SR are present in all excitable cells, providing effective mechanisms for cross-talk between Ca2+ channels/transporters in the plasma membrane and Ca2+ release channels in intracellular membranes6–10. A central focus in cardiovascular research is to understand the basic mechanisms that underlie the control of Ca2+ signaling in the heart, and to search for ways to correct the defective Ca2+ signaling process associated with arrhythmogenesis and heart failure.
In the heart, entry of extracellular Ca2+ via the L-type Ca2+ channel triggers opening of the ryanodine receptor (RyR) located in the SR membrane through the Ca2+ induced Ca2+ release (CICR) mechanism11–13. In skeletal muscle, it is membrane depolarization, rather than external Ca2+, that triggers Ca2+ release as the close proximity between the transverse-tubular invagination of PM and the terminal cisternae of SR permits direct relay of the depolarizing signal via voltage-induced Ca2+ release (VICR)14–16. In this case, CICR represents a secondary mechanism for amplification of VICR in skeletal muscle. While many studies have focused on understanding the mechanisms that contribute to control of CICR and VICR, the detailed ionic-flux events that take place across the SR/ER during Ca2+ release are largely un-known. The ER/SR Ca2+ load is maintained by the Ca2+ uptake and release processes, both of which are electrogenic events. The efflux of Ca2+ through the RyR channels will lead to generation of a negative potential inside the SR/ER lumen, and this would be expected to limit the release of Ca2+ from the SR/ER. Likewise, active uptake of Ca2+ into the ER/SR would lead to accumulation of a positive potential within the SR/ER lumen which would tend to inhibit Ca2+-pumping function. Thus, robust counter-ion movements are essential to balance the SR/ER membrane potential and to maintain efficient Ca2+ release and uptake mechanisms17–22. While channels selective for monovalent cations have been reported in SR membrane17, 21, 23–25, searching for the molecular identity of these channels and other accessory proteins that modulate the operation of CICR and VICR has emerged as an important area of muscle physiology and cardiovascular research.
Takeshima and colleagues developed an immuno-proteomic approach to search for novel proteins involved in myogenesis, Ca2+ signaling and maintenance of membrane integrity in striated muscle cells26. A combination of monoclonal antibody-immunohistochemistry, cDNA library screening and gene knockout techniques was employed to identify a group of novel proteins termed mitsugumins (MG) that play important roles in muscle physiology and cardiovascular diseases. For example, MG29, one protein isolated from this immuno-proteomic library, is a synaptophysin-related membrane protein that is essential for the maturation and development of the triad structure in skeletal muscle27–29. MG29 may act as a molecular marker of aging that can shield skeletal muscle against aging-related decreases in Ca2+ homeostatic capacity30–33. Another gene isolated using the same approach was junctophilin (JP) that physically links the transverse-tubule to the SR membrane34–38, allowing the formation of triad junctions which provide the structural framework for E-C coupling. Recent studies have also linked JP dysfunction and JP polymorphisms to the development of various cardiovascular diseases39–43. More recently, another MG protein, MG53, has been shown to be a muscle-specific member of the TRIM family of proteins that plays an important role in the repair of injuries to the plasma membrane of muscle cells44–49. Defects in MG53 function are associated with muscular dystrophy and cardiac dysfunction46, 50, 51. Encouragingly, recombinant MG53 protein can be used to modulate membrane repair, which would have important implication for the treatment of muscular dystrophy and other human diseases associated with membrane repair defects44, 52.
In 2007, we discovered TRIC channels located at the SR/ER of multiple cell type53. In the human and mouse genomes, two isoforms of TRIC were identified: TRIC-A - a subtype predominantly expressed in SR of muscle cells, and TRIC-B - a ubiquitous subtype expressed in ER of all tissues. TRIC-A is also present in the nuclear membrane (Fig. S1 of ref 53), but its biological function in regulation of gene transcription has yet to be examined. As a first step towards understanding the physiological function of TRIC, we generated knockout mice carrying deletion of either Tric-a or Tric-b. While mutant mice lacking TRIC-A survive past their adolescent age, homozygous ablation of Tric-b is lethal as the Tric-b−/− mice died at the neonatal stage. Moreover, aggravated embryonic lethality is observed with the Tric-a−/−Tric-b−/− mice, demonstrating the essential role of TRIC in development53. Our collaborative studies established that both TRIC-A and TRIC-B can function as K+-permeable channels with distinct conductance and regulatory properties53, 54. We found that genetic ablation of TRIC-A or TRIC-B lead to compromised K+-permeability and Ca2+ release across the SR/ER membrane, supporting the hypothesis that TRIC could function as counter-ion channels that provide the flow of K+ ions into the SR during the acute phase of Ca2+ release53, 55, 56 (Fig. 1A). In addition, TRIC may also participate in modulating ER/SR membrane potential between Ca2+ release events. In other words, TRIC likely helps maintain overall Ca2+ homeostasis and its function may become particularly important during periods of repetitive release events.
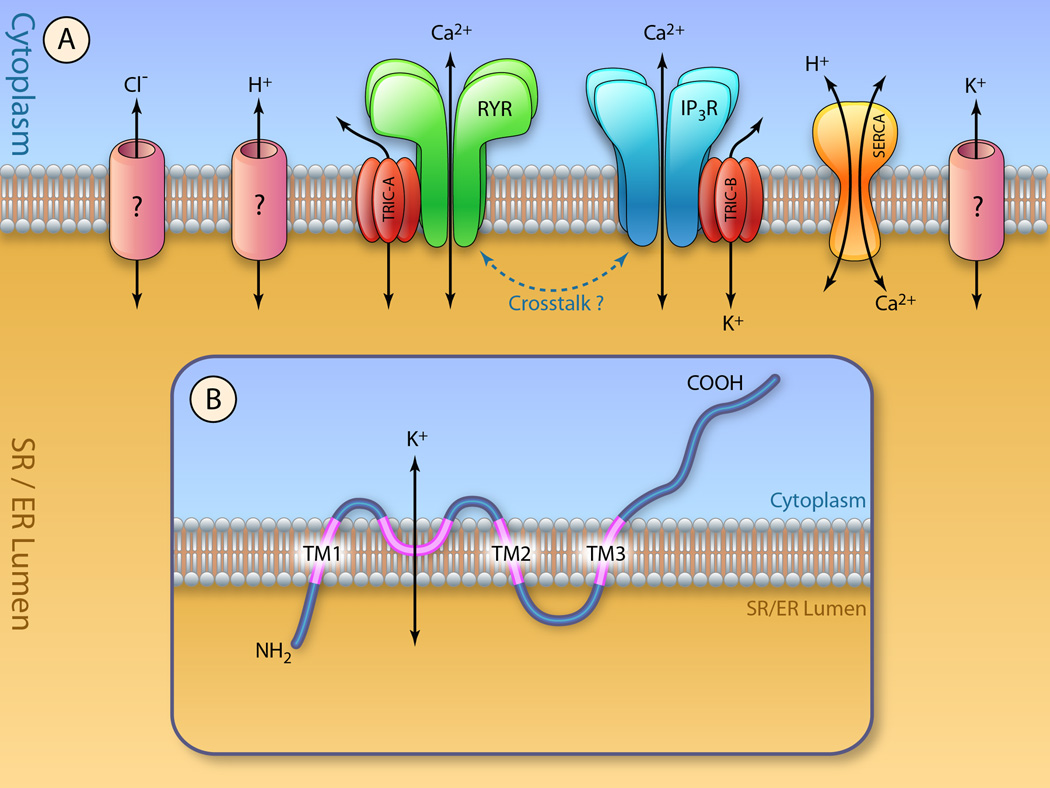
Figure 1. Model for TRIC function in Ca2+ signaling.
(A) TRIC-A and TRIC-B are two isoforms of the trimeric intracellular cation channels. Both TRIC-A and TRIC-B channels can conduct monovalent cations to provide the flow of counter currents associated with release of Ca2+ ions from intracellular stores. TRIC-A modulates RyR-mediated Ca2+ release from the SR, and TRIC-B facilitates IP3R-mediated Ca2+ release from the ER. Whether or not there is a cross-talk between TRIC-A and TRIC-B mediated intracellular Ca2+ signaling remains to be explored. Molecular identify of other channels located on the ER/SR membranes are not known. (B) Topology model of TRIC channels on the SR/ER membrane. (Illustration Credit: Ben Smith).
Studies from Fill and colleagues showed that the RyR channel could provide certain amount of counter ion movement associated with Ca2+ release from the SR membrane, due to the high permeability of the RyR channel to monovalent cations57–59. Clearly, further studies are required to define the contribution of the intrinsic K+-permeability of the RyR channel and its relationship to the TRIC channel and the overall Ca2+ signaling across the SR/ER membrane. In this review article, we summarize key properties of TRIC-A and TRIC-B in controlling intracellular Ca2+ homeostasis and signaling, and provide some recent evidence supporting the role for TRIC in modulating the RyR Ca2+ release channel and operation of store-overload induced Ca2+ release from the SR membrane. These findings highlight the important role of TRIC in cardiac physiology and disease.
Biochemical and biophysical properties of TRIC channels
TRIC subtypes are composed of ~300 amino acid residues. TRIC-A and TRIC-B share patches of sequence identities and similar hydropathy profiles that suggest the existence of multiple transmembrane segments53 (Fig. 1B). In their primary structures, three or four ER/SR membrane-spanning segments are proposed by protein-structural analysis using computer algorithms. In limited proteolysis of muscle SR vesicles, the amino-terminal region of TRIC-A was resistant to protease digestion, while the carboxyl-terminal tail was sensitive to digestion. The amino and carboxyl-terminal regions are therefore assigned to the SR/ER luminal and cytoplasmic sides, respectively. Additional observations in epitope-tagged TRIC-A proteins expressed in cultured cells support the existence of three transmembrane segments in the primary structure. Moreover, the hydrophobic loop connecting putative transmembrane segments TM1 and TM2 is likely associated with membranous environments at the cytoplasmic side and may constitute the channel pore region. The proposed transmembrane topology of TRIC subtypes thus bears an overall resemblance to that of glutamate receptor channels60, 61.
Solubilized TRIC-A protein can be purified using an affinity resin conjugated with a specific monoclonal antibody. Application of chemical cross-linkers to the SR vesicles containing endogenous TRIC-A and purified recombinant TRIC-A protein produced dimeric and trimeric products of TRIC-A. Combined computer algorithms which collect, classify, and average electron-microscopic (EM) images of purified TRIC-A particles allow us to reconstruct their three-dimensional volumes at a high resolution. TRIC-A reconstruction based on negatively-stained EM images of purified TRIC-A preparations, shows a bullet-shaped homo-trimeric structure53. Although homo-trimeric channels are uncommon, P2X receptor and bacterial porin channels are known as trimeric channels62, 63.
As mentioned above, TRIC-A and TRIC-B were identified as SR/ER-resident membrane proteins bearing several biochemical and structural features consistent with ion channels. To test this possibility, recombinant TRIC-A and TRIC-B proteins were purified from cDNA-transfected yeast cells and reconstituted into artificial lipid bilayer membranes. The reconstitution experiments demonstrated that both TRIC-A and TRIC-B form voltage-dependent cation channels, permeable to monovalent cations K+ and Na+ and impermeable to anions and divalent cations54,64. Both TRIC subtypes are much more active at positive (cytosolic side positive relative to the SR luminal side) holding potentials than at negative potentials. The channel characteristics observed in the purified TRIC preparations bear close resemblance to the SR K+ channel previously identified by Christopher Miller and colleagues17, 24, 65, indicating that TRIC-A and TRIC-B are subtypes of the SR K+ channel. Although detailed electrophysiological features remain to be defined, our current results on TRIC subtypes suggest that they are ideally suited to carry counter currents in response to loss of positive charge within the lumen of the SR/ER during Ca2+ release and/or to compensate for charge movements during SR/ER Ca2+ uptake.
Role of TRIC-A in SR Ca2+ homeostasis in skeletal and smooth muscles
As a first step towards understanding the physiological function of TRIC subtypes, we produced knockout mice carrying deletion of either the Tric-a or Tric-b gene. Perhaps not totally unexpected due to its ubiquitous expression pattern, homozygous ablation of TRIC-B is lethal; Tric-b−/− mice died at the neonatal stage. Interestingly, mutant mice lacking the muscle-specific subtype of TRIC-A, Tric-a−/−, survive past their adolescent age. Thus, Tric-a−/− can provide a useful animal model for studying the role of TRIC in muscle function in adults.
Skeletal muscle contains TRIC-A and TRIC-B isoforms as predominant and minor components, respectively. TRIC-A is approximately 10 fold more than TRIC-B in skeletal muscle54. Using microsomal membrane vesicles derived from rabbit skeletal muscle, we showed that TRIC-A is abundantly expressed in skeletal muscle, which is ~4-fold higher than that of the RyR (e.g. TRIC-A/RyR=5, in rabbit skeletal muscle)66. Ultrastructural analysis using electron microscopy showed that Tric-a−/− skeletal muscle frequently develops vacuolated SR elements with the formation of Ca2+ deposits that are rarely observed in wild-type muscle (Fig. 2A). The RyR activator caffeine could still be able to release Ca2+ from the overloaded SR in Tric-a−/− muscle, whereas elemental Ca2+ release events, for example, osmotic stress-induced Ca2+ sparks67, were significantly reduced. Moreover, isolated Tric-a−/− muscle often displayed “alternans” behavior reflected by the transient and alternate increases of contractile force during fatigue stimulations66 (Fig. 2B). Thus, TRIC-A deficiency impairs RyR-mediated Ca2+ release, resulting in SR Ca2+ overload. TRIC-A channels thus probably function as counter-ion channels to support physiological Ca2+ release across the SR in skeletal muscle.
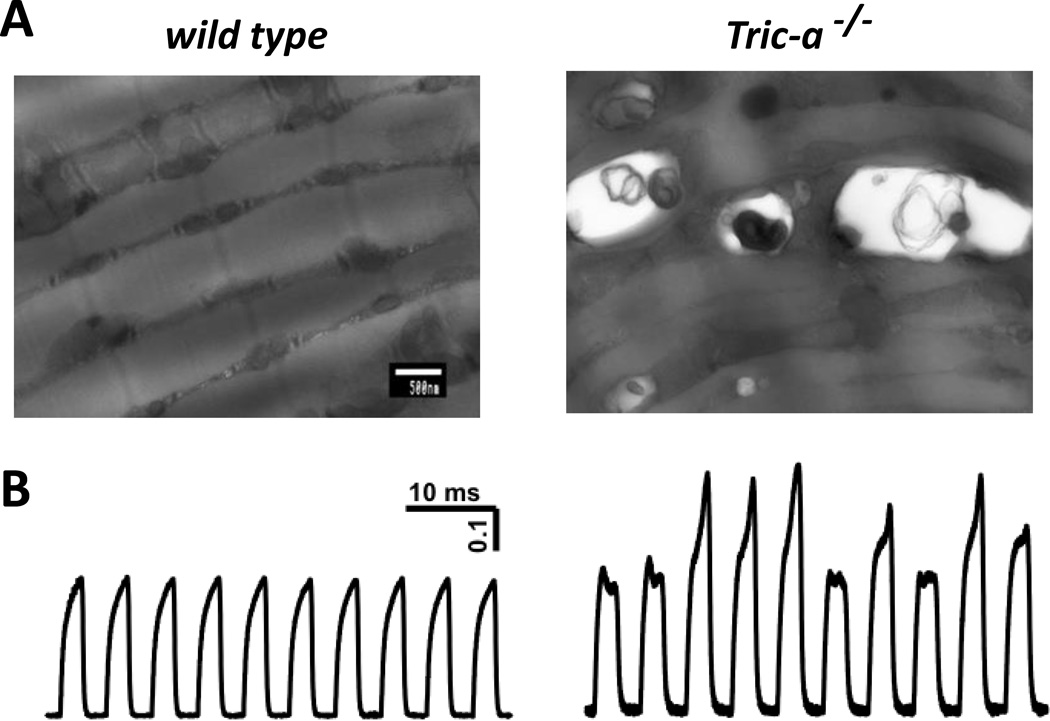
Figure 2. Ablation of TRIC-A leads to SR Ca2+ overload and muscle dysfunction.
(A) Transmission EM image reveals Ca2+-overloaded and swollen SR in Tric-a−/− skeletal muscle. Arrows designate electron dense Ca2+ deposits in the large-sized vacuoles. (B) Skeletal muscle derived from the Tric-a−/− mice showed alternans behavior following fatigue stimulation. Isolated muscle bundle from wild type mice showed constant contractile force after a fatiguing stimulation (left), whereas muscle bundle derived from the Tric-a−/− mice displayed irregular patterns of contractile force following similar fatigue stimulation (right). Such fatigue-induced irregular contractile force may reflect instability of SR Ca2+ handling in the Tric-a−/− muscle. Modified from Zhao et al66, Ca2+ overload and sarcoplasmic reticulum instability in tric-a null skeletal muscle. J Biol Chem. 2010;285:37370–37376.
Alternans also occurs in cardiac muscle, but the exact mechanism underlying cardiac alternans is unknown. Altered coupling between RyR-mediated intracellular Ca2+ release and various Ca2+ influxes across the sarcolemmal membrane, including L-type Ca2+ channel and Na+/Ca2+ exchanger, may contribute to the alternan behavior in cardiac muscle68–71. Furthermore, SR Ca2+ content is thought to be an important determinant of Ca2+ alternans72. Thus, we speculate that the mechanical alternans observed in the Tric-a−/− skeletal muscle may represent instability of the SR Ca2+ release machinery due to overload of the SR Ca2+ store and reduced membrane permeability for K+ ions.
While the pathological changes that took place in the Tric-a−/− muscle are consistent with a role for TRIC channels in providing counter-ion movements associated with Ca2+ release, it is also possible that TRIC may participate in limiting the electronegative influence of Ca2+ release from the SR, especially under conditions of repetitive stimulations where a succession of fast Ca2+ release events would lead to accumulation of negative potential inside the SR lumen. Without the TRIC-mediated K+ movement, the SR will be more electronegative than normal, which would electrically favor Ca2+ uptake. At the same time, the reduced electrochemical driving force for Ca2+ would work to reduce Ca2+ leak that would promote Ca2+ overload inside the SR. Smaller driving force means smaller Ca2+ currents through the RyR channel, which would make CICR less likely57–59. This model would explain why TRIC ablation resulted in SR Ca2+ overload and abnormal CICR. A direct test of this model would require quantitative assessment of the changes in electrical potential across the ER/SR membrane as a function of the Ca2+ release flux under controlled stimulation conditions.
Tric-a−/− mice also develop hypertension even at young-adult stages due to elevated resting tonus of vascular smooth muscle cells (VSMCs)73 (Fig. 3). There are two Ca2+ release mechanisms known to regulate VSMCs activities: one is local Ca2+ sparks generated by spontaneous RyR opening that activate cell-surface Ca2+-dependent K+ channels and lead to hyperpolarization; and the other is the agonist-induced IP3 receptor (IP3R) activation that evokes global Ca2+ transients, which frequently accompany Ca2+ waves and oscillations and induce contraction. Cross-talk between IP3R-mediated Ca2+ release and RyR-mediated Ca2+ release in smooth muscle has been reported by other investigators74, 75, however a role for TRIC channels in facilitating the respective Ca2+ release processes has not been examined before. In Tric-a−/− VSMCs, RyR-mediated Ca2+ sparks are significantly compromised, depressing the hyperpolarization signaling and elevating resting membrane potential. Under such depolarized conditions, voltage-dependent L-type Ca2+ channels are highly activated, leading to increased cytoplasmic Ca2+ concentration thus elevating resting myogenic tone in Tric-a−/− VSMCs73. On the other hand, agonist-induced Ca2+-release through IP3Rs is facilitated due to SR Ca2+ overloading in the Tric-a−/− VSMCs. In Tric-a−/− condition, RyR mediated Ca release is compromised while IP3Rs function normally, therefore, TRIC-A channels seem to selectively support RyR-mediated Ca2+ -release, while IP3R-mediated Ca2+ release might be maintained by TRIC-B channels in Tric-a−/− VSMCs. This conclusion is further supported by transgenic mice overexpressing TRIC-A in smooth muscle76. In contrast to the phenotype of Tric-a−/− mice, the transgenic mice develop persistent hypotension. In VSMSs overexpressing TRIC-A, Ca2+ spark generation is highly facilitated and Ca2+-dependent K+ channels are thus activated. Under such hyperpolarizing conditions, the L-type Ca2+ channels are inactivated and resting tonus is decreased in Tric-a-overexpressing VSMCs76.
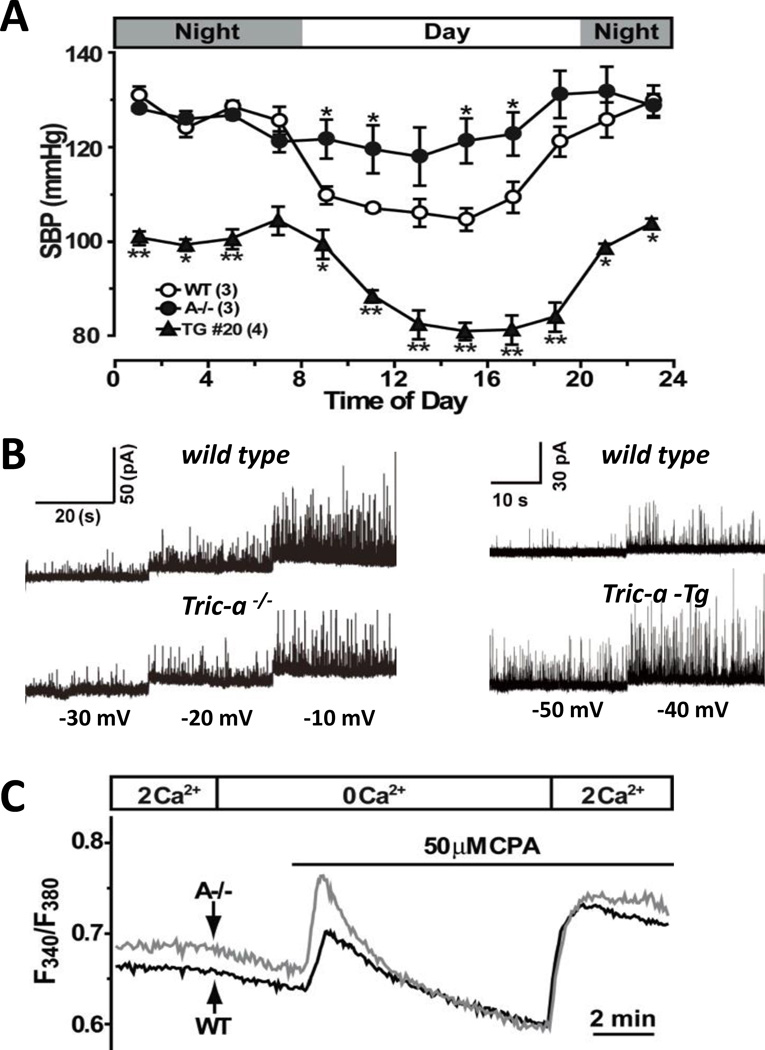
Figure 3. Tric-a−/− mice show hypertension while transgenic Tric-a mice show hypotension phenotypes.
(A) Systolic blood pressure (SBP) was monitored by telemetric recording. Tric-a-knockout and Tric-a-transgenic mice exhibited daytime hypertension and hypotension, respectively. (B) The membrane potential of isolated VSMCs was controlled by the whole-cell patch-clamp technique to monitor spontaneous transient outward currents (STOCs). Tric-a-knockout VSMCs exhibit insufficient STOCs due to impaired Ca2+ sparks; whereas Tric-a-transgenic (TG#20) VSMCs show facilitated STOCs due to enhanced Ca2+ spark generation. (C) Fura-2 Ca2+ imaging demonstrated elevated resting Ca2+ levels and store Ca2+ overloading in Tric-a−/− VSMCs.
Role of TRIC-B in IP3 receptor-mediated Ca2+ signaling in non-muscle cells
Tric-b−/− neonatal mice are cyanotic and die shortly after birth due to respiratory failure (Fig. 4). In mutant neonates, the lung alveolus was deflated and surfactant phospholipids were insufficient in extracellular space77. In immunoblotting analysis, the expression of TRIC-B and IP3R channels was observed in the wild-type lung, but TRIC-A and RyR channels were undetectable. There are mainly two cell types in alveolar epithelium: one is the squamous type I cells that surround the alveolar spaces, and the other is the cylindrical type II cells that contribute to surfactant phospholipid secretion. In the alveolus of Tric-b−/− neonates, excess glycogen deposits and insufficient phospholipid lamellar bodies could be observed indicating ultrastructural defects in the type II cells. Type II cells preserve glycogen during the late embryonic stage, and stored glycogen is converted into surfactant lipids just before delivery. It is likely that the metabolic conversion of glycogen into phospholipids is disrupted in the mutant type II cells, leading to defective surfactant secretion into the alveolar space in the Tric-b−/− lung77.
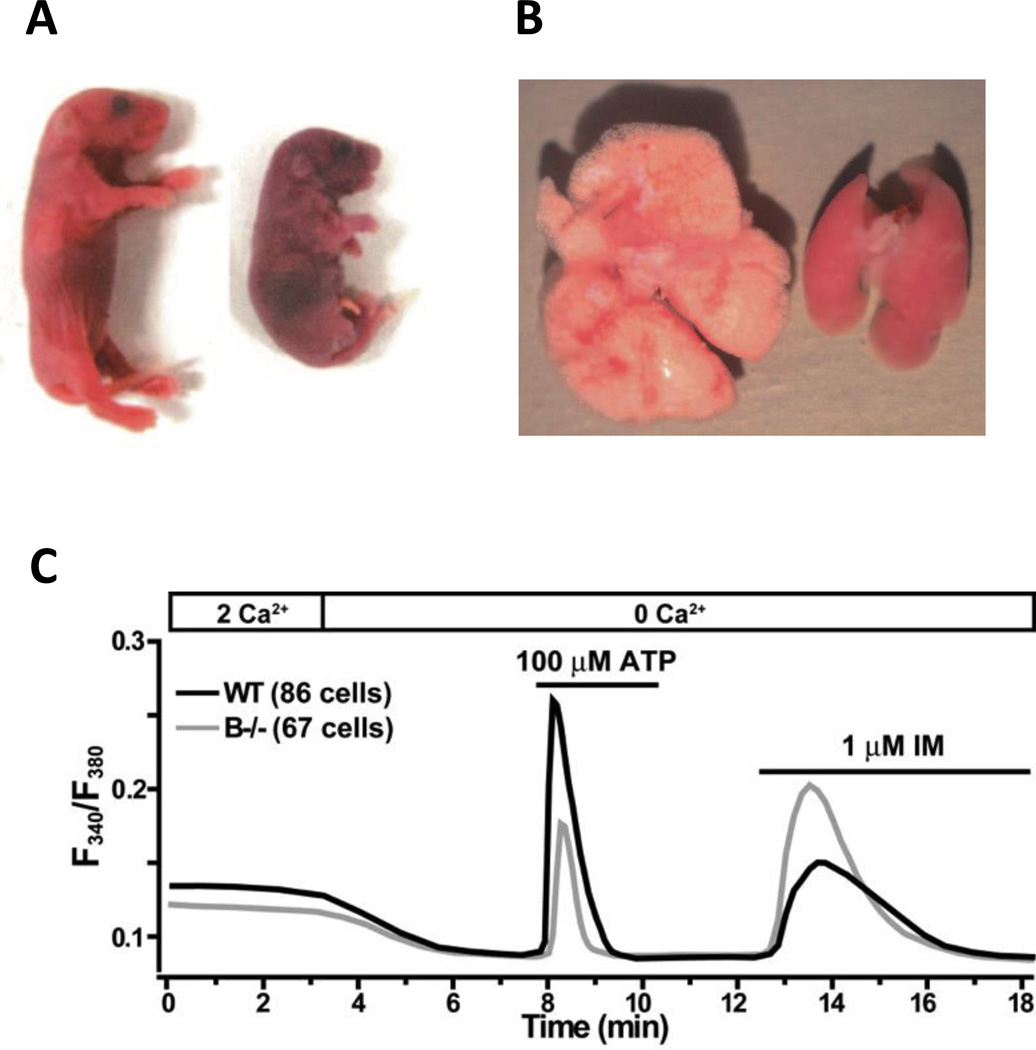
Figure 4. Tric-b knockout shows respiratory lethality phenotype.
(A) Tric-b−/− neonates were cyanotic and died shortly after birth due to respiratory failure. (B) The lungs from Tric-b−/− neonates were deflated, because the synthesis and secretion of surfactant phospholipids were impaired in alveolar type II epithelial cells. (C) Fura-2 Ca2+ imaging showed impaired ATP-evoked transients and facilitated ionomycin-induced responses in cultured alveolar type II cells from Tric-b−/− neonates, indicating IP3R-mediated Ca2+ release is disturbed despite the ER stores are overloaded with Ca2+. Modified from Yamazaki et al76, Facilitated hyperpolarization signaling in vascular smooth muscle-overexpressing tric-a channels. J Biol Chem. 2013;288:15581–15589
Since both the metabolic conversion and surfactant secretion are activated in Ca2+ -dependent manners, impaired Ca2+ release was proposed in Tric-b−/− type II alveolar cells. Indeed, IP3R-mediated Ca2+ release was compromised and intracellular Ca2+ stores were overloaded in the mutant type II cells77 (Fig. 4C). TRIC-B channels therefore seem to facilitate agonist-induced Ca2+ release by providing counter-K+ ions to the ER in type II alveolar cells, and enable them to exert the specialized cellular functions required for neonatal breathing.
As in alveolar epithelial cells, hepatocytes contain TRIC-B and IP3R channels, but not TRIC-A and RyR channels. Hepatocytes also preserve glycogen during the late embryonic development, and supply glucose to peripheral tissues after birth. Activation of the Ca2+/calmodulin-dependent enzyme phosphorylase requires both cAMP generated by glucagon and Ca2+ release triggered by adrenaline to liberate glucose units from glycogen in hepatocytes. Excess glycogen deposits were detected in the mutant liver of Tric-b−/− neonates, suggesting that Ca2+-dependent glucose release is inhibited in the mutant hepatocytes (D.Y. and H.T. unpublished observation). Based on these observations with Tric-b−/− neonates, we can reasonably hypothesize that TRIC-B channels exert a major impact on IP3-sensitive Ca2+ stores of non-excitable cells.
Contribution of TRIC-A and TRIC-B to Ca2+ signaling in cardiac development and contraction
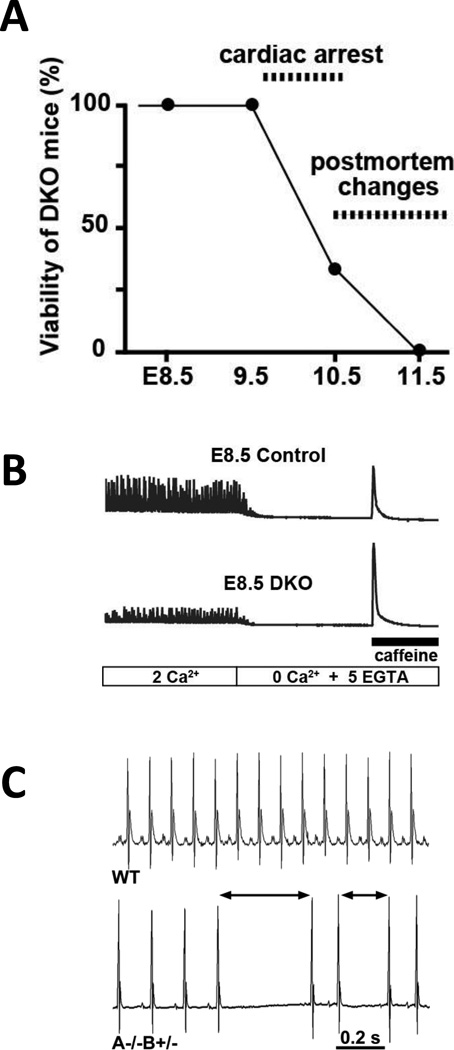
Through cross-breeding of the Tric-a−/− and Tric-b+/− mice, we found an aggravated embryonic lethality in the TRIC-A and TRIC-B double knockout (DKO) mice, e.g. the DKO embryos die between E9 and E11, perhaps as a result of abnormal heart function (Fig. 5A). This aggravated lethality suggests that TRIC-A and TRIC-B subtypes share a complementary function in the heart. In the looped cardiac tube from E8.5-9.5 DKO embryos, irregular cytoplasmic vacuoles were formed. EM observations revealed extensively swollen SR/ER structures in DKO myocytes. Such structures were not present in animals that carried a single functional copy of the TRIC-B gene. As in high Ca2+-containing organelles, fixative solutions containing oxalate form electron-dense calcium-oxalate deposits, were detectable in EM analysis. Such deposits were frequently detected in the SR/ER in DKO myocytes, but not in Tric-a+/− and Tric-b+/− DHE (double heterozygotes) myocytes. Fluorometric Ca2+ imaging of cardiac myocytes from the DKO embryos shows that the amplitudes of spontaneous Ca2+ oscillations were depressed at E8.5 (Fig. 5B). However, remarkably larger caffeine-evoked Ca2+ transients were observed in E8.5 DKO myocytes. The elevated caffeine-evoked Ca2+ transients, together with the insoluble deposits in EM observations, indicate severe SR/ER Ca2+-overloading in DKO myocytes.
Figure 5. Deletion of both Tric-a and Tric-b leads to embryonic lethality.
(A) TRIC-DKO embryos exhibited weak heartbeats at E9.5, stopped beating at ~E10.5, and postmortem autolysis and discoloration at ~E11.5. (B) Defective Ca2+ signaling in TRIC-DKO cardiomyocytes (bottom) compared normal Ca2+ signaling in DHE (double heterozygotes, control, top) cardiomyocytes (modified from Yazawa et al53). (C) Telemetry ECG recording of mice under non-treated conditions. Tric-a−/−Tric-b+/− mice developed bradycardia with occasional arrhythmic events.
In embryonic cardiomyocytes bearing immature intracellular stores, Ca2+ signaling or spontaneous Ca2+ oscillations are predominantly composed of Ca2+ influx, but significant contribution of RyR2-mediated CICR is also detectable78. RyR2 is the major cardiac Ca2+ release channel that regulates intracellular Ca2+ homeostasis. Lately, results from studies of the inducible, cardiac-specific RyR2 knockdown mice demonstrate that RyR2 loss-of-function can lead to fatal arrhythmias, which exemplifies the important contribution of RyR2 to cardiac arrhythmia and sudden death in humans13, 79. TRIC-DKO and RyR2-knockout mice show cardiac arrest at similar embryonic stages, and share similar characteristic phenotypes of swollen and Ca2+-overloaded SR/ER in embryonic cardiac myocytes. Further biochemical studies showed that myocytes derived from the DKO embryos retained normal expression of major SR Ca2+ store-related proteins. Thus, CICR mediated by RyR2 in Tric-DKO cardiomyocytes appear to be impaired. Insufficient RyR2 function could lead to SR Ca2+ overload and further disrupt cellular homeostasis in TRIC-DKO cardiomyocytes.
The viable nature of the Tric-a−/−Tric-b+/− mice allows us to examine the physiological roles of TRIC subtypes in adult cardiac function. Compared with wild-type mice, the Tric-a−/− Tric-b+/− mice show bradycardia and arrhythmic heart beats (Fig. 5C), which is partly linked to the activated baroreflex response under hypertensive condition and may also reflect an intrinsic defect in cardiac muscle function. Even under non-stressful conditions, frequent AV block was observed with the Tric-a−/− Tric-b+/− mice. Moreover, physiological stress applied to these mice, such as treatment with isoproterenol, caused high incidence of sudden death when compared to wild-type mice (unpublished observations, Yamazaki et al). It would be interesting to know whether this phenotype is linked to altered Ca2+ signaling in the cardiomyocytes.
TRIC control of store-overload induced Ca2+ release (SOICR)
Ca2+ release from the SR in cardiomyocytes is normally controlled by voltage-dependent Ca2+ influx via the L-type Ca2+ channel through the CICR mechanism11. In addition to CICR, it has long been recognized that SR Ca2+ release can occur spontaneously under conditions of SR Ca2+ overload70, 72, 80, 81. Considering its dependence on SR Ca2+ load and independence on membrane depolarization, this spontaneous SR Ca2+ release has been referred to as store-overload-induced Ca2+ release (SOICR) 82, 83, 4.
A number of conditions, including increased extracellular Ca2+ concentrations, high frequency stimulations, excessive beta-adrenergic activation, or digitalis intoxication, can lead to Ca2+ overloading of the SR and subsequently SOICR in cardiac cells68, 81. It is also well recognized that SOICR in the form of Ca2+ waves can enhance the activity of the Na+/Ca2+ exchanger, leading to delayed after depolarizations (DADs) and triggered activities3, 71, 84. These SOICR-evoked DADs and triggered activities are a major cause of ventricular tachyarrhythmias and sudden death in patients with catecholaminergic polymorphic ventricular tachycardia (CPVT) and heart failure3,71. Thus, understanding the molecular basis and regulation of SOICR is critical for the understanding and treatment of Ca2+-triggered cardiac arrhythmias and other diseases associated with Ca2+ dysregulation.
It is of interest to note that there appears to be no spontaneous Ca2+ waves (SOICR) in cardiomyocytes isolated from the TRIC-A and TRIC-B double knockout mice, despite the heavily overloaded SR. It is unknown why the Ca2+ overloaded SR in DKO cardiomyocytes does not lead to SOICR. It is possible that TRIC may be required to control the response of RyR2 channels to SR luminal Ca2+, and that TRIC-deficiency somehow renders RyR2 channels less sensitive to luminal Ca2+ activation. Reduced luminal Ca2+ sensitivity of RyR2 may provide an explanation for the lack of SOICR and the built-up of SR Ca2+ load in the DKO cells. This possible regulation of RyR2 by TRIC in cardiomyocytes has yet to be characterized.
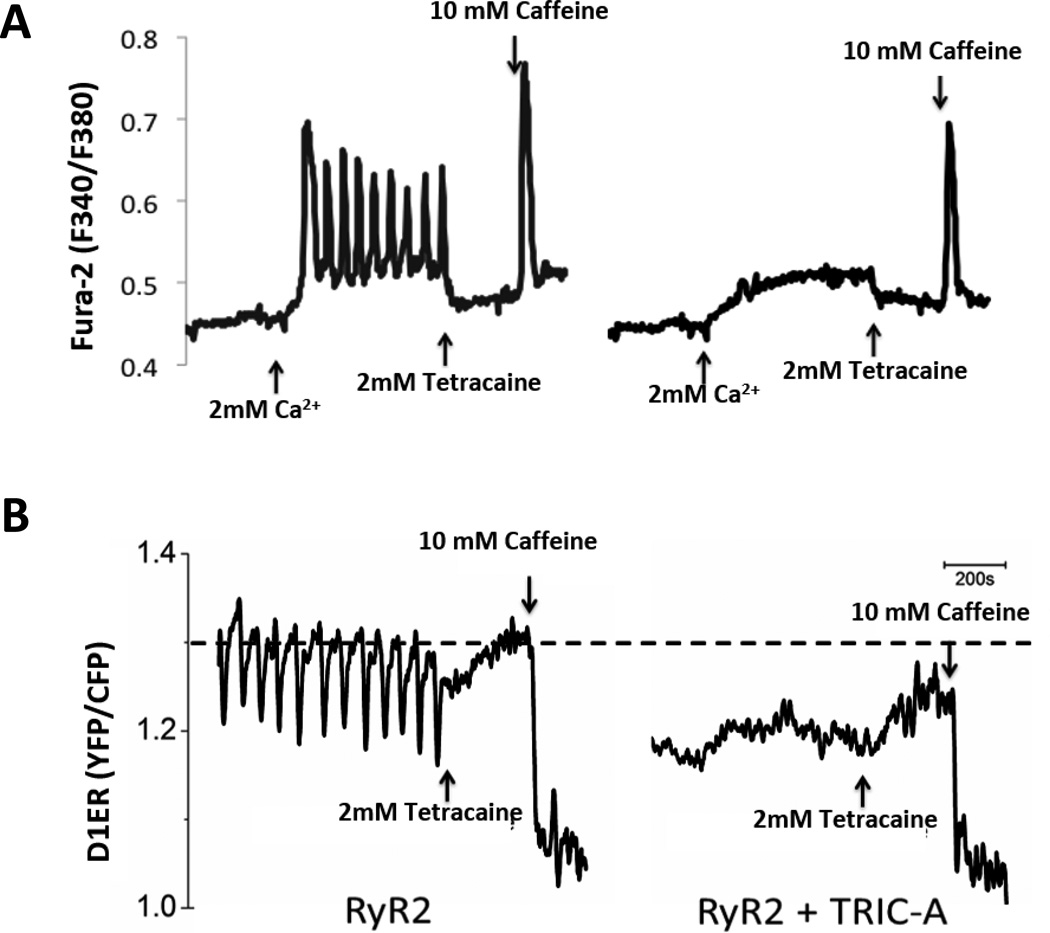
To gain some initial insights into the relationship between TRIC-A and RyR2, we employed stable, inducible HEK293 cells expressing the RyR2. In this cellular model, elevation of extracellular Ca2+ ([Ca]o) can lead to increased Ca2+ store inside the ER, which triggers the opening of the RyR2 channel, leading to SOICR in the form of Ca2+ oscillations82, 83, 85. Co-expression of TRIC-A and RyR2 in HEK293 cells led to the apparent suppression of SOICR (Fig. 6A). A direct measurement of ER luminal Ca2+ dynamics using the ER luminal Ca2+ indicator, D1ER83, 86, 87, revealed a markedly reduced ER Ca2+ store (Fig. 6B) This reduced ER Ca2+ store is unlikely to be due to Ca2+ leakage from the TRIC channel, because over-expression of TRIC-A or TRIC-B channel alone in HEK293 cells does not reduce ER Ca2+ content. The SOICR inhibitory effect of TRIC-A appears to be specific to TRIC-A, as co-expression of RyR2 with TRIC-B did not affect SOICR in HEK293 cells. These observations support a specific link between TRIC-A and RyR2. It remains to be determined how overexpression of TRIC-A in RyR2-expressing cells reduces ER Ca2+ content. TRIC-A may involve in maintaining a normal level of ER Ca2+ content by promoting counter ion movement, thus preventing store Ca2+ overload that triggers SOICR. Alternatively, TRIC-A may directly interact with RyR2, regulate its response to store/luminal Ca2+, thus controlling the occurrence of SOICR.
Figure 6. SOICR measurement in HEK293 cells expressing RyR2 and TRIC-A.
(A) Fluorometric Ca2+ imaging to determine cytosolic Ca2+ concentration. HEK293 cells expressing RyR2 alone show spontaneous Ca2+ oscillations caused by SOICR, while co-expression of RyR2 with TRIC-A suppressed SOICR. (B) FRET-based Ca2+ sensing protein D1ER was used to determine ER luminal Ca2+ concentration. FRET recordings from representative cells expressing RyR2, or RyR2 plus TRIC-A, were measured with 2 mM [Ca]o. 2 mM tetracaine inhibited ER Ca2+ oscillations and 10 mM caffeine caused depletion of ER Ca2+.
Genetic variations of TRIC and their roles in human diseases
Since TRIC subtypes are involved in various biological functions as described above, it is possible that TRIC channels may have important pathophysiological roles in human diseases. The hypertension phenotype of Tric-a-knockout73 and the hypotension phenotype of Tric-a-transgenic76 demonstrate that the expression level of TRIC-A channels in vascular smooth muscle cells (VSMCs) sets the resting blood pressure at the whole animal level. Gene association analysis in a Japanese population identified several single nucleotide polymorphisms (SNPs) around the Tric-a gene that increase hypertension risk and diminish the efficacy of antihypertensive drugs73. These risk SNPs are likely to be associated with a relatively low expression of the Tric-a gene in VSMCs. Therefore, Tric-a SNPs can provide biomarkers for the diagnosis and personalized treatment of essential hypertension. Moreover, the TRIC-A protein is a potential pharmaceutical target for malignant hypertension that is resistant to common depressors, since activators of TRIC-A channels are thought to directly stimulate hyperpolarization signaling and to reduce resting tonus in VSMCs.
Osteogenesis imperfecta (OI) is a monogenic hereditary disease characterized by low bone mass with abnormal bone microarchitecture, leading to increased bone fragility and deformity. OI has divergent phenotypic manifestations, and the heterogeneity of clinical symptoms suggests several OI-responsible genes in the human genome 88. Most of the OI cases (~90%) result from defected type I collagen; structural mutations and altered posttranslational modifications lead to its functional impairments. However, OI-causing mutations are also linked to collagen-unrelated genes, and a homozygous deletion mutation in the Tric-b (also referred to as TMEM38B) locus was recently identified in Saudi Arabic and Bedouin Israeli OI pedigrees 89 90. This mutation encodes a truncated form of TRIC-B lacking the third transmembrane segment in our topology model, and may severely impair the channel activity in various cell types. However, the pathological mechanism underlying this form of OI remains to be investigated. We recently detected OI-like abnormalities in Tric-b−/− neonatal mice; for example, insufficient bone density as revealed in quantitative tomography scanning, and impaired mineralization as demonstrated in histological analysis (D.Y. and H.T. unpublished observation). Therefore, Tric-b−/− mice may provide a useful animal model for studying OI associated with the Tric-b mutation. In addition to hypertension and OI, altered expression and genetic mutations of TRIC genes may be associated with other human disorders. We need to further examine Tric-mutant mice to define new phenotypes related to health and disease, and also to investigate corresponding pathologies in human diseases.
Conclusion and Discussion
The discovery of TRIC channels has potential importance for our understanding of Ca2+ signaling and homeostasis in the heart and other tissues. One question that requires further investigation is the extent to which TRIC contributes to ion flux across the ER/SR membrane during the Ca2+ release and uptake processes. In addition to TRIC, additional molecules are also likely to be involved in balancing the ion fluxes across the SR/ER membrane (Fig. 1A). For example, high H+ permeability is detected in the SR/ER membrane and is, in part, responsible for the counter-transport of H+ and Ca2+ mediated by Ca2+ pumps91. Along with the SR K+ channel, several other K+ and Cl−-selective currents were detected in intracellular organelles, whereas their molecular identities remain to be solved. Recent studies from Fill and colleagues suggested that RyR and IP3R channels can provide certain extent of counter-current movement due to the non-selective nature of the Ca2+ release channels. In their recent publication, Guo et al57 used pharmacological inhibitors of the SR K+ channel and concluded that TRIC-mediated counter ion movement does not contribute to the overall SR Ca2+ release property in cardiac muscle. Note that their experiments were conducted using the replacement of K+ ions with Cs+ ions that resulted in only ~70% inhibition of the cation current through the SR K+ channel, which may not be sufficient to cause detectable impact on the SR Ca2+ release property.
Aside from a role for TRIC channels in modulating the acute phase of Ca2+ release from the SR/ER store, TRIC-mediated movement of K+ ions could also help in limiting the electronegative influence of Ca2+ release on the overall Ca2+ homeostasis inside the SR/ER. Under conditions of repetitive stimulations with fast Ca2+ release in succession, TRIC may in effect function as a biologic “voltage clamp” for the ER/SR membrane that helps normalize SR/ER potential and thus sustain normal Ca2+ uptake and release. Direct evaluation of the role of both TRIC channel subtypes in cardiac Ca2+ signaling will require the use of specific and potent pharmacological inhibitors that can produce complete inhibition of the TRIC channels. Alternatively, tissue specific manipulations of TRIC-A or TRIC-B expression in transgenic mice are needed to examine the physiological function of these channels in cardiovascular physiology or disease. For overcoming the lethality of the germline ablation of Tric-b and Tric-a genes, inducible or targeted siRNA silencing of both Tric-a and Tric-b may be required in order to define the physiological function of TRIC subtypes in adult muscle and heart cells. Such studies would help define the role for TRIC channels in controlling the maturation of SR and the integrity of intracellular Ca2+ release associated with developmental function of the heart.
It is clear that TRIC-A and TRIC-B have differential functions in regulating SR and ER Ca2+ homeostasis. TRIC-B is ubiquitously expressed in all tissues and, considering the lethal phenotype produced by TRIC-B ablation, one can envision that TRIC-B may play an essential role in maintaining normal ER cellular function in a wide variety of cell types. In contrast, TRIC-A expression is predominantly targeted to tissues containing excitable cell types, such as the brain and muscles. Thus, TRIC-A may function to meet particular kinetic demands of Ca2+ release within those excitable cells. A reduction in either TRIC protein would likely lead to instability of ER/SR function and thus could have wide reaching effects in cellular physiology. One possibility is that TRIC-A may interact with the RyR channel and TRIC-B may associate with the IP3R channel to modulate the overall SR and ER Ca2+ homeostasis. While many studies have shown that cross-talk between IP3R and RyR can modulate Ca2+ signaling in muscle and heart cells in response to physiological and pathological stresses74, 75, 92–96, understanding the potential role of TRIC-A and TRIC-B in mediating IP3R/RyR cross-talk for regulation of Ca2+ signaling will certainly require further studies.
While many studies have suggested that altered function of store-overload induced Ca2+ release from the SR in cardiomyocytes may contribute to the development of cardiac arrhythmias, searching for accessory proteins that modulate the RyR2 channel function and SR Ca2+ homeostasis should yield important clues to the function of SOICR in physiological and pathophysiological settings. Since TRIC-A can potentially modulate RyR2-mediated SOICR, one can envision that potential therapeutic interventions can be introduced to target the TRIC/RyR interaction for restoring defective Ca2+ signaling in cardiovascular and potentially other human diseases.
Several lines of evidence have linked mutations in TRIC-B to bone and pulmonary diseases, and mutations in TRIC-A to hypertension and muscular diseases. Expansion of these research efforts should provide new insights into the physiological function of TRIC channels in human health and disease. For example, one area of cardiovascular research may focus on establishing the link of genetic mutations in TRIC-A or TRIC-B to the development of arrhythmia and other stress-induced heart diseases, and whether or not these are correlated with the altered intracellular Ca2+ signaling and homeostasis in the cardiovascular system.
Acknowledgments
SOURCES OF FUNDING
This work was supported by grants from the US National Institute of Health to JM, HT and SRWC, the Canadian Institutes of Health Research to SRWC, and the Ministry of Education, Culture, Sports, Science, and Technology of Japan (Core-to-core Program and Platform for Drug Design, Discovery, and Development) to HT.
Nonstandard Abbreviations and Acronyms
- TRIC
trimeric intracellular cation channels
- ER
endoplasmic reticulum
- SR
sarcoplasmic reticulum
- RyR
ryanodine receptor
- IP3R
IP3 receptor
- CICR
Ca2+ induced Ca2+ release
- VICR
voltage induced Ca2+ release
- VSMC
vascular smooth muscle cell
- SOICR
store overload induced Ca2+ release
Footnotes
DISCLOSURE: None
REFERENCES
- 1.Stutzmann GE, Mattson MP. Endoplasmic reticulum ca(2+) handling in excitable cells in health and disease. Pharmacol Rev. 2011;63:700–727. doi: 10.1124/pr.110.003814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.MacLennan DH. Ca2+ signalling and muscle disease. Eur J Biochem. 2000;267:5291–5297. doi: 10.1046/j.1432-1327.2000.01566.x. [DOI] [PubMed] [Google Scholar]
- 3.Bers DM. Calcium and cardiac rhythms: Physiological and pathophysiological. Circ Res. 2002;90:14–17. [PubMed] [Google Scholar]
- 4.Priori SG, Chen SR. Inherited dysfunction of sarcoplasmic reticulum ca2+ handling and arrhythmogenesis. Circ Res. 2011;108:871–883. doi: 10.1161/CIRCRESAHA.110.226845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Capes EM, Loaiza R, Valdivia HH. Ryanodine receptors. Skelet Muscle. 2011;1:18. doi: 10.1186/2044-5040-1-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yamazaki D, Yamazaki T, Takeshima H. New molecular components supporting ryanodine receptor-mediated ca(2+) release: Roles of junctophilin and tric channel in embryonic cardiomyocytes. Pharmacol Ther. 2009;121:265–272. doi: 10.1016/j.pharmthera.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 7.Komazaki S, Ito K, Takeshima H, Nakamura H. Deficiency of triad formation in developing skeletal muscle cells lacking junctophilin type 1. FEBS Lett. 2002;524:225–229. doi: 10.1016/s0014-5793(02)03042-9. [DOI] [PubMed] [Google Scholar]
- 8.Takeshima H. Intracellular ca2+ store in embryonic cardiac myocytes. Front Biosci. 2002;7:d1642–d1652. doi: 10.2741/takeshim. [DOI] [PubMed] [Google Scholar]
- 9.Ma J, Pan Z. Junctional membrane structure and store operated calcium entry in muscle cells. Front Biosci. 2003;8:d242–d255. doi: 10.2741/977. [DOI] [PubMed] [Google Scholar]
- 10.Maack C, O'Rourke B. Excitation-contraction coupling and mitochondrial energetics. Basic Res Cardiol. 2007;102:369–392. doi: 10.1007/s00395-007-0666-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bers DM. Cardiac excitation-contraction coupling. Nature. 2002;415:198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- 12.Cannell MB, Kong CH. Local control in cardiac e-c coupling. J Mol Cell Cardiol. 2012;52:298–303. doi: 10.1016/j.yjmcc.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 13.Bround MJ, Asghari P, Wambolt RB, Bohunek L, Smits C, Philit M, Kieffer TJ, Lakatta EG, Boheler KR, Moore ED, Allard MF, Johnson JD. Cardiac ryanodine receptors control heart rate and rhythmicity in adult mice. Cardiovasc Res. 2012;96:372–380. doi: 10.1093/cvr/cvs260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rios E, Ma JJ, Gonzalez A. The mechanical hypothesis of excitation-contraction (ec) coupling in skeletal muscle. J Muscle Res Cell Motil. 1991;12:127–135. doi: 10.1007/BF01774031. [DOI] [PubMed] [Google Scholar]
- 15.Fill M, Copello JA. Ryanodine receptor calcium release channels. Physiol Rev. 2002;82:893–922. doi: 10.1152/physrev.00013.2002. [DOI] [PubMed] [Google Scholar]
- 16.Klein MG, Schneider MF. Ca2+ sparks in skeletal muscle. Prog Biophys Mol Biol. 2006;92:308–332. doi: 10.1016/j.pbiomolbio.2005.05.016. [DOI] [PubMed] [Google Scholar]
- 17.Coronado R, Miller C. Decamethonium and hexamethonium block k+ channels of sarcoplasmic reticulum. Nature. 1980;288:495–497. doi: 10.1038/288495a0. [DOI] [PubMed] [Google Scholar]
- 18.Volpe P, Palade P, Costello B, Mitchell RD, Fleischer S. Spontaneous calcium release from sarcoplasmic reticulum. Effect of local anesthetics. J Biol Chem. 1983;258:12434–12442. [PubMed] [Google Scholar]
- 19.Best PM, Abramcheck CW. Potassium efflux from single skinned skeletal muscle fibers. Biophys J. 1985;48:907–913. doi: 10.1016/S0006-3495(85)83853-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tomlins B, Williams AJ, Montgomery RA. The characterization of a monovalent cation-selective channel of mammalian cardiac muscle sarcoplasmic reticulum. J Membr Biol. 1984;80:191–199. doi: 10.1007/BF01868775. [DOI] [PubMed] [Google Scholar]
- 21.Fink RH, Veigel C. Calcium uptake and release modulated by counter-ion conductances in the sarcoplasmic reticulum of skeletal muscle. Acta Physiol Scand. 1996;156:387–396. doi: 10.1046/j.1365-201X.1996.212000.x. [DOI] [PubMed] [Google Scholar]
- 22.Fink RH, Stephenson DG. Ca2+-movements in muscle modulated by the state of k+-channels in the sarcoplasmic reticulum membranes. Pflugers Arch. 1987;409:374–380. doi: 10.1007/BF00583791. [DOI] [PubMed] [Google Scholar]
- 23.Coronado R, Miller C. Conduction and block by organic cations in a k+-selective channel from sarcoplasmic reticulum incorporated into planar phospholipid bilayers. J Gen Physiol. 1982;79:529–547. doi: 10.1085/jgp.79.4.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coronado R, Rosenberg RL, Miller C. Ionic selectivity, saturation, and block in a k+-selective channel from sarcoplasmic reticulum. J Gen Physiol. 1980;76:425–446. doi: 10.1085/jgp.76.4.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sokabe M, Kasai M, Nomura K, Naruse K. Electrophysiological analysis of structural aspects of voltage-dependent sr k+ channel. Comp Biochem Physiol C. 1991;98:23–30. [PubMed] [Google Scholar]
- 26.Weisleder N, Takeshima H, Ma J. Immuno-proteomic approach to excitation--contraction coupling in skeletal and cardiac muscle: Molecular insights revealed by the mitsugumins. Cell Calcium. 2008;43:1–8. doi: 10.1016/j.ceca.2007.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takeshima H, Shimuta M, Komazaki S, Ohmi K, Nishi M, Iino M, Miyata A, Kangawa K. Mitsugumin29, a novel synaptophysin family member from the triad junction in skeletal muscle. Biochem J. 1998;331(Pt 1):317–322. doi: 10.1042/bj3310317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Komazaki S, Nishi M, Takeshima H, Nakamura H. Abnormal formation of sarcoplasmic reticulum networks and triads during early development of skeletal muscle cells in mitsugumin29-deficient mice. Dev Growth Differ. 2001;43:717–723. doi: 10.1046/j.1440-169x.2001.00609.x. [DOI] [PubMed] [Google Scholar]
- 29.Nishi M, Komazaki S, Kurebayashi N, Ogawa Y, Noda T, Iino M, Takeshima H. Abnormal features in skeletal muscle from mice lacking mitsugumin29. J Cell Biol. 1999;147:1473–1480. doi: 10.1083/jcb.147.7.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pan Z, Yang D, Nagaraj RY, Nosek TA, Nishi M, Takeshima H, Cheng H, Ma J. Dysfunction of store-operated calcium channel in muscle cells lacking mg29. Nat Cell Biol. 2002;4:379–383. doi: 10.1038/ncb788. [DOI] [PubMed] [Google Scholar]
- 31.Weisleder N, Brotto M, Komazaki S, Pan Z, Zhao X, Nosek T, Parness J, Takeshima H, Ma J. Muscle aging is associated with compromised ca2+ spark signaling and segregated intracellular ca2+ release. J Cell Biol. 2006;174:639–645. doi: 10.1083/jcb.200604166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weisleder N, Ma J. Altered ca2+ sparks in aging skeletal and cardiac muscle. Ageing Res Rev. 2008;7:177–188. doi: 10.1016/j.arr.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao X, Weisleder N, Thornton A, Oppong Y, Campbell R, Ma J, Brotto M. Compromised store-operated ca2+ entry in aged skeletal muscle. Aging Cell. 2008;7:561–568. doi: 10.1111/j.1474-9726.2008.00408.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ito K, Komazaki S, Sasamoto K, Yoshida M, Nishi M, Kitamura K, Takeshima H. Deficiency of triad junction and contraction in mutant skeletal muscle lacking junctophilin type 1. J Cell Biol. 2001;154:1059–1067. doi: 10.1083/jcb.200105040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nishi M, Mizushima A, Nakagawara K, Takeshima H. Characterization of human junctophilin subtype genes. Biochem Biophys Res Commun. 2000;273:920–927. doi: 10.1006/bbrc.2000.3011. [DOI] [PubMed] [Google Scholar]
- 36.Takeshima H, Komazaki S, Nishi M, Iino M, Kangawa K. Junctophilins: A novel family of junctional membrane complex proteins. Mol Cell. 2000;6:11–22. doi: 10.1016/s1097-2765(00)00003-4. [DOI] [PubMed] [Google Scholar]
- 37.Woo JS, Hwang JH, Ko JK, Kim do H, Ma J, Lee EH. Glutamate at position 227 of junctophilin-2 is involved in binding to trpc3. Mol Cell Biochem. 2009;328:25–32. doi: 10.1007/s11010-009-0070-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hirata Y, Brotto M, Weisleder N, Chu Y, Lin P, Zhao X, Thornton A, Komazaki S, Takeshima H, Ma J, Pan Z. Uncoupling store-operated ca2+ entry and altered ca2+ release from sarcoplasmic reticulum through silencing of junctophilin genes. Biophys J. 2006;90:4418–4427. doi: 10.1529/biophysj.105.076570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Landstrom AP, Weisleder N, Batalden KB, Bos JM, Tester DJ, Ommen SR, Wehrens XH, Claycomb WC, Ko JK, Hwang M, Pan Z, Ma J, Ackerman MJ. Mutations in jph2-encoded junctophilin-2 associated with hypertrophic cardiomyopathy in humans. J Mol Cell Cardiol. 2007;42:1026–1035. doi: 10.1016/j.yjmcc.2007.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van Oort RJ, Garbino A, Wang W, Dixit SS, Landstrom AP, Gaur N, De Almeida AC, Skapura DG, Rudy Y, Burns AR, Ackerman MJ, Wehrens XH. Disrupted junctional membrane complexes and hyperactive ryanodine receptors after acute junctophilin knockdown in mice. Circulation. 2011;123:979–988. doi: 10.1161/CIRCULATIONAHA.110.006437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Landstrom AP, Kellen CA, Dixit SS, van Oort RJ, Garbino A, Weisleder N, Ma J, Wehrens XH, Ackerman MJ. Junctophilin-2 expression silencing causes cardiocyte hypertrophy and abnormal intracellular calcium-handling. Circ Heart Fail. 2011;4:214–223. doi: 10.1161/CIRCHEARTFAILURE.110.958694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang HB, Li RC, Xu M, Xu SM, Lai YS, Wu HD, Xie XJ, Gao W, Ye H, Zhang YY, Meng X, Wang SQ. Ultrastructural uncoupling between t-tubules and sarcoplasmic reticulum in human heart failure. Cardiovasc Res. 2013;98:269–276. doi: 10.1093/cvr/cvt030. [DOI] [PubMed] [Google Scholar]
- 43.Wei S, Guo A, Chen B, Kutschke W, Xie YP, Zimmerman K, Weiss RM, Anderson ME, Cheng H, Song LS. T-tubule remodeling during transition from hypertrophy to heart failure. Circ Res. 2010;107:520–531. doi: 10.1161/CIRCRESAHA.109.212324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weisleder N, Takizawa N, Lin P, Wang X, Cao C, Zhang Y, Tan T, Ferrante C, Zhu H, Chen PJ, Yan R, Sterling M, Zhao X, Hwang M, Takeshima M, Cai C, Cheng H, Takeshima H, Xiao RP, Ma J. Recombinant mg53 protein modulates therapeutic cell membrane repair in treatment of muscular dystrophy. Sci Transl Med. 2012;4:139ra185. doi: 10.1126/scitranslmed.3003921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weisleder N, Takeshima H, Ma J. Mitsugumin 53 (mg53) facilitates vesicle trafficking in striated muscle to contribute to cell membrane repair. Commun Integr Biol. 2009;2:225–226. doi: 10.4161/cib.2.3.8077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cai C, Weisleder N, Ko JK, Komazaki S, Sunada Y, Nishi M, Takeshima H, Ma J. Membrane repair defects in muscular dystrophy are linked to altered interaction between mg53, caveolin-3, and dysferlin. J Biol Chem. 2009;284:15894–15902. doi: 10.1074/jbc.M109.009589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cai C, Masumiya H, Weisleder N, Matsuda N, Nishi M, Hwang M, Ko JK, Lin P, Thornton A, Zhao X, Pan Z, Komazaki S, Brotto M, Takeshima H, Ma J. Mg53 nucleates assembly of cell membrane repair machinery. Nat Cell Biol. 2009;11:56–64. doi: 10.1038/ncb1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lin P, Zhu H, Cai C, Wang X, Cao C, Xiao R, Pan Z, Weisleder N, Takeshima H, Ma J. Nonmuscle myosin iia facilitates vesicle trafficking for mg53-mediated cell membrane repair. FASEB J. 2012;26:1875–1883. doi: 10.1096/fj.11-188599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhu H, Lin P, De G, Choi KH, Takeshima H, Weisleder N, Ma J. Polymerase transcriptase release factor (ptrf) anchors mg53 protein to cell injury site for initiation of membrane repair. J Biol Chem. 2011;286:12820–12824. doi: 10.1074/jbc.C111.221440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cao CM, Zhang Y, Weisleder N, Ferrante C, Wang X, Lv F, Zhang Y, Song R, Hwang M, Jin L, Guo J, Peng W, Li G, Nishi M, Takeshima H, Ma J, Xiao RP. Mg53 constitutes a primary determinant of cardiac ischemic preconditioning. Circulation. 2010;121:2565–2574. doi: 10.1161/CIRCULATIONAHA.110.954628. [DOI] [PubMed] [Google Scholar]
- 51.Wang X, Xie W, Zhang Y, Lin P, Han L, Han P, Wang Y, Chen Z, Ji G, Zheng M, Weisleder N, Xiao RP, Takeshima H, Ma J, Cheng H. Cardioprotection of ischemia/reperfusion injury by cholesterol-dependent mg53-mediated membrane repair. Circ Res. 2010;107:76–83. doi: 10.1161/CIRCRESAHA.109.215822. [DOI] [PubMed] [Google Scholar]
- 52.He B, Tang RH, Weisleder N, Xiao B, Yuan Z, Cai C, Zhu H, Lin P, Qiao C, Li J, Mayer C, Ma J, Xiao X. Enhancing muscle membrane repair by gene delivery of mg53 ameliorates muscular dystrophy and heart failure in delta-sarcoglycan-deficient hamsters. Mol Ther. 2012;20:727–735. doi: 10.1038/mt.2012.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yazawa M, Ferrante C, Feng J, Mio K, Ogura T, Zhang M, Lin PH, Pan Z, Komazaki S, Kato K, Nishi M, Zhao X, Weisleder N, Sato C, Ma J, Takeshima H. Tric channels are essential for ca2+ handling in intracellular stores. Nature. 2007;448:78–82. doi: 10.1038/nature05928. [DOI] [PubMed] [Google Scholar]
- 54.Pitt SJ, Park KH, Nishi M, Urashima T, Aoki S, Yamazaki D, Ma J, Takeshima H, Sitsapesan R. Charade of the sr k+-channel: Two ion-channels, tric-a and tric-b, masquerade as a single k+-channel. Biophys J. 2010;99:417–426. doi: 10.1016/j.bpj.2010.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Venturi E, Sitsapesan R, Yamazaki D, Takeshima H. Tric channels supporting efficient ca(2+) release from intracellular stores. Pflugers Arch. 2013;465:187–195. doi: 10.1007/s00424-012-1197-5. [DOI] [PubMed] [Google Scholar]
- 56.Zhao X, Yamazaki D, Kakizawa S, Pan Z, Takeshima H, Ma J. Molecular architecture of ca2+ signaling control in muscle and heart cells. Channels (Austin) 2011;5:391–396. doi: 10.4161/chan.5.5.16467. [DOI] [PubMed] [Google Scholar]
- 57.Guo T, Nani A, Shonts S, Perryman M, Chen H, Shannon T, Gillespie D, Fill M. Sarcoplasmic reticulum k(+) (tric) channel does not carry essential countercurrent during ca(2+) release. Biophys J. 2013;105:1151–1160. doi: 10.1016/j.bpj.2013.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gillespie D, Chen H, Fill M. Is ryanodine receptor a calcium or magnesium channel? Roles of k+ and mg2+ during ca2+ release. Cell Calcium. 2012;51:427–433. doi: 10.1016/j.ceca.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gillespie D, Fill M. Intracellular calcium release channels mediate their own countercurrent: The ryanodine receptor case study. Biophys J. 2008;95:3706–3714. doi: 10.1529/biophysj.108.131987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dani JA, Mayer ML. Structure and function of glutamate and nicotinic acetylcholine receptors. Curr Opin Neurobiol. 1995;5:310–317. doi: 10.1016/0959-4388(95)80043-3. [DOI] [PubMed] [Google Scholar]
- 61.Bennett JA, Dingledine R. Topology profile for a glutamate receptor: Three transmembrane domains and a channel-lining reentrant membrane loop. Neuron. 1995;14:373–384. doi: 10.1016/0896-6273(95)90293-7. [DOI] [PubMed] [Google Scholar]
- 62.Nicke A. Homotrimeric complexes are the dominant assembly state of native p2×7 subunits. Biochem Biophys Res Commun. 2008;377:803–808. doi: 10.1016/j.bbrc.2008.10.042. [DOI] [PubMed] [Google Scholar]
- 63.Feher VA, Randall A, Baldi P, Bush RM, de la Maza LM, Amaro RE. A 3-dimensional trimeric beta-barrel model for chlamydia momp contains conserved and novel elements of gram-negative bacterial porins. PLoS One. 2013;8:e68934. doi: 10.1371/journal.pone.0068934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Venturi E, Matyjaszkiewicz A, Pitt SJ, Tsaneva-Atanasova K, Nishi M, Yamazaki D, Takeshima H, Sitsapesan R. Tric-b channels display labile gating: Evidence from the tric-a knockout mouse model. Pflugers Arch. 2013;465:1135–1148. doi: 10.1007/s00424-013-1251-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Garcia AM, Miller C. Channel-mediated monovalent cation fluxes in isolated sarcoplasmic reticulum vesicles. J Gen Physiol. 1984;83:819–839. doi: 10.1085/jgp.83.6.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhao X, Yamazaki D, Park KH, Komazaki S, Tjondrokoesoemo A, Nishi M, Lin P, Hirata Y, Brotto M, Takeshima H, Ma J. Ca2+ overload and sarcoplasmic reticulum instability in tric-a null skeletal muscle. J Biol Chem. 2010;285:37370–37376. doi: 10.1074/jbc.M110.170084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang X, Weisleder N, Collet C, Zhou J, Chu Y, Hirata Y, Zhao X, Pan Z, Brotto M, Cheng H, Ma J. Uncontrolled calcium sparks act as a dystrophic signal for mammalian skeletal muscle. Nat Cell Biol. 2005;7:525–530. doi: 10.1038/ncb1254. [DOI] [PubMed] [Google Scholar]
- 68.Lakatta EG, Guarnieri T. Spontaneous myocardial calcium oscillations: Are they linked to ventricular fibrillation? J Cardiovasc Electrophysiol. 1993;4:473–489. doi: 10.1111/j.1540-8167.1993.tb01285.x. [DOI] [PubMed] [Google Scholar]
- 69.Tao T, O'Neill SC, Diaz ME, Li YT, Eisner DA, Zhang H. Alternans of cardiac calcium cycling in a cluster of ryanodine receptors: A simulation study. Am J Physiol Heart Circ Physiol. 2008;295:H598–H609. doi: 10.1152/ajpheart.01086.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Marban E, Robinson SW, Wier WG. Mechanisms of arrhythmogenic delayed and early afterdepolarizations in ferret ventricular muscle. J Clin Invest. 1986;78:1185–1192. doi: 10.1172/JCI112701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pogwizd SM, Bers DM. Cellular basis of triggered arrhythmias in heart failure. Trends Cardiovasc Med. 2004;14:61–66. doi: 10.1016/j.tcm.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 72.Orchard CH, Eisner DA, Allen DG. Oscillations of intracellular ca2+ in mammalian cardiac muscle. Nature. 1983;304:735–738. doi: 10.1038/304735a0. [DOI] [PubMed] [Google Scholar]
- 73.Yamazaki D, Tabara Y, Kita S, Hanada H, Komazaki S, Naitou D, Mishima A, Nishi M, Yamamura H, Yamamoto S, Kakizawa S, Miyachi H, Yamamoto S, Miyata T, Kawano Y, Kamide K, Ogihara T, Hata A, Umemura S, Soma M, Takahashi N, Imaizumi Y, Miki T, Iwamoto T, Takeshima H. Tric-a channels in vascular smooth muscle contribute to blood pressure maintenance. Cell Metab. 2011;14:231–241. doi: 10.1016/j.cmet.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 74.Zhang WM, Lin MJ, Sham JS. Endothelin-1 and ip3 induced ca2+ sparks in pulmonary arterial smooth muscle cells. J Cardiovasc Pharmacol. 2004;44(Suppl 1):S121–S124. doi: 10.1097/01.fjc.0000166226.03712.4f. [DOI] [PubMed] [Google Scholar]
- 75.Zhang WM, Yip KP, Lin MJ, Shimoda LA, Li WH, Sham JS. Et-1 activates ca2+ sparks in pasmc: Local ca2+ signaling between inositol trisphosphate and ryanodine receptors. Am J Physiol Lung Cell Mol Physiol. 2003;285:L680–L690. doi: 10.1152/ajplung.00067.2003. [DOI] [PubMed] [Google Scholar]
- 76.Tao S, Yamazaki D, Komazaki S, Zhao C, Iida T, Kakizawa S, Imaizumi Y, Takeshima H. Facilitated hyperpolarization signaling in vascular smooth muscle-overexpressing tric-a channels. J Biol Chem. 2013;288:15581–15589. doi: 10.1074/jbc.M112.435396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yamazaki D, Komazaki S, Nakanishi H, Mishima A, Nishi M, Yazawa M, Yamazaki T, Taguchi R, Takeshima H. Essential role of the tric-b channel in ca2+ handling of alveolar epithelial cells and in perinatal lung maturation. Development. 2009;136:2355–2361. doi: 10.1242/dev.036798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Takeshima H, Komazaki S, Hirose K, Nishi M, Noda T, Iino M. Embryonic lethality and abnormal cardiac myocytes in mice lacking ryanodine receptor type 2. EMBO J. 1998;17:3309–3316. doi: 10.1093/emboj/17.12.3309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Billman GE. Does the 'coupled clock' make the heart tick? Cardiovasc Res. 2012;96:343–344. doi: 10.1093/cvr/cvs300. [DOI] [PubMed] [Google Scholar]
- 80.Fabiato A. Two kinds of calcium-induced release of calcium from the sarcoplasmic reticulum of skinned cardiac cells. Adv Exp Med Biol. 1992;311:245–262. doi: 10.1007/978-1-4615-3362-7_18. [DOI] [PubMed] [Google Scholar]
- 81.Lakatta EG. Functional implications of spontaneous sarcoplasmic reticulum ca2+ release in the heart. Cardiovasc Res. 1992;26:193–214. doi: 10.1093/cvr/26.3.193. [DOI] [PubMed] [Google Scholar]
- 82.Jiang D, Wang R, Xiao B, Kong H, Hunt DJ, Choi P, Zhang L, Chen SR. Enhanced store overload-induced ca2+ release and channel sensitivity to luminal ca2+ activation are common defects of ryr2 mutations linked to ventricular tachycardia and sudden death. Circ Res. 2005;97:1173–1181. doi: 10.1161/01.RES.0000192146.85173.4b. [DOI] [PubMed] [Google Scholar]
- 83.Jiang D, Xiao B, Yang D, Wang R, Choi P, Zhang L, Cheng H, Chen SR. Ryr2 mutations linked to ventricular tachycardia and sudden death reduce the threshold for store-overload-induced ca2+ release (soicr) Proc Natl Acad Sci U S A. 2004;101:13062–13067. doi: 10.1073/pnas.0402388101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Schlotthauer K, Bers DM. Sarcoplasmic reticulum ca(2+) release causes myocyte depolarization. Underlying mechanism and threshold for triggered action potentials. Circ Res. 2000;87:774–780. doi: 10.1161/01.res.87.9.774. [DOI] [PubMed] [Google Scholar]
- 85.Jiang D, Chen W, Wang R, Zhang L, Chen SR. Loss of luminal ca2+ activation in the cardiac ryanodine receptor is associated with ventricular fibrillation and sudden death. Proc Natl Acad Sci U S A. 2007;104:18309–18314. doi: 10.1073/pnas.0706573104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Palmer AE, Tsien RY. Measuring calcium signaling using genetically targetable fluorescent indicators. Nat Protoc. 2006;1:1057–1065. doi: 10.1038/nprot.2006.172. [DOI] [PubMed] [Google Scholar]
- 87.Palmer AE, Jin C, Reed JC, Tsien RY. Bcl-2-mediated alterations in endoplasmic reticulum ca2+ analyzed with an improved genetically encoded fluorescent sensor. Proc Natl Acad Sci U S A. 2004;101:17404–17409. doi: 10.1073/pnas.0408030101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Byers PH, Pyott SM. Recessively inherited forms of osteogenesis imperfecta. Annu Rev Genet. 2012;46:475–497. doi: 10.1146/annurev-genet-110711-155608. [DOI] [PubMed] [Google Scholar]
- 89.Shaheen R, Alazami AM, Alshammari MJ, Faqeih E, Alhashmi N, Mousa N, Alsinani A, Ansari S, Alzahrani F, Al-Owain M, Alzayed ZS, Alkuraya FS. Study of autosomal recessive osteogenesis imperfecta in arabia reveals a novel locus defined by tmem38b mutation. J Med Genet. 2012;49:630–635. doi: 10.1136/jmedgenet-2012-101142. [DOI] [PubMed] [Google Scholar]
- 90.Volodarsky M, Markus B, Cohen I, Staretz-Chacham O, Flusser H, Landau D, Shelef I, Langer Y, Birk OS. A deletion mutation in tmem38b associated with autosomal recessive osteogenesis imperfecta. Hum Mutat. 2013;34:582–586. doi: 10.1002/humu.22274. [DOI] [PubMed] [Google Scholar]
- 91.Bublitz M, Musgaard M, Poulsen H, Thogersen L, Olesen C, Schiott B, Morth JP, Moller JV, Nissen P. Ion pathways in the sarcoplasmic reticulum ca2+-atpase. J Biol Chem. 2013;288:10759–10765. doi: 10.1074/jbc.R112.436550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zima AV, Blatter LA. Inositol-1,4,5-trisphosphate-dependent ca(2+) signalling in cat atrial excitation-contraction coupling and arrhythmias. J Physiol. 2004;555:607–615. doi: 10.1113/jphysiol.2003.058529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Domeier TL, Zima AV, Maxwell JT, Huke S, Mignery GA, Blatter LA. Ip3 receptor-dependent ca2+ release modulates excitation-contraction coupling in rabbit ventricular myocytes. Am J Physiol Heart Circ Physiol. 2008;294:H596–H604. doi: 10.1152/ajpheart.01155.2007. [DOI] [PubMed] [Google Scholar]
- 94.Kockskamper J, Zima AV, Roderick HL, Pieske B, Blatter LA, Bootman MD. Emerging roles of inositol 1,4,5-trisphosphate signaling in cardiac myocytes. J Mol Cell Cardiol. 2008;45:128–147. doi: 10.1016/j.yjmcc.2008.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Li X, Zima AV, Sheikh F, Blatter LA, Chen J. Endothelin-1-induced arrhythmogenic ca2+ signaling is abolished in atrial myocytes of inositol-1,4,5-trisphosphate(ip3)-receptor type 2-deficient mice. Circ Res. 2005;96:1274–1281. doi: 10.1161/01.RES.0000172556.05576.4c. [DOI] [PubMed] [Google Scholar]
- 96.Tjondrokoesoemo A, Li N, Lin PH, Pan Z, Ferrante CJ, Shirokova N, Brotto M, Weisleder N, Ma J. Type 1 inositol (1,4,5)-trisphosphate receptor activates ryanodine receptor 1 to mediate calcium spark signaling in adult mammalian skeletal muscle. J Biol Chem. 2013;288:2103–2109. doi: 10.1074/jbc.M112.425975. [DOI] [PMC free article] [PubMed] [Google Scholar]