Abstract
Background
Fullerenes are molecules being investigated for a wide range of therapeutic applications. We have shown previously that certain fullerene derivatives (FD) inhibit mast cell (MC) function in vitro, and here we examine their in vivo therapeutic effect on asthma, a disease in which MC play a predominant role.
Objective
To determine whether if an efficient MC-stabilizing FD (TGA) can inhibit asthma pathogenesis in vivo and to examine its in vivo mechanism of action.
Methods
Asthma was induced in mice and animals were treated intranasally (i.n.) with TGA either simultaneously with treatment or following induction of pathogenesis. Efficacy of TGA was determined through the measurement of airway inflammation, bronchoconstriction, serum IgE, bronchoalveolar lavage (BALF) cytokine and eicosanoid levels.
Results
We find that TGA treated mice have significantly reduced airway inflammation, eosinophilia, and bronchoconstriction. The TGA treatments are effective even when given after disease is established. Moreover, we report a novel inhibitory mechanism as TGA stimulate the production of an anti-inflammatory P-450 eicosanoid metabolites (epoxyeicosatrienoic acids; EET’s) in the lung. Inhibitors of these anti-inflammatory EET reversed TGA inhibition. In human lung MC incubated with TGA there was a significant upregulation of CYP1B gene expression while TGA also reduced IgE production from B cells. Lastly, MC incubated with EET and challenged through FcεRI had a significant blunting of mediator release compared to non-treated cells.
Conclusion
The inhibitory capabilities of TGA reported here suggest that FD may be used a platform for developing treatments for asthma.
Keywords: fullerene derivative, eicosanoids, asthma, airway inflammation, bronchoconstriction, allergy
INTRODUCTION
In asthma the massive influx of immune cells, particularly eosinophils, causes airway thickening and reduced airflow into the lungs 1. Eicosanoids, including leukotrienes and prostaglandins, are rapidly produced by immune cells to initiate inflammation 2. Cytokines maintain chronic inflammation as IL-4 and IL-13 stimulate B cell class switching to IgE, IL-13 also promotes goblet cell metaplasia and mucus overproduction, and IL-5 recruits and stimulates eosinophils 3. The airways also become hyperreactive with smooth muscle contraction and MC activation. Breathing difficulty manifests as wheezing manifests which can become life threatening in severe cases. While the causative allergens are not always identified, in allergic asthma degranulated MC are found in lung tissue and patient serum contains elevated antigen-specific IgE and tryptase levels 4. Mast cells are thought to play an important role in pathogenesis as significant numbers are recruited to the airways and MC degranulation products are found in the broncholaveolar lavage fluid 5. In the mouse asthma models that most closely mimic human disease, MC deficient mice have reduced airway inflammation and bronchoconstriction in response to allergen challenge 6, 7.
Fullerene derivatives (FD) are nanospheres of carbon that have a unique ability to catalytically scavenge large numbers of oxygen free radicals making them potentially useful for treating disease 8–10. These robust antioxidants can reduce cellular damage and inflammation, and their therapeutic value has been suggested for the treatment of neurodegenerative and inflammatory diseases 11, 12. Previous research has found that polyhydroxylated FD can enter human lung MC and suppress degranulation and inflammatory cytokine production following IgE crosslinking 13. Further studies have demonstrated that the biological function of FD depends on the structure of the chemical moieties added to the carbon cage 14. Given that MC play a role in the pathogenesis of allergic asthma, and FD can stabilize MC when challenged with activating stimuli, we hypothesized FD could prevent or possibly reverse the mechanisms leading to asthma.
To test this hypothesis we used an ovalbumin challenge model of asthma to assess the in vivo functionality of a novel FD (TGA) previously demonstrated to be an efficient in vitro MC stabilizer 14. We find that whether TGA is given before or after pathogenesis develops, it can significantly dampen airway inflammation in mice. In addition to reductions in eosinophil recruitment, airway hyperresponsiveness, and overall airway inflammation, significant reductions in IL-4 and IL-5 levels and serum IgE were also observed. TGA also suppressed IgE production by activated B cells. Further, we have discovered a novel mechanism of action for TGA through the upregulation of the anti-inflammatory eicosanoid 11, 12-cis-epoxyeicosatrienoic acid (EET) and discovered these molecules can inhibit human MC mediator release in response to FcεRI challenge. TGA treatment causes no acute toxicity to mice as liver and kidney function are unaltered. Thus, our results suggest rationally designed FD may provide an effective therapeutic option for the treatment of asthma and that induction of anti-inflammatory EET’s represent a new strategy for asthma regulation.
Methods
Mice and Reagents
Chicken egg ovalbumin (OVA), decamethonium bromide, and acetyl-β-methylcholine chloride (methacholine) were purchased from Sigma-Aldrich (St. Louis, MO). Aluminum hydroxide (alum) was purchased from Pierce (Rockford, IL). Fullerene C70-tetraglycolate (TGA) was synthesized and tested at Luna Innovations Incorporated as described previously 14. The 11, 12-epoxyeicosatrienoic acid (EET) inhibitor 14,15-EE-5(Z)-E (EEZE, an EET-specific antagonist)15 and EET’s were obtained from Cayman Chemicals (Ann Arbor, MI) and 6-(2-propargyloxyphenyl) hexanoic acid (PPOH; a selective inhibitor of epoxygenation catalyzed by CYP450 isozymes)16 were obtained from Sigma-Aldrich. Female 8–12 week old C57BL/6 and Balb/c mice were purchased from Jackson Laboratory (Bar Harbor, ME).
Murine asthma induction and tissue collection
Acute asthma was induced as described by Williams and Galli7 as MC are known to be important for this model. Mice were given 20 µg TGA intranasally (i.n.) every three days throughout the experiment. In some experiments the EET inhibitors (14,15-EEZE and 6-2-PPOH) were given i.n. 15 minutes prior to TGA inhalation using 0.128 mg/kg in 20µl. PPOH was also given 2 hours following TGA inhalation at the same dose. Mice were sacrificed on day 47 and bronchoalveolar lavage (BAL) fluid was collected by flushing the lungs with 1 mL PBS. Supernatants were saved for cytokine analysis. Pelleted cells were spun onto slides and stained with Hema 3 stain set (Fisher diagnostics, Middletown, VA). Percentages were determined by counting at least 100 leukocytes per slide. Lung tissue was fixed with 10% buffered formalin phosphate (fisher) and embedded in paraffin at the VCU pathology core. Five µM sections were cut onto slides and stained with hematoxylin and eosin (H&E). A Nikon eclipse with a SPOT Flex Shifting Pixel Color Mosaic (Diagnostic Instrumental Inc., Sterling Heights, MI) camera was used to photograph lung sections. All mouse protocols were approved by the VCU Institutional Care and Use Committee.
A modified asthma model similar to that described by Williams and Galli 7 was utilized to assess bronchoconstriction. In this model, mice were sensitized with four injections of 50 µg OVA then challenged on days 22, 25, and 28 with 200 µg OVA. Mice were sacrificed on day 29 and bronchoconstriction was assessed using the Flexivent system (Scireq, Montreal, QC, Canada). Mice were anesthetized and a 19 gauge blunt end cannula was inserted into the trachea; ventilation began immediately. Mice were paralyzed by i.p. injection of 0.5 mg decamethonium bromide. Lung function was assessed once it was determined that breathing was completely by mechanical ventilation. Responsiveness to methacholine was determined by exposing mice to aerosolized PBS and then to increasing doses (10, 25, 50, 100 mg/ml) of methacholine. Maximum airway resistance (RL) in response to each methacholine dose was determined by averaging the three highest values.
Established asthma induction
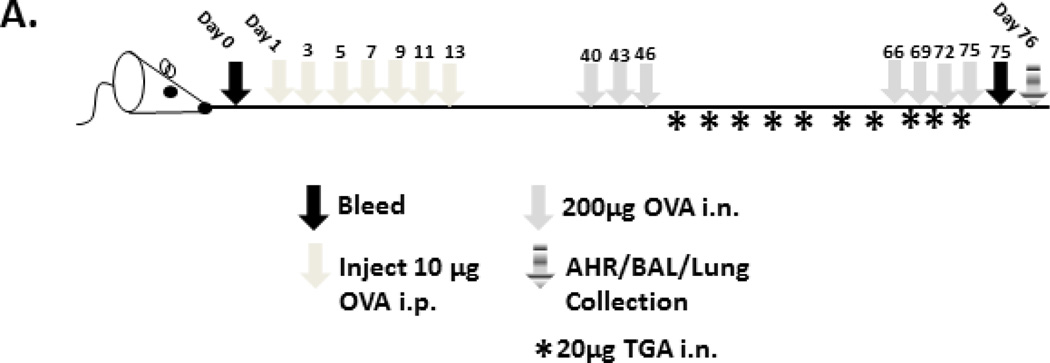
To determine if TGA could reverse established disease, mice were sensitized i.p. and challenged i.n. as in Figure 1b. However, i.n. treatment with 20 µg TGA began on day 47 and continued every three days following. Mice were challenged again with 200 µg OVA i.n. on days 66, 69, 72, and 75. On day 76 mice were sacrificed and AHR, BALF and lung tissues were collected and assessed as above.
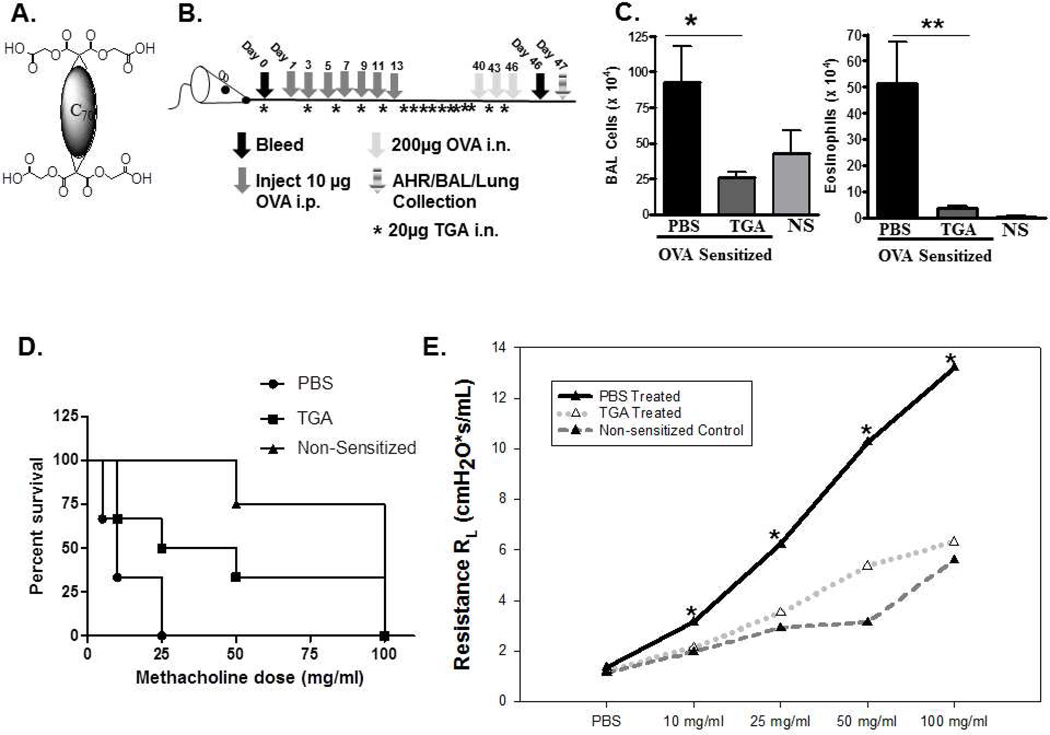
Figure 1. A novel fullerene derivative, tetraglycolate, inhibits airway inflammation and bronchoconstriction in a murine model of asthma.
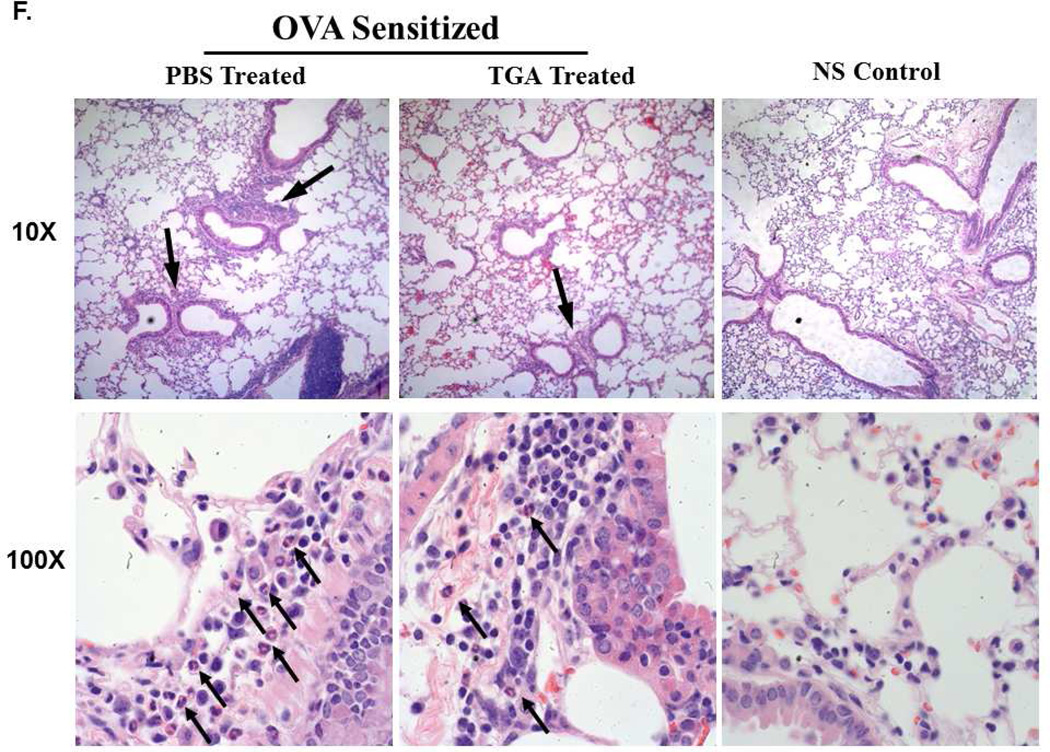
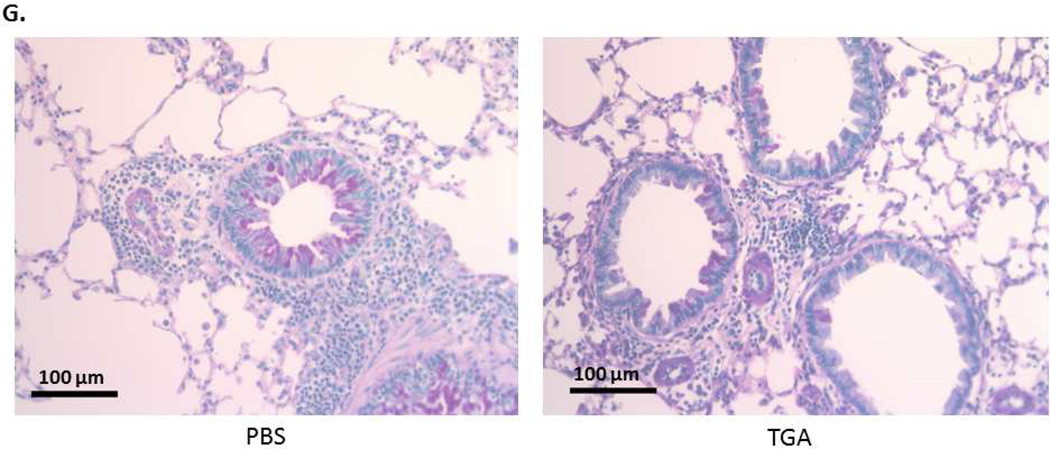
(A) Fullerene C70 –Tetraglycolate (TGA) consists of four glycolic acids covalently bonded to the fullerene C70 core, creating a water soluble molecule. (B) Schematic of ovalbumin challenge acute asthma model. Allergic lung inflammation was induced by i.p. sensitization with OVA followed by three i.n. OVA challenges. Tetraglycolate was given i.n. every three days starting on day -1. (C) Following OVA i.n. challenge BAL fluid and lung tissues were collected and total number of cells (left) were counted with trypan blue and total number of eosinophils (right) in the BAL cells were counted by Hema 3. Non-sensitized (NS) controls are shown for reference. n = 6–10 animals per group. * = p<.05 or ** = p<0.001 as determined by student t-test. TGA treated mice survived to a higher dose of methacholine (D) and were less responsive to aerosolized methacholine compared to PBS treated animals (E). In separate experiments using this model lung tissues were fixed, sectioned and stained with (F) H&E and (G) PAS. Representative photographs show cellular infiltration into airways of OVA sensitized mice treated with PBS or TGA. Arrows indicate areas of inflammation (10X) and eosinophils (100X).
Human lung MC preparation and mediator release
Human lung MC were purified as described previously (21;22). Tissue procurement and IRB approval were obtained from the Cooperative Human Tissue Network. Purified cells were incubated for 16 hours (a time point determined to be optimal for inhibition–data not shown) with or without three different EET’s, washed, and incubated with optimal concentrations of anti-FcεRI stimuli (1µg/ml). Mediator release (degranulation and cytokine production) was determined as previously described 17.
B cell IgE production and measurement
Naïve B cells isolated from Balb/c spleen 18 were treated with or without TGA (1 µg/ml) before challenge with IL-4 (20 ng/ml) and anti-CD40 Abs (1 µg/ml) for 8 days and IgE measured by ELISA.
Quantitative analysis of eicosanoids via HPLC ESI-MS/MS
To 300 µl of centrifuged BALF 300 µl of ethanol is added together with 10 ng each deuterated standard. 10 µl of this mixture was resolved in a 30 minute reversed-phase HPLC method. A Kinetex C18 column (100×2.1mm, 2.6µ) was used to separate the eicosanoids at a flow rate of 200 µl/min at 50°C. Prior to sample injection, the col umn was equilibrated with 100% Solvent A [acetonitrile:water:formic acid (40:60:0.02, v/v/v)]. 100% Solvent A was used for the first minute of elution. Solvent B [acetonitrile:isopropanol (50:50, v/v)] was increased following a linear gradient to 25% Solvent B by 3 minutes, 45% between 3 and 11 minutes, 60% between 11 and 13 minutes, 75% between 13 and 18 minutes, and 100% between 18 and 20 minutes. The gradient was maintained at 100% Solvent B from 20 to 25 minutes, and was then decreased to 0% by 26 minutes, and held at 0% until 30 minutes. The eluting eicosanoids were analyzed using an inline hybrid linear ion trap triple quadrapole tandem mass spectrometer (ABI 4000 Q-Trap®, Applied Biosystems) equipped with an electrospray ionization source operating in negative ion multiple-reaction monitoring mode. Eicosanoids were monitored using relevant precursor → product MRM pairs. Additional mass spectrometric parameters used are as follows: Curtain Gas: 30; CAD: High; Ion Spray Voltage: −3500V; Temperature: 500°C; Gas 1: 4 0; Gas 2: 60; Declustering Potential, Collision Energy, and Cell Exit Potential vary per transition.
ELISA and multiplex cytokine assay
Muc5AC protein was measured by ELISA, as described 19. Bronchoalveolar lavage fluid was diluted and 75 µl was incubated with bicarbonate-carbonate buffer (75µl) at 40° in a 96 well plate (Nunc) for a total volume/well of 150µl until dry. Plates were washed three times with PBS and blocked with 2% BSA (Sigma) for 1 hour at room temperature. Plates were again washed three times with PBS, then incubated with 50µl mouse monoclonal Muc5AC antibody (1:100), diluted with PBS containing 0.05% Tween 20, for one hour. Plates were washed three times with PBS and 100µl horseradish peroxidase-goat anti-mouse IgG conjugate (1:10,000) diluted in blocking solution was added for an hour and washed. Color reaction was developed with 3,3’,5,5’-tetramethylbenzidine (TMB) peroxidase solution, stopped with 0.18M H2SO4 and measured at 450 nm. Cytokine levels in BALF was measured using a multiplex cytokine assay (Biorad, Hercules, CA). Total mouse IgE was measured as described previously 20. The liver enzymes aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were measured as described previously 21. Serum creatinine levels were measured according to manufacturer’s instructions (Arbor Assays, Ann Arbor, MI).
RESULTS
Fullerene derivative TGA inhibits airway eosinophilia and bronchoconstriction
A panel of FD was developed with the objective of finding compounds capable of inhibiting MC responses14. The C70-based FD with 4 glycolic acids attached (TGA; Fig. 1A) was one of the most efficient inhibitors of MC degranulation and cytokine production in vitro, revealing its potential as an inhibitor of MC driven diseases. Because asthma pathogenesis is strongly influenced by MC activation and mediator release, we examined the therapeutic potential of TGA in a mouse model of asthma. To determine if TGA could inhibit disease onset we used an i.n. ovalbumin challenge protocol as described in Fig. 1B. TGA given i.n. throughout OVA sensitization and challenge resulted in a reduction in both total inflammatory cell numbers and eosinophil infiltration of the airways (Fig. 1C). Eosinophil percentage dropped from approximately 50% (±SD 7%) to 13% (± SD 4%) with TGA treatment. Additionally, TGA-treated animals were more resistant to methacholine induced death (Fig. 1D) as they were able to survive higher doses of methacholine than untreated mice. Further, TGA-treated mice had significantly less methacholine-induced airway resistance compared to vehicle (PBS) treated control mice (Fig. 1E). In lung sections stained with H&E, TGA treated animals had only a few small areas of mild inflammation (Fig. 1F, top panel). Within these areas eosinophil infiltration is clearly reduced in TGA treated animals (Fig. 1F, bottom, arrows indicate eosinophils). In contrast, several large areas of inflammation are seen in PBS treated mice (Fig 1F, top) and many eosinophils are found within this inflammation (Fig. 1f, bottom, arrows indicate eosinophils). Periodic acid Schiff (PAS) staining is marginally reduced in TGA treated animals (Fig 1G), however no difference in Muc5 RNA or protein levels were seen (not shown). These data indicate that TGA is able to dampen multiple features of IgE-dependent asthma pathogenesis leading us to further investigate its mechanism of action.
TGA dampens established disease
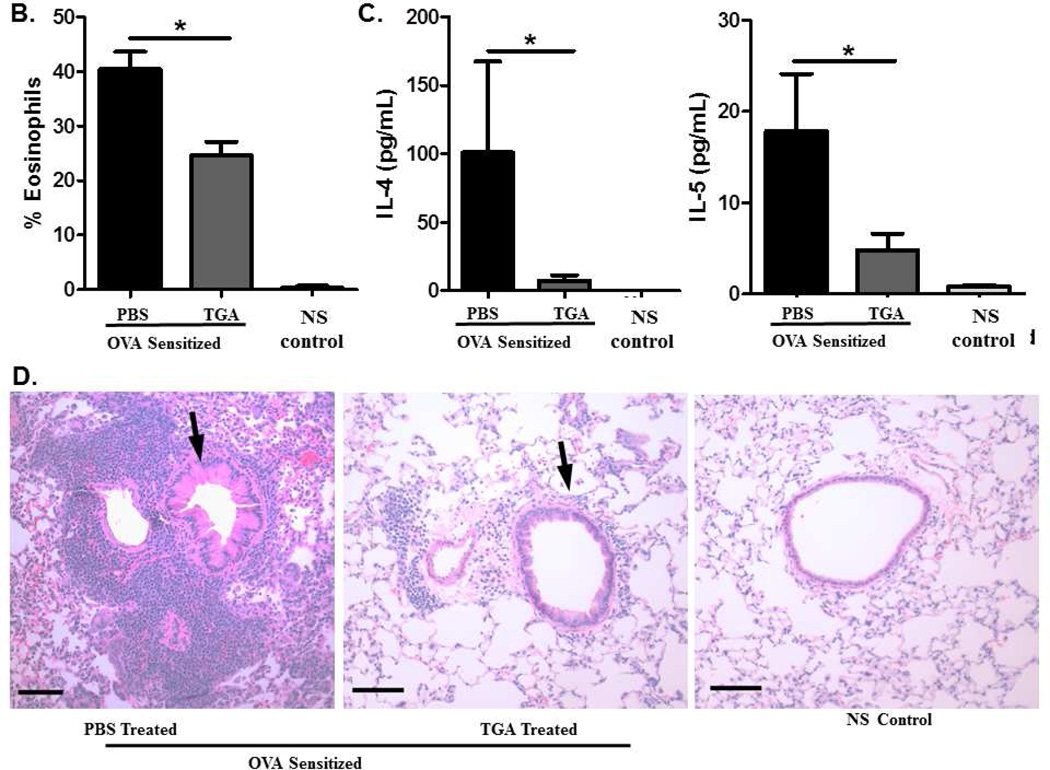
Because asthma therapeutics are given only after symptoms are established, we have developed a model to examine the effect of TGA on established disease. In this model (Fig. 2A) mice are sensitized and challenged with ovalbumin prior to the initiation of TGA treatment. Following disease initiation, mice are treated with TGA every three days for twenty days total. Mice then underwent a secondary ovalbumin challenge and were subsequently sacrificed; BALF and lung tissues were assessed as before. Even after disease is established, TGA administration significantly reduced eosinophil infiltration of the BALF and lung tissue (Fig. 2B). Further, IL-4 and IL-5 levels were significantly reduced in the BAL fluid suggesting an overall reduction in disease pathogenesis (Fig. 2C). Lung tissue sections show a dramatic reduction in cellular infiltration following TGA treatment (Fig. 2D). Thus, TGA has the ability to inhibit asthma pathogenesis even after disease is established.
Figure 2. Tetraglycolate dampens cellular infiltration, eosinophilia, and inflammatory cytokine levels in a murine model of established asthma.
(A) An established asthma model was used as described in methods. After the first set of i.n. OVA challenges mice began treatment with 20 µg TGA every three days. (B) Cells in the BAL fluid were stained with Hema 3 stain set. Inflammatory cells in the BAL were counted and eosinophils are represented as a percentage of total inflammatory cells. (C) Cytokine levels in the BAL fluid were measured using a multiplex cytokine assay. (D) Lung tissue was sectioned and stained with H&E. Arrows indicate airways. Representative photographs are shown. n = 6 –12 mice per group. *=p<.05 as determined by student t-test when variances were normal or Mann-Whitney test in the case of unequal variances.
TGA inhibits BAL cytokine levels and serum IgE, increases anti-inflammatory eicosanoid levels
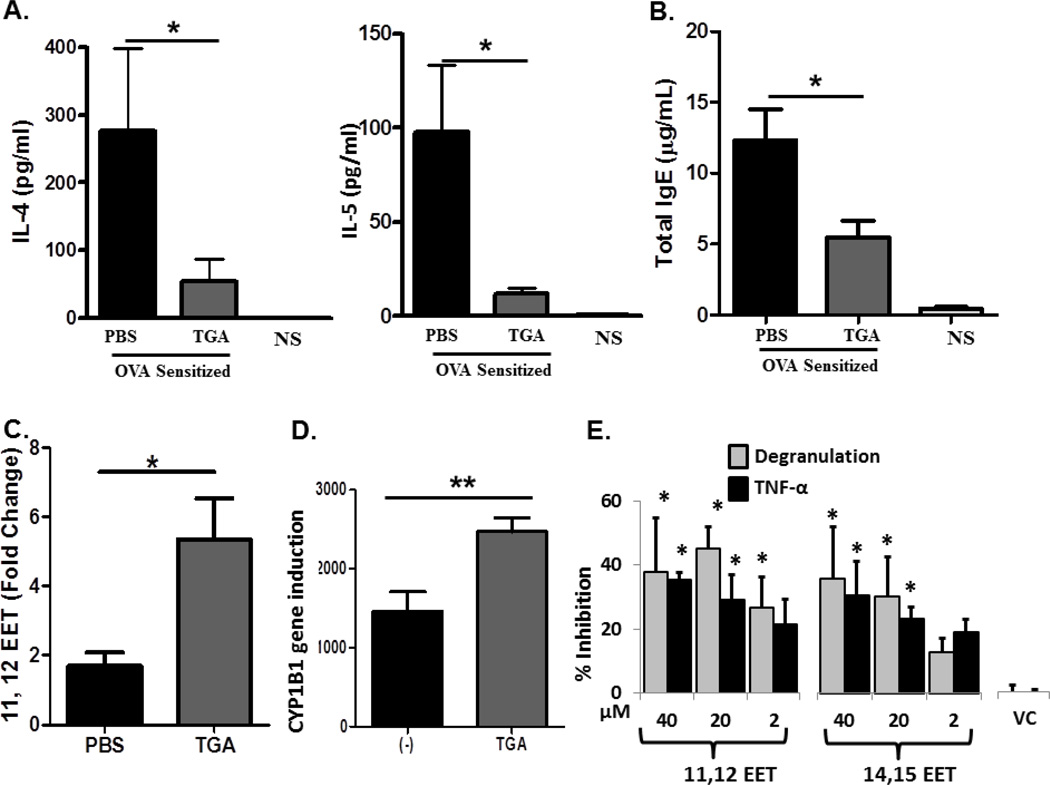
We next assessed the ability of TGA to inhibit several mediators of asthma pathogenesis in the disease model described in 1B. In the BAL fluid, asthma promoting cytokines IL-4 and IL-5 were significantly reduced in TGA treated mice to levels approaching that of non-sensitized animals (Fig. 3A). Additionally, serum IgE levels were significantly reduced in TGA treated animals (Fig. 3B). Epoxeicosatrienoic acids (EET) are anti-inflammatory derivatives of arachidonic acid 32. We used mass spectrometry to quantify EET levels in BALF of control and TGA-treated mice. Surprisingly, we found 11,12-EET levels significantly upregulated in the BAL fluid of TGA-treated mice compared to PBS-treated controls (Fig. 3C). This finding is mirrored in human lung MC, where we find a significant increase in CYP1B1 RNA, a cytochrome P450 capable of producing the HETE’s and EET’s 22, following TGA treatment (Fig. 3D). Further experiments using purified human lung MC demonstrated that both 11,12-EET and 14,15-EET significantly stabilized MC challenged through FcεRI inhibiting both degranulation and cytokine production compared to non-EET treated cells (Fig. 3E). In contrast, the 8,9-EET did not demonstrate any inhibition of MC mediator release (not shown). While TGA-induced increases in 11,12 EET paralleled inhibition of in vivo asthma induction and stabilized human MC mediator release alone, other arachidonic acid derivatives were not upregulated in vivo (Supplemental Table 1). Lastly, when B cells were incubated with TGA before challenge with IgE-producing stimuli there was a significant inhibition of IgE levels in TGA treated cells compared to non-TGA-treated cells (Figure 3F-left) that was not due to reduced B cell viability (Figure 3F-right). Similar inhibition of IgE production was observed with EET’s (data not shown). Together, these data indicate that TGA suppresses asthma by directly targeting lung MC activation or through reduced B cell IgE production.
Figure 3. Tetraglycolate significantly reduces inflammatory cytokines, eicosanoids, and serum IgE.
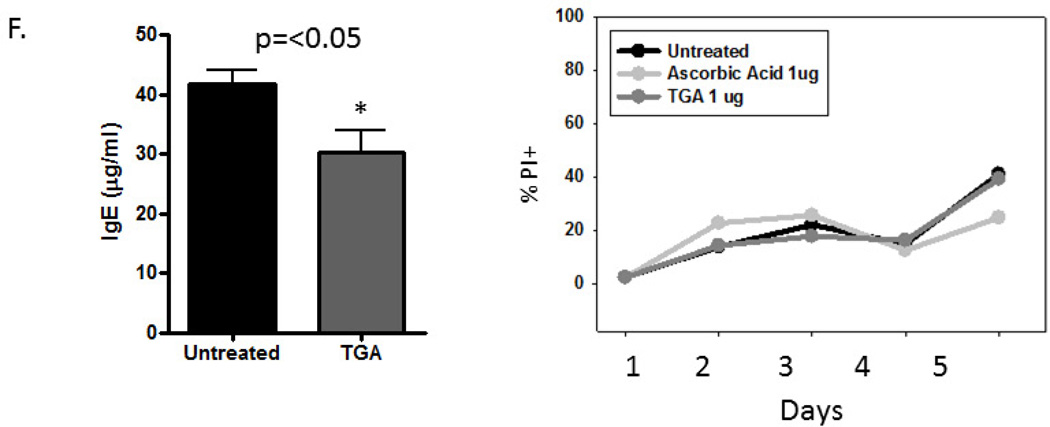
Asthma induction and fullerene treatment is described in Fig 1b. (A) Inflammatory cytokine levels were measured in the BAL fluid using a multiplex cytokine assay. (B) Serum was collected following OVA i.n. challenge and total IgE was measured by ELISA. (C) Levels of 11, 12-EET were measured in BALF by mass spectrometry. To account for variation between experiments, these values were standardized by calculating fold change relative to non-sensitized animals. n = 6 to 9 mice per group. (D) Human lung MC were pretreated with 10 µg/ml TGA overnight and activated 16 h later with anti-FcεRI antibodies (1µg/ml). Gene microarray was performed 14 and relative gene expression of CYP1B1 is shown. Each condition was performed in triplicate. * = p<.05 as determined by student t-test when variances were normal or Mann-Whitney test in the case of unequal variances. (E) Epoxygenases reduce degranulation from human lung MC after anti-FcεRI activation. Cells were cultured with fixed concentrations of the indicated EET or Vehicle Control (VC: Ethanol 40 µM), washed and stimulated for 20 min with optimal concentrations of anti-FcεRI Abs (3B4; 1mg/ml). Cells were centrifuged and β-hexosaminidase release and cytokine production determined. The data shown are the average of two separate experiments performed in triplicate with means ± SE shown. The asterisk indicated statistical significance compared to VC. (F). TGA inhibits IgE production by cultured B cells. B cells were incubated with or without TGA before being activated with IL4/anti-CD40 for 8 days. Cells were centrifuged and IgE in the supernatants measured by ELISA. B cell viability was assessed using propidium iodide (PI) staining of dead cells. The percentage PI+ cells was assessed using a flow cytometer for the first five days of incubation, after which viability was dramatically reduced in all groups. Experiments were performed at least three times in duplicate and representative data is shown. There are no toxic effects on these cells as compared to B cells that are left untreated or those treated with a non-toxic antioxidant, ascorbic acid.
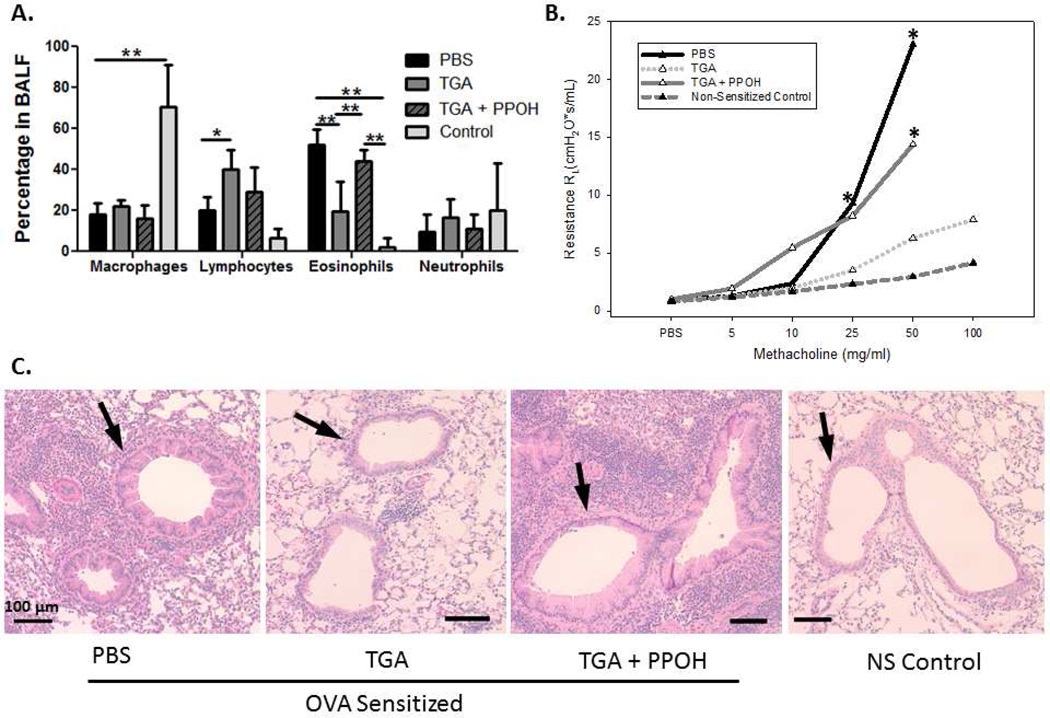
In order to confirm that the elevation of EET’s was relevant to the reduced asthmatic response that was seen, we examined the effect of blocking the synthesis of EET’s using the inhibitor 6-(2-propargyloxyphenyl) hexanoic acid (PPOH) and the model described in Figure1B. The PPOH is a potent and selective inhibitor of arachidonic acid epoxidation 16 and is more selective for EET inhibition compared to other terminal acetylenic compounds. Additionally, we used an antagonist of EET activity, 14,15-EE-5(Z)E (EEZE). As seen in Figure 4 there was a significant increase in the BAL eosinophils (Fig. 4A) and airway constriction (Fig. 4B) in TGA treated mice given the EET inhibitor PPOH over those given TGA alone. Similar results were obtained with the EET receptor antagonist EEZE (data not shown). Lung sections show increased cellular infiltration in PPOH treated mice compared to those given TGA alone (Fig. 4C). These data indicate that TGA induced production of anti-inflammatory 11, 12-EET is largely responsible for inhibition of the asthma phenotype seen in TGA treated mice.
Figure 4. Inhibiting the induction of 11, 12-epoxyeicosatrienoic acid by TGA restores the asthma phenotype.
Effects of the inhibitor of EET 6-(2-Propargyloxyphenyl) hexanoic acid (PPOH) on airway inflammation and bronchoconstriction were assessed. (A) Cells in the BAL fluid were stained with Hema 3 stain set. Inflammatory cells in the BAL were counted and each cell type is shown as a percentage of total inflammatory cells. (B) Airway resistance (RL) was measured in response to increasing doses of methacholine. (C) Representative lung sections stained with H&E. Arrows indicate airways. Bar measures 100 µm. n = 6 mice per group. *=p<.05
FD do not accumulate in major organs or alter liver or kidney function
The toxicity of FD is still widely debated and varies based on the specific moieties added to the fullerene core23. Therefore, in vivo toxicity of TGA was assessed via several methods. To assess accumulation, a TGA molecule containing gadolinium (Gd) within the fullerene sphere 24 was administered i.n. to mice every three days for 30 days. Twenty-four hours after the last dosage Gd levels were measured in serum, lung, spleen, liver, kidney, and brain tissue. Gadolinium was detected only in the lung tissue, where less than 10 percent of that injected remained indicating the TGA is cleared from the lung. No Gd was detected in any other tissue examined (data not shown). These enzymes are present at low levels in healthy individuals and large increases would suggest liver toxicity 25. No significant differences in serum levels of AST/ALT were observed between treated, untreated, and non-sensitized control animals (Supplemental Fig. 1A). No significant differences in serum creatinine levels 26 were seen between treated, untreated, and non-sensitized animals (Supplemental Fig. 1B). These initial studies suggest that TGA does not accumulate within the body and is not acutely toxic to the liver or kidney.
DISCUSSION
There is a strong need for novel therapeutics to treat asthmatic disease; indeed up to fifty-five percent of patients receiving treatment for asthma have uncontrolled symptoms 27. In this study we have shown that TGA is able to suppress both disease onset and reverse established disease. The latter is especially important as human asthma treatment always involves established disease. Mast cells play an important role in human disease, and previous publications have shown that MC-deficient mice sensitized with OVA lacking adjuvant and challenged with OVA alone, as performed in this study, have diminished airway inflammation and bronchoconstriction 7, 28. Additionally, we developed a model of established asthma to further emulate pathogenesis of human disease, as therapeutics are given following the initiation of symptoms. While murine and human anatomy differs, these models were chosen to best represent human pathogenesis and thus suggest that TGA (or similar FD) could have therapeutic efficacy in the treatment of human disease.
Specifically, it is demonstrated that mice treated with TGA throughout OVA challenge have significantly less airway inflammation and bronchoconstriction compared to untreated animals. In fact, total inflammation and bronchoconstriction in TGA treated animals is not only significantly reduced, but is similar to that seen in non-sensitized controls. Thus, symptoms of disease were largely reversed in these animals. Note that these studies used a model previously shown to utilize MC 28. In studies not shown, a different asthma model that is IgE, but not MC dependent 29 was also found to be inhibited by TGA. However, when a non-allergic airway inflammation model which develops in both MC deficient and IgE deficient mice 18 was used, TGA had no effect on eosinophilia or cytokine production (data not shown). In the established disease model, as when mice were treated throughout disease development, we found TGA dampens eosinophilia and cytokine levels significantly in the BAL fluid. Lung sections show massive cellular infiltration in untreated animals, while those receiving TGA have minimal cellular infiltration surrounding the airways. Airway hyperresponsiveness trended towards reduction in TGA treated animals, but due to high variability between mice significant differences were not observed.
Importantly, we have demonstrated that TGA activity has novel mechanisms of action. While previously published in vitro studies suggested that MC inhibition may be the predominant mechanism of FD inhibition, these in vivo studies suggest multipotent effects of these unique compounds. TGA treatment reduced the levels of BAL Th2 pro-inflammatory cytokines and reduced lung inflammation. While IL-4 stimulates IgE production by B cells, IL-5 both recruits and activates eosinophils at the site of inflammation. Tetraglycolate treatment significantly reduces both IL-4 and IL-5 in the BAL fluid. Additionally, serum IgE levels were significantly reduced following TGA treatment and TGA suppressed IgE production from B cells. In contrast, a non-allergic lung inflammation model was not influenced by TGA treatment.
Several eicosanoids derived from the cytochrome P450 pathway are relatively stable and thus we measured these molecules in BAL fluid samples using mass spectrometry. The EET’s are consistently associated with relaxation of the bronchi and other anti-inflammatory actions in vivo 30–32. Intriguingly, 11, 12-EET was consistently upregulated in BALF from TGA treated mice. Further in vivo studies demonstrated that the EET’s play a major role in dampening the asthma phenotype. Specifically, selective inhibitors of EET production as well as inhibitors of EET activity reversed the TGA-induced modulation of the OVA-induced asthma model.
The in vivo results with EET’s led us to examine possible mechanisms in vitro. We show for the first time that EET’s stabilize human lung MC through the inhibition of FcεRI-induced mediator release. The actual concentration of 11,12-EET in the BALFs of TGA treated animals averaged 40 ng/ml (Figure 3C) which was determined after diluting approximately 4–5 times when PBS is used to flush out the BAL. Based on this calculation, the concentrations of EET observed in the BALF (approximately 160–200 ng/ml) is approximately the same as that observed for maximal MC inhibition (20 µM). The 11,12-EET also showed a small but significant decrease in IgE production from IL-4/CD40-stimulated B cells (data not shown). These results suggest a possible mechanism as to how EET’s could block the asthmatic response. We are currently examining the mechanisms of how EET’s could block both MC and B-cell responses focusing on common signaling molecules in these two cell types.
The cellular source of EET is not certain at present. However, we found that TGA upregulates expression of human lung MC CYP1B1, a gene involved in the production of EET’s suggesting MC could be the source of 11,12-EET. Other studies have demonstrated that EET’s may be produced by lung epithelial and endothelial cells and can relax histamine-precontracted guinea pig and human bronchi 30. Further, they can inhibit the upregulation of VCAM-1, E-selectin, and ICAM-1, thus potentially limiting cellular infiltration of the lung 31. Consequently, 11, 12-EET upregulation is playing a significant role in dampening airway inflammation and bronchoconstriction in these models. Together, these results suggest that TGA/EETs suppress the asthmatic response through a variety of mechanisms, targeting both B cells and MC. In addition, our results implicate EET’s as a heretofore undiscovered mechanism for controlling asthma and suggest that strategies that induce the production of EET’s may be a viable therapeutic strategy for treating asthmatics. We are currently identifying the cell types that produce EET’s in response to TGA and are also exploring BAL lung fluids from asthmatics to compare their EET levels to non-asthmatics. The antagonist data indicates that EET’s induced by TGA are directly involved in the suppression seen in the asthma response. However, we recognize the possibility that TGA could have indirect effects by shunting arachidonic acid away from production of proinflammatory eicosanoids like the cysteinyl leukotrienes. Further studies will be required to determine if the TGA also alters the relative levels of proinflammatory eicosanoids as well.
Levels of liver enzymes AST and ALT, which are indicative of liver toxicity when present at high levels in the serum, were not different between treated and untreated animals. Tetraglycolate treated animals had creatinine levels similar to those seen in untreated animals, and all were within the normal range. Thus, liver and kidney function appear to be unaffected by short-term local administration of TGA. In addition, a TGA-like compound containing Gd within the fullerene core was developed so that its presence in tissue could be detected using ICP neutron bombardment. After one month of intranasal inhalations, followed by no challenge for up to a week, only lung tissue contained detectable amounts of Gd. Thus TGA does not appear to build up in the body tissues, limiting the possibility of toxicity. Further testing will be necessary to determine the safety of these compounds in humans.
In conclusion, these studies are the first to suggest the efficacy of FD for the treatment of asthma through a previously undescribed mechanism involving the upregulation of anti-inflammatory EET’s. Evidence presented here suggests that specific FD compounds have the potential to become novel therapeutics for the treatment of asthma and pave the way to new research efforts focusing on the role of EET’s in asthma.
Supplementary Material
Key Messages.
TGA may represent a new therapeutic option for the treatment of asthma.
Inhibition of asthma pathogenesis through the upregulation of 11, 12-EET represents a novel inhibitory mechanism. Thus, TGA may provide therapeutic benefit to patients with refractory asthma and increased EET levels may be an efficacy marker for asthma therapies.
EET stabilize MC in response to FcεRI challenge.
Acknowledgements
Microscopy was performed at the VCU Department of Neurobiology and Anatomy microscopy facility, supported in part with funding from NIH-NINDS center core grant 5P3ONS047463. We wish to thank Drs. Stephen Galli and Mang Yu at Stanford University for their training and assistance with the MC dependent model as well as various other techniques for asthma assessment.
We acknowledge grants 1R01GM083274-01C and R21 ESO15696-01A1 to CLK, grant 1U19AI077435-project 2 to DHC and the AHA predoctoral award #10PRE4170025 to SN for supporting this work.
Abbreviations
- FD
Fullerene derivative
- MC
mast cell
- i.p.
intraperitoneal
- i.n.
intranasal
- EET
cis-epoxyeicosatreinoic acid
- OVA
ovalbumin
- TGA
C70-tetraglycolate
- AST
aspartate aminotransferase
- ALT
alanine aminotransferase
- H&E
Hematoxylin and Eosin
- BAL
bronchoalveolar lavage
- Gd
Gadolinium
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Boyce JA, Bochner B, Finkelman FD, Rothenberg ME. Advances in mechanisms of asthma, allergy, and immunology in 2011. J Allergy Clin Immunol. 2012;129:335–341. doi: 10.1016/j.jaci.2011.12.968. [DOI] [PubMed] [Google Scholar]
- 2.Ogawa Y, Calhoun WJ. The role of leukotrienes in airway inflammation. J Allergy Clin Immunol. 2006;118:789–798. doi: 10.1016/j.jaci.2006.08.009. quiz 99–800. [DOI] [PubMed] [Google Scholar]
- 3.Broide DH. Immunologic and inflammatory mechanisms that drive asthma progression to remodeling. J Allergy Clin Immunol. 2008;121:560–570. doi: 10.1016/j.jaci.2008.01.031. quiz 71–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bachelet I, Munitz A, Levi-Schaffer F. Tryptase as an inflammatory marker in allergic disease and asthma. Expert Rev Clin Immunol. 2005;1:63–73. doi: 10.1586/1744666X.1.1.63. [DOI] [PubMed] [Google Scholar]
- 5.Holgate ST. Pathogenesis of asthma. Clinical and Experimental Allergy. 2008;38:872–897. doi: 10.1111/j.1365-2222.2008.02971.x. [DOI] [PubMed] [Google Scholar]
- 6.Casale TB, Wood D, Richerson HB, Zehr B, Zavala D, Hunninghake GW. Direct evidence of a role for mast cells in the pathogenesis of antigen-induced bronchoconstriction. J Clin Invest. 1987;80:1507–1511. doi: 10.1172/JCI113234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Williams CM, Galli SJ. Mast cells can amplify airway reactivity and features of chronic inflammation in an asthma model in mice. J Exp Med. 2000;192:455–462. doi: 10.1084/jem.192.3.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilson SR, Schuster DI, Nuber B, Meier M, Prato M, Taylor R. In: Fullerenes: Chemistry, Physics, and Technology. Kadish K, Ruoff R, editors. John Wiley & Sons; NY: 2000. [Google Scholar]
- 9.Jensen AW, Wilson SR, Schuster DI. Biological applications of fullerenes. Bioorg.Med.Chem. 1996;4:767–779. doi: 10.1016/0968-0896(96)00081-8. [DOI] [PubMed] [Google Scholar]
- 10.Djordjevic A, Bogdanovic G, Dobric S. Fullerenes in biomedicine. J BUON. 2006;11:391–404. [PubMed] [Google Scholar]
- 11.Basso AS, Frenkel D, Quintana FJ, Costa-Pinto FA, Petrovic-Stojkovic S, Puckett L, et al. Reversal of axonal loss and disability in a mouse model of progressive multiple sclerosis. J.Clin.Invest. 2008;118:1532–1543. doi: 10.1172/JCI33464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dugan LL, Turetsky DM, Du C, Lobner D, Wheeler M, Almli CR, et al. Carboxyfullerenes as neuroprotective agents. Proc.Natl.Acad.Sci.U.S.A. 1997;19(94):9434–9439. doi: 10.1073/pnas.94.17.9434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ryan JJ, Bateman HR, Stover A, Gomez G, Norton SK, Zhao W, et al. Fullerene nanomaterials inhibit the allergic response. J Immunol. 2007;179:665–672. doi: 10.4049/jimmunol.179.1.665. [DOI] [PubMed] [Google Scholar]
- 14.Norton SK, Dellinger A, Zhou Z, Lenk R, Macfarland D, Vonakis B, et al. A new class of human mast cell and peripheral blood basophil stabilizers that differentially control allergic mediator release. Clin Transl Sci. 2010;3:158–169. doi: 10.1111/j.1752-8062.2010.00212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gauthier KM, Deeter C, Krishna UM, Reddy YK, Bondlela M, Falck JR, et al. 14,15-Epoxyeicosa-5(Z)-enoic acid: a selective epoxyeicosatrienoic acid antagonist that inhibits endothelium-dependent hyperpolarization and relaxation in coronary arteries. Circ Res. 2002;90:1028–1036. doi: 10.1161/01.res.0000018162.87285.f8. [DOI] [PubMed] [Google Scholar]
- 16.Wang MH, Brand-Schieber E, Zand BA, Nguyen X, Falck JR, Balu N, et al. Cytochrome P450-derived arachidonic acid metabolism in the rat kidney: characterization of selective inhibitors. J Pharmacol Exp Ther. 1998;284:966–973. [PubMed] [Google Scholar]
- 17.Kepley CL, Taghavi S, Mackay G, Zhu D, Morel PA, Zhang K, et al. Co-aggregation of FcgammaRII with FcepsilonRI on human mast cells inhibits antigen-induced secretion and involves SHIP-Grb2-Dok complexes. J Biol Chem. 2004;279:35139–35149. doi: 10.1074/jbc.M404318200. [DOI] [PubMed] [Google Scholar]
- 18.Mathews JA, Ford J, Norton S, Kang D, Dellinger A, Gibb DR, et al. A potential new target for asthma therapy: a disintegrin and metalloprotease 10 (ADAM10) involvement in murine experimental asthma. Allergy. 2011;66:1193–1200. doi: 10.1111/j.1398-9995.2011.02614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takeyama K, Dabbagh K, Lee HM, Agusti C, Lausier JA, Ueki IF, et al. Epidermal growth factor system regulates mucin production in airways. Proc Natl Acad Sci U S A. 1999;96:3081–3086. doi: 10.1073/pnas.96.6.3081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Caven TH, Shelburne A, Sato J, Chan-Li Y, Becker S, Conrad DH. IL-21 dependent IgE production in human and mouse in vitro culture systems is cell density and cell division dependent and is augmented by IL-10. Cell Immunol. 2005;238:123–134. doi: 10.1016/j.cellimm.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 21.Reitman S, Frankel S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am J Clin Pathol. 1957;28:56–63. doi: 10.1093/ajcp/28.1.56. [DOI] [PubMed] [Google Scholar]
- 22.Choudhary D, Jansson I, Stoilov I, Sarfarazi M, Schenkman JB. Metabolism of retinoids and arachidonic acid by human and mouse cytochrome P450 1b1. Drug Metab Dispos. 2004;32:840–847. doi: 10.1124/dmd.32.8.840. [DOI] [PubMed] [Google Scholar]
- 23.Kolosnjaj J, Szwarc H, Moussa F. Toxicity studies of fullerenes and derivatives. Adv.Exp.Med.Biol. 2007;620:168–180. doi: 10.1007/978-0-387-76713-0_13. [DOI] [PubMed] [Google Scholar]
- 24.MacFarland DK, Walker KL, Lenk RP, Wilson SR, Kumar K, Kepley CL, et al. Hydrochalarones: a novel endohedral metallofullerene platform for enhancing magnetic resonance imaging contrast. J Med Chem. 2008;51:3681–3683. doi: 10.1021/jm800521j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giboney PT. Mildly elevated liver transaminase levels in the asymptomatic patient. Am Fam Physician. 2005;71:1105–1110. [PubMed] [Google Scholar]
- 26.Steinitz K. A simple bedside test for renal function; the semiquantitative determination of blood creatinine. Harefuah. 1947;32:174. [PubMed] [Google Scholar]
- 27.Peters SP, Jones CA, Haselkorn T, Mink DR, Valacer DJ, Weiss ST. Real-world Evaluation of Asthma Control and Treatment (REACT): findings from a national Web-based survey. J Allergy Clin Immunol. 2007;119:1454–1461. doi: 10.1016/j.jaci.2007.03.022. [DOI] [PubMed] [Google Scholar]
- 28.Williams CM, Galli SJ. Mast cells can amplify airway reactivity and features of chronic inflammation in an asthma model in mice. J Exp.Med. 2000;192:455–462. doi: 10.1084/jem.192.3.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oettgen HC, Martin TR, Wynshaw-Boris A, Deng C, Drazen JM, Leder P. Active anaphylaxis in IgE-deficient mice. Nature. 1994;370:367–370. doi: 10.1038/370367a0. [DOI] [PubMed] [Google Scholar]
- 30.Dumoulin M, Salvail D, Gaudreault SB, Cadieux A, Rousseau E. Epoxyeicosatrienoic acids relax airway smooth muscles and directly activate reconstituted KCa channels. Am J Physiol. 1998;275:L423–L431. doi: 10.1152/ajplung.1998.275.3.L423. [DOI] [PubMed] [Google Scholar]
- 31.Node K, Huo Y, Ruan X, Yang B, Spiecker M, Ley K, et al. Anti-inflammatory properties of cytochrome P450 epoxygenase-derived eicosanoids. Science. 1999;285:1276–1279. doi: 10.1126/science.285.5431.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zeldin DC, Plitman JD, Kobayashi J, Miller RF, Snapper JR, Falck JR, et al. The rabbit pulmonary cytochrome P450 arachidonic acid metabolic pathway: characterization and significance. J Clin Invest. 1995;95:2150–2160. doi: 10.1172/JCI117904. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.