Abstract
Transcutaneous electric nerve stimulation (TENS) is widely used for the treatment of pain. TENS produces an opioid-mediated antinociception that utilizes the rostroventromedial medulla (RVM). Similarly, antinociception evoked from the periaqueductal grey (PAG) is opioid-mediated and includes a relay in the RVM. Therefore, we investigated whether the ventrolateral or dorsolateral PAG mediates antinociception produced by TENS in rats. Paw and knee joint mechanical withdrawal thresholds were assessed before and after knee joint inflammation (3% kaolin/carrageenan), and after TENS stimulation (active or sham). Cobalt chloride (CoCl2; 5 mM) or vehicle was microinjected into the ventrolateral periaqueductal grey (vlPAG) or dorsolateral periaqueductal grey (dlPAG) prior to treatment with TENS. Either high (100 Hz) or low (4 Hz) frequency TENS was then applied to the inflamed knee for 20 min. Active TENS significantly increased withdrawal thresholds of the paw and knee joint in the group microinjected with vehicle when compared to thresholds prior to TENS (P<0.001) or to sham TENS (P<0.001). The increases in withdrawal thresholds normally observed after TENS were prevented by microinjection of CoCl2 into the vlPAG, but not the dlPAG prior to TENS and were significantly lower than controls treated with TENS (P<0.001). In a separate group of animals, microinjection of CoCl2 into the vlPAG temporarily reversed the decreased mechanical withdrawal threshold suggesting a role for the vlPAG in the facilitation of joint pain. No significant difference was observed for dlPAG. We hypothesize that the effects of TENS are mediated through the vlPAG that sends projections through the RVM to the spinal cord to produce an opioid-mediated analgesia.
Keywords: pain, TENS, hyperalgesia, opioid, inflammation, analgesia
The midbrain periaqueductal grey (PAG) surrounds the midbrain aqueduct (Osborne et al., 1996) and is implicated in a wide variety of functions including opioid-mediated analgesia (Gebhart et al., 1988; Fields et al., 1991; Osborne et al., 1996; Vaughan and Christie, 1997). Two separate, and distinct, nociceptive modulatory systems operate in the caudal PAG: a dorsal system which encompasses the dorsomedial, dorsolateral and lateral subdivisions of the PAG; and a ventral system which includes the ventrolateral PAG and dorsal raphe (reviewed by Morgan, 1991).
Opioid administration into the vlPAG in the rat (Jensen and Yaksh, 1989; Krzanowska and Bodnar, 1999; Tershner et al., 2000) and cat (Oliveras et al., 1974), as well as electrical stimulation of the PAG in humans (Hosobuchi et al., 1977) produces antinociception. Interestingly, opioids appear to interact exclusively with the ventral system, as antinociception produced by electrical stimulation of the ventral, but not dorsal, PAG is attenuated by the opioid antagonist naloxone (Cannon et al., 1982). Furthermore, microinjection of the opioid agonist morphine produces antinociception when microinjected into the vlPAG (Yaksh et al., 1976). Although morphine produces explosive motor behavior when injected into the lateral PAG, this behavior is also accompanied by antinociception (Jacquet and Lajtha, 1974; Jensen and Yaksh, 1986; Morgan et al., 1998). This antinociception occurs even when the aversive reactions are blocked (Morgan et al., 1987).
The PAG produces antinociception through a relay in the rostroventral medial medulla (RVM), a region which encompasses the nucleus raphe magnus and the adjacent reticular formation. In rat, as many as 18% of PAG neurons project to the RVM (Osborne et al., 1996) and are distributed throughout the dorsomedial, lateral and ventrolateral PAG divisions, but are absent in the dorsolateral PAG division (Reichling and Basbaum, 1991). Inactivation of the RVM disrupts antinociception mediated by stimulation of the PAG (Prieto et al., 1983; Sandkuhler and Gebhart, 1984). Further, microinjection of morphine into the RVM produces antinociception (Jensen and Yaksh, 1986; Morgan et al., 1998; Morgan and Whitney, 2000). Thus, opioid-mediated analgesia activates a pathway with neurons that project from the vlPAG to the RVM, and subsequently to the spinal cord dorsal horn (Bagley et al., 2005).
Transcutaneous electric nerve stimulation (TENS) is a non-pharmacological treatment for pain that produces antinociception through activation of opioid receptors in the spinal cord and RVM (Sluka et al., 1999; Kalra et al., 2001). Specifically, low (4 Hz) frequency TENS activates μ-opioid receptors and high (100 Hz) frequency TENS activates δ-opioid receptors (Sluka et al., 1999; Kalra et al., 2001). Further, repeated application of TENS, low or high frequency, produces analgesic tolerance and a cross-tolerance to μ- and δ-opioid receptors spinally, respectively (Chandran and Sluka, 2003). As TENS produces an opioid-mediated antinociception that utilizes the RVM (Kalra et al., 2001) and antinociception evoked from the PAG is opioid-mediated and includes a relay in the RVM, we hypothesized that the PAG mediates the antinociception produced by TENS.
EXPERIMENTAL PROCEDURES
All experiments were approved by Animal Care and Use Committee at the University of Iowa (Iowa City, IA, USA) and are in accordance with the guidelines of National Institutes of Health on use of laboratory animals. This study used the minimum number of animals to obtain statistical significance. Adult male Sprague–Dawley rats (n=64; 225–350 g, Harlan, Indianapolis, IN, USA) were used for this study. The animals were housed in a 12-h light/dark cycle, and the testing was done only in the light cycle. Food and water were available to the animals ad libitum.
Induction of inflammation
Immediately after baseline behavioral measurements that are described below, rats were anesthetized with isoflurane (2–4%) and the left knee joint was injected intra-articularly with a mixture of 3% carrageenan and 3% kaolin (0.1 ml in sterile saline, pH 7.4) (Sluka and Westlund, 1993). The inflammation is considered acute for the first 24 h, when there is neutrophil infiltration. By 1 week, the inflammation converts to chronic, as identified histologically by macrophage infiltration. This model is used to mimic arthritic conditions and shows good predictability for drug effects (Radhakrishnan et al., 2003). After induction of knee inflammation, the rats were returned to their cages and allowed to recover for 24 h. Within 24 h, the animals exhibit signs of inflammation such as edematous and warm knee joints and also behavioral signs such as guarding and decreased weight bearing on the inflamed limb (Sluka and Westlund, 1993).
Cannula implantation and microinjections
Intracerebral guide cannulae were placed in the ventrolateral (vlPAG) or dorsolateral (dlPAG) periaqueductal grey 3 to 5 days before induction of knee joint inflammation. The rats were anesthetized with an i.p. injection of sodium pentobarbital (Nembutal, 50 mg/kg, Ovation Pharmaceucticals, Deerfield, IL, USA) and secured in a stereotaxic head holder to implant the guide cannula (17.5 mm in length, 26 gauge; Plastics One, Roanoke, VA, USA). After the midline incision, the skull was exposed, and a small hole drilled for placement of the guide cannula. The guide cannula was 1 mm above the vlPAG, using the following coordinates: interaural: 1.7 mm; mediolateral: +0.6 mm; and dorsoventral: −5.0 mm below the skull surface. For dlPAG, the guide cannula was 1 mm above the dlPAG, using the following coordinates: interaural: 1.7 mm; mediolateral: +0.6 mm; and dorsoventral: −4.8 mm below the skull surface (Paxinos and Watson, 2005). Cannulae were secured to the skull by stainless-steel screws and dental cement (Urban and Smith, 1994). Cannula was implanted ipsilateral to the inflamed knee joint. A dummy cannula (33 gauge, Plastics One) was inserted into the guide cannula to maintain its patency. All rats were allowed to recover 3 to 5 days after surgery before behavioral testing.
To examine placement of the cannula into the vlPAG or dlPAG, an equivalent volume of methylene blue dye was injected through the cannula at the end of the experiment. Rats were then euthanized with an overdose of sodium pentobarbital (150 mg/kg i.p.) and transcardially perfused with 4% paraformaldehyde. After this, the brain was removed, stored in 30% sucrose solution, frozen, cross-sectioned at 40 µm on a cryostat and examined under a light microscope for placement of the cannula.
Drug administration
Vehicle (0.5 µl, 0.9% sterile saline) or 5 mM CoCl2 solution (0.5 µl, dissolved in 0.9% sterile saline, Fisher Scientific, NJ, USA) was microinjected into vlPAG or dlPAG through the guide cannula. The dose of CoCl2 was selected from a prior study (Cavun et al., 2004) and through preliminary experiments. Microinjections of CoCl2 in discrete brain areas have been used for reversible functional inactivation (Kretz, 1984; Nuseir et al., 1999; Fisk and Wyss, 2000; Pajolla et al., 2005). Co2+ obstructs the ionophore of the voltage-gated Ca2+ channel (Hagiwara and Byerly, 1981) and thus induces blockade of Ca2+-dependent release of neurotransmitter from presynaptic terminals (Kretz, 1984). This blockade of neurotransmitter release therefore causes a reversible blockade of neuronal pathways that synapse in the targeted area (Kretz, 1984) and fibers of passage are not affected by CoCl2 (Kretz, 1984).
A 33-gauge injection cannula was connected to a 10-µl Hamilton syringe through PE10 tubing backfilled with sterile saline. The microinjection (0.5 µl) of either CoCl2 or vehicle was performed over a 2-min period and the travel of the air bubble in the tubing was carefully observed to ensure that the drug solution entered the injection cannula. The needle was left in position for a minute to allow diffusion of drug before the needle was withdrawn. TENS application was performed 1 h after injection of CoCl2, a time when preliminary studies show a maximal effect of CoCl2.
Behavioral assessment
The paw withdrawal threshold and the joint withdrawal threshold were tested for all groups of rats. Paw and joint withdrawal thresholds were assessed before and 24 h after induction of inflammation, and 1 h after TENS application. Rats were tested for PWT with von Frey filaments applied to the paw. Initially, the animals were maintained in their home cages in the behavior room for 30 min to acclimate to the environment. Then, the animals were placed in transparent Lucite cubicles over a wire mesh and acclimated for another 30 min before testing. A series of von Frey filaments with increasing bending forces (9.4–495.8 mN) was applied to the plantar surface of the hind paw until the rat withdrew from the stimulus (Gopalkrishnan and Sluka, 2000). Each filament was applied twice. The lowest force at which the rat withdrew its paw from one of two applications was recorded as the paw withdrawal threshold for mechanical hyperalgesia. A reduction in mechanical withdrawal threshold was interpreted as cutaneous hyperalgesia. This testing method has shown significant statistical test–retest reliability (Sluka et al., 1999).
Rats were also tested for knee joint withdrawal thresholds with a pair of forceps applied to the knee joints as previously described (Vance et al., 2007; DeSantana et al., 2008). Rats were acclimated in a restraining device three times a day 1 h apart for 2 days, each acclimatizing session consisting of 5 min (two days prior to the induction of inflammation). The forceps were equipped with two strain gauges to measure force. To measure the knee joint withdrawal threshold, animals were placed in the restrainer, and the experimenter compressed the knee joint with the tip of the forceps while the hind limb was extended. Compression was continued until the animal withdrew the leg. The maximum force applied at withdrawal was recorded as the joint withdrawal threshold. Three trials 5 min apart at each time period were performed and averaged to obtain one reading per time period. A decrease in withdrawal threshold of the knee joint was interpreted as joint hyperalgesia.
We previously showed that (1) both low and high frequency TENS reduce hyperalgesia induced by kaolin and carrageenan for 12–24 h after administration, (2) application of halothane without TENS has no effect on the paw withdrawal latency to heat induced by joint inflammation, and (3) application of TENS to a non-inflamed knee joint has no effect on the paw withdrawal latency (Sluka et al., 1998).
Administration of TENS
EMPI Select TENS units with an asymmetrical biphasic square wave (EMPI Inc., MN, USA) and half-inch circular electrodes were used. Under isoflurane anesthesia, the left knee joint was shaved and round pre-gelled surface electrodes were applied to the medial and lateral aspects of the inflamed knee joint in the groups receiving active TENS or sham TENS. Animals were observed continuously during TENS to ensure adequate anesthesia and to ensure that the electrodes remained in contact with the skin.
Either high (100 Hz) or low (4 Hz) frequency TENS was administered keeping other parameters constant, i.e. pulse duration (100 µs), sensory intensity and 20 min for stimulation. This strategy allowed a comparison of frequency differences without confounding differences in pulse duration or amplitude. Sensory intensity was determined by increasing the intensity until a muscle contraction was visibly observed and then reducing the intensity to just below this level. The parameters were selected to model those used clinically (Sluka et al., 1998). The sham TENS group was anesthetized with 1–2% isoflurane and electrodes were placed on their shaved knee joint, but did not receive TENS treatment. Importantly, three rats always were anesthetized with same vaporizer; at least one rat receiving the sham TENS treatment and one rat receiving the active TENS treatment were anesthetized at the same time. This procedure ensured that there always were animals in the sham TENS treatment groups that received the same dose of anesthesia as the active TENS groups.
Experimental design
Baseline paw and knee joint withdrawal thresholds were measured bilaterally prior to the induction of the knee joint inflammation. Twenty-four hours after induction of knee joint inflammation, paw and knee joint withdrawal thresholds were reassessed and then, the animals were microinjected with either CoCl2 or saline. An hour following the microinjection, the rats were lightly anesthetized with 1–2% isoflurane for placement of the electrodes. TENS was then applied for 20 min.
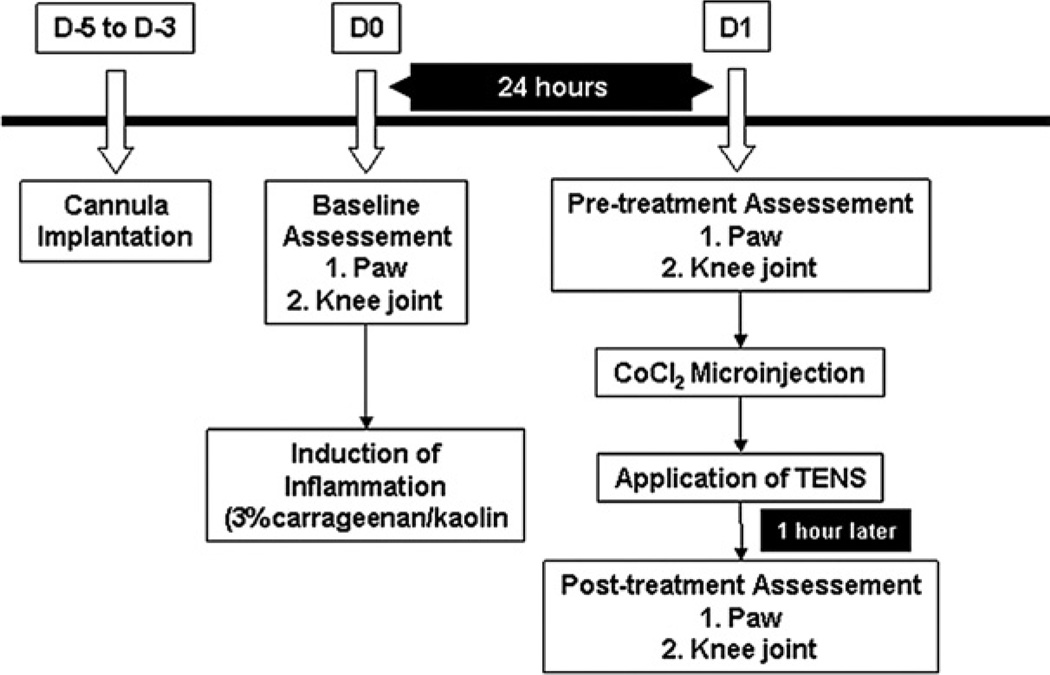
Following baseline and post-inflammation (24 h) withdrawal thresholds, rats (n=64) were randomly divided into 12 groups, six for vlPAG: (1) Sham TENS+vehicle (n=6); (2) Sham TENS+CoCl2 (n=6); (3) High TENS+vehicle (n=6); (4) High TENS+CoCl2 (n=6); (5) Low TENS+vehicle (n=6); (6) Low TENS+CoCl2 (n=6); and six for dlPAG: (7) Sham TENS+vehicle (n=5); (8) Sham TENS+CoCl2 (n=4); (9) High TENS+vehicle (n=6); (10) High TENS+CoCl2 (n=5); (11) Low TENS+vehicle (n=4); (12) Low TENS+CoCl2 (n=4). One hour after application of TENS, animals were retested for withdrawal thresholds. Importantly, all behavioral tests were done by the same experimenter who was blinded to the drug injection and to the TENS group. All experimental design is shown in Fig. 1.
Fig. 1.
Time line for the experiment.
Statistical analysis
Since the data for mechanical withdrawal thresholds of the paw were not evenly distributed, and were on a discontinuous logarithmic scale, non-parametric analysis with the Kruskal–Wallis test examined differences between groups. Joint withdrawal threshold data were evenly distributed and on a continuous scale and were thus examined for differences with a repeated measures ANOVA for differences across time and between groups. Post hoc testing between individual groups was performed with a Tukey’s test (parametric) or signed rank test (nonparametric) as appropriate. Parametric t-test and non-parametric Wilcoxon matched pairs test were used to analyze changes in the paw and joint withdrawal thresholds within groups, respectively. P value <0.05 was considered significant.
RESULTS
Effects of CoCl2 microinjection into the vlPAG on withdrawal thresholds
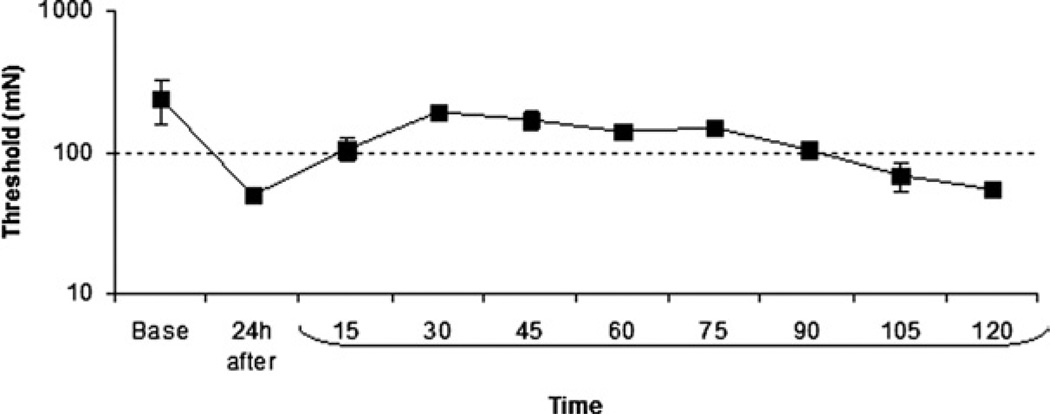
Joint inflammation significantly decreased the withdrawal thresholds of the paw 24 h after injection of kaolin and carrageenan (Fig. 2). In preliminary experiments (n=6), microinjection of 5 mM CoCl2 into the vlPAG ipsilaterally 24 h after induction of inflammation significantly increased the withdrawal threshold to mechanical stimulation of the paw (Fig. 2). The effect of CoCl2 peaked 30 min after microinjection and lasted through 90 min. Withdrawal thresholds returned to pre-CoCl2 values 2 h after microinjection of CoCl2 (Fig. 2). Thus, we applied TENS for 20 min beginning 1 h after CoCl2 to have an adequate block of synaptic transmission during TENS, and to test behavioral responses 1 h after the end of the TENS treatment so that the effects of CoCl2 were no longer present. The analgesia produced by TENS would still be present at this time as the effects of TENS last a minimum of 12 h (Sluka et al., 1998).
Fig. 2.
Microinjection of cobalt chloride (CoCl2) into the vlPAG 24 h after induction of inflammation increased the mechanical withdrawal threshold of the paw. The effect of CoCl2 peaked 30 min after microinjection and lasted through 90 min.
Distribution of microinjection sites in the vlPAG and dlPAG
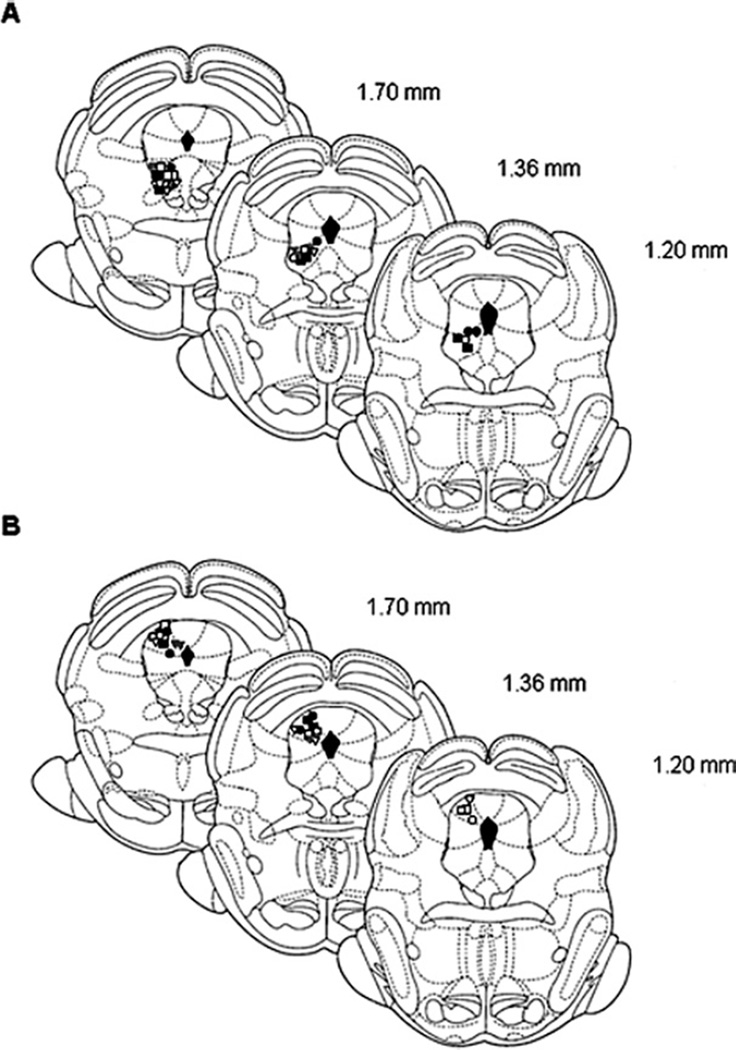
Histological analysis showed that microinjection sites were distributed in the vlPAG or the dlPAG. Fig. 3A displays the injection sites in all groups in the vlPAG, and Fig. 3B shows sites in the dlPAG. Sites outside the vlPAG or dlPAG including the lateral PAG (n=2), superior colliculus (n=6) and aqueduct (n=1) were removed from analysis. The sites plotted show the area of maximum concentration of the dye.
Fig. 3.
Schematic coronal sections of the rat brain adapted from Paxinos and Watson (2005) illustrating approximate sites of microinjections into the vlPAG (A) and dlPAG (B). Numbers indicate that distance from the interaural in millimeters. Only rats with injection sites in or immediately adjacent to the vlPAG or dlPAG were included in data analysis. Symbols represent the microinjection sites; filled symbols indicate animals microinjected with CoCl2 and open symbols, vehicle. Animals were stimulated with high frequency TENS (squares), low frequency TENS (circles) or sham TENS (triangles).
Effects of the blockade of the vlPAG and dlPAG on TENS antinociception
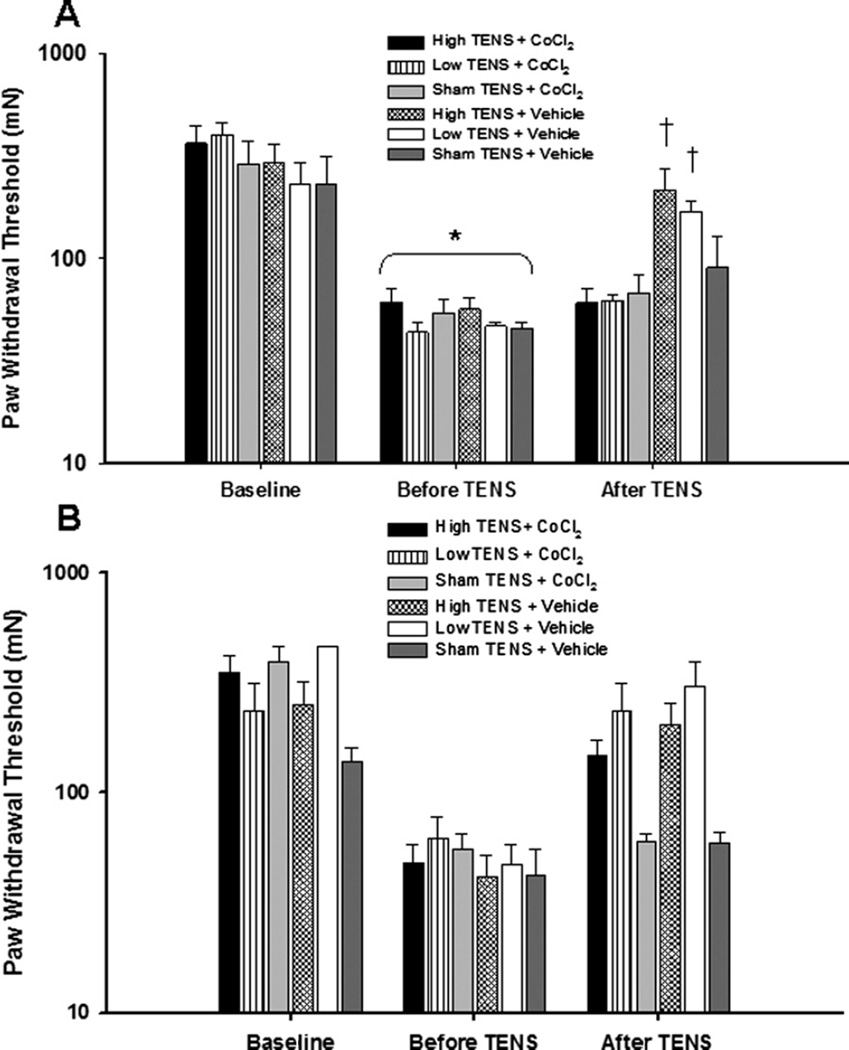
There were no significant differences between groups for mechanical withdrawal threshold of the paw or knee joint either before or 24 h after induction of inflammation. Twentyfour hours after the induction of inflammation there was a significant decrease in both paw and knee joint withdrawal thresholds (P<0.01; Figs. 4A and 5A).
Fig. 4.
Bar graph representing mechanical withdrawal threshold of the paw from animals microinjected with either CoCl2 or vehicle into the (A) vlPAG and (B) dlPAG. Mechanical withdrawal thresholds are illustrated prior to induction of inflammation (Baseline), before application of TENS, and after microinjection of the vlPAG or dlPAG. Data are represented as mean±SEM. P value 0.05 was considered statistically significant. * Significantly different from baseline time; † significantly different from vehicle control groups.
Fig. 5.
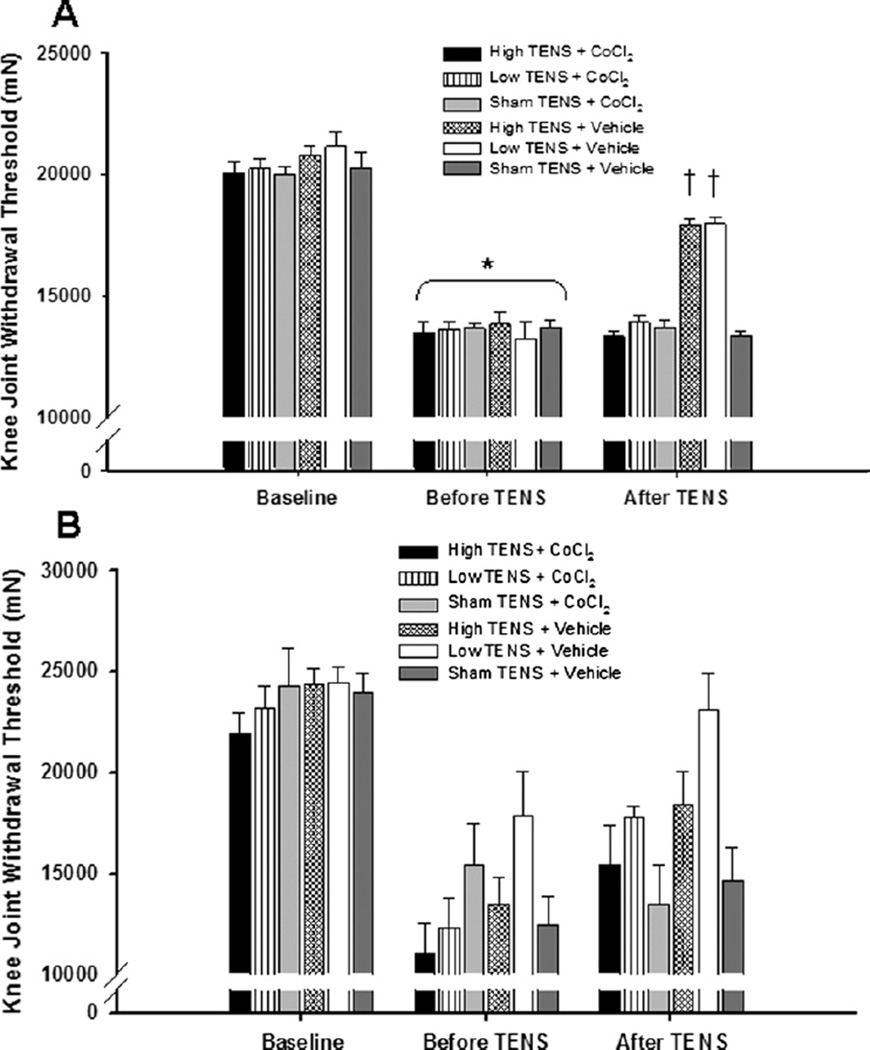
Bar graph representing mechanical withdrawal threshold of the knee from animals microinjected with either CoCl2 or vehicle into the (A) vlPAG or (B) dlPAG. Mechanical withdrawal thresholds are illustrated prior to induction of inflammation (Baseline), before application of TENS, and after microinjection of the vlPAG or dlPAG. Data are represented as mean±SEM. P value <0.05 was considered statistically significant. * Significantly different from baseline time; † significantly different from vehicle control groups.
In the group of rats microinjected with saline, either high or low frequency TENS significantly reversed the primary (knee joint) and secondary (paw) hyperalgesia when compared to the withdrawal thresholds prior to TENS treatment (P<0.001) or with sham TENS treatment (P<0.001; Fig. 4A). However, microinjection of CoCl2 into the vlPAG prior to application of either high or low frequency TENS prevented the increases in withdrawal thresholds normally observed by TENS. Withdrawal threshold of the paw and knee joint was significantly lower in the groups treated with TENS and CoCl2 into the vlPAG when compared to the group treated with TENS and vehicle; and was not significantly different from sham TENS groups or from the pre-TENS withdrawal thresholds (Fig. 5A). However, microinjection of CoCl2 into the dlPAG prior to application of either high or low frequency TENS had no effect on the antihyperalgesia produced by TENS. All the TENS groups after treatment were different from sham after treatment with TENS for both the muscle and paw withdrawal threshold. However no difference between cobalt and vehicle for each frequency was observed for the muscle (145±18% HF+CoCl2 vs. 143±18%, HF+Vehicle; 152±20% LF+CoCl2 vs. 132±8%, LF+Vehicle), or the paw (355±66% HF+CoCl2 vs. 606±166%, HF+Vehicle; 439±112% LF+CoCl2 vs. 682±143% LF+Vehicle) (Figs. 4B and 5B).
DISCUSSION
In the current study, we injected CoCl2 into the PAG to investigate if the PAG was involved in the antinociceptive pathway activated by stimulation with TENS. Our data demonstrated a complete blockade of the effects of high and low frequency TENS following microinjection of CoCl2 into the vlPAG, but not the dlPAG. These data are in agreement with prior data from our laboratory showing that TENS produces analgesia through activation of opioid receptors in the RVM (Kalra et al., 2001).
Opioid analgesia in the PAG
There is a growing literature indicating distinct dorsolateral and ventral antinociceptive systems within the PAG (Behbehani, 1995; Cannon et al., 1982; Morgan, 1991; Morgan et al., 1989). Numerous studies show that morphine microinjection into the ventral PAG produces analgesia (Jacquet and Lajtha, 1976; Yaksh et al., 1976; Lewis and Gebhart, 1977; Jensen and Yaksh, 1986; Siuciak and Advokat, 1987; Behbehani, 1995). The behavioral antinociception produced by microinjection of morphine into the vlPAG, but not the dlPAG, decreases with repeated administration (Jacquet and Lajtha, 1976; Lewis and Gebhart, 1977; Siuciak and Advokat, 1987; Tortorici et al., 1999, 2001; Morgan et al., 2005a,b), a phenomenon known as opioid tolerance. This opioid tolerance is restricted to the vlPAG, and does not occur with administration in the dlPAG (Tortorici et al., 1999). As with morphine, repeated application of TENS results in analgesic tolerance by the fourth day with a cross-tolerance at opioid receptors in the spinal cord (Chandran and Sluka, 2003) further supporting a role for the opioid-analgesic system in TENS analgesia.
Similar to morphine, microinjection of δ-opioid receptor agonists produces antinociception as measured by the hot plate and tail-flick test (Jensen and Yaksh, 1986). Moreover, microinjection of a δ-opioid receptor antagonist into the vlPAG prevented the antinociception produced by electrical stimulation of the amygdala further supporting a role for δ-opioid receptors in the PAG (Tershner and Helmstetter, 2000). These data suggest that activation of the ventral PAG produces an opioid-mediated analgesia that utilizes μ- and δ-opioid receptors.
The PAG does not have a major projection to the spinal cord (Basbaum and Fields, 1984), but produces its effects through a relay in the RVM, i.e. the nucleus raphe magnus (NRM) and adjacent structures (Kuypers and Maisky, 1975; Castiglioni et al., 1978; Mantyh and Peschanski, 1982; Urban and Smith, 1994). The morphine-induced analgesia from the PAG produces antinociception by activation of both μ- and δ-opioid receptors in the RVM (Kiefel et al., 1993; Urban and Smith, 1994). The RVM in turn projects to the spinal cord and reduces activity of nociceptive dorsal horn neurons, to result in an analgesic effect (Zhuo and Gebhart, 1997; Venegas and Schaible, 2004). The antinociception produced by microinjection of morphine in the vlPAG is prevented by blockade of NMDA, and both μ- and δ-opioid receptors in the RVM (Kiefel et al., 1993; Spinella et al., 1996). These effects of activation of the vlPAG inhibitory pathway by morphine modulate neuronal activity in the RVM such that there was an increase in off-cell activity and a decrease in on-cell activity (Cheng et al., 1986). Thus, the PAG projects through the RVM to produce inhibition. The RVM, then projects to the spinal cord using serotoninergic and non-serotoninergic cells to inhibit dorsal horn neurons (Zhuo and Gebhart, 1991; Venegas and Schable, 2004).
The PAG and RVM work synergistically to produce analgesia. Coadministration of μ-opioid agonists into the RVM and the PAG results in a profound synergistic interaction (Rossi et al., 1994). Coadministration of DAMGO into one region with deltorphin in the other also results in a significant synergy, whereas, if DAMGO and deltorphin are coadministered together in the same brain area there is an additive effect. These findings suggest the existence of mu/mu and mu/delta synergy between the PAG and RVM (Rossi et al., 1994).
We therefore hypothesize that TENS utilizes the opioid analgesia system originating in the vlPAG which projects through the RVM to the spinal cord. Both high and low frequency TENS produce their analgesia effects by activation of μ- and δ-opioid receptors in the RVM and the spinal cord (Sluka et al., 1999; Kalra et al., 2001). Further, low frequency TENS releases 5-HT in the spinal cord and activates serotoninergic receptors, 5-HT2 and 5-HT3 (Radhakrishnan et al., 2003; Sluka et al., 2006). High frequency TENS does not utilize 5-HT but releases GABA that activates GABAA receptors in the spinal cord (Radhakrishnan et al., 2003; Maeda et al., 2007). Both high and low frequency TENS reduce dorsal horn neuron sensitization after inflammation (Ma and Sluka, 2001), and the consequent hyperalgesia (Sluka et al., 1999). Thus, TENS, both high and low frequency requires activation of neurons in the vlPAG, the RVM, and spinal cord, and utilizes opioid mechanisms to produce analgesia.
Although the PAG and the RVM are clearly involved in the analgesia produced by both low and high frequency TENS, other mechanisms including peripheral, segmental spinal analgesia, or systemic effects are also possible. Indeed prior studies show that effects of low and/or high frequency TENS can be prevented by blockade of opioid or α-2 noradrenergic receptors at the site of stimulation (King et al., 2005; Resende et al., 2004). Spinally, there is activation and release of GABA, activation of muscarinic receptors, and activation of opioid receptors, all of which could be related to either spinal segmental inhibition or descending inhibitory pathways (Maeda et al., 2007; Radharkshinan and Sluka, 2003; Sluka et al., 1999). Thus, multiple mechanisms acting in concert or independently could be responsible for the analgesia produced by TENS.
Facilitation from the PAG
The current study showed that microinjection of CoCl2 into the vlPAG reversed the hyperalgesia induced by knee joint inflammation, suggesting a role for the PAG in facilitating nociception after injury. Recent studies support a role for the PAG in facilitation of nociception (Heinricher et al., 2004; Guo et al., 2006). This facilitatory effect from the PAG involves prostaglandin E2 and BDNF (Heinricher et al., 2004; Guo et al., 2006). Microinjection of prostaglandin E2 decreases the paw withdrawal latency to heat, and activates facilitatory cells in the RVM, i.e. on-cells (Heinricher et al., 2004). Further there are increases in BDNF in the PAG, the BDNF receptor TrkB in the RVM after inflammation, and blockade of TrkB receptors in the RVM reverses hyperalgesia (Guo et al., 2006). There is substantial evidence that the RVM mediates facilitation of nociception after injury in numerous animal models including joint inflammation (Urban et al., 1996), pancreatitis (Vera-Portocarrero et al., 2006), neuropathic pain (Burgess et al., 2002), visceral pain (Zhuo and Gebhart, 2002), and inflammatory pain (Guan et al., 2004; Sugiyo et al., 2005). Thus, there is emerging evidence that the vlPAG facilitates nociception, and this facilitation is mediated through the RVM.
Clinical significance
The use of TENS can therefore be thought of as a non-pharmacological tool to engage our endogenous analgesic system. It utilizes endogenous opioids acting on their receptors to produce analgesia without side effects normally observed with exogenous opioids. Early clinical studies show that low frequency TENS utilizes opioid receptors to produce analgesia (Sjolund and Eriksonn, 1979). Further high frequency TENS increases the concentration of β-endorphins increase in the bloodstream and cerebrospinal fluid, and methionine–enkephalin in the cerebrospinal fluid, in human subjects (Han et al., 1991; Salar et al., 1981). Together, these clinical studies in human subjects, along with animals studies on mechanisms support a role for activation of opioid-mediated analgesia utilizing the PAG–RVM pathway for both high and low frequency TENS. Since TENS utilizes known pharmacological pathways, particularly opioids, TENS should be administered with similar principles. Clinicians should be aware of the potential for the development of tolerance, as well as potential interactions of TENS with the patient’s pharmacological therapy. For example, if subjects have been taking opioids long enough to develop tolerance then low frequency TENS, which utilizes μ-opioid receptors, should be avoided. Preclinical studies in rats support this since low frequency TENS is ineffective in rats that were made previously tolerant to opioids (Sluka et al., 2000). Further, combining pharmacological agents with TENS could enhance the effectiveness of the treatment, and reduce side effects of the drug. For example combining morphine or clonidine with TENS enhances the analgesic effect so that a lower dose of the drug produces a similar degree of analgesia (Sluka, 2000; Sluka and Chandran, 2002). Thus, future studies should examine the effects of combining common pharmaceutical agents for treatment of pain with TENS in both animal and human subjects, and should be aimed at developing mechanisms to prevent tolerance (Hingne and Sluka, 2008; DeSantana et al., 2008).
Acknowledgment
This study is supported by a competitive grant from EMPI, Inc. and National Institutes of Health AR052316. No commercial party having a direct financial interest in the results of the research supporting this article has or will confer a benefit upon the authors or upon any organization with which the authors are associated.
Abbreviations
- dlPAG
dorsolateral periaqueductal grey
- PAG
periaqueductal grey
- RVM
rostroventral medial medulla
- TENS
transcutaneous electric nerve stimulation
- vlPAG
ventrolateral periaqueductal grey
REFERENCES
- Bagley EE, Chieng BC, Christie MJ, Connor M. Opioid tolerance in periaqueductal gray neurons isolated from mice chronically treated with morphine. Br J Pharmacol. 2005;146(1):68–76. doi: 10.1038/sj.bjp.0706315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basbaum AI, Fields HL. Endogenous pain control systems: brainstem spinal pathways and endorphin circuitry. Annu Rev Neurosci. 1984;7:309–338. doi: 10.1146/annurev.ne.07.030184.001521. [DOI] [PubMed] [Google Scholar]
- Behbehani MM. Functional characteristics of the midbrain periaqueductal gray. Prog Neurobiol. 1995;46:575–605. doi: 10.1016/0301-0082(95)00009-k. [DOI] [PubMed] [Google Scholar]
- Burgess SE, Gardell LR, Ossipov MH, Malan TP, Jr, Vanderah TW, Lai J, Porreca F. Time-dependent descending facilitation from the rostral ventromedial medulla maintains, but does not initiate, neuropathic pain. J Neurosci. 2002;22(12):5129–5136. doi: 10.1523/JNEUROSCI.22-12-05129.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon JT, Prieto GJ, Lee A, Liebeskind JC. Evidence for opioid and non-opioid forms of stimulation-produced analgesia in the rat. Brain Res. 1982;243:315–321. doi: 10.1016/0006-8993(82)90255-4. [DOI] [PubMed] [Google Scholar]
- Castiglioni AJ, Gallaway MC, Coulter JD. Spinal projections from the midbrain in monkey. J Comp Neurol. 1978;178:329–346. doi: 10.1002/cne.901780208. [DOI] [PubMed] [Google Scholar]
- Cavun S, Goktalay G, Millington WR. The hypotension evoked by visceral nociception is mediated by delta opioid receptors in the periaqueductal gray. Brain Res. 2004;1019(1–2):237–245. doi: 10.1016/j.brainres.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Chandran P, Sluka KA. Development of opioid tolerance with repeated transcutaneous electrical nerve stimulation administration. Pain. 2003;102(1–2):195–201. doi: 10.1016/s0304-3959(02)00381-0. [DOI] [PubMed] [Google Scholar]
- Cheng ZF, Fields HL, Heinricher MM. Morphine microinjected into the periaqueductal gray has differential effects on 3 classes of medullary neurons. Brain Res. 1986;375(1):57–65. doi: 10.1016/0006-8993(86)90958-3. [DOI] [PubMed] [Google Scholar]
- DeSantana JM, Santana-Filho VJ, Sluka KA. Modulation between high- and low-frequency transcutaneous electric nerve stimulation delays the development of analgesic tolerance in arthritic rats. Arch Phys Med Rehabil. 2008;89(4):754–760. doi: 10.1016/j.apmr.2007.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields HL, Heinricher MM, Mason P. Neurotransmitters in nociceptive modulatory circuits. Annu Rev Neurosci. 1991;14:219–245. doi: 10.1146/annurev.ne.14.030191.001251. [DOI] [PubMed] [Google Scholar]
- Fisk GD, Wyss JM. Descending projections of infralimbic cortex that mediate stimulation-evoked changes in arterial pressure. Brain Res. 2000;859(1):83–95. doi: 10.1016/s0006-8993(00)01935-1. [DOI] [PubMed] [Google Scholar]
- Gebhart GF, Jones SL, Besson JM. Effects of morphine given in the brain stem on the activity of dorsal horn nociceptive neurons. In: Fields HL, editor. Progress in brain research: pain modulation. Amsterdam: Elsevier; 1988. pp. 229–243. [DOI] [PubMed] [Google Scholar]
- Gopalkrishnan P, Sluka KA. Effect of varying frequency, intensity, and pulse duration of transcutaneous electrical nerve stimulation on primary hyperalgesia in inflamed rats. Arch Phys Med Rehabil. 2000;81(7):984–990. doi: 10.1053/apmr.2000.5576. [DOI] [PubMed] [Google Scholar]
- Guan Y, Guo W, Robbins MT, Dubner R, Ren K. Changes in AMPA receptor phosphorylation in the rostral ventromedial medulla after inflammatory hyperalgesia in rats. Neurosci Lett. 2004;366(2):201–205. doi: 10.1016/j.neulet.2004.05.051. [DOI] [PubMed] [Google Scholar]
- Guo W, Robbins MT, Wei F, Zou S, Dubner R, Ren K. Supraspinal brain-derived neurotrophic factor signaling: a novel mechanism for descending pain facilitation. J Neurosci. 2006;26(1):126–137. doi: 10.1523/JNEUROSCI.3686-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S, Byerly L. Calcium channel. Annu Rev Neurosci. 1981;4:69–125. doi: 10.1146/annurev.ne.04.030181.000441. [DOI] [PubMed] [Google Scholar]
- Han JS, Chen XH, Sun SL, Xu XJ, Yuan Y, Yan SC, Hao JX, Terenius L. Effect of low and high frequency TENS on met-enkephalin-arg-phe and dynorphin a immunoreactivity in human lumbar CSF. Pain. 1991;47:295–298. doi: 10.1016/0304-3959(91)90218-M. [DOI] [PubMed] [Google Scholar]
- Heinricher MM, Martenson ME, Neubert MJ. Prostaglandin E2 in the midbrain periaqueductal gray produces hyperalgesia and activates pain-modulating circuitry in the rostral ventromedial medulla. Pain. 2004;110(1–2):419–426. doi: 10.1016/j.pain.2004.04.026. [DOI] [PubMed] [Google Scholar]
- Hingne PM, Sluka KA. Blockade of NMDA receptors prevents analgesic tolerance to repeated transcutaneous electrical nerve stimulation (TENS) in rats. J Pain. 2008;9(3):217–225. doi: 10.1016/j.jpain.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosobuchi Y, Adams JE, Linchitz R. Pain relief by electrical stimulation of the central gray matter in humans and its reversal by naloxone. Science. 1977;197:183–186. doi: 10.1126/science.301658. [DOI] [PubMed] [Google Scholar]
- Jacquet YF, Lajtha A. The periaqueductal gray: site of morphine analgesia and tolerance as shown by 2-way cross tolerance between systemic and intracerebral injections. Brain Res. 1976;103:501–513. doi: 10.1016/0006-8993(76)90448-0. [DOI] [PubMed] [Google Scholar]
- Jensen TS, Yaksh TL. Comparison of the antinociceptive effect of morphine and glutamate at coincidental sites in the periaqueductal gray and medial medulla in rats. Brain Res. 1989;476(1):1–9. doi: 10.1016/0006-8993(89)91529-1. [DOI] [PubMed] [Google Scholar]
- Jensen TS, Yaksh TL. Comparison of the antinociceptive action of mu and delta opioid receptor ligands in the periaqueductal gray matter, medial and paramedial ventral medulla in the rats as studied by the microinjection technique. Brain Res. 1986;372:301–312. doi: 10.1016/0006-8993(86)91138-8. [DOI] [PubMed] [Google Scholar]
- Kalra A, Urban MO, Sluka KA. Blockade of opioid receptors in rostral ventral medulla prevents antihyperalgesia produced by transcutaneous electrical nerve stimulation (TENS) J Pharmacol Exp Ther. 2001;298(1):257–263. [PubMed] [Google Scholar]
- Kiefel JM, Rossi GC, Bodnar RJ. Medullary mu and delta opioid receptors modulate mesencephalic morphine analgesia in rats. Brain Res. 1993;624(1–2):151–161. doi: 10.1016/0006-8993(93)90073-v. [DOI] [PubMed] [Google Scholar]
- King EW, Audette K, Athman GA, Nguyen HO, Sluka KA, Fairbanks CA. Transcutaneous electrical nerve stimulation activates peripherally located alpha-2A adrenergic receptors. Pain. 2005;115(3):364–373. doi: 10.1016/j.pain.2005.03.027. [DOI] [PubMed] [Google Scholar]
- Kretz R. Local cobalt injection: a method to discriminate presynaptic axonal from postsynaptic neuronal activity. J Neurosci Methods. 1984;11(2):129–135. doi: 10.1016/0165-0270(84)90030-x. [DOI] [PubMed] [Google Scholar]
- Krzanowska EK, Bodnar RJ. Morphine antinociception elicited from the ventrolateral periaqueductal gray is sensitive to sex and gonadectomy differences in rats. Brain Res. 1999;821:224–230. doi: 10.1016/s0006-8993(98)01364-x. [DOI] [PubMed] [Google Scholar]
- Kuypers HGJM, Maisky VA. Retrograde axonal transport of horseradish peroxidase from spinal cord to brainstem cell groups in cats. Neurosci Lett. 1975;1:9–14. doi: 10.1016/0304-3940(75)90004-x. [DOI] [PubMed] [Google Scholar]
- Lewis VA, Gebhart GF. Morphine-induced and stimulation-produced analgesias at coincident periaqueductal central gray loci: evaluation of analgesic congruence, tolerance, and cross-tolerance. Exp Neurol. 1977;57:934–955. doi: 10.1016/0014-4886(77)90119-4. [DOI] [PubMed] [Google Scholar]
- Ma YT, Sluka KA. Reduction in inflammation-induced sensitization of dorsal horn neurons by transcutaneous electrical nerve stimulation in anesthetized rats. Exp Brain Res. 2001;137(1):94–102. doi: 10.1007/s002210000629. [DOI] [PubMed] [Google Scholar]
- Maeda Y, Lisi TL, Vance CG, Sluka KA. Release of GABA and activation of GABA(A) in the spinal cord mediates the effects of TENS in rats. Brain Res. 2007;1136(1):43–50. doi: 10.1016/j.brainres.2006.11.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantyh PW, Peschanski M. Spinal projections from the periaqueductal grey and dorsal raphe in the rat, cat and monkey. Neuroscience. 1982;7(11):2769–2776. doi: 10.1016/0306-4522(82)90099-9. [DOI] [PubMed] [Google Scholar]
- Morgan MM, Clayton CC, Boyer-Quick JS. Differential susceptibility of the PAG and RVM to tolerance to the antinociceptive effect of morphine in the rat. Pain. 2005a;113:91–98. doi: 10.1016/j.pain.2004.09.039. [DOI] [PubMed] [Google Scholar]
- Morgan MM, Depaulis A, Liebeskind JC. Diazepam dissociates the analgesic and aversive effects of periaqueductal gray stimulation in the rats. Brain Res. 1987;423(1–2):395–398. doi: 10.1016/0006-8993(87)90870-5. [DOI] [PubMed] [Google Scholar]
- Morgan MM, Tierney BW, Ingram SL. Intermittent dosing prolongs tolerance to the antinociceptive effect of morphine microinjection into the periaqueductal gray. Brain Res. 2005b;1059:173–178. doi: 10.1016/j.brainres.2005.08.024. [DOI] [PubMed] [Google Scholar]
- Morgan MM. Differences in antinociception evoked from dorsal and ventral regions of the caudal periaqueductal gray matter. In: DePaulis A, Bandler R, editors. The midbrain periaqueductal gray mattered. New York: Plenum; 1991. pp. 139–150. [Google Scholar]
- Morgan MM, Whitney PK. Immobility accompanies the antinociception mediated by the rostral ventromedial medulla of the rat. Brain Res. 2000;872(1–2):276–281. doi: 10.1016/s0006-8993(00)02502-6. [DOI] [PubMed] [Google Scholar]
- Morgan MM, Whitney PK, Gold MS. Immobility and flight associated with antinociception produced by activation of the ventral and lateral/dorsal regions of the rat periaqueductal gray. Brain Res. 1998;804:159–166. doi: 10.1016/s0006-8993(98)00669-6. [DOI] [PubMed] [Google Scholar]
- Morgan MM, Sohn JH, Liebeskind JC. Stimulation of the periaqueductal gray matter inhibits nociception at the supraspinal as well as spinal level. Brain Res. 1989;502:61–66. doi: 10.1016/0006-8993(89)90461-7. [DOI] [PubMed] [Google Scholar]
- Nuseir K, Heidenreich BA, Proudfit HK. The antinociception produced by microinjection of a cholinergic agonist in the ventromedial medulla is mediated by noradrenergic neurons in the A7 catecholamine cell group. Brain Res. 1999;822:1–7. doi: 10.1016/s0006-8993(98)01195-0. [DOI] [PubMed] [Google Scholar]
- Oliveras JL, Besson JM, Guilbaud G, Liebeskind JC. Behavioral and electrophysiological evidence of pain inhibition from midbrain stimulation in the cat. Exp Brain Res. 1974;20:32–44. doi: 10.1007/BF00239016. [DOI] [PubMed] [Google Scholar]
- Osborne PB, Vaughan CW, Wilson HI, Christie MJ. Opioid inhibition of rat periaqueductal grey neurones with identified projections to rostral ventromedial medulla in vitro. J Physiol. 1996;490(2):383–389. doi: 10.1113/jphysiol.1996.sp021152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pajolla GP, Tavares RF, Pelosi GG, Corrêa FM. Involvement of the periaqueductal gray in the hypotensive response evoked by L-glutamate microinjection in the lateral hypothalamus of unanesthetized rats. Auton Neurosci. 2005;122(1–2):84–93. doi: 10.1016/j.autneu.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson SJ. The rat brain, in stereotaxic coordinates. San Diego: Elsevier Academic Press; 2005. [Google Scholar]
- Prieto GJ, Cannon JT, Liebeskind JC. N. raphe magnus lesions disrupt stimulation-produced analgesia from ventral but not dorsal midbrain areas in the rat. Brain Res. 1983;261:53–57. doi: 10.1016/0006-8993(83)91282-9. [DOI] [PubMed] [Google Scholar]
- Radhakrishnan R, Moore SA, Sluka KA. Unilateral carrageenan injection into muscle or joint induces chronic bilateral hyperalgesia in rats. Pain. 2003;104(3):567–577. doi: 10.1016/s0304-3959(03)00114-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radhakrishinan R, Sluka KA. Spinal muscarinic receptors are activated during low and high frequency TENS-induced antihyperalgesia in rats. Neuropharmacology. 2003;45:1111–1119. doi: 10.1016/s0028-3908(03)00280-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichling DB, Basbaum AI. Collateralization of periaqueductal gray neurons to forebrain or diencephalon and to the medullary nucleus raphe magnus in the rat. Neuroscience. 1991;42(1):183–200. doi: 10.1016/0306-4522(91)90158-k. [DOI] [PubMed] [Google Scholar]
- Resende MA, Sabino GG, Cândido CR, Pereira LS, Francischi JN. Local transcutaneoous electrical stimulation (TENS) effects in experimental inflammatory edema and pain. Eur J Pharmacol. 2004;504(3):217–222. doi: 10.1016/j.ejphar.2004.09.055. [DOI] [PubMed] [Google Scholar]
- Rossi GC, Pasternak GW, Bodnar RJ. Mu and delta opioid synergy between the periaqueductal gray and the rostro-ventral medulla. Brain Res. 1994;665:85–93. doi: 10.1016/0006-8993(94)91155-x. [DOI] [PubMed] [Google Scholar]
- Salar G, Job I, Mingrino S, Bosio A, Trabucchi M. Effect of transcutaneous electrotherapy on CSF β-endorphin content in patients without pain problems. Pain. 1981;10:169–172. doi: 10.1016/0304-3959(81)90192-5. [DOI] [PubMed] [Google Scholar]
- Sandkuhler J, Gebhart GF. Relative contributions of the nucleus raphe magnus and adjacent medullary reticular formation to the inhibition by stimulation in the periaqueductal gray of a spinal nociceptive reflex in the pentobarbital-anesthetized rat. Brain Res. 1984;305:77–87. doi: 10.1016/0006-8993(84)91121-1. [DOI] [PubMed] [Google Scholar]
- Siuciak JA, Advokat C. Tolerance to morphine microinjections in the periaqueductal gray (PAG) induces tolerance to systemic, but not intrathecal morphine. Brain Res. 1987;424:311–319. doi: 10.1016/0006-8993(87)91476-4. [DOI] [PubMed] [Google Scholar]
- Sjolund BH, Eriksson MB. The influence of naloxone on analgesia produced by peripheral conditioning stimulation. Brain Res. 1979;173(2):295–301. doi: 10.1016/0006-8993(79)90629-2. [DOI] [PubMed] [Google Scholar]
- Sluka KA, Deacon M, Stibal A, Strissel S, Terpstra A. Spinal blockade of opioid receptors prevents the analgesia produced by TENS in arthritic rats. J Pharmacol Exp Ther. 1999;289(2):840–846. [PubMed] [Google Scholar]
- Sluka KA, Lisi TL, Westlund KN. Increased release of serotonin in the spinal cord during low, but not high, frequency transcutaneous electric nerve stimulation in rats with joint inflammation. Arch Phys Med Rehabil. 2006;87(8):1137–1140. doi: 10.1016/j.apmr.2006.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sluka KA, Bailey K, Bogush J, Olson R, Ricketts A. Treatment with either high or low frequency TENS reduces the secondary hyperalgesia observed after injection of kaolin and carrageenan into the knee joint. Pain. 1998;77(1):97–102. doi: 10.1016/S0304-3959(98)00090-6. [DOI] [PubMed] [Google Scholar]
- Sluka KA, Judge MA, McColley MM, Reveiz PM, Taylor BM. Low frequency TENS is less effective than high frequency TENS at reducing inflammation-induced hyperalgesia in morphine-tolerant rats. Eur J Pain. 2000;4(2):185–193. doi: 10.1053/eujp.2000.0172. [DOI] [PubMed] [Google Scholar]
- Sluka KA, Chandran P. Enhanced reduction in hyperalgesia by combined administration of clonidine and TENS. Pain. 2002;100(1–2):183–190. doi: 10.1016/s0304-3959(02)00294-4. [DOI] [PubMed] [Google Scholar]
- Sluka KA. Systemic morphine in combination with TENS produces an increased antihyperalgesia in rats with acute inflammation. J Pain. 2000;1(3):204–211. doi: 10.1054/jpai.2000.7149. [DOI] [PubMed] [Google Scholar]
- Sluka KA, Westlund KN. Behavioral and immunohistochemical changes in an experimental arthritis model in rats. Pain. 1993;55(3):367–377. doi: 10.1016/0304-3959(93)90013-F. [DOI] [PubMed] [Google Scholar]
- Spinella M, Cooper ML, Bodnar RJ. Excitatory amino acid antagonists in the rostral ventromedial medulla inhibit mesencephalic morphine analgesia in rats. Pain. 1996;64(3):545–552. doi: 10.1016/0304-3959(95)00192-1. [DOI] [PubMed] [Google Scholar]
- Sugiyo S, Takemura M, Dubner R, Ren K. Trigeminal transition zone/rostral ventromedial medulla connections and facilitation of orofacial hyperalgesia after masseter inflammation in rats. J Comp Neurol. 2005;493(4):510–523. doi: 10.1002/cne.20797. [DOI] [PubMed] [Google Scholar]
- Tershner SA, Helmstetter FJ. Antinociception produced by mu opioid receptor activation in the amygdala is partly dependent on activation of mu opioid and neurotensin receptors in the ventral periaqueductal gray. Brain Res. 2000;865(1):17–26. doi: 10.1016/s0006-8993(00)02179-x. [DOI] [PubMed] [Google Scholar]
- Tershner SA, Mitchell JM, Fields HL. Brainstem pain modulating circuitry is sexually dimorphic with respect to mu and kappa opioid receptor function. Pain. 2000;85:153–159. doi: 10.1016/s0304-3959(99)00257-2. [DOI] [PubMed] [Google Scholar]
- Tortorici V, Morgan MM, Vanegas H. Tolerance to repeated microinjection of morphine into the periaqueductal gray is associated with changes in the behavior of off- and on-cells in the rostral ventromedial medulla of rats. Pain. 2001;89:237–244. doi: 10.1016/s0304-3959(00)00367-5. [DOI] [PubMed] [Google Scholar]
- Tortorici VT, Robbins CS, Morgan MM. Tolerance to the antinociceptive effect of morphine microinjections into the ventral but not lateral-dorsal periaqueductal gray of the rat. Behav Neurosci. 1999;113:833–839. doi: 10.1037//0735-7044.113.4.833. [DOI] [PubMed] [Google Scholar]
- Urban MO, Smith DJ. Nuclei within the rostral ventromedial medulla mediating morphine antinociception from the periaqueductal gray. Brain Res. 1994;652(1):9–16. doi: 10.1016/0006-8993(94)90311-5. [DOI] [PubMed] [Google Scholar]
- Urban MO, Jiang MC, Gebhart GF. Participation of central descending nociceptive facilitatory systems in secondary hyperalgesia produced by mustard oil. Brain Res. 1996;737(1–2):83–91. doi: 10.1016/0006-8993(96)00631-2. [DOI] [PubMed] [Google Scholar]
- Vance CG, Radhakrishnan R, Skyba DA, Sluka KA. Transcutaneous electrical nerve stimulation at both high and low frequencies reduces primary hyperalgesia in rats with joint inflammation in a time-dependent manner. Phys Ther. 2007;87(1):44–51. doi: 10.2522/ptj.20060032. [DOI] [PubMed] [Google Scholar]
- Vaughan CW, Christie MJ. Presynaptic inhibitory action of opioids on synaptic transmission in the rat periaqueductal grey in vitro. J Physiol. 1997;498(2):463–472. doi: 10.1113/jphysiol.1997.sp021872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vera-Portocarrero LP, Xie JY, Kowal J, Ossipov MH, King T, Porreca F. Descending facilitation from the rostral ventromedial medulla maintains visceral pain in rats with experimental pancreatitis. Gastroenterology. 2006;130(7):2155–2164. doi: 10.1053/j.gastro.2006.03.025. [DOI] [PubMed] [Google Scholar]
- Yaksh TL, Yeung JC, Rudy TA. Systematic examination in the rat of brain sites sensitive to the direct application of morphine: observation of differential effects within the periaqueductal gray. Brain Res. 1976;114:83–103. doi: 10.1016/0006-8993(76)91009-x. [DOI] [PubMed] [Google Scholar]
- Zhuo M, Gebhart GF. Facilitation and attenuation of a visceral nociceptive reflex from the rostroventral medulla in the rat. Gastroenterology. 2002;122(4):1007–1019. doi: 10.1053/gast.2002.32389. [DOI] [PubMed] [Google Scholar]
- Zhuo M, Gebhart GF. Spinal serotonin receptors mediate descending facilitation of a nociceptive reflex from the nuclei reticularis gigantocellularis and gigantocellularis pars alpha in the rat. Brain Res. 1991;550(1):35–48. doi: 10.1016/0006-8993(91)90402-h. [DOI] [PubMed] [Google Scholar]
- Zhuo M, Gebhart GF. Biphasic modulation of spinal nociceptive transmission from the medullary raphe nuclei in the rat. J Neurophysiol. 1997;78(2):746–758. doi: 10.1152/jn.1997.78.2.746. [DOI] [PubMed] [Google Scholar]