Abstract
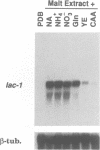
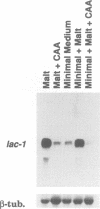
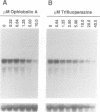
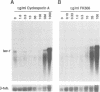
The gene lac-1, encoding the enzyme laccase, is one of several genes of the chestnut blight fungus, Cryphonectria parasitica, that are suppressed by virulence-attenuating mycoviruses of the hypovirus group. Two antagonistic regulatory pathways have been shown to govern the activity of the lac-1 promoter: a positive pathway that stimulates transcription and a negative pathway that represses transcription. We now report that these two regulatory pathways respond independently to specific changes in the nutritional environment. These newly defined conditions were used to confirm that a hypovirus suppresses the activity of the positive regulatory pathway, and to implicate calmodulin and calcineurin as components of the signal transduction cascades regulating lac-1 transcription. Significantly, lac-1 transcript accumulation was shown to be affected by amino acid availability. Further analysis revealed that transcriptional repression mediated by the negative regulatory pathway is relieved under conditions of amino acid deprivation. Thus, by blocking the positive pathway, hypovirus infection prevents increased lac-1 transcript accumulation in response to amino acid deficiency. These observations are consistent with the hypothesis that hypoviruses alter the transcriptional response of the host fungus to changes in nutrient availability.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anagnostakis S. L. Biological control of chestnut blight. Science. 1982 Jan 29;215(4532):466–471. doi: 10.1126/science.215.4532.466. [DOI] [PubMed] [Google Scholar]
- Carney D. H., Scott D. L., Gordon E. A., LaBelle E. F. Phosphoinositides in mitogenesis: neomycin inhibits thrombin-stimulated phosphoinositide turnover and initiation of cell proliferation. Cell. 1985 Sep;42(2):479–488. doi: 10.1016/0092-8674(85)90105-9. [DOI] [PubMed] [Google Scholar]
- Carpenter C. E., Mueller R. J., Kazmierczak P., Zhang L., Villalon D. K., Van Alfen N. K. Effect of a virus on accumulation of a tissue-specific cell-surface protein of the fungus Cryphonectria (Endothia) parasitica. Mol Plant Microbe Interact. 1992 Jan-Feb;5(1):55–61. doi: 10.1094/mpmi-5-055. [DOI] [PubMed] [Google Scholar]
- Chen B., Choi G. H., Nuss D. L. Attenuation of fungal virulence by synthetic infectious hypovirus transcripts. Science. 1994 Jun 17;264(5166):1762–1764. doi: 10.1126/science.8209256. [DOI] [PubMed] [Google Scholar]
- Cheung W. Y. Calmodulin: an overview. Fed Proc. 1982 May;41(7):2253–2257. [PubMed] [Google Scholar]
- Choi G. H., Larson T. G., Nuss D. L. Molecular analysis of the laccase gene from the chestnut blight fungus and selective suppression of its expression in an isogenic hypovirulent strain. Mol Plant Microbe Interact. 1992 Mar-Apr;5(2):119–128. doi: 10.1094/mpmi-5-119. [DOI] [PubMed] [Google Scholar]
- Choi G. H., Nuss D. L. A viral gene confers hypovirulence-associated traits to the chestnut blight fungus. EMBO J. 1992 Feb;11(2):473–477. doi: 10.1002/j.1460-2075.1992.tb05077.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi G. H., Nuss D. L. Hypovirulence of chestnut blight fungus conferred by an infectious viral cDNA. Science. 1992 Aug 7;257(5071):800–803. doi: 10.1126/science.1496400. [DOI] [PubMed] [Google Scholar]
- Cockcroft S., Howell T. W., Gomperts B. D. Two G-proteins act in series to control stimulus-secretion coupling in mast cells: use of neomycin to distinguish between G-proteins controlling polyphosphoinositide phosphodiesterase and exocytosis. J Cell Biol. 1987 Dec;105(6 Pt 1):2745–2750. doi: 10.1083/jcb.105.6.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen P. The structure and regulation of protein phosphatases. Annu Rev Biochem. 1989;58:453–508. doi: 10.1146/annurev.bi.58.070189.002321. [DOI] [PubMed] [Google Scholar]
- Craven M. G., Pawlyk D. M., Choi G. H., Nuss D. L. Papain-like protease p29 as a symptom determinant encoded by a hypovirulence-associated virus of the chestnut blight fungus. J Virol. 1993 Nov;67(11):6513–6521. doi: 10.1128/jvi.67.11.6513-6521.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downes C. P., Michell R. H. The polyphosphoinositide phosphodiesterase of erythrocyte membranes. Biochem J. 1981 Jul 15;198(1):133–140. doi: 10.1042/bj1980133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan W. M., Corthésy B., Bram R. J., Crabtree G. R. Nuclear association of a T-cell transcription factor blocked by FK-506 and cyclosporin A. Nature. 1991 Aug 29;352(6338):803–807. doi: 10.1038/352803a0. [DOI] [PubMed] [Google Scholar]
- Froehner S. C., Eriksson K. E. Induction of Neurospora crassa laccase with protein synthesis inhibitors. J Bacteriol. 1974 Oct;120(1):450–457. doi: 10.1128/jb.120.1.450-457.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson P. I., Schulman H. Neuronal Ca2+/calmodulin-dependent protein kinases. Annu Rev Biochem. 1992;61:559–601. doi: 10.1146/annurev.bi.61.070192.003015. [DOI] [PubMed] [Google Scholar]
- Larson T. G., Choi G. H., Nuss D. L. Regulatory pathways governing modulation of fungal gene expression by a virulence-attenuating mycovirus. EMBO J. 1992 Dec;11(12):4539–4548. doi: 10.1002/j.1460-2075.1992.tb05555.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson T. G., Nuss D. L. Cyclophilin-dependent stimulation of transcription by cyclosporin A. Proc Natl Acad Sci U S A. 1993 Jan 1;90(1):148–152. doi: 10.1073/pnas.90.1.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung P. C., Graves L. M., Tipton C. L. Characterization of the interaction of ophiobolin A and calmodulin. Int J Biochem. 1988;20(12):1351–1359. doi: 10.1016/s0020-711x(98)90003-9. [DOI] [PubMed] [Google Scholar]
- Marzluf G. A. Regulation of nitrogen metabolism and gene expression in fungi. Microbiol Rev. 1981 Sep;45(3):437–461. doi: 10.1128/mr.45.3.437-461.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthukumar G., Nickerson K. W. Ca(II)-calmodulin regulation of fungal dimorphism in Ceratocystis ulmi. J Bacteriol. 1984 Jul;159(1):390–392. doi: 10.1128/jb.159.1.390-392.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan R. D., Kanai K., Clark R. B., Giles W. Selective block of calcium current by lanthanum in single bullfrog atrial cells. J Gen Physiol. 1988 Apr;91(4):549–572. doi: 10.1085/jgp.91.4.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuss D. L. Biological control of chestnut blight: an example of virus-mediated attenuation of fungal pathogenesis. Microbiol Rev. 1992 Dec;56(4):561–576. doi: 10.1128/mr.56.4.561-576.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paranjape V., Roy B. G., Datta A. Involvement of calcium, calmodulin and protein phosphorylation in morphogenesis of Candida albicans. J Gen Microbiol. 1990 Nov;136(11):2149–2154. doi: 10.1099/00221287-136-11-2149. [DOI] [PubMed] [Google Scholar]
- Pieterse C. M., Verbakel H. M., Spaans J. H., Davidse L. C., Govers F. Increased expression of the calmodulin gene of the late blight fungus Phytophthora infestans during pathogenesis on potato. Mol Plant Microbe Interact. 1993 Mar-Apr;6(2):164–172. doi: 10.1094/mpmi-6-164. [DOI] [PubMed] [Google Scholar]
- Powell W. A., Jr, Van Alfen N. K. Two nonhomologus viruses of Cryphonectria (Endothia) parasitica reduce accumulation of specific virulence-associated polypeptides. J Bacteriol. 1987 Nov;169(11):5324–5326. doi: 10.1128/jb.169.11.5324-5326.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell W. A., Van Alfen N. K. Differential accumulation of poly(A)+ RNA between virulent and double-stranded RNA-induced hypovirulent strains of Cryphonectria (Endothia) parasitica. Mol Cell Biol. 1987 Oct;7(10):3688–3693. doi: 10.1128/mcb.7.10.3688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigling D., Van Alfen N. K. Regulation of laccase biosynthesis in the plant-pathogenic fungus Cryphonectria parasitica by double-stranded RNA. J Bacteriol. 1991 Dec;173(24):8000–8003. doi: 10.1128/jb.173.24.8000-8003.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabie F. T., Gadd G. M. Involvement of a Ca2+-calmodulin interaction in the yeast-mycelial (Y-M) transition of Candida albicans. Mycopathologia. 1989 Oct;108(1):47–54. doi: 10.1007/BF00436783. [DOI] [PubMed] [Google Scholar]
- Schreiber S. L. Chemistry and biology of the immunophilins and their immunosuppressive ligands. Science. 1991 Jan 18;251(4991):283–287. doi: 10.1126/science.1702904. [DOI] [PubMed] [Google Scholar]
- Schreiber S. L., Crabtree G. R. The mechanism of action of cyclosporin A and FK506. Immunol Today. 1992 Apr;13(4):136–142. doi: 10.1016/0167-5699(92)90111-J. [DOI] [PubMed] [Google Scholar]
- Schreiber S. L. Immunophilin-sensitive protein phosphatase action in cell signaling pathways. Cell. 1992 Aug 7;70(3):365–368. doi: 10.1016/0092-8674(92)90158-9. [DOI] [PubMed] [Google Scholar]
- Schäfer W., Straney D., Ciuffetti L., VAN Etten H. D., Yoder O. C. One enzyme makes a fungal pathogen, but not a saprophyte, virulent on a new host plant. Science. 1989 Oct 13;246(4927):247–249. doi: 10.1126/science.246.4927.247. [DOI] [PubMed] [Google Scholar]
- Scott-Craig J. S., Panaccione D. G., Cervone F., Walton J. D. Endopolygalacturonase is not required for pathogenicity of Cochliobolus carbonum on maize. Plant Cell. 1990 Dec;2(12):1191–1200. doi: 10.1105/tpc.2.12.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slivka S. R., Insel P. A. Alpha 1-adrenergic receptor-mediated phosphoinositide hydrolysis and prostaglandin E2 formation in Madin-Darby canine kidney cells. Possible parallel activation of phospholipase C and phospholipase A2. J Biol Chem. 1987 Mar 25;262(9):4200–4207. [PubMed] [Google Scholar]
- Stahl D. J., Schäfer W. Cutinase is not required for fungal pathogenicity on pea. Plant Cell. 1992 Jun;4(6):621–629. doi: 10.1105/tpc.4.6.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straney D. C., VanEtten H. D. Characterization of the PDA1 promoter of Nectria haematococca and identification of a region that binds a pisatin-responsive DNA binding factor. Mol Plant Microbe Interact. 1994 Mar-Apr;7(2):256–266. doi: 10.1094/mpmi-7-0256. [DOI] [PubMed] [Google Scholar]
- Varley D. A., Podila G. K., Hiremath S. T. Cutinase in Cryphonectria parasitica, the chestnut blight fungus: suppression of cutinase gene expression in isogenic hypovirulent strains containing double-stranded RNAs. Mol Cell Biol. 1992 Oct;12(10):4539–4544. doi: 10.1128/mcb.12.10.4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamma A., Tamaru H., Harashima T., Inoue H. Isolation and characterization of mutants defective in production of laccase in Neurospora crassa. Mol Gen Genet. 1993 Aug;240(2):231–237. doi: 10.1007/BF00277061. [DOI] [PubMed] [Google Scholar]