Introduction
Serious depressive states, whether unipolar or bipolar, have a lifetime prevalence of 15–20 %, and are projected to become the second leading cause of disability by 2020 (Murray and Lopez, 1996). The Sequenced Treatment Alternatives to Relieve Depression (STAR*D) Study found that less than one-third of patients achieved full remission after four months of traditional antidepressant treatment (Thase et al., 2005). Although definitions of “treatment-resistant depression” (TRD) vary (Berlim and Turecki, 2007), clinicians agree that a significant proportion of patients are antidepressant refractory despite adequate dosage, duration, and compliance. A subgroup of these patients is refractory to multiple monotherapy medications, combination and augmentation strategies, and the use of mood stabilizers. Many of these patients receive Electroconvulsive Therapy (ECT) but some of these patients even become refractory to ECT. These patients may be referred for treatment using Deep Brain Stimulation (DBS). A new, effective, and less intrusive treatment (than ECT or DBS) for TRD patients would be a significant advance in alleviating suffering and disability for these patients and their families.
Recent research has focused on the involvement of the glutamatergic system in mood disorders. Altered glutamate levels and glutamate receptors have been reported in a variety of human studies of depression (Krystal et al., 2002; Zarate et al., 2002; Scarr et al., 2003; Sanacora et al., 2004; Yildez-Yesiloglu and Ankerst, 2006; Hashimoto et al., 2007; Hasler et al., 2007), with one study even relating “treatment resistance” to abnormal glutamate/glutamine/gamma-amino butyric acid cycling (Price et al., 2009). Drugs that target this system, specifically ketamine, have been shown to have a rapid onset of antidepressant effects. Although its exact mechanism of action remains unclear, it is postulated that by blocking the N-methyl-D-aspartate (NMDA) receptors, ketamine simultaneously activates and potentiates alpha-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid (AMPA) throughput (Maeng and Zarate, 2007; Maeng et al., 2008; Li et al., 2010; Zarate et al., 2010; Koike et al., 2011). In addition, ketamine increases the release of brain-derived neurotrophic factor (BDNF; Garcia et al., 2008) and stimulates the mammalian target of rapamycin (mTOR; Li et al., 2010), both of which may play a part in its antidepressant effects.
Subanasthetic dosages of ketamine have produced rapid antidepressant effects in animal models of depression (Maeng et al., 2008; Yilmaz et al., 2002;, Hashimoto et al., 2009), as well as in patients who have a treatment-resistant form of depression (Berman et al., 2000; Diazgranados et al., 2010; Messer and Haller, 2010). Antidepressant effects have been reported within hours of the infusion with a sustained benefit lasting days (Messer and Haller, 2010; aan het Rot et al., 2008; Messer et al., 2010). These rapid and sustained antidepressant effects have been demonstrated in patients with TRD (Berman et al., 2000; Zarate et al., 2006; Phelps et al., 2009) and treatment-resistant bipolar depression (Diazgranados et al., 2010), as well as in patients with TRD and comorbid alcohol dependency, pain syndrome, and advanced cancer (Kudoh et al., 2002; Liebrenz et al., 2007; 2009; Stefanczyk-Sapieha et al., 2008; Irwin and Iglewicz, 2010). In addition, a single dose of ketamine has been shown to reduce suicidal ideation within 24 hours (Price et al., 2009) and repeat dosages of ketamine (every other day for two weeks) have shown prolonged benefit, lasting up to three weeks (Messer et al., 2010; Messer and Haller, 2010; aan het Rot et al., 2010).
Although the research supports an acute effect by ketamine, it remains to be seen if repeat dosages over a longer period of time will have the same effects. In this report, we present three case reports of repeat intravenous ketamine infusions in TRD patients over a 12-month period.
Methods
Three adult patients with a Major Depressive Episode, current, without psychotic features, as determined by a psychiatric clinical interview and confirmed by the Mini Neuropsychiatric International Interview (MINI; Sheehan et al., 1998), were admitted to the inpatient psychiatric unit and offered the option of another course of ECT or intravenous (IV) ketamine infusions. Patients understood that this was not a Food and Drug Administration-approved use of ketamine. Patients consented to IV ketamine infusions after a full description of a procedure approved by Lutheran Hospital’s Departments of Anesthesia, Medicine, Nursing, Pharmacy, and Medical Executive Committee. Institutional Review Board approval was not required for this naturalistic observation study, but it approved the consent form. Per standard of care, a toxicology screen and medical clearance was obtained prior to the first infusion.
In line with previous research (Berman et al., 2000; Zarate et al., 2006; aan het Rot et al., 2008; Diazgranados et al., 2010; Messer and Haller, 2010; Messer et al., 2010), ketamine was administered at 0.5 mg/kg of ideal body weight over 40 minutes. Vital signs were monitored throughout each infusion, as well as any side effects of the treatment. No medication changes or washouts occurred prior to the initiation of treatment. Severity of depressive symptoms was measured by the Montgomery-Asberg Depression Rating Scale (MADRS; Montgomery and Asberg, 1979). During the acute phase, outcome measures were completed prior to the infusion, 4-hours post-infusion, and 24-hours post infusion. Infusions were performed every other day, for up to six infusions. Once the patient reported a reduction in depressive symptoms, they were discharged and given infusions on an outpatient basis. The outpatient infusion schedule was individualized as described below. Outcome measures were completed prior to each infusion and 24-hours post-infusion via a telephone conversation. Remission of the depressive symptoms was defined as a MADRS score of ≤ 8 (Hawley et al., 2002).
Results
Patient 1
This patient was hospitalized after a suicide attempt by hanging. They were diagnosed with Major Depressive Disorder (MDD) at the age of 17, with co-morbid Panic Disorder without Agoraphobia, and had a history of multiple suicide attempts with over 20 hospitalizations. The patient had no history of an Axis II disorder, chemical dependency, or major medical illness. The family history was significant for mood disorders. Patient 1 had failed greater than 20 antidepressants, mood stabilizers, and augmentation treatments, and had received 35 ECT treatments in three separate acute trials with good benefit. However, significant cognitive symptoms developed after each trial, severe enough to cause job loss with the last two trials.
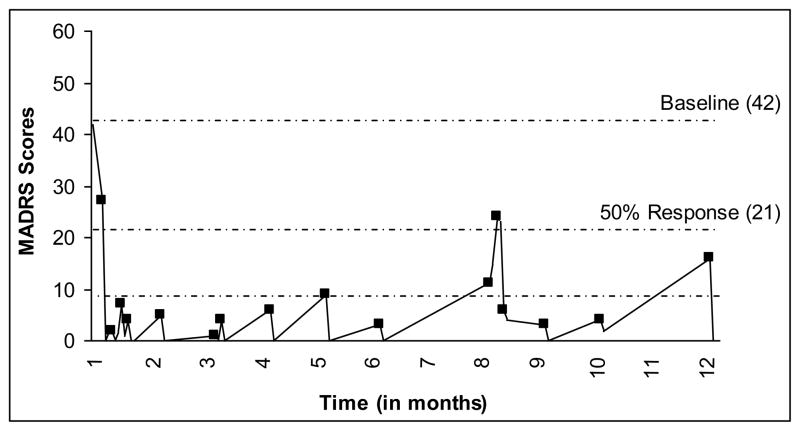
At the time of the ketamine trial, the patient was taking fluoxetine 80 mg, quetiapine 200 mg, lamotrigine 50 mg, and lorazepam 0.5 mg. The patient received 27 mg of ketamine throughout the 12-month course of treatment. Pre-treatment MADRS scores were severe. Four hours post-infusion 1, the MADRS score dropped to zero. The patient remained stable, was given the second infusion four days later, and discharged. The third infusion took place eight days after the second. Patient 1 reported mild depressive symptoms prior to the infusion, which resolved post-infusion. A week was added to each subsequent infusion until ketamine was being infused approximately every six to seven weeks. The patient experienced a moderate relapse in month 8, precipitated by anxiety related to school, work, and a failed relationship. However, they responded to an acute series of three ketamine treatments. The patient reported no cognitive issues and had become more functional in all facets of life. At the 12-month point, the medication regime included fluoxetine 60 mg, quetiapine 50 mg, and lorazepam as needed. The number of treatments totaled 16 (see Figure 1).
Fig. 1.
Patient 1’s response to ketamine infusions; each marker indicates an infusion (pre-treatment score) and the lowest line indicates remission (MADRS ≤ 8).
Patient 2
This patient was hospitalized after reporting suicidal ideation to their outpatient psychiatrist. The patient’s depression began in 2008 after surgical complications which led to a cascade of losses including job, car, and home. Patient 2 had failed 20 antidepressants, mood stabilizers, and augmentation treatments and had 15 ECTs with little improvement, resulting in seven hospitalizations and five serious suicide attempts. Family history was significant for mood disorders. There is no history of an Axis II disorder, chemical dependency, or major medical illness.
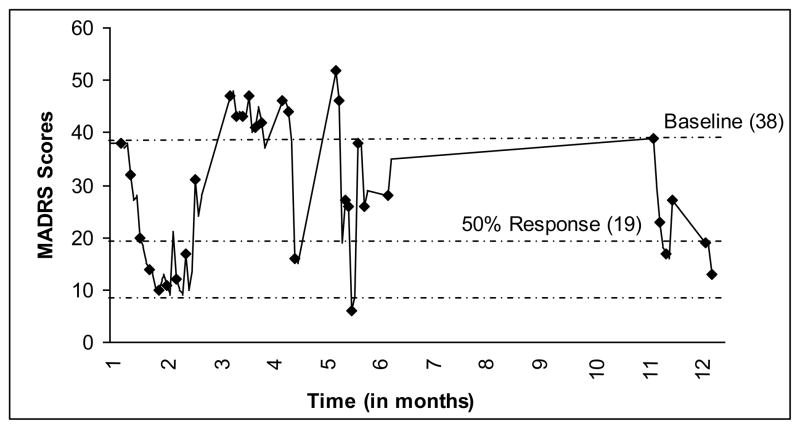
At the time of the ketamine trial, the patient was taking nortriptyline 100 mg and quetiapine 200 mg. At screening and prior to infusion 1, the patient scored severely depressed on the MADRS. No reduction in depressive symptoms occurred until 4 hours post-infusion 4, when the scores dropped to the moderate range. Patient 2 received 30 mg of ketamine throughout their first 12 treatments, at which point the patient relapsed, triggered by cancellation of their health insurance. A response was produced by seven infusions of 40 mg of ketamine. However, the patient terminated treatment and, one month later, made a serious suicide attempt by polydrug overdose. Patient 2 was hospitalized and started on 35 mg of ketamine for another seven infusions and responded. The patient then experienced a diminished response to the treatment and also expressed some cognitive difficulties (driving and forgetting to take the exit, word-finding difficulties, and concentration difficulties), as well as significant insomnia. These complaints were present prior to the ketamine treatments, but to a milder degree. A clinical decision was made to stop the ketamine treatments and begin treatment with a monoamine oxidase-inhibitor (MAO-I). The patient responded and had five months without ketamine. The patient was hospitalized in month 9 for a suicidal relapse due to persistent insomnia, managed by the addition of chlorpromazine, and again in month 11, during which ketamine treatments were again initiated. The patient again responded to the treatments. The patient was able to secure housing, helping to stabilize their mood. At the 12-month point, the patient was being maintained on a regime of phenelzine 45 mg, gabapentin 400 mg, temazepam 30 mg, and melatonin 3 mg. Their number of treatments totaled 34 (see Figure 2).
Fig. 2.
Patient 2’s response to ketamine infusions; each marker indicates an infusion (pre-treatment score) and the lowest line indicates remission (MADRS ≤ 8).
Patient 3
This patient was hospitalized for suicidal ideation brought about by an unremitting depression since 2003. There were numerous suicide attempts and parasuicidal behaviors throughout the years. Patient 3 carried diagnoses of MDD, Bulimia Nervosa, and Bipolar II Disorder, with cluster c traits, but no chemical dependency or major medical illness. The family history was significant for mood disorders. The patient had over 250 ECTs with only partial improvement, and had failed greater than 20 trials of antidepressants, mood stabilizers, and augmentation techniques.
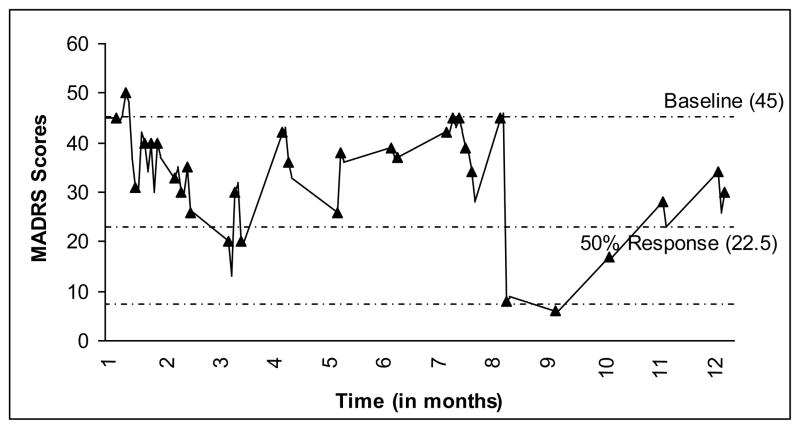
At the time of the ketamine trial, the patient was on quetiapine 800 mg, lamotrigine 200 mg, and bupropion 200 mg. The patient received 35 mg of ketamine throughout their course of treatment. At screening and prior to infusion 1, the patient scored severely depressed on the MADRS. There was no reduction in depressive symptoms until infusion 3 when the score dropped to the moderate range. The patient had an acute series of seven infusions before starting maintenance treatments. Although MADRS scores indicated a moderate level of depression, the staff’s clinical impression and the patient’s own evaluation supported a significant degree of functional improvement. After infusion 12, the patient entered a hypomanic state, consisting of insomnia, hyperactivity, and racing thoughts. The patient’s medications were changed to lithium 900 mg, quetiapine 400 mg, lamotrigine 200 mg, temazepam 15 mg, and clonazepam 0.5 mg prn. At month 3, after infusion 13, the patient made a polydrug overdose after arguing with their son. The patient was discharged after two days with no medication changes. Ketamine treatments were resumed two weeks later. No response to the ketamine was reported on the MADRS, but once again improved functionality in their daily life was described. During month 7, hospitalization occurred for excessive quetiapine use after an argument. They denied that this was a suicide attempt. Patient 3 was started on another acute series of infusions and, in month 9, after the 27th infusion, reported a dramatic decrease in all of their depression symptoms. They stated that they finally felt that “the darkness had lifted.” However, the patient was now feeling emotions that they had not felt in years and found this to be intense and overwhelming, especially in regard to unresolved traumatic events from childhood and their first marriage that had never been revealed. There were two short-term (1–2 days) hospitalizations during months 10 and 11, involving parasuicidal behavior. Mid-month 11, they switched into a second manic phase and further treatment was cancelled. On the day of their outpatient psychiatric appointment, they reported that they took an overdose of alprazolam and hydrocodone/acetaminophen in an effort to “appear less manic” to their psychiatrist. The patient was briefly hospitalized and olanzepine 10 mg was added to their medication regime. At the end of month 11, they developed olanzepine-induced dyskinesias and was again hospitalized. Propranolol was added, olanzepine was discontinued, and they received two ketamine treatments, for which they reported some relief. The patient was discharged and, at month 12 was taking lithium 900 mg, quetiapine 400 mg, alprazolam 0.5 mg as needed, and melatonin 3 mg. Their treatments totaled 32 (see Figure 3).
Fig. 3.
Patient 3’s response to ketamine infusions; each marker indicates an infusion (pre-treatment score) and the lowest line indicates (MADRS ≤ 8).
Discussion
Interest has increased regarding the involvement of the glutamatergic system in Major Depression and the rapid antidepressant effect of medications targeting this system. Single dosages and an acute series of ketamine infusions have been shown to be a rapid and safe alternative to more traditional management of treatment-resistant depression (Kudoh et al., 2002; Zarate et al., 2006; Liebrenz et al., 2007; aan het Rot et al., 2008; Stefanczyk-Sapieha et al., 2008; Liebrenz et al., 2009; Phelps et al., 2009; aan het Rot et al., 2010; Irwin and Iglewicz, 2010; Messer and Haller, 2010; Messer et al., 2010). In line with recent research, the cases described in this report demonstrate beneficial results during the acute phase of treatments, with no significant physiological or psychological side effects. Despite these immediate results, these cases illustrate three different courses of illness and treatment over a 12-month period and indicate that the benefit of long-term IV ketamine therapy remains an open question.
Much of the literature describing the benefits of IV ketamine infusions have been based on single infusions. The National Institute of Mental Health is currently conducting studies that are double-blind and placebo-controlled to determine if the benefit can be extended by repeat ketamine infusions. First, a long-term maintenance protocol needs to be established. Messer and Haller (2010) described a maintenance protocol where the patient received IV ketamine every three weeks. This was based on the patient’s own remission and relapse pattern. As seen in our report, every patient had a different response to ketamine and treatment needed to be individualized in order to receive maximum benefit.
Second, there is a need to identify ketamine responders and non-responders. Already described biomarkers of successful response to ketamine include increased cortical activity in the anterior cingulate cortex (Salvadore et al., 2009) and family history of alcohol use disorders (Phelps et al., 2009). Although biomarkers can be determined through neuroimaging techniques, advanced neuroimaging is unavailable on the clinical front line. Establishing both rapid and less-invasive procedures to determine useful biomarkers will prove beneficial.
Third, some research has indicated that those who have a response to ketamine will do so within 230 minutes post infusion (Zarate et al., 2006). This is not always the case. Two of our patients did not demonstrate significant responses to ketamine until after the fourth and seventh infusions, respectively. It is possible that there are subgroups within the responders: those with an immediate response and those who respond after “x” number of infusions. Future research needs to explore this possibility.
Fourth, studies are needed to determine which medications can be used in conjunction with ketamine to sustain remission. It has been reported that pre-ketamine treatment with lamotrigine did not extend the antidepressant benefits of ketamine and post-treatment with riluzole did not prevent relapse (Mathew et al., 2010). In our case report, a patient responded to a MAO-I after failing multiple other treatments. Further research into ketamine combinations with other antidepressants, as well as augmenting therapies, will be valuable.
Even though IV ketamine has proven to be a relatively safe and efficacious treatment for TRD, caution is warranted. Repeated exposure to ketamine may increase the risk of psychotic and dissociative episodes, as well as severe emotional states (either distress or euphoria) (Dillon et al., 2003). Further, additional infusions of ketamine may prove to be less efficacious than the initial infusion in some patients (Liebrenz et al., 2009). Moreover, the rapid activation of mTOR signaling pathways, which may play a role in the antidepressant effects of ketamine, is not without concern. A single dose of ketamine in rats activated the mTOR pathways, which led to an increase in synaptic signaling and synaptic spinal growth within 24 hours (Li et al., 2010). Li and colleagues (2011) also demonstrated the same pathway (activating mTOR) was responsible for reversing behavioral and synaptic deficits caused by chronic stress exposure. However, it has also been reported that alterations in mTOR signaling may be associated with the development of cancer (Arachchige Don and Zheng, 2010). More research is needed in order to assess the neurobiological risks and benefits of mTOR and ketamine.
The major limitation to the present study was that this was an open-label naturalistic observation without blinding, randomization, or a placebo drug. However, the treatment-resistant nature of these patients’ depression, along with the antidepressant effects and/or functional improvements that we have seen, offer support that IV ketamine infusion can provide relief in this difficult subset of patients. The effects that were observed in this naturalistic observation require validation in a blinded, randomized, and controlled trial.
Acknowledgments
Role of the funding source
No funding source
The authors wish to provide no acknowledgements.
Footnotes
Conflicts of Interest
Dr. Dale has received speaker’s fees from Astra Zeneca. All other authors declare that they have no conflicts of interest.
Contributors
All authors were involved in the design of the study and in the writing of the protocol. Ms. Szymkowicz and Ms. Finnegan managed the data collection, and Ms. Szymkowicz and Dr. Dale managed the literature searches and wrote the first draft of the manuscript. All authors contributed to and have approved the final manuscript.
References
- aan het Rot M, Charney DS, Mathew SJ. Intravenous ketamine for treatment resistant major depressive disorder. Primary Psychiatry. 2009;15:39–47. [Google Scholar]
- aan het Rot M, Collins KA, Murrough JW, Perez AM, Reich DL, Charney DS, Mathew SJ. Safety and efficacy of repeated-dose intravenous ketamine for treatment-resistant depression. Biol Psychiatry. 2010;67:139–145. doi: 10.1016/j.biopsych.2009.08.038. [DOI] [PubMed] [Google Scholar]
- Arachchige Don AS, Zheng XS. Recent clinical trials of mTOR-targeted cancer therapies. Rev Recent Clin Trials. 2010;6:24–35. doi: 10.2174/157488711793980147. [DOI] [PubMed] [Google Scholar]
- Berlim MT, Turecki G. What is the meaning of treatment resistant/refractory major depression (TRD)? A systematic review of current randomized trails. Eur Neuropsychopharmacol. 2007;17:696–707. doi: 10.1016/j.euroneuro.2007.03.009. [DOI] [PubMed] [Google Scholar]
- Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, Krystal JH. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47:351–354. doi: 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- Diazgranados N, Ibrahim L, Brutsche NE, Newberg A, Kronstein P, Khalife S, Kammerer WA, Quezado Z, Luckenbaugh DA, Salvadore G, Machado-Vieira R, Manji HK, Zarate CA. A randomized add-on trial of an N-methyl-D-aspartate antagonist in treatment-resistant bipolar depression. Arch Gen Psychiatry. 2010;67:793–802. doi: 10.1001/archgenpsychiatry.2010.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon P, Copeland J, Jansen K. Patterns of use and harms associated with non-medical ketamine use. Drug Alcohol Depend. 2003;69:23–28. doi: 10.1016/s0376-8716(02)00243-0. [DOI] [PubMed] [Google Scholar]
- Garcia LSB, Comim CM, Valvassori SS, Reus GZ, Barbosa LM, Andreazza AC, Stertz L, Fries GR, Gavioli EC, Kapczinski F, Quevedo J. Acute administration of ketamine induces antidepressant-like effects in a forced swimming test and increases BDNF levels in the rat hippocampus. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:140–144. doi: 10.1016/j.pnpbp.2007.07.027. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Sawa A, Iyo M. Increased levels of glutamate in brains from patients with mood disorders. Biol Psychiatry. 2007;62:1310–1316. doi: 10.1016/j.biopsych.2007.03.017. [DOI] [PubMed] [Google Scholar]
- Hashimoto K. Emerging role of glutamate in the pathophysiology of major depressive disorder. Brain Res Rev. 2009;61:105–123. doi: 10.1016/j.brainresrev.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Hasler G, Van Der Veen JW, Tumonis T, Meyers N, Shen J, Drevets WC. Reduced prefrontal glutamate/glutamine and gamma-aminobutyric acid levels in major depression determined using proton magnetic resonance spectroscopy. Arch Gen Psychiatry. 2007;64:193–200. doi: 10.1001/archpsyc.64.2.193. [DOI] [PubMed] [Google Scholar]
- Hawley CJ, Gale TM, Sivakumaran T. Defining remission by cut off score on the MADRS: Selecting the optimal value. J Affect Disord. 2002;72:177–184. doi: 10.1016/s0165-0327(01)00451-7. [DOI] [PubMed] [Google Scholar]
- Irwin SA, Iglewicz A. Oral ketamine for the rapid treatment of depression and anxiety In patients receiving hospice care. J Palliat Med. 2010;13:903–908. doi: 10.1089/jpm.2010.9808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike H, Iijima M, Chaki S. Involvement of AMPA receptor in both the rapid and sustained antidepressant-like effects of ketamine in animal models of depression. Behav Brain Res. 2011;224:107–111. doi: 10.1016/j.bbr.2011.05.035. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Sanacora G, Blumberg H, Anand A, Charney DS, Marek G, Epperson CN, Goddard A, Mason GF. Glutamate and GABA systems as targets for novel antidepressant and mood-stabilizing treatments. Mol Psychiatry. 2002;7(suppl 1):S71–S80. doi: 10.1038/sj.mp.4001021. [DOI] [PubMed] [Google Scholar]
- Kudoh A, Takahira Y, Katagai H, Takazawa T. Small-dose ketamine improves the postoperative state of depressed patients. Anesth Analg. 2002;95:114–118. doi: 10.1097/00000539-200207000-00020. [DOI] [PubMed] [Google Scholar]
- Li N, Lee B, Liu R, Banasr M, Dwyer JM, Iwata M, Li X, Aghajanian G, Duman RS. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329:959–964. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Liu R, Dwyer JM, Banasr M, Lee B, Son H, Li X, Aghajanian G, Duman RS. Glutamate N-methyl-D-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biol Psychiatry. 2011;69:754–761. doi: 10.1016/j.biopsych.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebrenz M, Borgeat A, Lesinger R, Stohler R. Intravenous ketamine therapy in a patient with a treatment-resistant major depression. Swiss Med Wkly. 2007;137:234–246. doi: 10.4414/smw.2007.11852. [DOI] [PubMed] [Google Scholar]
- Liebrenz M, Stohler R, Borgeat A. Repeated intravenous ketamine therapy in a patient with a treatment resistant major depression. World J Biol Psychiatry. 2009;10:640–643. doi: 10.1080/15622970701420481. [DOI] [PubMed] [Google Scholar]
- Maeng S, Zarate CA. The role of glutamate in mood disorders: results from the ketamine in major depression study and the presumed cellular mechanism underlying its antidepressant effects. Curr Psychiatry Rep. 2007;9:467–474. doi: 10.1007/s11920-007-0063-1. [DOI] [PubMed] [Google Scholar]
- Maeng S, Zarate CA, Du J, Schloesser RJ, McCammon J, Chen G, Manji HK. Cellular mechanisms underlying the antidepressant effects of ketamine: role of α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors. Biol Psychiatry. 2008;63:349–352. doi: 10.1016/j.biopsych.2007.05.028. [DOI] [PubMed] [Google Scholar]
- Mathew SJ, Murrough JW, aan het Rot M, Collins KA, Reich DL, Charney DS. Riluzole for relapse prevention following intravenous ketamine in treatment-resistant depression: A pilot randomized, placebo-controlled continuation trial. Int J Neuropsychopharmacol. 2010;13:71–82. doi: 10.1017/S1461145709000169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messer MM, Haller IV. Maintenance ketamine treatment produces long-term recovery from depression. Primary Psychiatry. 2010;17:48–50. [Google Scholar]
- Messer MM, Haller IV, Larson P, Pattison-Crisostomo J, Gessert CH. The use of a series of ketamine infusions in two patients with treatment-resistant depression. J Neuropsychiatry Clin Neurosci. 2010;22:442–444. doi: 10.1176/jnp.2010.22.4.442. [DOI] [PubMed] [Google Scholar]
- Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- Murray CJ, Lopez AD. Evidence-based health policy—lessons from the Global Burden of Disease Study. Science. 1996;27:740–743. doi: 10.1126/science.274.5288.740. [DOI] [PubMed] [Google Scholar]
- Phelps LE, Brutsche N, Moral JR, Luckenbaugh DA, Manji HK, Zarate CA. Family history of alcohol dependence an initial antidepressant response to an N-methyl-D-aspartate antagonist. Biol Psychiatry. 2009;65:181–184. doi: 10.1016/j.biopsych.2008.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price RB, Shungu DC, Mao X, Nestadt P, Kelly C, Collins KA, Murrough JW, Charney DS, Mathew SJ. Amino acid neurotransmitters assessed by proton magnetic resonance spectroscopy: relationship to treatment resistance in major depressive disorder. Biol Psychiatry. 2009;65:792–800. doi: 10.1016/j.biopsych.2008.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvadore G, Cornwell BR, Colon-Rosario V, Coppola R, Grillon C, Zarate CA, Manji HK. 3 Increased anterior cingulate cortical activity in response to fearful faces: a neurophysiological biomarker that predicts rapid antidepressant response to ketamine. Biol Psychiatry. 2009;65:289–295. doi: 10.1016/j.biopsych.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanacora G, Gueorguieva R, Epperson CN, Wu YT, Appel M, Rothman DL, Krystal JH, Mason GF. Subtype-specific alterations of gamma-animobutyric acid and glutamate in patients with major depression. Arch Gen Psychiatry. 2004;61:705–713. doi: 10.1001/archpsyc.61.7.705. [DOI] [PubMed] [Google Scholar]
- Scarr E, Pavey G, Sundram S, MacKinnon A, Dean B. Decreased hippocampal NMDA, but not kainite or AMPA receptors in bipolar depression. Bipolar Disord. 2003;5:257–264. doi: 10.1034/j.1399-5618.2003.00024.x. [DOI] [PubMed] [Google Scholar]
- Sheehan D, Lecrubier Y, Harnett-Sheehan L, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar G. The Mini International Neuropsychiatric Interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview. J Clin Psychiatry. 1998;59:22–33. [PubMed] [Google Scholar]
- Stefanczyk-Sapieha L, Oneschuk D, Demas M. Intravenous ketamine “burst” for refractory depression in a patient with advanced cancer. J Palliat Med. 2008;11:1268–1271. doi: 10.1089/jpm.2008.9828. [DOI] [PubMed] [Google Scholar]
- Thase ME, Haight BR, Richard N, Rockett CB, Mitton M, Modell JG, Vanmeter S, Harriett AE, Yang Y. Remission rates following antidepressant therapy with buproprion or selective serotonin reuptake inhibitors: a meta-analysis of original date from 7 randomized controlled trials. J Clin Psychiatry. 2005;66:974–981. doi: 10.4088/jcp.v66n0803. [DOI] [PubMed] [Google Scholar]
- Yildiz-Yesiloglu A, Ankerst DP. Neurochemical alterations of the br4ain in bipolar disorder and their implications for pathophysiology: a systematic review of the in vivo proton magnetic resonance spectroscopy findings. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:969–995. doi: 10.1016/j.pnpbp.2006.03.012. [DOI] [PubMed] [Google Scholar]
- Yilmaz A, Schulz D, Aksoy A, Canbeyli R. Prolonged effect of an anesthetic dose of ketamine on behavioral despair. Pharmacol Biochem Behav. 2002;71:341–344. doi: 10.1016/s0091-3057(01)00693-1. [DOI] [PubMed] [Google Scholar]
- Zarate CA, Quiroz J, Payne J, Manji HK. Modulators of the glutamatergic system: implications for the development of improved therapeutics in mood disorders. Psychopharmacol Bull. 2002;36:35–83. [PubMed] [Google Scholar]
- Zarate CA, Singh JB, Carlson PH, Brutsche NE, Ameli R, Luckenbaugh DA, Charney DS, Manji HK. A randomized trial of an N-methly-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63:856–864. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- Zarate C, Machado-Vieira R, Henter I, Ibrahim L, Diazgranados N, Salvadore G. Glutamatergic modulators: the future of treating mood disorders? Harv Rev Psychiatry. 2010;18:293–303. doi: 10.3109/10673229.2010.511059. [DOI] [PMC free article] [PubMed] [Google Scholar]