Abstract
Pavlovian conditioning processes contribute to the etiology of nicotine dependence. Conditioning involving interoceptive stimuli is increasingly recognized as playing a role in many diseases and psychopathologies, including drug addiction. Previous animal research on diminishing the influence of interoceptive conditioning has been limited to antagonism and non-reinforced exposures to the drug stimulus. The goal of the present research was to determine if interoceptive conditioning with a nicotine stimulus could be diminished through an unconditioned stimulus (US) devaluation procedure. In two separate experiments, male Sprague-Dawley rats received nicotine injections (0.4 mg base/kg) followed by intermittent sucrose (26%) access in a conditioning chamber. On intermixed saline sessions, sucrose was withheld. Conditioning was demonstrated by a reliable increase in head entries in the dipper receptacle on nicotine versus saline sessions. Following conditioning, rats in a devaluation condition were given access to sucrose in their home cages immediately followed by a lithium chloride (LiCl) injection on 3 consecutive days. On subsequent test days, nicotine-evoked conditioned responding was significantly attenuated. Within-subject (Experiment 1) and between-subject (Experiment 2) controls revealed that the diminished responding was not due to mere exposure to the sucrose US in the devaluation phase. Experiment 2 included a LiCl-alone control group. Repeated illness induced by LiCl did not reduce later nicotine-evoked responding. These findings suggest that there is a direct association between the interoceptive stimulus effects of nicotine and the appetitive sucrose US (i.e., stimulus-stimulus) rather than a stimulus-response association.
Keywords: Pavlovian conditioning, interoception, smoking, drug discrimination, stimulus-reinforcer learning
Introduction
Nicotine dependence is tenacious; 28.6 percent of adults in the U.S. are current users of tobacco (Department of Health and Human Services, 2008). Nearly half of tobacco users report a desire to quit (National Institutes of Health, 1998). However, 95% of the individuals that attempt to quit without intervention will relapse within a year (National Institutes of Health, 1998). Numerous cognitive-behavioral and pharmacological strategies for cessation have been developed to aid these individuals (Eisenberg et al, 2008; Gonzales et al, 2006; Hughes et al, 2007; Jorenby et al, 2006). Although pharmacotherapies such as bupropion (Zyban®) and varenicline (Chantix®) increase long-term abstinence from tobacco when compared to control groups receiving placebo, successful quit rates remain quite low [17% bupropion, average of meta-analysis including 36 clinical trials (Hughes et al, 2007); 26% varenicline, average of meta-analysis including 13 clinical trials (Eisenberg et al, 2008)]. The effectiveness of cognitive-behavioral therapies developed to assist in smoking cessation also remains in question (Conklin and Tiffany, 2002; Niaura et al, 1999). A more thorough understanding of how nicotine acquires control of behavior may provide insight into ways to increase the efficacy of current behavioral and pharmacological approaches to treating chronic tobacco use and nicotine dependence.
One potential factor that likely contributes to the tenacity of the nicotine addiction is interoceptive conditioning involving the nicotine stimulus (Bevins and Murray, 2011). Conceptually, interoceptive conditioning refers to nicotine as a complex perceptible internal conditioned stimulus (CS) that is available for modification through learning if the nicotine CS is reliably paired with either an appetitive or aversive unconditioned stimulus (US). To a smoker, for example, appetitive events or USs may include socialization during a work break, peer acceptance, a filling meal, or other drugs like caffeine or alcohol that reliably co-occur with nicotine (Bevins, 2009; Bevins et al, 2012). Research investigating interoceptive conditioning with the nicotine stimulus is quite limited. One approach to studying the behavioral and neural processes involved in interoceptive conditioning in rats has been the discriminated goal-tracking task (Charntikov et al, 2012; Reichel et al, 2007). In this task, interoceptive conditioning with nicotine as the CS involves intermixed exposure to 2 different types of daily sessions; nicotine and saline. On nicotine days, rats are injected with nicotine and then given intermittent access to liquid sucrose in a conditioning chamber. On intermixed days, saline is administered but sucrose is withheld. Nicotine comes to control an increase in approach and head entry into the dipper receptacle [termed goal-tracking; see (Farwell and Ayres, 1979)] compared to saline. One focus of our research on interoceptive conditioning has been to investigate approaches to diminishing nicotine’s control over the appetitive approach behavior. Identifying means of reducing the control the nicotine stimulus has on appetitive behavior could provide insight into how interoceptive conditioning may contribute to chronic tobacco use and the associated high relapse rate.
One approach we have taken to decrease nicotine-evoked goal-tracking is pharmacological blockade. This method has also helped provide insight into the neuropharmacological mechanisms mediating the CS effects of nicotine. For example, pretreatment with mecamylamine, a relatively non-selective nicotinic acetylcholine receptor (nAChR) antagonist, reliably reduces nicotine controlled goal-tracking when administered before testing (Besheer et al, 2004; Struthers et al, 2009). A more specific mechanism has been implicated by the demonstration that the nAChR antagonist DHβE, which is selective for α4-containing nAChRs, also blocks nicotine-evoked responding (Struthers et al, 2009). Although pharmacological blockade helps reveal underlying mechanism, this effect is transient and nicotine-evoked behavior returns if nicotine is administered in the absence of the ligand.
A second approach to decreasing nicotine-evoked goal-tracking is extinction. In extinction, nicotine after training as a CS is repeatedly presented except sucrose is not accessible. The goal-tracking conditioned response decreases across the non-reinforced nicotine sessions (Besheer et al, 2004; Murray and Bevins, 2007). A third approach that is a variant of extinction was termed “transfer of extinction learning” by Reichel et al. (2010). In this approach, repeated non-reinforced presentations of a ligand that shares stimulus effects with nicotine (e.g., varenicline or nornicotine) also attenuate subsequent responding evoked by the nicotine stimulus. That is, following training of the nicotine CS, rats are given the alternate ligand in place of nicotine during an extinction phase. If the nicotine stimulus is again tested after extinction, some, but not all, drugs that share stimulus effects with nicotine weaken conditioned responding to the nicotine CS; this weakening tends not to be as complete as when the nicotine stimulus itself is used in the extinction phase (Reichel et al, 2010).
An alternative, yet currently unstudied approach to weakening responding evoked by the nicotine CS is devaluation. In a typical devaluation study, a CS such as onset of a tone is repeatedly paired with an appetitive US such as a food pellet. For the devaluation phase, the appetitive US is paired with sickness like that induced by an injection of lithium chloride (LiCl). When the CS is re-tested, conditioned responding to the CS is reduced (Holland and Straub, 1979; Holland and Rescorla, 1975). Research in this field has focused on diminishing appetitive responding controlled by exteroceptive stimuli such as a brief tone, illumination of a light, or a familiar context. To our knowledge, devaluation of interoceptive conditioning has never been studied. Accordingly, the goal of the present research was to investigate whether interoceptive conditioning and nicotine’s control of appetitive behavior was susceptible to US devaluation.
General Method
Subjects
Forty experimentally naive male Sprague-Dawley rats ordered at 275–299 g from Harlan (Indianapolis, IN, USA) were housed individually in clear polycarbonate cages (48.3 × 26.7 × 20.3 cm; length × width × height) lined with wood shavings. Rats had ad libitum access to water in home cages, except when noted. Following acclimation to the colony, rats were handled for a minimum of 2 min per day for 3 consecutive days before access to food (Harlan Teklad Rodent Diet) was restricted to maintain rats at 85% of their free-feeding body weight. The colony room was temperature and humidity controlled. All experimental sessions were conducted during the light portion of a 12 hour light/dark cycle. Protocols were approved by the University of Nebraska-Lincoln Institutional Animal Care and Use Committee.
Apparatus
Eight conditioning chambers (ENV-008CT; Med Associates, Inc., Georgia, VT, USA) measuring 30.5 × 24.1 × 21.0 (length × width × height) cm were enclosed in sound and light attenuating cubicles fitted with an exhaust fan to provide airflow and mask noise. The front, back, and ceiling of the chambers were clear polycarbonate; side walls were aluminum. A recessed receptacle (5.2 × 5.2 × 3.8 cm; length × width × depth) was on one of the side walls. A dipper arm raised a 0.1-ml cup of sucrose (26% w/v) into the receptacle. To record head entries into the dipper, the chambers were equipped with an emitter/detector unit placed 1.2 cm into the recessed receptacle and 3 cm above the rod floor of the chamber. A personal computer with Med Associates interface and software (Med-PC for Windows, version IV) controlled sucrose deliveries and recorded dipper entries.
Drugs
(−)-Nicotine hydrogen tartrate and lithium chloride (LiCl) were purchased from Sigma (St. Louis, MO, USA). Nicotine was dissolved in 0.9% saline and adjusted to a pH of 7.0 ± 0.2 using a dilute NaOH solution. Nicotine injections were given subcutaneously (SC); dose is reported as the base. LiCl was dissolved in distilled water and injected interperitoneally (IP); dose is reported as the salt. All injections were given at a volume of 1 ml/kg.
Experiment 1
Acquisition
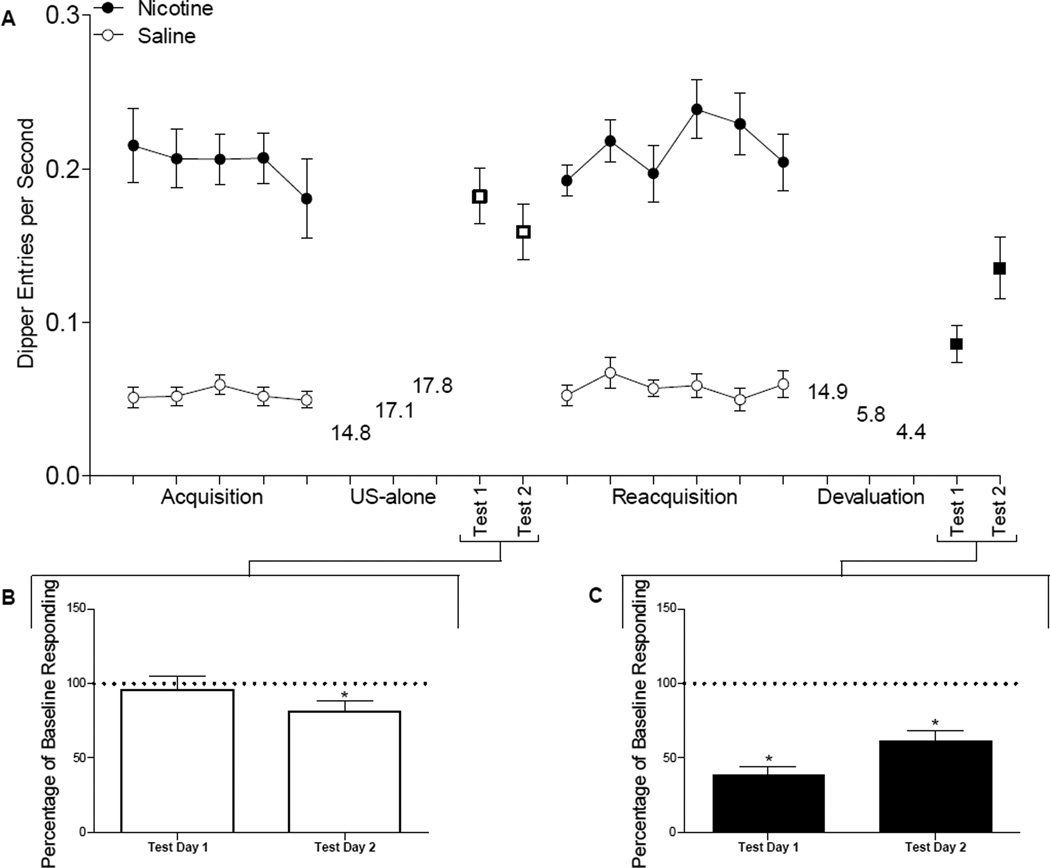
To minimize the initial locomotor suppressant effects of nicotine, rats (n=16) received daily injections of 0.4 mg/kg nicotine in their home cages for the 3 days immediately before the start of the experiment (Bevins et al, 2001). Discrimination training consisted of 32 or 44 daily sessions; nicotine sessions and saline sessions were intermixed (see figure 1 for experimental timeline). The experiment was conducted in two separate replications (replication 1, n=7; replication 2, n=9). Due to experimenter error, rats in the first replication received 44 acquisition sessions, whereas rats in the second replication received 32 sessions. Responding at the end of acquisition did not differ significantly between replications (Fs<2.25, p=0.156). Accordingly, the two replications were combined for all analyses. The order of the sessions was pseudo-randomly assigned with the stipulation that rats received no more than 2 consecutive days with the same type of session. Nicotine sessions consisted of a 0.4 mg/kg SC nicotine injection 5 min before placement in the chamber for a 20-min session. During each nicotine session, rats had access to 36 deliveries of 26% (w/v) sucrose (4 s each). The first sucrose delivery ranged from 124 to 152 s with an average of 137 s from the start of the session; subsequent sucrose deliveries were presented on average every 25 s (range = 4 to 80 s). On saline sessions, rats received a SC saline injection 5 min before placement in the conditioning chamber; sucrose was withheld during saline sessions. Past research has demonstrated that this training protocol would produce a robust discrimination between saline and nicotine as evidenced by increased goal-tracking, before the US is presented, during nicotine compared to saline sessions (e.g., Murray et al, 2007; Wilkinson et al, 2006).
Figure 1.

Displays the experimental timeline of Experiment 1.
US-alone Exposure
For the 3 days following acquisition, rats remained in the colony and received exposure to the sucrose US in the home cage. This protocol provided a within subjects test of whether mere exposure to the sucrose US would decrease later responding to the nicotine CS (Rescorla, 1973). Specifically, water was removed 30 min before the introduction of sucrose. Rats then received 15-min access to 100 ml of 26% sucrose in a standard water bottle. Immediately following sucrose access, rats were injected IP with saline. Sucrose consumption was recorded for each rat. Water was returned 30 min after the sucrose was removed from the home cage.
Testing following US-Alone Exposure
For the 2 days following US-alone exposure, rats were tested for conditioned responding evoked by the nicotine CS. On day 1 of testing, 0.4 mg/kg nicotine was administered 5 min before placement in the chamber for a 20-min session. During this test session, there was no access to sucrose to assess persistence of responding without the US. On day 2 of testing, 0.4 mg/kg nicotine was again administered five min before a 20-min session. During this session, sucrose was delivered on a schedule that matched acquisition training. This test allowed us to investigate how conditioned responding was affected when the US was reintroduced.
Reacquisition
Reacquisition commenced 24 h after the 2nd test. Reacquisition was identical to acquisition and consisted of 6 saline and 6 nicotine sessions intermixed as previously described.
Devaluation and Testing
Following 24 h after the last reacquisition session, rats received devaluation training. This phase was similar to the US-alone phase except that immediately following each 15-min access to sucrose, rats were injected IP with 127.2 mg/kg LiCl. Devaluation training occurred for 3 consecutive days. Following the last devaluation session, rats were again tested in the chambers for conditioned responding evoked by the nicotine CS. Testing was identical to testing following US-alone training.
Statistical Analyses
Dependent measures
The dependent measure during acquisition and reacquisition training was the rate of dipper entries per second before the first sucrose delivery or an equivalent time from the start of the session if no sucrose was available in the session. Using only dipper entries before any access to sucrose avoids any influence of US exposure on our measure of learning. For test sessions, a percentage-of-baseline-responding was calculated for each rat by dividing the rate of dipper entries before the first sucrose delivery during testing or an equivalent time in a test session without sucrose by the mean response rate for the last 3 nicotine training sessions that preceded testing, times 100. Sucrose consumption during US-alone exposure and devaluation training was measured in milliliters. Total number of dipper entries during each 5-min bin of the test sessions was also recorded to investigate how goal-tracking varied within test sessions.
Data Analyses
In acquisition and reacquisition, dipper entries were analyzed with a two-way within-subjects repeated measure analysis of variance (ANOVA) with Drug (nicotine versus saline) as one factor and Session as the repeated measure. Significant interactions were followed by paired t-tests with Bonferonni’s correction to analyze differences between saline and nicotine sessions. Test sessions were analyzed using one-sample t-tests that compared percentage of responding to a hypothetical mean of 100%; the value expected if there was no effect of the manipulation. Repeated measures ANOVAs were used to analyze difference in sucrose consumption during home cage US-alone as well as devaluation training. To analyze how goal-tracking varied within test sessions, we used a two-way within-subjects repeated measure ANOVA with Condition (US-alone versus devalue training) as one factor and 5-min Bin as the repeated measure. Paired t-tests with Bonferonni’s correction were used for post-hoc comparisons when prompted by a significant interaction. Statistical significance was declared using p<0.05 for all analyses.
Results
Acquisition
By the end of acquisition, rats discriminated between saline and nicotine sessions (last five sessions are shown in Figure 2A). Dipper entries were higher on nicotine than on saline sessions, F(1,15)=108.6, p<0.001. Neither the main effect of Session nor the Drug × Session interaction was significant, Fs<1.
Figure 2.
Panel A shows dipper entries per second (±SEM) before the first sucrose delivery (nicotine sessions) or during a comparable time (saline sessions) for the last 5 nicotine and saline acquisition sessions, US-Alone test days (nicotine sessions only), reacquisition, and devaluation test days (nicotine sessions only). Sucrose consumption (ml) during US-alone and devaluation training is also reported on Panel A. Panel B shows the percentage of baseline responding (±SEM) on the test days following US-alone training. * denotes significant differences (p<o.05) from baseline (100%; dotted line). Panel C shows the percentage of baseline responding (±SEM) on the test days following devaluation training. * denotes significant differences (p<o.05) from baseline (100%; dotted line).
US-alone Exposure and Testing
The mean sucrose consumption for the 3 days of US-alone exposure is shown in Figure 2A. There was a trend for an increase in sucrose intake across the 3 days. However, the main effect of Day did not meet the criterion for statistical significance, F(2,15)=3.3, p=0.051. On the first test of the nicotine stimulus following US-alone training (see Figure 2B), rats did not significantly differ in responding compared to their acquisition baseline,n t<1. On day 2 of testing, conditioned responding was significantly reduced at the start of the session compared to baseline levels, t(15)=2.69, p<0.05, reflecting some extinction of conditioned responding.
Reacquisition
Rats continued to discriminate between saline and nicotine sessions (Figure 2A). Responding on nicotine sessions was significantly higher than on saline sessions, F(1,5)=178.1, p<0.001. Neither the main effect of Session nor the Session × Drug interaction was significant, Fs≤1.58, p≥0.169.
Devaluation Training and Testing
Sucrose consumption decreased significantly across days (Figure 2A), F(2,15)=55.23, p<0.001.Consumption on day 2 and day 3 were significantly lower than day 1. There was no difference between day 2 and 3. Figure 2C shows conditioned responding to nicotine during devaluation testing as a percentage of baseline responding from the reacquisition phase. Following devaluation training, appetitive goal-tracking was reduced from baseline levels on both test days, ts(15)≥5.40, ps<0.001.
Within-Test Session Responding
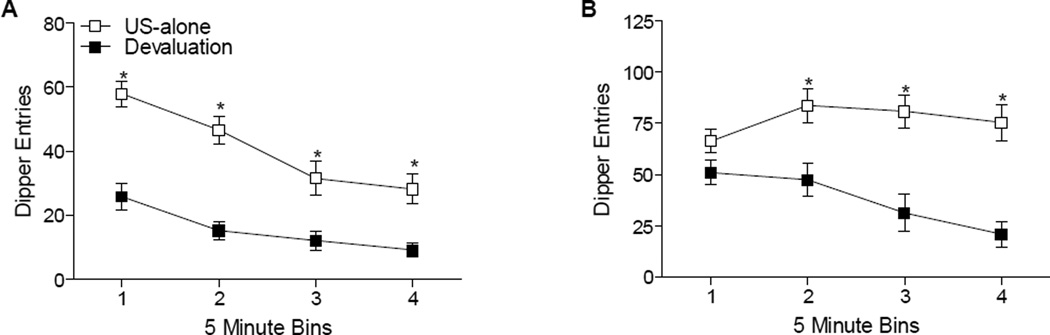
Figure 3A shows total dipper entries in 5-min bins for the first test of nicotine-evoked responding following US-alone and devaluation training. Recall that sucrose was not available during these 20-min tests so as to allow for measurement of conditioned responding without the sucrose US itself affecting responding. There were significant main effects of Condition, F(1,15)=35.39, p<0.001, and of Bin, F(3,15)=28.74, p<0.001, as well as a Condition × Bin interaction, F(3,90)=3.39, p<0.05. Conditioned responding on the first test after devaluation was lower than after US alone throughout the session. Figure 3B shows total dipper entries in 5-min bins for the second test of nicotine-evoked responding following US-alone and devaluation training, sucrose was available on the second test days. There were significant main effects of Condition, F(1,15)=14.74, p<0.001, and of Bin, F(3,15)=10.52, p<0.001, as well as a Condition × Bin interaction, F(3,90)=15.50, p<0.001. Post-hoc tests revealed no difference in the first 5 min of testing. However, dipper entries did differ for the remainder of the test session; responding following devaluation was lower than responding following US-alone conditioning. This data pattern suggests that once the sucrose was accessed later goal-tracking was deterred.
Figure 3.
Panel A shows total dipper entries (±SEM) in 5 minute bins during day 1 of testing (sucrose unavailable) following US-alone and devaluation training. * denotes significant differences (p<.05) between responding during US-alone and devaluation testing. Panel B shows total dipper entries (±SEM) in 5 minute bins during day 2 of testing (sucrose available) following US-alone and devaluation training. * denotes significant differences (p<.05) between responding during US-alone and devaluation testing.
Experiment 2
Experiment 1 used a within-subjects design to investigate the effect of devaluation of an appetitive US on interoceptive conditioning with the nicotine stimulus. We found that the nicotine-evoked conditioned response was weakened when the sucrose US was paired repeatedly with LiCl in the home cage. We saw no effect on the appetitive goal-tracking response if rats were merely exposed to sucrose without illness (US-alone phase). These findings suggest that the goal-tracking behavior controlled by the nicotine CS reflects an excitatory association between the nicotine stimulus and the sucrose US (Holland et al, 1979; Holland et al, 1975). However, before this conclusion can be accepted, an alternative account must be evaluated. There is the possibility that 3 consecutive days of illness induced by the LiCl, and not a conditioned aversion to the sucrose, reduced conditioned responding on subsequent test days. The goal of Experiment 2 was to test this alternative hypothesis. To do so, we utilized a between-subjects design with 3 groups. One group received sucrose devaluation, whereas the other two groups controlled for sucrose exposure and LiCl exposure (i.e., repeated illness). If attenuation of nicotine-evoked goal tracking in Experiment 1 was due to a devaluation of the sucrose, then only the devaluation group will show a reduction in dipper entries on the test days.
Acquisition
Acquisition training in Experiment 2 (n=24) was identical to Experiment 1 (see figure 4 for experimental timeline).
Figure 4.
Displays the experimental timeline of Experiment 2.
Devaluation
Following acquisition training, rats were randomly assigned to one of 3 groups (sucrose-LiCl; sucrose-saline; water-LiCl), with the stipulation that the groups did not significantly differ in responding at the end of acquisition. The three groups were designed to test the effects of sucrose devaluation (sucrose-LiCl), access to the US alone (sucrose-saline), or illness alone (water-LiCl). For three consecutive days, rats received 15-min access to 100 ml of their designated solution (sucrose or tap water) in the home cage, immediately followed by an injection of LiCl (sucrose-LiCl and water-LiCl groups) or saline (sucrose-saline group). As in the earlier experiment, water bottles were removed 30 min before training and then returned 30 min after the injection.
Testing
Following the devaluation phase, nicotine’s control of conditioned responding was assessed on two separate days. In each test, 0.4 mg/kg nicotine was administered 5 min before placement in the chamber for a 20-min session. On day 1 of testing, sucrose deliveries were withheld. On the second test day, sucrose was delivered on a schedule identical to that during acquisition. This testing protocol matches that of devaluation testing in Experiment 1.
Statistical Analyses
The analyses of Experiment 2 were identical to Experiment 1 with 2 exceptions. First, a repeated measures ANOVA was used to analyze difference in sucrose consumption between groups during devaluation training. Second, a two-way (between subjects) repeated measure ANOVAs with Group (sucrose-LiCl; sucrose-saline; water-LiCl) as one factor and Bin as the repeated measure was used to analyze group differences in within session responding during test 1 and test 2.
Results
Acquisition
Responding during the last five sessions of acquisition is shown in Figure 5A. Rats readily discriminated between saline and nicotine sessions displaying more responding during nicotine sessions, F(1,23)=79.45, p<0.001. Although there was a main effect of Session, F(4,184)=4.13, p<0.01, the Drug × Session interaction was not significant F<1.
Figure 5.
Panel A Shows dipper entries per second (±SEM) before the first sucrose delivery (nicotine sessions) or during a comparable time (saline sessions) for the last 5 nicotine and 5 saline acquisition sessions and for testing following devaluation training. Panel B shows liquid consumption (ml) during the 3 devaluation exposure days. Panel C shows the percentage of baseline responding (±SEM) on test day 1 following devaluation training * denotes significant differences (p<0.05) from baseline (100%; dotted line). Panel D shows the percentage of baseline responding (±SEM) on test day 2 following devaluation training. * denotes significant differences (p<0.05) from baseline (100%; dotted line).
Devaluation Training
Mean liquid consumption on each test day is shown in Figure 5B (inset into Figure 5A). There was a main effect of Group, F(2,42)=153.0, p<s0.001, a main effect of Day, F(2,42)=9.80, p<0.001, and a Group × Day interaction, F(4,42)=5.06, p<0.01. Intake was significantly lower on all days in the water-LiCl group compared to the sucrose-saline group and lower than the sucrose-LiCl group on day 1 and 2. The sucrose-LiCl and sucrose-saline group had similar levels of consumption on day 1. However, consumption in the sucrose-LiCl group was reduced in comparison to the both sucrose-saline group on day 2 and 3, as well as their own consumption level on day 1.
Devaluation Testing
Nicotine-evoked goal-tracking behavior following devaluation is shown in Figure 5C. On day 1, the group that had the US devalued (sucrose-LiCl) displayed significantly lower levels of responding early in the session compared to their nicotine baseline levels from the end of acquisition, t(7)=11.20, p<0.001. In contrast, the US-alone group (sucrose-saline), t(7)=1.86, p=0.106, and the illness only group (water-LiCl), t(7)1.54, p=0.168, did not differ significantly from their respective baselines. A similar pattern was seen on day two of testing (Figure 5D). The sucrose-LiCl group continued to display attenuated levels of responding compared to baseline, t(7)=2.50, p=0.04. The sucrose-saline and water-LiCl groups did not differ from their baseline, ts<1.
Within-Test Session Responding
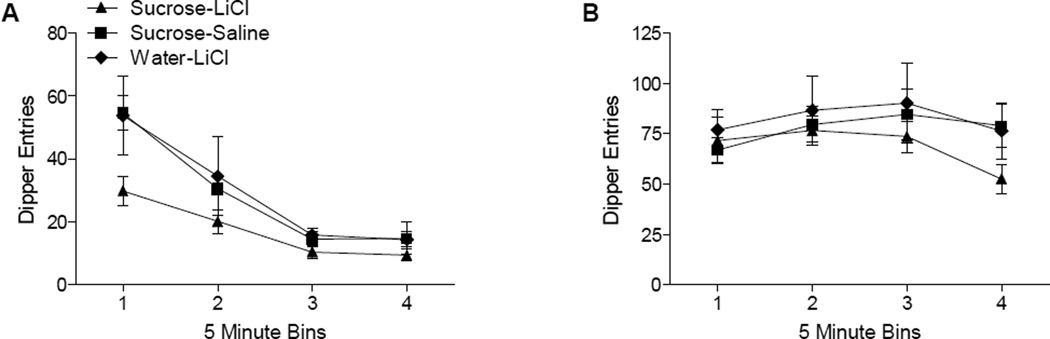
Analysis of dipper entries across the day 1 test session (Figure 6A), with no sucrose available, revealed that responding decreased across the 5 min bins, F(3, 63)=4.95, p<0.001, indicating sensitivity to removal of the US. Although responding was lower in sucrose-LiCl group, especially in the first 2 bins, there was no main effect of Group, F(2,63)=2.49, p=0.107, and no Group × Bin interaction, F(6,63)=1.24, p=0.297. Analysis of dipper entries across the day 2 test session, with sucrose available, (Figure 6B) revealed a main effect of Bin, F(3,63)=4.95, p<0.01, but no main effect of Group, F<1, or a Group × Bin interaction, F(6,63)=1.69, p=0.138. For Experiment 2, the devaluation effects appeared to be strongest early in the session (i.e., with the first few minutes; recall Figure 5C and 5D. This pattern differed from Experiment 1 where the effect was longer lasting whether sucrose was or was not available during the test session. One possible explanation for these differences is the within- vs. between-subjects design of Experiment 1 and Experiment 2, respectively. Perhaps experiencing sucrose alone in the home cage across several days has a long lasting effect when it is later devalued in the same cage. Of course, to ascertain the nature of this would require further experimentation on an issue that is tangential to the primary goal of the present studies— determining whether interoceptive conditioning with a nicotine stimulus is susceptible to devaluation.
Figure 6.
Panel A shows total dipper entries by group (± SEM) in 5 minute bins during day 1 of testing (sucrose unavailable) following devaluation training. Panel B shows total dipper entries by group (±SEM) in 5 minute bins during day 2 of testing (sucrose available) following devaluation training.
Discussion
The role of interoceptive conditioning in drug addiction more generally, and nicotine dependence more specifically, remains a major theoretical and empirical area requiring much more inquiry (Bevins et al, 2011; Verdejo-Garcia et al, 2012). Diminishing control of interoceptive conditioning over acquired appetitive behaviors seems of particular importance to advancing our understanding of the behavioral and neural processes involved in interoceptive drug conditioning. Previous research has demonstrated attenuation of nicotine-controlled behavior utilizing a variety of tactics that include extinction (Besheer et al, 2004), pharmacological blockade (Besheer et al, 2004; Murray et al, 2007; Struthers et al, 2009), and transfer of extinction learning (Bevins et al, 2012; Reichel et al, 2010). An alternative behavioral approach to attenuating nicotine’s control over acquired behavior examined in the present report was US devaluation. We found in two separate experiments that when the sucrose US was repeatedly paired with LiCl-induced illness that the subsequent control of conditioned responding by the nicotine stimulus was attenuated. To our knowledge, this set of experiments is the first demonstration of alteration of conditioned responding to an interoceptive stimulus using a US devaluation approach.
These findings suggest that the goal-tracking behavior evoked by the nicotine CS reflects an excitatory association between the nicotine stimulus and the sucrose US. This supports the notion that the interoceptive Pavlovian association is a stimulus-stimulus association, and not a stimulus-response association (see discussion below). Before we accept this conclusion, however, several alternative accounts should be considered. The first alternative account for a reduction in conditioned responding is that attenuation may be produced by extensive and repeated exposure to the sucrose [i.e., US habituation; cf. (Rescorla, 1973)]. This alternate account seems unlikely given that nicotine-controlled behavior was not reduced on day 1 of testing following US-alone exposure in a between or within-subjects design, but was attenuated following devaluation. A second alternate account is that illness on 3 consecutive days affected subsequent goal-tracking during test sessions independent of a conditioned aversion to the sucrose US. To test this account, Experiment 2 included a control group that received LiCl alone (i.e., the water-LiCl group) across 3 consecutive days. Nicotine-controlled behavior was not reduced in the LiCl alone group, providing evidence that the reduction in goal-tracking behavior early in the test sessions for the devalued condition was not from sickness alone.
As noted earlier, devaluation research over the past 40 years has focused on exteroceptive stimuli. Take the seminal work by Holland and Rescorla (1975) as an example. In this research, rats had an exteroceptive CS (tone) repeatedly paired with an appetitive US (sucrose) during initial training. This excitatory conditioning was followed by pairing the sucrose US with high speed rotation (illness) in a separate context from the training environment. Subsequent tests revealed that sucrose-illness pairings reduced conditioned response controlled by the tone CS. The authors concluded that the CS elicits a “representation” of the US which can be devalued by subsequent pairings with sickness. It is the devaluation of this “representation” that produces diminished responding to the CS (cf. Holland and Rescorla, 1975; Ostlund and Balleine, 2007; Reichelt et al., 2011).
Notably, the present set of experiments extends the utility of devaluation procedure to an interoceptive stimulus. The US devaluation effect, seen across both studies, suggests that interoceptive conditioning involving the nicotine CS in the discriminated goal-tracking task reflects a nicotine stimulus-sucrose reinforcer (US) association. The current experiments focused on attenuation of nicotine-controlled behavior by pairing sucrose with illness. This is just one approach to US devaluation. Future research could examine whether a US satiation procedure also diminishes the acquired appetitive behavior controlled by the nicotine stimulus; this could be achieved by giving prolonged sucrose access immediately before CS testing. Future research could also investigate the possibility of revaluation to increase the value of the US by pairing it with a second reinforcer following CS-US training (Rescorla, 1974). If successful, goal-tracking behavior controlled by the nicotine CS would be potentiated. Also of interest in future studies is the underlying neural mechanisms involved with devaluation of a US associated with an interoceptive CS. Previous research with exteroceptive stimuli indicates a role for the orbitofrontal cortex and the basolateral amygdala in the alteration of incentive value of stimuli (Gallagher et al, 1999; Hatfield et al, 1996; Pickens et al, 2003). Whether similar or distinct neural processes also play a role when the CS is an interoceptive stimulus has yet to be determined.
The experiments reported here have advanced our understanding of interoceptive conditioning involving the nicotine stimulus. Namely, the present research provides the best evidence to date that nicotine has acquired appetitive effects that reflect a direct association between the nicotine stimulus and the appetitive sucrose US. We do not necessarily believe that practitioners should directly translate this devaluation protocol and apply it to individuals trying to quit their tobacco use habit. Rather, these finding should prompt awareness of the potential role interoceptive conditioning may have in the addiction process, as well as reflection on how cognitive-behavioral and pharmacological strategies (Conklin et al, 2002; Eisenberg et al, 2008; Gonzales et al, 2006; Hughes et al, 2007; Jorenby et al, 2006; Niaura et al, 1999) could be improved in light of what we know about interoceptive conditioning (Bevins & Murray, 2011).
Acknowledgements
The authors would like to thank Lindsey C. Zeplin for her assistance in conducting the experiments, as well as Scott T. Barrett and Sergey Charntikov for their insightful conversations regarding the experiments and there design. The research and Rick A. Bevins were partially supported by the United States Public Health Service Grant DA018114, DA023951, and DA034389. All Med-PC programs used in this research are available upon request.
References
- Besheer J, Palmatier MI, Metschke DM, Bevins RA. Nicotine as a signal for the presence or absence of sucrose reward: a Pavlovian drug appetitive conditioning preparation in rats. Psychopharmacology (Berl) 2004;172(1):108–117. doi: 10.1007/s00213-003-1621-9. [DOI] [PubMed] [Google Scholar]
- Bevins RA. Altering the motivational function of nicotine through conditioning processes. Nebr Symp Motiv. 2009;55:111–129. doi: 10.1007/978-0-387-78748-0_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevins RA, Barrett ST, Polewan RJ, Pittenger ST, Swalve N, Charntikov S. Disentangling the nature of the nicotine stimulus. Behav Processes. 2012;90(1):28–33. doi: 10.1016/j.beproc.2011.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevins RA, Besheer J, Pickett KS. Nicotine-conditioned locomotor activity in rats: dopaminergic and GABAergic influences on conditioned expression. Pharmacol Biochem Behav. 2001;68(1):135–145. doi: 10.1016/s0091-3057(00)00451-2. [DOI] [PubMed] [Google Scholar]
- Bevins RA, Murray JE. Internal stimuli generated by abused substances: Associative learining and conditioning and its implications for drug addiction. In: Schachtman TR, Reilly SS, editors. Associative Learning and Conditioning Theory: Human and Non-Human Applications. New York: Oxford University Press; 2011. [Google Scholar]
- Charntikov S, Tracy ME, Zhao C, Li M, Bevins RA. Conditioned response evoked by nicotine conditioned stimulus preferentially induces c-Fos expression in medial regions of caudate-putamen. Neuropsychopharmacology. 2012;37(4):876–884. doi: 10.1038/npp.2011.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin CA, Tiffany ST. Applying extinction research and theory to cue-exposure addiction treatments. Addiction. 2002;97(2):155–167. doi: 10.1046/j.1360-0443.2002.00014.x. [DOI] [PubMed] [Google Scholar]
- Eisenberg MJ, Filion KB, Yavin D, Bélisle P, Mottillo S, Joseph L, et al. Pharmacotherapies for smoking cessation: a meta-analysis of randomized controlled trials. CMAJ. 2008;179(2):135–144. doi: 10.1503/cmaj.070256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farwell BJ, Ayres JJB. Stimulus-reinforcer and response-reinforcer relations in the control of conditioned appetitive headpoking ('goal tracking') in rats.: Learning and Motivation. 1979;Vol 10:295–312. [Google Scholar]
- Gallagher M, McMahan RW, Schoenbaum G. Orbitofrontal cortex and representation of incentive value in associative learning. J Neurosci. 1999;19(15):6610–6614. doi: 10.1523/JNEUROSCI.19-15-06610.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales D, Rennard SI, Nides M, Oncken C, Azoulay S, Billing CB, et al. Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: a randomized controlled trial. JAMA. 2006;296(1):47–55. doi: 10.1001/jama.296.1.47. [DOI] [PubMed] [Google Scholar]
- Hatfield T, Han JS, Conley M, Gallagher M, Holland P. Neurotoxic lesions of basolateral, but not central, amygdala interfere with Pavlovian second-order conditioning and reinforcer devaluation effects. J Neurosci. 1996;16(16):5256–5265. doi: 10.1523/JNEUROSCI.16-16-05256.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institutes of Health. U.S. Department of Health and Human Services; 1998. Tobacco Addiction. Vol NIH Publication Number 09-4342. [Google Scholar]
- Holland PC, Straub JJ. Differential effects of two ways of devaluing the unconditioned stimulus after Pavlovian appetitive conditioning. J Exp Psychol Anim Behav Process. 1979;5(1):65–78. doi: 10.1037//0097-7403.5.1.65. [DOI] [PubMed] [Google Scholar]
- Holland PC, Rescorla RA. The effect of two ways of devaluing the unconditioned stimulus after first- and second-order appetitive conditioning. J Exp Psychol Anim Behav Process. 1975;1(4):355–363. doi: 10.1037//0097-7403.1.4.355. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Stead LF, Lancaster T. Antidepressants for smoking cessation. Cochrane Database Syst Rev. 2007;(1) doi: 10.1002/14651858.CD000031.pub3. CD000031. [DOI] [PubMed] [Google Scholar]
- Jorenby DE, Hays JT, Rigotti NA, Azoulay S, Watsky EJ, Williams KE, et al. Efficacy of varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained-release bupropion for smoking cessation: a randomized controlled trial. JAMA. 2006;296(1):56–63. doi: 10.1001/jama.296.1.56. [DOI] [PubMed] [Google Scholar]
- Murray JE, Bevins RA. Behavioral and neuropharmacological characterization of nicotine as a conditional stimulus. Eur J Pharmacol. 2007;561(1–3):91–104. doi: 10.1016/j.ejphar.2007.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niaura R, Abrams DB, Shadel WG, Rohsenow DJ, Monti PM, Sirota AD. Cue exposure treatment for smoking relapse prevention: a controlled clinical trial. Addiction. 1999;94(5):685–695. doi: 10.1046/j.1360-0443.1999.9456856.x. [DOI] [PubMed] [Google Scholar]
- Ostlund SB, Balleine BW. The contribution of orbitofrontal cortex to action selection. Ann N Y Acad Sci. 2007;1121:174–192. doi: 10.1196/annals.1401.033. [DOI] [PubMed] [Google Scholar]
- Pickens CL, Saddoris MP, Setlow B, Gallagher M, Holland PC, Schoenbaum G. Different roles for orbitofrontal cortex and basolateral amygdala in a reinforcer devaluation task. J Neurosci. 2003;23(35):11078–11084. doi: 10.1523/JNEUROSCI.23-35-11078.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichel CM, Linkugel JD, Bevins RA. Nicotine as a conditioned stimulus: impact of attention-deficit/hyperactivity disorder medications. Exp Clin Psychopharmacol. 2007;15(5):501–509. doi: 10.1037/1064-1297.15.5.501. [DOI] [PubMed] [Google Scholar]
- Reichel CM, Murray JE, Barr JD, Bevins RA. Extinction with varenicline and nornicotine, but not ABT-418, weakens conditioned responding evoked by the interoceptive stimulus effects of nicotine. Neuropharmacology. 2010;58(8):1237–1245. doi: 10.1016/j.neuropharm.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichelt AC, Lin TE, Harrison JJ, Honey RC, Good MA. Differential role of the hippocampus in response-outcome and context-outcome learning: evidence from selective satiation procedures. Neurobiol Learn Mem. 2011;96(2):248–253. doi: 10.1016/j.nlm.2011.05.001. [DOI] [PubMed] [Google Scholar]
- Rescorla RA. Effect of US habituation following conditioning. J Comp Physiol Psychol. 1973;82(1):137–143. doi: 10.1037/h0033815. [DOI] [PubMed] [Google Scholar]
- Rescorla RA. Effects of inflation of the unconditioned stimulus value following conditioning. Journal of Comparative and Physiological Psychology. 1974;86 [Google Scholar]
- Department of Health and Human Services. Results from the 2007 National Survey on Drug Use and Health: National Findings. Rockville, MD: Office of Applied Studies; 2008. Vol NSDUH Series H-34. [Google Scholar]
- Struthers AM, Wilkinson JL, Dwoskin LP, Crooks PA, Bevins RA. Mecamylamine, dihydro-beta-erythroidine, and dextromethorphan block conditioned responding evoked by the conditional stimulus effects of nicotine. Pharmacol Biochem Behav. 2009;94(2):319–328. doi: 10.1016/j.pbb.2009.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdejo-Garcia A, Clark L, Dunn BD. The role of interoception in addiction: a critical review. Neurosci Biobehav Rev. 2012;36(8):1857–1869. doi: 10.1016/j.neubiorev.2012.05.007. [DOI] [PubMed] [Google Scholar]
- Wilkinson JL, Murray JE, Li C, Wiltgen SM, Penrod RD, Berg SA, et al. Interoceptive Pavlovian conditioning with nicotine as the conditional stimulus varies as a function of the number of conditioning trials and unpaired sucrose deliveries. Behav Pharmacol. 2006;17(2):161–172. doi: 10.1097/01.fbp.0000197456.63150.cd. [DOI] [PubMed] [Google Scholar]