Abstract
Hematopoiesis is regulated by the bone marrow (BM) niche microenvironment. We recently found that post transplant administration of AMD3100 (a specific and reversible CXCR4 antagonist) enhanced donor cell engraftment and promoted recovery of all donor cell lineages in a congeneic mouse transplant model. We hypothesized that AMD3100 enhances donor cell reconstitution in part by modulating the levels and constitution of soluble factors in the niche microenvironment. In the current study, the effects of the BM extracellular fluid (supernatant) from AMD3100-treated transplant recipient mice on colony forming units (CFUs) were examined. A semi-quantitative, mass spectrometry-based proteomics approach was used to screen for differentially expressed proteins between the BM supernatants of PBS-treated transplant mice and AMD3100-treated transplant mice. A total of 178 proteins were identified in the BM supernatants. Thioredoxin was among the 32 proteins that displayed >2-fold increase in spectral counts in the BM supernatant of AMD3100-treated transplant mice. We found that thioredoxin increased CFUs in a dose-dependent manner. Thioredoxin improved hematopoiesis in irradiated mice and protected mice from radiation-related death. Furthermore, ex vivo exposure to thioredoxin for 24 hours enhanced the long-term repopulation of hematopoietic stem cells. Additionally, combined post transplant administration of thioredoxin and AMD3100 improved hematological recovery in primary and secondary transplant recipient mice. Our studies demonstrated that factors in the BM niche microenvironment play a critical role in hematopoiesis. Identifying these factors provides clues on potential novel targets that can be used to enhance hematological recovery in hematopoietic stem cell transplantation.
Keywords: mass spectrometry, proteomic analysis, niche microenvironment, CXCR4 antagonist, thioredoxin, hematopoietic stem cell transplantation, radioprotection
INTRODUCTION
Hematopoietic stem cell transplantation (HCT) provides a potentially curative treatment approach for a wide variety of diseases. HCT, however, is associated with high incidence of morbidity and mortality. About 25% of patients die within one year of transplant from transplant-related complications. The speed of donor cell engraftment and hematopoietic recovery has been correlated with patients’ overall survival and transplant outcomes. Recombinant colony stimulating factor-granulocytes is commonly used in HCT to enhance neutrophil recovery [1]; however, its effects are limited to myeloid cell lineage [2]. Furthermore, over the last few decades we have seen advances in identifying molecules that regulate HSC expansion and reconstitution[3–7], however, genetically modifying hematopoietic stem cells (HSCs) with these molecules has several limitations and is not readily translatable into the clinic. There is an unmet medical need to develop novel approaches for enhancing hematological recovery in HCT.
Following HCT, donor HSCs home to the bone marrow (BM), engraft, and eventually reconstitute the entire hematological and immunological repertoire of the recipient [8, 9]. HSC homing is not a random event, and transplanted HSCs are not evenly distributed in the BM space. BM space is compartmentalized as individual specialized microenvironments, termed “niches” [10], that provide cellular and molecular support for HSCs to reside, proliferate, and differentiate [11]. HSCs in the niches are regulated by: i). Cytokines and chemokines [12–14]; ii). Supportive cellular elements such as mesenchymal stem cells and endothelial cells and their secreted, soluble molecules or membrane-bound factors [11]; iii). The nervous system and neurotransmitters [15, 16]; and iv). Extracellular matrix.
The interaction between stromal-derived factor-1 (SDF-1, now termed CXCL12) and the CXCR4 chemokine receptor plays a critical role in hematopoiesis after HCT. The SDF-1/CXCR4 interaction is important in attracting HSCs into the niches, anchoring HSCs in the niches, and maintaining HSCs in a quiescent state [17–20]. Plerixafor (Genzyme Corp, Brandname for AMD3100), is a specific and reversible CXCR4 antagonist and is currently approved by the FDA to be used in pre-transplant setting for stem cell collection. We recently found that giving AMD3100 subcutaneously every other day beginning at day +2 after transplant selectively enhanced donor cell engraftment and promoted recovery of all donor cell lineages in a congeneic mouse transplant model [21]. Additionally, we found that post transplant administration of AMD3100 significantly improved animal survival, likely resulting from a significant reduction in the levels of pro-inflammatory cytokines/chemokines [21]. Enhancing donor cell engraftment and reducing transplant-related mortality are very important goals in HCT and our approach offers a potentially effective, novel approach to improve the care and outcome of HCT patients. We have recently initiated a two medical center (Duke University and Medical University of South Carolina), phase I/II clinical trial to test the safety and efficacy of post transplant administration of plerixafor in myeloablative allogeneic HCT patients (ClinicalTrials.gov: NCT01280955).
The detailed mechanisms underlying the enhanced donor cell engraftment with post transplant administration of AMD3100 are not completely understood. CXCR4 is expressed not only on HSCs, but also on mesenchymal stem cells, endothelial cells, T cells, dendritic cells, neurons, and other cells. All of these cells constitute BM niche microenvironments and regulate hematopoiesis either through cell-cell interaction or through secretion of soluble factors including cytokines and chemokines. In the current study, we aimed to understand the effects of post transplant administration of AMD3100 on the BM niche microenvironement and to identify the soluble factors that can potentially be used to enhance hematological recovery in HCT.
METHODS AND MATERIALS
Animals
Eight to twelve-week-old C57BL/6 CD45.2 mice were purchased from the Jackson Laboratories (Bar Harbor, ME). Breeding pairs of C57BL/6 CD45.1 (BSJL) mice were purchased from the Jackson Laboratories and their offspring produced and maintained in our pathogen-free animal facility at the Medical University of South Carolina (MUSC). Studies were performed in accordance with MUSC Institutional Animal Care and Use Committee-approved procedures.
Antibodies and reagents
AMD3100 and thioredoxin-1 (Trx) were purchased from Sigma-Aldrich (St Louis, MO). Biotin-conjugated anti- mouse CD3e (145-2C11), CD4 (RM4-5), CD5 (53-7.3), CD8a (53-6.7), CD11b (M1/70), B220 (RA3-6B2), Gr-1 (RB6-8C5), TER-119 (TER119); Allophycocyanin (APC)-conjugated anti-mouse CD117 (c-Kit, 2B8); Phycoerythrin (PE)-conjugated anti- mouse Sca-1 (D7); APC-conjugated anti- mouse CD3 (145-2C11); APC-Cy7-conjugated anti- mouse Gr-1 (RB6-8C5); Fluorescein isothiocyanate (FITC)-conjugated anti- mouse CD45.2 (104); PE-conjugated anti- mouse CD45.1 (A20); and APC-conjugated anti- mouse CD45.1-PE (A20) were purchased from BD Pharmingen (San Diego, CA). PE-Cy5.5-conjugated anti- mouse B220 (RA3-6B2) was purchased from eBiosciences (San Diego, CA). The secondary antibodies were FITC-labeled streptavidin (BD Pharmingen) and Brilliant Violet 605-labeled streptavidin (Biolegend). Anti-mouse thioredoxin antibody was purchased from Cell Signaling (Danvers, MA).
Preparation of BM supernatants from PBS- or AMD3100- treated transplant recipient mice
Red blood cell (RBC)-depleted BM cells were obtained from C57BL/6 CD45.1 mice and enriched for Lineage negative (Lin−) cells using murine lineage cell depletion kit (Miltenyi Biotec, Auburn, CA). Lin− BM cells were subsequently stained with a cocktail of primary monoclonal antibodies consisting of biotin-conjugated anti- mouse CD3, CD4, CD5, CD8a, CD11b, B220, Gr-1, anti-TER-119, as well as APC-conjugated anti- mouse c-Kit and PE-conjugated anti-mouse Sca-1 antibodies, followed by secondary staining with FITC-labeled streptavidin. Propidium iodide (1 µg/ml) was added to the cell suspension to exclude dead cells. The stained BM cells were sorted for Lin−/lowSca-1+c-Kit+ (LSK) HSCs using MoFlo cell sorter (Beckman). Sorted LSK cells were then injected via tail vein to lethally irradiated (10.5 Gy) C57BL/6 CD45.2 mice within 4 hours after irradiation (250 LSK cells per recipient mouse).
The transplant recipient mice were injected subcutaneously with PBS buffer or AMD3100 at 5mg/kg body weight in a volume of 100 µl every other day beginning at day +2 post transplant for a total of 3 doses. The mice were then sacrificed at day +7 post transplant and the two femurs and two tibias of each mouse were harvested. We have performed 3 separate sets of experiments with 10 mice in each group of each experiment. In our first set of experiments, all femurs and tibias from the 10 mice of each treatment group were combined and flushed with 500 µl of PBS. In our second and third sets of experiments, the mice were divided into 4 groups and 5 femurs and 5 tibias were combined and flushed with 300 µl of PBS. The flushed BM suspension was then centrifuged at 15,000 rpm for 5 mins, and the BM supernatants collected for colony forming unit (CFU) assay and for proteomics analysis. The BM cell pellets were used for real time (RT)-PCR measurement of thioredoxin as described below.
CFU assays
The effects of BM supernatants and thioredoxin on CFUs were performed similarly. Briefly, BM mononuclear cells (MNCs) were isolated from sublethally irradiated (5.5 Gy) C57BL/6 mice and plated (5 × 105 cells/dish) in Methocult® GF 3434 (StemCell Technology, Vancouver, Canada) that contained 30 µl/dish of RPMI1640 medium, RPMI1640 medium with 0.78 µg/ml AMD3100, BM supernatant from PBS- treated transplant recipient mice, BM supernatant from AMD3100- treated transplant recipient mice, or various concentrations of thioredoxin. CFUs were performed in triplicates. BM CFUs from primary and secondary transplant recipient mice were also performed at 5 × 104 cells/dish. CFUs-granulocyte and monocyte (GM), Burst forming units-erythroid (BFUs-E) and CFUs-granulocyte, erythrocyte, monocyte, megakaryocyte (GEMM) were counted at day 7, day 9 and day 12, respectively.
Proteomic analysis
Protein concentration in the supernatant was determined using the Pierce 660nm protein assay (Thermo Scientific). A total of 20 µg protein from each pooled sample was resuspended in 150 µl ammonium bicarbonate and 0.12% Rapigest (Waters) for a final protein concentration of 0.13µg/µl. The samples were reduced in 5mM DTT at 60°C for 30 min and alkylated in 15mM iodoacetamide for 30min. Trypsin (Promega) was added to the sample at 1:20 and incubated overnight at 37°C. Tryptic digestion was halted by acidifying the sample by adding 1.8ml 0.1% formic acid. Peptides were isolated using solid phase extraction cartridges (Phenomenex Strata-X, 60mg). Cartridges were conditioned with methanol and 0.1% formic acid and the sample was passed through the cartridge, washed twice with 0.1% formic acid, followed by a wash containing 5% acetonitrile. Peptides were eluted stepwise in 0.1% formic acid solution containing 20%, 25%, 30%, 35%, 40%, 60% acetonitrile. Each fraction was dried using a vacuum concentrator and resuspended in 50µl mobile phase A (MPA; 98% water/2% acetonitrile/ 0.1% formic acid). Peptide concentration was estimated by absorbance at 280nM to ensure that no more than 2µg peptide was injected per fraction. Five microliters of each fraction was injected by autosampler and loaded onto an Acclaim PepMap 100 c18 trap column (100 µm ID x 2 cm, C18, 5 µm, 100 Å; Thermo Scientific). Trapped peptides were washed with 15µl MPA prior to separation on an Acclaim PepMap100 analytical column (75 µm ID x 15 cm, C18, 3 µm, 100 Å; Thermo Scientific). Peptides were separated using a three step gradient at 300nl/min: step 1) mobile phase B (MPB; 2% water/98% acetonitrile/0.1% formic acid) was increased from 5% to 35% over 30 minutes, step 2) MPB was increased from 35% to 60% over 12 minutes, step 3) MPB was increased from 60% to 80% over 1 minute and held at 80% MPB for 4 minutes. Peptides were eluted into the mass spectrometer source using 10µm nanospray tips (New Objective) and tandem mass spectrometry was performed using an AB SCIEX Triple TOF 5600 mass spectrometer. Parent ion TOF scans were conducted across 400–1800 m/z with an accumulation time of 0.25 seconds. Product ion scans of parent ions between 400–1250 m/z were conducted using the information dependent acquisition mode. Briefly, 20 candidate parent ions were monitored per cycle in high sensitivity mode with exclusion of former target ions for 10 seconds after one occurrence. Product ion data were collected in high sensitivity mode between 75 – 2000 m/z with an accumulation time of 0.05 seconds. Mass tolerance was set to 50 mDa and isotopes were excluded within 4 Da. Rolling collision energy was selected. Total cycle time was 1.3 seconds.
Data files for each run were converted to Mascot generic format (MGF) using a conversion tool provided by AB SCIEX version 1.1beta. MGF files were merged and searched using Mascot (version 2.3.02) against the mouse protein database (UniprotKB/Swiss-Prot version 2012_03, 24,410 entries) with addition of common contaminants. Digestion enzyme was set to trypsin allowing for 2 missed cleavages, parent ion tolerance was set to 10 ppm, and fragment tolerance was set to 0.25 Da. Fixed modification was +57 on C (carbamidomethyl) and variable modifications included: +16 on M (oxidation), +1 on N/Q (deamidation), and −17 on Q n-terminal (pyro-Glu). Mascot search results were uploaded to SCAFFOLD (version 3.5.1) for viewing. Spectral count data was normalized using the quantitative value tool. Protein identifications were included for analysis at two levels of false discovery. A conservative list was generated for proteins identified with 3 unique peptides, with a threshold of 80%, and with a protein threshold of 99% to ensure a conservative false discovery rate (less than 0.1%). Normalized spectral count fold change was calculated using normalized spectral counts and proteins that displayed a two-fold change in spectral count data were listed.
The data associated with this manuscript may be downloaded from the ProteomeCommons.org Tranche network using the following hash: XselMgl3o2tPEVec4osCyCwmg4zwjJNJ0vscXO79Tim16UtLenDsxm3JS9OJxarK1Y8js4 1dmfs7Wl79SyRWMnz5tgwAAAAAAYm8AA==.
Thioredoxin quantitative RT-PCR
BM cells were harvested from PBS- or AMD3100- treated transplant recipient mice and total RNA extracted. Quantitative RT-PCR was performed using iQ SYBR green supermix (Bio-Rad, Hercules, CA). The primers to amplify thioredoxin were 5’-GCCAAAATGGTGAAGCTGAT and 5’-TGATCATTTTGCAAGGTCCA. The actb was used as reference gene and amplified with the primers, 5’-GATCTGGCACCACACCTTCT and 5’-GGGGTGTTGAAGGTCTCAAA.
Thioredoxin for radioprotection
C57BL/6 mice were given 9.5 Gy total body irradiation using Cesium irradiator. Two hours after the irradiation, the mice were injected intraperitoneally with PBS buffer or thioredoxin at 32 µg/mouse in a volume of 100 µl and then every other day for a total of 5 doses. Animal survival was monitored daily. Blood samples were collected at day +7 post irradiation and peripheral blood cell counts were measured using Beckman automatic cell counter.
BM transplantation with thioredoxin- pretreated HSCs
LSK cells were obtained from C57BL/6 CD45.1 mice and cultured in StemPro®-34 SFM media (Invitrogen) containing stem cell factor (SCF, 100ng/ml) and thrombopoietin (TPO-1, 100ng/ml) with or without thioredoxin (10µg/ml) for 24 hrs. Cells were then harvested, washed, counted, and injected via tail vein to lethally irradiated (9.5Gy) C57BL/6 CD45.2 mice (2,000 LSK cells per recipient mouse). Peripheral blood hematological recovery and donor cell engraftment were followed. At 11 weeks post transplant, the mice were sacrificed and BM harvested for CFU assay, flow cytometry analysis, and secondary transplantation.
BM transplantation with post transplant administration of thioredoxin and/or AMD3100
LSK cells were isolated from C57BL/6 CD45.1 mice and cultured in StemPro®-34 SFM media with SCF (100ng/ml) and TPO-1 (100ng/ml) for 24 hrs. The cells were then injected intravenously to lethally irradiated (9.5Gy) C57BL/6 CD45.2 mice (2,000 cells/recipient). At Day +2 post transplant, the recipient mice were injected with: 1) PBS buffer subcutaneously (100µl/mouse every other day for 8 weeks); 2) AMD3100 subcutaneously (5 mg/kg body weight in a volume of 100 µl every other day for 8 weeks); 3) thioredoxin intraperitoneally (32 µg/mouse in a volume of 100 µl every other day for a total of 6 doses); or 4) AMD3100 plus thioredoxin (i.e., AMD3100 subcutaneously at 5 mg/kg body weight every other day for 8 weeks + thioredoxin intraperitoneally at 32 µg/mouse every other day for a total of 6 doses).
Secondary BM transplantation
The primary transplanted recipient mice were sacrificed at 11 weeks post transplant and BM cells were collected. The BM cells were injected into lethally irradiated C57BL/6 CD45.2 mice (6´106 BM cells/recipient, 8 mice/group, one primary to one secondary matched transplant).
Measurement of donor cell engraftment and hematological recovery
Peripheral blood donor cell engraftment was measured as described previously [21]. Briefly, 50µl of whole blood was collected at various time-points following transplant and stained with a combination of cell subset-specific antibodies for 15 minutes at room temperature. The stained blood samples were then processed in BD FACS™ Lysing solution. Flow-Count fluorospheres (50 µL; Beckman-Coulter) was added into the samples and the samples were analyzed using BD Fortessa analyzer. Whole blood cell counts including white blood cell (WBC) count, RBC count, hemoglobin count and platelet count were measured using a Scil plus hematology analyzer (Gurnee, IL) as per the manufacturer's instructions.
BM donor cell engraftment was determined in primary and secondary transplanted recipient mice at 11 and12 weeks post transplant, respectively. RBC-depleted BM cells from 1 tibia and 1 femur were counted and 2×106 cells were stained with indicated antibodies and analyzed for donor CD45.1+ LSK cells. The absolute number of donor LSK cells in 2 femurs and 2 tibias was calculated and shown.
Statistical Analysis
The values were reported as Mean ± SEM of combined data of multiple experiments or as Mean ± SD from a representative experiment. All experiments were performed at least 2 times. Differences were analyzed by Student's t test. p<0.05 was regarded as significant and indicated in the text or figure legends.
RESULTS
1. The BM supernatant of AMD3100- treated transplant recipient mice contains factors that promote the growth of hematopoietic progenitor cells
To test if post transplant administration of AMD3100 affected the BM niche microenvironment, we collected the BM extracellular fluids (also named as BM supernatants) at day +7 post transplant by flushing the bones with PBS and centrifuging the bone marrow suspension. We chose day +7 post transplant because our previous studies indicated that at this time-point, the plasma cytokine profile was clearly affected by AMD3100 treatment [21]. The BM supernatants were then tested for their effects on hematopoietic progenitors by adding them to the CFU assay using marrow cells isolated from sublethally (5.5 Gy) irradiated mice. We used BM cells harvested from irradiated mice for the CFU assay to more directly evaluate the influence of the BM microenvironment on HSCs that have been radiated, thus mimicking the setting of radiation preconditioning.
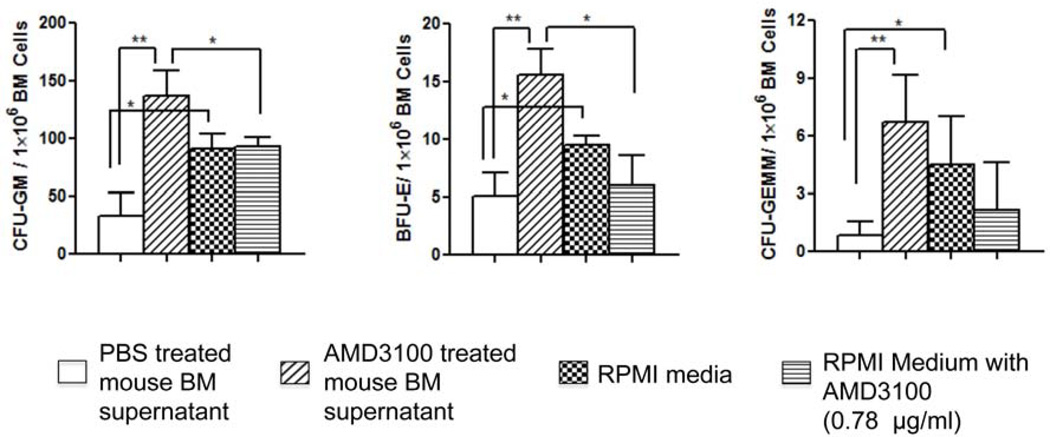
As shown in Fig. 1, compared to those that had no BM supernatants added (i.e., RPMI medium only), the number of CFUs was significantly reduced when BM supernatants from PBS-treated transplant recipient mice were added, suggesting that the BM microenvironment in the lethally irradiated transplant mice contains factors that inhibit hematopoiesis. In contrast, the BM supernatants from AMD3100-treated transplant recipient mice significantly increased CFUs. Since the last dose of AMD3100 was given 24 hrs before the mice were sacrificed, we estimated that the concentration of AMD3100 remaining in the BM supernatant was ∼0.78 µg/ml (5 mg/kg of AMD3100 was injected in a 20 gram mouse that had ∼2 ml blood distribution and the half-life of AMD3100 was 4 hrs; 0.1mg/2ml/26 = 0.78 µg/ml). AMD3100 at 0.78 µg/ml did not affect CFUs: results were similar in CFUs when medium only or medium containing 0.78µg/ml AMD3100 was added (Fig. 1). These data suggest that in the early phase of transplant following lethal total body irradiation, the BM microenvironment of transplant recipient mice contains soluble factors that inhibit hematopoiesis. Post transplant administration of AMD3100 promotes hematopoiesis likely by reducing these inhibitors or increasing factors that enhance hematopoiesis.
Fig. 1. BM supernatant of AMD3100-treated transplant recipient mice increases CFUs.
C57Bl/6 mice were sublethally irradiated (5.5 Gy) and BM cells harvested 24 hours later. The BM cells were plated in triplicates in MethoCult® GF 3434 (5 × 105 cells/1.1ml/dish). Marrow supernatants from PBS- or AMD3100-treated transplant recipient mice, RPMI1664 medium only, or RPMI1664 medium containing 0.78 µg/ml of AMD3100 were added to MethoCult®GF 3434 (30µl/dish). CFUs (CFU-GM, BFU-E and CFU-GEMM) were then measured. Data shown were the combined results of 3 independent sets of experiments (Mean ± SEM; * p< 0.05; ** p< 0.01).
2. Proteomic analysis screens for proteins that are differentially expressed between AMD3100-treated BM supernatant and PBS-treated BM supernatant
Identifying the proteins in the BM supernatants that were affected by AMD3100 treatment will provide important insights into the regulation of hematopoietic reconstitution following HCT. To this end, we used a liquid chromatography tandem mass spectrometry approach [22] to screen for proteins that were differentially expressed in the BM supernatants at day +7 post transplant between PBS-injected transplant recipient mice and AMD3100-treated transplant recipient mice. A conservative list was generated for proteins identified with 3 unique peptides, with a threshold of 80%, and with a protein threshold of 99% to ensure a conservative false discovery rate (less than 0.1%). Proteins that displayed a two-fold change between PBS-treated transplant recipient mice and AMD3100-treated transplant recipient mice were considered to be potential candidate proteins affected by AMD3100 treatment.
Using this conservative proteomics approach, we identified a total of 178 proteins in the marrow supernatants of lethally irradiated transplant recipient mice. Gene ontology analysis of these 178 proteins suggested that the top 3 categories of the molecular functions were molecular function (169), binding (133), and catalytic activity (79). The biological processes with the 3 largest numbers of identified proteins were cellular process (138), metabolic process (107), and biological regulation (94). The majority of the proteins present in the BM supernatants were from cytoplasm (122), intracellular organelle (96), and extracellular region (72).
Among the 178 proteins identified in the BM supernatants, 51 proteins displayed a twofold change in spectral counts between PBS-treated transplant recipient mice and AMD3100-treated transplant recipient mice. Thirty-two proteins showed a > 2-fold increase in spectral counts in AMD3100-treated transplant recipient mice (Table 1), and 19 proteins showed a > 2-fold decrease in spectral counts after post transplant AMD3100 treatment (Table 2).
Table 1.
List of proteins that displayed >2 fold increase in spectral counts in AMD3100 treated- transplant recipient mice.
| Protein | UniProt ID | Fold change |
Localization |
|---|---|---|---|
| Myomesin-1 | Q62234 | 110 | Cp, Cs, Io, Op |
| Plectin | Q9QXS1 | 67 | Cp, Cs, Io |
| Myristoylated alanine-rich C- kinase substrate | P26645 | 9.8 | Cp, Cs, Io, Mb, Nu, Op |
| Myosin-9 | Q8VDD5 | 9.8 | Cp, Cs, Io, Mb, Nu, Op, Pm |
| Alpha-actinin-3 | O88990 | 5.9 | Cp, Cs, Io, Op |
| Titin | A2ASS6 | 4.7 | Cp, Cs, Io, Nu, Op |
| Chitinase-3-like protein 3 | O35744 | 4.5 | Cp, Ec, Io |
| Talin-1 | P26039 | 4.3 | Cp, Cs, Io, Mb, Op, Pm |
| Galectin | P16045 | 4.3 | Cp, Ec, Io, Nu |
| Filamin-C | Q8VHX6 | 3.7 | Cp, Cs, Io, Mb |
| Tyrosine -protein phosphatase | P29351 | 3.7 | Cp, Io, Mb, Nu, Pm |
| Complement component C9 | P06683 | 3.7 | Ec, Mb, Pm |
| Far upstream element-binding protein | Q3U0V1 | 3.7 | Cp, Io, Nu |
| Ribonuclease inhibitor | Q91V17 | 3.7 | Cp |
| Fibronectin | P11276 | 3.4 | Ec, Mb, Pm |
| Alpha-actinin 4 | P57780 | 2.9 | Cp, Cs, Io, Nu, Op |
| Proteasome subunit beta type-2 | Q9R1P3 | 2.7 | Cp, Io, Nu |
| Antithrombin-III | P32261 | 2.6 | Ec |
| Ferritin heavy chain | P09528 | 2.5 | Cp, Io, Mc |
| Serum Amyloid A-1 protein | P05366 | 2.5 | Ec |
| Clathrin heavy chain 1 | Q68FD5 | 2.5 | Ga, Cp, Cs, Io, Mb, Mc, Op, Om, Pm |
| NSFL1 cofactor p47 | Q9CZ44 | 2.5 | Ga, Cp, Io, Nu, Op |
| Thioredoxin | P10639 | 2.3 | Cp, Ec, Io, Nu, Mc |
| Rab GDP dissociation inhibitor beta | Q61598 | 2.3 | Ga, Cp, Io, Mb |
| Ig gamma-2B chain C region | P01867 | 2.3 | Ec, Mb, Pm |
| Histidine-rich glycoprotein | Q9ESB3 | 2.1 | |
| Serum Amyloid A-2 protein | P05367 | 2.1 | Ec |
| Cytosolic non-specific dipeptidase | Q9D1A2 | 2.1 | Cp |
| Glutathione perioxidase 3 | P46412 | 2.1 | Ec |
| Transketolase | P40142 | 2.1 | Cp, Er, Io, Mb, Om, Op |
| Alpha-2-antiplasmin | Q61247 | 2.1 | Ec |
| Apolipoprotein E | P08226 | 2.1 | Ga, Cp, Cs, Es, Ec, Io, Mb, Op, Pm |
Gene Ontology Abbreviations: Ga: Golgi apparatus; Cp: cytoplasm; Cs: cytoskeleton; Er: endoplasmic reticulum; Es: endosome; Ec: extracellular region; Io: intracellular organelle; Mb: membrane; Mc: mitochondrion; Nu: nucleus; Om: organelle membrane; Op: organelle part; Pm: plasma membrane
Table 2.
List of proteins that displayed >2 fold decrease in spectral counts in AMD3100 treated- transplant recipient mice.
| Protein | UniProt ID | Fold change |
Localization |
|---|---|---|---|
| Beta-enolase | P21550 | 0.4 | Cp |
| C4b-binding protein | P08607 | 0.4 | Ec |
| 6-phosphogluconolactonase | Q9CQ60 | 0.4 | Cp |
| C-1-tetrahydrofolate synthase | Q922D8 | 0.4 | Cp, Io, Mc |
| Alpha-2-HS-glycoprotein | P29699 | 0.4 | Ec |
| Four and a half LIM domain protein 1 | P97447 | 0.3 | Cp, Io, Nu |
| T-complex protein 1 subunit eta | P80303 | 0.3 | Cp, Cs, Io, Nu, Op |
| Glutathione S-transferase Mu 2 | P15626 | 0.3 | Cp |
| Vinculin | Q64727 | 0.3 | Cp, Cs, Io, Mb, Op, Pm |
| Glycerol-3-phophate dehydrogenase | P13707 | 0.3 | Cp, Io, Mc |
| 6-phosphofructokinase | P47857 | 0.2 | Cp |
| Aspartate aminotransferase | P05201 | 0.2 | Cp, Io |
| Elongation factor 2 | P58252 | 0.2 | Cp |
| Keratin, type II | P50446 | 0.2 | Cp, Io, Op |
| Ras-related protein Rab-7a | P51150 | 0 | Ga, Cp, Es, Io |
| Adenylosuccinate synthetase isozyme | P28650 | 0 | Cp, Mb |
| Fatty acid synthase | P19096 | 0 | Ga, Cp, Io, Nu |
| Plasma Kallikrein | P26262 | 0 | Cp, Ec |
| Keratin, type I | Q6IFX2 | 0 | Cp, Cs, Io, Op |
Gene Ontology Abbreviations: Ga: Golgi apparatus; Cp: cytoplasm; Cs: cytoskeleton; Es: endosome; Ec: extracellular region; Io: intracellular organelle; Mb: membrane; Mc: mitochondrion; Nu: nucleus; Op: organelle part; Pm: plasma membrane
3. Thioredoxin increases BM CFUs and is up-regulated with AMD3100 treatment
We were interested in proteins that are localized extracellularly by gene ontology analysis but can enter cells and function intracellularly. These types of proteins will be an excellent candidate for systemic administration or for ex vivo cell culture. Thioredoxin (TRX) was among the top 5 protein candidates that met our criteria of interest.
Thioredoxin is a ubiquitous, small (12-kD) oxidoreductase protein secreted by a variety of normal cell types including hepatocytes, fibroblasts, activated monocytes, and lymphocytes using a leaderless export mechanism [23–25]. It is present in plasma, but can easily cross cell membranes and enter living cells within 24 hrs [26, 27]. Thioredoxin functions both intracellularly and extracellularly, and plays a crucial role in preserving tissue redox homeostasis, metabolic functions, and cellular integrity [28–30]. Deletion of thioredoxin gene is lethal in utero [31, 32]. However, very little is known about the roles of thioredoxin in hematopoiesis or in radioprotection.
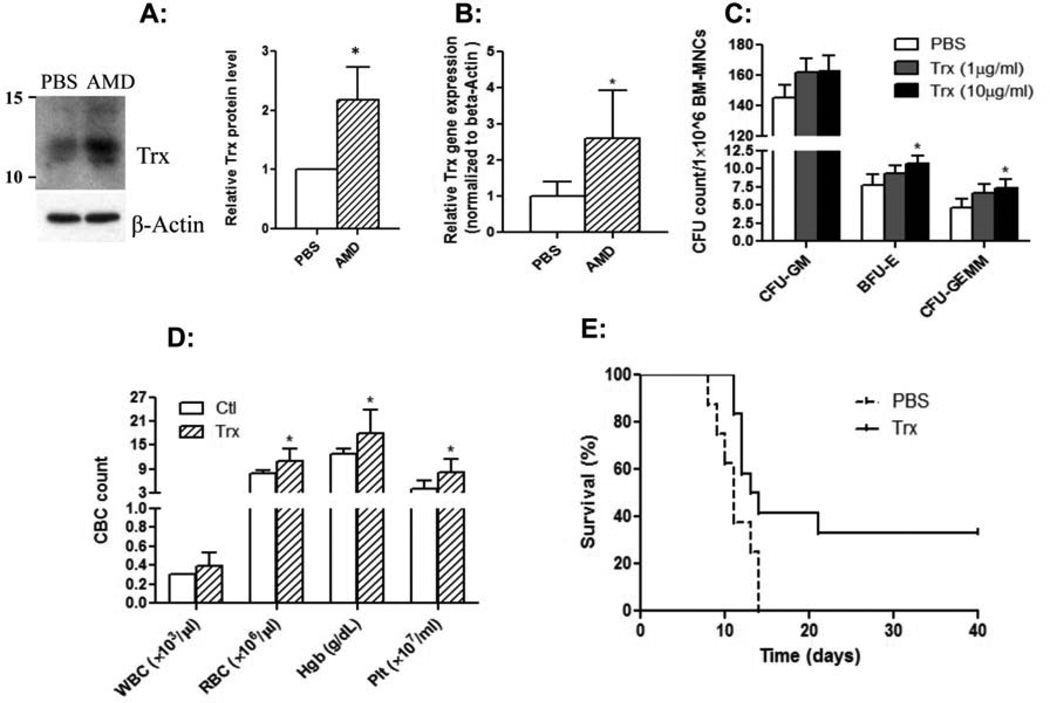
We first performed immunoblot of the BM supernatants to validate the increase in thioredoxin seen with our proteomic analysis. Indeed, AMD3100-treated transplant mice had ∼2 fold higher thioredoxin level in the BM supernatants compared to the BM supernatants from PBS-treated transplant recipient mice (Fig. 2A). We also asked if AMD3100 treatment affected thioredoxin gene expression in BM cells. We measured thioredoxin mRNA expression level in the BM cells of AMD3100-treated or PBS-treated transplant recipient mice at day +7 post transplant. As shown in Fig. 2B, thioredoxin mRNA expression in BM was increased by > 2 fold with post transplant administration of AMD3100 (p< 0.05), consistent with the increase seen with our proteomic and immunoblot analyses.
Fig. 2. BM thioredoxin expression and the protective effects of thioredoxin on radiation-related injury.
A: Thioredoxin protein level in the BM supernatants was increased with AMD3100 treatment. Mice were transplanted and treated with PBS or AMD3100 as described in Methods and Materials. At day +7 post transplant, BM supernatants were harvested and total protein concentration was quantified. The BM supernatants (60 µg of each sample) were processed for immunoblot, and probed with anti-mouse thioredoxin antibody. The picture on the left shows our first set of experiment with BM supernatants pooled from 10 mice in each group. The bar graph on the right shows quantitative densitometric results of thioredoxin protein expression of three independent sets experiments using NIH image J program (*p<0.05). B: Thioredoxin mRNA is up-regulated with AMD3100 treatment. At day +7 post transplant, the BM cells were harvested and thioredoxin mRNA measured by quantitative RT-PCR. Expression was normalized against β-actin (n= 5/group, *p<0.05). C: Thioredoxin enhances CFUs. CFUs were measured using BM cells from sublethally irradiated mice as described in Fig. 1. (n=3, *p< 0.05). D and E: Thioredoxin mitigates radiation-induced hematological injury and protects mice from radiation-related death. C67Bl/6 mice were irradiated with 9.5 Gy and then treated with PBS or thioredoxin intraperitoneally (32 µg on day 0 and then every other day for a total of 5 doses). D: Peripheral blood cell counts were measured at day 7 post irradiation. E: Animal survival was monitored daily. Data shown are Kaplan-Meyer survival curve combining 2 separate sets of experiments (n=13 for thioredoxin-treated mice and 12 for PBS-injected mice, *p< 0.05).
We next tested if thioredoxin had any effects on hematopoietic progenitor cells’ clonogenic activity by directly adding recombinant human thioredoxin to CFU assay. Various concentrations of thioredoxin were added to the CFU assay using marrow cells isolated from sublethally (5.5 Gy) irradiated mice. Thioredoxin at the concentration of 10 µg/ml slightly but significantly increased the CFUs-GEMM and BFUs-E (Fig. 2C).
4. Thioredoxin protects mice against radiation-related injury
Thioredoxin has been shown to defend several organs/tissues against oxidative and inflammatory injury [33]. Additionally, administration of recombinant thioredoxin protected mice from cytokine- or bleomycin-induced acute lung injury [34]. These data suggested that thioredoxin could act as a powerful cytoprotective reagent against acute oxidative stress. We thus tested if thioredoxin could mitigate radiation-induced injury. We irradiated C57Bl/6 mice with 9.5 Gy and then injected thioredoxin intraperitoneally with 32 µg per mouse beginning at 2 hours after irradiation and then every other day for a total of 5 doses. As shown in Fig. 2D, thioredoxin significantly protected the mice from radiation-induced hematological injury: thioredoxin-treated mice had significantly higher numbers of red blood cells (RBCs), hemoglobin (Hb), and platelets (Plt) than those in PBS-injected mice. Furthermore, all the irradiated mice receiving PBS control buffer died within 2 weeks following irradiation. In contrast, 35% of the irradiated mice receiving thioredoxin treatment survived the radiation injury (Fig. 2E).
5. Ex vivo treatment of HSCs with thioredoxin enhances long-term hematological reconstitution
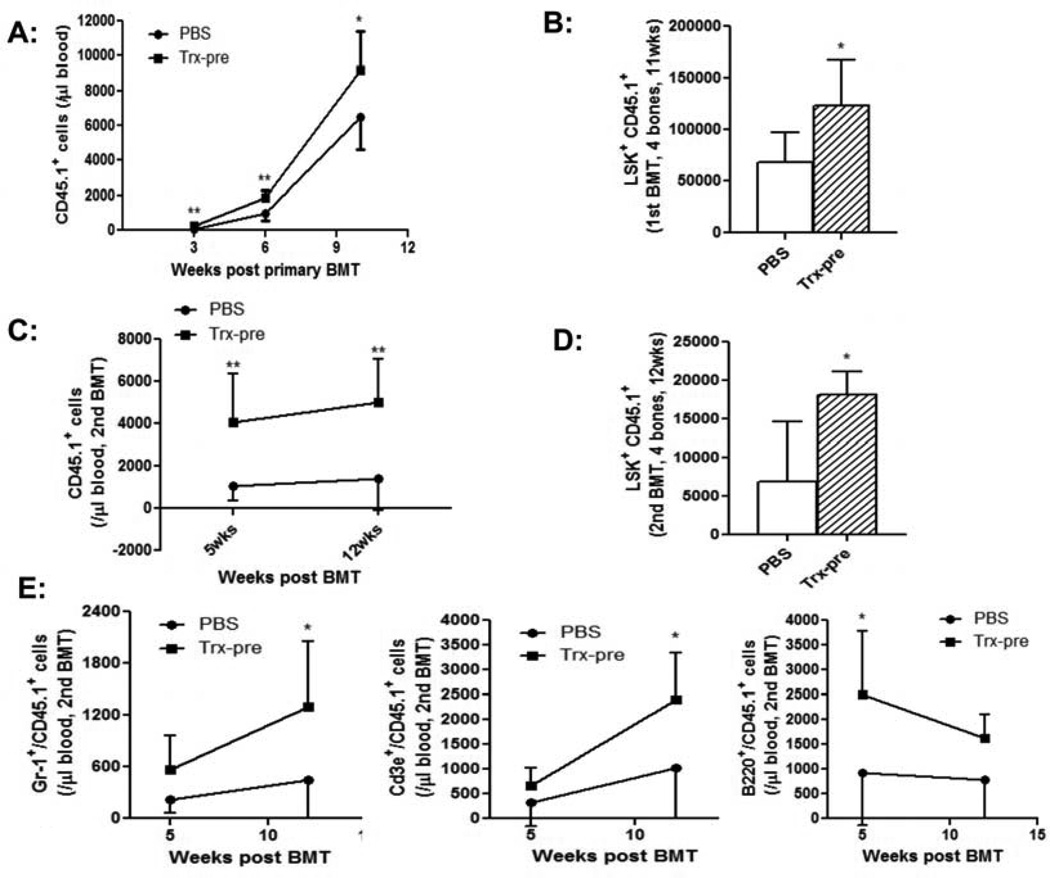
We next investigated if ex vivo treatment of HSCs with thioredoxin could enhance hematopoiesis. For this purpose, we sorted LSK HSCs from CD45.1 C57BL/6 mice and cultured the cells with PBS or 10 µg/ml thioredoxin for 24 hrs in stem cell media supplemented with growth factors. The cells were then transplanted into lethally irradiated CD45.2 congenic mice. Blood samples were collected from the transplant recipient mice at various time-points post transplant, and peripheral CD45.1+ donor derived mononuclear cells (MNCs) were quantified using flow cytometry. As shown in Fig. 3A, the mice transplanted with thioredoxin-treated HSCs had significantly higher numbers of CD45.1+ donor- derived MNCs. We sacrificed the mice at 11 weeks post transplant and measured BM CD45.1+ donor-derived LSK cells. The mice transplanted with thioredoxin- treated HSCs had a significantly higher number of CD45.1+ LSKs in the BM (Fig. 3B). These data suggested an enhanced hematopoiesis with ex vivo thioredoxin treatment.
Fig. 3. Ex vivo treatment of HSCs with thioredoxin enhances hematological reconstitution in primary and secondary transplant recipient mice.
A and B: primary transplantation. CD45.1 LSK cells were cultured ex vivo with PBS or thioredoxin for 24 hrs, and transplanted into lethally irradiated C57Bl/6 CD45.2 mice (2000 cells/mouse). A: Peripheral blood CD45.1+ donor derived MNCs were measured at various time-points post transplant. B: Mice were sacrificed at 11 weeks post transplant and BM CD45.1+ donor derived LSK cells were quantified (n=8 mice/group, *p<0.05, **p<0.01). C - E: secondary transplantation. BM cells were obtained from the primary transplant recipient mice at 11 weeks post transplant, and injected to lethally irradiated C57Bl/6 CD45.2 mice with one primary to one secondary transplant (6×106 cells/mouse). C: The numbers of peripheral CD45.1+ donor derived MNCs were determined by flow cytometry at various time-points post transplant (n=8/group, **p<0.01). D: Mice were then sacrificed at 4 months post transplant and BM CD45.1+ donor derived LSK cells quantified (n=8 mice/group, *p<0.05, **p<0.01). E. Absolute numbers of CD45.1+ Gr-1+ granulocytes (left panel), CD45.1+ CD3+ T cells (middle panel), and CD45.1+ B220+ B cells (right panel) were measured at various time-points post transplant (n=8/group, *p<0.05, **p<0.01).
To determine the effects of thioredoxin on HSC long-term repopulating capacity, we performed secondary BM transplant using BM cells from the primary transplant recipient mice (shown in Fig. 3B). Peripheral CD45.1+ donor-derived MNCs in the secondary transplant recipient mice were measured at different time-points post transplant. As shown in Fig. 3C, the numbers of CD45.1+ donor-derived MNCs were significantly higher in the secondary transplant mice receiving thioredoxin-treated HSCs than those in the secondary control transplant recipient mice. Furthermore, the secondary transplant mice receiving thioredoxin treated- HSCs had significantly higher numbers of CD45.1+ donor-derived BM LSKs harvested at 4 months post-secondary transplant (Fig. 3D). Peripheral blood cell subset analysis demonstrated an increased CD45.1 donor cell contribution in Gr-1+ granulocytes, CD3+ T cells and B220+ B cells in thioredoxin-treated HSC secondary transplant recipients (Fig. 3E). These results demonstrated that ex vivo treatment of thioredoxin enhanced the long-term repopulating capacity of multi-potent HSCs. It is noteworthy that we only cultured the LSK cells with thioredoxin for 24 hours and achieved such long-lasting effects.
6. Combined post transplant administration of thioredoxin and AMD3100 improves HSC long-term repopulating capacity
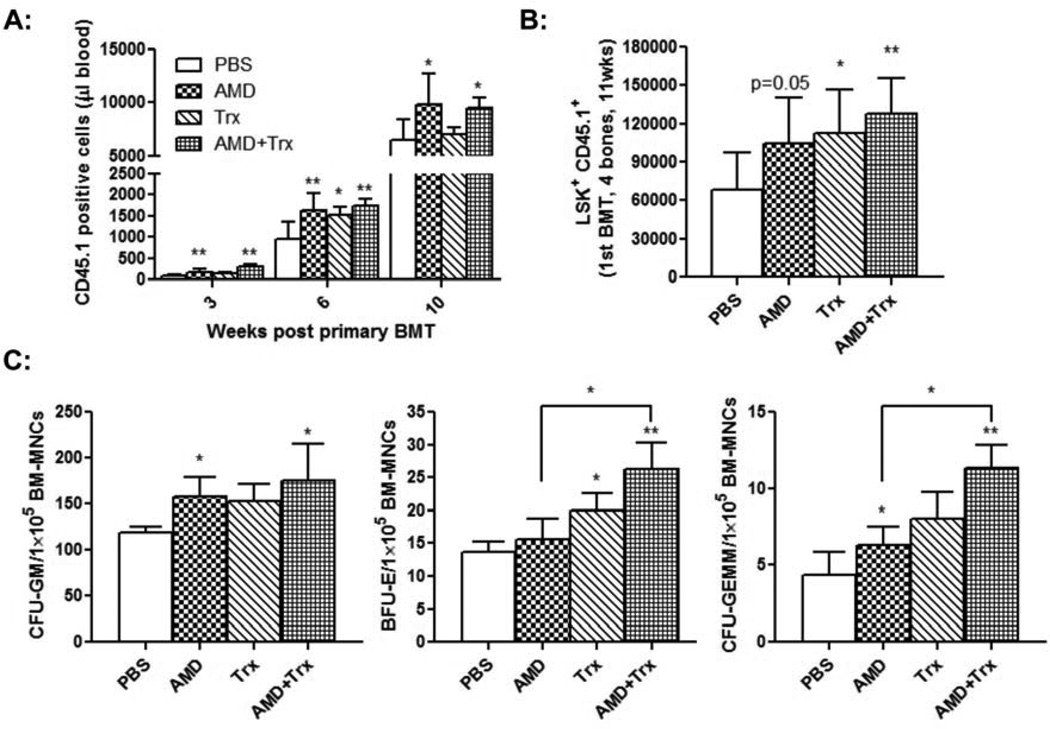
We next examined the effects of post transplant co-administration of thioredoxin and AMD3100 on hematological reconstitution following HCT. CD45.1 LSK cells were transplanted into lethally irradiated C57Bl/6 CD45.2 mice and at day +2 post transplant the mice were injected with AMD3100, thioredoxin, or a combination of both reagents. Consistent with our previous observations [21], post transplant administration of AMD3100 improved donor cell engraftment: AMD3100 treatment increased peripheral CD45.1+ donor cells, BM CD45.1+ LSK cells (p=0.05), BM CFUs-GM, and BM CFUs-GEMM (Fig. 4). Post transplant administration of thioredoxin for 6 doses increased BM donor-derived CD45.1+ LSK cells (Fig. 4B) and BM BFUs-E (Fig. 4C). Compared to PBS injection, post transplant co-administration of thioredoxin and AMD3100 significantly increased all the parameters measured for donor cell engraftment, including peripheral CD45.1+ donor cell numbers, BM CD45.1+ LSK cells, BM CFUs-GM, BM BFUs-E, and BM CFUs-GEMM (Fig. 4).
Fig. 4. Co-administration of AMD3100 and thioredoxin enhances hematological reconstitution in primary transplant recipient mice.
CD45.1 LSK cells were injected into lethally irradiated C57Bl/6 CD45.2 mice. Beginning at day +2 post transplant, the mice were given PBS buffer, AMD3100 alone, thioredoxin alone, or combination of AMD3100 and thioredoxin, as described in Materials and Methods. A: Peripheral CD45.1+ donor derived MNCs were determined by flow cytometry at various time-points post transplant (n=8 mice/group, *p<0.05, **p<0.01). B: Mice were sacrificed at 11 weeks post transplant and BM CD45.1+ donor derived LSK cells were quantified (n=8 mice/group, *p<0.05, **p<0.01). C: BM CFUs were measured as described (n=3 with triplicates). Results were compared to PBS-treated control mice unless indicated (*p<0.05, **p<0.01).
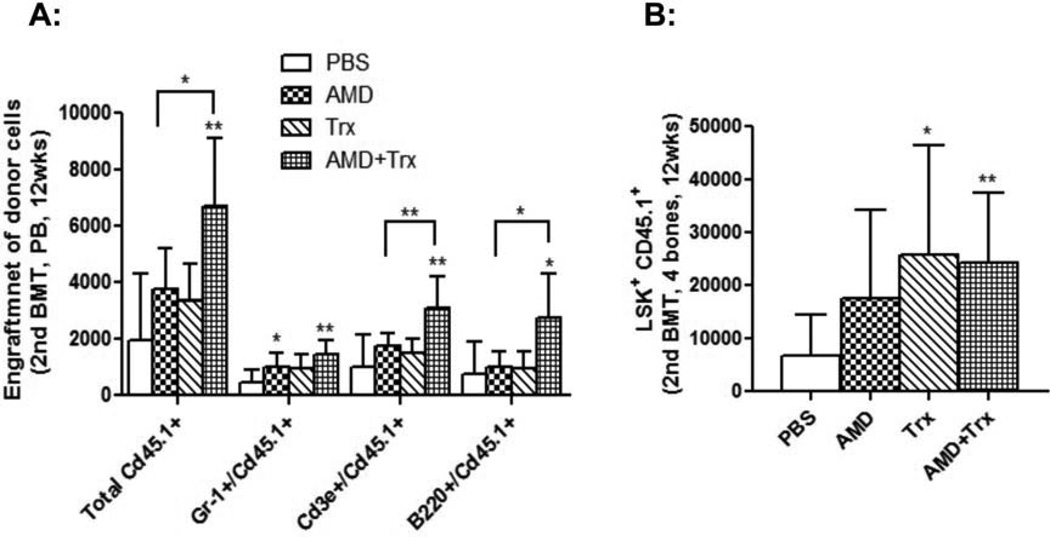
The effects of combined treatment of thioredoxin and AMD3100 on HSC long-term repopulating capacity were investigated in a secondary BM transplant model. Consistent with our previous findings [21], post transplant administration of AMD3100 alone did not affect HSC long-term repopulation. Peripheral donor cell number and BM CD45.1 LSK cells in AMD3100-treated secondary transplant recipient mice were not significantly different from those in PBS-treated secondary transplant recipient mice (Fig. 5). Thioredoxin administration alone increased BM CD45.1 LSK cells, but did not enhance peripheral donor cell contribution in secondary transplant mice. In contrast, co-administration of thioredoxin and AMD3100 resulted in significantly enhanced peripheral donor cell recovery in multiple cell lineages (Fig. 5A). BM CD45.1 LSK cells were also significantly higher in transplant mice receiving co-administration of thioredoxin and AMD3100 (Fig. 5B). These data suggested that co-administration of AMD3100 and thioredoxin significantly improved long-term repopulating HSC engraftment and recovery.
Fig. 5. Co-administration of AMD3100 and thioredoxin enhances HSC long-term repopulating capacity.
BM cells were obtained from the primary transplant recipient mice at 11 weeks post transplant as described in Fig. 4, and injected to lethally irradiated C57Bl/6 CD45.2 mice. A: The numbers of peripheral CD45.1+ donor derived MNCs, CD45.1+ Gr-1+ granulocytes, CD45.1+ B220+ B cells, and CD45.1+ CD3+ T cells were determined by flow cytometry at various time-points post transplant. B: Mice were then sacrificed at 4 months post transplant and BM CD45.1+ donor derived LSK cells quantified (n=8 mice per group). Results were compared to PBS-treated control mice unless indicated (*p<0.05, **p<0.01).
DISCUSSION
Our current study extended from our previous observation that post transplant administration of a CXCR4 antagonist (i.e., AMD3100) enhanced donor cell recovery in HCT [21]. The goal of our study was to understand the effects of post transplant administration of AMD3100 on the BM niche microenvironment. Previously, we have shown that post transplant administration of AMD3100 increases niche opening for donor cell engraftment and induces greater donor cell division [21]. Since CXCR4 is widely expressed in many cell types including mesenchymal stem cells, endothelial cells, osteoblasts and other stromal cells that constitute the BM niche microenvironment, we hypothesized that post transplant administration of AMD3100 likely affects the niche microenvironment. Winkler, et al. recently investigated the effects of AMD3100 on osteoblasts, CD11b+ F4/80+ Ly-6G+ BM macrophages, and 3 cytokines (i.e., CXCR12, c-Kit ligand, and angiopoietin-1) in normal C57BL/6 mice, and found no changes on these measurements [35]. In contrast, in the current study we utilized a MS based, label-free proteomics analysis to compare the marrow supernatnant between PBS- treated transplant recipient mice and AMD3100- treated mice. We demonstrated that post transplant administration of AMD3100 modulated the soluble factors in the BM supernatants. Additionally, we identified ∼51 proteins that were affected by AMD3100. Our study has important implications in HCT and provides a platform for future efforts to identify potential protein targets/molecules that can be used to improve donor cell reconstitution.
Proteomic analysis provides important, complementary information to that obtained from mRNA profiling by microarray, and has been increasingly used to identify disease-specific protein biomarkers. For instance, using a 2D differential in-gel electrophoresis comparing plasma from patients with skin GvHD to plasma from patients without GvHD, Dr. Ferrara’s group has identified several biomarkers (including Elafin) that are involved in the pathogenesis of skin GvHD [36]. Using conventional 2D electrophoresis combined with Matrix-assisted mass spectrometry, Wang, et al. identified peroxiredoxin 2 in the BM microenvironment regulated H2O2 level during aging [37]. Our proteomic analysis allows for simultaneous, relative quantification of protein differences in BM supernatants upon AMD3100 treatment. A significant number of the proteins we identified were similar to those previously reported [37, 38], indicating the reliability of our approach.
The disadvantages of our MS-based proteomic analysis include the inability to detect the changes in protein phosphorylation. Protein phosphorylation plays important roles in protein cellular functions. Additionally, our MS-based proteomic study has detection limitation for proteins present in the range of pictograms/ml. As a result, many of the cytokines and chemokines were not detected or identified using our conservative proteomic criteria. We are currently performing experiments using multiplex Luminex cytokine assay to compare the cytokine/chemokine levels in the BM supernatants of PBS-treated or AMD3100-treated transplant recipient mice.
We chose to focus on thioredoxin for several reasons. Although thioredoxin has diverse biological functions, its key role in mammals is to function as an antioxidant or reactive oxygen species (ROS) scavenger [39–41]. Persistent oxidative stress is one of the major causes for HSC injury during total body irradiation [42] and for graft-vs-host disease during HCT [43]. ROS are unavoidable byproducts of mammalian metabolism. At physiological levels, ROS are important mediators for diverse biological functions. At high levels, ROS are detrimental to cellular functions. It was found that long-term repopulating HSCs have a lower level of ROS and that increased intracellular ROS led to HSC senescence and the loss of self-renewal capacity [42, 44, 45]. N-acetyl-L-cysteine and glutathione have been shown to be able to protect HSCs from oxidative stress and to promote the engraftment of long-term repopulating HSCs [46, 47]. Our data further support the potential of harnessing redox homeostasis to improve transplant outcomes. Second, thioredoxin is an IFN-γ-induced factor [48] and may contribute to the enhanced donor cell engraftment seen with continuous in vivo infusion of IFN-g [49]. Third, thioredoxin not only reduces oxidized proteins, but also together with peroxiredoxins, detoxifies H2O2. Wang, et al. previously showed that peroxiredoxin 2 plays an important role in regulating HSC senescence and aging [37]. Furthermore, recombinant human thioredoxin is readily available and can be administered intravenously [50, 51], offering the possibility of translating our findings into clinical applications.
There are still questions to be answered. For instance, it is unclear how AMD3100 increases thioredoxin expression. This study could be quite challenging because AMD3100 may affect the level of thioredoxin indirectly through other mediators in the niche microenvironment. Thioredoxin activates NF-κB p50 pathway [52] and apoptosis signal-regulated kinase-1 (ASK-1) pathway [53]. However, it remains to be seen if thioredoxin promotes the expansion and functions of HSCs through the same molecular mechanisms. In summary, our current study demonstrated that post transplant administration of AMD3100 modulates the bone marrow niche microenvironment. We also found that thioredoxin is a potentially highly effective agent in promoting hematological recovery and in mitigating radiation injury.
ACKNOWLEDGEMENT
We thank Richard Peppler and Haiqun Zeng at the HCC Flow Cytometry Core for performing flow cytometry analysis. We are grateful to Dr. Henning Schade and Mr. Dzmitry Haviazheu for their technical assistance. This work is supported by MUSC Hollings Cancer Center Startup Fund, Hollings Cancer Center ACS IRG, ASCO Conquer Cancer Foundation Career Development Award, NIH 1K08HL 103780-01A1, and NIH 3P30CA138313-01S3. This manuscript is the result of work supported with resources and the use of facilities at the Ralph H Johnson VA medical center. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or other funding agents.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURE
The authors declare no competing financial interests.
AUTHOR CONTRIBUTIONS
N.A. performed all experiments and analyzed the data. M.J. and A.B performed the proteomic experiments and data analysis. J.L. and J.A. participated in research design. N.A. and Y.K. designed research and wrote the paper.
REFERENCE
- 1.Dekker A, Bulley S, Beyene J, Dupuis LL, Doyle JJ, Sung L. Meta-Analysis of Randomized Controlled Trials of Prophylactic Granulocyte Colony-Stimulating Factor and Granulocyte-Macrophage Colony-Stimulating Factor After Autologous and Allogeneic Stem Cell Transplantation. J Clin Oncol. 2006;24(33):5207–5215. doi: 10.1200/JCO.2006.06.1663. [DOI] [PubMed] [Google Scholar]
- 2.Battiwalla M, McCarthy PL. Filgrastim support in allogeneic HSCT for myeloid malignancies: a review of the role of G-CSF and the implications for current practice. Bone Marrow Transplant. 2009;43(5):351–356. doi: 10.1038/bmt.2008.443. [DOI] [PubMed] [Google Scholar]
- 3.Almeida-Porada GD. Stem cell gene manipulation and delivery as systemic therapeutics. Adv Drug Deliv Rev. 2010;62(12):1139–1140. doi: 10.1016/j.addr.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 4.Yu X, Zou J, Ye Z, Hammond H, Chen G, Tokunaga A, Mali P, Li YM, Civin C, Gaiano N, et al. Notch signaling activation in human embryonic stem cells is required for embryonic, but not trophoblastic, lineage commitment. Cell Stem Cell. 2008;2(5):461–471. doi: 10.1016/j.stem.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crispino JD. GATA1 in normal and malignant hematopoiesis. Semin Cell Dev Biol. 2005;16(1):137–147. doi: 10.1016/j.semcdb.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 6.Jude CD, Gaudet JJ, Speck NA, Ernst P. Leukemia and hematopoietic stem cells: balancing proliferation and quiescence. Cell Cycle. 2008;7(5):586–591. doi: 10.4161/cc.7.5.5549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goldman DC, Bailey AS, Pfaffle DL, Al Masri A, Christian JL, Fleming WH. BMP4 regulates the hematopoietic stem cell niche. Blood. 2009;114(20):4393–4401. doi: 10.1182/blood-2009-02-206433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chavakis E, Urbich C, Dimmeler S. Homing and engraftment of progenitor cells: A prerequisite for cell therapy. Journal of Molecular and Cellular Cardiology. 2008;45(4):514–522. doi: 10.1016/j.yjmcc.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 9.Voermans C, van Hennik PB, van der Schoot CE. Homing of Human Hematopoietic Stem and Progenitor Cells: New Insights, New Challenges? Journal of Hematotherapy & Stem Cell Research. 2001;10(6):725–738. doi: 10.1089/152581601317210827. [DOI] [PubMed] [Google Scholar]
- 10.Schofield R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells. 1978;4:7–25. [PubMed] [Google Scholar]
- 11.Li L, Xie T. Stem cell niche: structure and function. Annu Rev Cell Dev Biol. 2005;21:605–631. doi: 10.1146/annurev.cellbio.21.012704.131525. [DOI] [PubMed] [Google Scholar]
- 12.Wilson A, Trumpp A. Bone-marrow haematopoietic-stem-cell niches. Nat Rev Immunol. 2006;6(2):93–106. doi: 10.1038/nri1779. [DOI] [PubMed] [Google Scholar]
- 13.Yin T, Li L. The stem cell niches in bone. The Journal of Clinical Investigation. 2006;116(5):1195–1201. doi: 10.1172/JCI28568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eliasson P, Jönsson J-I. The hematopoietic stem cell niche: Low in oxygen but a nice place to be. Journal of Cellular Physiology. 2010;221(1):17–22. doi: 10.1002/jcp.21908. [DOI] [PubMed] [Google Scholar]
- 15.Spiegel A, Kalinkovich A, Shivtiel S, Kollet O, Lapidot T. Stem cell regulation via dynamic interactions of the nervous and immune systems with the microenvironment. Cell Stem Cell. 2008;3(5):484–492. doi: 10.1016/j.stem.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 16.Spiegel A, Shivtiel S, Kalinkovich A, Ludin A, Netzer N, Goichberg P, Azaria Y, Resnick I, Hardan I, Ben-Hur H, et al. Catecholaminergic neurotransmitters regulate migration and repopulation of immature human CD34+ cells through Wnt signaling. Nat Immunol. 2007;8(10):1123–1131. doi: 10.1038/ni1509. [DOI] [PubMed] [Google Scholar]
- 17.Peled A, Petit I, Kollet O, Magid M, Ponomaryov T, Byk T, Nagler A, Ben-Hur H, Many A, Shultz L, et al. Dependence of Human Stem Cell Engraftment and Repopulation of NOD/SCID Mice on CXCR4. Science. 1999;283(5403):845–848. doi: 10.1126/science.283.5403.845. [DOI] [PubMed] [Google Scholar]
- 18.Bowie MB, McKnight KD, Kent DG, McCaffrey L, Hoodless PA, Eaves CJ. Hematopoietic stem cells proliferate until after birth and show a reversible phase-specific engraftment defect. The Journal of Clinical Investigation. 2006;116(10):2808–2816. doi: 10.1172/JCI28310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cashman J, Clark-Lewis I, Eaves A, Eaves C. Stromal-derived factor 1 inhibits the cycling of very primitive human hematopoietic cells in vitro and in NOD/SCID mice. Blood. 2002;99(3):792–799. doi: 10.1182/blood.v99.3.792. [DOI] [PubMed] [Google Scholar]
- 20.Cashman J, Dykstra B, Clark-Lewis I, Eaves A, Eaves C. Changes in the Proliferative Activity of Human Hematopoietic Stem Cells in NOD/SCID Mice and Enhancement of Their Transplantability after In Vivo Treatment with Cell Cycle Inhibitors. J Exp Med. 2002;196(9):1141–1150. doi: 10.1084/jem.20010916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kang Y, Chen BJ, Deoliveira D, Mito J, Chao NJ. Selective enhancement of donor hematopoietic cell engraftment by the CXCR4 antagonist AMD3100 in a mouse transplantation model. PLoS One. 2010;5(6):e11316. doi: 10.1371/journal.pone.0011316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu H, Sadygov RG, Yates JR., 3rd A model for random sampling and estimation of relative protein abundance in shotgun proteomics. Anal Chem. 2004;76(14):4193–4201. doi: 10.1021/ac0498563. [DOI] [PubMed] [Google Scholar]
- 23.Holmgren A. Thioredoxin and glutaredoxin systems. J Biol Chem. 1989;264(24):13963–13966. [PubMed] [Google Scholar]
- 24.Wollman EE, d'Auriol L, Rimsky L, Shaw A, Jacquot JP, Wingfield P, Graber P, Dessarps F, Robin P, Galibert F, et al. Cloning and expression of a cDNA for human thioredoxin. J Biol Chem. 1988;263(30):15506–15512. [PubMed] [Google Scholar]
- 25.Rubartelli A, Bajetto A, Allavena G, Wollman E, Sitia R. Secretion of thioredoxin by normal and neoplastic cells through a leaderless secretory pathway. J Biol Chem. 1992;267(34):24161–24164. [PubMed] [Google Scholar]
- 26.Spector A, Yan GZ, Huang RR, McDermott MJ, Gascoyne PR, Pigiet V. The effect of H2O2 upon thioredoxin-enriched lens epithelial cells. J Biol Chem. 1988;263(10):4984–4990. [PubMed] [Google Scholar]
- 27.Kondo N, Ishii Y, Kwon YW, Tanito M, Horita H, Nishinaka Y, Nakamura H, Yodoi J. Redox-sensing release of human thioredoxin from T lymphocytes with negative feedback loops. J Immunol. 2004;172(1):442–448. doi: 10.4049/jimmunol.172.1.442. [DOI] [PubMed] [Google Scholar]
- 28.Ito W, Kobayashi N, Takeda M, Ueki S, Kayaba H, Nakamura H, Yodoi J, Chihara J. Thioredoxin in allergic inflammation. Int Arch Allergy Immunol. 2011;155(Suppl 1):142–146. doi: 10.1159/000327501. [DOI] [PubMed] [Google Scholar]
- 29.Sengupta R, Holmgren A. The role of thioredoxin in the regulation of cellular processes by S-nitrosylation. Biochim Biophys Acta. 2011 doi: 10.1016/j.bbagen.2011.08.012. [DOI] [PubMed] [Google Scholar]
- 30.Burke-Gaffney A, Callister ME, Nakamura H. Thioredoxin: friend or foe in human disease? Trends Pharmacol Sci. 2005;26(8):398–404. doi: 10.1016/j.tips.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 31.Nordberg J, Arner ES. Reactive oxygen species, antioxidants, and the mammalian thioredoxin system. Free Radic Biol Med. 2001;31(11):1287–1312. doi: 10.1016/s0891-5849(01)00724-9. [DOI] [PubMed] [Google Scholar]
- 32.Matsui M, Oshima M, Oshima H, Takaku K, Maruyama T, Yodoi J, Taketo MM. Early embryonic lethality caused by targeted disruption of the mouse thioredoxin gene. Dev Biol. 1996;178(1):179–185. doi: 10.1006/dbio.1996.0208. [DOI] [PubMed] [Google Scholar]
- 33.Nakamura H, Hoshino Y, Okuyama H, Matsuo Y, Yodoi J. Thioredoxin 1 delivery as new therapeutics. Adv Drug Deliv Rev. 2009;61(4):303–309. doi: 10.1016/j.addr.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 34.Hoshino T, Nakamura H, Okamoto M, Kato S, Araya S, Nomiyama K, Oizumi K, Young HA, Aizawa H, Yodoi J. Redox-active protein thioredoxin prevents proinflammatory cytokine- or bleomycin-induced lung injury. Am J Respir Crit Care Med. 2003;168(9):1075–1083. doi: 10.1164/rccm.200209-982OC. [DOI] [PubMed] [Google Scholar]
- 35.Winkler IG, Pettit AR, Raggatt LJ, Jacobsen RN, Forristal CE, Barbier V, Nowlan B, Cisterne A, Bendall LJ, Sims NA, et al. Hematopoietic stem cell mobilizing agents G-CSF, cyclophosphamide or AMD3100 have distinct mechanisms of action on bone marrow HSC niches and bone formation. Leukemia. 2012;26(7):1594–1601. doi: 10.1038/leu.2012.17. [DOI] [PubMed] [Google Scholar]
- 36.Paczesny S, Braun TM, Levine JE, Hogan J, Crawford J, Coffing B, Olsen S, Choi SW, Wang H, Faca V, et al. Elafin is a biomarker of graft-versus-host disease of the skin. Sci Transl Med. 2010;2(13):13ra12. doi: 10.1126/scitranslmed.3000406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang W, Gou L, Xie G, Tong A, He F, Lu Z, Yao Y, Liu K, Li J, Tang M, et al. Proteomic analysis of interstitial fluid in bone marrow identified that peroxiredoxin 2 regulates H(2)O(2) level of bone marrow during aging. J Proteome Res. 2010;9(8):3812–3819. doi: 10.1021/pr901180w. [DOI] [PubMed] [Google Scholar]
- 38.Chen C, Lorimore SA, Evans CA, Whetton AD, Wright EG. A proteomic analysis of murine bone marrow and its response to ionizing radiation. Proteomics. 2005;5(16):4254–4263. doi: 10.1002/pmic.200401295. [DOI] [PubMed] [Google Scholar]
- 39.Zschauer TC, Matsushima S, Altschmied J, Shao D, Sadoshima J, Haendeler J. Interacting with Thioredoxin-1 - disease or no disease? Antioxid Redox Signal. 2012 doi: 10.1089/ars.2012.4822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mitsui A, Hamuro J, Nakamura H, Kondo N, Hirabayashi Y, Ishizaki-Koizumi S, Hirakawa T, Inoue T, Yodoi J. Overexpression of human thioredoxin in transgenic mice controls oxidative stress and life span. Antioxid Redox Signal. 2002;4(4):693–696. doi: 10.1089/15230860260220201. [DOI] [PubMed] [Google Scholar]
- 41.Collet JF, Messens J. Structure, function, and mechanism of thioredoxin proteins. Antioxid Redox Signal. 2010;13:1205–1216. doi: 10.1089/ars.2010.3114. [DOI] [PubMed] [Google Scholar]
- 42.Wang Y, Liu L, Pazhanisamy SK, Li H, Meng A, Zhou D. Total body irradiation causes residual bone marrow injury by induction of persistent oxidative stress in murine hematopoietic stem cells. Free Radic Biol Med. 2010;48(2):348–356. doi: 10.1016/j.freeradbiomed.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Amer J, Weiss L, Reich S, Shapira MY, Slavin S, Fibach E. The oxidative status of blood cells in a murine model of graft-versus-host disease. Ann Hematol. 2007;86(10):753–758. doi: 10.1007/s00277-007-0321-7. [DOI] [PubMed] [Google Scholar]
- 44.Yahata T, Takanashi T, Muguruma Y, Ibrahim AA, Matsuzawa H, Uno T, Sheng Y, Onizuka M, Ito M, Kato S, et al. Accumulation of oxidative DNA damage restricts the self-renewal capacity of human hematopoietic stem cells. Blood. 2011;118(11):2941–2950. doi: 10.1182/blood-2011-01-330050. [DOI] [PubMed] [Google Scholar]
- 45.Jang YY, Sharkis SJ. A low level of reactive oxygen species selects for primitive hematopoietic stem cells that may reside in the low-oxygenic niche. Blood. 2007;110(8):3056–3063. doi: 10.1182/blood-2007-05-087759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ito K, Hirao A, Arai F, Takubo K, Matsuoka S, Miyamoto K, Ohmura M, Naka K, Hosokawa K, Ikeda Y, et al. Reactive oxygen species act through p38 MAPK to limit the lifespan of hematopoietic stem cells. Nat Med. 2006;12(4):446–451. doi: 10.1038/nm1388. [DOI] [PubMed] [Google Scholar]
- 47.Tothova Z, Kollipara R, Huntly BJ, Lee BH, Castrillon DH, Cullen DE, McDowell EP, Lazo-Kallanian S, Williams IR, Sears C, et al. FoxOs are critical mediators of hematopoietic stem cell resistance to physiologic oxidative stress. Cell. 2007;128(2):325–339. doi: 10.1016/j.cell.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 48.Kim SH, Oh J, Choi JY, Jang JY, Kang MW, Lee CE. Identification of human thioredoxin as a novel IFN-gamma-induced factor: mechanism of induction and its role in cytokine production. BMC Immunol. 2008;9:64. doi: 10.1186/1471-2172-9-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li X, Yang Y, Yuan J, Hong P, Freie B, Orazi A, Haneline LS, Clapp DW. Continuous in vivo infusion of interferon-gamma (IFN-gamma) preferentially reduces myeloid progenitor numbers and enhances engraftment of syngeneic wild-type cells in Fancc-/- mice. Blood. 2004;104(4):1204–1209. doi: 10.1182/blood-2004-03-1094. [DOI] [PubMed] [Google Scholar]
- 50.Nakamura T, Hoshino Y, Yamada A, Teratani A, Furukawa S, Okuyama H, Ueda S, Wada H, Yodoi J, Nakamura H. Recombinant human thioredoxin-1 becomes oxidized in circulation and suppresses bleomycin-induced neutrophil recruitment in the rat airway. Free Radic Res. 2007;41(10):1089–1098. doi: 10.1080/10715760701487682. [DOI] [PubMed] [Google Scholar]
- 51.Hattori I, Takagi Y, Nakamura H, Nozaki K, Bai J, Kondo N, Sugino T, Nishimura M, Hashimoto N, Yodoi J. Intravenous administration of thioredoxin decreases brain damage following transient focal cerebral ischemia in mice. Antioxid Redox Signal. 2004;6(1):81–87. doi: 10.1089/152308604771978372. [DOI] [PubMed] [Google Scholar]
- 52.Schulze-Osthoff K, Schenk H, Droge W. Effects of thioredoxin on activation of transcription factor NF-kappa B. Methods Enzymol. 1995;252:253–264. doi: 10.1016/0076-6879(95)52028-7. [DOI] [PubMed] [Google Scholar]
- 53.Saitoh M, Nishitoh H, Fujii M, Takeda K, Tobiume K, Sawada Y, Kawabata M, Miyazono K, Ichijo H. Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. EMBO J. 1998;17(9):2596–2606. doi: 10.1093/emboj/17.9.2596. [DOI] [PMC free article] [PubMed] [Google Scholar]