Abstract
Sumoylation is an emerging modification associated with a variety of cellular processes including the regulation of transcriptional activities of nuclear receptors and their coregulators. As SUMO modifications are often associated with transcriptional repression, we examined if sumoylation was involved in modulation of the transcriptional repressive activity of scaffold attachment factor B1. Here we show that SAFB1 is modified by both the SUMO1 and SUMO2/3 family of proteins, on lysine’s K231 and K294. Further, we demonstrate that SAFB1 can interact with PIAS1, a SUMO E3 ligase which mediates SAFB1 sumoylation. Additionally, SENP1 was identified as the enzyme desumoylating SAFB1. Mutation of the SAFB1 sumoylation sites lead to a loss of transcriptional repression, at least in part due to decreased interaction with HDAC3, a known transcriptional repressor and SAFB1 binding partner. In summary, the transcriptional repressor SAFB1 is modified by both SUMO1 and SUMO2/3, and this modification is necessary for its full repressive activity.
Keywords: Scaffold Attachment Factor B1, sumoylation, transcriptional repression
INTRODUCTION
Sumoylation is the process of covalent post-translational modification by the small ubiquitin-like modifier (SUMO). This process is important in many different cellular processes [1; 2]. Sumoylation occurs through a three step enzymatic process similar to ubiquitination [3]. Modification typically occurs at the four amino motif ΨKXE (where Ψ is a large hydrophobic amino acid, K is the lysine of modification, X is any amino acid and E is glutamic acid) [3]. Regulation can occur by SUMO-E3 ligases such as a members of the PIAS family, RanBP2, or PC2 [3]. Desumoylation of target proteins occurs via the SENP family of proteins [4] which have various activities [5]. Sumoylation of transcription factors and coregulators is important for regulation of their transcriptional activities. Specifically, sumoylation has been indicated most commonly to negatively regulate transcriptional activity [6].
Scaffold attachment factor B1 (SAFB1) is a large multi-functional protein with functions in transcriptional repression, and RNA splicing. Recent suggestions indicate that it might also play a role in chromatin organization [7; 8]. SAFB1 is known to play a role in a variety of cellular processes [9; 10; 11]. It is part of a protein family, together with the scaffold attachment factor B2 (SAFB2) and scaffold-like transcription modulator (SLTM) [12; 13]. SAFB1 contains an N-terminal SAF-Box, a centrally located RNA recognition motif (RRM), and a C-terminal repression domain [14]. These domains are highly conserved among the family members, especially between SAFB1 and SAFB2.
The most well studied function of SAFB1 is its role in transcriptional repression. Originally described as a repressor for the estrogen receptor (ER) [15] it is now known that SAFB1 can also regulate activity of other nuclear receptors, and potentially regulate other non-receptor proteins [16]. SAFB1 also contains an intrinsic and transferable C-terminal repression domain allowing for transcriptional repression independently of association with nuclear receptors. Additionally, SAFB1 is known to interact with other important transcriptional repressive proteins such as NCoR and HDAC3 [17]. Physiologically, SAFB1 plays an important role as SAFB1−/− mice have multiple defects [18]. While the physiological role of SAFB1 and its functions within the cellular context have been studied in detail, there is currently no data available on how its repressive activity is regulated.
Here we report that SAFB1 is a sumoylated protein modified by both SUMO-1 and SUMO2/3. In silico analysis revealed two potential sumoylation sites (K231/294) which corresponded to the consensus sumoylation motif, ΨKXE [19]. Sumoylation at those sites is necessary for SAFB1’s repressive activity, at least in part due to mediating interaction with HDAC3. Finally, we show that sumoylation of SAFB1 is regulated by the E3 ligase PIAS1, and by SENP1. Thus, our data place SAFB1 into a growing group of transcriptional (co)-repressors whose activity is modified by sumoylation.
MATERIALS AND METHODS
In silico sumoylation and sequence analysis
In silico sumoylation analysis was performed using the SUMOsp 2.0 sumoylation site prediction tool [19]. The SAFB1 sequence used for analysis is NCBI Reference Sequence: NM_002967.2. Conservation analysis of the SAFB1 sumoylation sites with SAFB1 orthologs in other organisms, and with its family members SAFB2 and STLM were performed using the Clustal web tool.
Cell culture, transfections and luciferase reporter assays
MCF7 and HEK293T cells were maintained in Dulbecco’s modified Eagle’s medium (Mediatech) supplemented with 5% fetal bovine serum (PAA Laboratories) and 2mM glutamine, 100 IU/ml penicillin and 100 ug/ml streptomycin (Mediatech). Transfections were performed using Lipofeactamine 2000 (Invitrogen) according to the manufacturer’s instructions. Reporter assays were performed as previously described [14]. Luciferase activity was normalized to Renilla luciferase or B-gal activity. All reporter assays were performed in triplicate and errors bars indicate standard deviation.
Plasmids and mutagenesis
pcDNA3.1-SAFB1-HA and pEGFP-SAFB1 has been previously described [14]. Mutagenesis for changing K231 and K294 to arginine was performed using the Quikchange XL Site-Directed mutagenesis kit (Stratagene) according to the manufacturer’s instructions. pcDNA3.1-SUMO1/2/3-HA, pCMX-Flag-SUMO1/3, and pcDNA3-myc-SUMO1/3 were kindly provided by Dr. Ronald Hay (University of Dundee, Dundee, Scotland), Dr. Narasimhaswamy Belaguli (Baylor College of Medicine, Houston, TX), and Dr. Christopher Glass (University of California San Diego, San Diego, CA), respectively. The Flag-SENP1 and Flag-SENP1 mutant (R630L, K631M) plasmids were a kind gift from Dr. Edward TH Yeh (University of Texas MD Anderson Cancer Center, Houston, TX).
Antibodies, Immunoblotting and Protein Half-Life Measurements
Antibodies were purchased from the following manufacturers: anti-HA (Covance), anti-Flag (Stratagene), anti-myc (Abcam), anti-GFP (Invitrogen), anti-HDAC3 (Abcam) and anti-NCoR (Santa Cruz). Generation of SAFB1 antibody has previously been described (21). For immunoblotting, proteins were separated by SDS-PAGE and transferred to nitrocellulose membranes (Whatman). For detection either HRP conjugated secondary antibodies (GE Healthcare) with chemiluminescent detection (Pierce) or fluorescent conjugated secondary antibodies (Rockland) were used. For Western blot analysis, cells with lysed in either 5% SDS or modified RIPA buffer (10mM Tris pH 7.5, 150mM NaCl, 1% NP-40, 1% deoxycholate and 0.1% SDS) as indicated. For interaction of SAFB1 and NCoR cells were fixed with 1% formaldehyde for 10 minutes and nuclear extraction was performed prior to lysis. For protein half-life measurements cells were treated with 10ug/ml cycloheximide, collected at indicated times and immunoblotted for HA.
Immunoprecipitation assay
For immunoprecipitation (IP), cells were plated in 15cm dishes, transfected, and then lysed in modified RIPA buffer containing 10mM NEM (Pierce). One milligram of protein lysate was subjected to IP. Antibody bound immunocomplexes were precipitated with Protein G beads (Invitrogen). Precipitates were washed three times with RIPA buffer, boiled in SDS-PAGE loading buffer containing 1% SDS and subjected to SDS-PAGE followed by immunoblotting.
Immunofluorescent Staining and Confocal Microscopy
HEK293T cells cultured on poly-D-lysine coated coverslips in DMEM were transfected with GFP tagged SAFB1-WT or K231,294R as indicated. 48 hours after transfection cells were washed twice with PBS, fixed in 4% formaldehyde diluted in PBS for 10min, washed twice with PBS, permeabilized with 0.5% Triton X100 diluted in PBS for 5 minutes, washed twice with PBS, and then incubated with 1% BSA in PBS for 1 hour. Cells were then washed twice with PBS and then mounted with mounting medium containing DAPI (Vectashield). Confocal images were obtained on a Leica TCS SP5 II confocal microscope with a 100x objective (Leica Microsystems).
RESULTS
SAFB1 and SAFB2 contain two highly conserved consensus sumoylation sites
Analysis of SAFB1 using the SUMOplot algorithm for identification of potential consensus sumoylation sites (ΨKxD/E) identified two consensus sumoylation sites where modification could potentially occur. The lysine’s of potential modification reside at amino acid positions 231 and 294 within SAFB1 (Figure 1A).
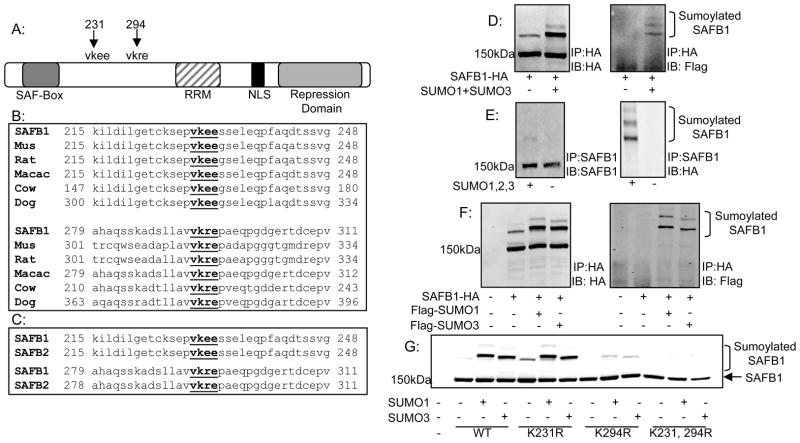
Figure 1. Identification and conservation of SAFB1 sumoylation sites.
A: Schematic of SAFB1 showing major domains and lysine’s at amino acid positions 231 and 294 which conform to the consensus sumoylation sequence. B: Alignment of region containing SAFB1 sumoylation sites using the ClustalW web tool indicating conservation of the sumoylation sites with lower organisms. C: Alignment as described in B indicating conservation of SAFB1 sumoylation sites in paralog SAFB2. D: 293T cells were transfected with constructs for Flag-SUMO1 and Flag-SUMO3 with the addition of either SAFB1-HA or SAFB2-GFP. Cells were collected and immunoprecipitated for either HA or GFP as indicated. Immunoblotting for HA or GFP and FLAG revealed two sumoylated SAFB1 bands. E: Performed as in D but with IP of endogenous SAFB1. F: 293T cells were transfected with SAFB1-HA and Flag-SUMO1 or Flag-SUMO3. Cells were collected and immunoprecipitated for HA. Immunoblotting for Flag revealed sumoylation of SAFB1 by both SUMO1 and SUMO3. G: 293T cells were transfected with varying SAFB1 constructs and my-SUMO1 or SUMO3 as indicated. Cells were collected and immunoblotted for HA. Results indicate complete loss of sumoylated forms with mutation of lysine’s 231 and 294.
Conservation analysis revealed that the potential sumoylation sites as well as the surrounding amino acid composition are highly conserved among SAFB1 orthologs in different species (Figure 1B), especially in mammals and conserved in lower organisms (data not shown).
Additional sequence analysis was performed identifying 100% conservation of the potential sumoylation sites between SAFB1 and its human paralog SAFB2 (Figure 1C). Unlike the analysis of SAFB1 in lower organisms, the amino acid sequence flanking the potential sumoylation sites maintains 100% sequence identity between SAFB1 and SAFB2.
SAFB1 and SAFB2 are modified by SUMO1 and SUMO2/3
We first tested whether SAFB1 could be modified by sumoylation in HEK293T cells transiently overexpressing Flag-SUMO1 and Flag-SUMO3. Immunoprecipitation of HA (SAFB1) followed by either immunoblotting for HA or Flag indicated two slower migrating bands (Figure 1D) whose approximate sizes corresponded to the estimated sizes of SAFB1 plus the addition of one or two ~20kDa SUMO proteins. Modification was examined and confirmed in a number of cell lines, including Hela, MCF7, and Saos2 (data not shown) thus indicating that sumoylation of SAFB1 is a common effect and not strictly cell line dependent. We also demonstrated that SAFB2 is sumoylated (data not shown). Modification of endogenous SAFB1 is shown in Figure 1E. Further, more detailed studies on sumoylation (described below) were focused on SAFB1, a well described transcriptional repressor.
To determine if SAFB1 could be equally modified by both SUMO1 and SUMO2/3, HEK293T cells transiently expressing HA-SAFB1 and either Flag-SUMO1 or Flag-SUMO3 were immunoprecipitated for HA followed by Western blotting for HA and Flag (Figure 1F). Two slower migrating bands corresponding to sumoylated SAFB1 were seen with both SUMO1 and SUMO3 expression indicating that SAFB1 can be modified by both the SUMO1 and SUMO2/3 family of proteins.
To examine if the predicted lysine’s were the sites of SUMO modification, SAFB1 constructs with lysine to arginine mutations (K231R, K294R and K231,294R) were generated and analyzed for sumoylation. HA immunoblotting of lysates from HEK293T cells expressing either HA-SAFB1 wild-type (WT), K231R, K294R or the K231/294R double mutant and SUMO1 or SUMO3 indicated that mutation of a single sumoylation site still allowed for SUMO modification, although mutation of K294 clearly resulted in decreased level of modification of SAFB1. Mutation of both lysines 231 and 294 totally abolished SUMO modification indicating SAFB1 is sumoylated at both lysines 231 and 294, with K294 being the more dominant sumoylation site (Figure 1G).
Regulation of SAFB1’s sumoylation
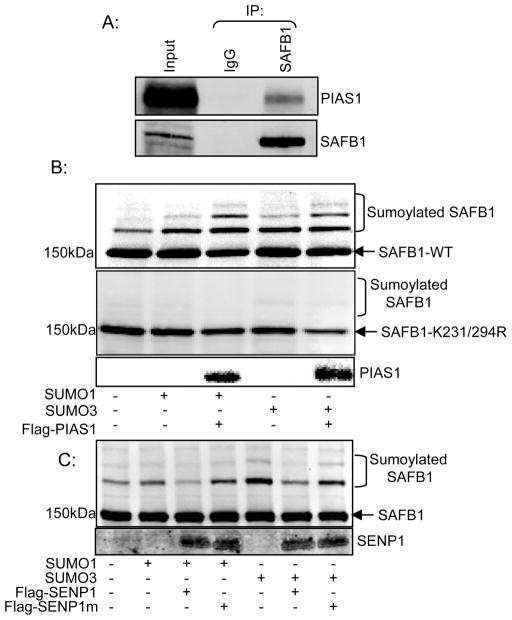
The role of PIAS1 as an E3 ligase for SAFB1 was examined based on its expression of PIAS1 in all cell lines where SAFB1 was shown to be modified (unpublished data) and described role in modification of transcriptional co-regulators [3]. First, we tested interaction of SAFB1 with PIAS1 by co-immunopreciptation from HEK293T cells transiently transfected with HA-PIAS1. Immunoprecipitation of SAFB1 and immunoblotting for HA indicated interaction between SAFB1 and PIAS1 (Figure 2A). Next, we examined the ability of PIAS1 to function as a SUMO E3 ligase for SAFB1. Addition of either SUMO1 (Lane 2) or SUMO3 (Lane 4) increased SAFB1-WT sumoylation (Figure 2B top panel) which was further increased with the addition of PIAS1 (Lanes 3 and 5 respectively), suggesting that PIAS1 can function as an E3 ligase for SAFB1. Addition of SUMO1 or SUMO3 in the presence of PIAS had no effect of the sumoylation status of SAFB1-K231,294R (Figure 2B, lower panel).
Figure 2. SAFB1 interaction and SUMO regulation by PIAS and SENP proteins.
A: 293T cells were transfected with construct for PIAS1-HA. Cells were collected and immunoprecipitated for SAFB1. Immunoblotting for HA revealed interaction between SAFB1 and PIAS1. B: 293T cells were transfected with SAFB1-WT-HA or SAFB1-K231,294R-HA, myc-SUMO1 or myc-SUMO2 and Flag-PIAS1 constructs as indicated. Cells were collected and immunoblotted for HA. Results show PIAS1 can increase sumoylation of SAFB1-WT by both SUMO1 and SUMO3 but has no effect on SAFB1-K231,294R. C: 293T cells were transfected with SAFB1-WT-HA, myc-SUMO1 or myc-SUMO3 and Flag-SENP1 or Flag-SENP1mutant. 48 hours after transfection cells were collected and immunoblotted for HA. Results indicated SENP1 can desumoylate SAFB1 modified by both SUMO1 and SUMO3. Enzyme deficient SENP1 (SENP1m) did not show an effect.
The role of SENP1 in desumoylation of SAFB1 was examined based on its known activities toward SUMO1 and SUMO2/3 modified proteins [5]. In HEK293T cells co-expressing SAFB1 and either SUMO1 or SUMO3 the addition of SENP1 led to decreased levels of sumoylated SAFB1 (Figure 2C; lanes 4 and 7). Addition of a catalytically inactive mutant SENP1 (SENP1m) did not decrease levels of sumoylated SAFB1 (Figure 2C; lanes 4 and 7). These results indicate that SENP1 acts as the desumoylating enzyme for SAFB1.
Sumoylation of SAFB1 regulates its transcriptional repressor activity
The most well defined function of SAFB1 is its ability to act as a transcriptional repressor for a number of transcription factors, and given the increasing evidence for sumoylation playing a critical role in repression [13], we next aimed to examine if sumoylation could regulate SAFB1’s repressor activity. We first examined the ability of SAFB1-WT or SAFB1-K231/294R to repress transcription of a Luciferase reporter driven by an estrogen response element – thymidine kinase promoter (ERE-TK), either in the absence of presence of estradiol. These assays were performed in MCF7 cells, and in HEK293T cells transfected with ERα (Figure 3A). The addition of SAFB1-WT led to transcriptional repression both in the absence and presence of estradiol. Addition of SAFB1-K231/294R led to a complete lack of transcriptional repression, causatively linking SAFB1 sumoylation and its repressor activity.
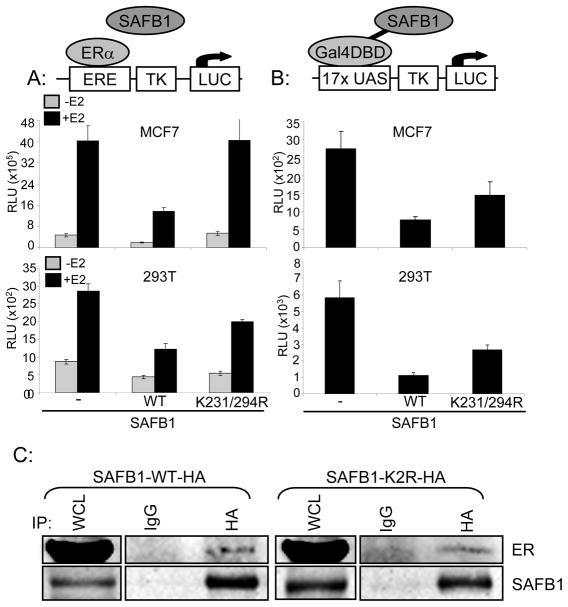
Figure 3. Sumoylation affects SAFB1 transcriptional repression activity.
A: Cells were transfected with either SAFB1-WT or K231,294R along with an ERE-Tk-Luciferase and B-gal reporter constructs. Cells were treated +/− 10−8M E2 and cells were collected for luciferase assay. Graphs represents mean of triplicates +/− standard deviation and indicated loss of transcriptional repression with K231,294R expression. Top panel MCF7 cells, bottom panel 293T cells (with co-transfection of ERα). B: Cells were transfected with Gal4-DBD-SAFB1-WT or K231,294R, 17xUAS-Tk-Luciferase and B-gal reporter constructs. 48 hours post transfection cells were collected for luciferase assay. Graph represents the mean of triplicates +/− standard deviation and indicates loss of transcriptional repression with K231,294R expression in an ERα independent manner. C: HEK293T cells were transfected with either SAFB1-WT or K231,294R-HA and Flag-ERα. Cells were collected and immunoprecipitated for HA. Immunoblotting for Flag indicated interaction between both SAFB1 WT and K231,294R and ERα.
Finally, to determine whether sumoylation would affect SAFB1’s repressive activity independent of its interaction with ERα we tested SAFB1 WT and K231,294R in a Gal4-DBD reporter assay. These assays were performed in both MCF7 and HEK293T cells and — as previously seen in the ERE-TK-Luc assays — addition of SAFB1-WT led to transcriptional repression compared to vector control and addition of SAFB1-K231,294R led to a partial loss of transcriptional repression (Figure 3B). Additionally, in HEK293T cells transiently expressing either HA-SAFB1 WT or K231,294R and Flag-ERα immunopreciptation of HA and immunoblotting for Flag indicated ERα interaction with both SAFB1 WT and K231,294R (Figure 3C). Together, these data suggest that the effect of sumoylation on SAFB1’s repressor activity is independent of its interaction with ERα.
Mutation of SAFB1 sumoylation sites leads to decreased binding to interaction partner HDAC3
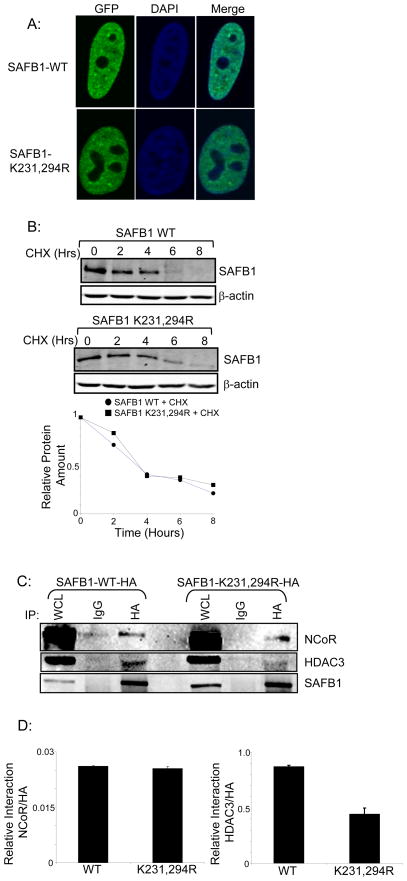
Sumoylation has been associated with transcriptional repression and multiple mechanisms for how modification alters protein function have been described. Mechanisms include changes in subcellular localization, alteration of protein half-life and changes in interaction with protein binding partners. We tested involvement of these mechanisms in altering SAFB1 repressor activity. Confocal microscopy of HEK293T cells transfected with GFP tagged SAFB1 WT or K231,294R did not indicate a change in subcellular localization with mutation of the SAFB1 sumoylation sites (Figure 4A). Further, we tested for a potential change in protein half-life with mutation of the SAFB1 sumoylation sites. HEK293T cells transfected with either SAFB1-WT-HA or K231,294R-HA were treated with the translation inhibitor cycloheximide. Cells were then collected and lysed at varying timepoints and immunoblotted for HA (Figure 4B). The results show that mutation of the SAFB1 sumoylation sites did not alter protein half-life of SAFB1. Quantification of blots was performed and plotted as relative protein amount over time. A protein half-life of approximately three hours was determined for both SAFB1 WT and SAFB1 K231,294R (Figure 4B lower panel).
Figure 4. Examination of mechanisms by which sumoylation affects SAFB1 repressive activity.
A: HEK293T cells were transfected with pEGFP-SAFB1-WT or K231,294R. Protein localization was examined by confocal microscopy and did not indicate a change with mutation of the SAFB1 sumoylation sites. B: Cells were transfected with SAFB1-WT or K231,294R, treated with cycloheximide and collected at the indicated timepoints. Lysates were immunoblotted for HA and indicate no change in protein half-life with mutation of sumoylation sites. C: Cells were transfected with SAFB1-WT or K231,294R lysed and immunoprecipitated for HA. Immunoblotting for NCoR indicated interaction with both SAFB1 WT and K231,294R. Immunoblotting for HDAC3 revealed decreased interaction of SAFB1 K231,294R with HDAC3 compared to SAFB1-WT. D: Quantification of the interaction blots. Denisitometry was performed on blots from two independent experiments using the FluorChem 8000 software and the graph represents the ratio of either the NCoR (left panel) or HDAC3 (right panel) signals to the corresponding HA signals for SAFB1-WT and K231, 294R interaction with NCoR and HDAC3. Graph represents average densitometry of the two experiments and error bars represent standard deviation.
Next, we tested whether changes in interaction between SAFB1 and known protein binding partners which could be involved in the repressive activity of SAFB1, such as HDAC3 and NCoR, were altered with mutation of the sumoylation sites. HEK293T cells were transfected with SAFB1 WT-HA or K231,294R-HA, lysed, immunoprecipitated with HA and immunoblotted for NCoR and HDAC3 (Figure 4C). While immunoblotting for NCoR did not indicate any change in interaction with mutation of the SAFB1 sumoylation sites, there was a significant decreased interaction between HDAC3 and SAFB1 K231,294R, compared to WT (Figure 4D). This indicates changes in protein-protein interaction as at least one mechanism by which sumoylation of SAFB1 affects its repressive activity.
DISCUSSION
In this report we have identified the transcriptional repressor SAFB1 as a new target of sumoylation. Modification occurred at two amino acid residues, lysines K231 and K294. Modification occurred by both the SUMO1 and SUMO2/3 family of proteins. This adds SAFB1 to a growing list of sumoylated proteins, with many modified proteins being nuclear receptors or their co-regulatory proteins. Modification of these proteins is most commonly associated with transcriptional repression, and our data also show that sumoylation of SAFB1 is critical for SAFB1’s repressive activity.
Numerous mechanisms have been associated with sumoylation and its link to transcriptional repression. Changes in protein turnover have been described as acting by sumoylation/ubiquitylation of the same target protein where sumoylation of the protein blocks ubiquitylation of the protein and prevents its degradation [3]. Change in localization to distinct nuclear bodies is also a frequent outcome of sumoylation [3]. Finally, changes in protein-protein interactions and association with repressor complexes have been associated with sumoylation of target proteins [3]. This change in protein-protein interaction is the putative mechanism that we identified to modify the repressive activity of SAFB1 when it is modified by sumoylation. While mutation of the SAFB1 sumoylation sites leads to decreased binding to the transcriptional repressor HDAC3 there was not complete loss of binding to HDAC3 with mutation of the sumoylation sites. This may suggest that there are additional potentially novel mechanisms that may be working cooperatively to give the loss of transcriptional repression activity witnessed with mutation of the sumoylation sites. Additionally, we found no change in interaction between SAFB1 and NCoR with mutation of the SAFB1 sumoylation sites. This indicates that there is a sumoylation-independent interaction between SAFB1 and NCoR but a sumoylation-dependent interaction between SAFB1and HDAC3.
We can speculate on other mechanisms by which sumoylation affects SAFB1 activity. There could be yet to be identified protein binding partners of SAFB1, which are important mediators of SAFB1 transcriptional repression and whose interactions with SAFB1 are SUMO dependent Additionally, it is known that SAFB1 can form large oligomerized complexes and sumoylation could potentially disrupt formation of these complexes leading to a loss of transcriptional repression activity. While these potential mechanisms provide interest as novel mechanisms linking SAFB1 sumoylation and transcriptional repression they do not address how sumoylation may be affecting the intrinsic repression ability of SAFB1. This is an important question to address and the focus of future studies.
The exon 7 region of SAFB1 containing the sumoylation sites is a very interesting region as it is highly conserved sharing 100% identity at the amino acid level with the paralog SAFB2. While it is highly conserved, suggesting evolutionarily it may be important, to date no function has been ascribed to this region. The identification of this region as the site of sumoylation is the first report to designate some function to this region. Of additional interest a number of putative phosphorylation sites have been identified within this region. As the exon 7 region of SAFB1 contains both phosphorylation and sumoylation sites we can speculate that this region is potentially a post translationally modified “control center” where coordinated modification from outside signals leads to regulation of SAFB1 activity. Also, examining the modification of lysine’s 231 and 294 the results suggest that it is not necessary for both sumoylation sites to be present for modification of SAFB1 to occur. While modification of each site occurred independently, the possibility of some mutual cooperation between the sites is still plausible. Interestingly, mutation of lysine 294 did result in slightly decreased sumoylation suggesting this site may be the more important site. While this is an interesting finding we do not have any other evidence as to the potential physiological importance to this finding but will be a focus of further studies.
In this report we have described the sumoylation of SAFB1 a multi-functional protein which can act as a transcriptional repressor for many nuclear receptors. SAFB1 is modified by both SUMO1 and SUMO2/3 family of proteins and this modification affects SAFB1’s transcriptional repression activity. These findings indicate that sumoylation plays a critical role in regulating SAFB1 repressive activity. We have also determined that one mechanism by which sumoylation affects SAFB1 repressive activity is through mediating interaction with a known transcriptional repressor HDAC3. Additionally, sumoylation of SAFB1 still needs to be placed in the appropriate cellular and physiological context of the numerous cellular processes in which SAFB1 plays an important role. These are important and interesting questions that will be addressed in the future and will continue to expand our knowledge of SAFB1 function and regulation.
Acknowledgments
We greatly appreciate the gift of pcDNA3.1-SUMO1/2/3-HA from Dr. Ronald Hay (University of Dundee, Dundee, Scotland), pCMX-Flag-SUMO1/3 from Dr. Narasimhaswamy Belaguli (Baylor College of Medicine, Houston, Texas) and pcDNA-myc-SUMO1/3 from Dr. Christopher Glass (University of California San Diego, San Diego, California). We would also like to thank Dr. Edward Yeh (University of Texas MD Anderson Cancer Center) for the gift of Flag-SENP1 and Flag-SENP1m. This work was supported by NIH R01CA CA097213 (SO) and DAMD W81XWH-06-1-0710 (JG). We would also like to thank Dr. Xia Lin (Baylor College of Medicine, Houston, Texas) for helpful suggestions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Geiss-Friedlander R, Melchior F. Concepts in sumoylation: a decade on. Nat Rev Mol Cell Biol. 2007;8:947–956. doi: 10.1038/nrm2293. [DOI] [PubMed] [Google Scholar]
- 2.Kerscher O, Felberbaum R, Hochstrasser M. Modification of Proteins by Ubiquitin and Ubiquitin-Like Proteins. Annual Review of Cell and Developmental Biology. 2006;22:159–180. doi: 10.1146/annurev.cellbio.22.010605.093503. [DOI] [PubMed] [Google Scholar]
- 3.Johnson ES. Protein modification by SUMO. Annual review of biochemistry. 2004;73:355–82. doi: 10.1146/annurev.biochem.73.011303.074118. [DOI] [PubMed] [Google Scholar]
- 4.Yeh ET. SUMOylation and De-SUMOylation: wrestling with life’s processes. The Journal of biological chemistry. 2009;284:8223–7. doi: 10.1074/jbc.R800050200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mukhopadhyay D, Dasso M. Modification in reverse: the SUMO proteases. Trends in biochemical sciences. 2007;32:286–95. doi: 10.1016/j.tibs.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 6.Gill G. Something about SUMO inhibits transcription. Current Opinion in Genetics & Development. 2005;15:536–541. doi: 10.1016/j.gde.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 7.Garee JP, Oesterreich S. SAFB1’s multiple functions in biological control—lots still to be done! Journal of Cellular Biochemistry. 109:312–319. doi: 10.1002/jcb.22420. [DOI] [PubMed] [Google Scholar]
- 8.Hammerich-Hille S, Kaipparettu BA, Tsimelzon A, Creighton CJ, Jiang S, Polo JM, Melnick A, Meyer R, Oesterreich S. SAFB1 Mediates Repression of Immune Regulators and Apoptotic Genes in Breast Cancer Cells. Journal of Biological Chemistry. 285:3608–3616. doi: 10.1074/jbc.M109.066431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee YB, Colley S, Norman M, Biamonti G, Uney JB. SAFB re-distribution marks steps of the apoptotic process. Experimental Cell Research. 2007;313:3914–3923. doi: 10.1016/j.yexcr.2007.06.023. [DOI] [PubMed] [Google Scholar]
- 10.Townson SM, Sullivan T, Zhang Q, Clark GM, Osborne CK, Lee AV, Oesterreich S. HET/SAF-B Overexpression Causes Growth Arrest and Multinuclearity and Is Associated with Aneuploidy in Human Breast Cancer. Clinical Cancer Research. 2000;6:3788–3796. [PubMed] [Google Scholar]
- 11.Weighardt F, Cobianchi F, Cartegni L, Chiodi I, Villa A, Riva S, Biamonti G. A novel hnRNP protein (HAP/SAF-B) enters a subset of hnRNP complexes and relocates in nuclear granules in response to heat shock. J Cell Sci. 1999;112:1465–1476. doi: 10.1242/jcs.112.10.1465. [DOI] [PubMed] [Google Scholar]
- 12.Chan CW, Lee YB, Uney J, Flynn A, Tobias JH, Norman M. A novel member of the SAF (scaffold attachment factor)-box protein family inhibits gene expression and induces apoptosis. Biochem J. 2007;407:355–362. doi: 10.1042/BJ20070170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Townson SM, Dobrzycka KM, Lee AV, Air M, Deng W, Kang K, Jiang S, Kioka N, Michaelis K, Oesterreich S. SAFB2, a New Scaffold Attachment Factor Homolog and Estrogen Receptor Corepressor. Journal of Biological Chemistry. 2003;278:20059–20068. doi: 10.1074/jbc.M212988200. [DOI] [PubMed] [Google Scholar]
- 14.Townson SM, Kang K, Lee AV, Oesterreich S. Structure-Function Analysis of the Estrogen Receptor α Corepressor Scaffold Attachment Factor-B1. Journal of Biological Chemistry. 2004;279:26074–26081. doi: 10.1074/jbc.M313726200. [DOI] [PubMed] [Google Scholar]
- 15.Oesterreich S, Zhang Q, Hopp T, Fuqua SAW, Michaelis M, Zhao HH, Davie JR, Osborne CK, Lee AV. Tamoxifen-Bound Estrogen Receptor (ER) Strongly Interacts with the Nuclear Matrix Protein HET/SAF-B, a Novel Inhibitor of ER-Mediated Transactivation. Mol Endocrinol. 2000;14:369–381. doi: 10.1210/mend.14.3.0432. [DOI] [PubMed] [Google Scholar]
- 16.Debril MB, Dubuquoy L, Feige JN, Wahli W, Desvergne B, Auwerx J, Gelman L. Scaffold attachment factor B1 directly interacts with nuclear receptors in living cells and represses transcriptional activity. J Mol Endocrinol. 2005;35:503–517. doi: 10.1677/jme.1.01856. [DOI] [PubMed] [Google Scholar]
- 17.Jiang S, Meyer R, Kang K, Osborne CK, Wong J, Oesterreich S. Scaffold Attachment Factor SAFB1 Suppresses Estrogen Receptor {alpha}-Mediated Transcription in Part via Interaction with Nuclear Receptor Corepressor. Mol Endocrinol. 2006;20:311–320. doi: 10.1210/me.2005-0100. [DOI] [PubMed] [Google Scholar]
- 18.Ivanova M, Dobrzycka KM, Jiang S, Michaelis K, Meyer R, Kang K, Adkins B, Barski OA, Zubairy S, Divisova J, Lee AV, Oesterreich S. Scaffold Attachment Factor B1 Functions in Development, Growth, and Reproduction. Mol Cell Biol. 2005;25:2995–3006. doi: 10.1128/MCB.25.8.2995-3006.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ren J, Gao X, Jin C, Zhu M, Wang X, Shaw A, Wen L, Yao X, Xue Y. Systematic study of protein sumoylation: Development of a site-specific predictor of SUMOsp 2.0. PROTEOMICS. 2009;9:3409–3412. doi: 10.1002/pmic.200800646. [DOI] [PubMed] [Google Scholar]