Abstract
High glucose concentrations due to diabetes increase leakage of plasma constituents across the endothelial permeability barrier. We sought to determine whether vitamin C, or ascorbic acid (ascorbate), could reverse such high glucose-induced increases in endothelial barrier permeability. Human umbilical vein endothelial cells and two brain endothelial cell lines cultured at 25 mM glucose showed increases in endothelial barrier permeability to radiolabeled inulin compared to cells cultured at 5 mM glucose. Acute loading of the cells for 30–60 min with ascorbate before the permeability assay prevented the high glucose-induced increase in permeability and decreased basal permeability at 5 mM glucose. High glucose-induced barrier leakage was mediated largely by activation of the receptor for advanced glycation end products (RAGE), since it was prevented by RAGE blockade and mimicked by RAGE ligands. Intracellular ascorbate completely prevented RAGE ligand-induced increases in barrier permeability. The high glucose-induced increase in endothelial barrier permeability was also acutely decreased by several cell-penetrant antioxidants, suggesting that at least part of the ascorbate effect could be due to its ability to act as an antioxidant.
Keywords: endothelial permeability, high glucose, RAGE, oxidative stress, ascorbate
Introduction
The endothelium presents a barrier to movement of cells and plasma constituents out of the blood vessel, the permeability of which varies greatly in different vascular beds and under different conditions of blood flow, hormonal activation, and disease state [1,2]. Among the conditions known to increase endothelial barrier permeability is the hyperglycemia of diabetes, where it is one of the earliest signs of endothelial dysfunction [3–5] and an established precursor to diabetes microvascular complications [6,7], including insulin resistance [8]. Several mechanisms may underlie high glucose-induced increases in endothelial barrier permeability. There is evidence that it is caused by increases in cellular oxidative stress and superoxide generation that are a consequence of excessive glucose metabolism [9] and protein kinase C activation [7,10,11]. High glucose also generates advanced glycation end-products (AGE) that along with other ligands produced by damaged cells (e.g., HMGB1, calgranulins) bind to the receptor for AGE (RAGE) and increase both its activation and expression [12,13]. RAGE activation in turn activates NADPH oxidase, which is associated with increased retinal endothelial permeability [14].
Agents known to reverse dysfunctional increases in endothelial permeability include increases in intracellular cyclic AMP [15], cyclic GMP [16,17], nitric oxide [1,18,19], prostaglandin E2 [20], and sphingosine 1-phosphate [21]. We have also found that an increase in intracellular vitamin C, or ascorbic acid (ascorbate), tightens the endothelial barrier as measured by decreased paracellular transfer of the polysaccharide inulin and of ascorbate itself across endothelial monolayers [22].
Whether and how ascorbate might reverse high glucose-induced increases in endothelial barrier permeability is unknown. In this work we tested this hypothesis in three types of endothelial cells: two immortalized microvascular brain endothelial cell lines and human umbilical vein endothelial cells (HUVECS).
Materials and Methods
Materials
Sigma/Aldrich Chemical Co. (St. Louis, MO) supplied the reagent chemicals, including ascorbate, N-2-hydroxyethylpiperazine N′-2-ethanesulfonic acid (Hepes), 4-hydroxy-2,2,6,6-tetramethylpiperidine 1-oxyl (4-hydroxy-TEMPO, Tempol), and (±)-6-hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid (Trolox). N-benzyl-4-chloro-N-cyclohexylbenzamide (FPS-ZM1) was purchased from EMD Millipore (Billerca, MA, catalog #553030). FPS-ZM1 was initially dissolved in a small amount of dimethylsulfoxide, and then diluted with culture medium such that the final dimethylsulfoxide concentration was 0.06% or less. HMGB1 was purchased from ProSpec (Ness Ziona, Israel, catalog # pro-610). AGE-conjugated bovine serum albumin (AGE-BSA) was purchased from BioVision, Inc. (Milpitas, CA, catalog #2221-10). Perkin-Elmer Life and Analytical Sciences, Inc. (Boston, MA) supplied the [carboxyl-14C]inulin (molecular weight range 5000–5500, 2 mCi/g).
Cell Culture
Early passage HUVECS were obtained from Sciencell Research Laboratories (Carlsbad, CA, catalog #8000) and cultured in Endothelial Cell Medium from the same company (catalog #1001) that contained 10% (v/v) heat-inactivated fetal bovine serum and the indicated glucose concentration.
The human cerebral microvascular endothelial cell line hCMEC/D3 [23] was a kind gift from Dr. Ashwath Jayagopal. Cells were cultured in Medium 131 (Gibco/Invitrogen, Carlsbad, CA, catalog # M-131-500) containing microvascular growth supplement (Gibco/Invitrogen catalog #S-005-25) after coating of the plate or filter with 0.1% gelatin (Gibco/Invitrogen catalog #S-006-100) according to the manufacturer’s instructions.
Murine brain endothelial cells (bEnd.3 cells) were obtained from ATTC (VA, USA) and cultured in a 1:1 ratio of Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal bovine serum and astrocyte conditioned media. Astrocyte-conditioned media from primary rat brain astrocytes is known to tighten the endothelial cell barrier [24].
Cells were cultured at 37 °C in humidified air containing 5% CO2.
Assay of intracellular ascorbate
To measure intracellular ascorbate, confluent cells cultured in 6-well plates were rinsed 3 times with Krebs-Ringer Hepes buffer (KRH) that consisted of 20 mM Hepes, 128 mM NaCl, 5.2 mM KCl, 1 mM NaH2PO4, 1.4 mM MgSO4, and 1.4 mM CaCl2, pH 7.4. After removal of the last rinse, the cells were triturated with 0.1 ml of 25 % (w/v) metaphosphoric acid with mixing. The lysate was partially neutralized with 0.35 ml of 0.1M Na2HPO4 and 0.05 mM EDTA, pH 8.0, centrifuged at 3 °C for 1 min at 13,000 × g, and the supernatant was taken for assay of ascorbate. Ascorbate assay was performed in duplicate by HPLC as previously described [25]. Intracellular ascorbate concentrations were calculated based on the measured intracellular distribution space of 3-O-methylglucose relative to protein: 3.0 μl/mg protein in hCMEC/D3 cells and 2.9 μL/mg protein in bEnd.3 cells [26]. The water space in HUVECs was taken as that previously measured in HUVEC-derived EA.hy926 endothelial cells (3.6 μl/mg protein) [26].
Assay of trans-endothelial inulin transfer
Endothelial cells were cultured to confluence in a 6-well format on polyethylene terephthalate cell culture inserts (0.4 micron pores at a density of 2 ± 0.2 × 106 pores per cm2, Falcon BD Biosciences, Franklin Lakes, NJ). After reaching confluence, cells were cultured for another 5–6 days with 1.7 ml of medium in the upper well and 2.8 ml of medium in the lower well. Agents were added above the cells/filter, followed by incubation at 37 °C for the times indicated. [Carboxyl-14C]inulin was added during the last hour of the transfer experiment. Aliquots of medium above and below the cells/filter were sampled for liquid scintillation counting of the radiolabeled inulin.
The permeability of the endothelial cell layer to [carboxyl-14C]inulin and was measured as previously described [27], with minor modification [22]. The permeability coefficient for [carboxyl-14C]inulin was corrected for [carboxyl-14C]inulin transfer across filters after removal of cells by treatment with ammonium hydroxide [28]. This adjusts for any changes in permeability due to deposition of matrix material by the cells during culture.
Measurement of trans-endothelial electrical resistance (TEER)
TEER, primarily reflecting the flux of ions through the endothelial barrier, was measured at room temperature across confluent cell monolayers using an EVOM resistance meter (EVOM2, World Precision Instruments, Sarasota, FL). Measurements were taken concomitantly with the inulin transfer experiments. Triplicate readings were taken for each well after the treatment time period. Once the cells were removed from the insert, blank measurements were recorded to adjust for changes due to deposition of matrix material by the cells during culture and to account for the insert itself. Values are reported in Ω × cm2.
Data Analysis
Results are shown as mean + standard error. Statistical comparisons were made using GraphPad Prism version 5.04 for Windows (GraphPad Software, San Diego, CA). Differences between treatments were assessed by one-way ANOVA with post-hoc testing, as appropriate.
Results
Intracellular accumulation of ascorbate in endothelial cells
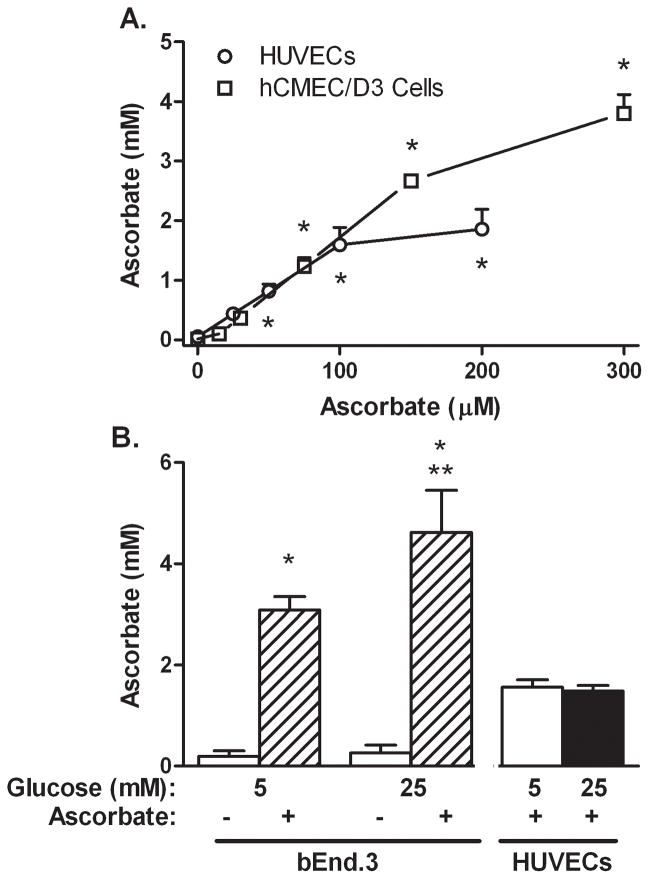
Early passage HUVECs, and both hCMEC/D3 cells and bEnd.3 cells contained only trace amounts of ascorbate in culture (Fig. 1). When incubated for 60 min with increasing amounts of ascorbate at 5 mM glucose, HUVECs and hCMEC/D3 cells accumulated intracellular ascorbate in a saturable manner to concentrations of about 2 mM for HUVECs and 4 mM for hCMEC/D3 cells (Fig. 1A). bEnd.3 cells also accumulated ascorbate against a concentration gradient (Fig. 1B). When bEnd.3 cells that had been cultured in 25 mM glucose were treated with 200 μM ascorbate for 60 min, intracellular ascorbate was modestly higher than at 5 mM glucose. This difference was not observed for HUVECs, however (Fig. 1B).
Figure 1. Ascorbate loading of endothelial cells in 5 mM and 25 mM glucose conditions.
Panel A. Cells (HUVECs, circles; HCMEC/D3 cells, squares) in culture at 5 mM glucose, were treated with the ascorbate concentrations noted for 60 min at 37 °C. Panel B. bEnd.3 cells in culture at 5 mM or 25 mM glucose were treated where indicated for 60 min with 200 μM ascorbate. HUVECs cultured in 5 or 25 mM glucose were treated with 150 μM ascorbate. Results are shown from 3–5 experiments with *p < 0.05 compared to no ascorbate control and **p < 0.05 compared to 5 mM glucose at the same ascorbate loading concentration.
Ascorbate reverses high glucose-induced increases in endothelial permeability
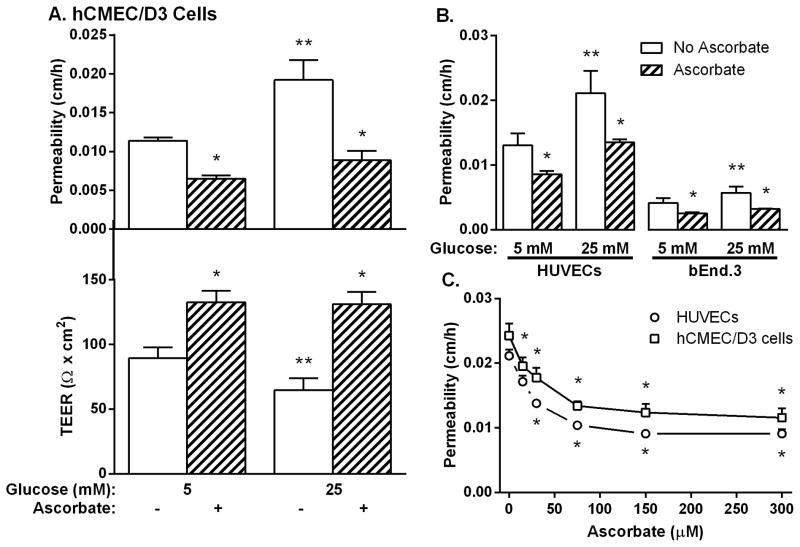
In hCMEC/D3 cells cultured at 5 mM glucose, a 30 min treatment with 150 μM ascorbate decreased transfer of [carboxyl-14C]inulin across the endothelial permeability barrier by about 40% (Fig. 2A, top panel). This was also reflected in a corresponding increase in TEER due to ascorbate (Fig. 2A, bottom panel). Culture of hCMEC/D3 cells for 6–7 days at 25 mM glucose increased endothelial permeability compared to that measured in cells cultured at 5 mM glucose and this increase was reversed by treatment with ascorbate (Fig. 2A, top panel). The glucose-induced increase in permeability was confirmed by a decrease in TEER, which was also reversed by ascorbate (Fig. 2A, bottom panel). Similar results were observed for HUVECs and bEnd.3 cells for [carboxyl-14C] inulin permeability (Fig. 2B). bEnd3 cells had tighter endothelial barriers to radiolabeled inulin transfer compared to HUVECs at both 5 mM and 25 mM glucose (Fig. 2B). The inhibitory effect of ascorbate on the glucose-induced increase in endothelial permeability to radiolabeled inulin was progressive and half-maximal at about 40 μM ascorbate for both hCMEC/D3 cells and HUVECs (Fig. 2C). Pre-loading each cell type at both glucose concentrations with similar concentrations of the oxidized form of ascorbate, dehydroascorbate, decreased endothelial permeability to the same extent as with ascorbate (results not shown).
Figure 2. Inhibition of high glucose-induced increases in endothelial permeability by ascorbate.
Panel A. HCMEC/D3 cells in culture with 5 mM or 25 mM glucose were treated with 150 μM ascorbate for 30 minutes and transfer of [carboxyl-14C] inulin (top) or TEER (bottom) measured after 60 min. Panel B. HUVECs and bEnd.3 cells cultured in 5 mM or 25 mM glucose were treated with 200 μM ascorbate for 30 min before addition of [carboxyl-14C] inulin. Panel C. HUVECs (circles) and hCMEC/D3 cells (squares) in culture for 6–7 days with 25 mM glucose were incubated for 30 min with the indicated concentrations of ascorbate, followed by the inulin transfer assay. Results are shown from 3–5 experiments with *p < 0.05 compared to no ascorbate and **p < 0.05 compared to 5 mM glucose at the same ascorbate concentration.
RAGE activation mediates high glucose-induced endothelial barrier leakage
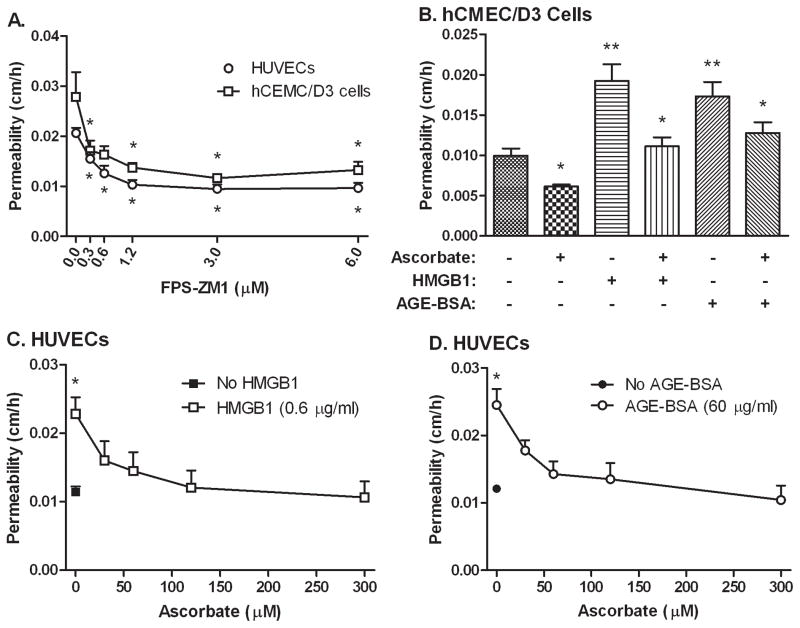
To determine whether RAGE activation is involved in the observed high glucose-induced increases in endothelial barrier permeability, HUVECs and hCMEC/D3 cells cultured at 25 mM glucose were treated for 60 min with increasing concentrations of the potent and specific RAGE inhibitor FPS-ZM1 [29] before the inulin transfer assay. As indicated for both cell types in Fig. 3A, FPS-ZM1 decreased endothelial permeability to levels similar to those observed at 5 mM glucose alone with a half-maximal effect at a concentration of 0.6 μM. These results suggest that RAGE activation mediates most, if not all, of the high glucose-induced increase in endothelial barrier permeability. FPS-ZM1 over this concentration range did not affect basal permeability in HUVECs cultured at 5 mM glucose (results not shown).
Figure 3. Ascorbate inhibits endothelial leakage due to RAGE activation.
Panel A: Cells (HUVECs, circles; hCMEC/D3 cells, squares) cultured at either 5 mM or 25 mM glucose for 6–7 days were treated with the indicated concentration of FPS-ZM1 for 60 min, followed by the radiolabeled inulin transfer assay. Results are from 6 experiments with *p < 0.05 compared to untreated control. Panel B. HCMEC/D3 cells cultured at 5 mM glucose were treated with for 24 h with 60 μg/ml AGE-BSA or 0.6 μg/ml HMGB1. On the next day, cells were treated for 30 min with 200 μM ascorbate before the inulin transfer assay. Results are from 6 experiments and *p < 0.05 compared to no ascorbate and **p < 0.05 compared to overall untreated control. Panels C and D. HUVECs cultured at 5 mM glucose were left untreated (closed symbols) or treated in culture for 24 h with 60 μg/ml AGE-BSA (Panel C) or 0.6 μg/ml HMGB1 (Panel D). On the next day, cells were treated for 30 min with the indicated concentrations of ascorbate, before the inulin transfer assay. Results are from 5 experiments with each RAGE ligand and *p < 0.05 compared to all other samples in the same experiment.
If culture of endothelial cells at high glucose concentrations increases endothelial barrier permeability by activating RAGE receptors, then known RAGE receptor ligands should also increase permeability. This hypothesis was tested with optimized concentrations of two RAGE ligands (Fig. 3B-D). In hCMEC/D3 cells, a 24 h incubation of either 0.6 μg/ml HMGB1 or 60 μg/ml AGE-BSA increased inulin transfer compared to control. Addition of 200 μM ascorbate for 30 min before the transfer assay both decreased basal inulin transfer and prevented the increases due to the RAGE ligands (Fig. 3B).
To determine the sensitivity of RAGE agonist-induced increases in inulin permeability in primary culture cells, HUVECs were cultured on trans-well filters at 25 mM glucose and treated in culture for 24 h with either AGE-BSA or HMGB1. As with hCMEC/D3 cells, both agents doubled basal inulin transfer (Fig. 3C and 3D, compare open and closed symbols at zero ascorbate). Control non-glycosylated BSA had no effect on permeability (results not shown). When cells were treated with increasing concentrations of ascorbate for 30 min before the inulin transfer assay, the endothelial permeability barrier progressively tightened to levels seen in the basal cells (Fig. 3C and 3D, open symbols). These effects of ascorbate mirror those seen in cells cultured at high glucose concentrations (Fig. 2), having half-maximal effects at ascorbate loading concentrations of about 25 μM.
Other antioxidants decrease permeability under high glucose conditions
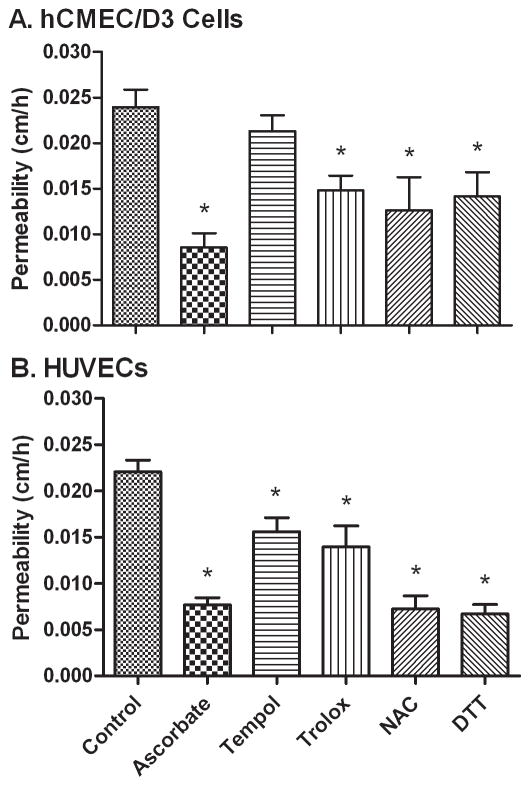
To assess whether other antioxidants might also acutely reverse the increase in endothelial permeability due to high glucose, endothelial cells cultured on filters in 25 mM glucose were treated for 60 min with several antioxidants with different mechanisms of action, followed by the endothelial permeability assay (Fig. 4). As shown for hCMEC/D3 cells in Fig. 4A, all of the antioxidants at the concentrations used except Tempol tightened the endothelial barrier. These agents were also tested in HUVECs (Fig. 4B), where all had significant decreases, although those observed with Tempol and Trolox were modest. These results suggest that the effect of intracellular ascorbate could relate to its function as an antioxidant.
Figure 4. Antioxidant reversal of high glucose-induced permeability in endothelial cells.
Panel A and B. HCMEC/D3 cells (Panel A) and HUVECs (Panel B) cultured for 6–7 days in 25 mM glucose were incubated without any additions (Control) or with additions as follows: ascorbate, 125 μM; Tempol, 300 μM; Trolox, 300 μM; N-acetyl cysteine (NAC), 600 μM; or dithiothreitol (DTT), 300 μM. After 60 min, radioactive inulin was added and the transfer assay was carried out. Results are from 6 experiments, with *p < 0.05 compared to 25 mM glucose control.
Discussion
As in previous studies in endothelial cells [3,30,31], culture of HUVECs, hCMEC/D3, and bEnd.3 cells at 25 mM glucose increased endothelial barrier permeability compared to that observed at 5 mM glucose. The present study shows that a relatively short 90–120 minute treatment of the endothelial cells with ascorbate reversed the increase in endothelial barrier permeability induced by culture at 25 mM glucose as well as that caused by 24 h of exposure to RAGE ligands.
Endothelial cells are typically cultured in medium lacking ascorbate and as shown in this work and in a previous study contain negligible concentrations of the vitamin [32]. However, they readily accumulate it over 60 min due to function of the ascorbate transporter SVCT2 [33]. A caveat regarding the brain endothelial cells is that they lack the SVCT2 in vivo [34], but rapidly develop it in culture [35].
The ability of ascorbate to decrease high glucose- and RAGE-induced endothelial permeability could involve several mechanisms. First, ascorbate might decrease endothelial permeability due to its function as an antioxidant, since both thiol and other antioxidants also partially or completely reversed high glucose-induced endothelial barrier leakage. Culture of endothelial cells in high glucose increases superoxide generation [36,37]. Subsequent increases in cellular hydrogen peroxide after the action of superoxide dismutase could then increase endothelial barrier leak [38–40]. In this scenario, scavenging of superoxide or its downstream products by low millimolar concentrations of ascorbate [41] could well decrease oxidant-induced increases in endothelial barrier permeability.
High glucose concentrations in culture also generate AGEs, which bind to and activate RAGE [42]. That RAGE ligands can contribute to high glucose-induced endothelial barrier leakage is clear from the results of this and previous studies [43,44]. Indeed, our finding that a specific RAGE inhibitor returned high glucose-induced increases in endothelial barrier permeability to baseline suggests that the RAGE pathway was a major cause of the glucose effects in HUVECs. The ability of ascorbate to acutely reverse RAGE ligand-mediated endothelial barrier leakage suggests that it was able to block one or more crucial features of this pathway. RAGE activation leads to multiple different signaling pathways, one of which involves an increase in intracellular reactive oxygen species due to the activation of NADPH oxidase [14]. Antioxidants and reactive oxygen species have been shown to have opposite acute effects on cell permeability by rearranging the cytoskeleton [45,46]. It is possible that the effects of ascorbate could alter the cytoskeleton within the time frame investigated, improving barrier stability.
In conclusion, culture of three separate endothelial cell lines at high glucose concentrations for several days increased RAGE-dependent leakage of radiolabeled inulin across the endothelial permeability barrier, an effect reversed by ascorbate loading of the cells. At least part of the ability of ascorbate to tighten the endothelial barrier to high glucose or RAGE activation is likely due to scavenging of radical species. These findings have relevance to microvascular disease caused by the hyperglycemia of diabetes, since replenishment of ascorbate depleted by oxidative stress could well tighten the endothelial permeability barrier and decrease capillary leak of plasma constituents.
HIGHLIGHTS.
Endothelial cells accumulate millimolar concentrations of ascorbate after one hour.
Ascorbate decreases permeability under basal and high glucose conditions.
Glucose-induced permeability is primarily due to RAGE activation.
Ascorbate reverses the increase in permeability due to RAGE activation.
Other antioxidants are able to decrease permeability under high glucose conditions.
Acknowledgments
This work was supported by National Institutes of Health grant DK 50435.
Abbreviations
- AGE
advanced glycation end-products
- FPS-ZM1
N-benzyl-4-chloro-N-cyclohexylbenzamide
- Hepes
N-2-hydroxyethylpiperazine-NN-2-ethanesulfonic acid
- HMGB1
high mobility group box 1
- KRH
Krebs-Ringer Hepes
- NAC
N-acetylcysteine
- RAGE
receptor for advanced glycation end-products
- SVCT2
sodium-dependent vitamin C transporter-2
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.van Hinsbergh WM. Endothelial permeability for macromolecules. Mechanistic aspects of pathophysiological modulation. Arterioscler Thromb Vasc Biol. 1997;17:1018–1023. doi: 10.1161/01.atv.17.6.1018. [DOI] [PubMed] [Google Scholar]
- 2.Vandenbroucke E, Mehta D, Minshall R, Malik AB. Regulation of endothelial junctional permeability. Ann N Y Acad Sci. 2008;1123:134–145. doi: 10.1196/annals.1420.016. [DOI] [PubMed] [Google Scholar]
- 3.Mimura K, Umeda F, Yamashita T, Kobayashi K, Hashimoto T, Nawata H. Effects of glucose and an aldose reductase inhibitor on albumin permeation through a layer of cultured bovine vascular endothelial cells. Horm Metab Res. 1995;27:442–446. doi: 10.1055/s-2007-979998. [DOI] [PubMed] [Google Scholar]
- 4.Yamashita T, Mimura K, Umeda F, Kobayashi K, Hashimoto T, Nawata H. Increased transendothelial permeation of albumin by high glucose concentration. Metabolism. 1995;44:739–744. doi: 10.1016/0026-0495(95)90186-8. [DOI] [PubMed] [Google Scholar]
- 5.Stauber WT, Ong SH, McCuskey RS. Selective extravascular escape of albumin into the cerebral cortex of the diabetic rat. Diabetes. 1981;30:500–503. doi: 10.2337/diab.30.6.500. [DOI] [PubMed] [Google Scholar]
- 6.Calles-Escandon J, Cipolla M. Diabetes and endothelial dysfunction: A clinical perspective. Endocr Rev. 2001;22:36–52. doi: 10.1210/edrv.22.1.0417. [DOI] [PubMed] [Google Scholar]
- 7.Hink U, Li H, Mollnau H, Oelze M, Matheis E, Hartmann M, Skatchkov M, Thaiss F, Stahl RA, Warnholtz A, Meinertz T, Griendling K, Harrison DG, Forstermann U, Munzel T. Mechanisms underlying endothelial dysfunction in diabetes mellitus. Circ Res. 2001;88:E14–E22. doi: 10.1161/01.res.88.2.e14. [DOI] [PubMed] [Google Scholar]
- 8.Pinkney JH, Stehouwer CD, Coppack SW, Yudkin JS. Endothelial dysfunction: Cause of the insulin resistance syndrome. Diabetes. 1997;46:S9–S13. doi: 10.2337/diab.46.2.s9. [DOI] [PubMed] [Google Scholar]
- 9.Baynes JW. Role of oxidative stress in development of complications of diabetes. Diabetes. 1991;40:405–412. doi: 10.2337/diab.40.4.405. [DOI] [PubMed] [Google Scholar]
- 10.Guzik TJ, Mussa S, Gastaldi D, Sadowski J, Ratnatunga C, Pillai R, Channon KM. Mechanisms of increased vascular superoxide production in human diabetes mellitus: role of NAD(P)H oxidase and endothelial nitric oxide synthase. Circulation. 2002;105:1656–1662. doi: 10.1161/01.cir.0000012748.58444.08. [DOI] [PubMed] [Google Scholar]
- 11.Yuan SY, Ustinova EE, Wu MH, Tinsley JH, Xu W, Korompai FL, Taulman AC. Protein kinase C activation contributes to microvascular barrier dysfunction in the heart at early stages of diabetes. Circ Res. 2000;87:412–417. doi: 10.1161/01.res.87.5.412. [DOI] [PubMed] [Google Scholar]
- 12.Goldin A, Beckman JA, Schmidt AM, Creager MA. Advanced glycation end products: sparking the development of diabetic vascular injury. Circulation. 2006;114:597–605. doi: 10.1161/CIRCULATIONAHA.106.621854. [DOI] [PubMed] [Google Scholar]
- 13.Hofmann MA, Drury S, Fu C, Qu W, Taguchi A, Lu Y, Avila C, Kambham N, Bierhaus A, Nawroth P, Neurath MF, Slattery T, Beach D, McClary J, Nagashima M, Morser J, Stern D, Schmidt AM. RAGE mediates a novel proinflammatory axis: a central cell surface receptor for S100/calgranulin polypeptides. Cell. 1999;97:889–901. doi: 10.1016/s0092-8674(00)80801-6. [DOI] [PubMed] [Google Scholar]
- 14.Warboys CM, Toh HB, Fraser PA. Role of NADPH oxidase in retinal microvascular permeability increase by RAGE activation. Invest Ophthalmol Vis Sci. 2009;50:1319–1328. doi: 10.1167/iovs.08-2730. [DOI] [PubMed] [Google Scholar]
- 15.Moy AB, Bodmer JE, Blackwell K, Shasby S, Shasby DM. cAMP protects endothelial barrier function independent of inhibiting MLC20-dependent tension development. Am J Physiol. 1998;274:L1024–L1029. doi: 10.1152/ajplung.1998.274.6.L1024. [DOI] [PubMed] [Google Scholar]
- 16.Draijer R, Atsma DE, van der LA, van Hinsbergh VW. cGMP and nitric oxide modulate thrombin-induced endothelial permeability. Regulation via different pathways in human aortic and umbilical vein endothelial cells. Circ Res. 1995;76:199–208. doi: 10.1161/01.res.76.2.199. [DOI] [PubMed] [Google Scholar]
- 17.Westendorp RG, Draijer R, Meinders AE, van Hinsbergh VW. Cyclic-GMP-mediated decrease in permeability of human umbilical and pulmonary artery endothelial cell monolayers. J Vasc Res. 1994;31:42–51. doi: 10.1159/000159030. [DOI] [PubMed] [Google Scholar]
- 18.May JM, Qu ZC. Nitric oxide mediates tightening of the endothelial barrier by ascorbic acid. Biochem Biophys Res Commun. 2011;404:701–705. doi: 10.1016/j.bbrc.2010.12.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Predescu D, Predescu S, Shimizu J, Miyawaki-Shimizu K, Malik AB. Constitutive eNOS-derived nitric oxide is a determinant of endothelial junctional integrity. Am J Physiol Lung Cell Mol Physiol. 2005;289:L371–L381. doi: 10.1152/ajplung.00175.2004. [DOI] [PubMed] [Google Scholar]
- 20.Birukova AA, Zagranichnaya T, Fu P, Alekseeva E, Chen W, Jacobson JR, Birukov KG. Prostaglandins PGE(2) and PGI(2) promote endothelial barrier enhancement via PKA- and Epac1/Rap1-dependent Rac activation. Exp Cell Res. 2007;313:2504–2520. doi: 10.1016/j.yexcr.2007.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mehta D, Konstantoulaki M, Ahmmed GU, Malik AB. Sphingosine 1-phosphate-induced mobilization of intracellular Ca2+ mediates rac activation and adherens junction assembly in endothelial cells. J Biol Chem. 2005;280:17320–17328. doi: 10.1074/jbc.M411674200. [DOI] [PubMed] [Google Scholar]
- 22.May JM, Qu ZC, Qiao H. Transfer of ascorbic acid across the vascular endothelium: mechanism and self-regulation. Am J Physiol Cell Physiol. 2009;297:C169–C178. doi: 10.1152/ajpcell.00674.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weksler BB, Subileau EA, Perriere N, Charneau P, Holloway K, Leveque M, Tricoire-Leignel H, Nicotra A, Bourdoulous S, Turowski P, Male DK, Roux F, Greenwood J, Romero IA, Couraud PO. Blood-brain barrier-specific properties of a human adult brain endothelial cell line. FASEB J. 2005;19:1872–1874. doi: 10.1096/fj.04-3458fje. [DOI] [PubMed] [Google Scholar]
- 24.Arthur FE, Shivers RR, Bowman PD. Astrocyte-mediated induction of tight junctions in brain capillary endothelium: an efficient in vitro model. Brain Res. 1987;433:155–159. doi: 10.1016/0165-3806(87)90075-7. [DOI] [PubMed] [Google Scholar]
- 25.May JM, Qu ZC, Mendiratta S. Protection and recycling of α-tocopherol in human erythrocytes by intracellular ascorbic acid. Arch Biochem Biophys. 1998;349:281–289. doi: 10.1006/abbi.1997.0473. [DOI] [PubMed] [Google Scholar]
- 26.Jones W, Li X, Perriott LM, Whitesell RR, May JM. Uptake, recycling, and antioxidant functions of α-lipoic acid in endothelial cells. Free Radic Biol Med. 2002;33:83–93. doi: 10.1016/s0891-5849(02)00862-6. [DOI] [PubMed] [Google Scholar]
- 27.Siflinger-Birnboim A, del Vecchio PJ, Cooper JA, Blumenstock FA, Shepard JM, Malik AB. Molecular sieving characteristics of the cultured endothelial monolayer. J Cell Physiol. 1987;132:111–117. doi: 10.1002/jcp.1041320115. [DOI] [PubMed] [Google Scholar]
- 28.Utoguchi N, Ikeda K, Saeki K, Oka N, Mizuguchi H, Kubo K, Nakagawa S, Mayumi T. Ascorbic acid stimulates barrier function of cultured endothelial cell monolayer. J Cell Physiol. 1995;163:393–399. doi: 10.1002/jcp.1041630219. [DOI] [PubMed] [Google Scholar]
- 29.Deane R, Singh I, Sagare AP, Bell RD, Ross NT, LaRue B, Love R, Perry S, Paquette N, Deane RJ, Thiyagarajan M, Zarcone T, Fritz G, Friedman AE, Miller BL, Zlokovic BV. A multimodal RAGE-specific inhibitor reduces amyloid beta-mediated brain disorder in a mouse model of Alzheimer disease. J Clin Invest. 2012;122:1377–1392. doi: 10.1172/JCI58642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hempel A, Maasch C, Heintze U, Lindschau C, Dietz R, Luft FC, Haller H. High glucose concentrations increase endothelial cell permeability via activation of protein kinase C alpha. Circ Res. 1997;81:363–371. doi: 10.1161/01.res.81.3.363. [DOI] [PubMed] [Google Scholar]
- 31.Dang L, Seale JP, Qu X. High glucose-induced human umbilical vein endothelial cell hyperpermeability is dependent on protein kinase C activation and independent of the Ca2+-nitric oxide signalling pathway. Clin Exp Pharmacol Physiol. 2005;32:771–776. doi: 10.1111/j.1440-1681.2005.04266.x. [DOI] [PubMed] [Google Scholar]
- 32.Ek A, Ström K, Cotgreave IA. The uptake of ascorbic acid into human umbilical vein endothelial cells and its effect on oxidant insult. Biochem Pharmacol. 1995;50:1339–1346. doi: 10.1016/0006-2952(95)02024-1. [DOI] [PubMed] [Google Scholar]
- 33.Seno T, Inoue N, Matsui K, Ejiri J, Hirata K, Kawashima S, Yokoyama M. Functional expression of sodium-dependent vitamin C transporter 2 in human endothelial cells. J Vasc Res. 2004;41:345–351. doi: 10.1159/000080525. [DOI] [PubMed] [Google Scholar]
- 34.García ML, Salazar K, Millán C, Rodríguez F, Montecinos H, Caprile T, Silva C, Cortes C, Reinicke K, Vera JC, Aguayo LG, Olate J, Molina B, Nualart F. Sodium vitamin C cotransporter SVCT2 is expressed in hypothalamic glial cells. Glia. 2005;50:32–47. doi: 10.1002/glia.20133. [DOI] [PubMed] [Google Scholar]
- 35.Qiao H, May JM. Development of ascorbate transport in brain capillary endothelial cells in culture. Brain Res. 2008;1208:79–86. doi: 10.1016/j.brainres.2008.02.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cosentino F, Hishikawa K, Katusic ZS, Luscher TF. High glucose increases nitric oxide synthase expression and superoxide anion generation in human aortic endothelial cells. Circulation. 1997;96:25–28. doi: 10.1161/01.cir.96.1.25. [DOI] [PubMed] [Google Scholar]
- 37.Cai S, Khoo J, Channon KM. Augmented BH4 by gene transfer restores nitric oxide synthase function in hyperglycemic human endothelial cells. Cardiovasc Res. 2005;65:823–831. doi: 10.1016/j.cardiores.2004.10.040. [DOI] [PubMed] [Google Scholar]
- 38.Cai H. Hydrogen peroxide regulation of endothelial function: origins, mechanisms, and consequences. Cardiovasc Res. 2005;68:26–36. doi: 10.1016/j.cardiores.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 39.Lee HS, Namkoong K, Kim DH, Kim KJ, Cheong YH, Kim SS, Lee WB, Kim KY. Hydrogen peroxide-induced alterations of tight junction proteins in bovine brain microvascular endothelial cells. Microvasc Res. 2004;68:231–238. doi: 10.1016/j.mvr.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 40.Siflinger-Birnboim A, Lum H, del Vecchio PJ, Malik AB. Involvement of Ca2+ in the H2O2-induced increase in endothelial permeability. Am J Physiol. 1996;270:L973–L978. doi: 10.1152/ajplung.1996.270.6.L973. [DOI] [PubMed] [Google Scholar]
- 41.Jackson TS, Xu AM, Vita JA, Keaney JF., Jr Ascorbate prevents the interaction of superoxide and nitric oxide only at very high physiological concentrations. Circ Res. 1998;83:916–922. doi: 10.1161/01.res.83.9.916. [DOI] [PubMed] [Google Scholar]
- 42.Hudson BI, Schmidt AM. RAGE: a novel target for drug intervention in diabetic vascular disease. Pharm Res. 2004;21:1079–1086. doi: 10.1023/b:pham.0000032992.75423.9b. [DOI] [PubMed] [Google Scholar]
- 43.Esposito C, Gerlach H, Brett J, Stern D, Vlassara H. Endothelial receptor-mediated binding of glucose-modified albumin is associated with increased monolayer permeability and modulation of cell surface coagulant properties. J Exp Med. 1989;170:1387–1407. doi: 10.1084/jem.170.4.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hirose A, Tanikawa T, Mori H, Okada Y, Tanaka Y. Advanced glycation end products increase endothelial permeability through the RAGE/Rho signaling pathway. FEBS Lett. 2010;584:61–66. doi: 10.1016/j.febslet.2009.11.082. [DOI] [PubMed] [Google Scholar]
- 45.Touil YS, Fellous A, Scherman D, Chabot GG. Flavonoid-induced morphological modifications of endothelial cells through microtubule stabilization. Nutr Cancer. 2009;61:310–321. doi: 10.1080/01635580802521346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kratzer E, Tian Y, Sarich N, Wu T, Meliton A, Leff A, Birukova AA. Oxidative stress contributes to lung injury and barrier dysfunction via microtubule destabilization. Am J Respir Cell Mol Biol. 2012;47:688–697. doi: 10.1165/rcmb.2012-0161OC. [DOI] [PMC free article] [PubMed] [Google Scholar]