Abstract
Background
The neuroanatomy of agitation and aggression in Alzheimer's disease is not well understood.
Methods
We analyzed 24 months of Alzheimer's Disease Neuroimaging Initiative data for patients with Alzheimer's disease, mild cognitive impairment-stable, and mild cognitive impairment-converter (n = 462) using the Neuropsychiatric Inventory Questionnaire Agitation and Aggression subscale. Magnetic resonance imaging regions of interest that correlated with Neuropsychiatric Inventory Questionnaire Agitation and Aggression subscale raw scores were included in mixed-model, repeated-measures analyses of agitation and aggression over time with age, sex, apolipoprotein E ε4 status, education, and Mini-Mental State Examination score as covariates.
Results
Neuropsychiatric Inventory Questionnaire Agitation and Aggression subscale scores worsened in patients with Alzheimer's disease and in mild cognitive impairment-converter (P <.05; trend for mild cognitive impairment, P =.0518). Greater agitation and aggression severity was associated with greater atrophy of frontal, insular, amygdala, cingulate, and hippocampal regions of interest (P < .05). Mini-Mental State Examination score was significant in mixed-effect model repeated measures only in mild cognitive impairment-converters for posterior regions of interest. Demographics and apolipoprotein ε4 were not associated with agitation and aggression.
Conclusions
Agitation and aggression in Alzheimer's disease and mild cognitive impairment is associated with neurodegeneration affecting the anterior salience network that may reduce capacity to process and regulate behaviors properly.
Keywords: MRI, Agitation and aggression, Alzheimer's disease, Mild cognitive impairment, Frontolimbic, Salience network
1. Introduction
Agitation and aggression are common neuropsychiatric symptoms (NPS) of Alzheimer's disease (AD) that result in caregiver distress, daily life disruption, and potential harm that affect the patient, family, and caregiver. These behaviors are likely to lead to caregiver distress and burden [1], and increased rates of institutionalization of community-dwelling patients with AD [2] where they continue to be problematic for caregivers in institutionalized settings. Cohen-Mansfield et al [3,4] described behavioral symptoms in AD as categories of verbal and physical agitation and aggression that involve a range of behaviors: motor restlessness, such as pacing and fidgeting; verbal agitation, such as repeating phrases and yelling out inappropriately; disinhibited and emotionally labile behaviors, such as irritability, temper outbursts, blurting embarrassing comments, and making inappropriate sexual advances; and hurtful behaviors such as belittling, cursing, pinching, punching, kicking, and biting. Rates of these symptoms vary with the measurement method, but have been reported to range from 48% to 80% of patients with AD [5], be persistent over months [6], and occur across all stages of AD [5,7,8]. Agitation and apathy are among the most common NPS to occur in mild cognitive impairment (MCI) compared with elderly control subjects [9], suggesting that noncognitive NPS begin during the earliest stages of this neurodegenerative disease process. Furthermore, agitation in MCI predicts earlier diagnosis of AD [10]. Symptoms of agitation and aggression may co-occur with other NPS such as psychotic or mood symptoms [7], although factor analyses report them as loading together, separate from other NPS [6,11]. An Alzheimer's Association research roundtable identified the need for further research into the biological underpinnings of NPS syndromes, including agitation, as a way to advance understanding and to target management efforts more effectively [12].
Neuroanatomic studies relevant to agitation and aggression are few, but point toward involvement of frontal and temporal areas. Hirono et al [13] compared dementia patient groups with and without agitation and aggression as measured on the Neuropsychiatric Inventory (NPI) using single photon-emission computed tomography (SPECT) neuroimaging and found hypoperfusion of left anterior temporal, bilateral dorsolateral prefrontal, and right superior parietal cortices in those with agitation and aggression. Autopsy of brains from patients with AD revealed that the burden of neurofibrillary tangles in the left orbitofrontal and left anterior cingulate correlated with A/A, and in the left orbitofrontal correlated with aberrant motor behavior as measured by NPI scores [14]. Aggression in patients with AD was associated with right medial temporal lobe (hippocampus, parahippocampus, and posterior amygdala) hypoperfusion on SPECT neuroimaging and also with greater motor agitation, as measured by the Behavioural Pathology in Alzheimer's Disease scale [15]. Magnetic resonance imaging (MRI) measures of medial temporal lobe atrophy using visual inspection of T1-weighted images and fluid attenuated inversion recovery-detected white matter hyperintensities did not correlate with any NPI score in patients with AD such that Staekenborg et al [16] concluded that other brain regions might be involved, such as amygdala or frontal cortex. Using fludeoxyglucose positron emission tomography scans, Sultzer et al [17] reported an association between agitation/disinhibition on the Neurobehavioral Rating Scale and hypometabolism in the frontal-temporal lobes. Bruen et al [18] found greater agitation in those with decreased gray matter density on MRI in the left insula and anterior cingulate bilaterally.
We studied measures of atrophy in selected anterior and posterior brain regions of interest (ROIs) using serial MRI data over 2 years from the Alzheimer's Disease Neuroimaging Initiative (ADNI) study for patients with MCI and AD with symptoms of agitation and aggression. We assessed the relationship between gray matter volume and cortical thickness in selected ROIs, and severity of agitation and aggression symptoms as measured on a four-item subscale of the NPI Questionnaire version (NPI-Q).
2. Methods
2.1. Subjects and design
We analyzed the ADNI data set released in September 2010 (http://adni.loni.ucla.edu/). The ADNI is a multisite, multistudy program funded by public and private partnership to investigate whether the combination of neuroimaging, biological markers, and clinical and neuropsychological assessments can track accurately the disease progression in the Alzheimer's disease [19]. Data are publicly available to the scientific community for analyses. As of September 2010, 819 subjects had been recruited: 229 elderly control subjects, 402 subjects diagnosed with amnestic MCI, and 188 subjects with mild AD. Mini-Mental State Examination (MMSE) scores of patients with AD were between 20 points and 26 points (inclusive), Clinical Dementia Rating Scale scores of either 0.5 point or 1.0 point, and all met National Institute of Neurological Disorders and Stroke/Alzheimer's Disease and Related Disorders Association (NINCDS/ ADRDA) criteria for probable AD. Mini-Mental State Examination scores of subjects with MCI were between 24 points and 30 points, and these subjects had a memory complaint, objective memory loss as measured by education-adjusted scores on the Wechsler Memory Scale Logical Memory II, a Clinical Dementia Rating Scale score of 0.5 point, an absence of significant levels of impairment in other cognitive domains, essentially preserved activities of daily living, and an absence of dementia. All subjects underwent clinical/cognitive assessments and 1.5-T structural MRI at specified intervals (6 months or 12 months) for 2 to 3 years. At each visit, subjects with MCI were assessed to determine whether they had clinically progressed to AD (MCI-converters) or regressed to normal.
For the MCI population, we excluded patients whose complaints were attributed to other identifiable etiologies such as frontotemporal dementia, Parkinson's disease, Huntington's disease, and so forth, and separated out the patients with MCI who converted to AD during the initial 2-year follow-up as a separate subgroup. For the AD population, we excluded subjects who had Parkinson's disease. We collected demographic, genetic, and cognitive measurements, including age, sex, years of education, apolipoprotein E (APOE) epsilon (ε) allele status (ε2, ε3, and ε4 alleles), and NPI and MMSE [20] scores.
We selected patients with AD and MCI from the ADNI database who had any evidence of agitation and aggression symptoms (at least 1 point on the subscale) at one or more study visits during a 2-year follow-up period to analyze the relationship between these symptoms and particular MRI measurements. Longitudinal data were analyzed for study visits that occurred at 6-month intervals, from baseline to 24 months (maximum of five study visits).
2.2. Procedures
2.2.1. Neuropsychiatric Inventory
The NPI-Q [21] was used in the ADNI instead of the standard version of the NPI. Both have the same 12 symptoms, but the NPI-Q differs from the NPI in that it rates only severity and not frequency in the ratings, and it is reformatted to be a self-administered written questionnaire instead of an interview of the caregiver. The caregiver chooses a yes or no answer for every subquestion for each item, and if any are selected for a symptom domain then the item is rated for severity (from 0–3 points). The NPI-Q correlates highly with the standard NPI (0.91 for total scale scores, 0.71–0.93 for items) and its maximum score is 36 points. Four items from the NPI-Q were chosen to capture a full range of verbal and physical agitation and aggression symptoms (agitation and aggression [A/A], irritability and emotional lability, aberrant motor behavior, and disinhibition) to comprise the NPI-Q-4-A/A subscale, which was our symptom outcome measure. The NPI-4-A/A has content validity based on factor analyses [7,11] and has been validated in part in an independent study [22].
2.2.2. MRI measurements
To study brain structure and atrophy associated with agitation and aggression in the AD and MCI populations, we used Freesurfer (http://surfer.nmr.mgh.harvard.edu) volumetric parcellations of the structural magnetic resonance images provided by the University of California at San Francisco, one of the funded MRI analysis sites in the ADNI consortium. Data files from September 2010 comprising numerical MRI end points and patient clinical and demographic variables were downloaded from the LONI website and combined by the Lilly study team. (A full list of subjects analyzed in this study is provided in Supplemental Data.) The preselected MRI ROIs that we analyzed are listed in Table 1 and were sorted into anterior ROI areas thought to be potentially related to agitation and aggression symptoms and posterior ROIs thought to be impaired in most patients with AD regardless of whether they have behavioral symptoms. We analyzed the left and right hemispheres separately in this study, and used both volumetric and cortical thickness measures (when available) for the selected ROIs.
Table 1.
Magnetic resonance imaging regions of interest are divided into two functional areas, include left and right hemispheres, and are modeled separately
| MRI anterior group ROIs | MRI posterior group ROIs |
|---|---|
| Volume | Volume |
| R and L ventral pallidum | R and L hippocampus |
| R and L insula | R and L splenium |
| R and L amygdala | R and L thalamus |
| R and L rostral anterior cingulate | R and L fusiform |
| R and L caudal anterior cingulate | R and L posterior cingulate |
| R and L medial orbitofrontal | R and L superior parietal |
| R and L lateral orbitofrontal | R and L precuneus |
| R and L rostral middle frontal | |
| R and L superior frontal | |
| Cortical thickness average | Cortical thickness average |
| R and L insula | R and L fusiform |
| R and L rostral anterior cingulate | R and L posterior cingulate |
| R and L caudal anterior cingulate | R and L superior parietal |
| R and L medial orbitofrontal | R and L precuneus |
| R and L medial orbitofrontal | |
| R and L rostral middle frontal | |
| R and L superior frontal |
Abbreviations: MRI, Magnetic resonance imaging; ROI, region of interest; R, right; L, left.
2.3. Statistical analyses
Data were analyzed using SAS (version 9.1) and JMP (version 8). Demographic, clinical, and scale data are reported as mean and standard deviation, and are reported for three subgroups: MCI-stable, MCI-converter, and AD.
2.3.1. Longitudinal modeling of NPI-Q-4-A/A
To understand the progression of the NPI-Q-4-A/A scores over time and its dependence on baseline covariates—age, MMSE score, sex, and ApoE genotype (binary variable)—we built longitudinal models separately for the AD, MCI-converter, and MCI-stable groups during a period of 2 years. For each ADNI subject, there were at most five time points during the 2-year follow-up (baseline and every 6-month visit). We implemented a linear model to capture the rate of NPI-Q-4-A/A changes in each group, as shown in equation 1.
| (1) |
where NPI4ij is the sum of NPI-Q-4-A/A scores for subject i at visit j; tij is the corresponding time elapsed since the baseline visit in years (0, 0.5, 1, 1.5, and 2 years); b0, bt, γi0, and γit are the fixed intercept, fixed slope, random intercept, and random slope; bage, bmmse, bapoe, and bsex are the weights of corresponding covariates, and εij is the individual error term with εij~N(0, σ2).
None of theseÞvariables were normalized. We examined the initial models built for the AD, MCI-converter, and MCI-stable groups and reduced these models further by leaving out variables that were not found to be significant (at a P value of .05) in any group. The final model includes significant variables only.
2.3.2. Correlation analysis of MRI measurements and NPI-Q-4-A/A
Next, to identify brain ROIs that are related to A/A symptoms in patients diagnosed with AD or MCI, we conducted nonparametric correlation analyses using the NPI-Q-4-A/A scores and MRI measurements in the selected brain ROIs across all longitudinal study visits using all subjects with AD and MCI. Volume/cortical thickness measurements of these brain ROIs, divided into primary and secondary functional groups as listed in Table 1, were used in this study. We used Spearman's ρ to calculate these correlations because the NPI-Q-4-A/A score is an ordinal variable. The correlation coefficients were calculated using raw measurements of brain ROIs (volume/cortical thickness) at each time point of the visit. An adjustment of raw MRI measurements using total intracranial volume did not generate significantly different results.
Based on these calculated correlation coefficients, we selected only MRI measurements with an absolute correlation coefficient greater than 0.09 across any visit as input to our subsequent mixed-effect model, repeated-measures (MMRM) analyses of the association between brain ROIs and A/A. These ROIs had P values ≤.15 to ensure we captured those with trends but also to delimit from those ROIs with correlations that were in a much lower range. Regions of interest clustered together clearly at ρ values either greater than 0.09 or much less than 0.09, so this value was chosen as the cutoff to select ROIs for the subsequent analyses. The method retains important features in the data set while avoiding issues such as multicolinearity and overfitting.
2.3.3. Association between brain ROIs and NPI-Q-4-A/A with MMRM modeling
Last, using the reduced set of MRI measurements, we modeled the association between NPI-Q-4-A/A and these selected brain ROIs during the period of 2 years with an MMRM approach. The model was built separately for each of the AD, MCI-converter, and MCI-stable subgroups, and for each of the MRI ROI groups. In each model, we assessed the degree to which the change in NPI-Q-4-A/A scores over time could be explained by the reduced set of MRI measurements over time, together with baseline covariates such as age, sex, MMSE score, ApoE genotype (binary as presence or absence of an ε4 allele), and patient educational level (measured in years of formal education). The MRI measurements were coded as percentage changes from baseline for normalization, and raw values were used for all other variables. In sum, using this model we attempted to determine which brain regions are associated with change in NPI-Q-4-A/A over an extended period of time in the AD and MCI populations.
3. Results
3.1. Subjects
During the 2-year study period, 139 of the 356 patients with MCI converted to AD, producing three study groups: AD (n = 179), MCI-converters (n = 139), and MCI-stable (n = 217). Of these subjects, 163 with AD, 122 MCI-converters, and 177 MCI-stable subjects whose NPI-Q-4-A/A score was nonzero for at least one time point during the 24-month follow-up were used in the analyses. Summary statistics of these three groups are shown in Table 2. There was no significant difference in age, but there were significant differences for sex, education, and MMSE score across groups. Note that there are 2 AD and 1 MCI-converter subjects whose MMSE scores did not meet the ADNI–specified inclusion criteria. Because they were assigned to clinical groups by the ADNI I study investigators and their MMSE scores were only slightly out of range, we still included them in this analysis.
Table 2.
Demographic and rating scale data for the three diagnostic groups
| Variable | Visit | AD (n = 163) | MCI converters (n = 122) | MCI stable (n = 177) |
|---|---|---|---|---|
| Age | Baseline | 75.3 ± 7.5 (57–91) | 74.6 ± 6.9 (55–89) | 74.4 ± 7.8 (55–88) |
| Sex, % male* | Baseline | 52.6% | 65.5% | 67.0% |
| Education, y† | Baseline | 14.7 (4–20) | 15.7 (6–20) | 15.7 (4–20) |
| MMSE‡ | Baseline | 23.3 ± 2.1 (18–27) | 26.6 ± 1.7 (23–30) | 27.2 ± 1.8 (24–30) |
| NPI-Q-4-A/A* | Baseline | 1.5 ± 1.6 (0–7) | 1.2 ± 1.8 (0–10) | 1 ± 1.5 (0–9) |
| NPI-Q-10‡ | Baseline | 3.2 ± 2.9 (0–13) | 2.2 ± 2.8 (0–15) | 1.8 ± 2.3 (0–15) |
| MMSE‡ | 24 mo | 18.4 ± 6.2 (0–28) | 22.4 ± 3.7 (8–30) | 27.1 ± 2.9 (8–30) |
| NPI-Q-4-A/A‡ | 24 mo | 2.2 ± 2.2 (0–11) | 1.7 ± 2.2 (0–11) | 1.2 ± 1.5 (0–7) |
| NPI-Q-10‡ | 24 mo | 4.8 ± 4.5 (0–25) | 3.6 ± 3.3 (0–15) | 2.4 ± 2.5 (0–10) |
Abbreviations: AD, Alzheimer's disease; MCI, mild cognitive impairment; MMSE, Mini-Mental State Examination; NPI-Q-4-A/A, Neuropsychiatric Inventory Questionnaire Agitation and Aggression; NPI-Q-10, Neuropsychiatric Inventory Questionnaire-10.
NOTE. Values are expressed as mean ± standard deviation unless otherwise specified. Ranges are in parentheses. P values are from one-way analysis of variance across all groups.
P ≤ .05.
P ≤ .01.
P ≤ .001.
As expected, mean MMSE score was the lowest in the AD group, with a range from 18 to 27 points at baseline that decreased at 2 years. At baseline, the AD group had the highest NPI-Q-10 and NPI-Q-4-A/A mean scores compared with the MCI-converter and MCI-stable groups. All groups' NPI mean scores were in the mild severity range, although some individuals had high scores. Both the NPI-Q-10 and NPI-Q-4-A/A scores increased from baseline to 24 months, indicating worsening NPS with progression of the neurodegenerative process.
3.2. Longitudinal modeling of NPI-Q-4-A/A
In Table 3, we summarize the parameters estimated with the reduced longitudinal model for NPI-Q-4-A/A scores for each group. The NPI-Q-4-A/A score increase significantly over time (P < .05) for all groups, with annual point increases of 0.37, 0.24, and 0.11 point for the AD, MCI-converter, and MCI-stable groups, respectively. Among all baseline covariates (age, MMSE score, sex, and ApoE genotype), the only one that was associated significantly with NPI-Q-4-A/A, after adjusting for progression over time, was sex in the MCI-converter group, in which females had less progression.
Table 3.
Coefficients that showed significance for relationships with Neuropsychiatric Inventory Questionnaire Agitation and Aggression scores over the 2-year period in the reduced longitudinal model are listed by group
| Variable | MCI stable | MCI converter | AD |
|---|---|---|---|
| Intercept | 1.33‡ | 1.58‡ | 1.54‡ |
| Sex (female) | −0.29 | −0.39* | 0.01 |
| Time | 0.11 | 0.24† | 0.37* |
Abbreviations: MCI, Mild cognitive impairment; AD, Alzheimer's disease.
P ≤ .05.
P ≤ .01.
P ≤ .001.
3.3. Correlation among MRI ROIs and NPI-Q-4-A/A scores
Twenty-three ROIs had Spearman correlations of at least 0.09 with the NPI-Q-4-A/A score at any visit (Table 4). Of the anterior ROIs that correlated with the NPI-Q-4-A/A score, 10 were volume measures (left and right insula, left and right rostral anterior cingulate, left and right amygdala, left and right pallidum, right caudal anterior cingulate, and left superior frontal), whereas four were cortical thickness measures (left rostral middle frontal, left superior frontal, medial orbitofrontal, and right superior frontal). Of the posterior ROIs that correlated with the NPI-Q-4-A/A score, six were volume measures (left and right fusiform, left and right hippocampus, left precuneus, and left superior parietal) whereas three were cortical thickness measures (left and right fusiform, and right posterior cingulate).
Table 4.
All anterior and posterior group region of interest values (Table 1) were entered into a correlation analysis with Neuropsychiatric Inventory Questionnaire Agitation and Aggression individual scores
| MRI ROI | Baseline ρ | 6-Month ρ | 12-Month ρ | 18-Month ρ | p 24-Month ρ |
|---|---|---|---|---|---|
| Anterior group | |||||
| R insula | 0.056 | 0.019 | −0.013 | −0.015 | −0.090 |
| L insula | 0.055 | 0.047 | 0.000 | −0.006 | −0.164 |
| R rostral anterior cingulate | 0.085 | 0.093 | −0.003 | 0.011 | −0.034 |
| L rostral anterior cingulate | −0.010 | 0.045 | −0.061 | −0.012 | −0.132 |
| R caudal anterior cingulate | 0.032 | 0.047 | −0.048 | −0.070 | −0.117 |
| R amygdala | 0.057 | 0.061 | 0.016 | 0.063 | −0.130 |
| L amygdala | 0.013 | 0.041 | −0.018 | 0.004 | −0.126 |
| R pallidum | 0.021 | 0.146 | 0.070 | −0.001 | 0.120 |
| L pallidum | 0.014 | 0.128 | 0.026 | 0.012 | 0.042 |
| R superior frontal* | −0.006 | −0.091 | −0.011 | 0.002 | −0.035 |
| L superior frontal | 0.044 | 0.009 | 0.008 | 0.017 | −0.092 |
| L superior frontal* | −0.010 | −0.100 | −0.033 | −0.017 | −0.061 |
| L rostral middle frontal* | −0.020 | −0.107 | −0.098 | −0.017 | −0.091 |
| R medial orbitofrontal* | 0.008 | −0.106 | −0.072 | −0.032 | −0.040 |
| Posterior group | |||||
| R fusiform | 0.094 | 0.049 | 0.001 | 0.015 | −0.096 |
| R fusiform* | −0.008 | −0.053 | −0.023 | 0.012 | −0.094 |
| L fusiform | 0.059 | 0.053 | 0.016 | −0.072 | −0.138 |
| L fusiform* | −0.042 | −0.060 | −0.041 | −0.040 | −0.136 |
| L precuneus | −0.002 | 0.025 | −0.022 | 0.069 | −0.111 |
| R hippocampus | 0.024 | 0.005 | −0.050 | 0.112 | −0.090 |
| L hippocampus | −0.020 | 0.007 | −0.073 | 0.065 | −0.104 |
| R posterior cingulate* | −0.017 | −0.063 | −0.070 | −0.094 | −0.041 |
| L superior parietal | 0.012 | 0.043 | 0.032 | 0.111 | −0.011 |
Abbreviations: MRI, Magnetic resonance imaging; ROI, region of interest; R, right; L, left.
NOTE. Those with Spearman correlation coefficients (ρ) values that were ≥0.09 at any visit over 2 years are listed. Regions of interest in bold type were then entered into the mixed-effect model, repeated-measures model (see Table 5). All patients with Alzheimer's disease and mild cognitive impairment were included in this analysis. Volumes are reported except when indicated by an asterisk.
Cortical thickness average.
3.4. Associations with NPI-Q-4-A/A in MMRM modeling
Baseline variables for demographics, ApoE ε4 allele status, and MMSE score were not related to agitation and aggression in MMRM modeling analyses with the exception that lower baseline MMSE scores were significant in the posterior ROIs analysis for the MCI-converter group (Table 5). In contrast, change over time for MRI structural measures from a number of brain regions was associated with change over time in agitation and aggression symptoms. These were predominantly frontolimbic regions rather than posterior ROIs and comprised many components of the salience network (Fig. 1).
Table 5.
Mixed-effect-model, repeated-measures modeling was performed for each diagnostic group for anterior and posterior group magnetic resonance imaging regions of interest
| MMRM model variable* | MCI stable P value |
MCI converters P value |
AD P value |
|---|---|---|---|
| Anterior group ROI | |||
| Age | NS | NS | NS |
| ApoE ε4+ status | NS | NS | NS |
| Sex, male | NS | NS | NS |
| Intercept | NS | NS | NS |
| Baseline MMSE | NS | .08 | NS |
| Education, y | NS | NS | NS |
| R pallidum | .06 | NS | NS |
| L pallidum | NS | NS | NS |
| R rostral anterior cingulate | NS | NS | NS |
| L rostral anterior cingulate | NS | .00003 | NS |
| R caudal anterior cingulate | NS | NS | NS |
| R superior frontal† | NS | NS | .01 |
| L superior frontal | NS | .04 | NS |
| L superior frontal† | NS | NS | NS |
| L rostral middle frontal† | .04 | NS | NS |
| R medial orbitofrontal† | .07 | .04 | NS |
| R insula | NS | NS | .01 |
| L insula | .02 | NS | .01 |
| R amygdala | NS | .02 | .0007 |
| L amygdala | NS | NS | NS |
| Posterior group ROI | |||
| Age | NS | NS | NS |
| ApoE ε4+ status | NS | NS | NS |
| Sex (male) | NS | NS | NS |
| Intercept | NS | NS | NS |
| Baseline MMSE | NS | .02 | NS |
| Education, y | NS | NS | NS |
| R posterior cingulate† | NS | .000005 | NS |
| R fusiform | NS | NS | NS |
| R fusiform† | NS | NS | NS |
| L fusiform | NS | NS | NS |
| L fusiform† | NS | NS | NS |
| R hippocampus | NS | NS | NS |
| L hippocampus | NS | .03 | .02 |
| L precuneus | NS | NS | NS |
| L superior parietal | NS | NS | NS |
Abbreviations: MMRM, Mixed-effect model repeated measures; MCI, mild cognitive impairment; AD, Alzheimer's disease; ROI, regions of interest; NS, not significant; ApoE, apolipoprotein E; MMSE, Mini-Mental State Examination; R, right; L, left.
NOTE. Mixed-effect-model, repeated-measures modeling was analyzed within each group and included age, sex, educational level, MMSE score, and binary ApoE ε4 allele status (heterozygous or homozygous) along with the ROIs. Regions of interest with significant P values are in bold type, trends are not in bold type, and nonsignificant values are denoted as NS. Volumes are reported unless followed by an dagger.
These ROIs were chosen for MMRM analyses if they showed a correlation of ≥0.09 with the Neuropsychiatric Inventory Questionnaire Agitation and Aggression subscale score at any time point during the 2-year study period (see Table 4).
Cortical thickness average.
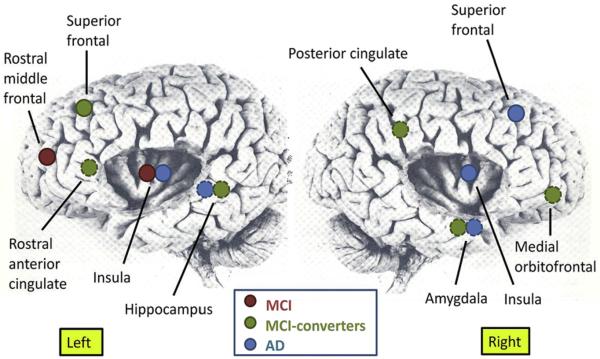
Fig. 1.
Neuroanatomic areas showing significant associations using mixed-effect model, repeated-measures modeling between increasing agitation and aggression as measured by the Neuropsychiatric Inventory Questionnaire Agitation and Aggression with increasing magnetic resonance region of interest atrophy (see text for details regarding volume or cortical thickness) during a 2-year period in Alzheimer's Disease Neuroimaging Initiative patients with Alzheimer's disease (AD) and mild cognitive impairment (MCI; n = 535). Dotted circles denote deep regions.
Increasing atrophy of two posterior regions—the left hippocampus in subjects with AD and MCI-converters, and the right posterior cingulate in MCI-converters—was associated with increasing agitation and aggression symptoms. Increasing atrophy of many frontal ROIs was associated with increasing agitation and aggression symptoms: left rostral middle frontal in MCI-stable, right superior frontal in AD, and right medial orbitofrontal, left rostral anterior cingulate, and left superior frontal in MCI-converters.
Atrophy of limbic structures—bilateral insula in the AD group, left insula in the MCI-stable group, and right amygdala in both the AD and MCI-converter groups—was related significantly to agitation and aggression. Amygdala atrophy (right sided) associations were contralateral to that of the hippocampus (left-sided).
4. Discussion
We investigated the relationship between agitation and aggression symptom severity over time and regional brain atrophy on MRI using longitudinal data in patients with MCI and AD from the ADNI database during a 2-year period. Our MMRM modeling analyses found a significant relationship for greater atrophy of frontolimbic regions, right posterior cingulate, and left hippocampus with greater severity of agitation and aggression. Volumetric and/or cortical thickness atrophy findings overshadowed educational, cognitive, and ApoE allele variables, suggesting that regional neurodegeneration was the dominating factor underlying these symptoms. There were more anterior ROI group relationships revealed by MMRM modeling than posterior. The frontolimbic ROIs are among those that represent components of the salience network [23] implicated in processing and reacting to complex social situations. Our findings suggest an important role for frontolimbic neural networks in the generation of agitation and aggression behaviors in each of our groups, although the specific components of these networks were affected variably at different disease stages. That these areas are affected early is supported by findings of decreased cortical thickness, decreased fractional anisotropy, and increased diffusivity in frontal and temporal regions in amnestic patients with MCI [24]. Our findings, taken in conjunction with the neuroimaging and autopsy literature [13–15,17,18], strongly support that neuroanatomic changes underlie abnormal behaviors in MCI and AD, even at early stages of the neurodegenerative disease process.
Using Freesurfer and voxel based morphometry analyses of atrophy per se via serial MRI data in the ADNI cohort, Risacher et al [25] reported larger percent changes for atrophy of frontal, temporal, and parietal ROIs in AD and MCI-converter groups but did not report behavioral indices. Furthermore, they reported that presence of one or more ApoE-4 alleles was associated significantly with higher annual mean rates of atrophy for various hippocampal and entorhinal cortex indices. In contrast, we did not find a relationship with ApoE-4 alleles in our models, and the literature on whether the ApoE4 allele is associated with agitation and aggression in AD is mixed [26–31]. This is interesting because in normal individuals the presence of the ApoE4 allele is associated with a faster breakdown of white matter integrity in late-myelinating regions (frontal and corpus callosal genu) than early-myelinating regions (splenium) [32,33], and the volume of the prefrontal callosal region shrinks faster with aging [34], which could be expected to affect neural signaling in frontolimbic brain regions implicated in behavioral changes in AD more in those with ApoE4. However, Agosta et al [35] found differentially more atrophy of gray matter in patients with AD with ApoE4-positive status in posterior ROIs than anterior ROIs. Although common in AD, agitation and aggression symptoms may only occur in those whose neurodegeneration affects certain anterior brain regions and circuitry regardless of ApoE allele status.
Our longitudinal model findings are consistent with cross-sectional reports of an association between agitation and aggression in AD with brain changes in frontal and limbic regions including amygdala, cingulate cortex, and insula [13–15,17]. Amygdaloid nuclei generate negative emotions like fear, disgust, and anxiety as well as affective memory, the latter occurring in close conjunction with the hippocampus, and become atrophied in AD. Amygdala outputs to insula may be abnormal in AD.
The dorsolateral prefrontal cortex subserves insight and executive functions and provides oversight and regulation over limbic outputs. Our findings of atrophy of superior frontal ROIs (ie, dorsolateral) is consistent with prior reports of impaired insight and executive functioning in patients with AD with agitation and aggression symptoms [36–38] and behavioral impairment. The orbitofrontal cortex (OFC) is important for social intelligence and comportment, and integrates limbic drives with social context. Dysfunctional OFC results in disinhibition, irritability, and other socially inappropriate behaviors, in part because limbic structures are dysregulated [39]. That right medial OFC atrophy was associated with agitation and aggression severity in MCI-converters suggests that impaired regulation of the amygdala by the OFC contributes to these symptoms. The OFC and rostral middle frontal cortices have a white matter connection to the amygdala via the uncinate fasciculus. Diffusion tensor imaging including tractography found significant abnormalities in the uncinate fasciculus in patients with AD compared with control subjects [40–42], consistent with disrupted connectivity that would exacerbate the neural communications between frontal–limbic regions where gray matter is also atrophied. Late- but not early-myelinating fibers, including uncinate, inferior, and superior longitudinal fascicule, stria terminalis, cingulum, and splenium, have significantly lower fractional anisotropy in patients with AD vs control subjects [43], supporting connectivity problems between frontal–limbic regions.
Our findings of bilateral insular atrophy associated with agitation and aggression are consistent with the report of Bruen et al [18] of left insular atrophy with agitation in AD and the occurrence of severe agitation with insular seizures. Furthermore, AD is associated with greater neurofibrillary tangle density in the agranular layer of the insula on autopsy, which is a more cytoarchitecturally primitive portion of the insula and interconnected reciprocally with the entorhinal region. The insula receives broad sensory inputs, has strong connections with the autonomic and limbic systems, and integrates external and internal sensations with emotion and memory such that it contributes to an awareness of oneself and how one feels [44]. The insula plays a fundamental role in human awareness and may contain interoceptive representations that link somatic and emotional feelings, whereas the anterior insula activates jointly with the anterior cingulate to integrate sensory, motor, and limbic inputs [45]. Therefore, insular pathology in AD may contribute to a disordered social sense of self and dysregulated social interactions evident in those with agitation and aggression in AD.
Related to insula function are Von Economo neurons (VENs). Von Economo neurons are only found in frontal–insular–anterior cingulate circuitry in primates and cetaceans that have large brains and live in complex social structures, and are most numerous in humans. Von Economo neurons are long, thin bipolar neurons that are larger than pyramidal neurons. They are implicated in mediating fast neurotransmission to support rapid, intuitive decision making and adjustment of behaviors, and thereby appear to subserve behavioral regulation in social contexts [46,47]. They are especially common in layer V of anterior cingulate and right frontoinsular cortices, as well as in the dorsolateral prefrontal cortex [48]. This anterior circuitry involving VENs is called the salience network [49], the activation of which is anticorrelated with activation of the posterior default mode network that is involved in introception [45]. Histopathology finds VENs to be dystrophic and atrophied in behavioral variant frontotemporal dementia, but not in AD [49], although patients with AD with agitation and aggression have not been studied separately. If VENs are preserved in patients with AD with agitation and aggression, then perhaps they are transmitting misinformation among atrophied amygdala and salience network regions, and may represent the altered neuroanatomy for the proposed frontal variant in AD [50].
Our data support that decreased prefrontal regulation of a dysfunctional amygdala that is receiving disrupted hippocampal inputs provides misinformation to an atrophied insula with an outflow for emotional information integration and autonomic stimulation that results in a range of agitated and aggressive behaviors in patients with MCI and AD. The prefrontal cortex loses its behavior-regulating capacity, which results in behavior and personality changes. These findings are consistent with a large body of neuropsychiatry literature describing a complex brain network of prefrontal, subcortical, and mesolimbic circuitry that mediates and regulates social behaviors and the frontoinsular circuitry that plays a crucial role in the processing of more complex social emotions such as empathy, compassion, and fairness.
Limitations of our study include its exploratory nature that used a low threshold for correlations between ROIs with symptoms to select ROIs initially for modeling. Our findings using longitudinal data did have consistency with prior cross-sectional literature, however, and contribute meaningfully to this poorly understood but common condition. Although agitation and aggression may co-occur with other NPS such as mood or psychosis, we did not covary for those in our multivariate analyses so as not to dilute the signal for our target symptoms, which are observable in a way that subjective symptoms are not. We selected an NPS phenotype measure based on face validity and results of large factor analyses [7,11,22]. The NPI-Q was the only measure of NPS available in the ADNI database.
5. Conclusions
An impaired modulatory interface of prefrontal regions with dysfunctional amygdalar–hippocampal and amygdalar–insular networks may result in disrupted behavioral control in a subset of patients with MCI and AD that expresses itself as symptoms of agitation and aggression. Magnetic resonance imaging indices of volumetric or cortical thickness found the left hippocampus and posterior cingulate as the only posterior ROIs associated with agitation and aggression, and these areas have important connectivity with amygdala and prefrontal cortex. These frontolimbic regions of the brain are known to subserve a range of functions such as insight, facial emotion recognition, and expression of negative emotions that are impaired in AD. Our exploratory MRI findings are consistent with other studies' findings of regional brain pathology in AD that affects both white and gray matter in more anterior brain areas and plausibly associated with agitation and aggression symptoms.
Supplementary Material
Acknowledgments
Data collection and sharing for this project was funded by the Alzheimer's Disease Neuroimaging Initiative (ADNI) (National Institutes of Health [NIH] grant U01 AG024904). The ADNI is funded by the National Institute on Aging and the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: Abbott; Alzheimer's Association; Alzheimer's Drug Discovery Foundation; Amorfix Life Sciences Ltd.; AstraZeneca; Bayer HealthCare; BioClinica, Inc; Biogen Idec Inc; Bristol-Myers Squibb Company; Eisai, Inc; Elan Pharmaceuticals, Inc; Eli Lilly and Company; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc; GE Healthcare; Innogenetics, N.V.; Janssen Alzheimer Immunotherapy Research & Development, LLC; Johnson & Johnson Pharmaceutical Research & Development LLC; Medpace, Inc; Merck & Co., Inc; Meso Scale Diagnostics, LLC; Novartis Pharmaceuticals Corporation; Pfizer, Inc; Servier; Synarc, Inc; and Takeda Pharmaceutical Company. The Canadian Institutes of Health Research is providing funds to support the ADNI clinical sites in Canada. Private-sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer's Disease Cooperative Study at the University of California, San Diego. The ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of California, Los Angeles. This research was also supported by NIH grants P30 AG010129 and K01 AG030514, and the Dana Foundation.
The principal investigator of the ADNI initiative is Michael W. Weiner, MD, VA Medical Center and University of California–San Francisco. The ADNI is the result of efforts of many co-investigators from a broad range of academic institutions and private corporations, and subjects have been recruited from more than 50 sites across the United States and Canada. The initial goal of the ADNI was to recruit 800 adults, ages 55 to 90, to participate in the research; approximately 200 cognitively normal older individuals to be followed for 3 years; 400 people with MCI to be followed for 3 years; and 200 people with early AD to be followed for 2 years. For up-to-date information, go to www.adni-info.org.
Footnotes
Data used in the preparation of this article were obtained from the Alzheimer's Disease Neuroimaging Initiative (ADNI) database (www.loni.ucla.edu/ADNI). As such, the investigators in the ADNI contributed to the design and implementation of the ADNI and/or provided data but did not participate in the analysis or writing of this report. A complete list of ADNI investigators is available at http://adni.loni.ucla.edu/wp-content/uploads/how_to_apply/ADNI_Acknowledgement_List.pdf.
Presented as a poster at the Alzheimer's Association International Conference on Alzheimer'sDisease annualmeeting in Paris, France, July 16–21, 2011.
References
- [1].Craig D, Mirakhur A, Hart DJ, McIlroy SP, Passmore AP. A cross-sectional study of neuropsychiatric symptoms in 435 patients with Alzheimer's disease. Am J Geriatr Psychiatry. 2005;13:460–8. doi: 10.1176/appi.ajgp.13.6.460. [DOI] [PubMed] [Google Scholar]
- [2].Gilley DW, Bienias JL, Wilson RS, Bennett DA, Beck TL, Evans DA. Influence of behavioral symptoms in rates of institutionalization for persons with Alzheimer's disease. Psychol Med. 2004;34:1129–35. doi: 10.1017/s0033291703001831. [DOI] [PubMed] [Google Scholar]
- [3].Cohen-Mansfield J, Deutsch LH. Agitation: subtypes and their mechanisms. Semin Clin Neuropsychiatry. 1996;1:325–39. doi: 10.1053/SCNP00100325. [DOI] [PubMed] [Google Scholar]
- [4].Cohen-Mansfield J, Werner P, Watson V, Pasis S. Agitation among elderly persons at adult day-care centers: the experiences of relatives and staff members. Int Psychogeriatr. 7:447–458. doi: 10.1017/s1041610295002195. 995. [DOI] [PubMed] [Google Scholar]
- [5].Tractenberg RE, Weiner MF, Thal LJ. Estimating the prevalence of agitation in community-dwelling persons with Alzheimer's Disease. J Neuropsychiatry Clin Neurosci. 2002;14:11–8. doi: 10.1176/jnp.14.1.11. [DOI] [PubMed] [Google Scholar]
- [6].Aalten P, de Vugt ME, Jaspers N, Jolles J, Verhey FRJ. The course of neuropsychiatric symptoms in dementia. Part I: findings from the two year longitudinal Maasbed study. Int J Geriatr Psychiatry. 2005;20:523–30. doi: 10.1002/gps.1316. [DOI] [PubMed] [Google Scholar]
- [7].Lyketsos CG, Sheppard J-M, Steinberg M, Tschanz JT, Steffens DC, Breitner JCS. Neuropsychiatric disturbance in Alzheimer's disease clusters into three groups: the Cache County Study. Int J Geriatr Psychiatry. 2001;16:1043–53. doi: 10.1002/gps.448. [DOI] [PubMed] [Google Scholar]
- [8].Steinberg M, Shao H, Zandi P, Lyketsos CG, Welsh-Bohmer KA, Norton MC, et al. Point and 5-year period prevalence of neuropsychiatric symptoms in dementia: the Cache County Study. Int J Geriatr Psychiatry. 2008;23:170–7. doi: 10.1002/gps.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Geda YE, Roberts RO, Knopman DS, Peterson RC, Christianson TJH, Pankratz VS, et al. Prevalence of neuropsychiatric symptoms in mild cognitive impairment and normal cognitive aging. Arch Gen Psychiatry. 2008;65:1193–8. doi: 10.1001/archpsyc.65.10.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Geda YE, Roberts RO, Knopman DS, Christianson TJH, Pankratz VS, Ivnik RJ, et al. Baseline neuropsychiatric symptoms and the risk of incident mild cognitive impairment: the Mayo Clinic Study of Aging. Presented at the annual meeting of American Academy of Neurology in Honolulu, Hawaii; April 9–16 2011. [Google Scholar]
- [11].Aalten P, Verhey FRJ, Boziki M, Bullock R, Byrne EJ, Camus V, et al. Neuropsychiatric syndromes in dementia: results from the European Alzheimer Disease Consortium: part 1. Dement Geriatr Cogn Disord. 2007;24:457–63. doi: 10.1159/000110738. [DOI] [PubMed] [Google Scholar]
- [12].Lyketsos CG, Carrillo MC, Ryan JM, Khachaturian AS, Trzepacz PT, Amatniek J, et al. Neuropsychiatric symptoms in Alzheimer's disease. Alzheimers Dementia. 2011;7:532–9. doi: 10.1016/j.jalz.2011.05.2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Hirono N, Mega MS, Dinov ID, Mishkin F, Cummings JL. Left frontotemporal hypoperfusion is associated with aggression in patients with dementia. Arch Neurol. 2000;57:861–6. doi: 10.1001/archneur.57.6.861. [DOI] [PubMed] [Google Scholar]
- [14].Tekin S, Mega M, Masterman DM, Chow T, Garakian J, Vinters HV, et al. Orbitofrontal and anterior cingulate cortex neurofibrillary tangle burden is associated with agitation in Alzheimer's disease. Ann Neurol. 2001;49:355–61. [PubMed] [Google Scholar]
- [15].Lancôt KL, Herrmann N, Nadkarni NK, Leibovitch FS, Caldwell CB, Black SE. Medial temporal hypoperfusion and aggression in Alzheimer's disease. Arch Neurol. 2004;61:1731–7. doi: 10.1001/archneur.61.11.1731. [DOI] [PubMed] [Google Scholar]
- [16].Staekenborg SS, Gillissen F, Romkes R, Pijnenburg YAL, Barkhof F, Scheltens P, et al. Behavioral and psychological symptoms are not related to white matter hyperintensities and medial temporal lobe atrophy in Alzheimer's disease. Int J Geriatr Psychiatry. 2008;23:387–92. doi: 10.1002/gps.1891. [DOI] [PubMed] [Google Scholar]
- [17].Sultzer DL, Mahler ME, Mandelkern MA, Cummings JL, Van Gorp WG, Hinkin CH, et al. The relationship between psychiatric symptoms and regional cortical metabolism in Alzheimer's disease. J Neuropsychiatry Clin Neurosci. 1995;7:476–84. doi: 10.1176/jnp.7.4.476. [DOI] [PubMed] [Google Scholar]
- [18].Bruen PD, McGeown WJ, Shanks MF, Venneria A. Neuroanatomical correlates of neuropsychiatric symptoms in Alzheimer's disease. Brain. 2008;131:2455–63. doi: 10.1093/brain/awn151. [DOI] [PubMed] [Google Scholar]
- [19].Weiner MW, Aisen PS, Jack CR, Jr, Jagust WJ, Trojanowski JQ, Shaw L, et al. The Alzheimer's Disease Neuroimaging Initiative: progress report and future plans. Alzheimers Dementia. 2010;6:202–11. doi: 10.1016/j.jalz.2010.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatry Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- [21].Kaufer DI, Cummings JL, Ketchel P, Smith V, MacMillan A, Shelley T, et al. Validation of the NPI-Q, a brief clinical form of the Neuropsychiatric Inventory. J Neuropsychiatry Clin Neurosci. 2000;12:233–9. doi: 10.1176/jnp.12.2.233. [DOI] [PubMed] [Google Scholar]
- [22].Dennehy EB, Kahle-Wrobleski K, Sarsour K, Milton DR. Derivation of a brief measure of agitation and aggression in Alzheimer's disease. Int J Geriatr Psychiatry Int J Geriatr Psychiatry. 2012 Apr;:18. doi: 10.1002/gps.3807. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- [23].Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct. 2010;214:655–67. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Wang L, Goldstein FC, Veledar E, Levey AI, Lah JJ, Meltzer CC, et al. Alterations in cortical thickness and white matter integrity in mild cognitive impairment measured by whole-brain cortical thickness mapping and diffusion tensor imaging. AJNR Am J Neuroradiol. 2009;30:893–9. doi: 10.3174/ajnr.A1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Risacher SL, Shen L, West JD, Kim S, McDonald BC, Beckett LA, et al. Longitudinal MRI atrophy biomarkers: relationship to conversion in the ADNI cohort. Neurobiol Aging. 2010;31:1401–18. doi: 10.1016/j.neurobiolaging.2010.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Hirono N, Mori E, Yasuda M, Imamura T, Shimomura T, Hashimoto M, et al. Lack of effect of apolipoprotein E ε4 allele on neuropsychiatric manifestations in Alzheimer's disease. J Neuropsychiatry Clin Neurosci. 1999;11:66–70. doi: 10.1176/jnp.11.1.66. [DOI] [PubMed] [Google Scholar]
- [27].Borroni B, Grassi M, Agosti C, Costanzi C, Archetti S, Franzoni S, et al. Genetic correlates of behavioral endophenotypes I Alzheimer's disease: role of COMT, 5-HTTLPR and ApoE polymorphisms. Neurobiol Aging. 2006;27:1595–603. doi: 10.1016/j.neurobiolaging.2005.09.029. [DOI] [PubMed] [Google Scholar]
- [28].Craig D, Hart DJ, McCool K, McIlroy SP, Passmore AP. Apolipoprotein E ε4 allele influences aggressive behavior in Alzheimer's disease. J Neurol Neurosurg Psychiatry. 2004;75:1327–30. doi: 10.1136/jnnp.2003.032276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].van der Flier WM, Staekenborg S, Pijnenburg YAL, Gillissen F, Romkes R, Kok A, et al. Apolipoprotein E genotype influences presence and severity of delusions and aggressive behavior in Alzheimer disease. Dement Geriatr Cogn Disord. 2007;23:41–6. doi: 10.1159/000096682. [DOI] [PubMed] [Google Scholar]
- [30].Del Prete M, Spaccavento S, Craca A, Fiore P, Angelelli P. Neuropsychiatric symptoms and the ApoE genotype in Alzheimer's disease. Neurol Sci. 2009;30:367–73. doi: 10.1007/s10072-009-0116-9. [DOI] [PubMed] [Google Scholar]
- [31].Woods DL, Bushnell B, Haesook K, Geschwind D, Cummings J. Apolipoprotein ε4 status is associated with behavioral symptoms in nursing home residents with dementia. Int Psychogeriatr. 2009;21:722–8. doi: 10.1017/S1041610209009235. [DOI] [PubMed] [Google Scholar]
- [32].Bartzokis G, Lu PH, Geschwind DH, Edwards N, Mintz J, Cummings JL. Apolipoprotein E genotype and age-related myelin breakdown in healthy individuals: implications for cognitive decline and dementia. Arch Gen Psychiatry. 2006;63:63–72. doi: 10.1001/archpsyc.63.1.63. [DOI] [PubMed] [Google Scholar]
- [33].Heise V, Filippini N, Ebmeier KP, Mackay CE. The ApoE ε4 allele modulates brain white matter integrity in healthy adults. Molecular Psychiatry. 2011;16:908–16. doi: 10.1038/mp.2010.90. [DOI] [PubMed] [Google Scholar]
- [34].Filippini N, Zarei M, Beckmann CF, Galluzzi S, Borsci G, Testa C, et al. Regional atrophy of transcallosal prefrontal connections in cognitively normal ApoE ε4 carriers. J Magn Reson Imaging. 2009;29:1021–6. doi: 10.1002/jmri.21757. [DOI] [PubMed] [Google Scholar]
- [35].Agosta F, Vossel KA, Miller BL, Migliaccio R, Bonasera SJ, Filippi M, et al. Apolipoprotein E ε4 is associated with disease-specific effects on brain atrophy in Alzheimer's disease and frontotemporal dementia. PNAS. 2010;106:2018–22. doi: 10.1073/pnas.0812697106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Harwood DG, Sultzer DL, Wheatley MV. Impaired insight in Alzheimer's disease: association with cognitive deficits, psychiatric symptoms and behavioral disturbances. Neuropsychiatry Neuropsychol Behav Neurol. 2000;13:83–8. [PubMed] [Google Scholar]
- [37].Chen ST, Sultzer DL, Hinkin CH, Mahler ME, Cummings JL. Executive dysfunction in Alzheimer's disease: association with neuropsychiatric symptoms and functional impairment. J Neuropsychiatry Clin Neurosci. 1988;10:426–32. doi: 10.1176/jnp.10.4.426. [DOI] [PubMed] [Google Scholar]
- [38].Weiss EM, Kohler CG, Vonbank J, Stadelmann E, Kemmler G, Hinterhuber H, et al. Impairment in emotion recognition abilities in patients with mild cognitive impairment, early and moderate Alzheimer disease compared with healthy comparison subjects. Am J Geriatr Psychiatry. 2008;16::974–80. doi: 10.1097/JGP.0b013e318186bd53. [DOI] [PubMed] [Google Scholar]
- [39].Mega MS, Cummings JL. Frontal–subcortical circuits and neuro-psychiatric disorders. J Neuropsychiatry Clin Neurosci. 1994;6:358–70. doi: 10.1176/jnp.6.4.358. [DOI] [PubMed] [Google Scholar]
- [40].Yasmin H, Nakata Y, Aoki S, Abe O, Sato N, Nemoto K, et al. Diffusion abnormalities of the uncinate fasciculus in Alzheimer's disease: diffusion tensor tract-specific analysis using a new method to measure the core of the tract. Neuroradiology. 2008;50:293–9. doi: 10.1007/s00234-007-0353-7. [DOI] [PubMed] [Google Scholar]
- [41].Taoka T, Iwasaki S, Sakamoto M, Nakagawa H, Fukusumi A, Myochin K, et al. Diffusion anisotropy and diffusivity of white matter tracts within the temporal stem in Alzheimer's disease: evaluation the tract of interest by diffusion tensor tractography. AJNR Am J Neuroradiol. 2006;27:1040–5. [PMC free article] [PubMed] [Google Scholar]
- [42].Zhang Y, Schuff N, Du A-T, Rosen HJ, Kramer JH, Gorno-Tempini MK, et al. White matter damage in frontotemporal dementia and Alzheimer's disease measured by diffusion MRI. Brain. 2009;132:2579–92. doi: 10.1093/brain/awp071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Striker NH, Schweinsburg BC, Delano-Wood L, Wierenga CE, Bangen KJ, Haaland KY, et al. Decreased white matter integrity late-myelinating fibre pathways in Alzheimer's disease supports retro-genesis. Neuroimage. 2009;45:10–6. doi: 10.1016/j.neuroimage.2008.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Bonthius DJ, Solodkin A, van Hoesen GW. Pathology of the insular cortex in Alzheimer's disease depends on cortical architecture. J Neuropathol Exp Neurol. 2005;64:910–22. doi: 10.1097/01.jnen.0000182983.87106.d1. [DOI] [PubMed] [Google Scholar]
- [45].Seeley WW, Allman JM, Carlin DA, Crawforf RK, Macedo MN, Grecious MD, et al. Divergent social functioning in behavioral variant frontotemporal dementia and Alzheimer disease: reciprocal networks and neuronal evolution. Alzheimer Dis Assoc Disord. 2007;21:S50–7. doi: 10.1097/WAD.0b013e31815c0f14. [DOI] [PubMed] [Google Scholar]
- [46].Allman JM, Tetreault NA, Hakeem AY, Park S. The von Economo neurons in apes and humans. Am J Human Biol. 2011;23:5–21. doi: 10.1002/ajhb.21136. [DOI] [PubMed] [Google Scholar]
- [47].Lamm C, Singer T. The role of the anterior insular cortex in social emotions. Brain Struct Funct. 2010;214:579–91. doi: 10.1007/s00429-010-0251-3. [DOI] [PubMed] [Google Scholar]
- [48].Fajardo C, Escobar MI, Buriticá, Arteaga G, Umbarila J, Casanova MF, et al. Von Economo neurons are present in the dorsolateral (dysgranular) prefrontal cortex of humans. Neurosci Lett. 2008;435:215–8. doi: 10.1016/j.neulet.2008.02.048. [DOI] [PubMed] [Google Scholar]
- [49].Zhou J, Grecious MD, Gennatas ED, Growdon ME, Jung JY, Rabinovici GD, et al. Divergent network connectivity changes in behavioural variant frontotemporal dementia and Alzheimer's disease. Brain. 2010;133:1352–67. doi: 10.1093/brain/awq075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Johnson JK, Head E, Kim R, Starr A, Cotman CW. Clinical and pathological evidence for a frontal variant of Alzheimer's disease. Arch Neurol. 1999;56:1233–9. doi: 10.1001/archneur.56.10.1233. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.