Abstract
Background:
Low- level laser therapy has been used to stimulate the orthodontic tooth movements (OTM) previously. Furthermore, in the orthodontic treatments accompanying tooth extractions, the adjacent teeth move towards the extraction sites and close the space in some cases. Then, the adjacent tooth movements must be prevented in the treatments requiring space. Laser stimulates and at some doses decelerates tooth movement; it also improves healing process and enhances osteogenesis. Hence, it can prevent movement by osteogenesis adjacent to the tooth. The present study investigated the effects of low-level laser therapy on the OTM and root resorption following artificial socket preservation.
Materials and Methods:
In this experimental animal trial, 16 male albino rabbits were selected with similar characteristics and randomly divided in two groups. Under general anesthesia, an artificial socket, 8 mm in height, was created in the mesial aspect of the first premolars of the rabbits and filled with demineralized freeze dried bone allograft (DFDBA). The first premolars were connected to the incisors using nickel titanium coil springs. In experimental group, gallium-aluminum-arsenide (GaAlAs) laser was irritated mesial to first premolar where artificial socket was created continuously (808 nm). The cycle was 10 days irritation, 14 days rest, 10 days irritation, 14 days rest (Biostimulation mode). Control group was not laser irradiated. All animals were sacrificed after 48 days and the distance between the distal aspect of the first premolars, and the mesial surface of the second premolars was measured with leaf gauge. The specimens underwent histological assessments. Integrity of root and its resorption was observed under microscope calibration. The size of resorption lacunae was calculated in mm2. Normality of data was proved according to Kolmogorov-Smirnov analysis, and Student's t-test was done. P value less than 0.05 was considered as significant.
Results:
The mean OTM were 5.68 ± 1.21 mm in the control group and 6.0 ± 0.99 mm in the laser irradiated teeth with no statistically significant differences(P > 0.75). The mean root resorption was 1.61 ± 0.43 mm2 and 0.18 ± 0.07 mm2 in the control and experimental groups respectively being significantly lower in the laser irradiated teeth (P < 0.0001).
Conclusion:
The findings of the present study show that GaAlAs irradiation together with the application of DFDBA led to limited amount of the stimulated OTM. The laser beam irradiation in combination with alloplastic materials used for socket preservation could reduce the degree of root resorption significantly.
Keywords: Low-level laser, orthodontic tooth movements, root resorption
INTRODUCTION
Long orthodontic treatments are associated with the decreased patients’ cooperation which negatively affects treatment outcomes in turn. The problem depends on the tooth movement during the orthodontic treatments to higher degrees.[1] However, tooth movements must occur very slowly in order to prevent negative effects of the orthodontic loads such as bone necrosis or root resorption.
Different factors cause bone remodeling to be changed altering the rate of tooth movements in the alveolar bone. These include Parathyroid hormone (PTH), estrogen, the applied force values, drugs injected, electrical stimulation or ultrasound. PTH increases bone formation while estrogen was found to decrease it. Furthermore, loads lower than 1000 μ strain are associated with the increased bone formation while loads more than 2000 μ strain leads in decreased bone formations and lower subsequent tooth movements. These factors have some side effects of uncontrolled tooth movements, root resorption, pain and patients’ discomfort together with increased tooth movements.[1,2,3,4,5]
Low-level laser therapy(LLLT) have been proposed to increase bone remodeling and tooth movement with the benefits like decreased pain and inflammation, collagen stimulation, and cell proliferation.[6] Low-level lasers can affect the bone cell activity and increased surface osteoblastic cells and osteoid width has been reported in the laser-irradiated areas.[3] Furthermore, lasers carry the advantages of having no systemic side effects of injected chemicals or orally-consumed medicaments on the patients’ health status. Low-level laser application possibly stimulates the orthodontic tooth movements (OTM) as it increases the alveolar bone formation without causing any damage to the teeth or surrounding tissues.[7] However, the effect of low-level laser therapy on tooth movement is reportedly controversial as different stimulatory, inhibitory and irrelevant effects have been shown in the literature. OTM is a complicated inflammatory procedure being influenced by different confounding factors; furthermore, bone resorption and regeneration are occurring simultaneously during orthodontic treatments requiring more investigations to be done in this field.[8]
Different laser modalities have been used in different doses and treatment protocols including helium-neon (632.8 nm wavelength), gallium-aluminum-arsenide (GaAlAs) (805 ± 25 nm wavelength) and gallium-arsenide (904 nm wavelength) previously.[9,10] GaAlAs diode laser has been repeatedly used in the last 10 years. This laser type has been shown to have higher depth of tissue penetration compared to other modalities, therefore, providing the clinicians a suitable penetrative instrument with a great efficiency in the orthodontic treatments.[11]
Two main forms of the graft materials including demineralized freeze dried bone allograft (DFDBA) and freeze dried bone allograft have been used for the healing of bone defects repeatedly. DFDBA has been studied in terms of bone induction properties or the treatment of periodontal lesions and different results have been reported for it; i.e., more bone induction following DFDBA use[12] or no significant differences being found between two graft materials concerning bone induction properties.[13]
Furthermore, in orthodontic treatments requiring tooth extractions, the adjacent teeth move towards the extraction sites and close the space in some cases following a biomechanical procedure. However, the adjacent tooth movements must be prevented in treatments requiring space. Given the positive properties of the low-level laser irradiation, it seems that the created space can be preserved by means of preventing tooth movements and consequent root resorption.
The present animal trial investigated the effects of LLLT using GaAlAs laser on the OTM and root resorption following artificial socket preservation.
MATERIALS AND METHODS
In this animal experimental trial, 16 New Zealand albino male rabbits were studied weighing 1.5 kg and aging 6 weeks. The animals were all healthy having suitable periodontal status and sound teeth. They had similar nutrition and housing conditions (23°C temperature, 12 h being in darkness and 12 h in daylight).
The animals underwent general anesthesia by intravenous injection of ketamin 10% (Alfasan, Woerden, Holland; 50 mg/kg) and xylazine 2% (Alfasan, Woerden, Holland; 4 mg/kg). An artificial socket, 8 mm in the height, was drilled on the mesial aspect of the first premolars using implant drill with 2.8 mm diameter and filled with DFDBA. The DFDBA powder (CenoBone®; Tissue Regeneration Corporation, Kish Island, Iran) was mixed with an equal volume of normal saline for socket preservation.
Using a modified mouth opening device, the animals’ mouths were opened completely and the left mandibular first premolars were subjected to orthodontic force applied to protract them mesially by a NiTi coil spring (Ormco®, USA) ligatured around them. The other end of the coil spring was ligated around the gingival aspect of mandibular incisors. The condition of the appliance, teeth, and gingivae was checked once a week and the force magnitude was measured every week.
The animals were assigned to two experimental and control groups randomly. In the experimental group, GaAlAs laser was irradiated on mesial of first premolar beside artificial socket in right angle. Every cycle was 90 seconds. It was performed according to the protocol: 10 days irritation, 14 days’ rest, 10 days irritation, 14 days’ rest (wavelength: 808 nm, energy: 6 J/cm2, continuous mode). No irradiation was done in the control group. In this study external control group is considered to omit systemic effect of LLLT.
After 48 days, the animals were sacrificed with the injection of ketamin 10% and the distance between the distal aspect of the first premolars and mesial surface of the second premolars were measured using leaf gauge with the accuracy of 0.05 mm.
The jaws were bisected and embedded in formalin 10% solution. The specimens were stored in nitric acid for 10 days for decalcification. After passage, they were embedded in formalin 10% solution for 24 h. Then, dehydration were completed in a graded series of alcohol solution, for which, the specimens were embedded into alcohol 70% for 1.5 h, alcohol 80% for 1.5 h, alcohol 96% for 2.5 h and alcohol 100% for 2.5 h. The specimens were kept in xylene for another 2 h and embedded in paraffin with 56°C-67°C for 8-18 h.
The embedded specimens were cut using the rotary microtome device. Five sections were obtained from each specimen. Sections included mesial portion of first premolar and the artificial socket and surrounding bone. After tissue sectioning, the specimens were stained with H and E. Then, the sections were assessed regarding root resorption using a light microscope (Nikon: E-400, Japan). Sections were observed under light microscope and a photograph from field was captured. The pictures were transferred to a measuring page and calibration was performed. Areas of root resorption lacunae were calculated.
Together with the normal distribution of data, as shown by one-sample Kolmogorov-Smirnov test, the statistical analysis was done using Student t-test between the experimental and control groups. P value < 0.05 was considered as significant.
RESULTS
The mean OTM was 5.68 ± 1.21 mm in the control teeth and 6.0 ± 0.99 mm in the laser irradiated teeth with no statistically significant difference between them (P > 0.57). Therefore, GaAlAs laser irradiation did not lead to significantly stimulated OTM [Table 1].
Table 1.
Orthodontic tooth movements and root resorption in the case and control animals
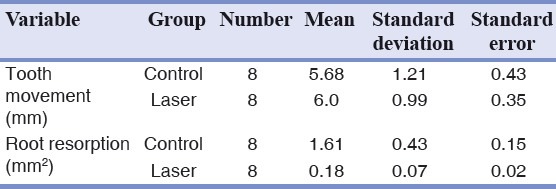
Furthermore, the mean root resorption were 1.61 ± 0.43 mm2 and 0.18 ± 0.07 mm2 in the control and experimental groups respectively. It was significantly lower in the laser irradiated teeth (P < 0.0001). Laser irradiation significantly reduced the degree of the root resorption [Table 1].
DISCUSSION
The mean OTM were slightly higher in the laser-irradiated teeth, although without any significant difference. Hence, GaAlAs low-level laser was not able to prevent tooth movements following artificial socket preservations.
Some investigations have reported that low-energy irradiation with GaAlAs diode laser affects OTM in the animals and humans, although, when using the optimal laser dosage.[5,14,15]
Coombe et al. showed low level laser therapy with GaAlAs diode laser (830-nm, 0.3-4 J/cm2) did not stimulate gross cell population in the short and long time intervals using the human osteosarcom cells culture.[16] Seifi et al. suggested that the values of tooth movement after LLLT with both pulsed 850-nm laser (Optodan, 8.1 J/cm2) and continuous 630-nm laser (potassium iodate, 27 J/cm2) to be diminished.[8] They suggested inhibitory effects of low level laser on the prostaglandins as an intermediate in the cell response to teeth movements as the involved mechanism for the findings.
Kawasaki and Shimizu showed higher values of OTM in the laser-irradiated rat teeth than control ones (1.3-fold).[5] In this study, total laser energy of 702 j was irradiated continuously during 13 days on rat's molars (output: 100 mw, wavelength: 830-nm). Furthermore, the tooth movement differences between the experimental and control groups was only 0.2 mm, suggesting less standard deviation values being responsible for the significant differences being explored as the differences was reported to be 0.32 mm in the present study.
Fujita et al. reported 50% increased rate of OTM following similar protocol used by Kawasaki and Shimizu.[15] Youssef et al. showed that the ratio of human canine retraction in the irradiated group to the control ones to be 1.98 (809-nm, 100-mW at 8 J/cm2).[14] Yamaguchi et al. concluded LLLT to significantly stimulate the OTM relative to control group.[17] Abtahi and Mohaseni Eghdam showed insignificant effects of LLLT (12.5 J/cm2) on the tooth movement induced by the separator loads on the human subjects.[18] Cruz et al. applied GaAlAs diode laser (5 J/cm2) for 10 s and found higher canine movements.[3] On the contrary, Limpanichkul et al. suggested LLLT with 25 J/cm2 densities not to induce faster OTM than the control group.[7]
In another study, Goulart et al. noted that the canine and premolars irradiated at 5.25 J/cm2 (780-nm) showed faster orthodontic movements initially, while the teeth irradiated at 35 J/cm2 (780-nm) showed slow movements.[19] Furthermore, Kim et al. showed higher accelerating effects on the experimental tooth movement using GaAlAs low-level laser (808-nm, 41.7 J/cm2) compared to the other studies.[20]
Different effects of laser irradiation have been reported on tooth movement using different laser irradiation period, density and wavelength. Furthermore, more accuracy of application methods, sample selection and statistical calculations are required in the assessment of low-level laser effects on the tooth movement. In the present study, GaAlAs laser (808-nm, 0.1 W and 6 J/cm2) was irradiated on the teeth with the continuous method for 10 days being followed by 14 days of no irradiation.
A reasonable energy density (J/cm2) is necessary to trigger biologic effects; therefore, low outputs cannot be fully compensated by the longer exposures. The dosage follows the Arndt-Schulz law; low dosages stimulate, while the higher dosages will have inhibitory effects.[20] As biological effects of laser irradiation depends on the laser parameters such as wavelength, output and laser density, different effects on tooth movements have been reported.[21]
Although, the exact mechanism of the low-energy laser application on the bone is not completely understood yet, it is possibly photochemical in nature, with the light increasing cell proliferation through the photochemical alterations, after the light at a lower radiation dosage is absorbed by the intracellular chromophores in the mitochondria.[22,23,24] This mechanism, is also multifactorial including promotion of angiogenesis,[25] collagen production,[26] osteogenic cell proliferation and differentiation,[27] mitochondrial respiration, and adenosine triphosphate synthesis.[28,29] LLLT can also enhance the local blood flow, increasing the supply of the circulating cells, nutrition, oxygen, and inorganic salts to the bone lesions.[30] In this regard, Kobu showed that in tissues treated with LLL application, intraosseous blood flow increased approximately 80% and oxygen tension by approximately 15%.[31] Kawasaki and Shimizu showed that low-energy laser application increased the number of osteoclasts in the pressure side during experimental tooth movement in rats.[5]
Together with the differences being found in the oxidation and consequent cellular pH change, a cell can demonstrate different responses to the specific laser types in different times, so that, the laser effects would be more complicated when complex mechanisms being present or more than one cell is involved in the specific time. Furthermore, in some occasions, the irradiation effects would be subjected to decrease or increase showing different laser natures, i.e., inhibitory or stimulatory effects being presented.[32]
Immunologic, neurologic and physiologic procedures involved in the bone formation, cartilage induction and defect repair are more complex, numerous and with interrelation effects, so that, a clear cause and effect relationship cannot be developed for a specific laser kind irradiation with the available knowledge. Furthermore, some interfering factors, including the host defensive system response against the laser irradiation, metabolic status and cellular pH during irradiation initiation, the contradictory and unclear influences of the tissue mediators and the more complicated effects of the different laser types remains unclear.[32] That is why different results have been reported in apparently similar in vitro studies. The role of laser output and density, irradiation mode, cellular culture technique and histological assessments must be added to the aforementioned justifications.
The mean root resorption was significantly lower in the laser-irradiated teeth than control specimens. It has been suggested that root resorption is the iatrogenic consequence of orthodontic movements being an idiopathic problem of the treatments. However, this degree of root resorption has no significant clinical effect.
Histophathological assessment of the control and experimental sections showed parakeratinized squamous epithelium. At their underlying connective tissue, collagen fibers, blood vessels and bony trabecules were evident together with the osteoblastic rim and osteocyte lacunae. The bone trabecules was normal around the tooth with no inflammation being observed. Angiogenesis and osteogenesis was observed around the first premolar in the laser irradiated sections [Figures 1 and 2].
Figure 1.
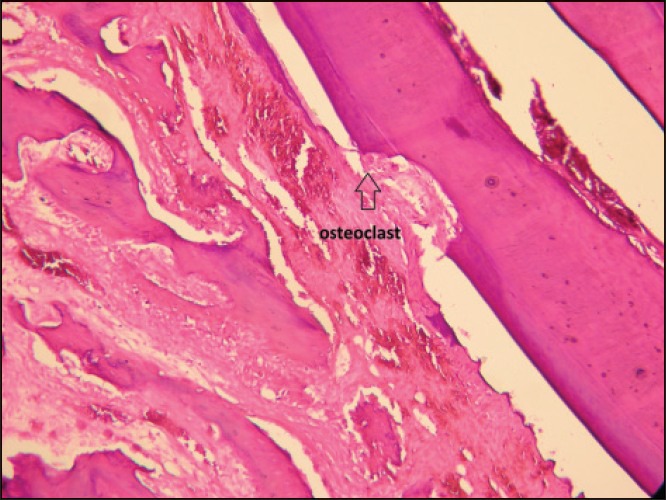
Root resorption and osteoclasts (control specimen)
Figure 2.
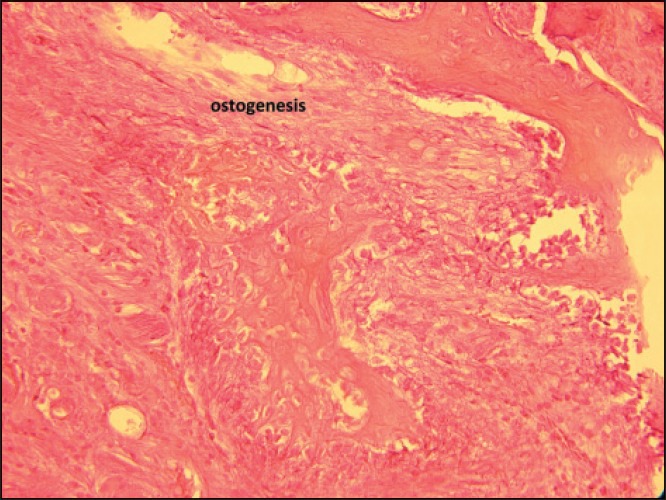
Angiogenesis and osteogenesis around the first premolar (laser-irradiated sections)
To prevent bias due to the possible systemic effect of LLLT, external control animalswere used. Systemic effects of LLLT have been reported in some studies.[33,34,35] It has been shown that LLLT can stimulate the release of the growth factors and cytokines in the circulatory system and consequently affect the untreated side of the studied animal or subject. Perhaps this is responsible for those studies being unable to show beneficial effects of LLLT using internal controls (e.g., the contralateral side of the same animal/patient).
Limitations of the present study were impossibility of measuring amount of tooth movement and root resorption in different periods after irradiation and impossibility of using different energies, wave lengths and power of GaAlAs in the process of artificial socket preservation, and last but not least, impossibility of determining primary and delayed effect of laser irradiation in artificial socket preservation.
CONCLUSION
Under the limitations of the present study, it was concluded that GaAlAs irradiation together with using DFDBA graft material led to limited amounts of stimulated OTM. The laser beam irradiation in combination with alloplastic materials used for socket preservation could reduce the amount of root resorption.
ACKNOWLEDGMENTS
The present research has been supported by Deputy of Research Affairs, Dental School, Shahid Beheshti University of Medical Sciences and is based on the doctoral dissertation of Dr. Atri under supervision of Prof. Seifi.
Footnotes
Source of Support: This study was as part of dissertation by Faezeh Atri under supervision of Massoud Seifi and was supported by Dental School (Deputy of Research Affairs) of Shahid Beheshti University of Medical Sciences
Conflict of Interest: None declared
REFERENCES
- 1.Seifi M, Eslami B, Saffar AS. The effect of prostaglandin E2 and calcium gluconate on orthodontic tooth movement and root resorption in rats. Eur J Orthod. 2003;25:199–204. doi: 10.1093/ejo/25.2.199. [DOI] [PubMed] [Google Scholar]
- 2.Roberts E. Tissue reactions in orthodontics. In: Graber TM, Vanarsdall RL, editors. Orthodontics Current Principals and Techniques. 3rd ed. Ch. 2. St. Louis: The C.V. Mosby Co; 2000. p. 218. [Google Scholar]
- 3.Cruz DR, Kohara EK, Ribeiro MS, Wetter NU. Effects of low-intensity laser therapy on the orthodontic movement velocity of human teeth: A preliminary study. Lasers Surg Med. 2004;35:117–20. doi: 10.1002/lsm.20076. [DOI] [PubMed] [Google Scholar]
- 4.Proffit WR, Fields HW. 3rd ed. St Louis: Mosby Inc; 2000. Contemporary Orthodontics; pp. 296–321. [Google Scholar]
- 5.Kawasaki K, Shimizu N. Effects of low-energy laser irradiation on bone remodeling during experimental tooth movement in rats. Lasers Surg Med. 2000;26:282–91. doi: 10.1002/(sici)1096-9101(2000)26:3<282::aid-lsm6>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 6.Mizutani K, Musya Y, Wakae K, Kobayashi T, Tobe M, Taira K, et al. A clinical study on serum prostaglandin E2 with low-level laser therapy. Photomed Laser Surg. 2004;22:537–9. doi: 10.1089/pho.2004.22.537. [DOI] [PubMed] [Google Scholar]
- 7.Limpanichkul W, Godfrey K, Srisuk N, Rattanayatikul C. Effects of low-level laser therapy on the rate of orthodontic tooth movement. Orthod Craniofac Res. 2006;9:38–43. doi: 10.1111/j.1601-6343.2006.00338.x. [DOI] [PubMed] [Google Scholar]
- 8.Seifi M, Shafeei HA, Daneshdoost SH, Mir M. Effects of two types of low-level-laser wave lengths (850 and 630 nm) on the orthodontic tooth movements in rabbits. Laser Med Sci. 2007;22:261–4. doi: 10.1007/s10103-007-0447-9. [DOI] [PubMed] [Google Scholar]
- 9.Basford JR. Low intensity laser therapy: Still not an established clinical tool. Lasers Surg Med. 1995;16:331–42. doi: 10.1002/lsm.1900160404. [DOI] [PubMed] [Google Scholar]
- 10.Walsh LJ. The current status of low level laser therapy in dentistry. Part 1. Soft tissue applications. Aust Dent J. 1997;42:247–54. doi: 10.1111/j.1834-7819.1997.tb00129.x. [DOI] [PubMed] [Google Scholar]
- 11.Khadra M, Kasem N, Haanaes HR, Ellingsen JE, Lyngstadaas SP. Enhancement of bone formation in rat calvarial bone defects using low-level laser therapy. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;97:693–700. doi: 10.1016/j.tripleo.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 12.Mellonig JT, Bowers GM, Bailey RC. Comparison of bone graft materials. Part I. New bone formation with autografts and allografts determined by Strontium-85. J Periodontol. 1981;52:291–6. doi: 10.1902/jop.1981.52.6.291. [DOI] [PubMed] [Google Scholar]
- 13.Rummelhart JM, Mellonig JT, Gray JL, Towle HJ. A comparison of freeze-dried bone allograft and demineralized freeze-dried bone allograft in human periodontal osseous defects. J Periodontol. 1989;60:655–63. doi: 10.1902/jop.1989.60.12.655. [DOI] [PubMed] [Google Scholar]
- 14.Youssef M, Ashkar S, Hamade E, Gutknecht N, Lampert F, Mir M. The effect of low-level laser therapy during orthodontic movement: A preliminary study. Lasers Med Sci. 2008;23:27–33. doi: 10.1007/s10103-007-0449-7. [DOI] [PubMed] [Google Scholar]
- 15.Fujita S, Yamaguchi M, Utsunomiya T, Yamamoto H, Kasai K. Low-energy laser stimulates tooth movement velocity via expression of RANK and RANKL. Orthod Craniofac Res. 2008;11:143–55. doi: 10.1111/j.1601-6343.2008.00423.x. [DOI] [PubMed] [Google Scholar]
- 16.Coombe AR, Ho CT, Darendeliler MA, Hunter N, Philips JR, Chapple CC, et al. The effects of low level laser irradiation on osteoblastic cells. Clin Orthod Res. 2001;4:3–14. doi: 10.1034/j.1600-0544.2001.040102.x. [DOI] [PubMed] [Google Scholar]
- 17.Yamaguchi M, Fujita S, Yoshida T, Oikawa K, Utsunomiya T, Yamamoto H, et al. Low-energy laser irradiation stimulates the tooth movement velocity via expression of M-CSF and c-fms. J.odw. 2007;66:139–48. [Google Scholar]
- 18.Abtahi SM, Mohaseni Eghdam H. The effects of low-level laser irradiation of the tooth movements applied by the separator. JQUMS. 2009;13:8–12. [Google Scholar]
- 19.Goulart CS, Nouer PR, Mouramartins L, Garbin IU, de Fátima Zanirato Lizarelli R. Photoradiation and orthodontic movement: Experimental study with canines. Photomed Laser Surg. 2006;24:192–6. doi: 10.1089/pho.2006.24.192. [DOI] [PubMed] [Google Scholar]
- 20.Kim SJ, Moon SU, Kang SG, Park YG. Effects of low-level laser therapy after Corticision on tooth movement and paradental remodeling. Lasers Surg Med. 2009;41:524–33. doi: 10.1002/lsm.20792. [DOI] [PubMed] [Google Scholar]
- 21.Sato S, Ogura M, Ishihara M, Kawauchi S, Arai T, Matsui T, et al. Nanosecond, high-intensity pulsed laser ablation of myocardium tissue at the ultraviolet, visible, and near-infrared wavelengths: In-vitro study. Lasers Surg Med. 2001;29:464–73. doi: 10.1002/lsm.10002. [DOI] [PubMed] [Google Scholar]
- 22.Luger EJ, Rochkind S, Wollman Y, Kogan G, Dekel S. Effect of low-power laser irradiation on the mechanical properties of bone fracture healing in rats. Lasers Surg Med. 1998;22:97–102. doi: 10.1002/(sici)1096-9101(1998)22:2<97::aid-lsm5>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 23.Friedmann H, Lubart R, Laulicht I, Rochkind S. A possible explanation of laser-induced stimulation and damage of cell cultures. J Photochem Photobiol B. 1991;11:87–91. doi: 10.1016/1011-1344(91)80271-i. [DOI] [PubMed] [Google Scholar]
- 24.Karu T. Photobiology of low-power laser effects. Health Phys. 1989;56:691–704. doi: 10.1097/00004032-198905000-00015. [DOI] [PubMed] [Google Scholar]
- 25.Khadra M, Rønold HJ. Low level laser effect on the osseointegration between implant and bone. J Dent Res. 2002;81:A–366. [Google Scholar]
- 26.Lam TS, Abergel RP, Meeker CA, Castel JC, Dwyer RM, Uitto J. Laser stimulation of collagen synthesis in human skin fibroblast cultures. Lasers Life Sci. 1986;1:61–77. [Google Scholar]
- 27.Yaakobi T, Maltz L, Oron U. Promotion of bone repair in the cortical bone of the tibia in rats by low energy laser (He-Ne) irradiation. Calcif Tissue Int. 1996;59:297–300. doi: 10.1007/s002239900126. [DOI] [PubMed] [Google Scholar]
- 28.Morimoto Y, Arai T, Kikuchi M, Nakajima S, Nakamura H. Effect of low-intensity argon laser irradiation on mitochondrial respiration. Lasers Surg Med. 1994;15:191–9. doi: 10.1002/lsm.1900150207. [DOI] [PubMed] [Google Scholar]
- 29.Yu W, Naim JO, McGowan M, Ippolito K, Lanzafame RJ. Photomodulation of oxidative metabolism and electron chain enzymes in rat liver mitochondria. Photochem Photobiol. 1997;66:866–71. doi: 10.1111/j.1751-1097.1997.tb03239.x. [DOI] [PubMed] [Google Scholar]
- 30.Chen JW, Zhou YC. Effect of low level carbon dioxide laser radiation on biochemical metabolism of rabbit mandibular bone callus. Laser Ther. 1989;2:83–7. [Google Scholar]
- 31.Kobu Y. Effects of infrared radiation on intraosseous blood flow and oxygen tension in the rat tibia. Kobe J Med Sci. 1999;45:27–39. [PubMed] [Google Scholar]
- 32.Karu T. Mechanism of low-power laser light action on cellular level. In: Simunovic Z, editor. Laser in Medicine and Dentistry. 1st ed. Ch 4. Rijeka: Vitgraf; 2000. pp. 98–116. [Google Scholar]
- 33.Inoue K, Nishioka J, Hukuda S. Suppressed tuberculin reaction in guinea pigs following laser irradiation. Lasers Surg Med. 1989;9:271–5. doi: 10.1002/lsm.1900090310. [DOI] [PubMed] [Google Scholar]
- 34.Schindl A, Heinze G, Schindl M, Pernerstorfer-Schön H, Schindl L. Systemic effects of low-intensity laser irradiation on skin microcirculation in patients with diabetic microangiopathy. Microvasc Res. 2002;64:240–6. doi: 10.1006/mvre.2002.2429. [DOI] [PubMed] [Google Scholar]
- 35.Braverman B, McCarthy RJ, Ivankovich AD, Forde DE, Overfield M, Bapna MS. Effect of helium-neon and infrared laser irradiation on wound healing in rabbits. Lasers Surg Med. 1989;9:50–8. doi: 10.1002/lsm.1900090111. [DOI] [PubMed] [Google Scholar]


