Abstract
Background:
The aim of the present study is to evaluate the effects of a fluoride-releasing composite resin bonding material on reducing enamel demineralization underneath and around orthodontic brackets and compare that with a conventional adhesive system.
Materials and Methods:
Buccal surfaces of 10 intact extracted premolar teeth were divided into two parts with nail varnish and stainless steel brackets were randomly bonded by two resin composite systems: (Transbond XT) and (Transbond XT plus Color Change) (3M, Unitek, Monrovia, CA, USA) on two sides of the teeth and then samples were placed in a demineralization solution. It is claimed that the second system has the ability of fluoride release. Elastic modulus and hardness of enamel were measured with nanoindentation test in 6 depths in 1-36 μm from the enamel surface and in 7 regions: Control (intact enamel surface), underneath the brackets and also 50 and 100 μm from the brackets edge. These nanomechanical features were evaluated in different regions and depths using analysis of variance and paired t-test (P < 0.05).
Results:
Considerable difference can be seen in different depths and regions in terms of hardness and elastic modulus. The region under the bracket with fluoridated adhesive shows similar results with intact enamel, whereas these parameters in fluoride less side show a significant reduction (P < 0.05).
Conclusion:
Results show that use of resin composite bonding system with the ability of fluoride release for bracket bonding, may reduce demineralization of enamel around brackets during orthodontic treatment.
Keywords: Elastic modulus, enamel demineralization, fluoride releasing adhesive, hardness nanoindentation
INTRODUCTION
Presence of orthodontic appliances inside the oral cavity accelerates plaque accumulation around them and can lead to an unwanted condition, which is the demineralization of enamel around orthodontic brackets.[1,2] This process, especially in patients with weak oral hygiene can finally lead to white spots that remain even after removing the appliances as an esthetic problem.[3,4] Prevalence of white spots during fixed orthodontics was different in studies from 50%[2] to 73-95%.[5,6] Studies show that these lesions can appear in just 1 month,[7,8,9] which is even shorter than the time between two sessions of meeting with the orthodontist.
In order to prevent this problem, orthodontists have tried different techniques and materials. Studies show that high oral hygiene and local use of fluoride with low concentration are the primary solutions for minimizing demineralization around the brackets.[10,11] Daily use of 0.05% sodium fluoride mouth rinse during orthodontic treatment significantly reduced enamel lesions, but in average, the cooperation of patients with this protocol was relatively low.[10] Thus, orthodontists searched for other methods, which did not need high cooperation of the patient. Orthodontic bonding materials with the ability of fluoride release show the potential of minimizing enamel demineralization, if the bond was strong enough and fluoride release was continuous.[12] The preventive effects of these orthodontic bonding materials containing fluoride on enamel adjacent to the brackets were studied in vivo[7,13] and in vitro[14,15,16,17] with quantitative assessment of demineralization depths and loss of mineral structure with different measurement techniques.
Between two major groups of orthodontic bonding materials (i.e., composite resins and glass ionomers), the second one was more successful in fluoride release, at least in in-vitro studies.[17,18] However, the efficiency of bond strength of these materials for clinical application is questionable.[19,20,21,22]
Potential advantage of a product with resin composite base and ability of fluoride release underneath and around fixed orthodontic attachments resulted in the development of these adhesives. However, the relationship between this low amount of fluoride release with expected protection against enamel demineralization was a matter of debate in different articles.[23,24,25,26,27]
Nanoindentation is one of the techniques, which presently allow quicker and much more accurate assessment of nanomechanical characteristics of low amounts of enamel in subsurface regions around orthodontic brackets. It has been shown that this technique is a useful method for very exact assessment of demineralization and remineralization of human enamel surface.[28,29,30,31]
The aim of the present study was to evaluate the nanohardness and elastic modulus of the enamel underneath and around orthodontic brackets using nanoindentation test after placing samples in a demineralization solution and compare these mechanical characteristics for two adhesive systems (conventional and fluoride releasing).
MATERIALS AND METHODS
Samples
In this in vitro experimental study, samples were chosen from 10 intact premolar teeth without caries and enamel hypoplasia that were extracted for orthodontic reasons. Every sample was kept in separate Hank's balanced salts solution (HBSS) (East Sages Inv. Co. Isfahan, Iran) in 37°C.[32]
Preparation process
Buccal surfaces of all teeth were cleaned by fluoride less pumice and polished using a rubber cup and completely washed and dried with oil-free air. Then, buccal surfaces of samples were divided into two equal parts from the cusp point to the cementoenamel junction by 2 mm nail varnish and this region under the varnish was used as control (intact enamel). Then, in each half of the tooth (left half and right half) a different bonding system was used, which was of the same composition with the other half, but one of them released fluoride (Transbond XT plus Color Change etch-and-rinse adhesive, 3 M, Unitek, CA, USA) and the other did not (Transbond XT etch-and-rinse adhesive, 3 M, Unitek, CA, USA). Lower incisor brackets (Opti-Mim, Ortho Organizers, CA, USA) were used because of their reduced area.
In short, the bonding method according to the manufacturer's instructions was: Acid phosphoric gel (35%) was used on buccal surfaces for 15 s and then washed with distilled water and completely dried with an oil-free air source. Then, a thin layer of primer was applied on etched surfaces and cured for 10 s. Lower incisor stainless steel brackets were randomly bonded under mild pressure on the left and right sides by an independent researcher in order to make the study blind and the adhesive residuals were removed around the brackets with a scaler and cured for 20 s. The mesiodistal distance from the nail varnish (midline) and the height from the cusp tip in each side were measured with a gauge to ensure that the brackets were placed in similar positions on each side.
Then, the samples were individually placed in a demineralization solution (50 cc 0.3% [W/V] citric acid at a pH of 2.35 adjusted with NaOH)[33] for 96 h in 37°C. Every sample was taken out of the solution in every 8 h for 1 h and washed with distilled water and kept in a separate HBSS solution in 37°C in order to simulate oral conditions and restore remineralization in some degrees. The samples were then washed completely with distilled water and divided into two occlusal and cervical halves with tooth sectioning machine (TC3000, Vafaei Industrial, Tehran, Iran) in such a way that the cut passed from the middle of the brackets. The cervical half was mounted in self-cure acryl (Dentsply, Addlestone, Surrey KT15 2PG, UK). All of the samples were polished in order to get a smooth and suitable surface for high quality of the nanoindentation experiment. Polishing was performed by abrasive paper disks (320, 600, 1200 grit) and then aluminum oxide powder with gradually subtler grades. The samples were stored in separate HBSS solution again until the time of nanoexperiment.
Nanoindentation experiment
After polishing of samples, the measuring points were investigated with a light microscope by an instructed engineer and if more polish was required, the samples were returned to the researcher; so that appropriate enamel surfaces were achieved for the machine in desired points. All nanoindentation tests were performed in 28°C with a peak load of 10 mN using Nanoindentation Tester (NHT, Peseux, Switzerland) in Central Laboratory of Industrial University of Isfahan. The test was constructed of 3 parts: 10 s of loading until the peak, 1 s holding in peak load and then 10 s of unloading.[34]
In this study, 42 indentations were performed for every sample consisting 6 depths in a distance of 1 μm from the outer surface of enamel to 36 μm (6 depths with equal distance of 7 μm) and also in 7 regions relatively similar to the study of Kohda et al.[34] [Figure 1]:
Figure 1.
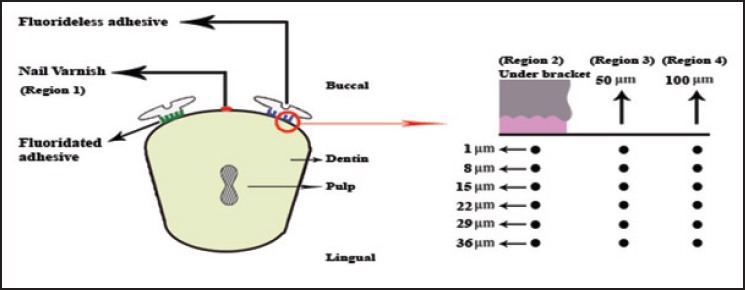
Schematic illustration of the cross-section of the specimen with the regions and depths on the fluoride less side of enamel, which studied by nanoindentation test. There are identical regions (5, 6, 7) and depths (1, 8, 15, 22, 29, 36 ƒÊm) on the fluoridated side, which are not shown
Region 1: Control (under nail varnish that implies intact enamel).
Region 2: Under the bracket with fluoride less (conventional) bonding.
Regions 3 and 4: 50 and 100 μm from the bracket edge with fluoride less bonding, respectively.
Region 5: Under the bracket with fluoridated bonding.
Regions 6 and 7: 50 and 100 μm from the bracket edge with fluoridated bonding, respectively.
The measurement method of elastic modulus and hardness with Nanoindentation machine and relating graphs and calculation formulas are available in Oliver et al.[28]
Statistical analysis
Measurements of hardness and elastic modulus in different regions and depths were extracted with the software companion of the machine and the results were analyzed by Statistical Package for Social Sciences (SPSS, Chicago, version 14.0). The tests of Kolmogorov-Smirnov and Leven variance homogeneity were used. The data showed a normal distribution, so there was homogeneity of variances among groups. Paired t-test, repeated measure of analysis of variance (ANOVA) was used for assessment of the effects of these two adhesives in different regions and depths of enamel surface and their interactions. Tukey post-hoc test was used for multiple comparisons. Level of significance was defined at P < 0.05.
RESULTS
Tables 1 and 2 show the means and standard deviations of hardness and elastic modulus in different regions and depths obtained by nanoindentation test; also the results of statistical comparisons among the 7 regions in total and individually in every 6 depths are shown in these tables.
Table 1.
Mean values and standard deviation for cross-sectional elastic modulus (GPAGp) of enamel in different regions and depths investigated by nanoindentation test
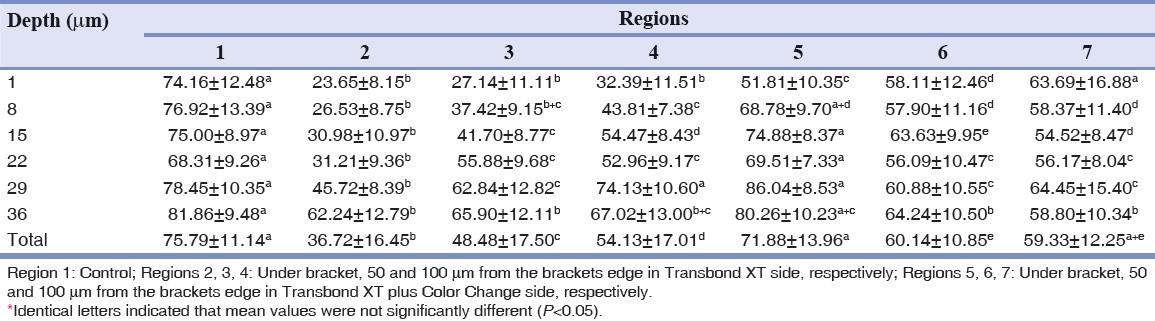
Table 2.
Mean values and standard deviation for cross-sectional hardness (GPGpA) of enamel in different regions and depths investigated by nanoindentation test
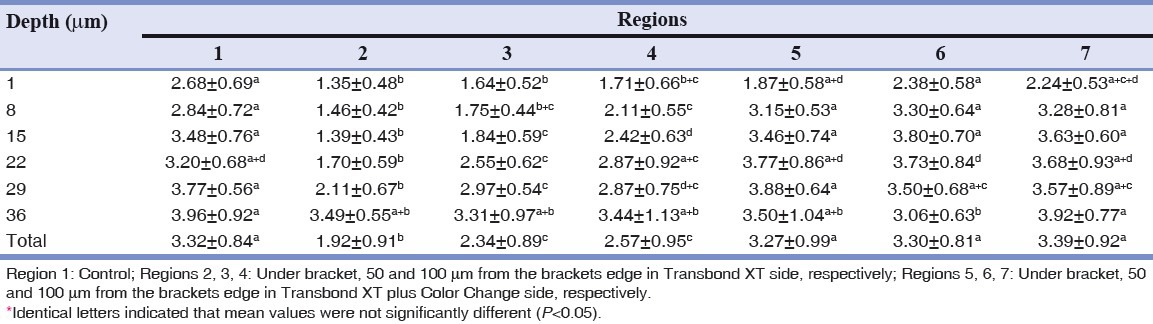
In all regions except for region 5 (region under the bracket with fluoridated adhesive) the elastic modulus reduced significantly compared to the intact enamel (region 1); but regarding hardness, significant reduction is only observable in the fluoride less side (regions 2, 3 and 4). Interestingly, total hardness average of region 7 is higher than average of this parameter in region 1. From the point of view of these two parameters, region 2 shows a significant reduction in comparison to other regions. In 50 and 100 μm from the bracket edge in the fluoride less side (regions 3, 4), investigated parameters increased, but in comparison to similar regions in the side with fluoride (regions 6, 7) both parameters show statistically significant reduction, except for the mean of elastic modulus in region 4 with 7, which were similar. Region 5 showed higher elastic modulus than all other 5 regions except for intact enamel; but regarding hardness, this increase was only noticeable in comparison to fluoride less regions (2, 3, 4). Regions 6 and 7 showed similar statistical amounts of elastic modulus and hardness.
Table 3 shows the results of ANOVA that indicate high statistical significance (P = 0.000) of depth, region and interaction of region and depth.
Table 3.
ANOVA results for elastic modulus (upper row) and hardness (lower row) investigated with nanoindentation test
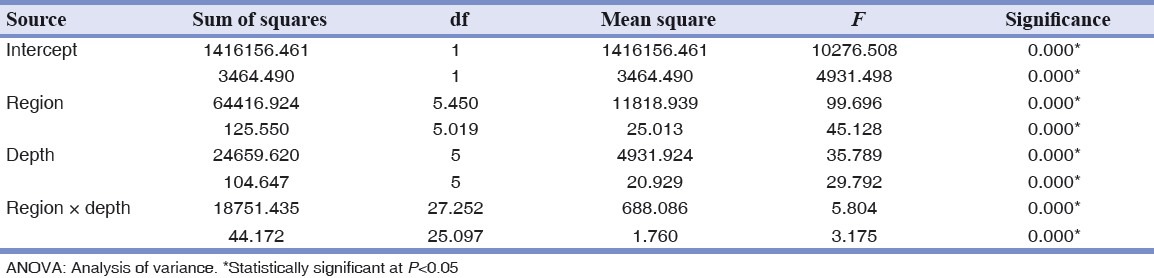
The post-hoc test showed these two parameters were significantly different between all depths of every region except for elastic modulus in regions 1, 6 and 7. But, in general, in all regions no statistical significant difference was observable in depths of (1 and 8 μm), (8 and 15 and 22 μm) and (29 and 36 μm) regarding elastic modulus and depths of (8 and 15 μm), (15 and 22 and 29 μm) and (29 and 36 μm) regarding hardness. Hardness of enamel in surface (1 μm depth) is significantly lower than deeper areas.
DISCUSSION
The hypothesis of this in vitro study is that demineralization of enamel around brackets can be avoided or reduced by using composite resin with the ability of fluoride releasing. There have been a lot of efforts to reduce enamel demineralization during orthodontic treatment. Adhesive systems can reduce demineralization by compounds such as fluoride, calcium phosphate or anti-bacterial materials.[35,36,37,38]
In this study, on one side of the samples, we used a conventional orthodontic bonding adhesive (Transbond XT) and on the other side, we used another product with similar composition from the same factory (3M, Unitek, Monrovia, CA, USA) that has the ability of fluoride releasing (Transbond XT plus Color Change). In addition, in order to understand initial mechanical characteristics of teeth and also realize the effects of etching and demineralization solutions on these characteristics, the enamel of the middle part of each tooth was covered with nail varnish from the beginning and used as control enamel. Considering the similarity of location and bond conditions of brackets in two sides of every tooth and also similarity of keeping, slicing and polishing conditions in two sides, the probability of equalizing basic conditions of research is higher. Since, there is a high statistical significance in the results, it can be concluded that the sample volume was enough.
Demineralization around the brackets can be studied by different tools such as electronic scanning microscope,[24,39] carries index,[16] polarized light microscope,[7] microhardness test[7,35] and photographic slide.[23,25,26] In the present study, we considered the mechanical characteristics of enamel such as elastic modulus and hardness by using the cross-sectional nanoindentation as an assessment of the amounts of minerals, because the mechanical characteristics of the enamel is usually related to its minerals and any change in these parameters can be caused by bonding process including etching and effects of used bonding materials, process of demineralization and also effects of keeping conditions.[32,33,40,41,42]
In recent years, nanoindentation test has been used for studying the human enamel characteristics and the average of hardness and elastic modulus amounts for intact enamel (region 1) in our study (hardness = 1.81-4.97 GPA, elastic modulus = 52.43-96.95 GPA) was consistent with other studies.[41,43,44]
Results of our in vitro study showed that in region 2, which was affected by etching solution and demineralization and then fluorideless bonding was used, in average in 6 depths absolutely significant reduction of elastic modulus and hardness is observed in comparison to the control region and also all the other regions. On the contrary, region 5 with same conditions except for fluoridated bonding had relatively similar parameters compared with intact enamel that shows releasing fluoride from bonding material may neutralize effects of etching and demineralization solution and restore the normal conditions of enamel.
These results are in contradiction to Kohda et al.,[34] which showed that Transbond plus Color Change is not sufficient for remineralization of etched enamel around brackets and they gained similar amounts for hardness and elastic modulus under and around brackets, which were bonded with these two kinds of bonding materials. Furthermore, Trimpeneers and Dermaut[23] didn't find a significant difference between a bonding system with the ability of fluoride releasing and resins with chemical curing without releasing of fluoride regarding decalcification amount. But another study, which investigated demineralization depth and presence of fluoride in two similar groups with our study concluded that Transbond plus group in comparison to Transbond XT had lower demineralization depth.[45] Dubroc et al., in another similar study in mice found similar findings to ours and stated that fluoride-releasing resin reduces demineralization of areas that the resin was applied and caries beyond the applied areas.[46]
After making distance from bracket edge in fluoride less side (regions 3, 4), mechanical characteristics of enamel increase and approach to similar regions in the side containing fluoride (regions 6, 7); although, there is still statistical significant difference, which can show that released fluoride effect is primarily limited to regions under bracket and regions completely close to that; this finding is similar to other studies about fluoride releasing.[35,47]
About the effects of etching and demineralization solutions and fluoride in enamel depth, it seems that investigated parameters have increased in the range of our study (1-36 μm) and became closer to normal amounts in such a way that no significant difference is seen between depth of 29 μm and 36 μm regarding elastic modulus and hardness; However, if investigated depths were more than this, we could have more accurate information. In other studies, Pascotto et al.,[13] de moura et al.,[48] and Kohda et al.,[34] found the depth of changes in 30, 70 and 21 μm from enamel surface, respectively.
In this study, the same behavior is seen in amounts of elastic modulus and hardness. The major difference is statistical similarity of hardness in region 1 with regions 6 and 7; although, elastic modulus is different in these regions. This general condition is similar to other studies in this field.[34,40,42]
Although the results of the study show better understanding of potential inhibitory characteristics of fluoride, but in vitro experimental conditions cannot simulate all the complexities of caries producing environment of the human mouth and the final result about efficiency of these products should be gained through controlled clinical trials. The weak point in the design of this study like studies with design of split mouth is the probability of cross over. In other words, released fluoride from the side of adhesive with fluoride has effects on the other side through transmission in demineralization solution.
CONCLUSION
Based on this in vitro study, we can conclude that:
Two bonding systems based on composite resin with different ability of fluoride release had different effects on hardness and elastic modulus of enamel under and around orthodontic brackets.
Etching enamel surface and keeping in demineralization solution was accompanied with clear reduction in mechanical characteristics of enamel. The released fluoride from adhesive had the capability of returning basic characteristics into the enamel. However, this capability was reduced with the increase in the distance from bracket edge.
Increasing depth from the enamel surface, reduced effects of etching and demineralization solutions and also fluoride.
Nanoindentation test is an excellent tool for fast and accurate assessment of site-specific characteristics of enamel.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Artun J, Brobakken BO. Prevalence of carious white spots after orthodontic treatment with multibonded appliances. Eur J Orthod. 1986;8:229–34. doi: 10.1093/ejo/8.4.229. [DOI] [PubMed] [Google Scholar]
- 2.Gorelick L, Geiger AM, Gwinnett AJ. Incidence of white spot formation after bonding and banding. Am J Orthod. 1982;81:93–8. doi: 10.1016/0002-9416(82)90032-x. [DOI] [PubMed] [Google Scholar]
- 3.Artun J, Thylstrup A. A 3-year clinical and SEM study of surface changes of carious enamel lesions after inactivation. Am J Orthod Dentofacial Orthop. 1989;95:327–33. doi: 10.1016/0889-5406(89)90166-2. [DOI] [PubMed] [Google Scholar]
- 4.Ogaard B. Prevalence of white spot lesions in 19-year-olds: A study on untreated and orthodontically treated persons 5 years after treatment. Am J Orthod Dentofacial Orthop. 1989;96:423–7. doi: 10.1016/0889-5406(89)90327-2. [DOI] [PubMed] [Google Scholar]
- 5.Richter AE, Arruda AO, Peters MC, Sohn W. Incidence of caries lesions among patients treated with comprehensive orthodontics. Am J Orthod Dentofacial Orthop. 2011;139:657–64. doi: 10.1016/j.ajodo.2009.06.037. [DOI] [PubMed] [Google Scholar]
- 6.Lovrov S, Hertrich K, Hirschfelder U. Enamel demineralization during fixed orthodontic treatment - Incidence and correlation to various oral-hygiene parameters. J Orofac Orthop. 2007;68:353–63. doi: 10.1007/s00056-007-0714-1. [DOI] [PubMed] [Google Scholar]
- 7.Gorton J, Featherstone JD. In vivo inhibition of demineralization around orthodontic brackets. Am J Orthod Dentofacial Orthop. 2003;123:10–4. doi: 10.1067/mod.2003.47. [DOI] [PubMed] [Google Scholar]
- 8.Ogaard B, Rølla G, Arends J. Orthodontic appliances and enamel demineralization. Part 1. Lesion development. Am J Orthod Dentofacial Orthop. 1988;94:68–73. doi: 10.1016/0889-5406(88)90453-2. [DOI] [PubMed] [Google Scholar]
- 9.O’Reilly MM, Featherstone JD. Demineralization and remineralization around orthodontic appliances: An in vivo study. Am J Orthod Dentofacial Orthop. 1987;92:33–40. doi: 10.1016/0889-5406(87)90293-9. [DOI] [PubMed] [Google Scholar]
- 10.Geiger AM, Gorelick L, Gwinnett AJ, Benson BJ. Reducing white spot lesions in orthodontic populations with fluoride rinsing. Am J Orthod Dentofacial Orthop. 1992;101:403–7. doi: 10.1016/0889-5406(92)70112-N. [DOI] [PubMed] [Google Scholar]
- 11.Sadowsky PL, Retief DH, Bradley EL., Jr Enamel fluoride uptake from orthodontic cements and its effect on demineralization. Am J Orthod. 1981;79:523–34. doi: 10.1016/s0002-9416(81)90463-2. [DOI] [PubMed] [Google Scholar]
- 12.Bounoure GM, Vezin JC. Orthodontic fluoride protection. J Clin Orthod. 1980;14:321–5. [PubMed] [Google Scholar]
- 13.Pascotto RC, Navarro MF, Capelozza Filho L, Cury JA. In vivo effect of a resin-modified glass ionomer cement on enamel demineralization around orthodontic brackets. Am J Orthod Dentofacial Orthop. 2004;125:36–41. doi: 10.1016/s0889-5406(03)00571-7. [DOI] [PubMed] [Google Scholar]
- 14.Corry A, Millett DT, Creanor SL, Foye RH, Gilmour WH. Effect of fluoride exposure on cariostatic potential of orthodontic bonding agents: An in vitro evaluation. J Orthod. 2003;30:323–9. doi: 10.1093/ortho/30.4.323. [DOI] [PubMed] [Google Scholar]
- 15.Hu W, Featherstone JD. Prevention of enamel demineralization: An in-vitro study using light-cured filled sealant. Am J Orthod Dentofacial Orthop. 2005;128:592–600. doi: 10.1016/j.ajodo.2004.07.046. [DOI] [PubMed] [Google Scholar]
- 16.Chung CK, Millett DT, Creanor SL, Gilmour WH, Foye RH. Fluoride release and cariostatic ability of a compomer and a resin-modified glass ionomer cement used for orthodontic bonding. J Dent. 1998;26:533–8. doi: 10.1016/s0300-5712(98)00017-7. [DOI] [PubMed] [Google Scholar]
- 17.Vorhies AB, Donly KJ, Staley RN, Wefel JS. Enamel demineralization adjacent to orthodontic brackets bonded with hybrid glass ionomer cements: An in vitro study. Am J Orthod Dentofacial Orthop. 1998;114:668–74. doi: 10.1016/s0889-5406(98)70199-4. [DOI] [PubMed] [Google Scholar]
- 18.Banks PA, Burn A, O’Brien K. A clinical evaluation of the effectiveness of including fluoride into an orthodontic bonding adhesive. Eur J Orthod. 1997;19:391–5. doi: 10.1093/ejo/19.4.391. [DOI] [PubMed] [Google Scholar]
- 19.Fajen VB, Duncanson MG, Jr, Nanda RS, Currier GF, Angolkar PV. An in vitro evaluation of bond strength of three glass ionomer cements. Am J Orthod Dentofacial Orthop. 1990;97:316–22. doi: 10.1016/0889-5406(90)70104-k. [DOI] [PubMed] [Google Scholar]
- 20.Wiltshire WA. Shear bond strengths of a glass ionomer for direct bonding in orthodontics. Am J Orthod Dentofacial Orthop. 1994;106:127–30. doi: 10.1016/S0889-5406(94)70029-X. [DOI] [PubMed] [Google Scholar]
- 21.Schanbel S, Kocjancic B. Cementing brackets with glass ionomer cements. Prakt Kieferorthop. 1991;5:149–52. [PubMed] [Google Scholar]
- 22.Evans R, Oliver R. Orthodontic bonding using glass ionomer cement: An in vitro study. Eur J Orthod. 1991;13:493–500. doi: 10.1093/ejo/13.6.493. [DOI] [PubMed] [Google Scholar]
- 23.Trimpeneers LM, Dermaut LR. A clinical evaluation of the effectiveness of a fluoride-releasing visible light-activated bonding system to reduce demineralization around orthodontic brackets. Am J Orthod Dentofacial Orthop. 1996;110:218–22. doi: 10.1016/s0889-5406(96)70112-9. [DOI] [PubMed] [Google Scholar]
- 24.Basdra EK, Huber H, Komposch G. Fluoride released from orthodontic bonding agents alters the enamel surface and inhibits enamel demineralization in vitro. Am J Orthod Dentofacial Orthop. 1996;109:466–72. doi: 10.1016/s0889-5406(96)70130-0. [DOI] [PubMed] [Google Scholar]
- 25.Wenderoth CJ, Weinstein M, Borislow AJ. Effectiveness of a fluoride-releasing sealant in reducing decalcification during orthodontic treatment. Am J Orthod Dentofacial Orthop. 1999;116:629–34. doi: 10.1016/s0889-5406(99)70197-6. [DOI] [PubMed] [Google Scholar]
- 26.Sonis AL, Snell W. An evaluation of a fluoride-releasing, visible light-activated bonding system for orthodontic bracket placement. Am J Orthod Dentofacial Orthop. 1989;95:306–11. doi: 10.1016/0889-5406(89)90163-7. [DOI] [PubMed] [Google Scholar]
- 27.Rogers S, Chadwick B, Treasure E. Fluoride-containing orthodontic adhesives and decalcification in patients with fixed appliances: A systematic review. Am J Orthod Dentofacial Orthop. 2010;138:390.e1–8. doi: 10.1016/j.ajodo.2010.02.025. [DOI] [PubMed] [Google Scholar]
- 28.Oliver WC, Pharr GM. An improved technique for determining hardness and elastic modulus using load and displacement sensing indentation experiments. J Mater Res. 1992;7:1564–83. [Google Scholar]
- 29.He LH, Swain MV. Influence of environment on the mechanical behaviour of mature human enamel. Biomaterials. 2007;28:4512–20. doi: 10.1016/j.biomaterials.2007.06.020. [DOI] [PubMed] [Google Scholar]
- 30.Alcock JP, Barbour ME, Sandy JR, Ireland AJ. Nanoindentation of orthodontic archwires: The effect of decontamination and clinical use on hardness, elastic modulus and surface roughness. Dent Mater. 2009;25:1039–43. doi: 10.1016/j.dental.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 31.Iijima M, Muguruma T, Brantley WA, Yuasa T, Uechi J, Mizoguchi I. Effect of mechanical properties of fillers on the grindability of composite resin adhesives. Am J Orthod Dentofacial Orthop. 2010;138:420–6. doi: 10.1016/j.ajodo.2008.08.039. [DOI] [PubMed] [Google Scholar]
- 32.Habelitz S, Marshall GW, Jr, Balooch M, Marshall SJ. Nanoindentation and storage of teeth. J Biomech. 2002;35:995–8. doi: 10.1016/s0021-9290(02)00039-8. [DOI] [PubMed] [Google Scholar]
- 33.Lippert F, Parker DM, Jandt KD. In vitro demineralization/remineralization cycles at human tooth enamel surfaces investigated by AFM and nanoindentation. J Colloid Interface Sci. 2004;280:442–8. doi: 10.1016/j.jcis.2004.08.016. [DOI] [PubMed] [Google Scholar]
- 34.Kohda N, Iijima M, Brantley W, Muguruma T, Yuasa T, Nakagaki S, et al. Effects of bonding materials on the mechanical properties of enamel around orthodontic brackets. Angle Orthod. 2012;82:187–95. doi: 10.2319/020411-78.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Uysal T, Amasyali M, Ozcan S, Koyuturk AE, Sagdic D. Effect of antibacterial monomer-containing adhesive on enamel demineralization around orthodontic brackets: An in-vivo study. Am J Orthod Dentofacial Orthop. 2011;139:650–6. doi: 10.1016/j.ajodo.2009.06.038. [DOI] [PubMed] [Google Scholar]
- 36.Uysal T, Amasyali M, Koyuturk AE, Sagdic D. Efficiency of amorphous calcium phosphate-containing orthodontic composite and resin modified glass ionomer on demineralization evaluated by a new laser fluorescence device. Eur J Dent. 2009;3:127–34. [PMC free article] [PubMed] [Google Scholar]
- 37.Imazato S, Torii M, Tsuchitani Y, McCabe JF, Russell RR. Incorporation of bacterial inhibitor into resin composite. J Dent Res. 1994;73:1437–43. doi: 10.1177/00220345940730080701. [DOI] [PubMed] [Google Scholar]
- 38.Øgaard B, Larsson E, Henriksson T, Birkhed D, Bishara SE. Effects of combined application of antimicrobial and fluoride varnishes in orthodontic patients. Am J Orthod Dentofacial Orthop. 2001;120:28–35. doi: 10.1067/mod.2001.114644. [DOI] [PubMed] [Google Scholar]
- 39.Fjeld M, Øgaard B. Scanning electron microscopic evaluation of enamel surfaces exposed to 3 orthodontic bonding systems. Am J Orthod Dentofacial Orthop. 2006;130:575–81. doi: 10.1016/j.ajodo.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 40.Zhou J, Hsiung LL. Depth-dependent mechanical properties of enamel by nanoindentation. J Biomed Mater Res A. 2007;81:66–74. doi: 10.1002/jbm.a.31012. [DOI] [PubMed] [Google Scholar]
- 41.Habelitz S, Marshall SJ, Marshall GW, Jr, Balooch M. Mechanical properties of human dental enamel on the nanometre scale. Arch Oral Biol. 2001;46:173–83. doi: 10.1016/s0003-9969(00)00089-3. [DOI] [PubMed] [Google Scholar]
- 42.Hairul Nizam BR, Lim CT, Chng HK, Yap AU. Nanoindentation study of human premolars subjected to bleaching agent. J Biomech. 2005;38:2204–11. doi: 10.1016/j.jbiomech.2004.09.023. [DOI] [PubMed] [Google Scholar]
- 43.Xu HH, Smith DT, Jahanmir S, Romberg E, Kelly JR, Thompson VP, et al. Indentation damage and mechanical properties of human enamel and dentin. J Dent Res. 1998;77:472–80. doi: 10.1177/00220345980770030601. [DOI] [PubMed] [Google Scholar]
- 44.Ge J, Cui FZ, Wang XM, Feng HL. Property variations in the prism and the organic sheath within enamel by nanoindentation. Biomaterials. 2005;26:3333–9. doi: 10.1016/j.biomaterials.2004.07.059. [DOI] [PubMed] [Google Scholar]
- 45.Zanarini M, Pazzi E, Bonetti S, Ruggeri O, Alessandri Bonetti G, Prati C. In vitro evaluation of the effects of a fluoride-releasing composite on enamel demineralization around brackets. Prog Orthod. 2012;13:10–6. doi: 10.1016/j.pio.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 46.Dubroc GC, Jr, Mayo JA, Rankine CA. Reduction of caries and of demineralization around orthodontic brackets: Effect of a fluoride-releasing resin in the rat model. Am J Orthod Dentofacial Orthop. 1994;106:583–7. doi: 10.1016/S0889-5406(94)70082-6. [DOI] [PubMed] [Google Scholar]
- 47.Primosch RE, Weatherell JA, Strong M. Distribution and retention of salivary fluoride from a sodium fluoride tablet following various intra-oral dissolution methods. J Dent Res. 1986;65:1001–5. doi: 10.1177/00220345860650070101. [DOI] [PubMed] [Google Scholar]
- 48.de Moura MS, de Melo Simplício AH, Cury JA. In-vivo effects of fluoridated antiplaque dentifrice and bonding material on enamel demineralization adjacent to orthodontic appliances. Am J Orthod Dentofacial Orthop. 2006;130:357–63. doi: 10.1016/j.ajodo.2004.12.026. [DOI] [PubMed] [Google Scholar]


