Abstract
Klinefelter syndrome (KS) (47, XXY) is the most abundant sex-chromosome disorder, and is a common cause of infertility and hypogonadism in men. Most men with KS go through life without knowing the diagnosis, as only 25% are diagnosed and only a few of these before puberty. Apart from hypogonadism and azoospermia, most men with KS suffer from some degree of learning disability and may have various kinds of psychiatric problems. The effects of long-term hypogonadism may be difficult to discern from the gene dose effect of the extra X-chromosome. Whatever the cause, alterations in body composition, with more fat and less muscle mass and diminished bone mineral mass, as well as increased risk of metabolic consequences, such as type 2 diabetes and the metabolic syndrome are all common in KS. These findings should be a concern as they are not simply laboratory findings; epidemiological studies in KS populations show an increased risk of both hospitalization and death from various diseases. Testosterone treatment should be offered to KS patients from early puberty, to secure a proper masculine development, nonetheless the evidence is weak or nonexisting, since no randomized controlled trials have ever been published. Here, we will review the current knowledge of hypogonadism in KS and the rationale for testosterone treatment and try to give our best recommendations for surveillance of this rather common, but often ignored, syndrome.
Keywords: androgen receptor, body composition, bone density, hypogonadism, infertility, Klinefelter syndrome, learning disorders, male, testosterone
INTRODUCTION
Despite being the most common sex chromosomal disorder affecting 1 in 600 men,1 Klinefelter syndrome (KS) (47, XXY) remains highly under-diagnosed because of substantial variation in the clinical presentation. The rate of diagnosis during childhood is extremely low, and only 10% of cases are identified before puberty reaching 25% by adulthood.1 The prevalence of KS may vary across different populations and recent studies have suggested a higher prevalence in Australia2 and perhaps in an Asian population,3 however, the prevalence from the last study was based on only three patients with KS and should be interpreted with caution.
While the ‘prototypic’ man with KS traditionally has been described as tall, with narrow shoulders, broad hips, sparse body hair, gynecomastia and small testes, with azoospermia and hypergonadothrophic hypogonadism,4,5 an alternative phenotype with fewer clinical features has now been recognized.6 In addition, no exclusive symptom defines the syndrome, however, the combination of small testes and elevated gonadothrophins is present in nearly all.7
During the past decades, much new information about the long-term consequences of the syndrome has emerged, describing many of the problems these men suffer from. KS is not just about testicular malfunction and increased height; it has marked effects on brain, behavior and psychiatric morbidity, body composition and insulin sensitivity, bone formation and fracture risk, and it affects general morbidity and mortality in a negative direction. Epidemiological studies in two cohorts of KS patients from UK and Denmark have shown that KS is associated with a range of long-term consequences not only affecting morbidity8 and mortality,9,10 but also affects the socioeconomic status in a negative way,11 as well as increased risk of being convinced for criminality.12
A substantial number of reviews concerning KS have been published during the past few years. Herein, we will focus on what is known about the consequences of hypogonadism in KS and discuss current knowledge on the effect of testosterone treatment.
TESTICULAR FUNCTION AND FERTILITY
KS affects testicular function. This has been known since the first description of the syndrome by Klinefelter et al. in 1942.4 Since then our knowledge about the development and mal-development of the testes in KS has expanded substantially. Beginning already in fetal life, a loss of germ cells that continues during infancy and accelerates through puberty leads to fibrosis and hyalinization of seminiferous tubules as well as hyperplasia of Leydig cells, which ultimately results in small firm testes (typically < 3 ml) seen in adults and azoospermia.13 At birth some KS boys may show signs of intrauterine hypogonadism such as micropenis or cryptorchism14,15 and although anecdotal, testosterone injections may alleviate this problem.14,16 Conflicting data regarding the surge in testosterone (mini-puberty) during the first 3 months has been published; demonstrating either low levels of testosterone,17 high-normal levels of testosterone18 or low-normal testosterone.19 If the mini-puberty during the first 3 months is blunted, a window for testosterone treatment has been proposed in order to reestablish normal testosterone levels and unconfirmed reports about such treatment to infants with KS, particularly in the USA, have been circulated for many years. Interestingly, a recent study by Samango-Sprouse et al.20 did find a better neuro-developmental outcome in KS boys (n = 34) treated with testosterone in infancy (because of micropenis) compared with KS nontreated boys (n = 67), but unfortunately, the treatment was not randomized or blinded, leaving the study with a great potential for selection bias. However, current knowledge does not support systematic treatment with testosterone in infancy except for cases of micropenis.16 Randomized controlled trials (RCTs) are needed to confirm a possible positive effect of testosterone treatment in infancy before treatment should be offered to all boys with KS.
At the beginning of puberty, which in general occurs at a normal age,21 testes grow a little but subsequently shrink and in parallel with this, the gonadotrophins rise to the greatly elevated levels seen in adults with KS.22 Most adults with KS have testosterone in the low-normal or sub-normal range but some may have very low levels, and some may even have normal levels of testosterone.23,24
Infertility without assisted reproduction is invariable, although a few spontaneous proven fatherhoods among KS men have been reported.25 Since the development of testicular sperm extraction (TESE), microdissecting TESE and microinsimination technique (ICSI), fatherhood is now a realistic possibility for a substantial part of KS males who have access to this technology. In a review by AksglÆde and Juul26 on published data on TESE and micro-TESE, success rates of sperm retrieval of 42% with TESE and of 57% with micro-TESE were demonstrated, however, the success rate regarding actual achieved fatherhoods was not recorded. It has been questioned whether testosterone treatment prior to micro-TESE could decrease the chance of retrieving spermatocytes,27 and some offer presurgery hormonal treatment with aromatase inhibitors, human choriogonadotrophin (hCG) or clomiphene, but presently, no controlled trials exists on this topic.26 A few KS patients may have spermatocytes in their ejaculate and may hence become fathers simply by ICSI, and a few such cases have been published.28,29 Concerns regarding an increased risk of aneuploidy in the offspring of KS fathers, based on findings of more hypehaploid spermatocytes and more aneuploid embryos found during preimplantation genetic diagnosis,30 have not been translated into more aneuploid outcomes of pregnancies, and only one pregnancy with a 47, XXY fetus in a triplet pregnancy has been reported.31
THE BRAIN, BEHAVIOR AND COGNITIVE FUNCTION
Knowledge about the neuropsychological phenotype of KS has expanded during the past decades resulting in a very comprehensive description, although the phenotype is very variable. The majority of boys and men with KS suffer in varying degree from cognitive disabilities, the most consistent finding being verbal deficits,32,33,34 but deficits in other cognitive abilities such as memory function35,36 and executive functions37,38,39 also seem to be common. Second, an increased psychiatric morbidity is seen in KS, including an increased prevalence of depression, autism, anxiety, attention-deficit/hyperactivity disorders and schizophrenia.37,40,41,42 KS is also associated with volumetric changes in global and regional brain volumes. Total brain volume, gray matter volume and white matter volume has been reported to be decreased in several studies.43,44,45 Decreased regional gray matter volumes have been reported in brain regions such as insula, caudate and putamen,44 hippocampus/parahippocampus,43 amydala,43,45,46 temporal pole and inferior frontal.44,45,46,47
Both a gene dosage effect due to the supernumerary X-chromosome and androgen deficiency or a combination of both have been proposed to cause the neuropsychological phenotype seen in KS. Many of the cognitive disabilities and psychological traits are already present in childhood,34 before hypergonadotropic hypogonadism develops. If androgen insufficiency were to account for these neuropsychological traits, it should be present prenatally, where testosterone is known to influence brain development.48 However, no study that we are aware of has measured the level of testosterone in fetus with KS. A few studies have investigated the level of testosterone in KS neonates, but have been equivocal reporting either decreased or normal/high testosterone levels.17,18 Some evidence for androgen deficits in KS prenatally has been indicated by the increased prevalence of microphallus and cryptorchidism16 seen in KS. Before any conclusion regarding the impact of testosterone deficiency in infancy on the cognitive and psychological phenotype can be drawn, more conclusive studies about testosterone levels in infants and follow up studies investigating the correlations between infant testosterone level and neuropsychological phenotype have to be performed.
Regarding the impact of hypogonadism on the changes in brain volumes seen in KS, very little is known. In our current study of structural brain volumes49 we found the same volumetric changes as a previous study on boys with KS,43 indicating that the difference in brain volumes seen in KS develop during fetal development either due to a gene-dosage effect or due to prenatal androgen deficiency. In favor of the first, the brain size of girls with congenital adrenal hyperplasia who are known to be exposed to high levels of androgens are not different from normal girls.50 Further support comes from a study in females with triple-X syndrome (47, XXX), who have been found to have smaller brain volumes than controls.51 Recently, testosterone was shown to influence gray matter volume specifically in the temporal, parietal and orbitofrontal regions,52 however, in our current study49 we did not find volumetric gray matter changes in these specific regions, giving further support for a gene dosage effect.
The modulating effect of testosterone therapy on the neuropsychological phenotype has also been investigated in adults with KS, however, with inconsistent conclusions. In traits such as behavior, energy level, well-being and learning capacity and verbal fluency testosterone therapy has been shown to have a positive impact.46,53,54 However, regarding the impact of testosterone on cognitive performance no difference has been reported between boys and men receiving testosterone therapy and untreated boys and men with KS,34,55,56 except for one study finding a positive effect of testosterone therapy on verbal fluency.46 Unfortunately, all studies used a cross-sectional design and the duration and dosage were not standardized, which may bias the results. Before we can conclude if there are effects of testosterone therapy, follow-up studies are needed.
Consistent conclusions regarding the effect of testosterone therapy on brain volumes in KS males are still lacking. One study reported smaller left temporal volumes in KS men not receiving testosterone therapy compared to controls, whereas KS males receiving testosterone treatment did not differ from controls.46 Other investigators, including ourselves,49,56 found no significant effect of testosterone treatment on brain volumes in KS patients.
In conclusion, the effect of prenatal and postnatal androgen deficiency on the neuropsychological phenotype and brain volumes in KS is putative and evidence for an effect of testosterone therapy on the neuropsychological phenotype is lacking, implying that the mechanism behind the neuropsychological phenotype and changes in brain volumes may instead be caused by the gene dosage effect of having a supernumerary X-chromosome. Regarding the modulating effect of testosterone therapy on the neuropsychological phenotype follow-up studies are needed.
BODY COMPOSITION AND INSULIN SENSITIVITY
KS patients have altered body composition with increased body fat and reduced muscle mass, but to date it is unknown whether these changes are a consequence of the specific KS genotype, the hormonal milieu or a combination of both. In men with normal chromosomes, hypogonadism independently predicts the development of abdominal adiposity,57 whereas testosterone treatment of middle-aged abdominal obese men reduces the amount of intraabdominal fat.58 Likewise, testosterone is inversely associated with visceral fat area in men receiving androgen deprivation therapy for prostate cancer.59 In KS, however, greater body fat mass is already present before puberty indicating that genetic factors may also impact on body fat distribution.60 In a recent study in KS patients, testosterone treatment only partly corrected the unfavorable muscle/fat ratio, but these findings may partly be the result of insufficient testosterone doses used.60 In addition to altered body composition, KS patients also have lower aerobic capacity24 and reduced muscle strength in both biceps and quadriceps muscles.61 At present, no studies have examined the effects of testosterone treatment on muscle strength or other measures of physical fitness in KS, but testosterone treatment of elderly hypogonadal men has been shown to increase hand grip strength and physical performance62 and to improve lower and upper body muscle strength.63 These findings, in addition to quality of life improvements, have recently been corroborated in a randomized placebo controlled study.64
A number of case reports have described an association between diabetes and KS, but reasons for this association remain unclear. Epidemiological studies on both morbidity8,10 and mortality in patients with KS9 have confirmed this increased risk of diabetes. We recently described a strikingly high incidence of the insulin resistance and the metabolic syndrome in 70 patients KS patients compared with age-matched controls. Almost half of the KS patients fulfilled criteria for the metabolic syndrome, whereas it was true for only 10% of the control group.24 These findings have been confirmed by others65,66,67 emphasizing that insulin resistance and metabolic syndrome may affect one-third to half of adult patients with KS. In a more recent study on 89 prepubertal KS boys, 37% were found to have elevated low-density lipoprotein (LDL) cholesterol, 24% insulin resistance and 7% meeting the criteria of the metabolic syndrome.68
Numerous prospective studies have shown that low levels of testosterone can predict the metabolic syndrome69 and type 2 diabetes,70 and cross-sectional studies have consistently reported an inverse relationship between plasma testosterone and insulin resistance in normal males.71 Type 2 diabetes is frequent in hypogonadal patients,24 and vice versa, hypogonadism is also more prevalent among type 2 diabetics than in age-matched controls.71 These findings, however, are minimized,72 or in some studies even abolished24,73 when correcting for measures of body composition, and it is likely that this association is largely mediated by adiposity rather than testosterone itself. The effects of testosterone treatment in hypogonadal patients, however, are conflicting, and generally, most studies on this topic only used rough or indirect measures of insulin sensitivity and body composition. Testosterone treatment in hypogonadal patients with type 2 diabetes, however, primarily improved insulin sensitivity in the obese,74,75 but not in the lean76,77 patients, and comparable results have been obtained with testosterone treatment of obese hypogonadal normo-glycemic men.58,78 Hence, improvements in insulin sensitivity of hypogonadal patients following testosterone treatment may therefore largely depend on a considerable amount of fat, especially visceral. Only few clinical studies, however, have demonstrated direct effects of testosterone on insulin sensitivity.79,80 Recently, a retrospective longitudinal study comparing KS patients with body mass index (BMI) and age-matched patients with idiopathic hypogonadotropic hypogonadism showed a significantly higher prevalence of diabetes among KS patients. Surprisingly, the prevalence increased markedly with testosterone treatment during a median follow up period of 4 years, but unfortunately, testosterone treatment only resulted in low to sub-normal testosterone levels in both groups and only crude measures of body composition changes were provided.66
Apart from the association between low testosterone, body composition, the metabolic syndrome and insulin sensitivity, hypogonadism has been associated with an adverse cardiovascular risk profile, with increased C-reactive protein (CRP), triglycerides and decreased high-density lipoprotein (HDL) cholesterol.81 Conversely, testosterone is negatively associated with the cardio-protective and antidiabetic adipokine adiponectin,82 and testosterone treatment has been shown to suppress (relatively elevated) adiponectin levels in both hypogonadal men and in castrated mice.83,84,85 Recently, we found normal (or nonsuppressed) adiponectin and normal blood pressure in our KS patients,24 albeit in the midst of other associated cardiac risk factors (increased weight, BMI, waist circumference, LDL cholesterol, triglycerides, CRP, fasting insulin and glucose and decreased HDL cholesterol). Likewise, the biological active high molecular weight subform of adiponectin was nonsuppressed.86 Although speculative, it seems possible that hypogonadism in KS contributes to development of the metabolic syndrome and increases numerous cardiac risk factors, but also protects against ischemic heart disease by increasing levels of adiponectin in concert with nonelevated blood pressure (Figure 1). This hypothesis is supported by the finding of decreased mortality risk from ischemic heart disease in KS patients,10 but findings from recent epidemiological studies in non-KS are contradictory and have demonstrated higher cardiovascular disease risk in men both in untreated hypogonadal men as well as in hypogonadal men after testosterone treatment.87,88 Thus, current knowledge supports the concept that multiple factors are involved in the development of hypogonadal metabolic dysfunction,89 and there is reason to believe that testosterone treatment of different hypogonadal populations with regard to age and etiology affects metabolism and body composition differently.90 Therefore, it is uncertain whether hypogonadal males and KS patients are comparable or whether genetic factors related to KS add another layer of complexity.
Figure 1.
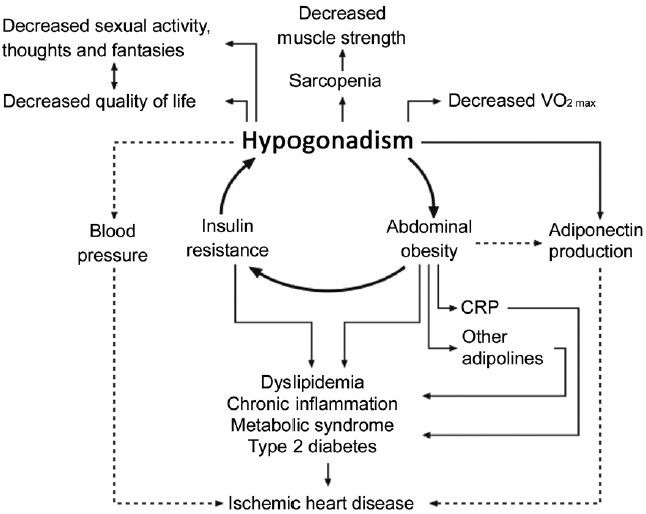
The vicious circle of hypogonadism – abdominal obesity – insulin resistance in Klinefelter syndrome, with secondary consequences. Solid arrows indicates promotion, broken arrows indicates inhibition. VO2max is a measure of maximal oxygen consumption, a measure of physical fitness. This figure is reproduced from Bojesen et al.7 with permission.
Collectively, substantial evidence points toward a dramatically increased risk of diabetes and metabolic syndrome in KS. Current knowledge, however, does not support hormone replacement therapy of KS patients with the primary aim of improving insulin sensitivity, but such effects may occur indirectly through favorable effects on body composition, resting energy expenditure and physical fitness. Unfortunately, prospective studies addressing these issues are not at hand in KS patients, but are deemed necessary to prove the postulated efficacy of testosterone therapy in preventing the occurrence of the metabolic syndrome and to guide clinicians in the clinical care of these patients.
BONES, OSTEOPOROSIS AND FRACTURE-RISK
Hypogonadism in both females and males are well known causes of low bone mineral density (BMD) and osteoporosis. In males, both testosterone and estrogen play a fundamental role in accretion and maintenance of bone mass. The androgen receptor (AR) is expressed in many bone cells, and testosterone is important in both bone growth and bone maintenance, and the effect of testosterone is both direct through AR and indirect through aromatisation to estrogens.91 During puberty, testosterone (together with estrogen) is very important for periostal bone formation91 to achieve peak BMD in the early twenties. In adulthood, testosterone is important for trabecular bone maintenance.91 In KS, substantial evidence for a general decrease in BMD has emerged during the past decades. Apart from one study,92 all studies have shown decreased BMD93,94,95,96 including recent studies on large cohorts of KS patients using modern DEXA scanning technology to measure bone density.61,97 These studies demonstrate that KS patients have decreased BMD at all investigated sites (Spine, hip and forearm), with a substantial proportion (>40%) having osteoporosis or osteopenia.97,98 The decreased BMD may not be present in childhood, but develops after puberty indicated by a study in prepubertal KS boys with normal lumbar BMD.60 The clinical importance of the reduced BMD is reflected in epidemiological studies describing an increased morbidity risk and mortality from fractures in KS patients.8,9 The role of hypogonadism (testosterone) in the development of low BMD has not been fully elucidated; no direct association of testosterone and BMD in KS has so far been described,61,97 but positive associations between BMD, muscle strength (an indirect effect of testosterone) and 25OH-vitamin D have been described.61
Longitudinal studies on KS patients regarding the effect of testosterone treatment on BMD have reported contradicting results; one study showing a positive effect if testosterone treatment was initiated before the age of 20 years,99 while another study showed a rather high prevalence of low BMD despite testosterone treatment.98
The role of estrogen in KS has not been investigated, but estrogen levels have been reported to be increased23 or normal,24,97 and the E/T relation is often described as elevated, compared with normal men. The high or normal to high level of estrogen may protect against excessive bone loss in KS.
The AR, and the role of the CAG repeat length polymorphism in the AR, has been quite extensively studied in KS. While the first study by Zitzmann et al. showed pronounced effect of this polymorphism on both anthropometric, pharmacogenetic and social measures, and a tendency for a nonrandom inactivation of the shortest allele (the most testosterone active allele),100 subsequent studies were not able to confirm these findings to the same extent. Two studies on BMD did not reveal associations between the length of the CAG repeat and BMD, and did not find the same tendency for a nonrandom inactivation pattern.97,101
It is important to stress that all results are based on cross-sectional studies, and neither longitudinal studies in KS examining the role of pubertal hormone increments on developing bones, nor RCTs evaluating the effect of early testosterone treatment have been published. Although no evidence exists, it is plausible that a diminished testosterone production in puberty and longstanding low- or sub-normal testosterone production hereafter may cause the unfavorable low BMD, which ultimately may cause the increased morbidity and mortality from fractures seen in KS. And although no substantial evidence exists for a positive effect of testosterone treatment in KS, we recommend that hormone replacement therapy with adequate doses of testosterone should be instituted early in puberty, to secure a proper development of bone and muscle bulk, and to prevent the later consequences of low BMD.
TESTOSTERONE SUBSTITUTION
Testosterone treatment in KS patients is recommended by most endocrinologists, but clear evidence for an effect of treatment is missing, since no randomized placebo controlled trials on testosterone treatment in KS has ever been published. A few nonrandomized studies have demonstrated some positive effects. Testosterone treatment in 30 KS patients had positive effects in more than 75% of men, including improvements in endurance and strength, fatigue, sleep, concentration and learning ability, mood, irritability and relations with other, although the methods of recording symptoms was not standardized.102 In another study using transdermal testosterone patches, improved sexual function, increased libido and decreased fatigue were recorded in 13 KS patients.103 Testosterone treatment in hypogonadal elderly men is associated with a possible range of benefits including improved sexual function, increased lean body mass and reduced fat mass and improved BMD.104 The general assumption is that the effects of testosterone treatment on hypogonadal males is equivalent in KS and there is a general consensus that testosterone treatment should be offered to the majority of patients with KS, starting from the peripubertal period to secure an optimal masculine development of sexual characteristics, muscle bulk and bone structure and in order to prevent the long-term negative consequences of hypogonadism.7
Several testosterone preparations for oral, transdermal and intramuscular administration with different costs and pharmacokinetics are available and are listed in Table 1.
Table 1.
Summary of different testosterone preparations
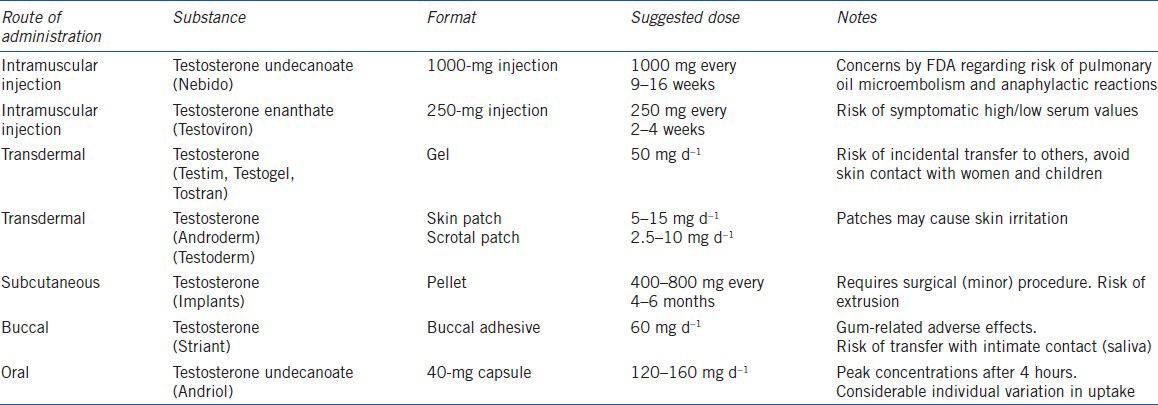
The oral route can be administered by undecanoated testosterone, being lipofile and therefore partly absorbed by the lymphatic system and bypassing the initial metabolism in the liver, decreasing the liver toxicity of oral testosterone.104 Oral administration should be dosed two to three times daily (40 mg each). Oral administration gives a peak concentration after about 4 hours but there is a considerable variability in the same individual on different days.105 Transdermal gel preparations containing 50, 75 or 100 mg testosterone is widely used with an absorption rate of about 10% within 24 hours. Patches is also available but is shown inferior to gel in normalizing serum testosterone and may cause skin irritation.106,107 Transdermal administration, bypasses the first pass metabolism in the lever, and gives a steady and physiological concentration but with an inter-individual variation. For testosterone gel the first few 4–6 hours after dermal administration, the patient should remain dry and avoid skin contact with especially females and children to avoid incidental transfer of testosterone to others.108 Transbuccal administration provides the absorption of testosterone through the oral mucosa, avoiding intestinal adsorption and liver inactivation, with main disadvantage being adverse gum-related event in 16% of treated men and concerns for transfer of testosterone via salvia in intimate contact.105,109 Testosterone enanthate or cypionate for intramuscular injection are the least costly and the preparation with the longest historical use. It can be administered intramuscular every 2–3 weeks and have serum peak level after 2–3 days and serum trough testosterone levels about 10 days after injection. Increasing the dose gives a greater peak and longer action time but the patients may experience ‘highs and lows’ symptoms.109 The long acting testosterone undecanoate preparation (1000 mg oil emulsion for intramuscular injection) is usually administered every 12 weeks but has recently raised concerns by Food and Drug Administration (FDA) about pulmonary oil microembolism and anaphylactic reactions and is therefore not approved in the USA.104 Testosterone pellets is also available but have about 1 month to peak, sustain 3–6 months and requires surgical incision. The pellets may extrude spontaneously.109
Monitoring of treatment should be based mainly on clinical effects (including libido, ejaculate volume, body hair growth, muscle mass and energy level), but should also be aimed at possible adverse effects. This is complemented with biochemical monitoring of serum testosterone (either steady state or trough concentrations depending on the formulation), and or LH values, which should be in the normal range.
Monitoring for side effects like increased hemoglobin, sleep apnea, acne and signs of too high or too low testosterone should be part of the routine outpatient program. Treatment of children and adolescents should be monitored carefully, with slow dose increments in order to mimic normal pubertal development. A recently proposed outpatient program110 is shown in Table 2.
Table 2.
Proposed assessment and follow-up program for at patient with Klinefelter syndrome

COMPETING INTERESTS
The authors disclose no financial conflicts of interests.
REFERENCES
- 1.Bojesen A, Juul S, Gravholt CH. Prenatal and postnatal prevalence of Klinefelter syndrome: a national registry study. J Clin Endocrinol Metab. 2003;88:622–6. doi: 10.1210/jc.2002-021491. [DOI] [PubMed] [Google Scholar]
- 2.Herlihy AS, Halliday JL, Cock ML, McLachlan RI. The prevalence and diagnosis rates of Klinefelter syndrome: an Australian comparison. Med J Aust. 2011;194:24–8. doi: 10.5694/j.1326-5377.2011.tb04141.x. [DOI] [PubMed] [Google Scholar]
- 3.Coffee B, Keith K, Albizua I, Malone T, Mowrey J, et al. Incidence of fragile X syndrome by newborn screening for methylated FMR1 DNA. Am J Hum Genet. 2009;85:503–14. doi: 10.1016/j.ajhg.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klinefelter HF, Reifenstein EC, Albright F. Syndrome characterized by gynecomastia, aspermatogenesis without a-leydigsm and increased secretion of follicle-stimulating hormone. J Clin Endocrinol Metab. 1942:615–22. [Google Scholar]
- 5.Smyth CM, Bremner WJ. Klinefelter syndrome. Arch Intern Med. 1998;158:1309–14. doi: 10.1001/archinte.158.12.1309. [DOI] [PubMed] [Google Scholar]
- 6.Simpson JL, de la CF, Swerdloff RS, Samango-Sprouse C, Skakkebaek NE, et al. Klinefelter syndrome: expanding the phenotype and identifying new research directions. Genet Med. 2003;5:460–8. doi: 10.1097/01.gim.0000095626.54201.d0. [DOI] [PubMed] [Google Scholar]
- 7.Bojesen A, Gravholt CH. Klinefelter syndrome in clinical practice. Nat Clin Pract Urol. 2007;4:192–204. doi: 10.1038/ncpuro0775. [DOI] [PubMed] [Google Scholar]
- 8.Bojesen A, Juul S, Birkebaek NH, Gravholt CH. Morbidity in Klinefelter Syndrome: a Danish register study based on hospital discharge diagnoses. J Clin Endocrinol Metab. 2006;91:1254–60. doi: 10.1210/jc.2005-0697. [DOI] [PubMed] [Google Scholar]
- 9.Bojesen A, Juul S, Birkebaek N, Gravholt CH. Increased mortality in klinefelter syndrome. J Clin Endocrinol Metab. 2004;89:3830–4. doi: 10.1210/jc.2004-0777. [DOI] [PubMed] [Google Scholar]
- 10.Swerdlow AJ, Higgins CD, Schoemaker MJ, Wright AF, Jacobs PA. Mortality in patients with Klinefelter syndrome in Britain: a cohort study. J Clin Endocrinol Metab. 2005;90:6516–22. doi: 10.1210/jc.2005-1077. [DOI] [PubMed] [Google Scholar]
- 11.Bojesen A, Stochholm K, Juul S, Gravholt CH. Socioeconomic trajectories affect mortality in Klinefelter syndrome. J Clin Endocrinol Metab. 2011;96:2098–104. doi: 10.1210/jc.2011-0367. [DOI] [PubMed] [Google Scholar]
- 12.Stochholm K, Bojesen A, Jensen AS, Juul S, Gravholt CH. Criminality in men with Klinefelter's syndrome and XYY syndrome: a cohort study. BMJ Open. 2012;2:e000650. doi: 10.1136/bmjopen-2011-000650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aksglaede L, Wikstrom M, Meyts ER, Dunkel L, Skakkebaek E, et al. Natural history of seminiferous tubule degeneration in Klinefelter syndrome. Hum Reprod Update. 2005;12:39–48. doi: 10.1093/humupd/dmi039. [DOI] [PubMed] [Google Scholar]
- 14.Ratcliffe SG, Butler GE, Jones M. Edinburgh study of growth and development of children with sex chromosome abnormalities. IV. Birth Defects Orig Artic Ser. 1990;26:1–44. [PubMed] [Google Scholar]
- 15.Lahlou N, Fennoy I, Ross JL, Bouvattier C, Roger M. Clinical and hormonal status of infants with nonmosaic XXY karyotype. Acta Paediatr. 2011;100:824–9. doi: 10.1111/j.1651-2227.2011.02280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fennoy I. Testosterone and the child (0-12 years) with Klinefelter syndrome (47XXY): a review. Acta Paediatr. 2011;100:846–50. doi: 10.1111/j.1651-2227.2011.02184.x. [DOI] [PubMed] [Google Scholar]
- 17.Lahlou N, Fennoy I, Carel JC, Roger M. Inhibin B and anti-Mullerian hormone, but not testosterone levels, are normal in infants with nonmosaic Klinefelter syndrome. J Clin Endocrinol Metab. 2004;89:1864–8. doi: 10.1210/jc.2003-031624. [DOI] [PubMed] [Google Scholar]
- 18.Aksglaede L, Petersen JH, Main KM, Skakkebaek NE, Juul A. High normal testosterone levels in infants with non-mosaic Klinefelter's syndrome. Eur J Endocrinol. 2007;157:345–50. doi: 10.1530/EJE-07-0310. [DOI] [PubMed] [Google Scholar]
- 19.Cabrol S, Ross JL, Fennoy I, Bouvattier C, Roger M, et al. Assessment of Leydig and Sertoli cell functions in infants with nonmosaic Klinefelter syndrome: insulin-like peptide 3 levels are normal and positively correlated with LH levels. J Clin Endocrinol Metab. 2011;96:E746–53. doi: 10.1210/jc.2010-2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Samango-Sprouse CA, Sadeghin T, Mitchell FL, Dixon T, Stapleton E, et al. Positive effects of short course androgen therapy on the neurodevelopmental outcome in boys with 47, XXY syndrome at 36 and 72 months of age. Am J Med Genet A. 2013;161:501–8. doi: 10.1002/ajmg.a.35769. [DOI] [PubMed] [Google Scholar]
- 21.Christiansen P, Andersson AM, Skakkebaek NE. Longitudinal studies of inhibin B levels in boys and young adults with Klinefelter syndrome. J Clin Endocrinol Metab. 2003;88:888–91. doi: 10.1210/jc.2002-021379. [DOI] [PubMed] [Google Scholar]
- 22.Aksglaede L, Wikstrom AM, Rajpert-De Meyts E, Dunkel L, Skakkebaek NE, et al. Natural history of seminiferous tubule degeneration in Klinefelter syndrome. Hum Reprod Update. 2006;12:39–48. doi: 10.1093/humupd/dmi039. [DOI] [PubMed] [Google Scholar]
- 23.Lanfranco F, Kamischke A, Zitzmann M, Nieschlag E. Klinefelter's syndrome. Lancet. 2004;364:273–83. doi: 10.1016/S0140-6736(04)16678-6. [DOI] [PubMed] [Google Scholar]
- 24.Bojesen A, Kristensen K, Birkebaek NH, Fedder J, Mosekilde L, et al. The metabolic syndrome is frequent in Klinefelter's syndrome and is associated with abdominal obesity and hypogonadism. Diabetes Care. 2006;29:1591–8. doi: 10.2337/dc06-0145. [DOI] [PubMed] [Google Scholar]
- 25.Laron Z, Dickerman Z, Zamir R, Galatzer A. Paternity in Klinefelter's syndrome: a case report. Arch Androl. 1982;8:149–51. doi: 10.3109/01485018208987032. [DOI] [PubMed] [Google Scholar]
- 26.Aksglaede L, Juul A. Testicular function and fertility in men with Klinefelter syndrome: a review. Eur J Endocrinol. 2013;168:R67–76. doi: 10.1530/EJE-12-0934. [DOI] [PubMed] [Google Scholar]
- 27.Schiff JD, Palermo GD, Veeck LL, Goldstein M, Rosenwaks Z, et al. Success of testicular sperm extraction [corrected] and intracytoplasmic sperm injection in men with Klinefelter syndrome. J Clin Endocrinol Metab. 2005;90:6263–7. doi: 10.1210/jc.2004-2322. [DOI] [PubMed] [Google Scholar]
- 28.Cruger D, Toft B, Agerholm I, Fedder J, Hald F, et al. Birth of a healthy girl after ICSI with ejaculated spermatozoa from a man with non-mosaic Klinefelter's syndrome: case report. Hum Reprod. 2001;16:1909–11. doi: 10.1093/humrep/16.9.1909. [DOI] [PubMed] [Google Scholar]
- 29.Komori S, Horiuchi I, Hamada Y, Hasegawa A, Kasumi H, et al. Birth of healthy neonates after intracytoplasmic injection of ejaculated or testicular spermatozoa from men with nonmosaic Klinefelter's syndrome: a report of 2 cases. J Reprod Med. 2004;49:126–30. [PubMed] [Google Scholar]
- 30.Staessen C, Tournaye H, Van Assche E, Michiels A, Van Landuyt L, et al. PGD in 47, XXY Klinefelter's syndrome patients. Hum Reprod Update. 2003;9:319–30. doi: 10.1093/humupd/dmg029. [DOI] [PubMed] [Google Scholar]
- 31.Ron-El R, Strassburger D, Gelman-Kohan S, Friedler S, Raziel A, et al. A 47, XXY fetus conceived after ICSI of spermatozoa from a patient with non-mosaic Klinefelter's syndrome: case report. Hum Reprod. 2000;15:1804–6. doi: 10.1093/humrep/15.8.1804. [DOI] [PubMed] [Google Scholar]
- 32.Bender BG, Linden MG, Robinson A. Neuropsychological impairment in 42 adolescents with sex chromosome abnormalities. Am J Med Genet. 1993;48:169–73. doi: 10.1002/ajmg.1320480312. [DOI] [PubMed] [Google Scholar]
- 33.Ratcliffe S. Long-term outcome in children of sex chromosome abnormalities. Arch Dis Child. 1999;80:192–5. doi: 10.1136/adc.80.2.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ross JL, Roeltgen DP, Stefanatos G, Benecke R, Zeger MP, et al. Cognitive and motor development during childhood in boys with Klinefelter syndrome. Am J Med Genet A. 2008;146A:708–19. doi: 10.1002/ajmg.a.32232. [DOI] [PubMed] [Google Scholar]
- 35.Fales CL, Knowlton BJ, Holyoak KJ, Geschwind DH, Swerdloff RS, et al. Working memory and relational reasoning in Klinefelter syndrome. J Int Neuropsychol Soc. 2003;9:839–46. doi: 10.1017/S1355617703960036. [DOI] [PubMed] [Google Scholar]
- 36.Graham JM, Jr, Bashir AS, Stark RE, Silbert A, Walzer S. Oral and written language abilities of XXY boys: implications for anticipatory guidance. Pediatrics. 1988;81:795–806. [PubMed] [Google Scholar]
- 37.Kompus K, Westerhausen R, Nilsson LG, Hugdahl K, Jongstra S, et al. Deficits in inhibitory executive functions in Klinefelter (47, XXY) syndrome. Psychiatry Res. 2011;189:135–40. doi: 10.1016/j.psychres.2011.02.028. [DOI] [PubMed] [Google Scholar]
- 38.Lee NR, Wallace GL, Clasen LS, Lenroot RK, Blumenthal JD, et al. Executive function in young males with klinefelter (XXY) syndrome with and without comorbid attention-deficit/hyperactivity disorder. J Int Neuropsychol Soc. 2011:1–9. doi: 10.1017/S1355617711000312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Temple CM, Sanfilippo PM. Executive skills in Klinefelter's syndrome. Neuropsychologia. 2003;41:1547–59. doi: 10.1016/s0028-3932(03)00061-7. [DOI] [PubMed] [Google Scholar]
- 40.Bender BG, Harmon RJ, Linden MG, Robinson A. Psychosocial adaptation of 39 adolescents with sex chromosome abnormalities. Pediatrics. 1995;96:302–8. [PubMed] [Google Scholar]
- 41.Boks MP, de Vette MH, Sommer IE, van Rijn S, Giltay JC, et al. Psychiatric morbidity and X-chromosomal origin in a Klinefelter sample. Schizophr Res. 2007;93:399–402. doi: 10.1016/j.schres.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 42.Bruining H, Swaab H, Kas M, van Engeland H. Psychiatric characteristics in a self-selected sample of boys with Klinefelter syndrome. Pediatrics. 2009;123:e865–70. doi: 10.1542/peds.2008-1954. [DOI] [PubMed] [Google Scholar]
- 43.Bryant DM, Hoeft F, Lai S, Lackey J, Roeltgen D, et al. Neuroanatomical phenotype of Klinefelter syndrome in childhood: a voxel-based morphometry study. J Neurosci. 2011;31:6654–60. doi: 10.1523/JNEUROSCI.5899-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Giedd JN, Clasen LS, Wallace GL, Lenroot RK, Lerch JP, et al. XXY (Klinefelter syndrome): a pediatric quantitative brain magnetic resonance imaging case-control study. Pediatrics. 2007;119:e232–40. doi: 10.1542/peds.2005-2969. [DOI] [PubMed] [Google Scholar]
- 45.Shen D, Liu D, Liu H, Clasen L, Giedd J, et al. Automated morphometric study of brain variation in XXY males. Neuroimage. 2004;23:648–53. doi: 10.1016/j.neuroimage.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 46.Patwardhan AJ, Eliez S, Bender B, Linden MG, Reiss AL. Brain morphology in Klinefelter syndrome: extra X chromosome and testosterone supplementation. Neurology. 2000;54:2218–23. doi: 10.1212/wnl.54.12.2218. [DOI] [PubMed] [Google Scholar]
- 47.Delisi LE, Maurizio AM, Svetina C, Ardekani B, Szulc K, et al. Klinefelter's syndrome (XXY) as a genetic model for psychotic disorders. Am J Med Genet B Neuropsychiatr Genet. 2005;135:15–23. doi: 10.1002/ajmg.b.30163. [DOI] [PubMed] [Google Scholar]
- 48.Knickmeyer RC, Baron-Cohen S. Fetal testosterone and sex differences in typical social development and in autism. J Child Neurol. 2006;21:825–45. doi: 10.1177/08830738060210101601. [DOI] [PubMed] [Google Scholar]
- 49.Skakkebæk A, Gravholt CH, Rasmussen PM, Bojesen A, Jensen JS, et al. Neuroanatomical correlates of Klinefelter syndrome studied in relation to the neuropsychological profile. NeuroImage: Clinical. 2013 doi: 10.1016/j.nicl.2013.10.013. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Merke DP, Fields JD, Keil MF, Vaituzis AC, Chrousos GP, et al. Children with classic congenital adrenal hyperplasia have decreased amygdala volume: potential prenatal and postnatal hormonal effects. J Clin Endocrinol Metab. 2003;88:1760–5. doi: 10.1210/jc.2002-021730. [DOI] [PubMed] [Google Scholar]
- 51.Warwick MM, Doody GA, Lawrie SM, Kestelman JN, Best JJ, et al. Volumetric magnetic resonance imaging study of the brain in subjects with sex chromosome aneuploidies. J Neurol Neurosurg Psychiatry. 1999;66:628–32. doi: 10.1136/jnnp.66.5.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lombardo MV, Ashwin E, Auyeung B, Chakrabarti B, Taylor K, et al. Fetal testosterone influences sexually dimorphic gray matter in the human brain. J Neurosci. 2012;32:674–80. doi: 10.1523/JNEUROSCI.4389-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Annell AL, Gustavson KH, Tenstam J. Symtomatology in schoolboys with positive sex chromatin (the klinefelter syndrome) Acta Psychiatr Scand. 1970;46:71–80. doi: 10.1111/j.1600-0447.1970.tb02102.x. [DOI] [PubMed] [Google Scholar]
- 54.Nielsen J, Pelsen B. Follow-up 20 years later of 34 Klinefelter males with karyotype 47, XXY and 16 hypogonadal males with karyotype 46, XY. Hum Genet. 1987;77:188–92. doi: 10.1007/BF00272390. [DOI] [PubMed] [Google Scholar]
- 55.Skakkebæk AP, Bojesen A, Kristensen MK, Hertz JM, Østergaard JR, et al. Personality, psychological distress and autism traits-key players in the neuropsychological phenotype of Klinefelter syndrome. Submitted 2013 [Google Scholar]
- 56.Itti E, Gaw Gonzalo IT, Pawlikowska-Haddal A, Boone KB, Mlikotic A, et al. The structural brain correlates of cognitive deficits in adults with Klinefelter's syndrome. J Clin Endocrinol Metab. 2006;91:1423–7. doi: 10.1210/jc.2005-1596. [DOI] [PubMed] [Google Scholar]
- 57.Tsai EC, Boyko EJ, Leonetti DL, Fujimoto WY. Low serum testosterone level as a predictor of increased visceral fat in Japanese-American men. Int J Obes Relat Metab Disord. 2000;24:485–91. doi: 10.1038/sj.ijo.0801183. [DOI] [PubMed] [Google Scholar]
- 58.Marin P, Holmang S, Jonsson L, Sjostrom L, Kvist H, et al. The effects of testosterone treatment on body composition and metabolism in middle-aged obese men. Int J Obes Relat Metab Disord. 1992;16:991–7. [PubMed] [Google Scholar]
- 59.Hamilton EJ, Gianatti E, Strauss BJ, Wentworth J, Lim-Joon D, et al. Increase in visceral and subcutaneous abdominal fat in men with prostate cancer treated with androgen deprivation therapy. Clin Endocrinol (Oxf) 2011;74:377–83. doi: 10.1111/j.1365-2265.2010.03942.x. [DOI] [PubMed] [Google Scholar]
- 60.Aksglaede L, Molgaard C, Skakkebaek NE, Juul A. Normal bone mineral content but unfavourable muscle/fat ratio in Klinefelter syndrome. Arch Dis Child. 2008;93:30–4. doi: 10.1136/adc.2007.120675. [DOI] [PubMed] [Google Scholar]
- 61.Bojesen A, Birkebaek N, Kristensen K, Heickendorff L, Mosekilde L, et al. Bone mineral density in Klinefelter syndrome is reduced and primarily determined by muscle strength and resorptive markers, but not directly by testosterone. Osteoporos Int. 2010;22:1441–50. doi: 10.1007/s00198-010-1354-7. [DOI] [PubMed] [Google Scholar]
- 62.Page ST, Amory JK, Bowman FD, Anawalt BD, Matsumoto AM, et al. Exogenous testosterone (T) alone or with finasteride increases physical performance, grip strength, and lean body mass in older men with low serum T. J Clin Endocrinol Metab. 2005;90:1502–10. doi: 10.1210/jc.2004-1933. [DOI] [PubMed] [Google Scholar]
- 63.Sheffield-Moore M, Dillon EL, Casperson SL, Gilkison CR, Paddon-Jones D, et al. A randomized pilot study of monthly cycled testosterone replacement or continuous testosterone replacement versus placebo in older men. J Clin Endocrinol Metab. 2011;96:E1831–7. doi: 10.1210/jc.2011-1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Srinivas-Shankar U, Roberts SA, Connolly MJ, O’Connell MD, Adams JE, et al. Effects of testosterone on muscle strength, physical function, body composition, and quality of life in intermediate-frail and frail elderly men: a randomized, double-blind, placebo-controlled study. J Clin Endocrinol Metab. 2010;95:639–50. doi: 10.1210/jc.2009-1251. [DOI] [PubMed] [Google Scholar]
- 65.Ishikawa T, Yamaguchi K, Kondo Y, Takenaka A, Fujisawa M. Metabolic syndrome in men with Klinefelter's syndrome. Urology. 2008;71:1109–13. doi: 10.1016/j.urology.2008.01.051. [DOI] [PubMed] [Google Scholar]
- 66.Jiang-Feng M, Hong-Li X, Xue-Yan W, Min N, Shuang-Yu L, et al. Prevalence and risk factors of diabetes in patients with Klinefelter syndrome: a longitudinal observational study. Fertil Steril. 2012;98:1331–5. doi: 10.1016/j.fertnstert.2012.07.1122. [DOI] [PubMed] [Google Scholar]
- 67.Yesilova Z, Oktenli C, Sanisoglu SY, Musabak U, Cakir E, et al. Evaluation of insulin sensitivity in patients with klinefelter's syndrome: a hyperinsulinemic euglycemic clamp study. Endocrine. 2005;27:11–6. doi: 10.1385/ENDO:27:1:011. [DOI] [PubMed] [Google Scholar]
- 68.Bardsley MZ, Falkner B, Kowal K, Ross JL. Insulin resistance and metabolic syndrome in prepubertal boys with Klinefelter syndrome. Acta Paediatr. 2011;100:866–70. doi: 10.1111/j.1651-2227.2011.02161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Laaksonen DE, Niskanen L, Punnonen K, Nyyssonen K, Tuomainen TP, et al. Testosterone and sex hormone-binding globulin predict the metabolic syndrome and diabetes in middle-aged men. Diabetes Care. 2004;27:1036–41. doi: 10.2337/diacare.27.5.1036. [DOI] [PubMed] [Google Scholar]
- 70.Stellato RK, Feldman HA, Hamdy O, Horton ES, McKinlay JB. Testosterone, sex hormone-binding globulin, and the development of type 2 diabetes in middle-aged men: prospective results from the Massachusetts male aging study. Diabetes Care. 2000;23:490. doi: 10.2337/diacare.23.4.490. [DOI] [PubMed] [Google Scholar]
- 71.Grossmann M, Thomas MC, Panagiotopoulos S, Sharpe K, Macisaac RJ, et al. Low testosterone levels are common and associated with insulin resistance in men with diabetes. J Clin Endocrinol Metab. 2008;93:1834–40. doi: 10.1210/jc.2007-2177. [DOI] [PubMed] [Google Scholar]
- 72.Grossmann M, Gianatti EJ, Zajac JD. Testosterone and type 2 diabetes. Curr Opin Endocrinol Diabetes Obes. 2010;17:247–56. doi: 10.1097/MED.0b013e32833919cf. [DOI] [PubMed] [Google Scholar]
- 73.Selvin E, Feinleib M, Zhang L, Rohrmann S, Rifai N, et al. Androgens and diabetes in men: results from the Third National Health and Nutrition Examination Survey (NHANES III) Diabetes Care. 2007;30:234–8. doi: 10.2337/dc06-1579. [DOI] [PubMed] [Google Scholar]
- 74.Heufelder AE, Saad F, Bunck MC, Gooren L. Fifty-two-week treatment with diet and exercise plus transdermal testosterone reverses the metabolic syndrome and improves glycemic control in men with newly diagnosed type 2 diabetes and subnormal plasma testosterone. J Androl. 2009;30:726–33. doi: 10.2164/jandrol.108.007005. [DOI] [PubMed] [Google Scholar]
- 75.Kapoor D, Goodwin E, Channer KS, Jones TH. Testosterone replacement therapy improves insulin resistance, glycaemic control, visceral adiposity and hypercholesterolaemia in hypogonadal men with type 2 diabetes. Eur J Endocrinol. 2006;154:899–906. doi: 10.1530/eje.1.02166. [DOI] [PubMed] [Google Scholar]
- 76.Gopal RA, Bothra N, Acharya SV, Ganesh HK, Bandgar TR, et al. Treatment of hypogonadism with testosterone in patients with type 2 diabetes mellitus. Endocr Pract. 2010;16:570–6. doi: 10.4158/EP09355.OR. [DOI] [PubMed] [Google Scholar]
- 77.Corrales JJ, Burgo RM, Garca-Berrocal B, Almeida M, Alberca I, et al. Partial androgen deficiency in aging type 2 diabetic men and its relationship to glycemic control. Metabolism. 2004;53:666–72. doi: 10.1016/j.metabol.2003.12.016. [DOI] [PubMed] [Google Scholar]
- 78.Marin P, Krotkiewski M, Bjorntorp P. Androgen treatment of middle-aged, obese men: effects on metabolism, muscle and adipose tissues. Eur J Med. 1992;1:329–36. [PubMed] [Google Scholar]
- 79.Yialamas MA, Dwyer AA, Hanley E, Lee H, Pitteloud N, et al. Acute sex steroid withdrawal reduces insulin sensitivity in healthy men with idiopathic hypogonadotropic hypogonadism. J Clin Endocrinol Metab. 2007;92:4254–9. doi: 10.1210/jc.2007-0454. [DOI] [PubMed] [Google Scholar]
- 80.Lapauw B, Ouwens M, t Hart LM, Wuyts B, Holst JJ, et al. Sex steroids affect triglyceride handling, glucose-dependent insulinotropic polypeptide, and insulin sensitivity: a 1-week randomized clinical trial in healthy young men. Diabetes Care. 2010;33:1831–3. doi: 10.2337/dc10-0515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Laaksonen DE, Niskanen L, Punnonen K, Nyyssonen K, Tuomainen TP, et al. Sex hormones, inflammation and the metabolic syndrome: a population-based study. Eur J Endocrinol. 2003;149:601–8. doi: 10.1530/eje.0.1490601. [DOI] [PubMed] [Google Scholar]
- 82.Nishizawa H, Shimomura I, Kishida K, Maeda N, Kuriyama H, et al. Androgens decrease plasma adiponectin, an insulin-sensitizing adipocyte-derived protein. Diabetes. 2002;51:2734–41. doi: 10.2337/diabetes.51.9.2734. [DOI] [PubMed] [Google Scholar]
- 83.Page ST, Herbst KL, Amory JK, Coviello AD, Anawalt BD, et al. Testosterone administration suppresses adiponectin levels in men. J Androl. 2005;26:85–92. [PubMed] [Google Scholar]
- 84.Xu A, Chan KW, Hoo RL, Wang Y, Tan KC, et al. Testosterone selectively reduces the high molecular weight form of adiponectin by inhibiting its secretion from adipocytes. J Biol Chem. 2005;280:18073–80. doi: 10.1074/jbc.M414231200. [DOI] [PubMed] [Google Scholar]
- 85.Lanfranco F, Zitzmann M, Simoni M, Nieschlag E. Serum adiponectin levels in hypogonadal males: influence of testosterone replacement therapy. Clin Endocrinol (Oxf) 2004;60:500–7. doi: 10.1111/j.1365-2265.2004.02007.x. [DOI] [PubMed] [Google Scholar]
- 86.Host C, Bojesen A, Frystyk J, Flyvbjerg A, Christiansen JS, et al. Effect of sex hormone treatment on circulating adiponectin and subforms in Turner and Klinefelter syndrome. Eur J Clin Invest. 2010;40:211–9. doi: 10.1111/j.1365-2362.2009.02250.x. [DOI] [PubMed] [Google Scholar]
- 87.Basaria S, Coviello AD, Travison TG, Storer TW, Farwell WR, et al. Adverse events associated with testosterone administration. N Engl J Med. 2010;363:109–22. doi: 10.1056/NEJMoa1000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hyde Z, Norman PE, Flicker L, Hankey GJ, McCaul KA, et al. Elevated LH predicts ischaemic heart disease events in older men: the Health in Men Study. Eur J Endocrinol. 2011;164:569–77. doi: 10.1530/EJE-10-1063. [DOI] [PubMed] [Google Scholar]
- 89.Traish AM, Miner MM, Morgentaler A, Zitzmann M. Testosterone deficiency. Am J Med. 2011;124:578–87. doi: 10.1016/j.amjmed.2010.12.027. [DOI] [PubMed] [Google Scholar]
- 90.Zitzmann M. Testosterone deficiency, insulin resistance and the metabolic syndrome. Nat Rev Endocrinol. 2009;5:673–81. doi: 10.1038/nrendo.2009.212. [DOI] [PubMed] [Google Scholar]
- 91.Sinnesael M, Claessens F, Boonen S, Vanderschueren D. Novel insights in the regulation and mechanism of androgen action on bone. Curr Opin Endocrinol Diabetes Obes. 2013;20:240–4. doi: 10.1097/MED.0b013e32835f7d04. [DOI] [PubMed] [Google Scholar]
- 92.Luisetto G, Mastrogiacomo I, Bonanni G, Pozzan G, Botteon S, et al. Bone mass and mineral metabolism in Klinefelter's syndrome. Osteoporos Int. 1995;5:455–61. doi: 10.1007/BF01626608. [DOI] [PubMed] [Google Scholar]
- 93.Foresta C, Ruzza G, Mioni R, Meneghello A, Baccichetti C. Testosterone and bone loss in Klinefelter syndrome. Horm Metab Res. 1983;15:56–7. doi: 10.1055/s-2007-1018630. [DOI] [PubMed] [Google Scholar]
- 94.Choi HR, Lim SK, Lee MS. Site-specific effect of testosterone on bone mineral density in male hypogonadism. J Korean Med Sci. 1995;10:431–5. doi: 10.3346/jkms.1995.10.6.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Smith DA, Walker MS. Changes in plasma steroids and bone density in Klinefelter's syndrome. Calcif Tissue Res. 1977;22(Suppl):225–8. doi: 10.1007/BF02064069. [DOI] [PubMed] [Google Scholar]
- 96.Horowitz M, Wishart JM, O’Loughlin PD, Morris HA, Need AG, et al. Osteoporosis and Klinefelter's syndrome. Clin Endocrinol (Oxf) 1992;36:113–8. doi: 10.1111/j.1365-2265.1992.tb02910.x. [DOI] [PubMed] [Google Scholar]
- 97.Ferlin A, Schipilliti M, Vinanzi C, Garolla A, Di Mambro A, et al. Bone mass in subjects with Klinefelter syndrome: role of testosterone levels and androgen receptor gene CAG polymorphism. J Clin Endocrinol Metab. 2011;96:E739–45. doi: 10.1210/jc.2010-1878. [DOI] [PubMed] [Google Scholar]
- 98.van den Bergh JP, Hermus AR, Spruyt AI, Sweep CG, Corstens FH, et al. Bone mineral density and quantitative ultrasound parameters in patients with Klinefelter's yndrome after long-term testosterone substitution. Osteoporos Int. 2001;12:55–62. doi: 10.1007/s001980170158. [DOI] [PubMed] [Google Scholar]
- 99.Kubler A, Schulz G, Cordes U, Beyer J, Krause U. The influence of testosterone substitution on bone mineral density in patients with Klinefelter's syndrome. Exp Clin Endocrinol. 1992;100:129–32. doi: 10.1055/s-0029-1211192. [DOI] [PubMed] [Google Scholar]
- 100.Zitzmann M, Depenbusch M, Gromoll J, Nieschlag E. X-chromosome inactivation patterns and androgen receptor functionality influence phenotype and social characteristics as well as pharmacogenetics of testosterone therapy in Klinefelter patients. J Clin Endocrinol Metab. 2004;89:6208–17. doi: 10.1210/jc.2004-1424. [DOI] [PubMed] [Google Scholar]
- 101.Bojesen A, Hertz JM, Gravholt CH. Genotype and phenotype in Klinefelter syndrome-impact of androgen receptor polymorphism and skewed X inactivation. Int J Androl. 2011;34:e642–8. doi: 10.1111/j.1365-2605.2011.01223.x. [DOI] [PubMed] [Google Scholar]
- 102.Nielsen J, Pelsen B, Sorensen K. Follow-up of 30 Klinefelter males treated with testosterone. Clin Genet. 1988;33:262–9. doi: 10.1111/j.1399-0004.1988.tb03447.x. [DOI] [PubMed] [Google Scholar]
- 103.Meikle AW, Dobs AS, Arver S, Caramelli KE, Sanders SW, et al. Androgen replacement in the treatment of Klinefelter's syndrome: efficacy and safety of a nonscrotal permeation-enhanced testosterone transdermal system. Endocr Pract. 1998;4:17–22. doi: 10.4158/EP.4.1.17. [DOI] [PubMed] [Google Scholar]
- 104.Lunenfeld B, Arver S, Moncada I, Rees DA, Schulte HM. How to help the aging male. Current approaches to hypogonadism in primary care? Aging Male. 2012;15:187–97. doi: 10.3109/13685538.2012.729110. [DOI] [PubMed] [Google Scholar]
- 105.Bhasin S, Cunningham GR, Hayes FJ, Matsumoto AM, Snyder PJ, et al. Testosterone therapy in men with androgen deficiency syndromes: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2010;95:2536–59. doi: 10.1210/jc.2009-2354. [DOI] [PubMed] [Google Scholar]
- 106.Steidle C, Schwartz S, Jacoby K, Sebree T, Smith T, et al. AA2500 Testosterone Gel Normalizes Androgen Levels in Aging Males with Improvements in Body Composition and Sexual Function. J Clin Endocrinol Metab. 2003;88:2673. doi: 10.1210/jc.2002-021058. [DOI] [PubMed] [Google Scholar]
- 107.McNicholas TA, Dean JD, Mulder H, Carnegie C, Jones NA. A novel testosterone gel formulation normalizes androgen levels in hypogonadal men, with improvements in body composition and sexual function. BJU Int. 2003;91:69–74. doi: 10.1046/j.1464-410x.2003.04016.x. [DOI] [PubMed] [Google Scholar]
- 108.de Ronde W. Hyperandrogenism after transfer of topical testosterone gel: case report and review of published and unpublished studies. Hum Reprod. 2009;24:425–8. doi: 10.1093/humrep/den372. [DOI] [PubMed] [Google Scholar]
- 109.Yin A, Swerdloff R. Treating hypogonadism in younger males. Expert Opin Pharmacother. 2010;11:1529–40. doi: 10.1517/14656561003742947. [DOI] [PubMed] [Google Scholar]
- 110.Groth KA, Skakkebaek A, Host C, Gravholt CH, Bojesen A. Clinical review: Klinefelter syndrome: a clinical update. J Clin Endocrinol Metab. 2012;98:20–30. doi: 10.1210/jc.2012-2382. [DOI] [PubMed] [Google Scholar]


