Abstract
Androgens have potent anabolic effects on skeletal muscle and decline with age in parallel to losses in muscle mass and strength. This loss of muscle mass and function, known as sarcopenia, is the central event in development of frailty, the vulnerable health status that presages adverse outcomes and rapid functional decline in older adults. The potential role of falling androgen levels in the development of frailty and their utility as function promoting therapies in older men has therefore attracted considerable attention. This review summarizes current concepts and definitions in muscle ageing, sarcopenia and frailty, and evaluates recent developments in the study of androgens and frailty. Current evidence from observational and interventional studies strongly supports an effect of androgens on muscle mass in ageing men, but effects on muscle strength and particularly physical function have been less clear. Androgen treatment has been generally well–tolerated in studies of older men, but concerns remain over higher dose treatments and use in populations with high cardiovascular risk. The first trials of selective androgen receptor modulators (SARMs) suggest similar effects on muscle mass and function to traditional androgen therapies in older adults. Important future directions include the use of these agents in combination with exercise training to promote functional ability across different populations of older adults, as well as more focus on the relationships between concurrent changes in hormone levels, body composition and physical function in observational studies.
Keywords: ageing, androgens, body composition, frailty, muscle, physical function, sarcopenia, selective androgen receptor modulators, sex hormones, testosterone
INTRODUCTION
Worldwide populations are ageing. The number of Europeans aged 65 years and over is predicted to almost double over the next 50 years, from 87 million in 2010 to 148 million in 2060.1 Similar trends are occurring throughout the developed and developing world. In this context, understanding ageing, and particularly why some older adults progress more quickly to disability and dependency, has become a leading research priority. Sarcopenia, the loss of muscle mass and function with advancing age, is a central event in the development of frailty, the vulnerable health status that precedes overt disability in older adults.2,3
The etiology of sarcopenia and frailty undoubtedly involves multiple mechanisms, one of which may be the age–related decline in anabolic hormone levels.4,5 Testosterone (T) is the primary androgenic hormone in men and has potent anabolic effects on skeletal muscle.6,7,8 The majority of T in the circulation is bound to albumin or sex hormone binding globulin (SHBG), the remaining unbound fraction is referred to as free T, this fraction combined with the albumin bound fraction can be described as bioavailable T. Total T levels decline modestly with age in men, while free T levels decline more steeply due to a concomitant increase in SHBG.9,10,11 These changes are influenced by health status and potentially modifiable risk factors, most notably obesity and smoking.9,10,12
In this context, the role of T in the development of frailty and in ameliorating this condition has attracted considerable attention. This article reviews current understanding of the anabolic effects of androgens, sarcopenia, frailty, and the preventative and therapeutic potentials of T treatment.
ANDROGEN EFFECTS ON MUSCLE
T supplementation is associated with dose dependent increases in muscle mass and reciprocal decreases in fat mass in young and older men.7,8 The increase in muscle mass is due to hypertrophy of type 1 (slow twitch) and type 2 (fast twitch) muscle fibers.13,14 Correspondingly, T treatment is associated with dose–dependent improvements in muscle strength and power, the product of the force and speed of contraction.15 Androgens, however may not affect other aspects of muscle function including fatigability and specific tension or muscle quality (the ratio of muscle strength to size).15,16
The anabolic effects of androgens are achieved through action on multiple cellular targets.17 T increases satellite cell replication and activation, the number of myonuclei and effects protein metabolism.14,18,19,20,21 In vitro studies suggest androgens modulate the differentiation of pluripotent mesenchymal cells preferentially towards the myogenic rather than the adipogenic lineage.22 Multiple signaling pathways are involved in these androgen–dependent myogenic effects on cellular differentiation and proliferation and muscle protein turnover.17 Androgen receptors, in the satellite cell as well as several other muscle cell types, are upregulated by androgens.23 Androgens binding to the androgen receptor promote translocation of β–catenin to the cell nucleus of mesenchymal pluripotent cells, leading to myogenic differentiation via follistatin signaling and inhibition of transforming growth factor–β.24 Similar mechanisms may be involved in androgen effects on satellite cell proliferation.25 Several studies also indicate a role for notch signaling in mediating androgen effects on satellite cell activation and proliferation.14,26,27 Other cellular mediators may include stimulation of protein synthesis via the Akt/4 mammalian target of rapamycin (mTOR) pathway and inhibition of forkhead box protein (FoxO) mediated protein breakdown, as well as upregulation of intramuscular insulin–like growth factor–1 signaling.28,29,30 The relative importance of these different subcellular mechanisms and their interaction with each other are currently not well–defined.
SARCOPENIA–MUSCLE AGEING
The loss of muscle mass is one of the most striking characteristics of the ageing process. Longitudinal estimates from the Health Aging and Body Composition Study (Health ABC), a large cohort of high functioning men and women aged 70–79 years at baseline, suggest an average decline in thigh muscle cross–sectional area of 6.8% in men and 3.2% in women over 5 years.31 The rate of decline may vary according to baseline fitness and body composition, as well as concurrent changes in body weight.31,32,33 The parallel loss of muscle strength greatly exceeds this decline in lean mass.31
The loss in lean mass is due to a reduction in the number of muscle fibers and a decrease in size of the remaining fibers.34 The primary mechanism of fiber loss is believed to be a progressive loss of limb motor neurons.35 Ageing is also accompanied by further changes in muscle morphology including an accumulation of shrunken muscle fibers and a clustering of fiber types, as well as an increase in muscle fat infiltration.36 This morphological degeneration partially explains the disproportionately greater loss in muscle strength with increasing age. However, changes in neural coordination and muscle fiber specific factors can also be relevant (for review see37).
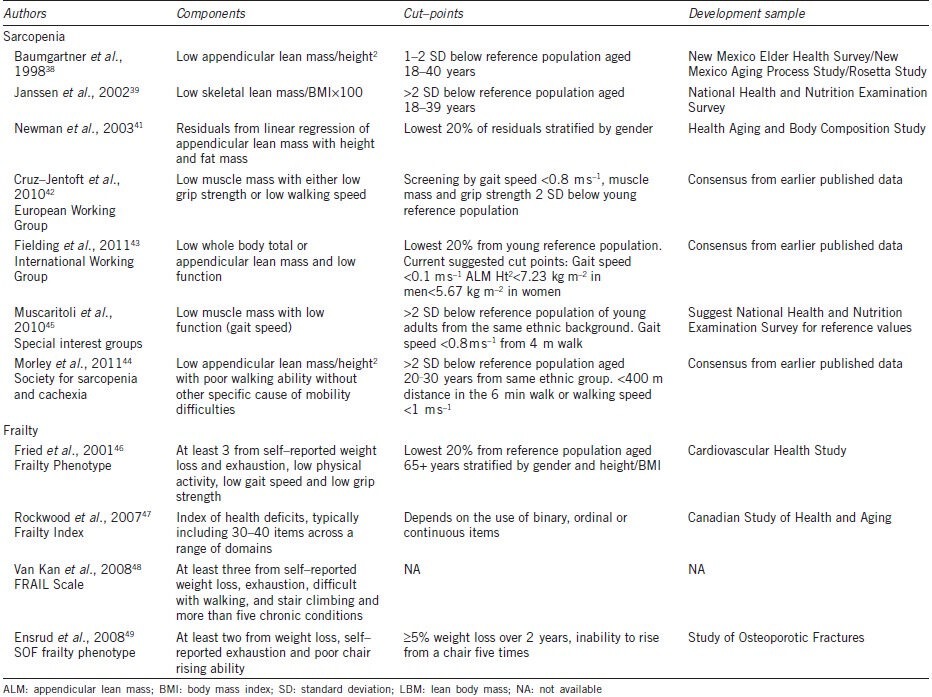
The term sarcopenia has been widely accepted to capture this ageing–related decline in muscle mass and function. Early definitions of sarcopenia focused on low levels of lean muscle mass relative to threshold levels derived from young reference populations.38,39 Definitions often focus on the lean mass of the limbs (appendicular lean mass (ALM)), reasoning that this most likely reflects functional skeletal muscle, rather than non–muscle lean tissues.38 The amount of lean mass is normally scaled to body size (height2), and some definitions also account for body fat.38,39,40,41 In recent years a number of international working groups have proposed new definitions of sarcopenia based not only on the presence of low muscle mass but also low muscle function.42,43,44,45 The European Working Group on Sarcopenia defined sarcopenia as low muscle mass combined with either slow gait speed or low grip strength.42 Similarly, consensus statements from the International Working Group on Sarcopenia and the special interest groups on ‘cachexia–anorexia in chronic wasting diseases’ and ‘nutrition in geriatrics’ propose definitions based on low muscle mass and low gait speed.43,45 Finally, the Society for Sarcopenia and Cachexia suggested sarcopenia with limited mobility should be considered a specific condition.44 A summary of these different definitions is shown in Table 1.38,39,41,42,43,44,45,46,47,48,49 Unsurprisingly the different definitions capture differing groups of older adults, highlighting the urgent need for a wider consensus on sarcopenia.50,51 The recent guidelines emphasize the clinical relevance of low muscle strength and physical function. However, decreases in muscle mass in ageing have a particular etiology and only partially explain declines in function.36,37,52 Moreover, skeletal muscle fulfills other important physiological functions including maintenance of insulin–mediated glucose homeostasis and providing a reservoir of proteins for use throughout the body.52,53 In this context, others have argued that ‘sarcopenia’ should be reserved for the decline in muscle mass, alongside terms such as ‘dynapenia’ and ‘kratopenia’ to capture the related declines in muscle function.54,55
Table 1.
Operational definitions of sarcopenia and frailty
FRAILTY
The age–related decline in muscle mass and function is a key process in the development and progression of frailty.2,3 However, frailty is currently conceptualized as a more general vulnerability, presaging adverse outcomes including falls, hospitalization, disability and death in older adults.1,4,56,57,58 This vulnerability arises when functional declines across multiple physiological systems lead to depleted homeostatic reserves and impaired responses to stressors.56,58 Numerous criteria have been proposed to describe this condition.59,60,61,62 Amongst these, the most widely accepted has been the phenotypic frailty model, proposed by Fried and colleagues, operationalized initially for the Cardiovascular Health Study (CHS).46 This model focuses on physical frailty and comprises five criteria drawn from a hypothetical cycle of decline: shrinking or sarcopenia, muscle weakness, slow gait speed, exhaustion and low physical activity.46 People with three or more criteria are considered frail and those with one to two are considered intermediate or prefrail. The model has been adapted for use across many ageing cohort studies.63,64,65,66,67 A pared down version incorporating weight loss, exhaustion and impaired chair rising has also been proposed by the Study of Osteoporotic Fractures (SOF) group.49,59
The second popular model proposed by Rockwood adopts a broader approach, grading frailty according to the number of ageing related health deficits summarized as a frailty index (FI).47,68 The health deficits may include any variable ranging from symptoms and physical signs to social isolation.47,68 Despite this flexibility, a remarkable degree of concordance has been shown between indices generated using many different deficits across different populations.69,70,71 A more recent approach, the Fatigue, Resistance, Ambulation, Illness and Loss of weight, or FRAIL Scale combines self–reported physical symptoms with the count of chronic conditions, and has utility where objective measures required for other frailty models are unavailable.48,72 It is self-evident that relationships with potential causal factors will be specific for each model of frailty studied and therefore not generalizable across different classifications. Table 1 summarizes the key features of some current frailty models.
RELATIONSHIPS BETWEEN ANDROGENS AND FRAILTY
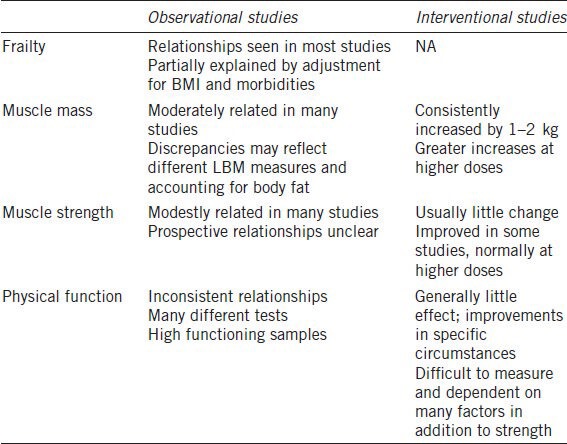
In recent years several studies have explored the relationships between T levels and defined frailty models. Alongside this, there has been an expansion of research on the relationships between androgens, body composition, muscle function and physical function. A summary of evidence from these studies is shown in Table 2.
Table 2.
Summary of testosterone effects on frailty
Frailty
Several recent studies have assessed the relationships between T levels and frailty using the CHS criteria. Low bioavailable T, but not total T, was related to frailty in cross–sectional analyses in men aged ≥65 years from the Osteoporotic fractures in men study (MrOS), this relationship was marginally nonsignificant in prospective analyses.73 Lower free and total T were associated with frailty in a sample of 552 Spanish men aged ≥65 years from the Toledo Study for Health and Aging,74 and in a sample of 54 Taiwanese men recruited from clinical and community settings.75 In a report from the Concord Health and Aging in Men Project (CHAMP), lower free and total T were related to higher levels of frailty in men aged 70–97 years, greater declines in T over 2 years also predicted progression of frailty at follow up in this study.76 Results were similar using the CHS criteria or the abbreviated SOF version.76 Finally, reports from the Massachusetts Male Aging Study (MMAS) and the Nutrition and Health Examination Survey (NHANES) showed relationships between low free T and physical frailty, that became nonsignificant after multivariate adjustment.77,78 No relationship with total T was seen in these studies.77,78
Studies using broader frailty models have also shown relationships between T levels and frailty.79,80 Lower baseline free T was related to the FRAIL Scale in both cross–sectional and prospective analyses in men aged 70–88 years from the Health in Men Study.79 Similarly, lower free T was related to higher levels of the FI in an analysis from the European Male Ageing Study.80 Weaker relationships with total T were seen in these studies.79,80
Body composition
Studies in adult men have frequently shown moderate positive associations between T levels and lean body mass, alongside negative associations with fat mass.81,82,83,84,85,86,87,88 Total and free T were correlated to arm and leg lean mass in men aged 24–90 years from the Baltimore Longitudinal Study on Ageing.89 Total T and free T were related to appendicular skeletal muscle (ASM) mass in men aged 65–97 years from the New Mexico Aging Process study.83 Free T indices, but not total T, were associated with relative ASM (RASM) in men aged 45–85 years from the MINOS study.86 Similarly, bioavailable T was associated with RASM in a sample of 142 men aged 64–92 years.85 In another study SHBG/T ratio, but not total T or free T, was related to lean body mass in 403 men aged 70 years and older.87 Free T, but not total T, was related to total and thigh lean mass in a sample of 101 men aged 60–70 years.90 Free T and bioavailable T were related to lean body mass in a sample of 335 Malaysian and Chinese men aged ≥40 years.84 Lastly, total and free T were related to RASM in 1489 men aged ≥65 years from the MrOS Hong Kong study.82
However, several recent studies did not find significant relationships between T levels and lean body mass: Vandenput et al., found no association between total or leg lean body mass in 2014 men aged 69–80 years from the MrOS Sweden sample.91 Similarly, Orwoll et al., found no difference in lean body mass index (BMI) across the range of bioavailable T levels in men from the American MrOS sample,92 while Maggio et al., found no difference in calf muscle area, measured by peripheral quantitative computed tomography (pQCT) scanning, across strata of total T levels in men from the Chinati study.93 Finally, a mild negative relationship between T levels and lean body mass was seen in men aged 20–90 years from the NHANES study,94 with similar, but nonsignificant, trends seen in men aged 30–79 years from the Boston Area Community Health Bone Survey.95
This discrepancy between studies may reflect sample differences in T levels or the different lean body mass parameters and/or measurement techniques used. Another possible explanation is differential scaling to body size and/or adjustment for fat mass across studies. Higher adiposity (and therefore BMI) is positively correlated with lean body mass and associated with reduced T levels, it is possible this comparatively strong effect may obscure the more modest positive effects of T on lean mass.81 Interestingly, a recent study in older Caribbean men suggested an inverse relationship between androgens and calf muscle fat infiltration, as well as a positive relationship with muscle density.96 This suggests an effect of androgens on muscle composition in addition to the influence of muscle mass and further highlights the potential confounding role of body fat in the relationship between lean mass and T, particularly as most techniques, including dual–energy X–ray absorptiometry (DXA), are not sensitive to muscle composition.
In addition to these cross–sectional studies, a recent prospective study found that higher baseline total T or bioavailable T was associated with less loss of lean mass over 4.5 years follow–up in 1183 men from the MrOS cohort, with strongest effects seen in the men who lost the most weight.97 Another study found a relationship between low baseline free T and increased likelihood of low muscle mass at 10 year follow up in Japanese men aged 40–79 years, weaker, nonsignificant trends were seen for total T.98
Muscle strength
Grip strength is the most widely used strength measurement in epidemiological studies, and is generally considered a good proxy for overall muscle strength and frailty.99 Cross–sectional studies in middle–aged and older men have shown modest positive associations between T levels and grip strength in most,82,83,84,87,92,93,100,101 but not all studies.81,102 In studies using more sophisticated muscle function measurements, total T and free T Index levels were correlated to upper and lower limb muscle strength in 262 men aged 24–90 years from the Baltimore Longitudinal Study of Ageing,89 while total and bioavailable T levels correlated to measures of upper and lower limb muscle strength in a small sample of older African American men.100 In a recent study, free, but not total T was weakly associated with knee extensor strength by isometric and isokinetic dynamometry in a sample of 101 men aged 60–70 years.90 Similarly, total and free T were modestly correlated with greater knee extensor strength in a sample of 403 men aged ≥70 years,87 while lower bioavailable T was associated with slightly lower leg power in men from the MrOs study.92 In a prospective study, baseline T levels were not associated with 3 year change in grip or knee extensor strength in men from the Longitudinal Aging Study Amsterdam (LASA) or the Health ABC study.103 Similarly, baseline T was not associated with 4.5 year change in grip strength or leg power in a report from the MrOs study, although there were trends towards greater losses at the lowest T levels among men who lost the most weight (≥2 kg) between waves.97
Physical function
Functional aspects of physical frailty are important in terms of determining clinical outcomes and quality of life. However, accurate assessment of physical function is methodologically challenging due to the large individual variability, effort dependence and practice effects. Studies on the relationships between androgens and physical function have shown equivocal results. In a small sample of black American men, total T levels were correlated with chair standing and door opening performance, but not with gait speed, timed up and go or simulated eating, while bioavailable T levels were correlated with gait speed, timed up and go and the doors task, but not with the other tasks.100 In a cross–sectional analysis from the LASA, bioavailable and free T, but not total T levels were correlated with better physical performance.101 A report from the MMAS found a relationship between total T and performance on the physical performance test (PPT) only below a threshold level estimated at 451 ng dl−1, and no relationship with chair rising performance.102 Similar relationships with bioavailable T were seen in this study.102 In men from the MrOS, low bioavailable T was associated with marginally poorer performance on tests of chair rising and walking ability.92 In men from the MrOS Hong Kong, higher total and free T were related to higher scores on a composite measure derived from several neuromuscular function tests; of the individual tests T levels were related to higher narrow walk speed and step length, but not to chair rising ability or gait speed.82 Higher free T, but not total T levels were related to faster gait speed and improved performance on the Short Physical Performance Battery (SPPB) in middle–aged and older men from the Framingham Offspring Study.104 In prospective analyses from this study, lower baseline free T was associated with self–reported mobility limitation at 6 year follow–up.104 Prospective studies using objective assessments of physical performance have shown largely negative results. No relationship between baseline T levels and 3 year change in performance of a composite physical score including gait speed, chair rising and tandem stands, in men from the LASA and Health ABC studies.103 Similarly, no overall relationship between baseline T and declines in chair standing and gait speed tests in men from the MrOS, although a mild relationship between lower T and less decline in chair stand performance was seen in men who lost the most weight between follow–up.97
Summary
In summary, a relationship between lower T levels (especially free T) and frailty constructs has been consistently, though not universally, found across studies. This relationship can be explained, at least partially, by confounding covariates, particularly age, BMI, morbidities and smoking, suggesting that low T may be a marker for these risk factors underlying frailty. Nevertheless, in most studies, some relationship with T persists even after adjustment for these confounders and it is plausible that lower T may be causally linked to frailty through its effects on muscle mass. The broadly consistent relationships with lean mass, including recent prospective data, offer some support for this pathway. T has been modestly, but consistently, related to muscle strength, but less clearly related to physical function. It is likely these effects are mediated through effects on lean mass, however in one study effects on strength persisted after adjustment for ALM.82 This may suggest additional mechanisms for androgen effects on strength that are not explained by changes in lean mass. Prospective data on progression of frailty or functional decline have shown equivocal relationships, although many studies included high functioning men.97,103 Few studies have simultaneously measured changes in T levels and functional measures across two or more time points and this is an important area for future research.
EFFECTS OF ANDROGEN TREATMENT ON FRAILTY
This section reviews studies on the effects of T treatment on components of frailty in healthy and frail older men. Particular attention is given to body composition, muscle strength and physical function. Table 2 gives an overview of the results from these interventional studies.
Body composition
T treatment at near physiological dosages for 3–36 months has been reliably found to increase lean body mass and reduce fat mass in both healthy and frail older men with low to low normal T levels.8,18,105,106,107,108,109,110,111,112,113,114,115,116,117,118 The magnitude of improvements in lean mass has been in the region of 1–2 kg in most trials depending on the dose and type of preparation used.105,106,107,108,110,113,114,116,117 Larger gains of around 4 kg have been seen in trials using injected T preparations.18,111 The minority of studies not to show an effect were limited by short treatment duration (1 month), use of the relatively insensitive bioelectrical impedance technique to assess lean mass changes, and inclusion of men with normal T levels.119,120,121 An increase in lean body mass in sarcopenic older men may lead to improvements in glucose metabolism, drug tolerance and whole body protein reserves.52 However, the important question is whether this gain in muscle mass translates to increases in strength and so functional ability.
Muscle strength
Studies in young and older men have suggested dose–dependent effects of T on muscle strength and power, without clear effects on muscle fatigability.15,122 While power maybe more closely related to physical function in older adults,123 it is more difficult to measure than strength,124 and the effects of T on muscle power are probably explained by the effects on strength. Due to the wider range of strength assessments available, most studies in older men have focused on this parameter, with varying improvements seen. Grip strength, the most widely used strength assessment, has been found to increase in some trials,62,111,125,126 but not others.106,113,114,115,116,117,120 Similarly, some studies have reported improvements in lower limb strength,18,114,127,128 while others have failed to show any effect of treatment.107,109,111,113,117 There are several reasons why gains in lean mass may not reliably lead to improvements in muscle strength. Firstly, it is a possible that lean mass gains may not reflect increases in myofibrillar protein content. Early gains (8 weeks treatment) in lean mass may reflect water retention or accumulation of noncontractile proteins.129 However, most studies have used longer treatment durations, and as discussed above, a variety of studies demonstrate effects of androgens on muscle protein synthesis, fiber size, myonuclear number and satellite cell activation.13,18,20,22 Another possibility is that gains in lean mass may be too small to lead to increases in strength: a recent study using several combinations of T and growth hormone treatments estimated that gains in lean mass of at least 1.6 kg were required to improve leg or chest press strength in a sample of healthy older men.130 This may explain the lack of effect in some studies,106,110 but in many others increases in lean mass were in or above this range.
Improvements in strength have most frequently been seen using 1 repetition maximum (1–RM) techniques and relatively high doses of T. A small study in healthy men (n = 12) showed an increase of 15 kg in leg press strength with treatment alongside a gain in lean mass of 4 kg.18 Larger studies have also shown improvements in 1–RM leg press strength alongside smaller (<2 kg) gains in lean mass following doses of 10 mg day−1 transdermal T in healthy and mobility limited older men.116,127 Improvements at lower doses (2.5–5 mg day−1) have been less clear.107,108,112 Studies using isokinetic dynamometry in healthy or frail older men have shown very limited to no improvement in lower limb strength compared to placebo, despite gains in lean mass of 1–4 kg and treatment for up to 3 years.107,113,114,117,128 In one of these studies, improvements in knee extension strength of 6% have been shown in frail men using isometric dynamometry.114
1–RM protocols define maximum strength as the terminal weight lifted in a series of incrementally higher loads, while dynamometry protocols typically define strength as the highest torque generated during a muscle contraction. Isokinetic dynamometry involves a contraction at a set speed over a defined range of motioned; whereas, isometric protocols involve static contractions at a fixed joint angle. Contraction velocity in 1–RM contractions is quite slow; it has been suggested that this may be more similar to that seen during isometric contractions.131 In older men, both the contraction velocity and rate of force development are reduced,132,133 it is therefore likely that older men find it difficult to perform the faster contraction speeds used in many isokinetic dynamometry protocols. Consequentially, it is possible that isokinetic dynamometry may not capture improvements in strength even when there are large gains in lean mass and improvements in other muscle function measures.111 While differences in strength assessments may partially account for small differences between studies, overall the effects of near physiological T treatments on strength can be said to be modest, with trends towards greater improvements at higher doses.
Physical function
T–induced improvements in physical function have been limited across many studies to date, with most failing to show any clear effects. Studies in healthy men have failed to show improvements in functional tasks including tests of balance, gait speed, chair rising, step height and functional reaching.106,109,113,120,128 One study did show an improvement on a composite physical performance test including rising from a chair, a fast walk, a step height test, a door task and a stair ascent and descent over 12–36 months treatment.111 Two studies in frailer men did not show any overall improvements in gait speed, mobility or activities of daily living tests following treatment for 6–24 months.107,114 Although in one of these studies improvements on some scales were seen in older (≥75 years) and frailer (≥2 of Fried's criteria) men.114 Finally, in the TOM trial, loaded stair climbing improved with treatment compared to placebo in mobility limited men (P = 0.05), there were also trends towards greater improvements in loaded gait speed, but not in unloaded tasks.127
It is possible that increases in strength were too small to lead to measureable improvements in function in some studies, particularly as relatively large improvements on functional tasks often occurred in both groups, consistent with learning effects.109,128 Another factor is likely to be the nonlinear relationship between muscle strength and physical function: at a certain level, dependent on the difficulty of the task used, the relationship plateaus, such that further increases in strength will not result in improvements in physical function.134,135,136 It is likely that in the majority of studies participants were functioning above the most sensitive strength ranges for the assessments used. Indeed, while improvements in strength and power are dose dependent, even supraphysiological doses of T may not improve performance on functional tasks in healthy older men.122 Finally, physical function is dependent upon a number of factors in addition to muscle function,137 with strength making a varying contribution to the performance of different tasks.123 T therapy might be expected to preferentially affect more strength–dependent tasks, in agreement improvements in loaded stair climbing and gait speed were correlated with increases in T and leg press strength in the TOM trial, but improvements in unloaded tasks were not.116 It is also likely that T alone may be relatively ineffective and may need to be combined with exercise or other functional training in order to engender broad spectrum functional improvements.138
Summary
In summary, T treatment reliably improves body composition and may be associated with modest increases in muscle strength, especially at higher (near supraphysiological) doses. Response in physical function may preferentially improve for strength dependent tasks, but such improvements will only be detectable using tasks appropriate to the baseline ability of participants.
TIME COURSE AND DURABILITY
Improvements in lean body mass and strength in response to T treatment are reached within 6 months and can be maintained without further increment for the duration of treatment (longest study to date is 3 years).111,113,117 Studies in healthy and frail older men suggest most of this benefit is lost within 3–6 months of discontinuing treatment, although in men experiencing the largest gains some residual benefits may remain at 3 months.139,140
SAFETY OF T THERAPY IN OLDER MEN
The use of T therapies in older men has been limited by concerns over adverse cardiovascular and prostatic effects. Several meta–analyses suggest T has been well–tolerated in the majority of studies in healthy older men.141,142,143 The most frequent adverse effect seen is increased hematocrit, which may lead to clinically significant erthrocytosis.141,142,143 T has also been shown to be well–tolerated in frailer older men, with only mild effects on hematocrit, prostate specific antigen (PSA) and blood lipids.114 In contrast to these findings, the TOM trial of T therapy in men with limited mobility was discontinued following an imbalance in cardiovascular events in T treated men compared to placebo.127 This discrepancy may be explained by the relatively high dose used in a comparatively high risk population: the strongest risk factor for cardiovascular events in this trial was the increase in free T.144 This is consistent with previous findings of greater frequencies of adverse events associated with higher T doses in healthy older men.8 Men included in the trial had a high mean BMI, as well as a very high frequency of hypertension, diabetes and hyperlipidemia.127 This experience sounds a salutary note of caution regarding the safety of treating frail elderly men with relatively high doses of T, highlighting the importance of careful patient or trial subject selection.
The effects of T on serious prostate events are currently unclear due to the relatively small size of the studies and short duration of exposure. A 2005 meta–analysis suggested that men treated with T experienced approximately double the rate of all prostate events including biopsies, cancers, increased symptoms, increments in PSA and urinary retention.141 However, this may be explained by monitoring bias.141 The effects of T on prostate and cardiovascular events will only be clearly established by larger scale, longer duration, appropriately powered clinical trials.
FUTURE DIRECTIONS IN ANDROGEN THERAPIES
As described, physiological androgen therapies have shown limited improvements in muscle function and concerns remain over the safety of higher doses of T in older men. Several new approaches with the potential to address these limitations have started to emerge.
Selective androgen receptor modulators (SARMs) have been developed, aimed at maximizing anabolic effects on muscle and bone without androgenic effects on other tissues, especially the prostate and hair follicles.145,146 The first trials of these compounds as function promoting therapies have recently been reported.147,148,149 Treatment with GTx–024 (Enobosarm) has been associated with increases in lean body mass and stair climbing ability, without virilizing effects, in healthy older men and women and in patients with cancer cachexia.147,148 In another trial, 6 months treatment with MK–0773 was well–tolerated and associated with increases in lean body mass, but not muscle strength or physical performance in older women with sarcopenia and mobility limitations.149 Finally, in a recent dose finding study another SARM, LGD–4033, increased lean body mass without effecting PSA levels in healthy young men.150 As demonstrated by these early studies, these agents will permit the use of androgen–based anabolic therapies in older women and raise the possibility of safely using more potent pharmacological doses to more reliably improve muscle strength not only in older adults but also in the broader context of cancer cachexia and posttraumatic and postoperative rehabilitation. In these latter indications, the shorter duration of treatment and the consistent positive effects on muscle mass (as opposed to strength and function) may well be the important primary therapeutic outcome.
Androgen therapy consistently increases lean body mass, but may not improve muscle function, while progressive resistance training may improve muscle function in older adults in the absence of gains in lean mass.151 Two recent studies have explored the potential of combining these interventions.152,153 The combination of 12 months progressive resistance training and T lead to greater improvements in body composition than either intervention alone in healthy older men, but did not provide additional improvements in muscle strength or physical function.152 Similarly, in the second study the addition of T therapy for the latter 12 weeks of a 24 week resistance training program increased muscle mass, but not muscle function over men engaged in training alone.153 Although this study may have lacked statistical power; the combined T and training group included only six men.153 Despite the lack of evidence of synergy between the two therapies, these studies confirm their differential effects on muscle mass and function. Combining novel androgen therapies with different exercise training programs will be an essential key area for future research in combating frailty.
CONCLUSIONS
The consistent effects on lean mass in interventional studies combined with the relationships seen in observational studies and the increasingly well–characterized mechanistic pathways all suggest T is an important promoter of muscle mass gain in older men. As such, falling T levels may contribute to the development of frailty, although the decline in strength with ageing involves many more mechanisms. Correspondingly, the functional effects of T are less clear. Much of the current research has involved high functioning older men; there is a need for more observational studies as well as interventional trials in frailer populations. The present confusion over the purported syndromes of sarcopenia and frailty presents a limitation for study design. A consensus on the most meaningful features of physical decline will assist in determining etiologies and future trial design. Focus on particular domains, such as mobility decline, may be preferable to the current syndromic definitions. More sophisticated analysis of parallel changes in hormone levels, body composition and functional outcomes over time will help to unravel the directionality of these relationships and so the true role of androgens in functional decline. The development of SARMs has the potential to limit the adverse effects of T, allow more potent functional promotion and extend the use of androgen therapies to broader populations. Deeper understanding of the molecular mechanisms underlying androgens’ anabolic effects will facilitate the development of further nonsteroidal agents. The application of these agents in combination with well–designed exercise training protocols represents an exciting new direction in this field.
COMPETING INTERESTS
Fred CW Wu has received honorarium for lectures from Bayer Schering and research grant funding from Eli Lilly and Merk.
REFERENCES
- 1. [Last accessed date on 12 August, 2012]. Available from: http: //ec.europa.eu/eurostat .
- 2.Cesari M, Leeuwenburgh C, Lauretani F, Onder G, Bandinelli S, et al. Frailty syndrome and skeletal muscle: Results from the Invecchiare in Chianti study. Am J Clin Nutr. 2006;83:1142–8. doi: 10.1093/ajcn/83.5.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gielen E, Verschueren S, O’Neill TW, Pye SR, O’Connell MD, et al. Musculoskeletal frailty: a geriatric syndrome at the core of fracture occurrence in older age. Calcif Tissue Int. 2012;91:161–77. doi: 10.1007/s00223-012-9622-5. [DOI] [PubMed] [Google Scholar]
- 4.Fried LP, Hadley EC, Walston JD, Newman AB, Guralnik JM, et al. From bedside to bench: research agenda for frailty. Sci Aging Knowledge Environ. 2005:24. doi: 10.1126/sageke.2005.31.pe24. [DOI] [PubMed] [Google Scholar]
- 5.O’Connell MD, Tajar A, Roberts SA, Wu FC. Do androgens play any role in the physical frailty of ageing men? Int J Androl. 2011;34:195–211. doi: 10.1111/j.1365-2605.2010.01093.x. [DOI] [PubMed] [Google Scholar]
- 6.Bhasin S, Storer TW, Berman N, Callegari C, Clevenger B, et al. The effects of supraphysiologic doses of testosterone on muscle size and strength in normal men. N Engl J Med. 1996;335:1–7. doi: 10.1056/NEJM199607043350101. [DOI] [PubMed] [Google Scholar]
- 7.Bhasin S, Woodhouse L, Casaburi R, Singh AB, Bhasin D, et al. Testosterone dose–response relationships in healthy young men. Am J Physiol Endocrinol Metab. 2001;281:E1172–81. doi: 10.1152/ajpendo.2001.281.6.E1172. [DOI] [PubMed] [Google Scholar]
- 8.Bhasin S, Woodhouse L, Casaburi R, Singh AB, Mac RP, et al. Older men are as responsive as young men to the anabolic effects of graded doses of testosterone on the skeletal muscle. J Clin Endocrinol Metab. 2005;90:678–88. doi: 10.1210/jc.2004-1184. [DOI] [PubMed] [Google Scholar]
- 9.Camacho EM, Huhtaniemi IT, O’Neill TW, Finn JD, Pye SR, et al. EMAS Group. Age–associated changes in hypothalamic–pituitary–testicular function in middle–aged and older men are modified by weight change and lifestyle factors: longitudinal results from the European Male Ageing Study. Eur J Endocrinol. 2013;168:445–55. doi: 10.1530/EJE-12-0890. [DOI] [PubMed] [Google Scholar]
- 10.Wu FC, Tajar A, Pye SR, Silman AJ, Finn JD, et al. European Male Aging Study Group. Hypothalamic–pituitary–testicular axis disruptions in older men are differentially linked to age and modifiable risk factors: the European Male Aging Study. J Clin Endocrinol Metab. 2008;93:2737–45. doi: 10.1210/jc.2007-1972. [DOI] [PubMed] [Google Scholar]
- 11.Yeap BB, Almeida OP, Hyde Z, Norman PE, Chubb SA, et al. In men older than 70 years, total testosterone remains stable while free testosterone declines with age. The Health in Men Study. Eur J Endocrinol. 2007;156:585–94. doi: 10.1530/EJE-06-0714. [DOI] [PubMed] [Google Scholar]
- 12.Travison TG, Araujo AB, Kupelian V, O’Donnell AB, McKinlay JB. The relative contributions of aging, health, and lifestyle factors to serum testosterone decline in men. J Clin Endocrinol Metab. 2007;92:549–55. doi: 10.1210/jc.2006-1859. [DOI] [PubMed] [Google Scholar]
- 13.Sinha-Hikim I, Artaza J, Woodhouse L, Gonzalez-Cadavid N, Singh AB, Lee MI, et al. Testosterone–induced increase in muscle size in healthy young men is associated with muscle fiber hypertrophy. Am J Physiol Endocrinol Metab. 2002;283:E154–64. doi: 10.1152/ajpendo.00502.2001. [DOI] [PubMed] [Google Scholar]
- 14.Sinha-Hikim I, Cornford M, Gaytan H, Lee ML, Bhasin S. Effects of testosterone supplementation on skeletal muscle fiber hypertrophy and satellite cells in community-dwelling older men. J Clin Endocrinol Metab. 2006;91:3024–33. doi: 10.1210/jc.2006-0357. [DOI] [PubMed] [Google Scholar]
- 15.Storer TW, Magliano L, Woodhouse L, Lee ML, Dzekov C, et al. Testosterone dose–dependently increases maximal voluntary strength and leg power, but does not affect fatigability or specific tension. J Clin Endocrinol Metab. 2003;88:1478–85. doi: 10.1210/jc.2002-021231. [DOI] [PubMed] [Google Scholar]
- 16.Schroeder ET, Terk M, Sattler FR. Androgen therapy improves muscle mass and strength but not muscle quality: results from two studies. Am J Physiol Endocrinol Metab. 2003;285:E16–24. doi: 10.1152/ajpendo.00032.2003. [DOI] [PubMed] [Google Scholar]
- 17.Dubois V, Laurent M, Boonen S, Vanderschueren D, Claessens F. Androgens and skeletal muscle: cellular and molecular action mechanisms underlying the anabolic actions. Cell Mol Life Sci. 2012;69:1651–67. doi: 10.1007/s00018-011-0883-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferrando AA, Sheffield–Moore M, Yeckel CW, Gilkison C, Jiang J, et al. Testosterone administration to older men improves muscle function: molecular and physiological mechanisms. Am J Physiol Endocrinol Metab. 2002;282:E601–7. doi: 10.1152/ajpendo.00362.2001. [DOI] [PubMed] [Google Scholar]
- 19.Ferrando AA, Tipton KD, Doyle D, Phillips SM, Cortiella J, et al. Testosterone injection stimulates net protein synthesis but not tissue amino acid transport. Am J Physiol. 1998;275:E864–71. doi: 10.1152/ajpendo.1998.275.5.E864. [DOI] [PubMed] [Google Scholar]
- 20.Sinha–Hikim I, Roth SM, Lee MI, Bhasin S. Testosterone–induced muscle hypertrophy is associated with an increase in satellite cell number in healthy, young men. Am J Physiol Endocrinol Metab. 2003;285:E197–205. doi: 10.1152/ajpendo.00370.2002. [DOI] [PubMed] [Google Scholar]
- 21.Urban RJ, Bodenburg YH, Gilkison C, Foxworth J, Coggan AR, et al. Testosterone administration to elderly men increases skeletal muscle strength and protein synthesis. Am J Physiol. 1995;269:E820–6. doi: 10.1152/ajpendo.1995.269.5.E820. [DOI] [PubMed] [Google Scholar]
- 22.Singh R, Artaza JN, Taylor WE, Gonzalez–Cadavid NF, Bhasin S. Androgens stimulate myogenic differentiation and inhibit adipogenesis in C3H 10T1/2 pluripotent cells through an androgen receptor–mediated pathway. Endocrinology. 2003;144:5081–8. doi: 10.1210/en.2003-0741. [DOI] [PubMed] [Google Scholar]
- 23.Sinha–Hikim I, Taylor WE, Gonzalez–Cadavid NF, Zheng W, Bhasin S. Androgen receptor in human skeletal muscle and cultured muscle satellite cells: Up–regulation by androgen treatment. J Clin Endocrinol Metab. 2004;89:5245–55. doi: 10.1210/jc.2004-0084. [DOI] [PubMed] [Google Scholar]
- 24.Singh R, Bhasin S, Braga M, Artaza JN, Pervin S, et al. Regulation of myogenic differentiation by androgens: cross talk between androgen receptor/β–Catenin and follistatin/transforming growth factor–β signaling pathways. Endocrinology. 2009;150:1259–68. doi: 10.1210/en.2008-0858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Braga M, Bhasin S, Jasuja R, Pervin S, Singh R. Testosterone inhibits transforming growth factor–β signaling during myogenic differentiation and proliferation of mouse satellite cells: potential role of follistatin in mediating testosterone action. Mol Cell Endocrinol. 2012;350:39–52. doi: 10.1016/j.mce.2011.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brown D, Hikim AP, Kovacheva EL, Sinha-Hikim I. Mouse model of testosterone–induced muscle fiber hypertrophy: involvement of p38 mitogen–activated protein kinase–mediated Notch signaling. J Endocrinol. 2009;201:129–39. doi: 10.1677/JOE-08-0476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kovacheva EL, Sinha Hikim AP, Shen R, Sinha I, Sinha–Hikim I. Testosterone supplementation reverses sarcopenia in aging through regulation of myostatin, c–Jun NH2–terminal kinase, notch, and akt signaling pathways. Endocrinology. 2010;151:628–38. doi: 10.1210/en.2009-1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Serra C, Bhasin S, Tangherlini F, Barton ER, Ganno M, et al. The Role of GH and IGF–I in mediating anabolic effects of testosterone on androgen–responsive muscle. Endocrinology. 2010;152:193–206. doi: 10.1210/en.2010-0802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.White JP, Gao S, Puppa MJ, Sato S, Welle SL, et al. Testosterone regulation of Akt/mTORC1/FoxO3a signaling in skeletal muscle. Mol Cell Endocrinol. 2013;365:174–86. doi: 10.1016/j.mce.2012.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu Y, Bauman WA, Blitzer RD, Cardozo C. Testosterone–induced hypertrophy of L6 myoblasts is dependent upon Erk and mTOR. Biochem Biophys Res Commun. 2010;400:679–83. doi: 10.1016/j.bbrc.2010.08.127. [DOI] [PubMed] [Google Scholar]
- 31.Delmonico MJ, Harris TB, Visser M, Park SW, Conroy MB, et al. Longitudinal study of muscle strength, quality, and adipose tissue infiltration. Am J Clin Nutr. 2009;90:1579–85. doi: 10.3945/ajcn.2009.28047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koster A, Ding J, Stenholm S, Caserotti P, Houston DK, et al. Health ABC study. Does the amount of fat mass predict age–related loss of lean mass, muscle strength, and muscle quality in older adults? J Gerontol A Biol Sci Med Sci. 2011;66A:888–95. doi: 10.1093/gerona/glr070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koster A, Visser M, Simonsick EM, Yu B, Allison DB, et al. Health, Aging and Body Composition Study. Association between fitness and changes in body composition and muscle strength. J Am Geriatr Soc. 2010;58:219–26. doi: 10.1111/j.1532-5415.2009.02681.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Narici MV, Maffulli N, Maganaris CN. Ageing of human muscles and tendons. Disabil Rehabil. 2008;30:1548–54. doi: 10.1080/09638280701831058. [DOI] [PubMed] [Google Scholar]
- 35.Tomlinson BE, Irving D. The numbers of limb motor neurons in the human lumbosacral cord throughout life. J Neurol Sci. 1977;34:213–9. doi: 10.1016/0022-510x(77)90069-7. [DOI] [PubMed] [Google Scholar]
- 36.Mitchell WK, Atherton PJ, Williams J, Larvin M, Lund JN, et al. Sarcopenia, dynapenia and the impact of advancing age on human skeletal muscle size and strength; a quantitative review. Front Physiol. 2012;3:260. doi: 10.3389/fphys.2012.00260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Russ D, Gregg–Cornell K, Conaway M, Clark BC. Evolving concepts on the age–related changes in “muscle quality”. J Cachexia Sarcopenia Muscle. 2012;3:95–109. doi: 10.1007/s13539-011-0054-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998;147:755–63. doi: 10.1093/oxfordjournals.aje.a009520. [DOI] [PubMed] [Google Scholar]
- 39.Janssen I, Heymsfield SB, Ross R. Low relative skeletal muscle mass (Sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc. 2002;50:889–96. doi: 10.1046/j.1532-5415.2002.50216.x. [DOI] [PubMed] [Google Scholar]
- 40.Delmonico MJ, Harris TB, Lee JS, Visser M, Nevitt M, et al. Health, Aging and Body Composition Study. Alternative definitions of sarcopenia, lower extremity performance, and functional impairment with aging in older men and women. J Am Geriatr Soc. 2007;55:769–74. doi: 10.1111/j.1532-5415.2007.01140.x. [DOI] [PubMed] [Google Scholar]
- 41.Newman AB, Kupelian V, Visser M, Simonsick E, Goodpaster B, et al. Health ABC Study Investigators. Sarcopenia: alternative definitions and associations with lower extremity function. J Am Geriatr Soc. 2003;51:1602–9. doi: 10.1046/j.1532-5415.2003.51534.x. [DOI] [PubMed] [Google Scholar]
- 42.Cruz–Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, et al. European Working Group on Sarcopenia in Older People. Sarcopenia: european consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39:412–23. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fielding RA, Vellas B, Evans WJ, Bhasin S, Morley JE, et al. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. international working group on sarcopenia. J Am Med Dir Assoc. 2011;12:249–56. doi: 10.1016/j.jamda.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Morley JE, Abbatecola AM, Argiles JM, Baracos V, Bauer J, et al. Society on Sarcopenia, Cachexia and Wasting Disorders Trialist Workshop. Sarcopenia with limited mobility: an international consensus. J Am Med Dir Assoc. 2011;12:403–9. doi: 10.1016/j.jamda.2011.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Muscaritoli M, Anker SD, Argilés J, Aversa Z, Bauer JM, et al. Consensus definition of sarcopenia, cachexia and pre–cachexia: joint document elaborated by Special Interest Groups (SIG) “cachexia–anorexia in chronic wasting diseases” and “nutrition in geriatrics”. Clin Nutr. 2010;29:154–9. doi: 10.1016/j.clnu.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 46.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, et al. Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–56. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 47.Rockwood K, Mitnitski A. Frailty in relation to the accumulation of deficits. J Gerontol A Biol Sci Med Sci. 2007;62:722–7. doi: 10.1093/gerona/62.7.722. [DOI] [PubMed] [Google Scholar]
- 48.Abellan van Kan G, Rolland Y, Bergman H, Morley JE, Kritchevsky SB, et al. The I.A.N.A. task force on frailty assessment of older people in clinical practice. J Nutr Health Aging. 2008;12:29–37. doi: 10.1007/BF02982161. [DOI] [PubMed] [Google Scholar]
- 49.Ensrud KE, Ewing SK, Taylor BC, Fink HA, Cawthon PM, et al. Comparison of 2 frailty indexes for prediction of falls, disability, fractures, and death in older women. Arch Intern Med. 2008;168:382–9. doi: 10.1001/archinternmed.2007.113. [DOI] [PubMed] [Google Scholar]
- 50.Abellan van Kan G, Cesari M, Gillette–Guyonnet S, Dupuy C, Nourhashémi F, et al. Sarcopenia and cognitive impairment in elderly women: results from the EPIDOS cohort. Age Ageing. 2013;42:196–202. doi: 10.1093/ageing/afs173. [DOI] [PubMed] [Google Scholar]
- 51.Bijlsma AY, Meskers CG, Ling CH, Narici M, Kurrle SE, et al. Defining sarcopenia: the impact of different diagnostic criteria on the prevalence of sarcopenia in a large middle aged cohort. Age (Dordr) 2013;35:871–81. doi: 10.1007/s11357-012-9384-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Narici MV, Maffulli N. Sarcopenia: characteristics, mechanisms and functional significance. Br Med Bull. 2010;95:139–59. doi: 10.1093/bmb/ldq008. [DOI] [PubMed] [Google Scholar]
- 53.Bijlsma AY, Meskers CG, van Heemst D, Westendorp RG, de Craen AM, et al. Diagnostic criteria for sarcopenia relate differently to insulin resistance. Age (Dordr) 2013;35:2367–75. doi: 10.1007/s11357-013-9516-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Clark BC, Manini TM. Sarcopenia =/= Dynapenia. J Gerontol A Biol Sci Med Sci. 2008;63:829–34. doi: 10.1093/gerona/63.8.829. [DOI] [PubMed] [Google Scholar]
- 55.Morley JE. Sarcopenia in the elderly. Fam Pract. 2012;29:i44–8. doi: 10.1093/fampra/cmr063. [DOI] [PubMed] [Google Scholar]
- 56.Bergman H, Ferrucci L, Guralnik J, Hogan DB, Hummel S, et al. Frailty: an emerging research and clinical paradigm issues and controversies. J Gerontol A Biol Sci Med Sci. 2007;62:731–7. doi: 10.1093/gerona/62.7.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fried LP, Ferrucci L, Darer J, Williamson JD, Anderson G. Untangling the concepts of disability, frailty, and comorbidity: implications for improved targeting and care. J Gerontol A Biol Sci Med Sci. 2004;59:255–63. doi: 10.1093/gerona/59.3.m255. [DOI] [PubMed] [Google Scholar]
- 58.Walston J, Hadley EC, Ferrucci L, Guralnik JM, Newman AB, et al. Research agenda for frailty in older adults: toward a better understanding of physiology and etiology: summary from the American Geriatrics Society/National Institute on Aging Research Conference on Frailty in Older Adults. J Am Geriatr Soc. 2006;54:991–1001. doi: 10.1111/j.1532-5415.2006.00745.x. [DOI] [PubMed] [Google Scholar]
- 59.Ensrud KE, Ewing SK, Cawthon PM, Fink HA, Taylor BC, et al. Osteoporotic Fractures in Men Research Group. A comparison of frailty indexes for the prediction of falls, disability, fractures, and mortality in older men. J Am Geriatr Soc. 2009;57:492–8. doi: 10.1111/j.1532-5415.2009.02137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ravaglia G, Forti P, Lucicesare A, Pisacane N, Rietti E, et al. Development of an easy prognostic score for frailty outcomes in the aged. Age Ageing. 2008;37:161–6. doi: 10.1093/ageing/afm195. [DOI] [PubMed] [Google Scholar]
- 61.Rolfson DB, Majumdar SR, Tsuyuki RT, Tahir A, Rockwood K. Validity and reliability of the edmonton frail scale. Age Ageing. 2006;35:526–9. doi: 10.1093/ageing/afl041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Strawbridge WJ, Shema SJ, Balfour JL, Higby HR, Kaplan GA. Antecedents of frailty over three decades in an older cohort. J Gerontol B Psychol Sci Soc Sci. 1998;53:S9–16. doi: 10.1093/geronb/53b.1.s9. [DOI] [PubMed] [Google Scholar]
- 63.Cawthon PM, Marshall LM, Michael Y, Dam TT, Ensrud KE, et al. Osteoporotic Fractures in Men Research Group. Frailty in older men: prevalence, progression, and relationship with mortality. J Am Geriatr Soc. 2007;55:1216–23. doi: 10.1111/j.1532-5415.2007.01259.x. [DOI] [PubMed] [Google Scholar]
- 64.Ensrud KE, Ewing SK, Taylor BC, Fink HA, Stone KL, et al. Study of Osteoporotic Fractures Research Group. Frailty and risk of falls, fracture, and mortality in older women: the study of osteoporotic fractures. J Gerontol A Biol Sci Med Sci. 2007;62:744–51. doi: 10.1093/gerona/62.7.744. [DOI] [PubMed] [Google Scholar]
- 65.Woods NF, LaCroix AZ, Gray SL, Aragaki A, Cochrane BB, et al. Women's Health Initiative. Frailty: emergence and consequences in women aged 65 and older in the women's health initiative observational study. J Am Geriatr Soc. 2005;53:1321–30. doi: 10.1111/j.1532-5415.2005.53405.x. [DOI] [PubMed] [Google Scholar]
- 66.O’Connell MD, Tajar A, O’Neill TW, Roberts SA, Lee DM, et al. Frailty is associated with impaired quality of life and falls in middle–aged and older european men. J Frailt Aging. 2013;2:77–83. doi: 10.14283/jfa.2013.12. [DOI] [PubMed] [Google Scholar]
- 67.Rochat S, Cumming RG, Blyth F, Creasey H, Handelsman D, et al. Frailty and use of health and community services by community–dwelling older men: the Concord Health and Ageing in Men Project. Age Ageing. 2010;39:228–33. doi: 10.1093/ageing/afp257. [DOI] [PubMed] [Google Scholar]
- 68.Searle S, Mitnitski A, Gahbauer E, Gill TM, Rockwood K. A standard procedure for creating a frailty index. BMC Geriatr. 2008;8:24. doi: 10.1186/1471-2318-8-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mitnitski A, Rockwood K. Decrease in the relative heterogeneity of health with age: a cross–national comparison. Mech Ageing Dev. 2006;127:70–2. doi: 10.1016/j.mad.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 70.Mitnitski A, Song X, Skoog I, Broe GA, Cox JL, et al. Relative fitness and frailty of elderly men and women in developed countries and their relationship with mortality. J Am Geriatr Soc. 2005;53:2184–9. doi: 10.1111/j.1532-5415.2005.00506.x. [DOI] [PubMed] [Google Scholar]
- 71.Rockwood K, Mitnitski A. Limits to deficit accumulation in elderly people. Mech Ageing Dev. 2006;127:494–6. doi: 10.1016/j.mad.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 72.Abellan van Kan G, Rolland YM, Morley JE, Vellas B. Frailty: toward a clinical definition. J Am Med Dir Assoc. 2008;9:71–2. doi: 10.1016/j.jamda.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 73.Cawthon PM, Ensrud KE, Laughlin GA, Cauley JA, Dam TT, et al. Osteoporotic Fractures in Men (MrOS) Research Group. Sex hormones and frailty in older men: the osteoporotic fractures in men (MrOS) study. J Clin Endocrinol Metab. 2009;94:3806–15. doi: 10.1210/jc.2009-0417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Carcaillon L, Blanco C, Alonso–Bouzón C, Alfaro–Acha A, Garcia–García FJ, et al. Sex differences in the association between serum levels of testosterone and frailty in an elderly population: the Toledo Study for Healthy Aging. PLoS One. 2012;7:e32401. doi: 10.1371/journal.pone.0032401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wu IC, Lin XZ, Liu PF, Tsai WL, Shiesh SC. Low serum testosterone and frailty in older men and women. Maturitas. 2010;67:348–52. doi: 10.1016/j.maturitas.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 76.Travison TG, Nguyen AH, Naganathan V, Stanaway FF, Blyth FM, et al. Changes in reproductive hormone concentrations predict the prevalence and progression of the frailty syndrome in older men: the concord health and ageing in men project. J Clinl Endocrinol Metab. 2011;96:2464–74. doi: 10.1210/jc.2011-0143. [DOI] [PubMed] [Google Scholar]
- 77.Eichholzer M, Barbir A, Basaria S, Dobs AS, Feinleib M, et al. Serum sex steroid hormones and frailty in older American men of the Third National Health and Nutrition Examination Survey (NHANES III) Aging Male. 2012;15:208–15. doi: 10.3109/13685538.2012.705366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mohr BA, Bhasin S, Kupelian V, Araujo AB, O’Donnell AB, et al. Testosterone, sex hormone–binding globulin, and frailty in older men. J Am Geriatr Soc. 2007;55:548–55. doi: 10.1111/j.1532-5415.2007.01121.x. [DOI] [PubMed] [Google Scholar]
- 79.Hyde Z, Flicker L, Almeida OP, Hankey GJ, McCaul KA, et al. Low free testosterone predicts frailty in older men: the health in men study. J Clin Endocrinol Metab. 2010;95:3165–72. doi: 10.1210/jc.2009-2754. [DOI] [PubMed] [Google Scholar]
- 80.Tajar A, O’Connell MD, Mitnitski AB, O’Neill TW, Searle SD, et al. European Male Aging Study Group. Frailty in relation to variations in hormone levels of the hypothalamic–pituitary–testicular axis in older men: results from the european male aging study. J Am Geriatr Soc. 2011;59:814–21. doi: 10.1111/j.1532-5415.2011.03398.x. [DOI] [PubMed] [Google Scholar]
- 81.Araujo AB, Travison TG, Bhasin S, Esche GR, Williams RE, et al. Association between testosterone and estradiol and age–related decline in physical function in a diverse sample of men. J Am Geriatr Soc. 2008;56:2000–8. doi: 10.1111/j.1532-5415.2008.01965.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Auyeung TW, Lee JS, Kwok T, Leung J, Ohlsson C, et al. Testosterone but not estradiol level is positively related to muscle strength and physical performance independent of muscle mass: a cross–sectional study in 1489 older men. Eur J Endocrinol. 2011;164:811–7. doi: 10.1530/EJE-10-0952. [DOI] [PubMed] [Google Scholar]
- 83.Baumgartner RN, Waters DL, Gallagher D, Morley JE, Garry PJ. Predictors of skeletal muscle mass in elderly men and women. Mech Ageing Dev. 1999;107:123–36. doi: 10.1016/s0047-6374(98)00130-4. [DOI] [PubMed] [Google Scholar]
- 84.Chin KY, Soelaiman IN, Naina Mohamed I, Shahar S, Teng NI, et al. Testosterone is associated with age–related changes in bone health status, muscle strength and body composition in men. Aging Male. 2012;15:240–5. doi: 10.3109/13685538.2012.724740. [DOI] [PubMed] [Google Scholar]
- 85.Iannuzzi–Sucich M, Prestwood KM, Kenny AM. Prevalence of sarcopenia and predictors of skeletal muscle mass in healthy, older men and women. J Gerontol A Biol Sci Med Sci. 2002;57:M772–7. doi: 10.1093/gerona/57.12.m772. [DOI] [PubMed] [Google Scholar]
- 86.Szulc P, Duboeuf F, Marchand F, Delmas PD. Hormonal and lifestyle determinants of appendicular skeletal muscle mass in men: the MINOS study. Am J Clin Nutr. 2004;80:496–503. doi: 10.1093/ajcn/80.2.496. [DOI] [PubMed] [Google Scholar]
- 87.van den Beld AW, de Jong FH, Grobbee DE, Pols HA, Lamberts SW. Measures of bioavailable serum testosterone and estradiol and their relationships with muscle strength, bone density, and body composition in elderly men. J Clin Endocrinol Metab. 2000;85:3276–82. doi: 10.1210/jcem.85.9.6825. [DOI] [PubMed] [Google Scholar]
- 88.Waters DL, Yau CL, Montoya GD, Baumgartner RN. Serum sex hormones, IGF–1, and IGFBP3 exert a sexually dimorphic effect on lean body mass in aging. J Gerontol A Biol Sci Med Sci. 2003;58:648–52. doi: 10.1093/gerona/58.7.m648. [DOI] [PubMed] [Google Scholar]
- 89.Roy TA, Blackman MR, Harman SM, Tobin JD, Schrager M, et al. Interrelationships of serum testosterone and free testosterone index with FFM and strength in aging men. Am J Physiol Endocrinol Metab. 2002;283:E284–94. doi: 10.1152/ajpendo.00334.2001. [DOI] [PubMed] [Google Scholar]
- 90.Folland JP, Mc Cauley TM, Phypers C, Hanson B, Mastana SS. The relationship of testosterone and AR CAG repeat genotype with knee extensor muscle function of young and older men. Exp Gerontol. 2012;47:437–43. doi: 10.1016/j.exger.2012.03.013. [DOI] [PubMed] [Google Scholar]
- 91.Vandenput L, Mellstrom D, Karlsson MK, Orwoll E, Labrie F, et al. Serum estradiol is associated with lean mass in elderly Swedish men. Eur J Endocrinol. 2010;162:737–45. doi: 10.1530/EJE-09-0696. [DOI] [PubMed] [Google Scholar]
- 92.Orwoll E, Lambert LC, Marshall LM, Blank J, Barrett–Connor E, et al. Endogenous testosterone levels, physical performance, and fall risk in older men. Arch Intern Med. 2006;166:2124–31. doi: 10.1001/archinte.166.19.2124. [DOI] [PubMed] [Google Scholar]
- 93.Maggio M, Ceda GP, Lauretani F, Bandinelli S, Metter EJ, et al. Gonadal status and physical performance in older men. Aging Male. 2011;14:42–7. doi: 10.3109/13685538.2010.518179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Trabert B, Graubard B, Nyante SJ, Rifai N, Bradwin G, et al. Relationship of sex steroid hormones with body size and with body composition measured by dual–energy X–ray absorptiometry in US men. Cancer Causes Control. 2012;23:1881–91. doi: 10.1007/s10552-012-0024-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gates MA, Mekary RA, Chiu GR, Ding EL, Wittert GA, et al. Sex steroid hormone levels and body composition in men. J Clin Endocrinol Metab. 2013;98:2442–50. doi: 10.1210/jc.2012-2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Miljkovic I, Cauley JA, Dressen AS, Gordon CL, Goodpaster BH, et al. Bioactive androgens and glucuronidated androgen metabolites are associated with subcutaneous and ectopic skeletal muscle adiposity among older black men. Metabolism. 2011;60:1178–85. doi: 10.1016/j.metabol.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.LeBlanc ES, Wang PY, Lee CG, Barrett–Connor E, Cauley JA, et al. Higher testosterone levels are associated with less loss of lean body mass in older men. J Clin Endocrinol Metab. 2011;96:3855–63. doi: 10.1210/jc.2011-0312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yuki A, Otsuka R, Kozakai R, Kitamura I, Okura T, et al. Relationship between low free testosterone levels and loss of muscle mass. Sci Rep. 2013;3:1818. doi: 10.1038/srep01818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Syddall H, Cooper C, Martin F, Briggs R, Aihie Sayer A. Is grip strength a useful single marker of frailty? Age Ageing. 2003;32:650–6. doi: 10.1093/ageing/afg111. [DOI] [PubMed] [Google Scholar]
- 100.Perry HM, 3rd, Miller DK, Patrick P, Morley JE. Testosterone and leptin in older African–American men: relationship to age, strength, function, and season. Metabolism. 2000;49:1085–91. doi: 10.1053/meta.2000.7710. [DOI] [PubMed] [Google Scholar]
- 101.Schaap LA, Pluijm SM, Smit JH, van Schoor NM, Visser M, et al. The association of sex hormone levels with poor mobility, low muscle strength and incidence of falls among older men and women. Clin Endocrinol (Oxf) 2005;63:152–60. doi: 10.1111/j.1365-2265.2005.02315.x. [DOI] [PubMed] [Google Scholar]
- 102.O’Donnell AB, Travison TG, Harris SS, Tenover JL, McKinlay JB. Testosterone, Dehydroepiandrosterone, and physical performance in older men: results from the massachusetts male aging study. J Clin Endocrinol Metab. 2006;91:425–31. doi: 10.1210/jc.2005-1227. [DOI] [PubMed] [Google Scholar]
- 103.Schaap LA, Pluijm SM, Deeg DJ, Penninx BW, Nicklas BJ, et al. Health ABC study. Low testosterone levels and decline in physical performance and muscle strength in older men: findings from two prospective cohort studies. Clin Endocrinol (Oxf) 2008;68:42–50. doi: 10.1111/j.1365-2265.2007.02997.x. [DOI] [PubMed] [Google Scholar]
- 104.Krasnoff JB, Basaria S, Pencina MJ, Jasuja GK, Vasan RS, et al. Free testosterone levels are associated with mobility limitation and physical performance in community–dwelling men: the Framingham Offspring Study. J Clin Endocrinol Metab. 2010;95:2790–9. doi: 10.1210/jc.2009-2680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Blackman MR, Sorkin JD, Munzer T, Bellantoni MF, Busby-Whitehead J, et al. Growth hormone and sex steroid administration in healthy aged women and men: a randomized controlled trial. JAMA. 2002;288:2282–92. doi: 10.1001/jama.288.18.2282. [DOI] [PubMed] [Google Scholar]
- 106.Emmelot-Vonk MH, Verhaar HJ, Nakhai Pour HR, Aleman A, Lock TM, et al. Effect of testosterone supplementation on functional mobility, cognition, and other parameters in older men: a randomized controlled trial. JAMA. 2008;299:39–52. doi: 10.1001/jama.2007.51. [DOI] [PubMed] [Google Scholar]
- 107.Kenny AM, Kleppinger A, Annis K, Rathier M, Browner B, et al. Effects of transdermal testosterone on bone and muscle in older men with low bioavailable testosterone levels, low bone mass, and physical frailty. J Am Geriatr Soc. 2010;58:1134–43. doi: 10.1111/j.1532-5415.2010.02865.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kenny AM, Prestwood KM, Gruman CA, Marcello KM, Raisz LG. Effects of transdermal testosterone on bone and muscle in older men with low bioavailable testosterone levels. J Gerontol A Biol Sci Med Sci. 2001;56:M266–72. doi: 10.1093/gerona/56.5.m266. [DOI] [PubMed] [Google Scholar]
- 109.Liu PY, Wishart SM, Handelsman DJ. A double–blind, placebo–controlled, randomized clinical trial of recombinant human chorionic gonadotropin on muscle strength and physical function and activity in older men with partial age–related androgen deficiency. J Clin Endocrinol Metab. 2002;87:3125–35. doi: 10.1210/jcem.87.7.8630. [DOI] [PubMed] [Google Scholar]
- 110.Nair KS, Rizza RA, O’Brien P, Dhatariya K, Short KR, et al. DHEA in elderly women and DHEA or testosterone in elderly men. N Engl J Med. 2006;355:1647–59. doi: 10.1056/NEJMoa054629. [DOI] [PubMed] [Google Scholar]
- 111.Page ST, Amory JK, Bowman FD, Anawalt BD, Matsumoto AM, et al. Exogenous testosterone (T) alone or with finasteride increases physical performance, grip strength, and lean body mass in older men with low serum T. J Clin Endocrinol Metab. 2005;90:1502–10. doi: 10.1210/jc.2004-1933. [DOI] [PubMed] [Google Scholar]
- 112.Sattler FR, Castaneda–Sceppa C, Binder EF, Schroeder ET, Wang Y, et al. Testosterone and growth hormone improve body composition and muscle performance in older men. J Clin Endocrinol Metab. 2009;94:1991–2001. doi: 10.1210/jc.2008-2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Snyder PJ, Peachey H, Hannoush P, Berlin JA, Loh L, et al. Effect of testosterone treatment on body composition and muscle strength in men over 65 years of age. J Clin Endocrinol Metab. 1999;84:2647–53. doi: 10.1210/jcem.84.8.5885. [DOI] [PubMed] [Google Scholar]
- 114.Srinivas–Shankar U, Roberts SA, Connolly MJ, O’Connell MD, Adams JE, et al. Effects of testosterone on muscle strength, physical function, body composition, and quality of life in intermediate-frail and frail elderly men: a randomized, double–blind, placebo–controlled study. J Clin Endocrinol Metab. 2010;95:639–50. doi: 10.1210/jc.2009-1251. [DOI] [PubMed] [Google Scholar]
- 115.Tenover JS. Effects of testosterone supplementation in the aging male. J Clin Endocrinol Metab. 1992;75:1092–8. doi: 10.1210/jcem.75.4.1400877. [DOI] [PubMed] [Google Scholar]
- 116.Travison TG, Basaria S, Storer TW, Jette AM, Miciek R, et al. Clinical meaningfulness of the changes in muscle performance and physical function associated with testosterone administration in older men with mobility limitation. J Gerontol A Biol Sci Med Sci. 2011;66A:1090–9. doi: 10.1093/gerona/glr100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wittert GA, Chapman IM, Haren MT, Mackintosh S, Coates P, et al. Oral testosterone supplementation increases muscle and decreases fat mass in healthy elderly males with low–normal gonadal status. J Gerontol A Biol Sci Med Sci. 2003;58:618–25. doi: 10.1093/gerona/58.7.m618. [DOI] [PubMed] [Google Scholar]
- 118.Frederiksen L, Højlund K, Hougaard DM, Brixen K, Andersen M. Testosterone therapy increased muscle mass and lipid oxidation in aging men. Age (Dorodr) 2012;34:145–56. doi: 10.1007/s11357-011-9213-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Brill KT, Weltman AL, Gentili A, Patrie JT, Fryburg DA, et al. Single and combined effects of growth hormone and testosterone administration on measures of body composition, physical performance, mood, sexual function, bone turnover, and muscle gene expression in healthy older men. J Clin Endocrinol Metab. 2002;87:5649–57. doi: 10.1210/jc.2002-020098. [DOI] [PubMed] [Google Scholar]
- 120.Clague JE, Wu FC, Horan MA. Difficulties in measuring the effect of testosterone replacement therapy on muscle function in older men. Int J Androl. 1999;22:261–5. doi: 10.1046/j.1365-2605.1999.00177.x. [DOI] [PubMed] [Google Scholar]
- 121.Giannoulis MG, Sonksen PH, Umpleby M, Breen L, Pentecost C, et al. The effects of growth hormone and/or testosterone in healthy elderly men: a randomized controlled trial. J Clin Endocrinol Metab. 2006;91:477–84. doi: 10.1210/jc.2005-0957. [DOI] [PubMed] [Google Scholar]
- 122.Storer TW, Woodhouse L, Magliano L, Singh AB, Dzekov C, et al. Changes in muscle mass, muscle strength, and power but not physical function are related to testosterone dose in healthy older men. J Am Geriatr Soc. 2008;56:1991–9. doi: 10.1111/j.1532-5415.2008.01927.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bean JF, Kiely DK, Herman S, Leveille SG, Mizer K, et al. The relationship between leg power and physical performance in mobility–limited older people. J Am Geriatr Soc. 2002;50:461–7. doi: 10.1046/j.1532-5415.2002.50111.x. [DOI] [PubMed] [Google Scholar]
- 124.Macaluso A, Vito G. Muscle strength, power and adaptations to resistance training in older people. Eur J Appl Physiol. 2004;91:450–72. doi: 10.1007/s00421-003-0991-3. [DOI] [PubMed] [Google Scholar]
- 125.Morley JE, Perry HM, 3rd, Kaiser FE, Kraenzle D, Jensen J, et al. Effects of testosterone replacement therapy in old hypogonadal males: a preliminary study. J Am Geriatr Soc. 1993;41:149–52. doi: 10.1111/j.1532-5415.1993.tb02049.x. [DOI] [PubMed] [Google Scholar]
- 126.Sih R, Morley JE, Kaiser FE, Perry HM, 3rd, Patrick P, et al. Testosterone replacement in older hypogonadal men: a 12–Month randomized controlled trial. J Clin Endocrinol Metab. 1997;82:1661–7. doi: 10.1210/jcem.82.6.3988. [DOI] [PubMed] [Google Scholar]
- 127.Basaria S, Coviello AD, Travison TG, Storer TW, Farwell WR, et al. Adverse events associated with testosterone administration. N Engl J Med. 2010;363:109–22. doi: 10.1056/NEJMoa1000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ly LP, Jimenez M, Zhuang TN, Celermajer DS, Conway AJ, et al. A double–blind, placebo–controlled, randomized clinical trial of transdermal dihydrotestosterone gel on muscular strength, mobility, and quality of life in older men with partial androgen deficiency. J Clin Endocrinol Metab. 2001;86:4078–88. doi: 10.1210/jcem.86.9.7821. [DOI] [PubMed] [Google Scholar]
- 129.Schroeder ET, He J, Yarasheski KE, Binder EF, Castaneda–Sceppa C, et al. Value of measuring muscle performance to assess changes in lean mass with testosterone and growth hormone supplementation. Eur J Appl Physiol. 2012;112:1123–31. doi: 10.1007/s00421-011-2077-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sattler F, Bhasin S, He J, Chou CP, Castaneda–Sceppa C, et al. Testosterone threshold levels and lean tissue mass targets needed to enhance skeletal muscle strength and function: the HORMA trial. J Gerontol A Biol Sci Med Sci. 2011;66:122–9. doi: 10.1093/gerona/glq183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Verdijk LB, van Loon L, Meijer K, Savelberg HH. One–repetition maximum strength test represents a valid means to assess leg strength in vivo in humans. J Sports Sci. 2009;27:59–68. doi: 10.1080/02640410802428089. [DOI] [PubMed] [Google Scholar]
- 132.Becker R, Berth A, Nehring M, Awiszus F. Neuromuscular quadriceps dysfunction prior to osteoarthritis of the knee. J Orthop Res. 2004;22:768–73. doi: 10.1016/j.orthres.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 133.Roos MR, Rice CL, Connelly DM, Vandervoort AA. Quadriceps muscle strength, contractile properties, and motor unit firing rates in young and old men. Muscle Nerve. 1999;22:1094–103. doi: 10.1002/(sici)1097-4598(199908)22:8<1094::aid-mus14>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 134.Buchner DM, Larson EB, Wagner EH, Koepsell TD, De Lateur BJ. Evidence for a non–linear relationship between leg strength and gait speed. Age Ageing. 1996;25:386–91. doi: 10.1093/ageing/25.5.386. [DOI] [PubMed] [Google Scholar]
- 135.Kwon IS, Oldaker S, Schrager M, Talbot LA, Fozard JL, et al. Relationship between muscle strength and the time taken to complete a standardized walk–turn–walk test. J Gerontol A Biol Sci Med Sci. 2001;56:B398–404. doi: 10.1093/gerona/56.9.b398. [DOI] [PubMed] [Google Scholar]
- 136.Rantanen T, Guralnik JM, Izmirlian G, Williamson JD, Simonsick EM, et al. Association of muscle strength with maximum walking speed in diisabled older women. Am J Phys Med Rehabil. 1998;77:299–305. doi: 10.1097/00002060-199807000-00008. [DOI] [PubMed] [Google Scholar]
- 137.Lord SR, Murray SM, Chapman K, Munro B, Tiedemann A. Sit–to–Stand performance depends on sensation, speed, balance, and psychological status in addition to strength in older people. J Gerontol A Biol Sci Med Sci. 2002;57:M539–43. doi: 10.1093/gerona/57.8.m539. [DOI] [PubMed] [Google Scholar]
- 138.Bhasin S. The brave new world of function–promoting anabolic therapies: testosterone and frailty. J Clin Endocrinol Metab. 2010;95:509–11. doi: 10.1210/jc.2009-2550. [DOI] [PubMed] [Google Scholar]
- 139.O’Connell MD, Roberts SA, Srinivas–Shankar U, Tajar A, Connolly MJ, et al. Do the effects of testosterone on muscle strength, physical function, body composition and quality of life persist six months post–treatment in intermediate–frail and frail elderly men? J Clin Endocrinol Metab. 2011;96:454–8. doi: 10.1210/jc.2010-1167. [DOI] [PubMed] [Google Scholar]
- 140.Sattler FR, Bhasin S, He J, Yarasheski KE, Binder EF, et al. Durability of the effects of testosterone and growth hormone supplementation in older community-dwelling men: the HORMA Trial. Clin Endocrinol. 2011;75:103–11. doi: 10.1111/j.1365-2265.2011.04014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Calof OM, Singh AB, Lee ML, Kenny AM, Urban RJ, et al. Adverse events associated with testosterone replacement in middle–aged and older men: a meta–analysis of randomized, placebo–controlled trials. J Gerontol A Biol Sci Med Sci. 2005;60:1451–7. doi: 10.1093/gerona/60.11.1451. [DOI] [PubMed] [Google Scholar]
- 142.Fernandez–Balsells MM, Murad MH, Lane M, Lampropulos JF, Albuquerque F, et al. Adverse effects of testosterone therapy in adult men: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2010;95:2560–75. doi: 10.1210/jc.2009-2575. [DOI] [PubMed] [Google Scholar]
- 143.Haddad RM, Kennedy CC, Caples SM, Tracz MJ, Bolona ER, et al. Testosterone and cardiovascular risk in men: a systematic review and meta–analysis of randomized placebo–controlled trials. Mayo Clin Proc. 2007;82:29–39. doi: 10.4065/82.1.29. [DOI] [PubMed] [Google Scholar]
- 144.Basaria S, Davda MN, Travison TG, Ulloor J, Singh R, et al. Risk factors associated with cardiovascular events during testosterone administration in older men with mobility limitation. J Gerontol A Biol Sci Med Sci. 2013;68:153–60. doi: 10.1093/gerona/gls138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Bhasin S, Jasuja R. Selective androgen receptor modulators as function promoting therapies. Curr Opin Clin Nutr Metab Care. 2009;12:232–40. doi: 10.1097/MCO.0b013e32832a3d79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Mohler ML, Bohl CE, Jones A, Coss CC, Narayanan R, et al. Nonsteroidal selective androgen receptor modulators (SARMs): dissociating the anabolic and androgenic activities of the androgen receptor for therapeutic benefit. J Med Chem. 2009;52:3597–617. doi: 10.1021/jm900280m. [DOI] [PubMed] [Google Scholar]
- 147.Dalton J, Barnette K, Bohl C, Hancock M, Rodriguez D, et al. The selective androgen receptor modulator GTx–024 (enobosarm) improves lean body mass and physical function in healthy elderly men and postmenopausal women: results of a double–blind, placebo–controlled phase II trial. J Cachexia Sarcopenia Muscle. 2011;2:153–61. doi: 10.1007/s13539-011-0034-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Dobs AS, Boccia RV, Croot CC, Gabrail NY, Dalton JT, et al. Effects of enobosarm on muscle wasting and physical function in patients with cancer: a double–blind, randomised controlled phase 2 trial. Lancet Oncol. 2013;14:335–45. doi: 10.1016/S1470-2045(13)70055-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Papanicolaou DA, Ather SN, Zhu H, Zhou Y, Lutkiewicz J, et al. A phase IIA randomized, placebo–controlled clinical trial to study the efficacy and safety of the selective androgen receptor modulator (SARM), MK-0773 in female participants with sarcopenia. J Nutr Health Aging. 2013;17:533–43. doi: 10.1007/s12603-013-0335-x. [DOI] [PubMed] [Google Scholar]
- 150.Basaria S, Collins L, Dillon EL, Orwoll K, Storer TW, Miciek R, et al. The safety, pharmacokinetics, and effects of LGD–4033, a novel nonsteroidal oral, selective androgen receptor modulator, in healthy young men. J Gerontol A Biol Sci Med Sci. 2013;68:87–95. doi: 10.1093/gerona/gls078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Raue U, Slivka D, Minchev K, Trappe S. Improvements in whole muscle and myocellular function are limited with high–intensity resistance training in octogenarian women. J Appl Physiol (1985) 2009;106:1611–7. doi: 10.1152/japplphysiol.91587.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Hildreth KL, Barry DW, Moreau KL, Vande Griend J, Meacham RB, et al. Effects of testosterone and progressive resistance exercise in healthy, highly functioning older men with low–normal testosterone levels. J Clin Endocrinol Metab. 2013;98:1891–900. doi: 10.1210/jc.2012-3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kvorning T, Christensen LL, Madsen K, Nielsen JL, Gejl KD, et al. Mechanical Muscle function and lean body mass during supervised strength training and testosterone therapy in aging men with low–normal testosterone levels. J Am Geriatr Soc. 2013;61:957–62. doi: 10.1111/jgs.12279. [DOI] [PubMed] [Google Scholar]


