Abstract
With increasing modernization and urbanization of Asia, much of the future focus of the obesity epidemic will be in the Asian region. Low testosterone levels are frequently encountered in obese men who do not otherwise have a recognizable hypothalamic-pituitary-testicular (HPT) axis pathology. Moderate obesity predominantly decreases total testosterone due to insulin resistance-associated reductions in sex hormone binding globulin. More severe obesity is additionally associated with reductions in free testosterone levels due to suppression of the HPT axis. Low testosterone by itself leads to increasing adiposity, creating a self-perpetuating cycle of metabolic complications. Obesity-associated hypotestosteronemia is a functional, non-permanent state, which can be reversible, but this requires substantial weight loss. While testosterone treatment can lead to moderate reductions in fat mass, obesity by itself, in the absence of symptomatic androgen deficiency, is not an established indication for testosterone therapy. Testosterone therapy may lead to a worsening of untreated sleep apnea and compromise fertility. Whether testosterone therapy augments diet- and exercise-induced weight loss requires evaluation in adequately designed randomized controlled clinical trials.
Keywords: androgens, hypogonadism, obesity, testosterone, weight loss
PREVALENCE AND CLINICAL SIGNIFICANCE OF OBESITY
Obesity, a worldwide epidemic, is on the rise. Populous developing Asian nations such as China and India have seen increases in the prevalence of overweight (body mass index (BMI) 25-29.9 kg m−2) and obesity (BMI ≥ 30 kg m−2) in adult men by more than 25% in the last 8 years according to WHO estimates1 (Table 1). In developed countries including Australia, over 75% of the adult male population is already overweight or obese. The number of overweight people is expected to increase from 937 million in 2005 to 1.35 billion in 2030.2 Similarly the number of obese people is projected to increase from 396 million in 2005 to 573 million in 2030. By 2030, China alone is predicted to have more overweight men and women than the traditional market economies combined.
Table 1.
World health organization estimates of overweight and obesity (BMI>25 kg m-2) in males aged 30–100 years in selected Asia-Pacific countries1
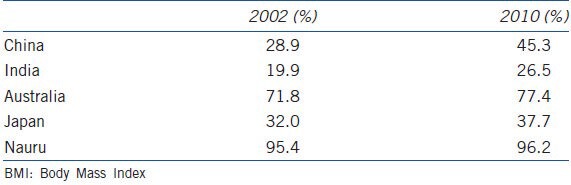
In addition to posing significant societal and environmental challenges, obesity is associated with a multitude of adverse health outcomes including cardiovascular disease, sleep apnea, osteoarthritis, increased risk of certain cancers, and in men, lowered testosterone levels.3 A recent systematic review and meta-analysis including 2.8 million people and 270 000 deaths reported increased overall mortality only in those with extreme obesity (BMI > 35 kg m−2, hazard ratio (HR) 1.29, 95% confidence interval (CI) 1.18–1.41), but not in grade 1 obesity (BMI 30–34.9 kg m−2, HR 0.95, 95% CI 0.88–1.01) compared to their non-obese counterparts.4 However, this meta-analysis has been subsequently criticized in a series of letters and commentaries.5,6,7 For example, the adverse effect of obesity may have been underestimated because the lean comparison group (BMI 18-25 kg m−2) included frail and elderly with serious illness and weight loss due to their disease. Therefore, the “obesity paradox” remains a hotly debated, but currently still unresolved issue. In addition, obesity, as measured by BMI, is a relatively crude indicator of metabolic risk with waist circumference providing a better indicator of all-cause (HR 1.19 vs 1.10 per standard deviation) and cardiovascular mortality (HR 1.33 vs 1.23 per standard deviation) compared to BMI.8 This is because excess weight stored as visceral adipose tissue (VAT) is more closely linked to cardiovascular outcomes than subcutaneous adipose tissue (SAT). Consistent with this, there is evidence that some individuals may be metabolically healthy despite a BMI in the obese range (MHO), because they have lower amounts of VAT. Conversely, others may present with a cluster of obesity-associated risk factors for diabetes and cardiovascular disease despite a BMI in the normal range, the so-called “metabolically obese but normal weight” (MONW). Indeed, a recent prospective cohort study from Korea has shown that MONW individuals have a higher mortality than MHO.9
The capacity to store excess energy in SAT vs VAT may be genetically regulated, providing a potential mechanistic explanation for the variability in metabolic risk at a given BMI. Interestingly, diacylglycerol O-acyltransferase 2 (DGAT2), mechanistically implicated in this differential storage,10 is regulated by dihydrotestosterone,11 suggesting a potential role for androgens to influence the genetic predisposition to either the MHO or MONW phenotype.
Unfortunately, obesity is a chronic condition that is difficult to treat. Public health measures, lifestyle interventions and pharmacotherapy adopted thus far have neither registered a marked impact on the prevalence of obesity, nor markedly reduced body weight-related disease burden.12 Although dietary restriction often results in initial weight loss, many obese dieters fail to maintain their reduced weight, and there is increasing evidence that weight is physiologically defended.13 While bariatric surgery achieves 10%–30% long-term weight loss in controlled studies,12 this therapy is expensive and currently not widely available.
OBESITY AND LOW TESTOSTERONE: EVIDENCE FROM POPULATION-BASED STUDIES
The fact that obese men have lower testosterone compared to lean men has been recognized for more than 30 years.14 Since then, multiple cross-sectional and prospective studies have consistently found negative linear correlations between both total and free testosterone levels and adiposity in men.15 In a cohort of 3219 men from the European Male Aging Study (EMAS), obesity was associated with an 8.7-fold and overweight with a 3.3-fold increased relative risk (RR) of secondary hypogonadism (defined as total testosterone of <10.5 nmol l−1 and normal luteinizing hormone (LH)), relative to normal weight3. Both total testosterone (5.9 nmol l−1) and free testosterone (54 pmol l−1) levels were lower in obese compared to lean men, with lesser but still significant reduction in overweight men (total T 2.3 nmol l−1, free T 18 pmol l−1)16. A cross-sectional study of 314 Chinese men similarly found that older obese men (defined by BMI >28 kg m−2 for these Asian men) had a 3 nmol l−1 lower testosterone level compared to age-matched lean men.17 The inverse association of BMI with low testosterone may be compounded by the presence of comorbidities: while one Australian study reported a relatively low 12% prevalence of low testosterone in obese, but otherwise healthy men;18 another study found a 57% prevalence in diabetic men attending tertiary hospital outpatient clinics.19 In a cross-sectional study of 1849 community-dwelling obese US American men 40% had low testosterone levels.20 Reductions in testosterone levels correlate with the severity of obesity and men with a BMI >35–40 kg m−2 have >50% reduction in total and free testosterone levels compared to lean men.15
Obesity can be an important confounder when testosterone levels are compared across different ethnic groups. For example, in a cross-sectional study, while Japanese and Hong Kong Asian men had higher unadjusted testosterone levels than Swedish and US men, these differences did not persist when adjusted for BMI.21 Similarly, in a multi-ethnic Malaysian population, the lower (11%) prevalence of low testosterone in Chinese compared Malay and Indian men (21%) was due to a higher burden of obesity and the metabolic syndrome in the latter.22
In summary, observational studies consistently show a strong association of obesity with low circulating testosterone levels in men. Indeed, epidemiological data suggest that the single most powerful predictor of low testosterone is obesity, and that obesity is a major contributor of the age-associated decline in testosterone levels.16 Conversely, there is increasing evidence that healthy ageing by itself is uncommonly associated with marked reductions in testosterone levels.23 This may be because age-related testicular dysfunction is, at least in part, compensated for by an age-associated increase in pituitary LH secretion.3 However, because obesity blunts this LH rise, obesity leads to hypothalamic-pituitary suppression irrespective of age which cannot be compensated for by physiological mechanisms.3
OBESITY AND LOW TESTOSTERONE: POTENTIAL MECHANISMS AND BIOLOGICAL PLAUSIBILITY
Overweight and moderate obesity is predominantly associated with reductions in total testosterone; whereas, free testosterone levels remain within the reference range, especially in younger men. Reductions in total testosterone levels are largely a consequence of reductions in sex hormone binding globulin (SHBG) due to obesity-associated hyperinsulinemia. Indeed, although controversial, measurement of free testosterone levels may provide a more accurate assessment of androgen status than the (usually preferred) measurement of total testosterone in situations where SHBG levels are outside the reference range.24 However, reference ranges for free testosterone levels are not well established, especially in older men whose SHBG increases with age. Some have argued that the measurement of free testosterone levels merely reintroduces age in a covert form.25
More marked obesity however is associated with an unequivocal reduction of free testosterone levels, where LH and follicle stimulating hormone (FSH) levels are usually low or inappropriately normal, suggesting that the dominant suppression occurs at the hypothalamic-pituitary level. This may be because adipose tissue, especially when in the inflamed, insulin-resistant state, expresses aromatase which converts testosterone to estradiol (E2). Adipose E2 in turn may feedback negatively to decrease pituitary gonadotropin secretion; although, partly due to assay limitations, confirmation of increased circulatory E2 concentrations is often elusive. In addition, local, tissue-specific increases of E2 may not be reflected in circulatory concentrations. However, this adipose tissue-aromatase hypothesis is not well supported by other data. Clinical studies showing that treatment of obese men with aromatase inhibitors can increase testosterone levels and restore fertility26 do not necessarily support the pathophysiological importance of this E2-mediated hypothalamic-pituitary-testicular (HPT) axis suppression, because gonadotropins and testosterone levels also rise with this treatment. Interestingly, more recent studies suggest that, diabetic obesity is associated with decreases in circulatory E2.27 Moreover, there is evidence from the EMAS that even in nondiabetic obese men, E2 is low and correlated with low testosterone levels.28,29 In addition to E2, increased visceral fat also releases increased amounts of pro-inflammatory cytokines, insulin and leptin; all of which may inhibit the activity of the HPT axis at multiple levels.30
Evidence that obesity leads to lower testosterone
Multiple observational studies in community-dwelling men suggest that obesity leads to decreased testosterone. In the prospective Massachusetts Male Aging Study (MMAS), moving from a non-obese to an obese state resulted in a decline of testosterone levels comparable to that of advancing 10 years in age.31 Similar findings have been reported in cohort studies of men from Europe3,32 and Australia.33 Finally, as discussed in more detail in section 5, weight loss, whether by diet or surgery, increases testosterone levels proportional to the amount of weight lost.19,34
Evidence that low testosterone promotes obesity
While the above discussed studies suggest that obesity leads to reduced testosterone, there is also ample evidence, both from experimental and human studies, to suggest the reverse. Evidence from studies in mice with genetic deletions of the androgen receptor (AR) (AR knockout (ARKO)) is discussed by Rana et al. in more detail elsewhere in this issue. Briefly, ARKO mice develop obesity with increased adipocyte numbers and visceral fat mass suggesting that fat is androgen-responsive.35 A study of mice with a targeted deletion of the AR in adipose tissue showed that compared to controls, higher visceral fat develops only in the setting of a high fat diet, but not with regular chow, suggesting that low testosterone may augment the effects of a hypercaloric diet.36 In support of this, transgenic mice with AR overexpression show reduction in adipose tissue volume37 due to reduction in adipocyte area and adipocyte size. Primate experiments in Japanese macaques show that androgen depletion via castration alters adipocytes size and appearance to an insulin-resistant phenotype which can be rescued by androgen replacement.38 Consistent with animal experiments linking low testosterone to increases in fat mass are in vitro studies showing that testosterone promotes commitment of pluripotent rodent stem cells to the myogenic lineage, but inhibits their differentiation into adipocytes via an androgen-receptor mediated pathway.39 In human male ex vivo adipose tissue, testosterone decreased adipocyte differentiation by 50%.40 Testosterone enhances catecholamine-induced lipolysis in vitro and reduces lipoprotein lipase activity and triglyceride uptake in human abdominal adipose tissue in vivo.19 Moreover, in men with prostate cancer receiving 12 months of androgen deprivation therapy, fat mass increased by 3.4 kg and abdominal VAT by 22%, with the majority of these changes established within 6 months.41 Experimental induction of hypogonadism in healthy young men with gonadotropin-releasing hormone analogue treatment increased fat mass within 10 weeks, suggesting that severe sex steroid deficiency can increase fat mass rapidly.42
While evidence reviewed so far suggests that relatively extreme manipulations of testosterone are required to effect changes in fat mass, more moderate variations on testosterone as seen in the majority of men can also impact on fat mass. For example, in a longitudinal study of community-dwelling Japanese-American men, lower baseline testosterone independently predicted increase in intra-abdominal fat after 7.5 years of follow-up.43 Finally, confirmation that testosterone treatment reduces fat mass has been verified in multiple randomized controlled trials (RCT) (see below).
Low testosterone and obesity: a self-perpetuating cycle
In summary, the current evidence suggests a bidirectional relationship between testosterone and obesity (Figure 1) in men initiating a self-perpetuating cycle, which may have treatment implications (see sections “TREATMENT OF OBESITY LEADING TO INCREASED TESTOSTERONE” and “INTERVENTION STUDIES LINKING EXOGENOUS TESTOSTERONE TO REDUCTION IN BODY FAT MASS” below). On the one hand, increasing body fat suppresses the HPT axis by multiple mechanisms30 via increased secretion of pro-inflammatory cytokines, insulin resistance and diabetes;19,44 while on the other hand low testosterone promotes further accumulation of total and visceral fat mass, thereby exacerbating the gonadotropin inhibition. Finally there is evidence, reviewed elsewhere,45 that obesity-associated comorbidities including obstructive sleep apnea and hypercortisolism may also suppress the HPT axis.
Figure 1.
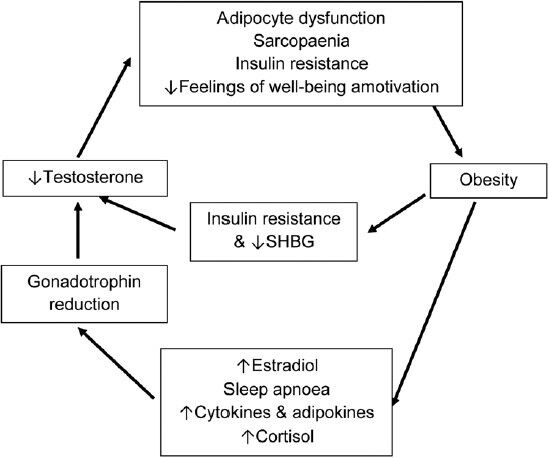
Bidirectional relationship between obesity and low testosterone.
In addition to lowered circulating serum total testosterone levels, obese individuals may have a propensity to lowered androgens in the local fat milieu. Belanger, et al.46 found a significant negative correlation of omental testosterone levels with waist circumference (r = −0.59, P <0.002). Increased activity of the DHT inactivating enzyme 3α/β-ketosteroid reductase (3α/β-HSD) in obese versus non-obese male omental fat biopsies coupled with rat studies showing increased expression of AR in VAT as opposed to SAT47 suggests that androgens may play a more significant role in VAT than SAT. Indeed, men undergoing androgen depletion for prostate cancer show more marked increases in visceral compared to subcutaneous fat following treatment.41 However, RCT studies of testosterone replacement have largely failed to show benefit of selective VAT reduction following testosterone treatment (see section “INTERVENTION STUDIES LINKING EXOGENOUS TESTOSTERONE TO REDUCTION IN BODY FAT MASS” below). In contrast, relatively little is known about the role of androgens in brown fat, since its potential role in energy expenditure in humans has been recognized only more recently. The recently-discovered hormone irisin, derived from muscle, induces brown fat-like properties in rodent white fat48 and its overexpression led to reduced weight and improved glucose homeostasis.49 Whether the action of androgens on fat is mediated in part via irisin is yet to be determined; although, molecular experiments suggest that androgens can act via the PPARγ-pathway37 which is implicated in the differentiation of precursor fat cells to the energy-consuming phenotype.50
LOW TESTOSTERONE AND OBESITY BEYOND TESTOSTERONE-FAT INTERACTIONS
Because of its association with sarcopenia,51 low testosterone may compound the effect of increasing fat mass by making it more difficult for obese men to lose weight via exercise. Conversely, obesity in itself contributes to loss of muscle mass and function, thus escalating the effects of sarcopenia on mobility disability and functional impairment, a concept known as ‘sarcopenic obesity’.52 Indeed, pro-inflammatory cytokines released by adipose tissue may contribute to loss of muscle mass and function, leading to inactivity and further weight gain in a vicious cycle.53,54 Sarcopenic obesity, a phenotype recapitulated in men receiving ADT for prostate cancer,55 may not only be associated with functional limitations, but also aggravate the metabolic risks of obesity; the association of low testosterone with sarcopenia may be an additional mechanism linking low testosterone to insulin resistance beyond its relationship to increased visceral fat.56
An important concern related to otherwise desirable weight loss induced by hypocaloric dieting, especially in older obese men who are already at risk of sarcopenia, is the concomitant loss of muscle mass causing altered function of muscle and physical functional decline.52,54 Although this accelerated loss of muscle mass can be attenuated by exercise,57 adherence to an exercise program is often difficult to achieve. Whether testosterone treatment will attenuate the catabolic effects of diet restriction on loss of muscle mass and function, requires further study. Consistent with this hypothesis is observational evidence associating higher endogenous testosterone with reduced loss of muscle mass and crude measures of muscle function in men losing weight.58
In addition to muscle effects, reduced testosterone levels may also lead to obesity via its effects on motivation to exercise. In a study of male mice lacking the androgen-receptor, spontaneous activity was reduced compared to wildtype mice,59 while another animal study reported a positive association between testosterone intake and amount of time spent on a running wheel.60 In a small RCT, men receiving testosterone undecanoate showed reduced fatigue although the effect of testosterone on exercise motivation and tolerance is to be determined.61
TREATMENT OF OBESITY LEADING TO INCREASED TESTOSTERONE
Observational evidence that weight changes are inversely associated with testosterone levels in community dwelling men have recently been reported in a longitudinal analysis of the EMAS cohort.34 Minor weight loss (<15%) over 4.4 years was associated with modest increases (+2 nmol l−1) in total testosterone, probably as a consequence of increases in SHBG; whereas, free testosterone did not change. However, a more substantial weight loss of >15% led not only to a more marked increase (+5.75 nmol l−1) of total testosterone, but was also associated with significant increase in free testosterone (+51.78 pmol l−1), likely because of HPT activation, evidenced by a significant rise in LH (+2 U l−1). This data suggests that while testosterone levels remain relatively stable with small fluctuations in weight, genuine reactivation of the HPT axis in obese men requires more substantial weight-loss, which may be difficult to achieve with lifestyle changes alone.
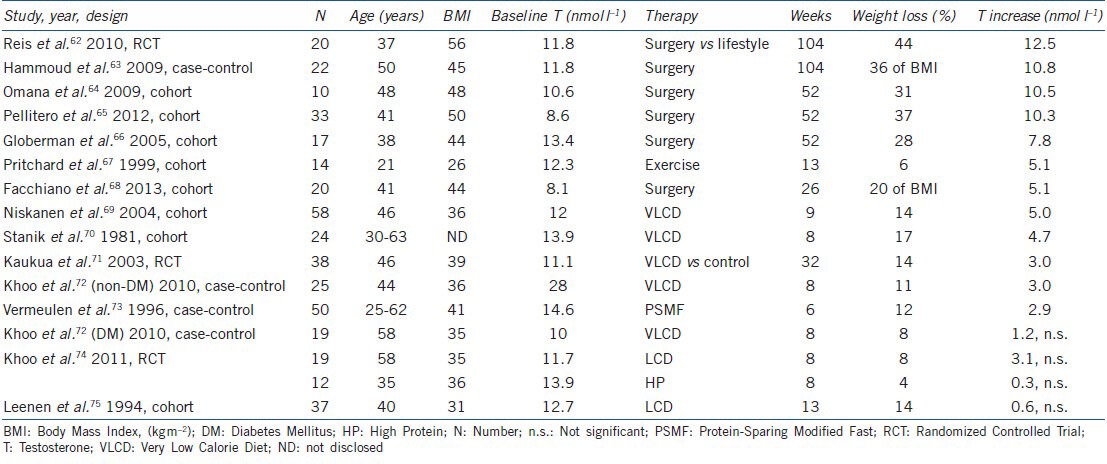
A number of intervention studies have confirmed that both diet- and surgically-induced weight losses are associated with increased testosterone, with the rise in testosterone generally proportional to the amount of weight lost (Figure 2). Table 2 lists 15 published trials that have assessed the effects of weight loss interventions on testosterone.62,63,64,65,66,67,68,69,70,71,72,73,74,75 The majority of the trials was single-arm cohort studies and included small numbers of subjects. Follow-up in the lifestyle trials was generally shorter than in the surgical trials. Overall, diet led to modest weight loss (6%–17%) with modest increases in testosterone (2.9–5.1 nmol l−1). In comparison to lifestyle, surgical intervention resulted in loss of weight of 28%–44% and increase of testosterone from 7.8 to 12.5 nmol l−1. While these studies were uncontrolled, a 32-week RCT comparing a very low calorie diet (VLCD) against no intervention reported a 14% weight loss and a 3.0 nmol l−1 increase in testosterone.71 Another small RCT comparing lifestyle modification with gastric bypass found that after prolonged follow-up of 2 years, bariatric surgery led to greater weight loss and greater testosterone increments compared to lifestyle.62 However, not all studies were positive, with a number of studies (Table 2) showing no increase in testosterone, possibly due to small reductions in weight (4%–8%)72,74 or modest baseline obesity in one study (BMI 31 kg m−2).75
Figure 2.
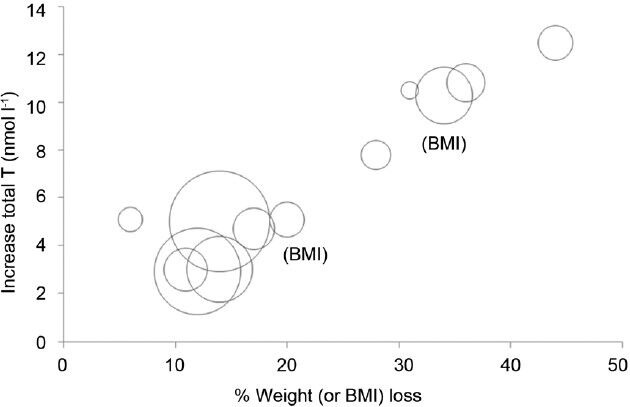
Effect of weight (or body mass index (BMI)) reduction on circulating total testosterone. Each circle represents a single longitudinal study. Size of the circle is proportional to study size. Adapted from Grossmann.19
Table 2.
Effect of weight loss on testosterone: clinical trials
A recent systematic review and meta-analysis of the effect of weight loss on testosterone reported that lifestyle changes achieve a mean weight reduction of 9.8% vs 32% for surgical intervention.76 Overall, diet therapy led to an increase in total testosterone of 2.87 vs 8.73 nmol l−1 in the surgical studies. While younger age and higher baseline BMI predicted greater gains in testosterone, in a stepwise logistic regression analysis, only the change in BMI was associated with change in testosterone. This suggests that men, regardless of obesity level, can benefit from the effect of weight loss.
INTERVENTION STUDIES LINKING EXOGENOUS TESTOSTERONE TO REDUCTION IN BODY FAT MASS
Testosterone replacement in men with authentic, pathologically-based hypogonadism reduces fat mass by 10%–15%.77,78 In a meta-analysis of RCTs of older men without confirmed hypogonadism (mean baseline serum testosterone 10.9 nmol l−1, BMI 29 kg m−2), testosterone treatment reduced total fat mass by 1.6 kg (95% CI 0.6–2.5), corresponding to a relative reduction of fat mass of 6.2% (95% CI 3.3–9.2).79 While these effects are relatively modest, more recent RCTs using long-acting testosterone undecanoate formulations in men with higher baseline BMI have found more pronounced effects on total fat mass, ranging from 2.5 to 6 kg.80,81,82,83 Uncontrolled studies recently reported larger benefits with progressive weight loss of up to 13% after 5 years of continuous testosterone undecanoate therapy in unselected patients.84
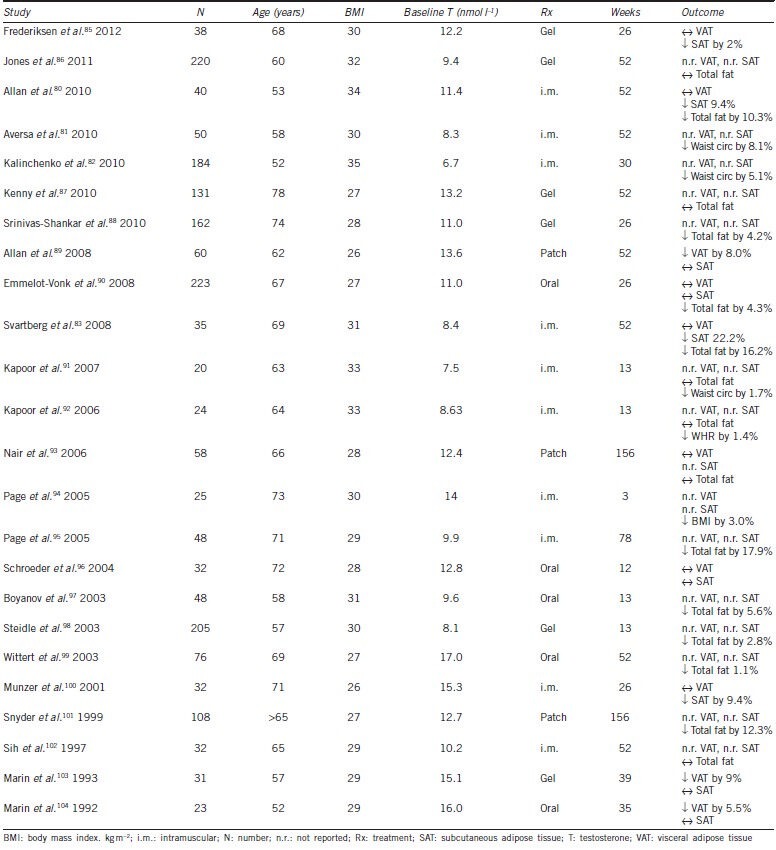
While RCTs consistently show that testosterone treatment reduces total body fat mass, effects of testosterone treatment on regional adipose tissue distribution have been less well-studied (Table 3).85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104 RCTs assessing effects of testosterone therapy on VAT have shown inconsistent results with one showing a reduction89 and others no change.80,90,93 These inconsistencies may be due to small trial size,96,100 use of oral testosterone therapy (which did not raise serum testosterone levels),90 or imprecise methodology to quantify VAT such as dual energy X-ray absorptiometry93 or ultrasound.90 Given that VAT is more closely related to insulin resistance and cardiovascular risk than SAT, inconsistent effect of testosterone on VAT may be one possible explanation as to why testosterone treatment, despite reduction of fat mass, has not consistently led to improvements in measures of glucose metabolism.19 Effects of testosterone therapy on glucose metabolism are discussed in more detail by Allan elsewhere in this issue.
Table 3.
Effect of testosterone therapy on body composition: randomized clinical trials
CLINICAL CONSEQUENCES OF LOW TESTOSTERONE IN OBESE MEN AND APPROACH TO THERAPY
While many obese men have low testosterone levels and nonspecific symptoms, it is unclear whether such symptoms are causally related to the hypotestosteronemia. In a study of 181 men with a low testosterone (<10.4 nmol l−1), less than half of men (n = 70) reported symptoms consistent with androgen deficiency and these men had higher BMIs than asymptomatic men.105 A cross-sectional study of older overweight men found that loss of libido occurred at a testosterone level <15 nmol l−1, poor concentration at <10 nmol l−] and erectile dysfunction at <8 nmol l−1. However, these thresholds confer neither sensitivity nor specificity for these symptoms, and a high specificity (> 90%) was achieved only when testosterone levels declined to <3.7–6.3 nmol l−1.106 In EMAS, while certain end-organ deficits compatible with androgen deficiency, such as reductions in muscle mass, hemoglobin and bone density; occurred more commonly in symptomatic men with a total testosterone of <12 nmol l−1, increased insulin resistance and the metabolic syndrome could only be demonstrated in men with testosterone <8 nmoll−1.29 Low testosterone either directly or via its metabolite E2 is a risk factor for osteoporotic fractures.107 While this may be counterbalanced by the protective effects of obesity on the skeleton,108 recent evidence suggests that increased VAT may have adverse consequences for skeletal health.56
One practical issue for the clinician is when and how to evaluate obese men with lowered testosterone for underlying intrinsic HPT axis pathology, rather than to assume that the lowered testosterone is a nonspecific consequence of the obesity. The probability of organic pathology is inversely related to age, BMI, number of comorbidities and testosterone level. We recommend a thorough clinical evaluation for symptoms and signs of androgen deficiency24 including assessment for end-organ deficits and pituitary pressure symptoms in all men. Mild, otherwise unexplained anemia,109 and trabecular-predominant osteopenia may be clues to organic androgen deficiency. Measurements of prolactin and where indicated, iron studies (hemochromatosis is less common in Asian men) should be performed in most men. Provided this evaluation is normal, pituitary imaging can be limited to men with a testosterone of repeatedly <5.2 nmol l−1 and non-raised gonadotropins.24
The presence of both obesity and low testosterone and can have negative impacts on fertility, sleep apnea, exercise ability, fatigue, mood and feelings of well-being. Weight loss can improve many of these features, and it is conceivable that the associated rise in testosterone may be responsible for salutary effects beyond those achieved by the weight loss itself. However, because of insufficient evidence regarding its risk-benefit ratio, testosterone treatment should not be used for the sole purpose of weight loss. Nevertheless, a reduction in fat mass may be a collateral benefit for men receiving testosterone therapy for treatment of established androgen deficiency. Potential safety concerns with testosterone treatment particularly relevant to obese, older men include sleep apnea and adverse cardiovascular disease and prostate events,110,111,112,113 in part because such comorbidities are common in this population. However, whether testosterone treatment increases risk is unknown because there are no adequately designed and powered RCTs that have assessed the long-term risk-benefit ratio of testosterone therapy. As testosterone treatment impairs spermatogenesis, it is contraindicated in obese men seeking to have children who are already at increased risk of impaired fertility.114 Other approaches using aromatase inhibitors, selective estrogen receptor modulators and gonadotropins may be considered instead. Studies, reviewed elsewhere have shown that use of such agents can improve the endocrine hormonal profile (increased gonadotropins and testosterone), semen parameters and sexual function.115 For example, in one study of men with secondary hypogonadism, clomiphene therapy increased testosterone levels and improved sexual function in 75%; younger men with less comorbidities were more likely to respond.116 However, their effects on fertility are not proven.115 In addition, because of the associated lowering of circulating E2 levels, long-term use of such agents may lead to reduced bone mineral density.117
SUMMARY AND CONCLUSIONS
The bidirectional, inverse relationship between increased fat mass and testosterone levels suggests that both weight loss as well as testosterone therapy have the potential to break this vicious cycle. While HPT axis reactivation is achievable with weight loss, the degree of weight loss required to achieve this may be difficult to achieve and to maintain, with usual lifestyle changes for many obese men. However, successful weight loss has many other health benefits and should be first priority. The preclinical and observational data reviewed here suggests that testosterone therapy has the potential to augment diet-induced weight loss, and that it may have additional benefits on other, androgen-responsive tissues beyond its effects on fat mass. While a small, uncontrolled study found that testosterone treatment augmented the reductions in central adiposity and insulin resistance achieved with lifestyle;118 a recent, preliminary RCT failed to find additive effects of dietary restriction and testosterone therapy on weight loss.112 Given the increasing prevalence of obesity-associated hypotestosteronemia, not only the underlying pathophysiology, but also the risk-benefit of testosterone therapy added to lifestyle intervention on long-term outcomes of obesity and its associated adverse health consequences require further study.
COMPETING INTERESTS
The authors are primary investigators of an investigator initiated trial “Effect of Testosterone and Diet on Weight”, ClinicalTrials.gov Identifier NCT01616732, supported by Bayer HealthCare.
ACKNOWLDGEMENTS
M Grossmann was supported by a National Health and Medical Research Council of Australia Career Development Fellowship (#1024139), M Ng Tang Fui was supported by a National Health and Medical Research Council of Australia Postgraduate Research Scholarship (#1055305) and P Dupuis was supported by Bourse du Comité des Médecins, Dentistes et Pharmaciens du Centre Hospitalier Universitaire de Quιbec.
REFERENCES
- 1.WHO. WHO Global infobase, estimated overweight and obesity, 2002-2010 in males aged 30-100 y. [Last accessed on June 2013]. Available from: http://www.apps.who.int/infobase/Comparisons.aspx .
- 2.Kelly T, Yang W, Chen CS, Reynolds K, He J. Global burden of obesity in 2005 and projections to 2030. Int J Obes (Lond) 2008;32:1431–7. doi: 10.1038/ijo.2008.102. [DOI] [PubMed] [Google Scholar]
- 3.Tajar A, Forti G, O’Neill TW, Lee DM, Silman AJ, et al. Characteristics of secondary, primary, and compensated hypogonadism in aging men: evidence from the European Male Ageing Study. J Clin Endocrinol Metab. 2010;95:1810–8. doi: 10.1210/jc.2009-1796. [DOI] [PubMed] [Google Scholar]
- 4.Flegal KM, Kit BK, Orpana H, Graubard BI. Association of all-cause mortality with overweight and obesity using standard body mass index categories: a systematic review and meta-analysis. JAMA. 2013;309:71–82. doi: 10.1001/jama.2012.113905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heymsfield SB, Cefalu WT. Does body mass index adequately convey a patient's mortality risk? JAMA. 2013;309:87–8. doi: 10.1001/jama.2012.185445. [DOI] [PubMed] [Google Scholar]
- 6.Hughes V. The big fat truth. Nature. 2013;497:428–30. doi: 10.1038/497428a. [DOI] [PubMed] [Google Scholar]
- 7.Willett WC, Hu FB, Thun M. Overweight, obesity, and all-cause mortality. JAMA. 2013;309:1681. doi: 10.1001/jama.2013.3075. [DOI] [PubMed] [Google Scholar]
- 8.Staiano AE, Reeder BA, Elliott S, Joffres MR, Pahwa P, et al. Body mass index versus waist circumference as predictors of mortality in Canadian adults. Int J Obes (Lond) 2012;36:1450–4. doi: 10.1038/ijo.2011.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choi KM, Cho HJ, Choi HY, Yang SJ, Yoo HJ, et al. Higher mortality in metabolically obese normal-weight people than in metabolically healthy obese subjects in elderly Koreans. Clinical Endocrinol (Oxf) 2013;79:364–70. doi: 10.1111/cen.12154. [DOI] [PubMed] [Google Scholar]
- 10.Alligier M, Gabert L, Meugnier E, Lambert-Porcheron S, Chanseaume E, et al. Visceral fat accumulation during lipid overfeeding is related to subcutaneous adipose tissue characteristics in healthy men. J Clin Endocrinol Metab. 2013;98:802–10. doi: 10.1210/jc.2012-3289. [DOI] [PubMed] [Google Scholar]
- 11.Gupta V, Bhasin S, Guo W, Singh R, Miki R, et al. Effects of dihydrotestosterone on differentiation and proliferation of human mesenchymal stem cells and preadipocytes. Mol Cell Endocrinol. 2008;296:32–40. doi: 10.1016/j.mce.2008.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Proietto J. Why is treating obesity so difficult? Justification for the role of bariatric surgery. Med J Aust. 2011;195:144–6. doi: 10.5694/j.1326-5377.2011.tb03242.x. [DOI] [PubMed] [Google Scholar]
- 13.Sumithran P, Prendergast LA, Delbridge E, Purcell K, Shulkes A, et al. Long-term persistence of hormonal adaptations to weight loss. N Engl J Med. 2011;365:1597–604. doi: 10.1056/NEJMoa1105816. [DOI] [PubMed] [Google Scholar]
- 14.Glass AR, Swerdloff RS, Bray GA, Dahms WT, Atkinson RL. Low serum testosterone and sex-hormone-binding-globulin in massively obese men. J Clin Endocrinol Metab. 1977;45:1211–9. doi: 10.1210/jcem-45-6-1211. [DOI] [PubMed] [Google Scholar]
- 15.Allan CA, McLachlan RI. Androgens and obesity. Curr Opin Endocrinol Diabetes Obes. 2010;17:224–32. doi: 10.1097/MED.0b013e3283398ee2. [DOI] [PubMed] [Google Scholar]
- 16.Wu FC, Tajar A, Pye SR, Silman AJ, Finn JD, et al. Hypothalamic-pituitary-testicular axis disruptions in older men are differentially linked to age and modifiable risk factors: the European Male Aging Study. J Clin Endocrinol Metab. 2008;93:2737–45. doi: 10.1210/jc.2007-1972. [DOI] [PubMed] [Google Scholar]
- 17.Cao J, Chen TM, Hao WJ, Li J, Liu L, et al. Correlation between sex hormone levels and obesity in the elderly male. Aging Male. 2012;15:85–9. doi: 10.3109/13685538.2012.666585. [DOI] [PubMed] [Google Scholar]
- 18.Allan CA, Strauss BJ, Burger HG, Forbes EA, McLachlan RI. The association between obesity and the diagnosis of androgen deficiency in symptomatic ageing men. Med J Aust. 2006;185:424–7. doi: 10.5694/j.1326-5377.2006.tb00639.x. [DOI] [PubMed] [Google Scholar]
- 19.Grossmann M. Low testosterone in men with type 2 diabetes: significance and treatment. J Clin Endocrinol Metab. 2011;96:2341–53. doi: 10.1210/jc.2011-0118. [DOI] [PubMed] [Google Scholar]
- 20.Dhindsa S, Miller MG, McWhirter CL, Mager DE, Ghanim H, et al. Testosterone concentrations in diabetic and nondiabetic obese men. Diabetes Care. 2010;33:1186–92. doi: 10.2337/dc09-1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Orwoll ES, Nielson CM, Labrie F, Barrett-Connor E, Cauley JA, et al. Evidence for geographical and racial variation in serum sex steroid levels in older men. J Clin Endocrin Metab. 2010;95:E151–60. doi: 10.1210/jc.2009-2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tan WS, Ng CJ, Khoo EM, Low WY, Tan HM. The triad of erectile dysfunction, testosterone deficiency syndrome and metabolic syndrome: findings from a multi-ethnic Asian men study (The Subang Men's Health Study) Aging Male. 2011;14:231–6. doi: 10.3109/13685538.2011.597463. [DOI] [PubMed] [Google Scholar]
- 23.Sartorius G, Spasevska S, Idan A, Turner L, Forbes E, et al. Serum testosterone, dihydrotestosterone and estradiol concentrations in older men self-reporting very good health: the healthy man study. Clin Endocrinol (Oxf) 2012;77:755–63. doi: 10.1111/j.1365-2265.2012.04432.x. [DOI] [PubMed] [Google Scholar]
- 24.Bhasin S, Cunningham GR, Hayes FJ, Matsumoto AM, Snyder PJ, et al. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010;95:2536–59. doi: 10.1210/jc.2009-2354. [DOI] [PubMed] [Google Scholar]
- 25.Handelsman DJ. An old emperor finds new clothing: rejuvenation in our time. Asian J Androl. 2011;13:125–9. doi: 10.1038/aja.2010.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Boer H, Verschoor L, Ruinemans-Koerts J, Jansen M. Letrozole normalizes serum testosterone in severely obese men with hypogonadotropic hypogonadism. Diabetes Obes Metab. 2005;7:211–5. doi: 10.1111/j.1463-1326.2004.00397.x. [DOI] [PubMed] [Google Scholar]
- 27.Dhindsa S, Furlanetto R, Vora M, Ghanim H, Chaudhuri A, et al. Low estradiol concentrations in men with subnormal testosterone concentrations and type 2 diabetes. Diabetes Care. 2011;34:1854–9. doi: 10.2337/dc11-0208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huhtaniemi IT, Tajar A, Lee DM, O’Neill TW, Finn JD, et al. Comparison of serum testosterone and estradiol measurements in 3174 European men using platform immunoassay and mass spectrometry; relevance for the diagnostics in aging men. Eur J Endocrinol. 2012;166:983–91. doi: 10.1530/EJE-11-1051. [DOI] [PubMed] [Google Scholar]
- 29.Tajar A, Huhtaniemi IT, O’Neill TW, Finn JD, Pye SR, et al. Characteristics of androgen deficiency in late-onset hypogonadism: results from the European Male Aging Study (EMAS) J Clin Endocrinol Metab. 2012;97:1508–16. doi: 10.1210/jc.2011-2513. [DOI] [PubMed] [Google Scholar]
- 30.Grossmann M, Gianatti EJ, Zajac JD. Testosterone and type 2 diabetes. Curr Opin Endocrinol Diabetes Obes. 2010;17:247–56. doi: 10.1097/MED.0b013e32833919cf. [DOI] [PubMed] [Google Scholar]
- 31.Travison TG, Araujo AB, Kupelian V, O’Donnell AB, McKinlay JB. The relative contributions of aging, health, and lifestyle factors to serum testosterone decline in men. J Clin Endocrinol Metab. 2007;92:549–55. doi: 10.1210/jc.2006-1859. [DOI] [PubMed] [Google Scholar]
- 32.Haring R, Ittermann T, Volzke H, Krebs A, Zygmunt M, et al. Prevalence, incidence and risk factors of testosterone deficiency in a population-based cohort of men: results from the study of health in Pomerania. Aging Male. 2010;13:247–57. doi: 10.3109/13685538.2010.487553. [DOI] [PubMed] [Google Scholar]
- 33.Yeap BB, Chubb SA, Hyde Z, Jamrozik K, Hankey GJ, et al. Lower serum testosterone is independently associated with insulin resistance in non-diabetic older men: the Health In Men Study. Eur J Endocrinol. 2009;161:591–8. doi: 10.1530/EJE-09-0348. [DOI] [PubMed] [Google Scholar]
- 34.Camacho EM, Huhtaniemi IT, O’Neill TW, Finn JD, Pye SR, et al. Age-associated changes in hypothalamic-pituitary-testicular function in middle-aged and older men are modified by weight change and lifestyle factors: longitudinal results from the European Male Ageing Study. Eur J Endocrinol. 2013;168:445–55. doi: 10.1530/EJE-12-0890. [DOI] [PubMed] [Google Scholar]
- 35.Rana K, Fam BC, Clarke MV, Pang TP, Zajac JD, et al. Increased adiposity in DNA binding-dependent androgen receptor knockout male mice associated with decreased voluntary activity and not insulin resistance. Am J Physiol Endocrinol Metab. 2011;301:E767–78. doi: 10.1152/ajpendo.00584.2010. [DOI] [PubMed] [Google Scholar]
- 36.McInnes KJ, Smith LB, Hunger NI, Saunders PT, Andrew R, et al. Deletion of the androgen receptor in adipose tissue in male mice elevates retinol binding protein 4 and reveals independent effects on visceral fat mass and on glucose homeostasis. Diabetes. 2012;61:1072–81. doi: 10.2337/db11-1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Semirale AA, Zhang XW, Wiren KM. Body composition changes and inhibition of fat development in vivo implicates androgen in regulation of stem cell lineage allocation. J Cell Biochem. 2011;112:1773–86. doi: 10.1002/jcb.23098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Varlamov O, White AE, Carroll JM, Bethea CL, Reddy A, et al. Androgen effects on adipose tissue architecture and function in nonhuman primates. Endocrinology. 2012;153:3100–10. doi: 10.1210/en.2011-2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Singh R, Artaza JN, Taylor WE, Gonzalez-Cadavid NF, Bhasin S. Androgens stimulate myogenic differentiation and inhibit adipogenesis in C3H 10T1/2 pluripotent cells through an androgen receptor-mediated pathway. Endocrinology. 2003;144:5081–8. doi: 10.1210/en.2003-0741. [DOI] [PubMed] [Google Scholar]
- 40.Blouin K, Nadeau M, Perreault M, Veilleux A, Drolet R, et al. Effects of androgens on adipocyte differentiation and adipose tissue explant metabolism in men and women. Clin Endocrinol (Oxf) 2010;72:176–88. doi: 10.1111/j.1365-2265.2009.03645.x. [DOI] [PubMed] [Google Scholar]
- 41.Hamilton EJ, Gianatti E, Strauss BJ, Wentworth J, Lim-Joon D, et al. Increase in visceral and subcutaneous abdominal fat in men with prostate cancer treated with androgen deprivation therapy. Clin Endocrinol (Oxf) 2011;74:377–83. doi: 10.1111/j.1365-2265.2010.03942.x. [DOI] [PubMed] [Google Scholar]
- 42.Mauras N, Hayes V, Welch S, Rini A, Helgeson K, et al. Testosterone deficiency in young men: marked alterations in whole body protein kinetics, strength, and adiposity. J Clin Endocrinol Metab. 1998;83:1886–92. doi: 10.1210/jcem.83.6.4892. [DOI] [PubMed] [Google Scholar]
- 43.Tsai EC, Boyko EJ, Leonetti DL, Fujimoto WY. Low serum testosterone level as a predictor of increased visceral fat in Japanese-American men. Int J Obes Relat Metab Disord. 2000;24:485–91. doi: 10.1038/sj.ijo.0801183. [DOI] [PubMed] [Google Scholar]
- 44.Grossmann M. Diagnosis and treatment of hypogonadism in older men: proceed with caution. Asian J Androl. 2010;12:783–6. doi: 10.1038/aja.2010.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mah PM, Wittert GA. Obesity and testicular function. Mol Cell Endocrinol. 2010;316:180–6. doi: 10.1016/j.mce.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 46.Belanger C, Hould FS, Lebel S, Biron S, Brochu G, et al. Omental and subcutaneous adipose tissue steroid levels in obese men. Steroids. 2006;71:674–82. doi: 10.1016/j.steroids.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 47.Rodriguez-Cuenca S, Monjo M, Proenza AM, Roca P. Depot differences in steroid receptor expression in adipose tissue: possible role of the local steroid milieu. Am J Physiol Endocrinol Metab. 2005;288:E200–7. doi: 10.1152/ajpendo.00270.2004. [DOI] [PubMed] [Google Scholar]
- 48.Pedersen BK, Febbraio MA. Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat Rev Endocrinol. 2012;8:457–65. doi: 10.1038/nrendo.2012.49. [DOI] [PubMed] [Google Scholar]
- 49.Bostrom P, Wu J, Jedrychowski MP, Korde A, Ye L, et al. A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481:463–8. doi: 10.1038/nature10777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Petrovic N, Walden TB, Shabalina IG, Timmons JA, Cannon B, et al. Chronic peroxisome proliferator-activated receptor gamma (PPARgamma) activation of epididymally derived white adipocyte cultures reveals a population of thermogenically competent, UCP1-containing adipocytes molecularly distinct from classic brown adipocytes. J Biol Chem. 2010;285:7153–64. doi: 10.1074/jbc.M109.053942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Baumgartner RN, Waters DL, Gallagher D, Morley JE, Garry PJ. Predictors of skeletal muscle mass in elderly men and women. Mech Ageing Dev. 1999;107:123–36. doi: 10.1016/s0047-6374(98)00130-4. [DOI] [PubMed] [Google Scholar]
- 52.Srikanthan P, Hevener AL, Karlamangla AS. Sarcopenia exacerbates obesity-associated insulin resistance and dysglycemia: findings from the National Health and Nutrition Examination Survey III. PloS one. 2010;5:e10805. doi: 10.1371/journal.pone.0010805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Waters DL, Baumgartner RN. Sarcopenia and obesity. Clin Geriatr Med. 2011;27:401–21. doi: 10.1016/j.cger.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 54.Jensen GL, Hsiao PY. Obesity in older adults: relationship to functional limitation. Curr Opin Clin Nutr Metab Care. 2010;13:46–51. doi: 10.1097/MCO.0b013e32833309cf. [DOI] [PubMed] [Google Scholar]
- 55.Grossmann M, Zajac JD. Management of side effects of androgen deprivation therapy. Endocrinol Metab Clin North Am. 2011;40:655–71. doi: 10.1016/j.ecl.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 56.Grossmann M, Cheung AS, Zajac DJ. Androgens and prostate cancer; pathogenesis and deprivation therapy. Best Pract Res Clin Endocrinol Metab. 2013 doi: 10.1016/j.beem.2013.05.001. In press. [DOI] [PubMed] [Google Scholar]
- 57.Chomentowski P, Dube JJ, Amati F, Stefanovic-Racic M, Zhu S, et al. Moderate exercise attenuates the loss of skeletal muscle mass that occurs with intentional caloric restriction-induced weight loss in older, overweight to obese adults. J Gerontol A Biol Sci Med Sci. 2009;64:575–80. doi: 10.1093/gerona/glp007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.LeBlanc ES, Wang PY, Lee CG, Barrett-Connor E, Cauley JA, et al. Higher testosterone levels are associated with less loss of lean body mass in older men. J Clin Endocrinol Metab. 2011;96:3855–63. doi: 10.1210/jc.2011-0312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fan W, Yanase T, Nomura M, Okabe T, Goto K, et al. Androgen receptor null male mice develop late-onset obesity caused by decreased energy expenditure and lipolytic activity but show normal insulin sensitivity with high adiponectin secretion. Diabetes. 2005;54:1000–8. doi: 10.2337/diabetes.54.4.1000. [DOI] [PubMed] [Google Scholar]
- 60.Wood RI. Oral testosterone self-administration in male hamsters: dose-response, voluntary exercise, and individual differences. Horm Behav. 2002;41:247–58. doi: 10.1006/hbeh.2002.1769. [DOI] [PubMed] [Google Scholar]
- 61.O’Connor DB, Archer J, Wu FC. Effects of testosterone on mood, aggression, and sexual behavior in young men: a double-blind, placebo-controlled, cross-over study. J Clin Endocrinol Metab. 2004;89:2837–45. doi: 10.1210/jc.2003-031354. [DOI] [PubMed] [Google Scholar]
- 62.Reis LO, Favaro WJ, Barreiro GC, de Oliveira LC, Chaim EA, et al. Erectile dysfunction and hormonal imbalance in morbidly obese male is reversed after gastric bypass surgery: a prospective randomized controlled trial. Int J Androl. 2010;33:736–44. doi: 10.1111/j.1365-2605.2009.01017.x. [DOI] [PubMed] [Google Scholar]
- 63.Hammoud A, Gibson M, Hunt SC, Adams TD, Carrell DT, et al. Effect of Roux-en-Y gastric bypass surgery on the sex steroids and quality of life in obese men. J Clin Endocrinol Metab. 2009;94:1329–32. doi: 10.1210/jc.2008-1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Omana J, Tamler R, Strohmayer E, Herron D, Kini S. Sex hormone levels in men undergoing bariatric surgery. J Am Coll Surg. 2009;209:S22–3. [Google Scholar]
- 65.Pellitero S, Olaizola I, Alastrue A, Martinez E, Granada ML, et al. Hypogonadotropic hypogonadism in morbidly obese males is reversed after bariatric surgery. Obes Surg. 2012;22:1835–42. doi: 10.1007/s11695-012-0734-9. [DOI] [PubMed] [Google Scholar]
- 66.Globerman H, Shen-Orr Z, Karnieli E, Aloni Y, Charuzi I. Inhibin B in men with severe obesity and after weight reduction following gastroplasty. Endocr Res. 2005;31:17–26. doi: 10.1080/07435800500228971. [DOI] [PubMed] [Google Scholar]
- 67.Pritchard J, Despres JP, Gagnon J, Tchernof A, Nadeau A, et al. Plasma adrenal, gonadal, and conjugated steroids following long-term exercise-induced negative energy balance in identical twins. Metabolism. 1999;48:1120–7. doi: 10.1016/s0026-0495(99)90125-7. [DOI] [PubMed] [Google Scholar]
- 68.Facchiano E, Scaringi S, Veltri M, Samavat J, Maggi M, et al. Age as a Predictive Factor of Testosterone Improvement in Male Patients After Bariatric Surgery: preliminary Results of a Monocentric Prospective Study. Obes Surg. 2013;23:167–72. doi: 10.1007/s11695-012-0753-6. [DOI] [PubMed] [Google Scholar]
- 69.Niskanen L, Laaksonen DE, Punnonen K, Mustajoki P, Kaukua J, et al. Changes in sex hormone-binding globulin and testosterone during weight loss and weight maintenance in abdominally obese men with the metabolic syndrome. Diabetes Obes Metab. 2004;6:208–15. doi: 10.1111/j.1462-8902.2004.00335.x. [DOI] [PubMed] [Google Scholar]
- 70.Stanik S, Dornfeld LP, Maxwell MH, Viosca SP, Korenman SG. The effect of weight loss on reproductive hormones in obese men. J Clin Endocrinol Metab. 1981;53:828–32. doi: 10.1210/jcem-53-4-828. [DOI] [PubMed] [Google Scholar]
- 71.Kaukua J, Pekkarinen T, Sane T, Mustajoki P. Sex hormones and sexual function in obese men losing weight. Obes Res. 2003;11:689–94. doi: 10.1038/oby.2003.98. [DOI] [PubMed] [Google Scholar]
- 72.Khoo J, Piantadosi C, Worthley S, Wittert GA. Effects of a low-energy diet on sexual function and lower urinary tract symptoms in obese men. Int J Obes (Lond) 2010;34:1396–403. doi: 10.1038/ijo.2010.76. [DOI] [PubMed] [Google Scholar]
- 73.Vermeulen A, Kaufman JM, Giagulli VA. Influence of some biological indexes on sex hormone-binding globulin and androgen levels in aging or obese males. J Clin Endocrinol Metab. 1996;81:1821–6. doi: 10.1210/jcem.81.5.8626841. [DOI] [PubMed] [Google Scholar]
- 74.Khoo J, Piantadosi C, Duncan R, Worthley SG, Jenkins A, et al. Comparing effects of a low-energy diet and a high-protein low-fat diet on sexual and endothelial function, urinary tract symptoms, and inflammation in obese diabetic men. J Sex Med. 2011;8:2868–75. doi: 10.1111/j.1743-6109.2011.02417.x. [DOI] [PubMed] [Google Scholar]
- 75.Leenen R, van der Kooy K, Seidell JC, Deurenberg P, Koppeschaar HP. Visceral fat accumulation in relation to sex hormones in obese men and women undergoing weight loss therapy. J Clin Endocrinol Metab. 1994;78:1515–20. doi: 10.1210/jcem.78.6.8200956. [DOI] [PubMed] [Google Scholar]
- 76.Corona G, Rastrelli G, Monami M, Saad F, Luconi M, et al. Body weight loss reverts obesity-associated hypogonadotropic hypogonadism: a systematic review and meta-analysis. Eur J Endocrinol. 2013;168:829–43. doi: 10.1530/EJE-12-0955. [DOI] [PubMed] [Google Scholar]
- 77.Katznelson L, Finkelstein JS, Schoenfeld DA, Rosenthal DI, Anderson EJ, et al. Increase in bone density and lean body mass during testosterone administration in men with acquired hypogonadism. J Clin Endocrinolo Metab. 1996;81:4358–65. doi: 10.1210/jcem.81.12.8954042. [DOI] [PubMed] [Google Scholar]
- 78.Brodsky IG, Balagopal P, Nair KS. Effects of testosterone replacement on muscle mass and muscle protein synthesis in hypogonadal men--a clinical research center study. J Clin Endocrinol Metab. 1996;81:3469–75. doi: 10.1210/jcem.81.10.8855787. [DOI] [PubMed] [Google Scholar]
- 79.Isidori AM, Giannetta E, Greco EA, Gianfrilli D, Bonifacio V, et al. Effects of testosterone on body composition, bone metabolism and serum lipid profile in middle-aged men: a meta-analysis. Clin Endocrinol (Oxf) 2005;63:280–93. doi: 10.1111/j.1365-2265.2005.02339.x. [DOI] [PubMed] [Google Scholar]
- 80.Allan CA, Strauss BJ, Forbes EA, McLachlan RI. P2. San Diego: 92nd Annual Meeting of the Endocrine Society; 2010. Testosterone Therapy Improves Body Composition and Metabolic Parameters in Obese Aging Men: Results of a RCT; p. 455. [Google Scholar]
- 81.Aversa A, Bruzziches R, Francomano D, Rosano G, Isidori AM, et al. Effects of testosterone undecanoate on cardiovascular risk factors and atherosclerosis in middle-aged men with late-onset hypogonadism and metabolic syndrome: results from a 24-month, randomized, double-blind, placebo-controlled study. J Sex Med. 2010;7:3495–503. doi: 10.1111/j.1743-6109.2010.01931.x. [DOI] [PubMed] [Google Scholar]
- 82.Kalinchenko SY, Tishova YA, Mskhalaya GJ, Gooren LJ, Giltay EJ, et al. Effects of testosterone supplementation on markers of the metabolic syndrome and inflammation in hypogonadal men with the metabolic syndrome: the double-blinded placebo-controlled Moscow study. Clinical Endocrinol. 2010;73:602–12. doi: 10.1111/j.1365-2265.2010.03845.x. [DOI] [PubMed] [Google Scholar]
- 83.Svartberg J, Agledahl I, Figenschau Y, Sildnes T, Waterloo K, et al. Testosterone treatment in elderly men with subnormal testosterone levels improves body composition and BMD in the hip. Int J Impot Res. 2008;20:378–87. doi: 10.1038/ijir.2008.19. [DOI] [PubMed] [Google Scholar]
- 84.Saad F, Haider A, Doros G, Traish A. Long-term treatment of hypogonadal men with testosterone produces substantial and sustained weight loss. Obesity (Silver Spring) 2013 doi: 10.1002/oby.20407. In press. [DOI] [PubMed] [Google Scholar]
- 85.Frederiksen L, Hojlund K, Hougaard DM, Mosbech TH, Larsen R, et al. Testosterone therapy decreases subcutaneous fat and adiponectin in aging men. Eur J Endocrinol. 2012;166:469–76. doi: 10.1530/EJE-11-0565. [DOI] [PubMed] [Google Scholar]
- 86.Jones TH, Arver S, Behre HM, Buvat J, Meuleman E, et al. Testosterone replacement in hypogonadal men with type 2 diabetes and/or metabolic syndrome (the TIMES2 study) Diabetes Care. 2011;34:828–37. doi: 10.2337/dc10-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kenny AM, Kleppinger A, Annis K, Rathier M, Browner B, et al. Effects of transdermal testosterone on bone and muscle in older men with low bioavailable testosterone levels, low bone mass, and physical frailty. J Am Geriatr Soc. 2010;58:1134–43. doi: 10.1111/j.1532-5415.2010.02865.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Srinivas-Shankar U, Roberts SA, Connolly MJ, O’Connell MD, Adams JE, et al. Effects of testosterone on muscle strength, physical function, body composition, and quality of life in intermediate-frail and frail elderly men: a randomized, double-blind, placebo-controlled study. J Clin Endocrinol Metab. 2010;95:639–50. doi: 10.1210/jc.2009-1251. [DOI] [PubMed] [Google Scholar]
- 89.Allan CA, Strauss BJ, Burger HG, Forbes EA, McLachlan RI. Testosterone therapy prevents gain in visceral adipose tissue and loss of skeletal muscle in nonobese aging men. J Clin Endocrinol Metab. 2008;93:139–46. doi: 10.1210/jc.2007-1291. [DOI] [PubMed] [Google Scholar]
- 90.Emmelot-Vonk MH, Verhaar HJ, Nakhai Pour HR, Aleman A, Lock TM, et al. Effect of testosterone supplementation on functional mobility, cognition, and other parameters in older men: a randomized controlled trial. JAMA. 2008;299:39–52. doi: 10.1001/jama.2007.51. [DOI] [PubMed] [Google Scholar]
- 91.Kapoor D, Clarke S, Stanworth R, Channer KS, Jones TH. The effect of testosterone replacement therapy on adipocytokines and C-reactive protein in hypogonadal men with type 2 diabetes. Eur J Endocrinol. 2007;156:595–602. doi: 10.1530/EJE-06-0737. [DOI] [PubMed] [Google Scholar]
- 92.Kapoor D, Goodwin E, Channer KS, Jones TH. Testosterone replacement therapy improves insulin resistance, glycaemic control, visceral adiposity and hypercholesterolaemia in hypogonadal men with type 2 diabetes. Eur J Endocrinol. 2006;154:899–906. doi: 10.1530/eje.1.02166. [DOI] [PubMed] [Google Scholar]
- 93.Nair KS, Rizza RA, O’Brien P, Dhatariya K, Short KR, et al. DHEA in elderly women and DHEA or testosterone in elderly men. N Engl J Med. 2006;355:1647–59. doi: 10.1056/NEJMoa054629. [DOI] [PubMed] [Google Scholar]
- 94.Page ST, Herbst KL, Amory JK, Coviello AD, Anawalt BD, et al. Testosterone administration suppresses adiponectin levels in men. J Androl. 2005;26:85–92. [PubMed] [Google Scholar]
- 95.Page ST, Amory JK, Bowman FD, Anawalt BD, Matsumoto AM, et al. Exogenous testosterone (T) alone or with finasteride increases physical performance, grip strength, and lean body mass in older men with low serum T. J Clin Endocrinol Metab. 2005;90:1502–10. doi: 10.1210/jc.2004-1933. [DOI] [PubMed] [Google Scholar]
- 96.Schroeder ET, Zheng L, Ong MD, Martinez C, Flores C, et al. Effects of androgen therapy on adipose tissue and metabolism in older men. J Clini Endocrinol Metab. 2004;89:4863–72. doi: 10.1210/jc.2004-0784. [DOI] [PubMed] [Google Scholar]
- 97.Boyanov MA, Boneva Z, Christov VG. Testosterone supplementation in men with type 2 diabetes, visceral obesity and partial androgen deficiency. Aging Male. 2003;6:1–7. [PubMed] [Google Scholar]
- 98.Steidle C, Schwartz S, Jacoby K, Sebree T, Smith T, et al. AA2500 testosterone gel normalizes androgen levels in aging males with improvements in body composition and sexual function. J Clin Endocrinol Metab. 2003;88:2673–81. doi: 10.1210/jc.2002-021058. [DOI] [PubMed] [Google Scholar]
- 99.Wittert GA, Chapman IM, Haren MT, Mackintosh S, Coates P, et al. Oral testosterone supplementation increases muscle and decreases fat mass in healthy elderly males with low-normal gonadal status. J Gerontol A Biol Sci Med Sci. 2003;58:618–25. doi: 10.1093/gerona/58.7.m618. [DOI] [PubMed] [Google Scholar]
- 100.Munzer T, Harman SM, Hees P, Shapiro E, Christmas C, et al. Effects of GH and/or sex steroid administration on abdominal subcutaneous and visceral fat in healthy aged women and men. J Clin Endocrinol Metab. 2001;86:3604–10. doi: 10.1210/jcem.86.8.7773. [DOI] [PubMed] [Google Scholar]
- 101.Snyder PJ, Peachey H, Hannoush P, Berlin JA, Loh L, et al. Effect of testosterone treatment on body composition and muscle strength in men over 65 years of age. J Clin Endocrinol Metab. 1999;84:2647–53. doi: 10.1210/jcem.84.8.5885. [DOI] [PubMed] [Google Scholar]
- 102.Sih R, Morley JE, Kaiser FE, Perry HM, 3rd, Patrick P, et al. Testosterone replacement in older hypogonadal men: a 12-month randomized controlled trial. J Clin Endocrinol Metab. 1997;82:1661–7. doi: 10.1210/jcem.82.6.3988. [DOI] [PubMed] [Google Scholar]
- 103.Marin P, Holmang S, Gustafsson C, Jonsson L, Kvist H, et al. Androgen treatment of abdominally obese men. Obes Res. 1993;1:245–51. doi: 10.1002/j.1550-8528.1993.tb00618.x. [DOI] [PubMed] [Google Scholar]
- 104.Marin P, Holmang S, Jonsson L, Sjostrom L, Kvist H, et al. The effects of testosterone treatment on body composition and metabolism in middle-aged obese men. Int J Obes Relat Metab Disord. 1992;16:991–7. [PubMed] [Google Scholar]
- 105.Hall SA, Esche GR, Araujo AB, Travison TG, Clark RV, et al. Correlates of low testosterone and symptomatic androgen deficiency in a population-based sample. J Clin Endocrinol Metab. 2008;93:3870–7. doi: 10.1210/jc.2008-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zitzmann M, Faber S, Nieschlag E. Association of specific symptoms and metabolic risks with serum testosterone in older men. J Clin Endocrinol Metab. 2006;91:4335–43. doi: 10.1210/jc.2006-0401. [DOI] [PubMed] [Google Scholar]
- 107.Mellstrom D, Vandenput L, Mallmin H, Holmberg AH, Lorentzon M, et al. Older men with low serum estradiol and high serum SHBG have an increased risk of fractures. J Bone Miner Res. 2008;23:1552–60. doi: 10.1359/jbmr.080518. [DOI] [PubMed] [Google Scholar]
- 108.De Laet C, Kanis JA, Oden A, Johanson H, Johnell O, et al. Body mass index as a predictor of fracture risk: a meta-analysis. Osteoporos Int. 2005;16:1330–8. doi: 10.1007/s00198-005-1863-y. [DOI] [PubMed] [Google Scholar]
- 109.Grossmann M, Zajac JD. Hematological changes during androgen deprivation therapy. Asian J Androl. 2012;14:187–92. doi: 10.1038/aja.2011.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Basaria S, Coviello AD, Travison TG, Storer TW, Farwell WR, et al. Adverse events associated with testosterone administration. N Engl J Med. 2010;363:109–22. doi: 10.1056/NEJMoa1000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Fernandez-Balsells MM, Murad MH, Lane M, Lampropulos JF, Albuquerque F, et al. Clinical review 1: adverse effects of testosterone therapy in adult men: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2010;95:2560–75. doi: 10.1210/jc.2009-2575. [DOI] [PubMed] [Google Scholar]
- 112.Hoyos CM, Killick R, Yee BJ, Grunstein RR, Liu PY. Effects of testosterone therapy on sleep and breathing in obese men with severe obstructive sleep apnoea: a randomized placebo-controlled trial. Clin Endocrinol (Oxf) 2012;77:599–607. doi: 10.1111/j.1365-2265.2012.04413.x. [DOI] [PubMed] [Google Scholar]
- 113.Zitzmann M, Mattern A, Hanisch J, Gooren L, Jones H, et al. IPASS: a study on the tolerability and effectiveness of injectable testosterone undecanoate for the treatment of male hypogonadism in a worldwide sample of 1,438 men. J Sex Med. 2013;10:579–88. doi: 10.1111/j.1743-6109.2012.02853.x. [DOI] [PubMed] [Google Scholar]
- 114.Cabler S, Agarwal A, Flint M, du Plessis SS. Obesity: modern man's fertility nemesis. Asian J Androl. 2010;12:480–9. doi: 10.1038/aja.2010.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hammoud AO, Meikle AW, Reis LO, Gibson M, Peterson CM, et al. Obesity and male infertility: a practical approach. Semin Reprod Med. 2012;30:486–95. doi: 10.1055/s-0032-1328877. [DOI] [PubMed] [Google Scholar]
- 116.Guay AT, Jacobson J, Perez JB, Hodge MB, Velasquez E. Clomiphene increases free testosterone levels in men with both secondary hypogonadism and erectile dysfunction: who does and does not benefit? Int J Impot Res. 2003;15:156–65. doi: 10.1038/sj.ijir.3900981. [DOI] [PubMed] [Google Scholar]
- 117.Burnett-Bowie SA, McKay EA, Lee H, Leder BZ. Effects of aromatase inhibition on bone mineral density and bone turnover in older men with low testosterone levels. J Clin Endocrinol Metab. 2009;94:4785–92. doi: 10.1210/jc.2009-0739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Heufelder AE, Saad F, Bunck MC, Gooren L. Fifty-two-week treatment with diet and exercise plus transdermal testosterone reverses the metabolic syndrome and improves glycemic control in men with newly diagnosed type 2 diabetes and subnormal plasma testosterone. J Androl. 2009;30:726–33. doi: 10.2164/jandrol.108.007005. [DOI] [PubMed] [Google Scholar]


