Abstract
Testosterone levels are lower in men with metabolic syndrome and type 2 diabetes mellitus (T2DM) and also predict the onset of these adverse metabolic states. Body composition (body mass index, waist circumference) is an important mediator of this relationship. Sex hormone binding globulin is also inversely associated with insulin resistance and T2DM but the data regarding estrogen are inconsistent. Clinical models of androgen deficiency including Klinefelter's syndrome and androgen deprivation therapy in the treatment of advanced prostate cancer confirm the association between androgens and glucose status. Experimental manipulation of the insulin/glucose milieu and suppression of endogenous testicular function suggests the relationship between androgens and insulin sensitivity is bidirectional. Androgen therapy in men without diabetes is not able to differentiate the effect on insulin resistance from that on fat mass, in particular visceral adiposity. Similarly, several small clinical studies have examined the efficacy of exogenous testosterone in men with T2DM, however, the role of androgens, independent of body composition, in modifying insulin resistance is uncertain.
Keywords: androgen, glucose metabolism, sex steroids, testosterone
INTRODUCTION
Male aging is associated with a decline in serum total testosterone (TT) beginning in the third decade. This was approximately 1%–2% per annum in cohorts of men followed for 7–10 years in the Massachusetts Male Aging Study (MMAS)1 and up to 30 years in the Baltimore Longitudinal Study of Aging.2 The decrease in testosterone, however, is not universal and men who remain in good health as they age may not experience this decline.3 One of the strongest correlates with falling testosterone levels in middle-aged and aging men is obesity,4 with a 30% reduction in obese compared with age-matched healthy weight men in the MMAS cohort.5 In the European Male Ageing Study, obesity was the most important predictor of low TT.6 In follow-up of the MMAS and EMAS cohorts, men who gained weight had a greater decline in TT.7,8 Conversely, in the EMAS cohort studied over 4 years, weight loss was associated with a proportional increase in serum testosterone.7 In a group of healthy Australian men aged 70 years or older, diabetes, in addition to increasing age, higher body mass index (BMI) and higher waist-to-hip ratio, was independently associated with lower TT levels.9
Aging is also associated with a reduction in glucose tolerance10 leading to an increased prevalence of impaired glucose tolerance (IGT) and Type 2 diabetes mellitus (T2DM) in men as a function of age.11 The impact of age on insulin sensitivity and beta-cell function is independent of intraabdominal fat.12
A causal role for testosterone in the relationship between insulin resistance, metabolic syndrome and T2DM in men as they age is suggested by epidemiological data, models of androgen deficiency and studies of testosterone therapy in men with and without T2DM. However, the mechanisms by which androgen action is mediated, and the contribution independent of the pivotal role of body composition, remain uncertain with conflicting data from in vivo studies and small clinical trials.
PREVALENCE OF LOW TESTOSTERONE LEVELS IN MEN WITH DIABETES MELLITUS
Serum testosterone levels and the prevalence of biochemical hypoandrogenism (defined according to an arbitrary testosterone cut-off) have been studied in populations of men attending hospital-based diabetes clinics with estimates that 30%–50% of men with T2DM have low testosterone.13 A total of 43% of men with T2DM (mean age 65 years) attending an Australian diabetes service had TT levels <10 nmol l−1; mean BMI was 30 kg m−2.14 In contrast, only 7% of men with T1DM (mean age 45 years) had a TT level <10 nmol l−1 with the mean BMI of this group 27 kg m−2. Similarly, in a younger US cohort (18–35 years), 33% of men with T2DM were considered hypogonadal based on free testosterone levels compared with 8% of men with T1DM. Mean TT levels were 11 and 23 nmol l−1, respectively.15 From the Hypogonadism in Males (HIM) US cohort,16 33% of lean, 44% of overweight and 46% of obese diabetic men aged >45 years had subnormal TT levels (<10.5 nmol l−1). TT was independently predicted by BMI, age and sex hormone-binding globulin (SHBG) levels. In a primary care setting in the UK, 4.4% of men with T2DM had TT levels <8.0 nmol l−1 and 32% had TT levels <12 nmol l−1 with BMI an independent predictor of low testosterone.17 Of 766 Taiwanese men with T2DM (mean age 62 years; mean BMI 26 kg m−2), 33% had a TT <10 nmol l−1. BMI and waist circumference (WC) were the major predictors of biochemical androgen deficiency.13 Similarly, one-third of adult diabetic men had TT levels <12 nmol l−1 in a general hospital setting in Nigeria.18 Finally, in the Japanese Saku Cohort Study Group, testosterone levels were lower in 215 diabetic men (aged 65 years; BMI 24 kg m−2) compared with controls who were of a similar age but lower BMI.19
SEX STEROIDS AND FUTURE RISK OF TYPE 2 DIABETES MELLITUS AND METABOLIC SYNDROME
Total testosterone
Cross-sectional studies document an association between lower testosterone levels and subsequent risk of T2DM. A meta-analysis of cross-sectional studies20 showed a difference of −2.66 nmol l−1 (95% CI −3.45 to −1.86) (P <0.001) in testosterone levels between men with and without T2DM. This difference remained after adjustment for BMI, waist-hip ratio (WHR), age, race and criteria for diagnosis of diabetes in studies conducted in a number of countries. It is noteworthy that in these studies, the mean testosterone level in those men with diabetes was above the cut-off (10 nmol l−1) considered to potentially represent a less than optimal androgen status.21
In a meta-analysis of prospective studies, there was a difference in testosterone of −2.48 nmol l−1 (95% CI −4.04 to −0.93) between men with and without T2DM (P = 0.02).20 A subset of four studies of more than 1000 men with TT levels 15.6–21.0 nmol l−1 described a 42% lower risk of developing diabetes when compared with their peers with TT levels 7.4–15.5 mol l−1 (RR 0.58 95%CI 0.39–0.87) over 5–11 years of follow-up.20 A summary of prospective cohort studies detailing the risk of developing T2DM as a function of TT levels is provided in Table 1. In the Tromso cohort with 9 years of follow-up,22 the risk of developing diabetes for the highest (>16.2 nmol l−1) compared with the lowest (<9.9 nmol l−1) quartile of testosterone was 0.41 (95% CI 0.20–0.84) but TT was no longer predictive when adjusted for WC.
Table 1.
Prospective cohort studies: testosterone and risk of developing diabetes
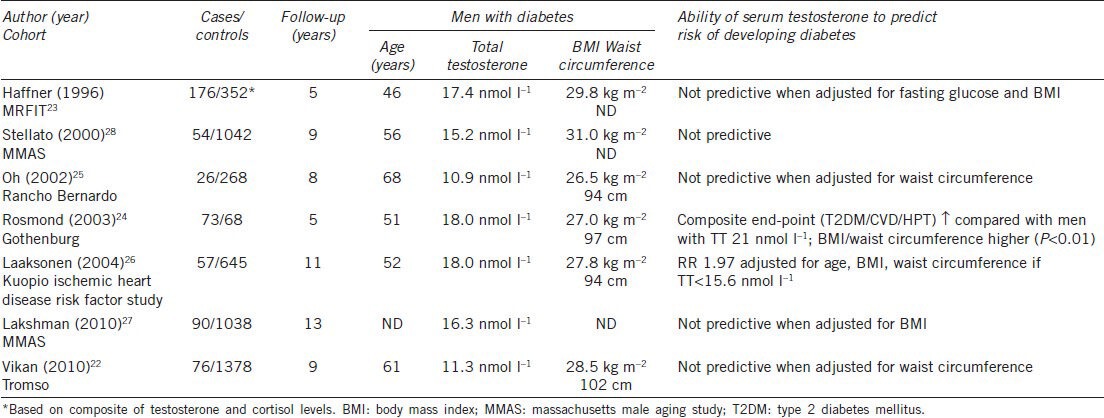
Similarly, in the MRFIT cohort,23 the association between testosterone and development of diabetes was not significant after BMI and baseline glucose were accounted for. In the Gothenburg cohort,24 men were classified according to hormonal profiles (an algorithm based on testosterone and cortisol), with those having a baseline TT of 18 nmol l−1 being more likely to develop a composite endpoint of hypertension, ischemic heart disease or T2DM than those with a TT of 21 nmol l−1. Those men with a lower TT also had higher BMI and WC at baseline. This is consistent with previous observations that elevated BMI, increased WC and low TT form an adverse metabolic phenotype but it does not clarify cause and effect.
In the Rancho Bernardo cohort, lower TT predicted increased insulin resistance (as measured by homeostatic model assessment-insulin resistance (HOMA-IR)) after adjusting for BMI and age; however, WC was not included in the model.25 Men with TT levels in the lowest quartile (<8.6 nmol l−1) had an OR 2.7 (95%CI 1.1–6.6) (P = 0.03) for developing diabetes when compared with men in the remaining quartiles. Baseline TT in the diabetic group was 10.9 nmol l−1, this is relatively lower than in other cohort studies.23,24,26,27,28 In the Kuopio 11-year follow-up study, men who developed diabetes had a mean baseline TT of 18 nmol l−1 and, within this group of 57 men, 11% had TT levels less than 11 nmol l−1. Men with TT levels <15.6 nmol l−1 were more likely to develop diabetes after adjusting for age, BMI and WC with an OR 1.97 (95%CI 1.03–3.77).26
In the MMAS cohort followed for 7–10 years, TT levels were lower in the 5% of men who developed diabetes but TT was not significant in a multivariate model that included BMI;28 this finding persisted after a total of 13 years of follow up.27
Consistent with the integral role played by body composition in mediating the relationship between TT and risk of diabetes, in data from 1736 men aged 65 years and older from the Cardiovascular Health Study, a prospective cohort study with a median of 12 years of follow-up, the hazard ratio (HR) for incident diabetes was 5.6 (95% CI 2.4–11.4) for a BMI of ≥28.7 kg m−2 compared with <23.3 kg m−2. For WC ≥104.6 cm the HR was 5.1 (95% CI 2.7–9.9) compared with WC <89.1 cm. In joint models of BMI and WC, both were independently associated with the risk of developing diabetes.29 Further evidence for the inter-relationship between adiposity, insulin resistance and testosterone is provided by a small study of men undergoing bariatric surgery whereby the degree of weight loss was paralleled by an increase in insulin sensitivity and free testosterone levels.30 In a meta-analysis of the effects of weight loss on testosterone levels achieved by low-calorie diets or bariatric surgery, the degree of weight loss was the best determinant of an increase in testosterone levels.31 Although men with diabetes appeared to have a lesser rise in TT, the authors note that this could be accounted for by the lower weight loss observed in diabetic men.
In a nonobese cohort from the MMAS, low TT levels were predictive of development of the metabolic syndrome; this relationship was strongest in the BMI <25 kg m−2 range32 raising the possibility that the role of testosterone in nonobese men may be ameliorated by adiposity in obese men. In cross-sectional studies of middle-aged and older men each 5.3 nmol l−1 increase in TT was associated with a 57% reduction in risk of a diagnosis of metabolic syndrome,33 independent of BMI and WC. A similar magnitude relationship was evident for TT and lower insulin sensitivity and higher fasting serum insulin levels. TT and insulin resistance were also inversely correlated in men ≥70 years independent of BMI and WC34 with the greatest risk of insulin resistance present in men with TT ≤8 mmol l−1.
An important limitation to these observational studies is the use of a single baseline measurement of testosterone. Differences in the way in which diabetes is diagnosed further confound comparison of the cohorts.
SEX HORMONE-BINDING GLOBULIN
Based on a number of prospective studies including TT and SHBG, it has been concluded that SHBG is the more important determinant of T2DM.27,35 Moreover, in men with T2DM, SHBG but not TT was independently associated with worse glycemic control.14
In a cohort of 170 men from the Physicians Health Study with newly diagnosed diabetes, SHBG levels were strongly inversely associated with risk of T2DM.36 This was independent of BMI although WC was not included in the models. Prospective studies confirm this inverse relationship23,27,28 but again did not include WC in the modeling. In the Tromso cohort, after accounting for WC, SHBG no longer predicted T2DM,22 and in the Kupio cohort with the addition of features of insulin resistance, strongly associated with WC, the strength of the relationship with SHBG was attenuated although the OR remained significant 2.74 (95% CI 1.42–5.29).26
In cross-sectional and longitudinal analyses from the Framingham Heart Study SHBG but not testosterone was associated with incident metabolic syndrome in men aged 61 years with BMI 28.8 kg m−2 after adjusting for age and BMI.37 SHBG was independently associated with metabolic syndrome after adjusting for both BMI and WC33 in men aged 40–80 years and was also associated with insulin sensitivity although the strength of the correlation was diminished when BMI and WC were added to the model; the cohort included BMIs ranging from normal to obese. The association of SHBG with insulin resistance was also independent of total and intraabdominal body fat as measured by dual-energy X-ray absorptiometry (DEXA) and computed tomography (CT) in men aged 45–65 years, 31% of whom were obese, from the Puget Sound Veteran's Affairs cohort.38 In nonobese men from a MMAS cohort, the strength of the association between SHBG and metabolic syndrome was greatest in men with BMI <25 kg m−2, similar to that seen with TT.32 Low SHBG levels did not predict metabolic syndrome in men with BMI ≥25 kg m−2 suggesting that adiposity is the dominant factor in these men. Furthermore SHBG was not associated with insulin resistance when adjusted for WC in men aged ≥70 years.34 Thus it may be that SHBG and adiposity interact as a function of age and baseline obesity status to contribute differentially to the development of metabolic syndrome in men.
FREE TESTOSTERONE
The utility of free testosterone in predicting risk of T2DM is uncertain and data interpretation is limited by methodological concerns regarding the way in which free testosterone is measured.39 In the Tromso22 and Rancho Bernardo25 cohorts, free testosterone (FT) was not predictive of T2DM. FT was a predictor in men in the MMAS cohort after 7–10 years (no WC data) but not after 13 years of follow-up;27 the influence of longer follow-up periods and a greater number of men diagnosed with T2DM is not known.
Estrogen
A correlation between estradiol levels and risk of T2DM in men after controlling for BMI36 and WC22 has been demonstrated in some studies but neither total nor bioavailable estradiol was able to predict IGT or T2DM in men in cross-sectional40 or prospective25 analyses of the Rancho Bernardo cohort. In the Framingham Heart Study estrone but not estradiol was correlated with T2DM after 7 years of follow-up after adjusting for BMI; WC was not included in the model.41
MODELS OF ANDROGEN DEFICIENCY AND INSULIN RESISTANCE/TYPE 2 DIABETES MELLITUS
Klinefelter's syndrome
Men with Klinefelter's Syndrome are at increased risk of both Type 1 and Type 2 diabetes42 with HR of 2.21 (T1DM) and 3.71 (T2DM), respectively. They have a reduced median survival of 2.1 years with diabetes as contributor to the cause-specific mortality (HR 1.6).43,44 In a cohort of 71 men with Klinefelter's syndrome, 44% had features of the metabolic syndrome compared with 10% of controls.45 After controlling for TT levels, truncal obesity is a major determinant of insulin resistance in Klinefelter's syndrome.46 Although expert clinical opinion advises testosterone therapy to improve body composition aiming to prevent metabolic syndrome and/or diabetes mellitus, the evidence base for this is lacking.47
Androgen deprivation therapy in advanced prostate cancer
Androgen deprivation therapy (ADT) is associated with increased insulin resistance, independent of age and BMI.48 The relationship between ADT and insulin resistance appears to be a continuum with short-term therapy resulting in reduced insulin sensitivity and longer term ADT leading to hyperglycemia and subsequently increasing the risk of metabolic syndrome and overt T2DM.49 Men treated with combined androgen blockade experienced increased fasting insulin levels and decreased insulin sensitivity after 12 weeks, although with no change in glucose levels,50 and a cross-sectional study of longer-term ADT demonstrated higher fasting glucose levels with ADT compared with men with nonmetastatic prostate cancer not treated with ADT and a noncancer control group. The ADT group also had higher fasting insulin levels and increased insulin resistance after adjustment for age and BMI.48 Thus it may be that the relatively rapid development of insulin resistance in the setting of ADT is a compensatory mechanism to maintain normal glucose levels, however, after prolonged treatment, the hyperinsulinemic response becomes inadequate to maintain euglycemia.51 ADT-associated adverse body composition changes are likely to further exacerbate insulin resistance.
T2DM appears to be more common with prolonged ADT. After an average of 45 months of therapy in 18 men, 44% satisfied the fasting glucose criterion for diabetes mellitus, compared with 11% of a noncancer control group (n = 18) and 12% of men with treated metastatic prostate cancer who did not have ADT (n = 17), although the numbers in this cross-sectional study were small.48 The risk of incident diabetes in large observational studies comparing men treated with ADT with those not receiving ADT have reported significantly elevated risks, with HR (adjusted for confounders) ranging from 1.16 to 1.44.52,53,54 Two of these studies included only men 66 years or older52,54 while the other included men of all ages with over 40% of the cohort younger than 66 years.53 The risk of developing diabetes with ADT was independent of known risk factors.55 Glycemic control may also deteriorate in men with known T2DM exposed to ADT. In a retrospective analysis of 77 men with preexisting T2DM treated with ADT, almost 20% experienced a minimum 10% increase in HbA1c.56
Experimental androgen deficiency/insulin resistance
Epidemiological studies examining the relationships between sex steroids and insulin resistance/T2DM are not able to adequately stratify according to baseline age, BMI, WC and insulin sensitivity. In turn, preexisting obesity and/or insulin resistance may influence the response of the hypothalamo-pituitary-testicular (HPT)-axis to perturbations in the glucose/insulin milieu. Additionally, such studies do not allow sufficiently for assessing relationships between testosterone and glucose/insulin independent of the effect of SHBG and to investigate the possibility that the relationship is bidirectional. Thus, in vivo studies manipulating androgen status and/or insulin sensitivity provide valuable insights into the cause and effect nature of the relationship.
In a small study of diazoxide-induced suppression of insulin secretion and worsening glucose tolerance, a lowering of total TT in obese but not normal weight men was observed.57 Using an alternative approach with a protocol of induced hypogonadism with a GnRH antagonist, followed by sequential stimulation of the HPT-axis with physiological doses of GnRH and human chorionic gonadotropin (as an LH substitute), in men with normal glucose tolerance, IGT, and diabetes mellitus, and with BMI range 24–46 kg m−2, it was demonstrated that insulin resistance was associated with a decrease in Leydig cell testosterone secretory capacity.58 There was no correlation between insulin sensitivity and either endogenous LH secretion or the LH response to exogenous GnRH, suggesting the deficit in the HPT-axis associated with insulin resistance occurs at the levels of the testis. An increase in TT levels was seen during the hyperinsulinemic phase of the glucose clamp perhaps suggesting that high levels of insulin are able to overcome insulin resistance in the testis. This is consistent with a small euglycemic-hyperinsulinemic clamp study whereby there was an inverse baseline relationship between insulin resistance and TT but a significant increase in TT in obese men with acute hyperinsulinemia.59 Interestingly, in both studies a greater response was seen in obese men. Glucose levels were maintained in the normal range during both clamp studies.
Evidence for the role of androgens in direct modulation of insulin sensitivity is suggested by the observation that, in a group of otherwise healthy young men with idiopathic hypogonadotrophic hypogonadism studied after withdrawal of testosterone replacement therapy,60 fasting insulin levels and HOMA-IR were increased in the absence of a change in body composition. Healthy young men administered a GnRH agonist and add-back testosterone therapy did not show a change in insulin sensitivity as a function of graded testosterone levels61 although the lowest TT level was approximately 9 nmol l−1 compared with castrate levels in the idiopathic hypogonadotrophic hypogonadism men,60 suggesting a threshold for testosterone effect.
THE IMPACT OF ACUTE CHANGES IN GLUCOSE AND INSULIN ON TESTOSTERONE
Whilst chronic hyperglycemia and hyperinsulinemia are associated with hypoandrogenism, and low testosterone levels may play a role in the development of T2DM, the impact of more acute changes in glucose and/or insulin on the HPT-axis is less well understood.
Following the observation that insulin-induced hypoglycemia in healthy young men led to a rapid decrease in serum TT levels,62 euglycemic and hypoglycemic clamp experiments, again in healthy young men, demonstrated hypoglycemia-mediated suppression of TT secretion and LH levels, suggesting impaired hypothalamo – pituitary action. Manipulation of insulin levels did not influence either testosterone or LH.63 It is not stipulated, however, it is probable that these subjects were of normal BMI and were not insulin-resistant.
Manipulation of the glucose milieu in clinical scenarios may also influence testosterone levels. It has been demonstrated that glucose administration impacts TT levels in men without preexisting androgen deficiency with implications for the biochemical categorization of androgen status. A standard 75-g OGTT resulted in a 25% reduction in serum TT levels across a range of BMIs and glucose tolerance with 15% of men recording a TT level of <9.7 nmol l−1 on at least one occasion during the 120-min sampling period,64 confirming earlier observations.65 SHBG and LH levels (measured at 5 time points) were unchanged, however, a deconvolution analysis study of 50 men with age range of 18–80 years and BMI range of 20–40 kg m−2 with normal baseline glucose tolerance66 demonstrated a glucose-induced fall in pulsatile LH secretion, which was exacerbated by higher fasting insulin concentrations.
Consistent with these observations, in an Australian study of over 300 men aged 40–97 years who were sampled on multiple occasions over a 3-month period, testosterone levels were higher after an overnight fast.3 More prolonged fasting, however, has been shown to reduce LH and testosterone secretion in men67 although with differential effects as a function of age.68
While the mechanisms underlying these observations remain to be elucidated, one practical implication is consideration of measuring testosterone in a fasted state, in addition to morning sampling,21 to avoid recording artefactually low testosterone readings.
ANDROGEN THERAPY AND INSULIN SENSITIVITY
The observations of inverse associations between endogenous testosterone levels and hyperinsulinemia69 and an increased likelihood of developing T2DM refer (Table 1) have led to the hypothesis that testosterone therapy may improve insulin sensitivity. Results from studies of testosterone therapy in nondiabetic men using gold standard methods are inconsistent and comparisons limited due to varied subject characteristics and dose and duration of androgen treatment.70 Young, lean subjects did not demonstrate any change in insulin sensitivity across a wide range of serum testosterone levels in a 20-week dose-response study despite a dose-related reduction in fat mass.61 Centrally obese middle-aged men receiving testosterone showed an improvement in insulin sensitivity (by hyperinsulinemic/euglycemic clamp studies) and a lowering of serum insulin levels,71 however, these results were not seen with dihydrotestosterone (DHT)72 or oxandrolone (when administered to a similarly obese cohort).73 In aging men, hCG administered for 3 months did not affect insulin sensitivity (as measured by euglycemic clamp).74 It was unclear from these studies as to whether changes in serum testosterone are able to mediate insulin sensitivity independent of their effect on fat mass (specifically visceral fat). Comparison of data sets is difficult as those middle-aged men showing improved insulin sensitivity had higher fat mass and greater WC at baseline71 than the aging men treated with hCG.74 Furthermore the middle-aged cohort had significant visceral fat loss with treatment71 and although total fat mass declined in the older men, there was no data regarding regional adipose tissue changes;74 it is also possible that the duration of hCG treatment was insufficient. In a 12-month placebo-controlled study of testosterone therapy in nonobese men (satisfying both BMI and WC criteria), visceral fat change was inversely related to the change in serum TT in the men receiving testosterone;75 insulin resistance did not change, however, only small numbers of metabolically healthy men were studied. While anabolic steroids (oxandrolone) demonstrate a significant reduction in abdominal fat they have been associated with insulin resistance, thought to be mediated through hepatotoxicity.73
TESTOSTERONE THERAPY IN MEN WITH TYPE 2 DIABETES MELLITUS
The first study to describe the impact of testosterone therapy on glycemic control in diabetes76 enrolled men aged 45–65 years with T2DM diabetes and TT <15.1 nmol l−1, and administered oral testosterone undecanoate (120 mg per day) for 3 months in an open-label trial. HbA1c was reduced by 1.8% (17% improvement) (P <0.05) and BMI and WC also decreased. The subjects continued on their usual diabetic medication (approximately one-third were treated with insulin). However, the study was not placebo-controlled and therefore the effect of participation per se could not be determined. Further, the reduction in HbA1c was somewhat greater than expected over this small time interval. A double-blind placebo-controlled trial studied slightly older men with lower baseline TT levels (requirement <12 nmol l−1 on two occasions) but better diabetes control.77 Again approximately 30% of men were using insulin therapy. Men were treated with 200 mg of intramuscular testosterone esters every 2 weeks and received six injections in total. HOMA-IR improved in the men not on insulin and HbA1c was reduced by −0.37% (5% reduction) (P = 0.03). WC was reduced (by 1.6 cm; P = 0.03) and there was a trend to reduction in percentage body fat in this group of men whose mean BMI was 33 kg m−2 at baseline. A single-blind study of 52 weeks duration78 also studied men with a TT <12 nmol l−1 on two occasions and a similar HbA1c at baseline (7.5%), although these men were newly diagnosed and were therefore not receiving concurrent treatment for T2DM. Additionally, as an entry criteria all men had metabolic syndrome. The longer duration of treatment, achieving a 47% increase in serum testosterone levels while maintaining them within a physiological range (TT increased from 10.5 to 15.4 nmol l−1), resulted in a further reduction in HbA1c of 0.8% (P <0.001) with the combination of testosterone, diet and exercise compared with diet and exercise alone (reduction in HbA1c 0.5%; P = NS). All men treated with testosterone recorded an HbA1c of <7.0% at the completion of the study. WC was reduced by approximately 10 cm in the testosterone group, a greater effect than with lifestyle intervention alone (nonsignificant effect). All men completed the study protocol and no adverse effects were recorded. For details of these studies refer to Table 2.
Table 2.
Effects of testosterone therapy in men with type 2 diabetes mellitus
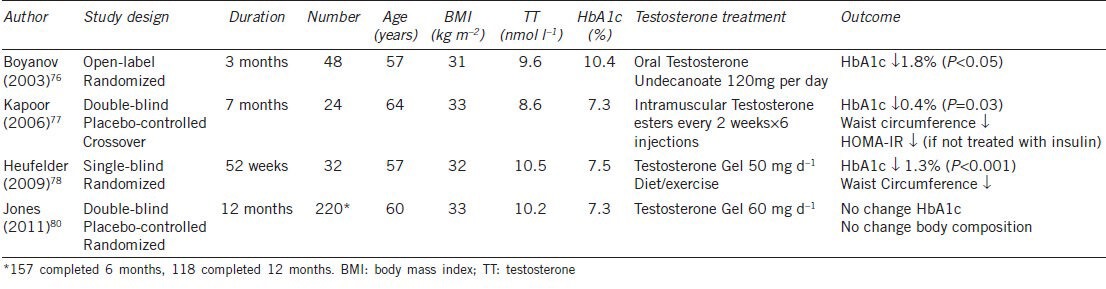
As reviewed by Jones,79 it is not clear from these studies whether the effect of testosterone on glycemic control is medicated by change in body composition or if there is a direct impact on insulin sensitivity. These men were all obese at baseline with low-normal range testosterone levels, consistent with their body composition.5,6 It remains to be determined whether the same outcomes would be observed in men with T2DM who are not obese. Furthermore, if these testosterone levels are ‘normal’ for men with T2DM and obesity, is testosterone therapy in these circumstances physiological or pharmacological? Finally, only small numbers of men were studied and only one study extended to 12 months.
The only double-blind, randomized, placebo-controlled study reported to date was conducted in 36 centers in Europe80 (Table 2). Men aged ≥ 40 years with TT ≤11 nmol l−1 or FT ≤255 pmol l−1 on two occasions with T2DM and/or metabolic syndrome were randomized to 2% transdermal testosterone gel for 12 months. Placebo-treated subjects did not receive advice regarding diet or exercise. A total of 62% of men had T2DM at baseline. Only 71% of men completed 6 months and 54% completed 12 months of the study protocol. The reasons for withdrawal were varied but no adverse cardiac or prostate events were reported in the testosterone treated group. TT levels increased by 19.5 nmol l−1 from a baseline of 9.2 nmol l−1 with the dose of testosterone gel adjusted to keep TT within the predetermined range of 17–52 nmmol l−1. Despite this arguably supraphysiological 12-month treatment regimen, and a baseline BMI of 33 kg m−2, no change in BMI, WC or percent body fat was achieved. Likewise there was no effect on total or low-density lipoprotein (LDL)-cholesterol. This is not consistent with previous studies of testosterone therapy on body composition and lipid parameters in nondiabetic men.81,82 There was no change in fasting insulin or glucose although HOMA-IR was reduced in men with T2DM with a similar (but nonsignificant) trend in men with metabolic syndrome. Although the data are extremely limited, the lack of efficacy of testosterone supplementation in the TIMES2 study in the absence of a favorable effect on body composition supports the notion that changes in glycmic/insulin parameters are secondary to a reduction in adiposity. A placebo-controlled trial currently underway in which obese men with low-normal testosterone levels and IGT are randomized to testosterone in addition to all men partaking in a weight loss program to prevent progression to T2DM (T4DM) (Australian New Zealand Clinical Trials Registry: ACTRN12612000287831) should provide valuable insights into the mechanisms by which androgen action is mediated.
CONCLUSIONS
An association between sex steroids – most importantly TT and SHBG – and insulin resistance, metabolic syndrome and T2DM has been demonstrated in epidemiological studies and clinical and experimental models of androgen deficiency. Body composition, most importantly visceral fat mass, is a critical determinant of the bidirectional relationship between androgens and insulin resistance, although limited evidence also supports a direct causal role. Ongoing clinical trials will aid in understanding the role of testosterone (as either physiological replacement or a pharmacological agent) in the treatment of men with insulin resistance/T2DM.
COMPETING INTERESTS
Carolyn Allan acted as an advisor to, and received research funding from, Eli Lilly (Australia) and Bayer Schering Pharma.
REFERENCES
- 1.Feldman HA, Longcope C, Derby CA, Johannes CB, Araujo AB, et al. Age trends in the level of serum testosterone and other hormones in middle-aged men: Longitudinal results from the Massachusetts male aging study. J Clin Endocrinol Metab. 2002;87:589–98. doi: 10.1210/jcem.87.2.8201. [DOI] [PubMed] [Google Scholar]
- 2.Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR Baltimore Longitudinal Study of A. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging. J Clin Endocrinol Metab. 2001;86:724–31. doi: 10.1210/jcem.86.2.7219. [DOI] [PubMed] [Google Scholar]
- 3.Sartorius G, Spasevska S, Idan A, Turner L, Forbes E, et al. Serum testosterone, dihydrotestosterone and estradiol concentrations in older men self-reporting very good health: the healthy man study. Clin Endocrinol (Oxf) 2012;77:755–63. doi: 10.1111/j.1365-2265.2012.04432.x. [DOI] [PubMed] [Google Scholar]
- 4.Allan CA, McLachlan RI. Androgens and obesity. Curr Opin Endocrinol Diabetes Obes. 2010;17:224–32. doi: 10.1097/MED.0b013e3283398ee2. [DOI] [PubMed] [Google Scholar]
- 5.Field AE, Colditz GA, Willett WC, Longcope C, McKinlay JB. The relation of smoking, age, relative weight, and dietary intake to serum adrenal steroids, sex hormones, and sex hormone-binding globulin in middle-aged men. J Clin Endocrinol Metab. 1994;79:1310–6. doi: 10.1210/jcem.79.5.7962322. [DOI] [PubMed] [Google Scholar]
- 6.Tajar A, Forti G, O’Neill TW, Lee DM, Silman AJ, et al. Characteristics of secondary, primary, and compensated hypogonadism in aging men: evidence from the European Male Ageing Study. J Clin Endocrinol Metab. 2010;95:1810–8. doi: 10.1210/jc.2009-1796. [DOI] [PubMed] [Google Scholar]
- 7.Camacho EM, Huhtaniemi IT, O’Neill TW, Finn JD, Pye SR, et al. Age-associated changes in hypothalamic-pituitary-testicular function in middle-aged and older men are modified by weight change and lifestyle factors: longitudinal results from the European Male Ageing Study. Eur J Endocrinol. 2013;168:445–55. doi: 10.1530/EJE-12-0890. [DOI] [PubMed] [Google Scholar]
- 8.Mohr BA, Bhasin S, Link CL, O’Donnell AB, McKinlay JB. The effect of changes in adiposity on testosterone levels in older men: longitudinal results from the Massachusetts Male Aging Study. Eur J Endocrinol. 2006;155:443–52. doi: 10.1530/eje.1.02241. [DOI] [PubMed] [Google Scholar]
- 9.Yeap BB, Alfonso H, Chubb SA, Handelsman DJ, Hankey GJ, et al. Reference ranges and determinants of testosterone, dihydrotestosterone, and estradiol levels measured using liquid chromatography-tandem mass spectrometry in a population-based cohort of older men. J Clin Endocrinol Metab. 2012;97:4030–9. doi: 10.1210/jc.2012-2265. [DOI] [PubMed] [Google Scholar]
- 10.Ahren B, Pacini G. Age-related reduction in glucose elimination is accompanied by reduced glucose effectiveness and increased hepatic insulin extraction in man. J Clin Endocrinol Metab. 1998;83:3350–6. doi: 10.1210/jcem.83.9.5107. [DOI] [PubMed] [Google Scholar]
- 11. [Last accessed date on 12 October 2013]. Available from: http: //www.cdc.gov/diabetes/statistics/incidence/fig3.htm .
- 12.Utzschneider KM, Carr DB, Hull RL, Kodama K, Shofer JB, et al. Impact of intra-abdominal fat and age on insulin sensitivity and beta-cell function. Diabetes. 2004;53:2867–72. doi: 10.2337/diabetes.53.11.2867. [DOI] [PubMed] [Google Scholar]
- 13.Liu RT, Chung MS, Wang PW, Chen CD, Lee JJ, et al. The prevalence and predictors of androgen deficiency in taiwanese men with type 2 diabetes. Urology. 2013;82:124–9. doi: 10.1016/j.urology.2013.03.023. [DOI] [PubMed] [Google Scholar]
- 14.Grossmann M, Thomas MC, Panagiotopoulos S, Sharpe K, Macisaac RJ, et al. Low testosterone levels are common and associated with insulin resistance in men with diabetes. J Clin Endocrinol Metab. 2008;93:1834–40. doi: 10.1210/jc.2007-2177. [DOI] [PubMed] [Google Scholar]
- 15.Chandel A, Dhindsa S, Topiwala S, Chaudhuri A, Dandona P. Testosterone concentration in young patients with diabetes. Diabetes Care. 2008;31:2013–7. doi: 10.2337/dc08-0851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dhindsa S, Miller MG, McWhirter CL, Mager DE, Ghanim H, et al. Testosterone concentrations in diabetic and nondiabetic obese men. Diabetes Care. 2010;33:1186–92. doi: 10.2337/dc09-1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anderson SG, Heald A, Younger N, Bujawansa S, Narayanan RP, et al. Screening for hypogonadism in diabetes 2008/9: results from the Cheshire Primary Care cohort. Prim Care Diabetes. 2012;6:143–8. doi: 10.1016/j.pcd.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 18.Ogbera OA, Sonny C, Olufemi F, Wale A. Hypogonadism and subnormal total testosterone levels in men with type 2 diabetes mellitus. J Coll Physicians Surg Pak. 2011;21:517–21. [PubMed] [Google Scholar]
- 19.Goto A, Morita A, Goto M, Sasaki S, Miyachi M, et al. Associations of sex hormone-binding globulin and testosterone with diabetes among men and women (the Saku Diabetes study): a case control study. Cardiovasc Diabetol. 2012;11:130. doi: 10.1186/1475-2840-11-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ding EL, Song Y, Malik VS, Liu S. Sex differences of endogenous sex hormones and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA. 2006;295:1288–99. doi: 10.1001/jama.295.11.1288. [DOI] [PubMed] [Google Scholar]
- 21.Bhasin S, Cunningham GR, Hayes FJ, Matsumoto AM, Snyder PJ, et al. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010;95:2536–59. doi: 10.1210/jc.2009-2354. [DOI] [PubMed] [Google Scholar]
- 22.Vikan T, Schirmer H, Njolstad I, Svartberg J. Low testosterone and sex hormone-binding globulin levels and high estradiol levels are independent predictors of type 2 diabetes in men. Eur J Endocrinol. 2010;162:747–54. doi: 10.1530/EJE-09-0943. [DOI] [PubMed] [Google Scholar]
- 23.Haffner SM, Shaten J, Stern MP, Smith GD, Kuller L. Low levels of sex hormone-binding globulin and testosterone predict the development of non-insulin-dependent diabetes mellitus in men. MRFIT Research Group. Multiple Risk Factor Intervention Trial. Am J Epidemiol. 1996;143:889–97. doi: 10.1093/oxfordjournals.aje.a008832. [DOI] [PubMed] [Google Scholar]
- 24.Rosmond R, Wallerius S, Wanger P, Martin L, Holm G, et al. A 5-year follow-up study of disease incidence in men with an abnormal hormone pattern. J Intern Med. 2003;254:386–90. doi: 10.1046/j.1365-2796.2003.01205.x. [DOI] [PubMed] [Google Scholar]
- 25.Oh JY, Barrett-Connor E, Wedick NM, Wingard DL, Rancho Bernardo S. Endogenous sex hormones and the development of type 2 diabetes in older men and women: the Rancho Bernardo study. Diabetes Care. 2002;25:55–60. doi: 10.2337/diacare.25.1.55. [DOI] [PubMed] [Google Scholar]
- 26.Laaksonen DE, Niskanen L, Punnonen K, Nyyssonen K, Tuomainen TP, et al. Testosterone and sex hormone-binding globulin predict the metabolic syndrome and diabetes in middle-aged men. Diabetes Care. 2004;27:1036–41. doi: 10.2337/diacare.27.5.1036. [DOI] [PubMed] [Google Scholar]
- 27.Lakshman KM, Bhasin S, Araujo AB. Sex hormone-binding globulin as an independent predictor of incident type 2 diabetes mellitus in men. J Gerontol A Biol Sci Med Sci. 2010;65:503–9. doi: 10.1093/gerona/glq002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stellato RK, Feldman HA, Hamdy O, Horton ES, McKinlay JB. Testosterone, sex hormone-binding globulin, and the development of type 2 diabetes in middle-aged men: prospective results from the Massachusetts male aging study. Diabetes Care. 2000;23:490–4. doi: 10.2337/diacare.23.4.490. [DOI] [PubMed] [Google Scholar]
- 29.Biggs ML, Mukamal KJ, Luchsinger JA, Ix JH, Carnethon MR, et al. Association between adiposity in midlife and older age and risk of diabetes in older adults. JAMA. 2010;303:2504–12. doi: 10.1001/jama.2010.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Botella-Carretero JI, Balsa JA, Gomez-Martin JM, Peromingo R, Huerta L, et al. Circulating free testosterone in obese men after bariatric surgery increases in parallel with insulin sensitivity. J Endocrinol Invest. 2013;36:227–32. doi: 10.3275/8469. [DOI] [PubMed] [Google Scholar]
- 31.Corona G, Rastrelli G, Monami M, Saad F, Luconi M, et al. Body weight loss reverts obesity-associated hypogonadotropic hypogonadism: a systematic review and meta-analysis. Eur J Endocrinol. 2013;168:829–43. doi: 10.1530/EJE-12-0955. [DOI] [PubMed] [Google Scholar]
- 32.Kupelian V, Page ST, Araujo AB, Travison TG, Bremner WJ. Low sex hormone-binding globulin, total testosterone, and symptomatic androgen deficiency are associated with development of the metabolic syndrome in nonobese men. J Clin Endocrinol Metab. 2006;91:843–50. doi: 10.1210/jc.2005-1326. [DOI] [PubMed] [Google Scholar]
- 33.Muller M, Grobbee DE, den Tonkelaar I, Lamberts SW, van der Schouw YT. Endogenous sex hormones and metabolic syndrome in aging men. J Clin Endocrinol Metab. 2005;90:2618–23. doi: 10.1210/jc.2004-1158. [DOI] [PubMed] [Google Scholar]
- 34.Yeap BB, Chubb SA, Hyde Z, Jamrozik K, Hankey GJ, et al. Lower serum testosterone is independently associated with insulin resistance in non-diabetic older men: the Health In Men Study. Eur J Endocrinol. 2009;161:591–8. doi: 10.1530/EJE-09-0348. [DOI] [PubMed] [Google Scholar]
- 35.Grossmann M. Low testosterone in men with type 2 diabetes: significance and treatment. J Clin Endocrinol Metab. 2011;96:2341–53. doi: 10.1210/jc.2011-0118. [DOI] [PubMed] [Google Scholar]
- 36.Ding EL, Song Y, Manson JE, Hunter DJ, Lee CC, et al. Sex hormone-binding globulin and risk of type 2 diabetes in women and men. N Engl J Med. 2009;361:1152–63. doi: 10.1056/NEJMoa0804381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bhasin S, Jasjua GK, Pencina M, D’Agostino R, Sr, Coviello AD, et al. Sex hormone-binding globulin, but not testosterone, is associated prospectively and independently with incident metabolic syndrome in men: the framingham heart study. Diabetes Care. 2011;34:2464–70. doi: 10.2337/dc11-0888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsai EC, Matsumoto AM, Fujimoto WY, Boyko EJ. Association of bioavailable, free, and total testosterone with insulin resistance: influence of sex hormone-binding globulin and body fat. Diabetes Care. 2004;27:861–8. doi: 10.2337/diacare.27.4.861. [DOI] [PubMed] [Google Scholar]
- 39.Rosner W, Auchus RJ, Azziz R, Sluss PM, Raff H. Position statement: utility, limitations, and pitfalls in measuring testosterone: an Endocrine Society position statement. J Clin Endocrinol Metab. 2007;92:405–13. doi: 10.1210/jc.2006-1864. [DOI] [PubMed] [Google Scholar]
- 40.Goodman-Gruen D, Barrett-Connor E. Sex differences in the association of endogenous sex hormone levels and glucose tolerance status in older men and women. Diabetes Care. 2000;23:912–8. doi: 10.2337/diacare.23.7.912. [DOI] [PubMed] [Google Scholar]
- 41.Jasuja GK, Travison TG, Davda M, Rose AJ, Zhang A, et al. Circulating estrone levels are associated prospectively with diabetes risk in men of the framingham heart study. Diabetes Care. 2013;36:2591–6. doi: 10.2337/dc12-2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bojesen A, Juul S, Birkebaek NH, Gravholt CH. Morbidity in Klinefelter syndrome: a Danish register study based on hospital discharge diagnoses. J Clin Endocrinol Metab. 2006;91:1254–60. doi: 10.1210/jc.2005-0697. [DOI] [PubMed] [Google Scholar]
- 43.Bojesen A, Juul S, Birkebaek N, Gravholt CH. Increased mortality in Klinefelter syndrome. J Clin Endocrinol Metab. 2004;89:3830–4. doi: 10.1210/jc.2004-0777. [DOI] [PubMed] [Google Scholar]
- 44.Swerdlow AJ, Higgins CD, Schoemaker MJ, Wright AF, Jacobs PA. United Kingdom Clinical Cytogenetics G. Mortality in patients with Klinefelter syndrome in Britain: a cohort study. J Clin Endocrinol Metab. 2005;90:6516–22. doi: 10.1210/jc.2005-1077. [DOI] [PubMed] [Google Scholar]
- 45.Bojesen A, Kristensen K, Birkebaek NH, Fedder J, Mosekilde L, et al. The metabolic syndrome is frequent in Klinefelter's syndrome and is associated with abdominal obesity and hypogonadism. Diabetes Care. 2006;29:1591–8. doi: 10.2337/dc06-0145. [DOI] [PubMed] [Google Scholar]
- 46.Bojesen A, Host C, Gravholt CH. Klinefelter's syndrome, type 2 diabetes and the metabolic syndrome: the impact of body composition. Mol Hum Reprod. 2010;16:396–401. doi: 10.1093/molehr/gaq016. [DOI] [PubMed] [Google Scholar]
- 47.Groth KA, Skakkebaek A, Host C, Gravholt CH, Bojesen A. Clinical review: klinefelter syndrome: a clinical update. J Clin Endocrinol Metab. 2013;98:20–30. doi: 10.1210/jc.2012-2382. [DOI] [PubMed] [Google Scholar]
- 48.Basaria S, Muller DC, Carducci MA, Egan J, Dobs AS. Hyperglycemia and insulin resistance in men with prostate carcinoma who receive androgen-deprivation therapy. Cancer. 2006;106:581–8. doi: 10.1002/cncr.21642. [DOI] [PubMed] [Google Scholar]
- 49.Basaria S. Androgen deprivation therapy, insulin resistance, and cardiovascular mortality: an inconvenient truth. J Androl. 2008;29:534–9. doi: 10.2164/jandrol.108.005454. [DOI] [PubMed] [Google Scholar]
- 50.Smith MR, Lee H, Nathan DM. Insulin sensitivity during combined androgen blockade for prostate cancer. J Clin Endocrinol Metab. 2006;91:1305–8. doi: 10.1210/jc.2005-2507. [DOI] [PubMed] [Google Scholar]
- 51.Shahani S, Braga-Basaria M, Basaria S. Androgen deprivation therapy in prostate cancer and metabolic risk for atherosclerosis. J Clin Endocrinol Metab. 2008;93:2042–9. doi: 10.1210/jc.2007-2595. [DOI] [PubMed] [Google Scholar]
- 52.Alibhai SM, Duong-Hua M, Sutradhar R, Fleshner NE, Warde P, et al. Impact of androgen deprivation therapy on cardiovascular disease and diabetes. J Clin Oncol. 2009;27:3452–8. doi: 10.1200/JCO.2008.20.0923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Keating NL, O’Malley AJ, Freedland SJ, Smith MR. Diabetes and cardiovascular disease during androgen deprivation therapy: observational study of veterans with prostate cancer. J Natl Cancer Inst. 2010;102:39–46. doi: 10.1093/jnci/djp404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Keating NL, O’Malley AJ, Smith MR. Diabetes and cardiovascular disease during androgen deprivation therapy for prostate cancer. J Clin Oncol. 2006;24:4448–56. doi: 10.1200/JCO.2006.06.2497. [DOI] [PubMed] [Google Scholar]
- 55.Keating NL, O’Malley A, Freedland SJ, Smith MR. Diabetes and cardiovascular disease during androgen deprivation therapy: observational study of veterans with prostate cancer. J Natl Cancer Inst. 2012;104:1518–23. doi: 10.1093/jnci/djs376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Derweesh IH, Diblasio CJ, Kincade MC, Malcolm JB, Lamar KD, et al. Risk of new-onset diabetes mellitus and worsening glycaemic variables for established diabetes in men undergoing androgen-deprivation therapy for prostate cancer. BJU Int. 2007;100:1060–5. doi: 10.1111/j.1464-410X.2007.07184.x. [DOI] [PubMed] [Google Scholar]
- 57.Pasquali R, Casimirri F, De Iasio R, Mesini P, Boschi S, et al. Insulin regulates testosterone and sex hormone-binding globulin concentrations in adult normal weight and obese men. J Clin Endocrinol Metab. 1995;80:654–8. doi: 10.1210/jcem.80.2.7852532. [DOI] [PubMed] [Google Scholar]
- 58.Pitteloud N, Hardin M, Dwyer AA, Valassi E, Yialamas M, et al. Increasing insulin resistance is associated with a decrease in Leydig cell testosterone secretion in men. J Clin Endocrinol Metab. 2005;90:2636–41. doi: 10.1210/jc.2004-2190. [DOI] [PubMed] [Google Scholar]
- 59.Pasquali R, Macor C, Vicennati V, Novo F, De lasio R, et al. Effects of acute hyperinsulinemia on testosterone serum concentrations in adult obese and normal-weight men. Metabolism. 1997;46:526–9. doi: 10.1016/s0026-0495(97)90189-x. [DOI] [PubMed] [Google Scholar]
- 60.Yialamas MA, Dwyer AA, Hanley E, Lee H, Pitteloud N, et al. Acute sex steroid withdrawal reduces insulin sensitivity in healthy men with idiopathic hypogonadotropic hypogonadism. J Clin Endocrinol Metab. 2007;92:4254–9. doi: 10.1210/jc.2007-0454. [DOI] [PubMed] [Google Scholar]
- 61.Singh AB, Hsia S, Alaupovic P, Sinha-Hikim I, Woodhouse L, et al. The effects of varying doses of T on insulin sensitivity, plasma lipids, apolipoproteins, and C-reactive protein in healthy young men. J Clin Endocrinol Metab. 2002;87:136–43. doi: 10.1210/jcem.87.1.8172. [DOI] [PubMed] [Google Scholar]
- 62.Cumming DC, Quigley ME, Yen SS. Acute suppression of circulating testosterone levels by cortisol in men. J Clin Endocrinol Metab. 1983;57:671–3. doi: 10.1210/jcem-57-3-671. [DOI] [PubMed] [Google Scholar]
- 63.Oltmanns KM, Fruehwald-Schultes B, Kern W, Born J, et al. Hypoglycemia, but not insulin, acutely decreases LH and T secretion in men. J Clin Endocrinol Metab. 2001;86:4913–9. doi: 10.1210/jcem.86.10.7892. [DOI] [PubMed] [Google Scholar]
- 64.Caronia LM, Dwyer AA, Hayden D, Amati F, Pitteloud N, et al. Abrupt decrease in serum testosterone levels after an oral glucose load in men: implications for screening for hypogonadism. Clin Endocrinol. 2013;78:291–6. doi: 10.1111/j.1365-2265.2012.04486.x. [DOI] [PubMed] [Google Scholar]
- 65.Wall JR, Jarrett RJ, Zimmet PZ, Bailes M, Ramage CM. Fall in plasma-testosterone levels in normal male subjects in response to an oral glucose load. Lancet. 1973;1:967–8. doi: 10.1016/s0140-6736(73)91601-2. [DOI] [PubMed] [Google Scholar]
- 66.Iranmanesh A, Lawson D, Veldhuis JD. Glucose ingestion acutely lowers pulsatile LH and basal testosterone secretion in men. Am J Physiol Endocrinol Metab. 2012;302:E724–30. doi: 10.1152/ajpendo.00520.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Aloi JA, Bergendahl M, Iranmanesh A, Veldhuis JD. Pulsatile intravenous gonadotropin-releasing hormone administration averts fasting-induced hypogonadotropism and hypoandrogenemia in healthy, normal weight men. J Clin Endocrinol Metab. 1997;82:1543–8. doi: 10.1210/jcem.82.5.3947. [DOI] [PubMed] [Google Scholar]
- 68.Bergendahl M, Aloi JA, Iranmanesh A, Mulligan TM, Veldhuis JD. Fasting suppresses pulsatile luteinizing hormone (LH) secretion and enhances orderliness of LH release in young but not older men. J Clin Endocrinol Metab. 1998;83:1967–75. doi: 10.1210/jcem.83.6.4856. [DOI] [PubMed] [Google Scholar]
- 69.Simon D, Charles MA, Nahoul K, Orssaud G, Kremski J, et al. Association between plasma total testosterone and cardiovascular risk factors in healthy adult men: the Telecom Study. J Clin Endocrinol Metab. 1997;82:682–5. doi: 10.1210/jcem.82.2.3766. [DOI] [PubMed] [Google Scholar]
- 70.Liu PY, Swerdloff RS, Veldhuis JD. Clinical review 171: the rationale, efficacy and safety of androgen therapy in older men: future research and current practice recommendations. J Clin Endocrinol Metab. 2004;89:4789–96. doi: 10.1210/jc.2004-0807. [DOI] [PubMed] [Google Scholar]
- 71.Marin P, Holmang S, Jonsson L, Sjostrom L, Kvist H, et al. The effects of testosterone treatment on body composition and metabolism in middle-aged obese men. Int J Obes Relat Metab Disord. 1992;16:991–7. [PubMed] [Google Scholar]
- 72.Marin P, Holmang S, Gustafsson C, Jonsson L, Kvist H, et al. Androgen treatment of abdominally obese men. Obes Res. 1993;1:245–51. doi: 10.1002/j.1550-8528.1993.tb00618.x. [DOI] [PubMed] [Google Scholar]
- 73.Lovejoy JC, Bray GA, Greeson CS, Klemperer M, Morris J, et al. Oral anabolic steroid treatment, but not parenteral androgen treatment, decreases abdominal fat in obese, older men. Int J Obes Relat Metab Disord. 1995;19:614–24. [PubMed] [Google Scholar]
- 74.Liu PY, Wishart SM, Celermajer DS, Jimenez M, Pierro ID, et al. Do reproductive hormones modify insulin sensitivity and metabolism in older men. A randomized, placebo-controlled clinical trial of recombinant human chorionic gonadotropin? Eur J Endocrinol. 2003;148:55–66. doi: 10.1530/eje.0.1480055. [DOI] [PubMed] [Google Scholar]
- 75.Allan CA, Strauss BJ, Burger HG, Forbes EA, McLachlan RI. Testosterone therapy prevents gain in visceral adipose tissue and loss of skeletal muscle in nonobese aging men. J Clin Endocrinol Metab. 2008;93:139–46. doi: 10.1210/jc.2007-1291. [DOI] [PubMed] [Google Scholar]
- 76.Boyanov MA, Boneva Z, Christov VG. Testosterone supplementation in men with type 2 diabetes, visceral obesity and partial androgen deficiency. Aging Male. 2003;6:1–7. [PubMed] [Google Scholar]
- 77.Kapoor D, Goodwin E, Channer KS, Jones TH. Testosterone replacement therapy improves insulin resistance, glycaemic control, visceral adiposity and hypercholesterolaemia in hypogonadal men with type 2 diabetes. Eur J Endocrinol. 2006;154:899–906. doi: 10.1530/eje.1.02166. [DOI] [PubMed] [Google Scholar]
- 78.Heufelder AE, Saad F, Bunck MC, Gooren L. Fifty-two-week treatment with diet and exercise plus transdermal testosterone reverses the metabolic syndrome and improves glycemic control in men with newly diagnosed type 2 diabetes and subnormal plasma testosterone. J Androl. 2009;30:726–33. doi: 10.2164/jandrol.108.007005. [DOI] [PubMed] [Google Scholar]
- 79.Jones TH. Effects of testosterone on Type 2 diabetes and components of the metabolic syndrome. J Diabetes. 2010;2:146–56. doi: 10.1111/j.1753-0407.2010.00085.x. [DOI] [PubMed] [Google Scholar]
- 80.Jones TH, Arver S, Behre HM, Buvat J, Meuleman E, et al. Testosterone replacement in hypogonadal men with type 2 diabetes and/or metabolic syndrome (the TIMES2 study) Diabetes Care. 2011;34:828–37. doi: 10.2337/dc10-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Allan CA, McLachlan RI. Age-related changes in testosterone and the role of replacement therapy in older men. Clin Endocrinol. 2004;60:653–70. doi: 10.1111/j.1365-2265.2004.02002.x. [DOI] [PubMed] [Google Scholar]
- 82.Isidori AM, Giannetta E, Greco EA, Gianfrilli D, Bonifacio V, et al. Effects of testosterone on body composition, bone metabolism and serum lipid profile in middle-aged men: a meta-analysis. Clinical Endocrinol. 2005;63:280–93. doi: 10.1111/j.1365-2265.2005.02339.x. [DOI] [PubMed] [Google Scholar]


