Abstract
Several testosterone preparations are used in the treatment of hypogonadism in the ageing male. These therapies differ in their convenience, flexibility, regional availability and expense but share their pharmacokinetic basis of approval and dearth of long-term safety data. The brevity and relatively reduced cost of pharmacokinetic based registration trials provides little commercial incentive to develop improved novel therapies for the treatment of late onset male hypogonadism. Selective androgen receptor modulators (SARMs) have been shown to provide anabolic benefit in the absence of androgenic effects on prostate, hair and skin. Current clinical development for SARMs is focused on acute muscle wasting conditions with defined clinical endpoints of physical function and lean body mass. Similar regulatory clarity concerning clinical deficits in men with hypogonadism is required before the beneficial pharmacology and desirable pharmacokinetics of SARMs can be employed in the treatment of late onset male hypogonadism.
Keywords: hypogonadism, late onset hypogonadism, selective androgen receptor modulators, testosterone
INTRODUCTION
As the population ages, longitudinal and cross-sectional studies have shown that a significant portion of men over the age of 60 have serum testosterone (T) levels below the lower limits of those seen in healthy younger men (20–30 years).1,2 Late onset hypogonadism (LOH) encompasses a broad list of signs and symptoms including sexual dysfunction, fatigue and reduced lean body mass and bone mineral density (BMD) that are associated with low T and are what typically drives the patient to seek medical attention.3 Due to the often varied intensity of these symptoms and their association with non-pathological general ageing processes, a significant effort has been made to clearly define LOH and thus patients who might benefit from therapy.4 Despite such efforts, LOH prevalence remains controversial as does the treatment of men who do not display overt hypothalamic-pituitary-gonadal pathology where the risk benefit profile of androgen supplementation is more clearly established.5 Diagnostic tools like ANDROTEST have estimated a disease incidence of 10%–40% in men aged over 45 years and suggest that up to 60 million men in the United States, Europe and Japan suffer from LOH.3,6,7 Conversely, more stringent criteria have placed LOH rates at as little as 2.1% in similarly aged men.8 Regardless of the diagnostic criteria employed, the current gold standard for the treatment of hypogonadism in older men is T therapy.3
TESTOSTERONE REPLACEMENT THERAPY FOR LATE ONSET HYPOGONADISM
The goal of T therapy in the treatment of LOH is to return serum T levels to the normal eugonadal range. Diagnosing LOH involves serial measurements of an individual's T levels.4 Based on a host of factors including body mass index (BMI), circulating sex hormone binding globulin SHBG and concurrent disease states, an individual's total circulating T may fall below the eugonadal range despite being asymptomatic. Decreased sex hormone binding globulin resulting from any number of separate pathologies may allow eugonadal levels of free T (unbound) despite hypogonadal levels of total T. Likewise, elevated levels of sex hormone binding globulin might result in hypogonadal symptoms, due to reduced levels of free T, despite eugonadal levels of total T. For these reasons it has been suggested that free T levels, along with associated symptoms of hypogonadism, be used in the positive diagnoses of LOH.3,8 Though it is accepted that eugonadal levels of total T are variable and patient specific, the range of approximately 300–1000 ng dl−1 (10.4–34.7 nmol l−1) has been adopted and is used to gauge the success of TRT. When T therapy is indicated, minimally therapeutic T levels are desired and the upper end of the eugonadal range for young men (1000 ng dl−1) is generally avoided as the risks associated with T therapy are thought to increase with the levels of exogenous T administered.9 Currently available T product labeling suggests monitoring serum T and dose adjustments to maintain appropriate T levels.
T therapy has well-established beneficial effects on body composition, including increases in lean body mass and decreases in fat mass.10,11 These effects are rapid (apparent after as little as 3 months of therapy) and durable (maintained after 3 years).12,13 Increases in muscle strength are less consistently reported due to training effects and technical challenges in measuring maximal muscle strength.14 However, durable muscle strength benefits are reported in placebo controlled TRT trials performed in a hypogonadal population.15 Improvements in lumbar spine and hip BMD require extended treatment, but have been reported following T therapy in a placebo controlled setting.16 Rapid improvements in sexual function are a hallmark of T administration with increases in sexual desire, motivation and performance in several studies.11,12,17 Likewise, durable improvements are reported in positive mood scores.13,18
The success of TRT in treating the symptoms of LOH is supported by a rapidly expanding market.3 Annual T prescriptions increased by nearly 300% in the US between the years of 2000 and 2008 mirroring a trend seen in European nations as well.19 Critics of this trend instead believe that TRT is overprescribed and more the result of successful marketing than successful therapeutic intervention.20,21 Detractors also cite a paucity of long-term safety data especially concerning cardiovascular and prostate disease risk. Current registration trials for TRT products are short (~3 months), driven by pharmacokinetic endpoints and of relatively low cost. The low development-cost barrier has resulted in a plethora of TRT delivery options, but provides no incentive to develop novel therapies addressing risks associated with prolonged androgen administration to older men. A new class of drugs currently being developed for acute muscle wasting conditions, selective androgen receptor modulators (SARMs), could provide once daily oral androgen supplement with reduced side effects for the treatment of LOH.
SELECTIVE ANDROGEN RECEPTOR MODULATOR DEVELOPMENT
SARMs were initially reported in the 1990s as nonsteroidal androgen receptor agonists.22 Early nonclinical work demonstrated these orally active agents had unique pharmacology operating as full agonists in anabolic tissues (muscle and bone), but partial agonists in androgenic tissues (prostate, skin and hair).23 The utility of this pharmacology in expanding androgen therapy to women and men at risk for prostate disease was recognized immediately and followed with extensive worldwide drug discovery efforts.24 The result of one such effort, GTx-024 or enobosarm, is a first in class SARM in late clinical development.
Enobosarm has been evaluated for its effect on body composition and physical function in three clinical studies. In a phase I study in 48 postmenopausal women, enobosarm 3.0 mg or placebo was administered for 12 weeks.25 Enobosarm 3.0 mg significantly increased total lean body mass compared to placebo (1.54 kg; P < 0.001). Enobosarm also increased thigh muscle volume by 0.17 L from baseline to day 84 compared to a decrease of 0.12 l in the placebo group. Correspondingly, a 22 lb increase in leg muscle strength was observed in the enobosarm treatment group relative to 1.5 lb in patients that received placebo.
A dose-response phase II study was conducted in 120 healthy elderly men (>60 years of age) and postmenopausal women.26 Subjects received placebo or enobosarm at a daily dose of 0.1, 0.3, 1.0 or 3.0 mg for 12 weeks. Enobosarm dose-dependently increased total lean body mass, while decreasing total fat mass. At 3.0 mg, enobosarm increased lean body mass by 1.3 kg (P < 0.001), which was accompanied by a decrease of approximately 0.6 kg (P = 0.049) in total fat mass relative to placebo. Subjects also exhibited a dose-dependent increase in stair climb power (P = 0.013) and an improvement in the time required to climb 12 steps.
A second phase II study was performed in 159 patients with a variety of cancers.27 After 4 months of treatment, subjects experienced a significant increase in total lean body mass at 1.0 mg (1.5 kg; P = 0.0012) and 3.0 mg (1.3 kg; P = 0.046). Additionally, an average increase of 18.0% for 1 mg (P = 0.001) and 21.7% for 3 mg (P = 0.001) was observed in stair climb power with significant improvements in the time required to climb 12 steps (P = 0.0019 at 1.0 mg; P = 0.0065 at 3.0 mg). Enobosarm 3.0 mg is currently being evaluated in two phase III studies in a larger patient population (n ≈ 650) with non-small cell lung cancer with key endpoints of total lean body mass and physical function (i.e., stair climb test) after 3 and 5 months of treatment.
The principal challenge in developing SARMs for the treatment of male hypogonadism is the definition of acceptable clinical endpoints. Unlike a T product registration trial, eugonadal levels of SARM cannot be used as a primary endpoint. Furthermore, though established improvement in body composition, muscle strength, BMD and sexual function follow successful T therapy; little agreement exists on what constitutes clinical deficits in these areas within a hypogonadal population let alone meaningful therapeutic amelioration.3 These challenges aside, SARMs’ unique pharmacology could provide therapeutic benefit in the treatment of male hypogonadism.
SELECTIVE ANDROGEN RECEPTOR MODULATORSS FOR THE TREATMENT OF LATE ONSET HYPOGONADISM
Pharmacokinetics
The vast majority of T products approved for the treatment of hypogonadism require parenteral administration.3 Through varied formulation and delivery techniques, often combined with chemical modifications to the parent steroid T, T levels can be restored to steady-state eugonadal levels in roughly a week's time. Though effective in restoring T levels, intramuscular (IM) injections of T esters (TE) result in large fluctuations in circulating T and are long acting (2–14 weeks), which prevents rapid dose adjustments or cessation in case of an adverse event. IM injections are also frequently associated with pain and irritation at the injection site and often require a doctor's visit increasing the end cost of therapy to the patient.28 Treatment frequency can be reduced with surgical implantation of slow release T pellets. However, this modality suffers from the same therapeutic rigidity as long-acting IM injections with increased invasiveness. Several transdermal preparations offer therapeutic flexibility, more tightly controlled physiologic T levels and noninvasive administration. Transdermal T is rapidly becoming the therapy of choice for many hypogonadal men.5,20 The disadvantages of these therapies are their increased cost, site irritation and contact transfer risk.5,29 The daily administration of a lipophilic T patch or gel can result in significant unintended drug transfer which is of particular concern when women and children are exposed to high levels of androgen.30,31,32
T undecanoate (TU) is available as an oral capsule though not in the US. Unmodified T is subject to extensive first pass metabolism and suffers from a half-life on the order of minutes.33 The increased lipophilicity of TU relative to other TEs, affords increased absorption by the intestinal lymphatic system without the well characterized overt hepatotoxicity associated with orally administered 17-alkylated androgens such as methyl T.34,35 Consistent therapeutic levels of T are often challenging to achieve following oral TU administration. Despite an improved formulation, 300 mg twice daily dosing of TU, or roughly 50 molar equivalents of the T generated daily by healthy testes, is required to maintain physiological nadir levels of serum T. The risk associated with the super physiological serum 5α-dihydrotestosterone (DHT) levels and increased androgen burden on the liver that result from this dosing regimen are disputed.35 However, the reduced utilization of oral TU relative to the multiple parenteral T formulations available suggests that the perceived risks outweigh the convenience of an oral dosage form in the minds of patients and their physicians.
The SARMs under current clinical development are orally bioavailable and capable of anabolic benefit at low milligram doses.26,36 These drugs also have prolonged elimination half-lives that results in minimal peak-to-trough fluctuations and makes them amenable to once-daily dosing.25 The multiple nonsteroidal SARM pharmacophores discovered to date offer a great deal of flexibility in that small chemical modifications can be made that are neutral to the drugs inherent activity, but have a large impact on its pharmaceutical properties.23,37 This situation differs greatly from the exhausted field of modified steroidal androgens, which produced the TEs in use today more than 50 years ago.33
Effects on prostate
The prostate is an androgen-dependent organ. The requirement of a functioning androgen axis for prostatic development and homeostasis is well-characterized. Androgens are also implicated in benign prostatic hyperplasia and prostate carcinogenesis, raising obvious concerns around the potential impact of TRT on prostatic disease.38 The effects of T supplementation on prostate cancer risk remain controversial. Retrospective studies evaluating 5 years of TRT found that men who developed prostate cancer while on TRT had steeper increases in prostate specific antigen (PSA); however, the total rate of disease was no different than the general population.39 In a prospective setting, multiple aspects of prostatic physiology were compared between TRT and placebo-treated controls with few differences in molecular markers and no differences in disease instance or severity reported.40 Alternatively, a meta-analysis of nearly 700 patients across 19 placebo-controlled trials reported a higher risk (odds ratio [OR] =1.78, 95% confidence interval [CI] = 1.07–2.95) of all prostatic events defined as prostate biopsies, prostate cancers, increase in International Prostate Symptom Score >4, PSA >4 ng ml−1 or PSA increment ≥1.5 ng ml−1 during treatment and acute urinary retention.41 The primary prostatic androgen, DHT, has been implicated in the development of prostate cancer by the clinical evaluation of several 5α-reductase inhibitors. 5α-reductase drives the conversion of T to the more potent androgen DHT. Prostatic levels of DHT are known to be several fold higher than prostatic levels of T, owing to elevated expression of 5α-reductase in the prostate, and an order of magnitude higher than circulating levels of DHT.16,40,42 5α-reductase inhibitor administration reduced the chance of at risk men developing prostate cancer by nearly 25% over a 4–7 year period, suggesting the importance of DHT formation in the natural disease course of prostate cancer.43,44 When T supplementation is combined with a 5α-reductase inhibitor, T-mediated increases in serum PSA and prostate volume were completely and partially reversed, respectively.16 Amory et al. and Page et al. demonstrated in a hypogonadal population that DHT is not required for the anabolic bone or muscle benefits associated with T supplementation and may instead provide a prostatic liability15,16 (Figures 1 and 2). Enobosarm is not subject to 5α-reduction and tissue selective pharmacology suggests its potential to stimulate diseases of benign prostatic hyperplasia is greatly reduced. To this end, enobosarm does not cause increases in serum PSA in healthy older men at anabolic doses (Figures 1 and 2).
Figure 1.
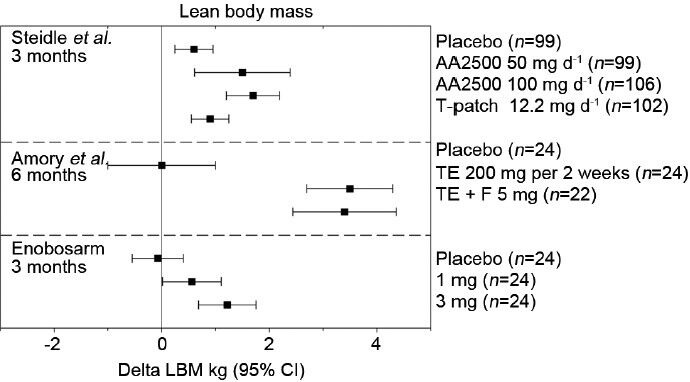
Changes in lean body mass (LBM) following testosterone or enobosarm treatment. Mean changes from baseline at the indicated treatment times are reported along with 95% confidence intervals (CIs) from Steidle et al.,12 Page et al.,15 and Dalton et al.26 AA2500: Testim© gel; F: Finasteride; T-patch, Androderm©; TE: Testosterone enanthanate injection.
Figure 2.
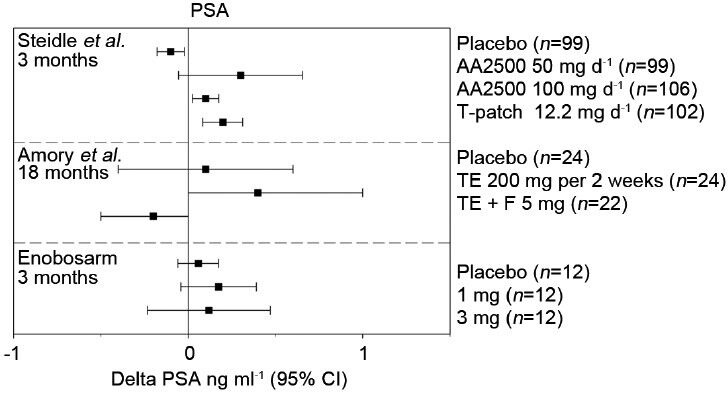
Changes in prostate specific antigen (PSA) following testosterone or enobosarm treatment. Mean changes from baseline at the indicated treatment times are reported along with 95% confidence intervals (CIs) from Steidle et al.,12 Page et al.,15 and Dalton et al.26 AA2500: Testim© gel; F: Finasteride; T-patch, Androderm©; TE: Testosterone enanthanate injection.
Cardiovascular and hematologic effects
The most common adverse event associated with T supplementation is increased erythrocytosis which can lead to increased blood viscosity and consequently ischemic events. Calof et al.'s survey of trials reported that T-treated men were nearly four times (OR = 3.69, 95% CI = 1.82–7.51) more likely to have a hematocrit value >50% when compared to placebo-treated men.41 Despite elevating this cardiovascular risk factor, cardiac event rates were similar between T-treated and placebo-treated men (33.2 per 1000 patient-years and 44.3 per 1000 patient-years, respectively). Steidle et al. reported at least one hematocrit value >55% in 3%, 6% and 1% of patients in the 50 mg per day AA2500, 100 mg per day AA2500 and T patch groups, respectively, compared to 1% in placebo. Similarly, Amory et al. reported dose reductions in 30% of subjects receiving TE injections based on hematocrit values >52%, but reported no significant cardiovascular events.16 Enobosarm minimally stimulated erythrocytosis in healthy older men and women and resulted in only one hematocrit value >50% (3-mg group) during 3 months of daily administration (Figure 3). Increased iron incorporation by erythrocytes results in the stimulation of hemoglobin production.33 As such, hemoglobin levels are elevated following T administration, closely resembling hematocrit inductions (Figure 4). Enobosarm's minimal effects on hemoglobin production in healthy men and women similarly reflected its effects on hematocrit.
Figure 3.
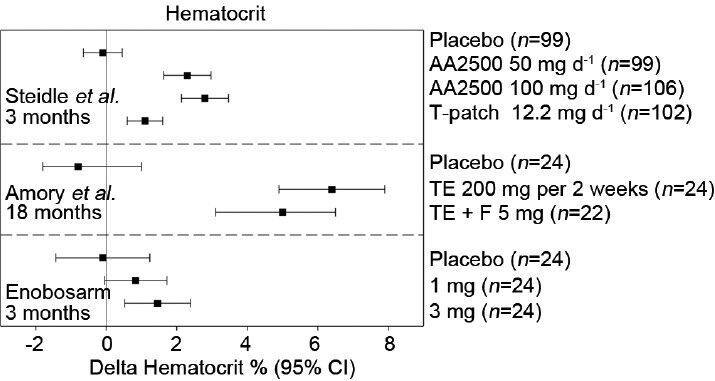
Changes in hematocrit following testosterone or enobosarm treatment. Mean changes from baseline at the indicated treatment times are reported along with 95% confidence intervals (CIs) from Steidle et al.,12 Page et al.,15 and Dalton et al.26 AA2500, Testim© gel; F: finasteride; T-patch, Androderm©; TE: testosterone enanthanate injection.
Figure 4.
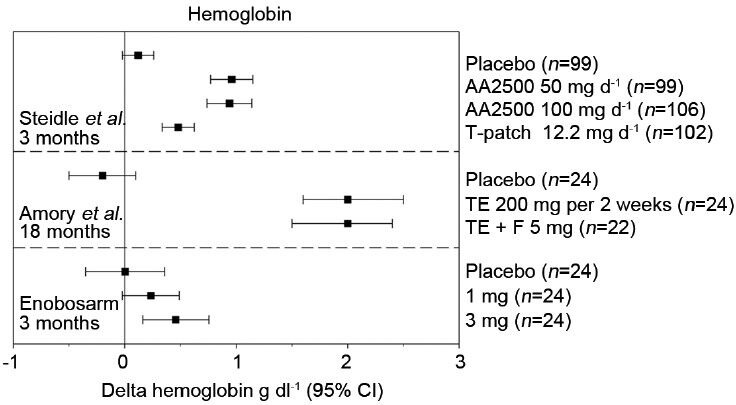
Changes in hemoglobin following testosterone or enobosarm treatment. Mean changes from baseline at the indicated treatment times are reported along with 95% confidence intervals (CIs) from Steidle et al.,12 Page et al.,15 and Dalton et al.26 AA2500, Testim© gel; F: Finasteride; T-patch, Androderm©; TE: Testosterone enanthanate injection.
The role of T and TRT in atherogenic cardiovascular disease is poorly understood and controversial. Increased cardiovascular events and mortality in men relative to women is dogmatically associated with increased endogenous circulating T levels despite ample evidence to the contrary.45,46 In a case-controlled comparison between healthy men with eugonadal levels of total T and age- and ethnicity-matched controls with low total T, an increased risk in all metabolic cardiovascular risk factors was reported for the low T group.47 This early study was limited in terms of its size (n = 50) and methodology, but its findings have been substantiated in larger studies evaluating free as well as total T.48,49 Despite being extensively characterized, the cardiovascular risk associated with exogenous T administration remains controversial. A recent meta-analysis of placebo-controlled trials included nearly 3000 patients experiencing a total of 180 cardiovascular events and found an increased risk associated with T therapy (OR–1.54, 95% CI=1.09–2.18).50 Alternatively, an analysis of over 1000 patients involved in placebo-controlled trials reported no association between T therapy and an increase in cardiovascular events.41 A recent trial involving hypogonadal community-dwelling men was terminated early due to treatment-related cardiovascular events.9 It was suggested by the investigators that the elevated total cardiac event rate in this study was related to the chronic disease prevalence and limited mobility of the test subjects. This assertion is supported by the reduced number of cardiac events reported in much larger trials using similar T gel products.12,13,18
The lack of consensus concerning T and cardiovascular outcomes is likely due in part to T's myriad effects on body composition. Low T levels are also associated with metabolic syndrome and increases in visceral fat.51 Increased adiposity is a well-characterized cardiovascular risk factor, but its status as a cause or an effect of hypogonadal T remains an open question.52 In either case, T administration has been shown to reduce fat mass and improve insulin sensitivity amongst other aspects of metabolic syndrome.53 Similarly, enobosarm administration to a healthy elderly population of men and women improved insulin resistance (according to the HOMA-IR criteria54) in the 1- and 3-mg treated groups (placebo: 2.6% ± 8.6%, 1 mg: -9.3% ± 5.5%, 3 mg: -27.5% ± 7.6%, P = 0.013 for 3 mg vs placebo).26
Compared to parenteral therapy, oral androgens are associated with greater effects on serum lipids, namely reductions in high density lipoprotein (HDL).55 Though colloquially considered ‘good cholesterol’, new research suggests that the cholesterol efflux capacity of circulating HDL is an improved predictor of atherosclerosis.56 Efflux capacity is a distinct metric from both total serum HDL, as is routinely measured, and HDL subfractionation. Several studies have evaluated the effects of T therapy on various HDL subfractions with widely varying results.57,58,59 The effects of therapy on the HDL efflux capacity of T-treated men are yet to be determined. Like other oral anabolic agents SARMs reduce total serum cholesterol and HDL dose dependently.36 Enobosarm treatment resulted in 17% and 27% reductions in HDL at the 1.0 and 3.0 mg dose levels, respectively. However, the average total cholesterol/HDL ratio in these treatment groups remained in the low risk category between 3.5 and 5.0 for the duration of the study.26
Role of aromatase
The composite pharmacologic actions of T include its conversion to estradiol (E2) as well as DHT. CYP19 (aromatase) is responsible for this largely peripheral conversion, however T administration does result in increased circulating E2.16 In rare cases, usually associated with long-acting IM T injections, E2 overproduction can even result in painful gynecomastia.28 The importance of E2 to the male skeleton is apparent in men who are naturally aromatase deficient and present with stunted bone development and greatly reduced BMD.60,61 Likewise, men who are hypogonadal often have low BMD putting them at greater risk for fractures and their associated comorbidities.62 Direct effects of androgens on both cortical and trabecular bone are suggested by knockout animal models and supported by several nonaromatizable, non-estrogenic SARMs demonstrating the ability to maintain bone in preclinical models of osteopenia.63,64,65,66 To date the clinical evaluation of SARMs has been of insufficient duration (3 months or less) to characterize effects on relatively slow changing bone parameters.
Sexual dysfunction is the primary cause of hypogonadal men seeking treatment.51 It follows that any broadly applicable therapy for LOH needs to alleviate sexual symptoms as well as improve body composition and physical function. Though the benefits of TRT on multiple sexual parameters is established, the relative contributions of E2 to this effect remains a rather poorly studied open question.67 To date no sexual parameters have been monitored clinically following SARM administration. Nonclinical evidence suggests nonaromatizable, non-estrogenic SARM administration benefits male sexual function.68
CONCLUSION
Despite registration trials based on purely pharmacokinetic endpoints, in practice TRT doses are more often adjusted based on symptoms and safety monitoring than on the levels of serum total T actually achieved in the patient.46 The inter-patient variability in the pharmacokinetics of T products and patient specific levels of effective TRT combine to all but obviate truly useful T monitoring. Thus, successful TRT is ultimately symptom and not pharmacokinetic driven. Unlike TRT, a SARMs approval for the treatment of LOH would likely require proven amelioration of hypogonadal symptoms in a large otherwise healthy cohort of older men. Agreement from regulatory bodies as to what constitutes clinically relevant efficacy would requisitely precede initiation of such a trial. The lack of consensus amongst physicians in diagnosing LOH suggests that broad agreement on clinically relevant efficacy in LOH remains out of reach. In light of this regulatory challenge, current SARM development is focused on acute and chronic muscle wasting diseases with defined clinical deficits in physical function and lean body mass. It stands to reason that the accumulation of efficacy and safety data, especially pertaining to benefits in physical function, will inform discussions with regulatory bodies as to acceptable clinical endpoints in an ageing hypogonadal population. SARM's beneficial pharmacology and desirable pharmacokinetics offer considerable promise in the treatment of LOH. The convenience of once daily oral therapy combined with defined safety margins surrounding a proven efficacious dosage form may one day challenge TRT as the gold standard in treating LOH.
COMPETING INTERESTS
All authors were employees of GTx Inc and hold stock options in GTx Inc at the time this article was written. GTx Inc. is developing GTx-024 (enobosarm) for the treatment of cachexia in late stage NSCLC patients.
REFERENCES
- 1.Araujo AB, Esche GR, Kupelian V, O’Donnell AB, Travison TG, et al. Prevalence of symptomatic androgen deficiency in men. J Clin Endocrinol Metab. 2007;92:4241–7. doi: 10.1210/jc.2007-1245. [DOI] [PubMed] [Google Scholar]
- 2.Wu FC, Tajar A, Pye SR, Silman AJ, Finn JD, et al. European Male Aging Study Group. Hypothalamic-pituitary-testicular axis disruptions in older men are differentially linked to age and modifiable risk factors: the European Male Aging Study. J Clin Endocrinol Metab. 2008;93:2737–45. doi: 10.1210/jc.2007-1972. [DOI] [PubMed] [Google Scholar]
- 3.Corona G, Rastrelli G, Vignozzi L, Maggi M. Emerging medication for the treatment of male hypogonadism. Expert Opin Emerg Drugs. 2012;17:239–59. doi: 10.1517/14728214.2012.683411. [DOI] [PubMed] [Google Scholar]
- 4.Wang C, Nieschlag E, Swerdloff R, Behre HM, Hellstrom WJ, et al. Investigation, treatment and monitoring of late-onset hypogonadism in males: ISA, ISSAM, EAU, EAA and ASA recommendations. Eur J Endocrinol. 2008;159:507–14. doi: 10.1530/EJE-08-0601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abadilla KA, Dobs AS. Topical testosterone supplementation for the treatment of male hypogonadism. Drugs. 2012;72:1591–603. doi: 10.2165/11635620-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 6.Corona G, Mannucci E, Petrone L, Balercia G, Fisher AD, et al. ANDROTEST: a structured interview for the screening of hypogonadism in patients with sexual dysfunction. J Sex Med. 2006;3:706–15. doi: 10.1111/j.1743-6109.2006.00262.x. [DOI] [PubMed] [Google Scholar]
- 7.Corona G, Rastrelli G, Forti G, Maggi M. Update in testosterone therapy for men. J Sex Med. 2011;8:639–54. doi: 10.1111/j.1743-6109.2010.02200.x. [DOI] [PubMed] [Google Scholar]
- 8.Wu FC, Tajar A, Beynon JM, Pye SR, Silman AJ, et al. EMAS Group. Identification of late-onset hypogonadism in middle-aged and elderly men. N Engl J Med. 2010;363:123–35. doi: 10.1056/NEJMoa0911101. [DOI] [PubMed] [Google Scholar]
- 9.Basaria S, Coviello AD, Travison TG, Storer TW, Farwell WR, et al. Adverse events associated with testosterone administration. N Engl J Med. 2010;363:109–22. doi: 10.1056/NEJMoa1000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Snyder PJ, Peachey H, Hannoush P, Berlin JA, Loh L, et al. Effect of testosterone treatment on body composition and muscle strength in men over 65 years of age. J Clin Endocrinol Metab. 1999;84:2647–53. doi: 10.1210/jcem.84.8.5885. [DOI] [PubMed] [Google Scholar]
- 11.Wang C, Swerdloff RS, Iranmanesh A, Dobs A, Snyder PJ, et al. Testosterone Gel Study Group. Transdermal testosterone gel improves sexual function, mood, muscle strength, and body composition parameters in hypogonadal men. J Clin Endocrinol Metab. 2000;85:2839–53. doi: 10.1210/jcem.85.8.6747. [DOI] [PubMed] [Google Scholar]
- 12.Steidle C, Schwartz S, Jacoby K, Sebree T, Smith T, et al. North American AA2500 T Gel Study Group. AA2500 testosterone gel normalizes androgen levels in aging males with improvements in body composition and sexual function. J Clin Endocrinol Metab. 2003;88:2673–81. doi: 10.1210/jc.2002-021058. [DOI] [PubMed] [Google Scholar]
- 13.Wang C, Cunningham G, Dobs A, Iranmanesh A, Matsumoto AM, et al. Long-term testosterone gel (AndroGel) treatment maintains beneficial effects on sexual function and mood, lean and fat mass, and bone mineral density in hypogonadal men. J Clin Endocrinol Metab. 2004;89:2085–98. doi: 10.1210/jc.2003-032006. [DOI] [PubMed] [Google Scholar]
- 14.LeBrasseur NK, Bhasin S, Miciek R, Storer TW. Tests of muscle strength and physical function: reliability and discrimination of performance in younger and older men and older men with mobility limitations. J Am Geriatr Soc. 2008;56:2118–23. doi: 10.1111/j.1532-5415.2008.01953.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Page ST, Amory JK, Bowman FD, Anawalt BD, Matsumoto AM, et al. Exogenous testosterone (T) alone or with finasteride increases physical performance, grip strength, and lean body mass in older men with low serum T. J Clin Endocrinol Metab. 2005;90:1502–10. doi: 10.1210/jc.2004-1933. [DOI] [PubMed] [Google Scholar]
- 16.Amory JK, Watts NB, Easley KA, Sutton PR, Anawalt BD, et al. Exogenous testosterone or testosterone with finasteride increases bone mineral density in older men with low serum testosterone. J Clin Endocrinol Metab. 2004;89:503–10. doi: 10.1210/jc.2003-031110. [DOI] [PubMed] [Google Scholar]
- 17.Loizides E, Swierzewski MJ, O’Neill C, Griesser J, Smith T. Early response time in sexual activity and mood following testosterone gel replacement in hypogonadal males from the testim (R) START study. Rev Urol. 2004;6(Suppl 6):S16–21. [PMC free article] [PubMed] [Google Scholar]
- 18.Dean JD, Carnegie C, Rodzvilla J, Smith T. Long-term effects of testim (r) 1% testosterone gel in hypogonadal men. Rev Urol. 2004;6 Suppl 6:S22–9. [PMC free article] [PubMed] [Google Scholar]
- 19.Mehta H. Androgel® (testosterone) BPCA Drug Use Review, NDA 21-015. 2009 [Google Scholar]
- 20.Gan EH, Pattman S, Pearce SH, Quinton R. A UK epidemic of testosterone prescribing, 2001-2010. Clin Endocrinol (Oxf) 2013 doi: 10.1111/cen.12178. [DOI] [PubMed] [Google Scholar]
- 21.Nigro N, Christ-Crain M. Testosterone treatment in the aging male: myth or reality? Swiss Med Wkly. 2012;142:w13539. doi: 10.4414/smw.2012.13539. [DOI] [PubMed] [Google Scholar]
- 22.Dalton JT, Mukherjee A, Zhu Z, Kirkovsky L, Miller DD. Discovery of nonsteroidal androgens. Biochem Biophys Res Commun. 1998;244:1–4. doi: 10.1006/bbrc.1998.8209. [DOI] [PubMed] [Google Scholar]
- 23.Mohler ML, Bohl CE, Jones A, Coss CC, Narayanan R, et al. Nonsteroidal selective androgen receptor modulators (SARMs): dissociating the anabolic and androgenic activities of the androgen receptor for therapeutic benefit. J Med Chem. 2009;52:3597–617. doi: 10.1021/jm900280m. [DOI] [PubMed] [Google Scholar]
- 24.Negro-Vilar A. Selective androgen receptor modulators (SARMs): a novel approach to androgen therapy for the new millennium. J Clin Endocrinol Metab. 1999;84:3459–62. doi: 10.1210/jcem.84.10.6122. [DOI] [PubMed] [Google Scholar]
- 25.Jones A, Coss CC, Steiner MS, Dalton JT. An overview of selective androgen receptor modulators: focus on enobosarm. Drugs Future. 2013;38:309–16. [Google Scholar]
- 26.Dalton JT, Barnette KG, Bohl CE, Hancock ML, Rodriguez D, et al. The selective androgen receptor modulator GTx-024 (enobosarm) improves lean body mass and physical function in healthy elderly men and postmenopausal women: results of a double-blind, placebo-controlled phase II trial. J Cachexia Sarcopenia Muscle. 2011;2:153–61. doi: 10.1007/s13539-011-0034-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dobs AS, Boccia RV, Croot CC, Gabrail NY, Dalton JT, et al. Effects of enobosarm on muscle wasting and physical function in patients with cancer: a double-blind, randomised controlled phase 2 trial. Lancet Oncol. 2013;14:335–45. doi: 10.1016/S1470-2045(13)70055-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu FC, Farley TM, Peregoudov A, Waites GM. Effects of testosterone enanthate in normal men: experience from a multicenter contraceptive efficacy study. World Health Organization Task Force on Methods for the Regulation of Male Fertility. Fertil Steril. 1996;65:626–36. [PubMed] [Google Scholar]
- 29.Mazer N, Fisher D, Fischer J, Cosgrove M, Bell D, et al. Transfer of transdermally applied testosterone to clothing: a comparison of a testosterone patch versus a testosterone gel. J Sex Med. 2005;2:227–34. doi: 10.1111/j.1743-6109.2005.20232.x. [DOI] [PubMed] [Google Scholar]
- 30.Yu YM, Punyasavatsu N, Elder D, D’Ercole AJ. Sexual development in a two-year-old boy induced by topical exposure to testosterone. Pediatrics. 1999;104:e23. doi: 10.1542/peds.104.2.e23. [DOI] [PubMed] [Google Scholar]
- 31.Merhi ZO, Santoro N. Postmenopausal virilization after spousal use of topical androgens. Fertil Steril. 2007;87:976.e13–5. doi: 10.1016/j.fertnstert.2006.07.1547. [DOI] [PubMed] [Google Scholar]
- 32.Bhowmick SK, Ricke T, Rettig KR. Sexual precocity in a 16-month-old boy induced by indirect topical exposure to testosterone. Clin Pediatr (Phila) 2007;46:540–3. doi: 10.1177/0009922806296651. [DOI] [PubMed] [Google Scholar]
- 33.Neischlag E, Behre HM. 3rd Edition. Cambridge University Press; 2004. Testosterone: Action, Deficiency, Substitution. [Google Scholar]
- 34.Bhasin S, Cunningham GR, Hayes FJ, Matsumoto AM, Snyder PJ, et al. Testosterone therapy in men with androgen deficiency syndromes: An Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010;95:2536–59. doi: 10.1210/jc.2009-2354. [DOI] [PubMed] [Google Scholar]
- 35.Yin AY, Htun M, Swerdloff RS, Diaz-Arjonilla M, Dudley RE, et al. Reexamination of pharmacokinetics of oral testosterone undecanoate in hypogonadal men with a new self-emulsifying formulation. J Androl. 2012;33:190–201. doi: 10.2164/jandrol.111.013169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Basaria S, Collins L, Dillon EL, Orwoll K, Storer TW, et al. The safety, pharmacokinetics, and effects of LGD-4033, a novel nonsteroidal oral, selective androgen receptor modulator, in healthy young men. J Gerontol A Biol Sci Med Sci. 2013;68:87–95. doi: 10.1093/gerona/gls078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang X, Sui Z. Deciphering the selective androgen receptor modulators paradigm. Expert Opin Drug Discov. 2013;8:191–218. doi: 10.1517/17460441.2013.741582. [DOI] [PubMed] [Google Scholar]
- 38.Bosland MC, Mahmoud AM. Hormones and prostate carcinogenesis: androgens and estrogens. J Carcinog. 2011;10:33. doi: 10.4103/1477-3163.90678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Coward RM, Simhan J, Carson CC., 3rd Prostate-specific antigen changes and prostate cancer in hypogonadal men treated with testosterone replacement therapy. BJU Int. 2009;103:1179–83. doi: 10.1111/j.1464-410X.2008.08240.x. [DOI] [PubMed] [Google Scholar]
- 40.Marks LS, Mazer NA, Mostaghel E, Hess DL, Dorey FJ, et al. Effect of testosterone replacement therapy on prostate tissue in men with late-onset hypogonadism: a randomized controlled trial. JAMA. 2006;296:2351–61. doi: 10.1001/jama.296.19.2351. [DOI] [PubMed] [Google Scholar]
- 41.Calof OM, Singh AB, Lee ML, Kenny AM, Urban RJ, et al. Adverse events associated with testosterone replacement in middle-aged and older men: a meta-analysis of randomized, placebo-controlled trials. J Gerontol A Biol Sci Med Sci. 2005;60:1451–7. doi: 10.1093/gerona/60.11.1451. [DOI] [PubMed] [Google Scholar]
- 42.Cai C, Balk SP. Intratumoral androgen biosynthesis in prostate cancer pathogenesis and response to therapy. Endocr Relat Cancer. 2011;18:R175–82. doi: 10.1530/ERC-10-0339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thompson IM, Goodman PJ, Tangen CM, Lucia MS, Miller GJ, et al. The influence of finasteride on the development of prostate cancer. N Engl J Med. 2003;349:215–24. doi: 10.1056/NEJMoa030660. [DOI] [PubMed] [Google Scholar]
- 44.Andriole GL, Bostwick DG, Brawley OW, Gomella LG, Marberger M, et al. REDUCE Study Group. Effect of dutasteride on the risk of prostate cancer. N Engl J Med. 2010;362:1192–202. doi: 10.1056/NEJMoa0908127. [DOI] [PubMed] [Google Scholar]
- 45.Tirabassi G, Gioia A, Giovannini L, Boscaro M, Corona G, et al. Testosterone and cardiovascular risk. Intern Emerg Med. 2013;8(Suppl 1):S65–9. doi: 10.1007/s11739-013-0914-1. [DOI] [PubMed] [Google Scholar]
- 46.Rhoden EL, Morgentaler A. Risks of testosterone-replacement therapy and recommendations for monitoring. N Engl J Med. 2004;350:482–92. doi: 10.1056/NEJMra022251. [DOI] [PubMed] [Google Scholar]
- 47.Simon D, Charles MA, Nahoul K, Orssaud G, Kremski J, et al. Association between plasma total testosterone and cardiovascular risk factors in healthy adult men: The Telecom Study. J Clin Endocrinol Metab. 1997;82:682–5. doi: 10.1210/jcem.82.2.3766. [DOI] [PubMed] [Google Scholar]
- 48.Hak AE, Witteman JC, de Jong FH, Geerlings MI, Hofman A, et al. Low levels of endogenous androgens increase the risk of atherosclerosis in elderly men: the Rotterdam study. J Clin Endocrinol Metab. 2002;87:3632–9. doi: 10.1210/jcem.87.8.8762. [DOI] [PubMed] [Google Scholar]
- 49.Debing E, Peeters E, Duquet W, Poppe K, Velkeniers B, et al. Men with atherosclerotic stenosis of the carotid artery have lower testosterone levels compared with controls. Int Angiol. 2008;27:135–41. [PubMed] [Google Scholar]
- 50.Xu L, Freeman G, Cowling BJ, Schooling CM. Testosterone therapy and cardiovascular events among men: a systematic review and meta-analysis of placebo-controlled randomized trials. BMC Med. 2013;11:108. doi: 10.1186/1741-7015-11-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Corona G, Rastrelli G, Morelli A, Vignozzi L, Mannucci E, et al. Hypogonadism and metabolic syndrome. J Endocrinol Invest. 2011;34:557–67. doi: 10.3275/7806. [DOI] [PubMed] [Google Scholar]
- 52.Marcellini F, Giuli C, Papa R, Tirabassi G, Faloia E, et al. Obesity and body mass index (BMI) in relation to life-style and psycho-social aspects. Arch Gerontol Geriatr. 2009;49(Suppl 1):195–206. doi: 10.1016/j.archger.2009.09.029. [DOI] [PubMed] [Google Scholar]
- 53.Corona G, Monami M, Rastrelli G, Aversa A, Tishova Y, et al. Testosterone and metabolic syndrome: a meta-analysis study. J Sex Med. 2011;8:272–83. doi: 10.1111/j.1743-6109.2010.01991.x. [DOI] [PubMed] [Google Scholar]
- 54.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 55.Thompson PD, Cullinane EM, Sady SP, Chenevert C, Saritelli AL, et al. Contrasting effects of testosterone and stanozolol on serum lipoprotein levels. JAMA. 1989;261:1165–8. [PubMed] [Google Scholar]
- 56.Khera AV, Cuchel M, de la Llera-Moya M, Rodrigues A, Burke MF, et al. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med. 2011;364:127–35. doi: 10.1056/NEJMoa1001689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zgliczynski S, Ossowski M, Slowinska-Srzednicka J, Brzezinska A, Zgliczynski W, et al. Effect of testosterone replacement therapy on lipids and lipoproteins in hypogonadal and elderly men. Atherosclerosis. 1996;121:35–43. doi: 10.1016/0021-9150(95)05673-4. [DOI] [PubMed] [Google Scholar]
- 58.Ozata M, Yildirimkaya M, Bulur M, Yilmaz K, Bolu E, et al. Effects of gonadotropin and testosterone treatments on Lipoprotein (a), high density lipoprotein particles, and other lipoprotein levels in male hypogonadism. J Clin Endocrinol Metab. 1996;81:3372–8. doi: 10.1210/jcem.81.9.8784099. [DOI] [PubMed] [Google Scholar]
- 59.Tan KC, Shiu SW, Pang RW, Kung AW. Effects of testosterone replacement on HDL subfractions and apolipoprotein A-I containing lipoproteins. Clin Endocrinol. 1998;48:187–94. [PubMed] [Google Scholar]
- 60.Morishima A, Grumbach MM, Simpson ER, Fisher C, Qin K. Aromatase deficiency in male and female siblings caused by a novel mutation and the physiological role of estrogens. J Clin Endocrinol Metab. 1995;80:3689–98. doi: 10.1210/jcem.80.12.8530621. [DOI] [PubMed] [Google Scholar]
- 61.Carani C, Qin K, Simoni M, Faustini-Fustini M, Serpente S, et al. Effect of testosterone and estradiol in a man with aromatase deficiency. N Engl J Med. 1997;337:91–5. doi: 10.1056/NEJM199707103370204. [DOI] [PubMed] [Google Scholar]
- 62.Meier C, Nguyen TV, Handelsman DJ, Schindler C, Kushnir MM, et al. Endogenous sex hormones and incident fracture risk in older men: the Dubbo Osteoporosis Epidemiology Study. Arch Intern Med. 2008;168:47–54. doi: 10.1001/archinternmed.2007.2. [DOI] [PubMed] [Google Scholar]
- 63.Kearbey JD, Gao W, Fisher SJ, Wu D, Miller DD, et al. Effects of selective androgen receptor modulator (SARM) treatment in osteopenic female rats. Pharm Res. 2009;26:2471–7. doi: 10.1007/s11095-009-9962-7. [DOI] [PubMed] [Google Scholar]
- 64.Furuya K, Yamamoto N, Ohyabu Y, Makino A, Morikyu T, et al. The novel non-steroidal selective androgen receptor modulator S-101479 has additive effects with bisphosphonate, selective estrogen receptor modulator, and parathyroid hormone on the bones of osteoporotic female rats. Biol Pharm Bull. 2012;35:1096–104. doi: 10.1248/bpb.b12-00054. [DOI] [PubMed] [Google Scholar]
- 65.Vajda EG, Hogue A, Griffiths KN, Chang WY, Burnett K, et al. Combination treatment with a selective androgen receptor modulator q (SARM) and a bisphosphonate has additive effects in osteopenic female rats. J Bone Miner Res. 2009;24:231–40. doi: 10.1359/jbmr.081007. [DOI] [PubMed] [Google Scholar]
- 66.Riggs BL, Khosla S, Melton LJ., 3rd Sex steroids and the construction and conservation of the adult skeleton. Endocr Rev. 2002;23:279–302. doi: 10.1210/edrv.23.3.0465. [DOI] [PubMed] [Google Scholar]
- 67.Kacker R, Traish AM, Morgentaler A. Estrogens in men: clinical implications for sexual function and the treatment of testosterone deficiency. J Sex Med. 2012;9:1681–96. doi: 10.1111/j.1743-6109.2012.02726.x. [DOI] [PubMed] [Google Scholar]
- 68.Miner JN, Chang W, Chapman MS, Finn PD, Hong MH, et al. An orally active selective androgen receptor modulator is efficacious on bone, muscle, and sex function with reduced impact on prostate. Endocrinology. 2007;148:363–73. doi: 10.1210/en.2006-0793. [DOI] [PubMed] [Google Scholar]


