Abstract
Plasma testosterone levels display circadian variation, peaking during sleep, and reaching a nadir in the late afternoon, with a superimposed ultradian rhythm with pulses every 90 min reflecting the underlying rhythm of pulsatile luteinizing hormone (LH) secretion. The increase in testosterone is sleep, rather than circadian rhythm, dependent and requires at least 3 h of sleep with a normal architecture. Various disorders of sleep including abnormalities of sleep quality, duration, circadian rhythm disruption, and sleep-disordered breathing may result in a reduction in testosterone levels. The evidence, to support a direct effect of sleep restriction or circadian rhythm disruption on testosterone independent of an effect on sex hormone binding globulin (SHBG), or the presence of comorbid conditions, is equivocal and on balance seems tenuous. Obstructive sleep apnea (OSA) appears to have no direct effect on testosterone, after adjusting for age and obesity. However, a possible indirect causal process may exist mediated by the effect of OSA on obesity. Treatment of moderate to severe OSA with continuous positive airway pressure (CPAP) does not reliably increase testosterone levels in most studies. In contrast, a reduction in weight does so predictably and linearly in proportion to the amount of weight lost. Apart from a very transient deleterious effect, testosterone treatment does not adversely affect OSA. The data on the effect of sleep quality on testosterone may depend on whether testosterone is given as replacement, in supratherapeutic doses, or in the context abuse. Experimental data suggest that testosterone may modulate individual vulnerability to subjective symptoms of sleep restriction. Low testosterone may affect overall sleep quality which is improved by replacement doses. Large doses of exogenous testosterone and anabolic/androgenic steroid abuse are associated with abnormalities of sleep duration and architecture.
Keywords: obesity, obstructive sleep apnea, shift work, sleep restriction, testosterone
INTRODUCTION
Normal sleep physiology
From a neurophysiological standpoint, there are two types of sleep: nonrapid eye movement (NREM) and rapid eye movement (REM) sleep. The first two phases of NREM sleep (phases 1 and 2) are light and often alternate with brief waking episodes. Two deeper phases of NREM sleep (phases 3 and 4) together known as slow wave sleep (SWS) tend to occur predominantly in the earlier part of the night and become lighter thereafter. Usually four to six cycles of REM sleep occur at intervals of approximately 90 min becoming longer and more frequent over the course of the night. The time between sleep onset and the first REM episode is termed REM latency. The term sleep architecture is used to describe the pattern of sleep that occurs through the night. From approximately middle-aged onwards, less time is spent in the deeper phases of sleep and there is more stage I sleep and more awakenings. The effect of ageing on REM sleep is more variable and it tends to be preserved until quite late in life.1
Sleep and the regulation of the hypothalamo-pituitary-gonadal axis
Plasma testosterone levels vary in a circadian manner, higher on waking and decreasing to a low point at the end of the day. Superimposed on this are burst-like increases in testosterone production that occur every 90 min or so.2 Plasma testosterone levels begin to increase with the onset of sleep, and in young men peak at the first REM sleep episode and remaining at that level until waking;3 the longer the REM sleep latency the slower the rise in testosterone.4
It was originally thought that there is an endogenous rhythm of testosterone production similar to what occurs for cortisol.5 More recently it has become clear that the production of testosterone is dependent on sleep generally reaching the peak during the first 3 h of uninterrupted sleep, and at least in young men at about the time of the first REM episode.4 Total fragmentation of normal sleep architecture throughout the night prevents the increase in testosterone.4 It has also been shown, at least in young men, that the sleep-dependent increase in testosterone occurs irrespective of whether the sleep occurs at night or for an equivalent duration during the day. The increase in testosterone with sleep time and a decrease during time awake is stable within an individual, but in turn there are large individual differences.6 It seems likely that there is an effect of ageing, since in healthy older men objectively measured differences in the amount of nighttime sleep are associated with the variability in the morning testosterone levels.7 Middle-aged men secrete less testosterone at night then healthy young men.1
Prevalence and nature of sleep disorders including short sleep, circadian rhythm disruption, and sleep-disordered breathing
Sleep restriction is an increasing problem of an electrified, digitalized, and constantly connected lifestyle. In the USA, average nocturnal sleep time is 6.9 h per night and 20% of adults sleep less than 6.5 h per night as compared to an average optimal sleep time of 8.2 h per night.8
They are a number of definitions of shift work. Typically it is defined as at least half of the work shift being outside the standard work time of 08:00–17:00 hours and this may apply to 25%–33% of the workforce. Of these, 5%–7% either work permanent nights or rotating shifts. In night workers or those working rotating shifts, as compared to night sleep, day sleep is associated with a reduction in SWS.9
There are a number of syndromes characterized by disordered breathing during sleep. One group of these relates to increased upper airway resistance. Vibration of the upper airways during the passage of air results in snoring. More severe obstruction of the upper airway during sleep results in the obstructive sleep apnea (OSA) or OSA syndrome (OSAS) when there are compatible symptoms present. Apneas are repetitive pauses in breathing lasting 10–40 s despite efforts to breathe. Hypopneas are defined as a 50% decrease in airflow for 10 s or 30% decrease when oxygen saturation falls or sleep arousal occurs.10 The presence and severity can be classified by a number of indices including the oxygen desaturation index (ODI), arousal index, or apnea hypopnea index (AHI). The AHI describes the number of apneas or hypopneas per hour of sleep. A normal AHI is less than 5.
Although originally considered to affect around 4% of middle-aged men, OSA has been shown to affect 41% of men in an urban Brazilian population where 60% were overweight or obese11 and 53% of men aged 40 and over from suburban Adelaide, Australia, where 72% were overweight or obese.(Submitted)
The severity of OSA is considerably worse during daytime sleep after night shift as compared to normal nighttime sleep and this may intensify the unfavorable health effects of OSAS.9
THE EFFECT OF SPECIFIC SLEEP ABNORMALITIES ON TESTOSTERONE
Sleep restriction
While studies confirm the effect of total sleep deprivation12,13 to lower testosterone, data on the effect of sleep restriction on the hypothalamo-pituitary-gonadal axis remains contradictory. The effect of sleep deprivation on testosterone may be dependent on age. In old as compared to young rats “paradoxical sleep deprivation” results in a greater decrease in testosterone and recovery is delayed.14 Other factors such as concomitant circadian shifts, changes to sex hormone binding globulin (SHBG), circadian rhythm disruption, induction of stress, depression, medication, and the use of self-reported as opposed to objectively assessed sleep duration may also explain differences between epidemiological studies. Moreover, the timing of sleep may be more important sleep length itself in determining testosterone levels.12,15 Although the physiology described above suggest that it is the first 3–4 h of sleep that are critical to determining the increase in testosterone a recent study has shown that restriction of sleep time to 4.5 h was associated with a lower morning testosterone level when sleep was permitted the first half rather than the second half of the night.12 This is probably not contradictory given that testosterone levels have been shown to decrease with increasing time awake.6 This may also, at least in part explain discrepant data in the literature relating to the effects of experimental sleep restriction in healthy young men. Restriction of sleep for eight nights to 5 h (00:30–05:30 hours) a night decreased testosterone levels by 10%–15%;16 although SHBG was not measured. In a subsequent study where sleep was restricted during the first half of the night and permitted from 0400–0800 hours for five nights there was no significant change in testosterone, although SHBG decreased.17
Circadian rhythm disruption
The most common form circadian rhythm disruption is that which occurs as a result of shift work. Shift work as such, appears not to have any adverse effect on morning testosterone levels. There is, however, a relationship between disturbed sleep and wakefulness, increased need for sleep and recovery, reduced morning cortisol, lower testosterone levels, and dissatisfaction with the shift system. The authors suggested that the reduced testosterone levels may be part of a mechanism of shift work maladjustment.18
Obstructive sleep apnea
Although it is commonly asserted that OSA is a direct cause of the decrease in pituitary gonadal function, exposure of C57BL/6 mice to either 8 or 24 weeks of chronic intermittent hypoxia had no effect on plasma testosterone levels at either time point,19 but the evidence in humans is mixed.
In 89 severely obese men with body mass index (BMI) 35 kg m−2 or more after correction for age and BMI, the severity of OSA; however, it was measured (hypopnea index, percent time below a SpO2 of 90%, and percent time below a SpO2 of 80%) was inversely related to the free testosterone level.20 In a case-control study, comparing 15 men with OSA all with an Epworth score greater than 10–15; age- and obesity-matched controls testosterone was inversely related to the severity of OSA as defined by the ODI independent of obesity.21 There is also a body of evidence suggesting that the low testosterone in men with OSA is primarily related to obesity.22 There may also be some effect of sleep fragmentation and hypoxia.22 Other data also suggests that BMI may be the primary determinant of testosterone in men with OSA,23 including a cross-sectional retrospective case study of 103 registries of OSAS patients from 2002 to 2009.24 In cohort of 1312 men aged 65 years and older at baseline and followed for 3.4 years it was shown that total testosterone levels were unrelated to age or duration of sleep, but was inversely related to the AHI and ODI. After adjusting for BMI or waist circumference significant associations were either absent or markedly attenuated.25
Effect of continuous positive airway pressure (CPAP) on testosterone
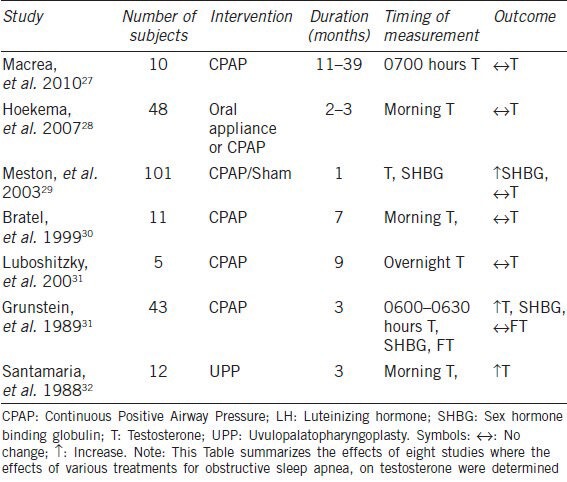
Although limited data suggests that treatment of OSA by CPAP or palatal surgery results in an increase in morning plasma testosterone levels at 3 months, the majority of other studies show that CPAP applied for durations that range from a single night26 to 39 months is without effect on luteinizing hormone (LH), follicle stimulating hormone (FSH), or testosterone even when compliance is assured.27 A summary of the studies looking at the effect of CPAP on plasma testosterone in men with OSA is shown in Table 1.27,28,29,30,31,32
Table 1.
The effect of treatment of obstructive sleep apnea on plasma testosterone in men
In contrast to the effect of CPAP, there is a linear relationship between a decrease in weight and an increase in plasma testosterone in obese men.33
Effect of testosterone on obstructive sleep apnea
Based largely on anecdotal evidence exogenous testosterone has been considered to have a deleterious effect on obstructive sleep apnea (OSA). Despite the paucity of evidence, current guidelines indicate that it is contraindicated in the presence of untreated OSA.34 In a recent clinical trial, testosterone undecanoate or placebo was administered intramuscularly, after baseline assessment and again at 6 and 12 weeks, to obese men with OSA. Testosterone treatment resulted in a mild worsening of the ODI at 7, but not 18 weeks. There were no relationships of ODI to the testosterone levels,35 but positive correlations were observed between changes in serum testosterone and hyperoxic ventilatory recruitment threshold and between changes in hyperoxic ventilatory recruitment threshold and time spent with oxygen saturations during sleep at 6–7 weeks but not at 18 weeks.36
Testosterone and sleep quality
Both insufficient and excessive testosterone levels have been shown to affect sleep. In a cohort study of men aged 65 years and over, those with lower testosterone levels had reduced sleep efficiency, increased nocturnal awakenings, and less time in SWS.25
In mice, the loss of testosterone following gonadectomy results in a very small reduction in the amount of SWS, which is increased by the replacement of testosterone. Testosterone replacement in these mice also increased the amount of SWS after 6 h of sleep deprivation.37
Healthy young men with higher endogenous testosterone levels have greater degradation of cognitive functioning and increased subjective sleepiness after 5 days of sleep restriction as compared to those with low endogenous testosterone levels.38 The administration of testosterone and the abuse of androgenic/anabolic steroids has been reported to be associated with reduce sleep time, insomnia and increased awakenings.39,40 Given acutely methyltestosterone increases arousal and diminished sleep changes attributed to activation of the brain serotonergic system.41,42
CONCLUSIONS
Testosterone is not subject to circadian variation in the same way that cortisol. There is sleep-dependent increase in testosterone that requires 3 h of SWS or perhaps a bit longer with increasing age. Testosterone remains elevated for the duration of sleep. The subsequent decrease in testosterone depends on the duration of wakefulness; decreasing more with prolonged wakefulness.
OSA per se is not a cause of low testosterone, rather it is due to obesity, and is increased by weight loss but not CPAP. Shift work does not affect testosterone and unless severely disrupted, sleep quality is not a determinant of testosterone. Testosterone deficiency may have a deleterious effect on sleep quality that may be improved with testosterone replacement. However, large doses of exogenous testosterone and anabolic/androgenic steroid abuse are associated with abnormalities of sleep duration and architecture. Further clarification is required about the relationships between sleep and testosterone in older age, psychiatric disease (depression, post a stress disorder, and psychotic disorders such as schizophrenia) as well as any interaction with the presence of other chronic diseases.
COMPETING INTERESTS
The author has received research support from Resmed Foundation, Bayer Schering, Eli Lilly, and Sanofi Aventis. The author is a member of the advisory board for Eli Lilly. The author has received speaking fees from Sanofi Aventis, Bayer Schering, Novartis, and Astra Zeneca. The author has received consulting fees and undertaken contract research for Lawley Pharmaceuticals.
REFERENCES
- 1.Luboshitzky R, Shen-Orr Z, Herer P. Middle-aged men secrete less testosterone at night than young healthy men. J Clin Endocrinol Metab. 2003;88:3160–6. doi: 10.1210/jc.2002-021920. [DOI] [PubMed] [Google Scholar]
- 2.Luboshitzky R, Aviv A, Hefetz A, Herer P, Shen-Orr Z, et al. Decreased pituitary-gonadal secretion in men with obstructive sleep apnea. J Clin Endocrinol Metab. 2002;87:3394–8. doi: 10.1210/jcem.87.7.8663. [DOI] [PubMed] [Google Scholar]
- 3.Luboshitzky R, Herer P, Levi M, Shen-Orr Z, Lavie P. Relationship between rapid eye movement sleep and testosterone secretion in normal men. J Androl. 1999;20:731–7. [PubMed] [Google Scholar]
- 4.Luboshitzky R, Zabari Z, Shen-Orr Z, Herer P, Lavie P. Disruption of the nocturnal testosterone rhythm by sleep fragmentation in normal men. J Clin Endocrinol Metab. 2001;86:1134–9. doi: 10.1210/jcem.86.3.7296. [DOI] [PubMed] [Google Scholar]
- 5.Miyatake A, Morimoto Y, Oishi T, Hanasaki N, Sugita Y, et al. Circadian rhythm of serum testosterone and its relation to sleep: comparison with the variation in serum luteinizing hormone, prolactin, and cortisol in normal men. J Clin Endocrinol Metab. 1980;51:1365–71. doi: 10.1210/jcem-51-6-1365. [DOI] [PubMed] [Google Scholar]
- 6.Axelsson J, Ingre M, Akerstedt T, Holmback U. Effects of acutely displaced sleep on testosterone. J Clin Endocrinol Metab. 2005;90:4530–5. doi: 10.1210/jc.2005-0520. [DOI] [PubMed] [Google Scholar]
- 7.Penev PD. Association between sleep and morning testosterone levels in older men. Sleep. 2007;30:427–32. doi: 10.1093/sleep/30.4.427. [DOI] [PubMed] [Google Scholar]
- 8.Banks S, Dinges DF. Behavioral and physiological consequences of sleep restriction. J Clin Sleep Med. 2007;3:519–28. [PMC free article] [PubMed] [Google Scholar]
- 9.Paciorek M, Korczynski P, Bielicki P, Byskiniewicz K, Zielinski J, et al. Obstructive sleep apnea in shift workers. Sleep Med. 2011;12:274–7. doi: 10.1016/j.sleep.2010.06.013. [DOI] [PubMed] [Google Scholar]
- 10.2nd ed. Westchester, Ill: American Academy of Sleep Medicine; 2005. American Academy of Sleep Medicine. The international classification of sleep disorders: diagnostic and coding manual; p. pxviii. 297. [Google Scholar]
- 11.Tufik S, Santos-Silva R, Taddei JA, Bittencourt LR. Obstructive sleep apnea syndrome in the Sao Paulo Epidemiologic Sleep Study. Sleep Med. 2010;11:441–6. doi: 10.1016/j.sleep.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 12.Schmid SM, Hallschmid M, Jauch-Chara K, Lehnert H, Schultes B. Sleep timing may modulate the effect of sleep loss on testosterone. Clin Endocrinol (Oxf) 2012;77:749–54. doi: 10.1111/j.1365-2265.2012.04419.x. [DOI] [PubMed] [Google Scholar]
- 13.Jauch-Chara K, Schmid SM, Hallschmid M, Oltmanns KM, Schultes B. Pituitary-gonadal and pituitary-thyroid axis hormone concentrations before and during a hypoglycemic clamp after sleep deprivation in healthy men. PloS One. 2013;8:e54209. doi: 10.1371/journal.pone.0054209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oh MM, Kim JW, Jin MH, Kim JJ, Moon du G. Influence of paradoxical sleep deprivation and sleep recovery on testosterone level in rats of different ages. Asian J Androl. 2012;14:330–4. doi: 10.1038/aja.2011.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Randler C, Ebenhoh N, Fischer A, Hochel S, Schroff C, et al. Chronotype but not sleep length is related to salivary testosterone in young adult men. Psychoneuroendocrinology. 2012;37:1740–4. doi: 10.1016/j.psyneuen.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 16.Leproult R, Van Cauter E. Effect of 1 week of sleep restriction on testosterone levels in young healthy men. JAMA. 2011;305:2173–4. doi: 10.1001/jama.2011.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reynolds AC, Dorrian J, Liu PY, Van Dongen HP, Wittert GA, et al. Impact of five nights of sleep restriction on glucose metabolism, leptin and testosterone in young adult men. PloS One. 2012;7:e41218. doi: 10.1371/journal.pone.0041218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Axelsson J, Akerstedt T, Kecklund G, Lindqvist A, Attefors R. Hormonal changes in satisfied and dissatisfied shift workers across a shift cycle. J Appl Physiol. 2003;95:2099–105. doi: 10.1152/japplphysiol.00231.2003. [DOI] [PubMed] [Google Scholar]
- 19.Soukhova-O’Hare GK, Shah ZA, Lei Z, Nozdrachev AD, Rao CV, et al. Erectile dysfunction in a murine model of sleep apnea. Am J Respir Crit Care Med. 2008;178:644–50. doi: 10.1164/rccm.200801-190OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hammoud AO, Walker JM, Gibson M, Cloward TV, Hunt SC, et al. Sleep apnea, reproductive hormones and quality of sexual life in severely obese men. Obesity (Silver Spring) 2011;19:1118–23. doi: 10.1038/oby.2010.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gambineri A, Pelusi C, Pasquali R. Testosterone levels in obese male patients with obstructive sleep apnea syndrome: relation to oxygen desaturation, body weight, fat distribution and the metabolic parameters. J Endocrinol Invest. 2003;26:493–8. doi: 10.1007/BF03345209. [DOI] [PubMed] [Google Scholar]
- 22.Luboshitzky R, Lavie L, Shen-Orr Z, Herer P. Altered luteinizing hormone and testosterone secretion in middle-aged obese men with obstructive sleep apnea. Obes Res. 2005;13:780–6. doi: 10.1038/oby.2005.88. [DOI] [PubMed] [Google Scholar]
- 23.Canguven O, Salepci B, Albayrak S, Selimoglu A, Balaban M, et al. Is there a correlation between testosterone levels and the severity of the disease in male patients with obstructive sleep apnea? Archives of Italian Urology and Andrology. 2010;82:143–7. [PubMed] [Google Scholar]
- 24.Molina FD, Suman M, Carvalho TB, Piatto VB, Taboga SR, et al. Evaluation of testosterone serum levels in patients with obstructive sleep apnea syndrome. Braz J Otorhinolaryngol. 2011;77:88–95. doi: 10.1590/S1808-86942011000100015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barrett-Connor E, Dam TT, Stone K, Harrison SL, Redline S, et al. The association of testosterone levels with overall sleep quality, sleep architecture, and sleep-disordered breathing. J Clin Endocrinol Metab. 2008;93:2602–9. doi: 10.1210/jc.2007-2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vlkova B, Mucska I, Hodosy J, Celec P. Short-term effects of continuous positive airway pressure on sex hormones in men and women with sleep apnoea syndrome. Andrologia. 2013 doi: 10.1111/and.12092. [DOI] [PubMed] [Google Scholar]
- 27.Macrea MM, Martin TJ, Zagrean L. Infertility and obstructive sleep apnea: the effect of continuous positive airway pressure therapy on serum prolactin levels. Sleep Breath. 2010;14:253–7. doi: 10.1007/s11325-010-0373-0. [DOI] [PubMed] [Google Scholar]
- 28.Hoekema A, Stel AL, Stegenga B, van der Hoeven JH, Wijkstra PJ, et al. Sexual function and obstructive sleep apnea-hypopnea: a randomized clinical trial evaluating the effects of oral-appliance and continuous positive airway pressure therapy. J Sex Med. 2007;4:1153–62. doi: 10.1111/j.1743-6109.2006.00341.x. [DOI] [PubMed] [Google Scholar]
- 29.Meston N, Davies RJ, Mullins R, Jenkinson C, Wass JA, et al. Endocrine effects of nasal continuous positive airway pressure in male patients with obstructive sleep apnoea. J Intern Med. 2003;254:447–54. doi: 10.1046/j.1365-2796.2003.01212.x. [DOI] [PubMed] [Google Scholar]
- 30.Bratel T, Wennlund A, Carlström K. Pituitary reactivity, androgens and catecholamines in bstructive sleep apnoea. Effects of continuous positive airway pressure treatment (CPAP) Respir Med. 1999;93:1–7. doi: 10.1016/s0954-6111(99)90068-9. [DOI] [PubMed] [Google Scholar]
- 31.Grunstein RR, Handelsman DJ, Lawrence SJ, Blackwell C, Caterson ID, et al. Neuroendocrine dysfunction in sleep apnea: reversal by continuous positive airways pressure therapy. J Clin Endocrinol Metab. 1989;68:352–8. doi: 10.1210/jcem-68-2-352. [DOI] [PubMed] [Google Scholar]
- 32.Santamaria JD, Prior JC, Fleetham JA. Reversible reproductive dysfunction in men with obstructive sleep apnoea. Clin Endocrinol (Oxf) 1988;28:461–70. doi: 10.1111/j.1365-2265.1988.tb03680.x. [DOI] [PubMed] [Google Scholar]
- 33.Grossmann M. Low testosterone in men with type 2 diabetes: significance and treatment. J Clin Endocrinol Metab. 2011;96:2341–53. doi: 10.1210/jc.2011-0118. [DOI] [PubMed] [Google Scholar]
- 34.Hanafy HM. Testosterone therapy and obstructive sleep apnea: is there a real connection? J Sex Med. 2007;4:1241–6. doi: 10.1111/j.1743-6109.2007.00553.x. [DOI] [PubMed] [Google Scholar]
- 35.Hoyos CM, Killick R, Yee BJ, Grunstein RR, Liu PY. Effects of testosterone therapy on sleep and breathing in obese men with severe obstructive sleep apnoea: a randomized placebo-controlled trial. Clin Endocrinol (Oxf) 2012;77:599–607. doi: 10.1111/j.1365-2265.2012.04413.x. [DOI] [PubMed] [Google Scholar]
- 36.Killick R, Wang D, Hoyos CM, Yee BJ, Grunstein RR, et al. The effects of testosterone on ventilatory responses in men with obstructive sleep apnoea: a randomised, placebo-controlled trial. J Sleep Res. 2013;22:331–6. doi: 10.1111/jsr.12027. [DOI] [PubMed] [Google Scholar]
- 37.Paul KN, Laposky AD, Turek FW. Reproductive hormone replacement alters sleep in mice. Neurosci Lett. 2009;463:239–43. doi: 10.1016/j.neulet.2009.07.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reynolds AC, Dorrian JA, Liu PY, Van Dongen HP, Wittert GA, et al. A pilot study on the relationship between sleep restriction, endogenous testosterone and cognitive performance. In: Kennedy GA, Sargent C, editors. Little clock, big clock: Molecular to physiological clocks. Melbourne, Australia: Australasian Chronobiology Society; 2011. pp. 11–6. [Google Scholar]
- 39.Liu PY, Yee B, Wishart SM, Jimenez M, Jung DG, et al. The short-term effects of high-dose testosterone on sleep, breathing, and function in older men. J Clin Endocrinol Metab. 2003;88:3605–13. doi: 10.1210/jc.2003-030236. [DOI] [PubMed] [Google Scholar]
- 40.Venancio DP, Tufik S, Garbuio SA, da Nobrega AC, de Mello MT. Effects of anabolic androgenic steroids on sleep patterns of individuals practicing resistance exercise. Eur J Appl Physiol. 2008;102:555–60. doi: 10.1007/s00421-007-0621-6. [DOI] [PubMed] [Google Scholar]
- 41.Daly RC, Su TP, Schmidt PJ, Pickar D, Murphy DL, et al. Cerebrospinal fluid and behavioral changes after methyltestosterone administration: preliminary findings. Arch Gen Psychiatry. 2001;58:172–7. doi: 10.1001/archpsyc.58.2.172. [DOI] [PubMed] [Google Scholar]
- 42.Daly RC, Su TP, Schmidt PJ, Pagliaro M, Pickar D, et al. Neuroendocrine and behavioral effects of high-dose anabolic steroid administration in male normal volunteers. Psychoneuroendocrinology. 2003;28:317–31. doi: 10.1016/s0306-4530(02)00025-2. [DOI] [PubMed] [Google Scholar]


