Abstract
The goal of our study was to evaluate the impact of the interval between prostate biopsy and magnetic resonance imaging (MRI) on the accuracy of simple tumor localization, which is essential information that enables nerve-sparing surgery. We also sought to determine the optimal timing of a post-biopsy MRI. A total of 184 patients who had undergone MRI before radical prostatectomy at an institution without a predetermined schedule for MRI after a prostate biopsy were enrolled. The mean interval from the biopsy to the MRI was 30.8 ± 18.6 days. The accuracy of the MRI for simplified tumor location (right, left, bilateral and none) was 44.6%. In the group with discordant pathologic and MRI findings, the most common reason recorded was ‘MRI predicted a unilateral lesion, but pathology revealed bilateral lesions’ (58.3%), followed by ‘MRI predicted no lesion, but pathology revealed the presence of a lesion’ (32.0%). Multivariable analysis showed that the discordant group had a shorter interval (25.0 ± 14.3 vs 38.1 ± 20.6 days, P < 0.01) preceding the MRI and a higher rate of hemorrhage as observed by MRI (80.4% vs 54.8%, P < 0.01) in comparison with the accordant group. In receiver operating characteristics analysis, the area under the curve of the MRI interval in accurate prediction of the tumor location was 0.707 (P < 0.001). At the MRI interval's cutoff of 28.5 days, the sensitivity was 73.2% and the specificity was 63.7%. When the MRI was performed within 28 days, the accumulated accuracy was only 26.1% (23/88); however, when it was performed after 28 days, the reversely accumulated accuracy was 61.5% (59/96). These data support a waiting period of at least 4 weeks after a biopsy before performing an MRI for the purposes of surgical refinement.
Keywords: magnetic resonance imaging, nerve preservation, prostate biopsy, radical prostatectomy
INTRODUCTION
In radical prostatectomy, preoperative localization of the tumor is essential to achieving the goal of the surgery, because nerve sparing (NS) is a key step that affects not only functional recovery but also the marginal status, and the degree of NS has been clinically associated with tumor distribution.1 Even in locally advanced disease, the use of contemporary surgical techniques enables an attempt at NS surgery, which is usually performed on the contralateral side of the tumor in order to minimize margin positivity. Hence, in planning the side and extent of NS surgery, the surgeon should consider the intraprostatic distribution of the tumor.
Although an magnetic resonance imaging (MRI) can depict the contours of the prostate, as well as its internal zonal anatomy; several situations, including post-biopsy hemorrhage, prostatitis, intraglandular dysplasia and benign prostatic hyperplasia, hinder its accuracy in detecting tumors by generating similar low-signal-intensity lesions in the peripheral zone.2,3,4,5 Above all, post-biopsy hemorrhage, which is generally observed during the first 8 weeks after the procedure,6,7 is a substantial limitation. However, waiting more than 2 months after a biopsy is likely to be impractical.
From the surgeon's standpoint, the most important preoperative information regarding the NS procedure is the intraprostatic distribution of the tumor, simply defined as right/left side, because the extracapsular anatomy of the prostate surrounding the apex and base can be clearly exposed and identified during surgical dissection. However, although several reports have proposed the optimal timing for an MRI, they have focused mainly on the observation of hemorrhage. No substantial recommendation on the radiologic accuracy of the prediction of pathologic tumor location has been made. In this study, we evaluated the impact of the interval between biopsy and MRI on the accuracy of intraprostatic tumor localization in preoperative MRI and determined the appropriate timing based on acceptable agreement between radiologic and pathologic findings.
MATERIALS AND METHODS
Patient selection and data collection
The study population comprised 184 patients with localized or locally advanced prostate cancer (clinical stage T1c to T3c) diagnosed via a template-based transrectal prostate biopsy conducted by a urologist. The patients underwent robot-assisted radical prostatectomy from July 2009 to December 2011 at our institution. Because of the potential bias on imaging workup and pathologic outcome, patients who underwent prior hormone treatments, radiotherapy or any ablative technique were excluded. Owing to the known limitations of tumor identification the central portions of the prostate using MRI,8,9 patients diagnosed with a transitional-zone tumor were also excluded.
After the transrectal biopsy, the enrolled patients underwent an MRI using the same protocol, mainly for the purpose of refining the surgical plan. Because of the anxiety of patients who wanted early surgery rather than waiting 1–3 months after the biopsy, along with recent data that do not support a relationship between the elapsed time after transrectal biopsy and operative outcome,10,11 our policy for radical prostatectomy is minimal delay after the confirmation of histologic diagnosis. Thus, we have no previously established waiting period for MRI after initial biopsy.
All robot-assisted radical prostatectomy procedures were performed by a single experienced surgeon. Based on information from each preoperative MRI, the surgeon decided whether to perform no, partial or full NS surgery for each lobe of the prostate, separately. Following prostatectomy and after inking of the margins, routine sections of all surgical margins, including the prostatic base, apex and peripheral zone, capsule and periprostatic soft tissue, seminal vesicle, urethra and bladder neck, were examined with permanent staining. Based on these findings, the tumor location was evaluated by a single full-faculty uropathologist who was unaware of the preoperative MRI findings. Following approval by the local institutional review board, the data for enrolled patients were evaluated and analyzed. In this study, the clinical stage was assigned according to the 1992 TNM staging system, based on digital rectal examination, transrectal ultrasound findings and MRI.
Protocol for pelvic magnetic resonance imaging and image interpretation
MRI was performed using a 3.0T whole-body MR scanner (Magnetom Tim Trio; Siemens Medical Systems, Erlangen, Germany) with a pelvic-phased array coil (3T Body Matrix TIM Coil; Siemens Medical Systems, Erlangen, Germany). The study protocol consisted of high-resolution transverse T2-weighted images (TR 3290 ms, TE 120 ms; echo train length, 17, matrix, 384 × 384; slice thickness, 4 mm; a 140° flip angle and field of view, 250 mm), transverse T1-weighted images (TR 440 ms, TE 11 ms; slice thickness, 4 mm; a 150° flip angle; field of view, 250 mm; and matrix, 448 × 359) and transverse diffusion-weighted images (b values of 0 and 1000 s mm−2; TR/TE, 3150/71; bandwidth of 2604 Hz pixel−1; matrix size of 128 × 104; field of view, 280 mm; slice thickness, 4 mm). Apparent diffusion-coefficient (ADC) maps were generated using a voxelwise calculation from the two diffusion-weighted imaging sequences acquired with b values of 0 and 1000 s m−2.
The MRIs were interpreted by an experienced radiologist who is also a board-certified urologist. The radiologist was aware that the patients had prostate cancer, but was unaware of all other clinical and histopathologic findings, including Gleason score, tumor location at biopsy, clinical stage and outcome of digital rectal examination. Hemorrhage was considered to be present when an area of high signal intensity within the prostate was observed on T1-weighted images. On MRI, lesions meeting the following criteria were regarded as prostate cancer: a nodular or mass-like homogeneous low-signal-intensity area in the peripheral zone relative to a normal peripheral zone, or an area with homogeneous low signal intensity, ill-defined margins, and an absent capsule in the transition zone on T2-weighted images and ADC maps.
The primary end point of this study was the accuracy between preoperative MRI and pathologic outcome, which was documented simply as right, left, bilateral or none. We considered a case as ‘accord’ only when these four categories in the preoperative depiction matched the final pathology. For multivariable analysis, a logistic regression model was used, including all variables listed in Tables 1 and 2. Statistical Package for Social Sciences (SPSS), version 20 for Windows (SPSS, Chicago, IL, USA) was used as a statistical program. A receiver operating characteristics-derived area under the curve was estimated in order to evaluate each clinical variable with regard to accuracy of prediction. In this study, statistical significance was set at P < 0.05, and all reported P values are two-sided.
Table 1.
Clinical variables related to the accuracy of tumor localization in MRI after prostate biopsy
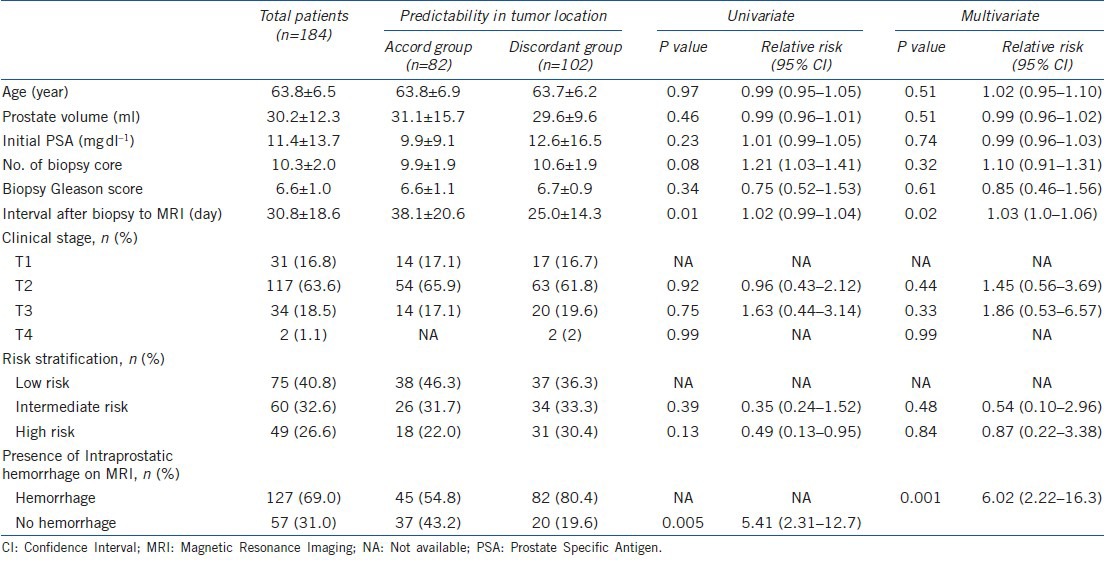
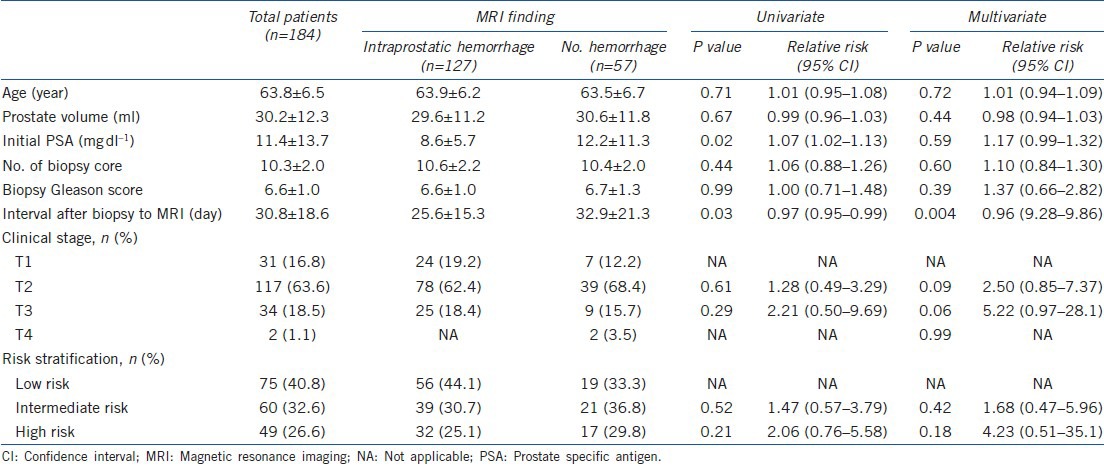
Table 2.
Clinical variables for intraprostatic hemorrhage in MRI after prostate biopsy
RESULTS
The mean ± standard deviation (s.d.) age of enrolled patients was 63.8 ± 6.5 years, with a prostate volume of 30.2 ± 12.3 ml and initial prostate specific antigen of 11.4 ± 13.7 ng dl-1. The number of biopsy cores was 10.3 ± 2.0. The mean (median) interval from biopsy to MRI was 30.8 ± 18.6 (29.5) days and intraprostatic hemorrhage was observed in 127 images (69.0%). In 47.8% of patients (88/184), an MRI was performed within 4 weeks after biopsy.
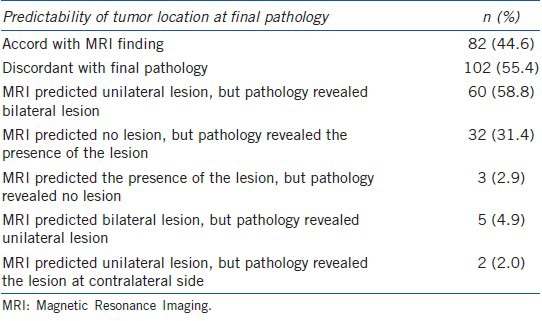
The estimated accuracy of MRI for tumor location was 44.6% (82/184). Among the patients with discord between MRI findings and final histologic outcome in the prediction of tumor location, the most common reason for the discordance was noted as ‘MRI predicted a unilateral lesion, but pathology revealed bilateral lesions’ (58.8%), followed by ‘MRI predicted no lesion, but pathology revealed the presence of a lesion’ (31.4%) (Table 3).
Table 3.
Accuracy of MRI in predicting intraprostatic tumor location and categories of discordance
As compared with the group with accordant MRI findings in the prediction of tumor location, in multivariable analysis, the discordant group had a significantly shorter interval (25.0 ± 14.3 vs 38.1 ± 20.6 days, P < 0.001) from prostate biopsy to MRI and a higher rate of hemorrhage on MRI (80.4% vs 54.8%, P < 0.01) (Table 1). When the entire patient group was divided into two groups based on the presence or absence of intraprostatic hemorrhage on MRI, in multivariable analysis, the interval between biopsy and MRI was the single significant variable (P = 0.004) (Table 2). In receiver operating characteristics analysis, the area under the curve of the MRI interval in accurate prediction of tumor location was 0.707 (P < 0.001, 95% CI: 0.632–0.783) (Figure 1a). At the MRI interval cutoff of 28.5 days, the sensitivity was 73.2% and the specificity was 63.7%. In the prediction of intraprostate hemorrhage, the area under the curve of the MRI interval was 0.648 (P = 0.012, 95% CI: 0.533–0.763) (Figure 1b); at a cutoff of 25 days, the sensitivity was 61.8% and the specificity was 66.7%.
Figure 1.
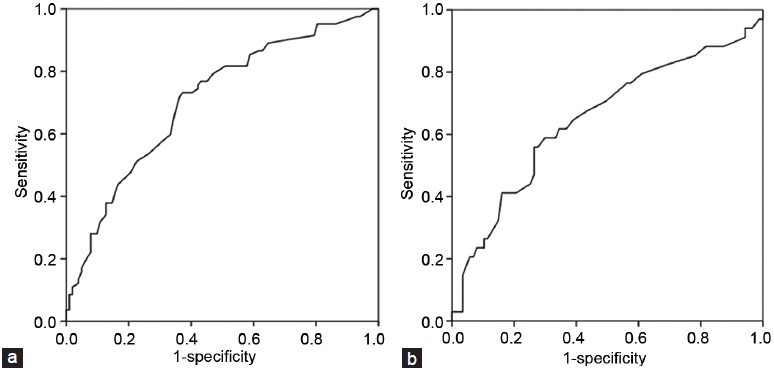
ROC curves for the prediction of (a) intraprostatic tumor location and (b) intraprostatic hemorrhage.
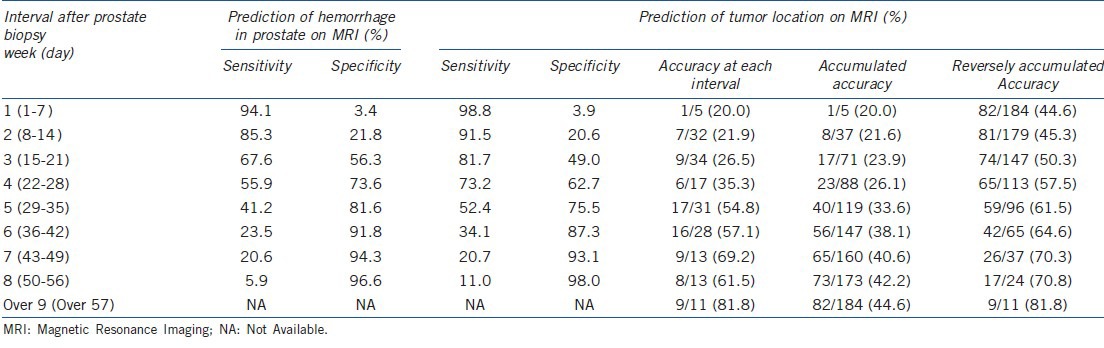
The effect of MRI interval on accuracy and accumulated accuracy in the prediction of tumor location at each week is summarized in Table 4. As the interval from MRI from prostate biopsy increased, the accuracy for each interval showed a significant increase (P = 0.01). When an MRI was performed within 28 days, the accumulated accuracy was only 26.1% (23/88); when it was performed after 28 days, the reversely accumulated accuracy was 61.5% (59/96). The overall margin positivity in this study was 11.4% (21/184): 5.9% (7/119) in pT2 and 21.5% (14/65) over pT3.
Table 4.
Accuracy of hemorrhage and tumor localization for each MRI interval
DISCUSSION
Preoperative knowledge of the tumor location influences the adjustments made in surgical techniques for reduction of positive margins and improvement of overall oncological outcomes. Some locations have a higher risk for extraglandular diffusion. These areas include the implant of the seminal vesicles, where the capsule insinuates itself, and the glandular apex, where the anatomic capsule no longer exists. Owing to the penetrating vessels and concern for erectile dysfunction induced by injury to the neurovascular bundle (NVB) at proximity, the lateral aspect of the prostate is one of the most frequent locations of positive surgical margins. Thus, to obtain margin negativity during the procedure, preoperative images showing the focus of the tumor can aid in decisions pertaining to preservation of NVB.
Combined T2-weighted and diffusion-weighted imaging has emerged as a promising method in detection of prostate cancer, replacing transrectal ultrasonography.12,13,14 In addition to the tumor location, MRI enables evaluation of tumor volume, capsular penetration, invasion of the neurovascular bundle and seminal vesicle involvement.9 These characteristics support the use of preoperative MRI in clinical practice, and indeed, MRI findings suggesting a change in the surgical plan, particularly for NVB preservation, have been reported in 27%–39% of procedures.15,16
The aim of this study was to identify the effect of clinical factors on accuracy in predicting tumor location, essential to surgeons for determining an NS strategy and a more accurate, individualized surgical plan. Multivariate analysis confirmed that the presence of hemorrhage on MRI and a shorter interval from prostate biopsy to MRI are obstacles to conclusive radiologic prediction. In a clinical setting, the use of MRI after prostate biopsy is associated with significant artifacts, which confounds the accuracy of MRI. In MRI, T2-weighted imaging provides high-resolution morphologic imaging of the gland in the three planes, and axial T1-weighted imaging is used for detection of post-biopsy hemorrhage. Although cancer has generally been identified as a low signal in T2-weighted images, there are numerous false positives (e.g., inflammation, scars and post-radiotherapy appearance), and infiltrating cancers may not show a typical appearance. Among these factors, post-biopsy hemorrhage is the most common obstacle.17,18 High-signal-intensity changes on post-biopsy T1-weighted imaging has been demonstrated in 28%–77% of patients.19,20 Subsequent post-biopsy hemorrhage presents a diagnostic challenge for accurate tumor detection using MRI because low T2 signals from a hemorrhage may cause overestimation of tumor presence in the initial postbiopsy period.21 In this study, hemorrhage was observed in 69% of cases, and the presence of intraprostatic hemorrhage was significantly higher in the discordant group than in the accordant group. In particular, the interval from prostate biopsy to MRI was the sole independent variable predicting the presence of hemorrhage, implying that delay of MRI probably results in a more accurate image. However, for both patients and clinicians, delay of decisive procedures solely to obtain more precise radiologic findings cannot be justified.
From a urologist's practical standpoint, this study suggests the optimal timing of MRI after prostate biopsy for the purpose of surgical refinement based on pathologic outcomes as a reference. Among various types of information that can be obtained from an MRI, we focused on the intraprostatic distribution of the tumor as a primary variable because this has an essential role in preoperative refinement for NS surgery that affects surgical and oncologic outcomes. Unlike that of most study in this regard, our data include results of MRIs performed early after prostate biopsy; about half of the patients underwent MRI within a month after biopsy. In prior studies, investigators recommended an interval of 3 weeks between biopsy and MRI,2 and, more recently, investigators suggested that a post-biopsy interval of 8 weeks before MRI might be more beneficial.11 In addition, there has been a recent trend toward increased prostate sampling during transrectal biopsy; currently, more than six core biopsies are performed routinely.22 In our study, with a mean number of biopsy cores of 10.3, 91.4% of the biopsies had more than the typical six cores. This increase in prostate sampling has raised new questions about the proper timing of MRI after transrectal biopsy. Given these limitations, it has recently been proposed that pre-biopsy MRI may obviate the potential confusion caused by residual blood products, potential distortion of native tissue and local inflammation.23,24
Our data showed that a minimum waiting period of 4 weeks is required in order to attain accuracy of more than 60% between radiologic localization of a tumor and pathologic outcome. The ROC curve indicated that 4 weeks (28.5 days) after biopsy is a reasonable cutoff for maintenance of high sensitivity and specificity in predicting intraprostatic tumor location. The accuracy of tumor localization after biopsy increased from 35.3% at 4 weeks to 54.8% at 5 weeks and was maintained at a higher level over the following weeks. Reversely accumulating accuracy, which indicates the accuracy after each specific time point, was constantly maintained at more than 60% after 4 weeks, as shown in Table 4.
We recognize several limitations of this study. First, because the distribution of the MRI interval was not normal, our patient group was slightly biased toward an earlier MRI. Thus, the recommended MRI waiting period of 4 weeks is still shorter than that advised by other researchers; in most studies, an MRI was performed with a routine delay of 3–8 weeks. Hence, the delay of 4 weeks extracted from our analysis is only a minimal waiting period for MRI, not a rigid cutoff. Second, we did not evaluate the degree of hemorrhage, mainly because we believe that there is no reliable tool for its quantification. Considering that degree of hemorrhage has shown a significant negative correlation with tumor size,25 development of a large hemorrhage from a small tumor, resulting in discordance of tumor location, is possible. In addition, because only a single radiologist reviewed the MRI, interobserver variability for detection of the tumor was not assessed. Finally, because the radiologist was aware that the patients had prostate cancer, there may have been a bias that led the radiologist to identify equivocal lesions as tumor foci.
In summary, our data suggest waiting a minimum of 4 weeks before performing an MRI after prostate biopsy to enable preoperative surgical refinement based on intraprostate tumor location.
AUTHOR CONTRIBUTIONS
YHK carried out the preliminary analysis, drafted the manuscript. PHS performed statistic analysis. KHM and HCJ participated in its design and coordination, and JC was the main surgeon of this stdy, participated in patient enrols. DJS supervised all of the study and also drafted the manuscript. All authors read and approved the final manuscript.
COMPETING INTERESTS
The authors declare that they have no competing interests.
ACKNOWLEDGMENTS
This work was supported by the 2013 Yeungnam University Research Grant.
REFERENCES
- 1.Bott SR, Kirby RS. Avoidance and management of positive surgical margins before, during and after radical prostatectomy. Prostate Cancer Prostatic Dis. 2002;5:252–63. doi: 10.1038/sj.pcan.4500612. [DOI] [PubMed] [Google Scholar]
- 2.White S, Hricak H, Forstner R, Kurhanewicz J, Vigneron DB, et al. Prostate cancer: effect of postbiopsy hemorrhage on interpretation of MR images. Radiology. 1995;195:385–90. doi: 10.1148/radiology.195.2.7724756. [DOI] [PubMed] [Google Scholar]
- 3.Schiebler ML, Schnall MD, Pollack HM, Lenkinski RE, Tomaszewski JE, et al. Current role of MR imaging in the staging of adenocarcinoma of the prostate. Radiology. 1993;189:339–52. doi: 10.1148/radiology.189.2.8210358. [DOI] [PubMed] [Google Scholar]
- 4.D’Amico AV, Schnall M, Whittington R, Malkowicz SB, Schultz D, et al. Endorectal coil magnetic resonance imaging identifies locally advanced prostate cancer in select patients with clinically localized disease. Urology. 1998;51:449–54. doi: 10.1016/s0090-4295(97)00630-4. [DOI] [PubMed] [Google Scholar]
- 5.Sommer FG, Nghiem HV, Herfkens R, McNeal J, Low RN. Determining the volume of prostatic carcinoma: value of MR imaging with an external-array coil. AJR Am J Roentgenol. 1993;161:81–6. doi: 10.2214/ajr.161.1.8517328. [DOI] [PubMed] [Google Scholar]
- 6.Zhang JQ, Loughlin KR, Zou KH, Haker S, Tempany CM. Role of endorectal coil magnetic resonance imaging in treatment of patients with prostate cancer and in determining radical prostatectomy surgical margin status: report of a single surgeon's practice. Urology. 2007;69:1134–7. doi: 10.1016/j.urology.2007.01.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schnall MD, Imai Y, Tomaszewski J, Pollack HM, Lenkinski RE, et al. Prostate cancer: local staging with endorectal surface coil MR imaging. Radiology. 1991;178:797–802. doi: 10.1148/radiology.178.3.1994421. [DOI] [PubMed] [Google Scholar]
- 8.Bartolozzi C, Crocetti L, Menchi I, Ortori S, Lencioni R. Endorectal magnetic resonance imaging in local staging of prostate carcinoma. Abdom Imaging. 2001;26:111–22. doi: 10.1007/s002610000142. [DOI] [PubMed] [Google Scholar]
- 9.Nayyar R, Kumar R, Kumar V, Jagannathan NR, Gupta NP, et al. Magnetic resonance spectroscopic imaging: current status in the management of prostate cancer. BJU Int. 2009;103:1614–20. doi: 10.1111/j.1464-410X.2009.08446.x. [DOI] [PubMed] [Google Scholar]
- 10.Park SY, Kim JJ, Kim TH, Lim SH, Han DH, et al. The role of endorectal magnetic resonance imaging in predicting extraprostatic extension and seminal vesicle invasion in clinically localized prostate cancer. Korean J Urol. 2010;51:308–12. doi: 10.4111/kju.2010.51.5.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qayyum A, Coakley FV, Lu Y, Olpin JD, Wu L, et al. Organ-confined prostate cancer: effect of prior transrectal biopsy on endorectal MRI and MR spectroscopic imaging. AJR Am J Roentgenol. 2004;183:1079–83. doi: 10.2214/ajr.183.4.1831079. [DOI] [PubMed] [Google Scholar]
- 12.Puech P, Huglo D, Petyt G, Lemaitre L, Villers A. Imaging of organ-confined prostate cancer: functional ultrasound, MRI and PET/computed tomography. Curr Opin Urol. 2009;19:168–76. doi: 10.1097/MOU.0b013e328323f5ed. [DOI] [PubMed] [Google Scholar]
- 13.Haider MA, van der Kwast TH, Tanguay J, Evans AJ, Hashmi AT, et al. Combined T2-weighted and diffusion-weighted MRI for localization of prostate cancer. AJR Am J Roentgenol. 2007;189:323–8. doi: 10.2214/AJR.07.2211. [DOI] [PubMed] [Google Scholar]
- 14.Lim HK, Kim JK, Kim KA, Cho KS. Prostate cancer: apparent diffusion coefficient map with T2-weighted images for detection: a multireader study. Radiology. 2009;250:145–51. doi: 10.1148/radiol.2501080207. [DOI] [PubMed] [Google Scholar]
- 15.McClure TD, Margolis DJ, Reiter RE, Sayre JW, Thomas MA, et al. Use of MR imaging to determine preservation of the neurovascular bundles at robotic-assisted laparoscopic prostatectomy. Radiology. 2012;262:874–83. doi: 10.1148/radiol.11103504. [DOI] [PubMed] [Google Scholar]
- 16.Hricak H, Wang L, Wei DC, Coakley FV, Akin O, et al. The role of preoperative endorectal magnetic resonance imaging in the decision regarding whether to preserve or resect neurovascular bundles during radical retropubic prostatectomy. Cancer. 2004;100:2655–63. doi: 10.1002/cncr.20319. [DOI] [PubMed] [Google Scholar]
- 17.Kim CK, Park BK, Kim B. Diffusion-Weighted MRI at 3 T for the Evaluation of Prostate Cancer. AJR Am J Roentgenol. 2010;194:1461–9. doi: 10.2214/AJR.09.3654. [DOI] [PubMed] [Google Scholar]
- 18.Lee HW, Seo SI, Jeon SS, Lee HM, Choi HY. Can we predict real T3 stage prostate cancer in patients with clinical T3 (cT3) disease before radical prostatectomy? Yonsei Med J. 2010;51:700–7. doi: 10.3349/ymj.2010.51.5.700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ikonen S, Kivisaari L, Vehmas T, Tervahartiala P, Salo JO, et al. Optimal timing of post-biopsy MR imaging of the prostate. Acta Radiol. 2001;42:70–3. doi: 10.1080/028418501127346260. [DOI] [PubMed] [Google Scholar]
- 20.Kaji Y, Kurhanewicz J, Hricak H, Sokolov DL, Huang LR, et al. Localizing prostate cancer in the presence of postbiopsy changes on MR images: role of proton MR spectroscopic imaging. Radiology. 1998;206:785–90. doi: 10.1148/radiology.206.3.9494502. [DOI] [PubMed] [Google Scholar]
- 21.Rosenkrantz AB, Kopec M, Kong X, Melamed J, Dakwar G, et al. Prostate cancer vs. post-biopsy hemorrhage: diagnosis with T2- and diffusion-weighted imaging. J Magn Reson Imaging. 2010;31:1387–94. doi: 10.1002/jmri.22172. [DOI] [PubMed] [Google Scholar]
- 22.Bauer JJ, Zeng J, Zhang W, McLeod DG, Sesterhenn IA, et al. Lateral biopsies added to the traditional sextant prostate biopsy pattern increases the detection rate of prostate cancer. Prostate Cancer Prostatic Dis. 2000;3:43–6. doi: 10.1038/sj.pcan.4500397. [DOI] [PubMed] [Google Scholar]
- 23.Ahmed HU, Kirkham A, Arya M, Illing R, Freeman A, et al. Is it time to consider a role for MRI before prostate biopsy? Nat Rev Clin Oncol. 2009;6:197–206. doi: 10.1038/nrclinonc.2009.18. [DOI] [PubMed] [Google Scholar]
- 24.Shimizu T, Nishie A, Ro T, Tajima T, Yamaguchi A, et al. Prostate cancer detection: the value of performing an MRI before a biopsy. Acta Radiol. 2009;50:1080–8. doi: 10.3109/02841850903216718. [DOI] [PubMed] [Google Scholar]
- 25.Tamada T, Sone T, Jo Y, Yamamoto A, Yamashita T, et al. Prostate cancer: relationships between postbiopsy hemorrhage and tumor detectability at MR diagnosis. Radiology. 2008;248:531–9. doi: 10.1148/radiol.2482070157. [DOI] [PubMed] [Google Scholar]


