Abstract
Testicular torsion (TT) is a serious urologic emergency that is observed in adolescent males and that can lead to infertility if left untreated. The ischemia-reperfusion (I/R) injury due to TT has been implicated in the pathogenesis of testicular damage. We investigated the effects of melatonin on oxidative damage in the ipsilateral and contralateral testes of rats induced by unilateral TT. A total of 21 prepubertal male Wistar albino rats were divided into three groups, each consisting of seven rats. In Group 1 (SHAM group): a sham operation to the left testis and bilateral orchiectomy were performed. In Group 2 (I/R group): I/R injury was created by rotating the left testis 720° in a clockwise direction for 2 h and detorsing the testis after 2 h. Group 3 (I/R + MEL group): rats were subjected to I/R injury and one-shot melatonin injection (50 mg kg−1, intraperitoneal (i.p.)). The testes of the rats were excised bilaterally in all groups. The testicular tissue activities of antioxidant catalase (CAT), superoxide dismutase (SOD) and glutathione peroxidase enzymes (GSH-Px), and the tissue levels of malondialdehyde (MDA), protein carbonyl (PC) and nitric oxide (NO) were determined. Administration of melatonin caused a significant decrease in lipid peroxidation and enzyme activities in the ipsilateral testis when compared with the control group (P < 0.05). All of the changes in the enzyme activities of the contralateral testis were insignificant (P > 0.05). MDA levels were significantly altered in the contralateral testis (P = 0.009). Melatonin administration decreased the deleterious effects of I/R injury in the ipsilateral torted testes of the rats. The contralateral testes were slightly affected by unilateral TT.
Keywords: antioxidant enzymes, ischemia-reperfusion (I/R), lipid peroxidation, melatonin, testicular torsion (TT)
INTRODUCTION
Testicular torsion (TT) is a serious urologic emergency that is mostly encountered in newborn and adolescent males. TT causes testicular injury, leading to potential serious sequelae of infertility and subfertility; thus, immediate diagnosis and intervention is mandatory.1,2 The duration and degree of twisting of the cord are closely related to the severity of the testicular injury.3 Although the main pathological mechanisms of testicular injury following TT is not completely understood, the overproduction of reactive oxygen species (ROS) generated during the ischemia-reperfusion (I/R) process has been implicated as one of the main factors in cellular and tissue damage.4
Ipsilateral testicular damage after spermatic cord torsion is clearly evident, but the status of the contralateral testis remains obscure. Although some studies have claimed that the contralateral testis is not affected by unilateral torsion, others have advocated the opposite view.5,6,7 Despite the controversy, there are several theories for the etiology of contralateral testicular damage after unilateral TT, such as immunological mechanisms, reflex vasoconstriction and decreased blood flow to the contralateral testis, previous episodes of silent intermittent torsion and congenital testicular dysplasia.8
Numerous prior experimental studies have used different antioxidant agents to prevent oxidative injury in both the ipsilateral and contralateral testicular tissues.9,10,11,12,13 Melatonin (MEL) (N-acetyl-5-methyl-tryptamine), which is the main secretory product of the pineal gland, is one of the most powerful antioxidant agents, and the ameliorative function of MEL in reducing tissue damage after I/R processes or transient hypoxia and reperfusion episodes in many organs have been demonstrated in previous studies.14,15 In the literature, very few reports have addressed the protective effects of MEL on testicular I/R injury.6,10,13,16,17
In this experimental study, we aimed to investigate the biochemical effects of I/R injury in the ipsilateral and contralateral testes of rats and the protective role of MEL. We experimentally induced I/R injury in the left testis of rats and determined the testicular tissue antioxidant enzyme activities, including catalase (CAT), superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px) in both ipsilateral torted and contralateral testicular tissues of rats. The testicular tissue levels of lipid peroxidation product malondialdehyde (MDA), protein denaturation product protein carbonyl (PC) and nitric oxide (NO) were also determined bilaterally.
MATERIALS AND METHODS
Animals and experimental design
The experimental protocols were approved by the Local Ethics Committee of Gaziosmanpasa University. The animal care and experimental procedures were executed in compliance with the principles of the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals (NIH publication No. 85–23, revised 1985). A total of 21 male Wistar albino rats, 5 - to 6-months-old and weighing between 230 and 270 g were used in the study. The animals were housed in cages with three animals per cage and allowed free access to standard rodent chow and tap water, ad libitum.
Surgical procedure and experimental groups
All of the rats were anesthetized with intramuscular ketamine (50 mg kg−1) and xylazine (10 mg kg−1). The surgical procedures were performed under sterile conditions through standard ilioinguinal incisions. The rats were randomly divided into three groups, each consisting of seven rats. Animals in Group 1 (SHAM, sham-operated control group, n = 7) underwent sham operation to determine the basal values for biochemical comparisons. Consistent with the related previous experimental studies, the left testis was exposed through the incision and replaced to the hemiscrotum, and the wound was closed with 4–0 silk suture. At the end of the 4 h experimental period, bilateral orchiectomies were performed.
Group 2 (I/R group, n = 7) was designed to study the effects of I/R injury on ipsilateral and contralateral testicular tissues. The left testis was rotated 720° in a clockwise direction and maintained for 2 h by fixing the left testis to the scrotum with a 4–0 silk suture. Detorsion was performed by untwisting the testis and maintaining the position for 2 h. At the end of the experiments, bilateral orchiectomies were performed. The left testicles of the animals in Group 3 (I/R + MEL group, ischemia + melatonin treatment + reperfusion group, n = 7) were also subjected to the same duration of ischemia (2 h) with the same operation technique; but 30 min before detorsion; one shot of melatonin (50 mg kg−1, intraperitoneal (i.p.)) was administered to the animals. After the same period of detorsion (2 h), bilateral orchiectomies were performed. At the end of the experimental procedures, all rats were sacrificed; and the testicular tissues were stored at –70°C pending biochemical analysis.
Biochemical analysis
To evaluate the effects of I/R injury and MEL administration on the ipsilateral and contralateral testis, the activities of the three free radical scavenger enzymes, CAT, SOD and GSH-Px, and the tissue levels of MDA and PC, which are tissue destruction products, were determined. The tissue levels of NO were also determined.
All of the testicular tissues were washed with cold saline solution and wet tissue weights were obtained. The tissues were cut into small pieces and placed into glass bottles. The tissues were then homogenized in ice-cold Tris-HCl buffer solution (0.2 mmol l–1 and 50/39.9 (v/v)) within a homogenizer (Ultra Turrax Type T25-B; IKA Labortechnic, Staufen, Germany) for 2 min at 11 200 g. The homogenate was centrifuged at 3500 g for 60 min, and a supernatant was obtained. The levels of CAT and GSH-Px were determined in the supernatant, and MDA and NO levels were studied in the homogenate. For a further extraction procedure, the supernatant was extracted in ethanol/chloroform mixture (5/3, v/v). After a second centrifugation at 3500 g (20 min), the clear upper layer (the ethanol phase) was taken and used in SOD activity determination. All procedures were performed at 4 °C, and icepacks were used to maintain the temperature during the homogenization procedure.
Catalase activity
The CAT activity determination method was based on the determination of the rate constant of the H2O2 decomposition rate at 240 nm, and the results are expressed as units per mg tissue protein.
Superoxide dismutase activity
The principle of the SOD activity determination method is based on the inhibition of nitroblue tetrazolium reduction by the xanthine-xanthine oxidase system as a superoxide radical generator. One unit of SOD was defined as the enzyme activity causing 50% inhibition of the nitroblue tetrazolium reduction rate. The SOD activity is expressed as units per mg protein.
Glutathione peroxidase enzymes activity
GSH-Px was measured by the enzymatic reaction that was initiated by addition of H2O2 to the reaction mixture containing reduced glutathione, nicotine adenine dinucleotide phosphate and glutathione reductase, and the change in the absorbance at 340 nm was monitored by spectrophotometer. The activity of the enzyme is given in units per mg tissue protein.
Tissue Malondialdehyde level
The MDA levels in testicular tissues were analyzed by a method based on the reaction with thiobarbituric acid at 90–100 °C. In the thiobarbituric acid test reaction, MDA or MDA-like substances and thiobarbituric acid react together to produce a pink pigment with an absorption maximum of 532 nm. The results are expressed as nanomoles per gram wet tissue, calculated using a standard graphics, which was prepared with serial dilutions of standard 1,1,3,3-tetramethoxypropane.
Tissue protein carbonyl determination
The PC contents were determined spectrophotometrically (GBC Cintra 10 E UV/visible spectrophotometry, Melbourne, Australia) with the reaction of the carbonyl group with 2,4-dinitrophenylhydrazine to form 2,4-dinitrophenylhydrazine. The results are given as nanomoles of PC per milligram of protein.
Tissue nitric oxide determination
Tissue nitrite (NO2-) and nitrate (NO3-) were estimated as an index of NO production. Quantitations of NO2- and NO3- were based on the Griess reaction, in which a chromophore with a strong absorbance at 540 nm was formed by the reaction of nitrite with a mixture of naphtylethylenediamine and sulfanilamide. The results are expressed as micromoles per gram wet tissue.
Statistical analysis
The data were analyzed by a commercially available Statistical Package for Social Sciences (SPSS) version 15.0 for windows software (SPSS Inc., Chicago, IL, USA). P < 0.05 was regarded as statistically significant. Distribution of the groups was analyzed with the Kolmogorov–Smirnov one-sample test. One-way analysis of variance test was performed, and post hoc multiple comparisons were conducted with least-squares differences.
RESULTS
The results of CAT, SOD and GSH-Px activities in the ipsilateral and contralateral testicular tissues of all groups are presented in Table 1. In the torted ipsilateral testis, SOD and GSH-Px activities increased in the I/R group (P = 0.0001) when compared with Group 1, but MEL injection caused a significant decrease in the SOD and GSH-Px activities in Group 3 (P = 0.0001). CAT activity in the ipsilateral testis decreased significantly in the I/R group (P = 0.0001), and MEL caused an increase in CAT activity (P = 0.099). The resultant SOD and GSH-Px levels in the ipsilateral testis of the treatment group (Group 3) compared with the SHAM group differed significantly for GSH-Px (P = 0.004), but did not differ with respect to SOD (P = 0.477). In the contralateral testis, CAT activity decreased in the I/R group (P = 0.215) and increased after MEL administration (P = 0.130). The difference in the CAT activity of the contralateral testis between the SHAM and treatment group was insignificant (P = 0.812). Increased SOD and GSH-Px activities due to I/R in the contralateral testis were decreased with MEL injection in Group 3 (P > 0.05).
Table 1.
Tissue CAT, SOD and GSH-Px activity and statistical comparisons between the groups

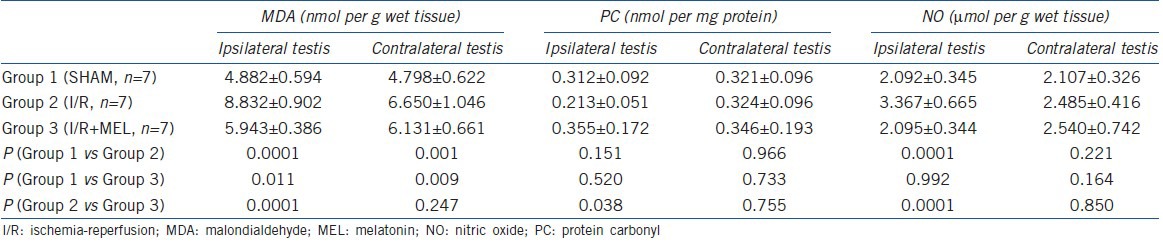
The tissue MDA, PC and NO levels in both the ipsilateral and contralateral testicular tissues of all groups are presented in Table 2. The MDA levels significantly increased in both the ipsilateral and contralateral testes (P = 0.0001 and 0.001, respectively), and MEL administration decreased these values. The decrease in the MDA level was significant in the ipsilateral testis (P = 0.0001), but was insignificant in the contralateral testis (P = 0.247). The decreased PC level due to I/R injury in the ipsilateral testis was increased with MEL treatment. The differences in the PC levels of the contralateral testis for all the groups were insignificant (P > 0.05). The tissue NO level was increased in the ipsilateral testis after I/R injury, and MEL treatment reduced NO levels (P = 0.992). The changes in the NO levels in the contralateral testis were insignificant for all groups (P > 0.05).
Table 2.
Tissue MDA, PC and NO levels and statistical analyses between the groups
DISCUSSION
Mammalian testes are highly sensitive to oxidative insult, and the most important component of post-torsion testicular damage is oxidative injury.1,4,5,18,19 The pathogenetic mechanism of I/R injury is mostly attributed to the over-generation of ROS in various organ models, including testis.3,4,5,9,10,11,12,18 During the ischemic phase of the I/R process, hypoxic conditions develop in the testicular tissue due to the disruption of blood flow. Consequently, hypoxic conditions prevail, and tissue ATP production decreases because of limited oxygen available in the tissue. Ca2+ influx into the intracellular compartment increases, leading to the conversion of hypoxanthine deoxygenase to xanthine oxidase, which is a superoxide generator enzyme.1,2,18 Additionally, ischemia stimulates chemotactic factors and leads to the migration of polymorphonuclear leukocytes to the ischemic region, which also generates ROS after reperfusion.18 Oxygen becomes abundant during the first 60–90 min of reperfusion, which causes the toxic burst of ROS from invading neutrophils, macrophages and residual parenchymal cells in the tissue.3 The enzymatic antioxidant defence system, which include SOD, CAT and GSH-Px, reacts to scavenge the free radicals to protect tissues from I/R injury.1,3,4
The clinical implications of these biochemical events can be summarized as follows: TT causes damage to the testicular tissue due to ischemic processes. After rescue or detorsion procedures, this damage is aggravated by reperfusion injury.19,20,21 After restoration of the oxygen supply to the tissues, the mitochondrial respiratory system causes an overproduction of free radicals. In the early phase, the production of ROS in excessive amounts reacts with membrane lipids and results in lipid peroxidation and eventually the loss of cellular components of the tissue.20,21 The biochemical markers and parameters are more sensitive in the acute phase of such injuries. The changes in the biochemical parameters occur earlier in comparison with morphologic changes, so for the evaluation of testicular damage after unilateral TT, biochemical analysis would be more informative than histological and morphological studies in the acute phase of the I/R processes.5 From this point of view, our aim was to investigate the early biochemical changes in both the ipsilateral and contralateral testicular tissues of rats subjected to unilateral TT (left side) and the ameliorative effects of MEL. MEL is a neuroregulatory hormone primarily synthesised and secreted with a circadian rhythm by the pineal gland that has powerful antioxidant effects.14,15,22 The absence of histologic examinations may be regarded as a limitation of this study. However, histological changes, especially in the contralateral testis, are likely to be non-informative and difficult to interpret in the acute phase of TT, which deterred us from analyzing the tissues histopathologically. Nevertheless, histopathological examination of the testis after such an injury warrants a chronic, well-designed experimental study in the future.
As a result of biochemical and statistical analysis, changes in the tissue antioxidant enzyme activities (CAT, SOD and GSH-Px) and changes in the tissue levels of MDA, PC and NO in the I/R group demonstrated evidence of I/R injury in the ipsilateral (torted), and to a lesser extent, contralateral (non-torted) testis. The most important indicator of tissue injury due to I/R injury was the MDA level. Although significant changes occurred in the MDA levels in the contralateral testis, the change or increase in the levels of MDA and the beneficial effects of MEL was more prominent in the ipsilateral testis than the contralateral testis. MDA is an indirect indicator of lipid peroxidation in cells due to ROS effects. The ROS causes chain reactions of lipid peroxidation in the cell membranes, which eventually leads to the generation of the major lipid peroxidation product MDA.2,4,5,10,16,23 As an indicator of ROS injury, MDA levels were elevated in comparison with the sham group in this study (P = 0.0001). As a free radical scavenger agent and powerful antioxidant hormone in the human body, MEL decreased the levels of MDA to nearly the levels of the sham group in Group 3 rats.
The changes in the tissue levels of PC could not be argued in the same way of MDA because various types of changes were determined in the tissue levels of PC both in the I/R and treatment groups of the ipsilateral and contralateral testicular tissues. According to our results, MEL acted as a prooxidative agent both in the ipsilateral and contralateral testes. In other words, MEL increased protein oxidation in testicular tissues. However, significant change in the PC levels of the MEL-treated group was observed only in the ipsilateral testis. All of the other comparisons between the groups with respect to PC levels were insignificant (P > 0.005).
NO is a water- and lipid-soluble free radical that plays an important role in modulating blood flow in normal and pathological states, and its levels seems to alter with I/R injury.5,7,11,12,24 Ischemia causes an increase in NO synthetase (NOS) activity and changes in NO levels.25 Reperfusion leads to the generation of the superoxide radical (O2-), and the interaction between NO and O2- produces a specific type of ROS, peroxynitrite (ONOO-), which further promotes cellular damage.24 Increases in NO levels both in the ipsilateral and contralateral testis by I/R injury and the restoration to near control levels in this study supported the ideas about the deleterious effects of NO production on testicular tissues due to I/R.
In the previous studies, the activities of antioxidant enzymes in different tissues were reported to change either positively or negatively with respect to the intensity of lipid peroxidation. In some of the studies, long-term I/R led to increases in the intensity of lipid peroxidation and the inactivation of antioxidant enzymes in the testicular tissues. Conversely, in some other studies, compensatory enhancement in enzyme activities were reported.1,2,5,9 This diversity in the changes of the enzyme activities was based upon the specific responses of the tissues and the tissue-specific activities of the antioxidant enzyme system.1,9,14 In this context, we observed diverse activities of the antioxidant enzymes in this study. Although SOD and GSH-Px activities increased with I/R injury, the CAT level decreased. However, all of these changes were restored to levels that were closer to control levels by MEL treatment (P < 0.05). Minor changes in the enzyme activities of the contralateral testis, demonstrating the modest effects of I/R injury in the contralateral testis, were also minimized by MEL treatment.
To date, various drugs and chemicals have been used to protect the testes against I/R injury.1,9,18,21,25,26 In the English literature, we found very few studies about the protective effects of MEL on testicular I/R injury. Melatonin, which is by far the most powerful endogenous antioxidant, exerts its effects directly by scavenging ROS or indirectly by stimulating several antioxidative enzyme systems.10,14,26 Having both lipophilic and hydrophilic properties, MEL easily passes through all biological membranes and enter cells and their subcellular compartments. Thus, MEL reduces oxidative damage in both the lipid and aqueous environments of the cell.14,26,27 In addition to these activities, MEL reduces PMNL cell migration, inhibits leukocyte adhesion to endothelial cells, increases blood flow and decreases edema during oxidative processes.27,28
In this study, the tissue levels of MDA were significantly increased in the ipsilateral testes of I/R group rats (P < 0.0001). The elevation in MDA levels confirmed the oxidative injury in the testicular tissues. The SOD and GSH-Px activities were significantly increased (P < 0.0001), and CAT activity was significantly decreased (P < 0.0001) after unilateral torsion, but with the exception of GSH-Px (P > 0.05), the levels of the enzyme activities were brought to near control levels with MEL administration (P < 0.0001). The mechanism of these changes is unclear, but may be regarded as a mechanism to protect the testis from injury because reductions to basal levels after the administration of a specific agent have been confirmed as indicative of potential anti-ROS activity in previous studies.
With the exception of MDA levels, our results did not reveal significant adverse effects of TT on the contralateral testes. Notwithstanding, the solely significant change in MDA levels predicted a mild noxious oxidative effect in the contralateral testis, which was also decreased with MEL. This result supports the protective role of MEL on the contralateral side. Despite suggestions of several possible etiologic factors, a clear reason for the occasional deterioration of the contralateral testis due to unilateral TT has not been established to date. However, Sarica et al.27 has suggested that the possible mechanism of the effect of unilateral TT on the contralateral testis is the reflexive decrease in contralateral testicular blood flow evidenced by electromagnetic and radioisotopic studies following unilateral TT.
In conclusion, MEL produced protective effects on the torted and detorted testicular tissues of rats. Thus, in clinical practice, medical treatment with a potential ROS scavenger antioxidant agent may help rescue the testes from subsequent subfertility problems after unilateral TT and detorsion.
AUTHOR CONTRIBUTIONS
BSP designed the study, drafted the manuscript, and coordinated and participated in every part of the experiments. DA participated in the design of the study and helped in the experiments and drafting of the manuscript. HO participated in the design, biochemical assays and statistical analysis of the study. YG participated in the experiments, coordinated among the authors and helped to draft the manuscript. AA performed the biochemical analysis. FE participated in the design of the study and drafting of the manuscript. NU participated in the experiments of the study. All of the authors read and approved the final manuscript.
COMEPETING INTERESTS
The authors declare that they have no competing interests.
REFERENCES
- 1.Çay A, Alver A, Küçük M, Işik O, Eminağaoğlu MS, et al. The effects of N-Acetylcysteine on antioxidant enzyme activities in experimental testicular torsion. J Surg Res. 2006;131:199–203. doi: 10.1016/j.jss.2005.11.572. [DOI] [PubMed] [Google Scholar]
- 2.Unsal A, Devrim E, Guven C, Eroglu M, Durak I, et al. Propofol attenuates reperfusion injury after testicular torsion and detorsion. World J Urol. 2004;22:461–5. doi: 10.1007/s00345-004-0451-7. [DOI] [PubMed] [Google Scholar]
- 3.Prillaman HM, Turner TT. Rescue of testicular function after acute experimental torsion. J Urol. 1997;157:340–5. [PubMed] [Google Scholar]
- 4.Dokmeci D, Inan M, Basaran UN, Yalcin O, Aydogdu N, et al. Protective effect of L-carnitine on testicular ischemia-reperfusion injury in rats. Cell Biochem Funct. 2006;25:611–8. doi: 10.1002/cbf.1355. [DOI] [PubMed] [Google Scholar]
- 5.Filho DW, Torres MA, Bordin AL, Crezcynski-Pasa TB, Boveris A. Spermatic cord torsion, reactive oxygen and nitrogen species and ischemia-reperfusion injury. Mol Aspects Med. 2004;25:199–210. doi: 10.1016/j.mam.2004.02.020. [DOI] [PubMed] [Google Scholar]
- 6.Jeong SJ, Choi WS, Chung JS, Baek M, Hong SK, et al. Preventive effects of cyclosporine a combined with prednisolone and melatonin on contralateral testicular damage after ipsilateral torsion-detorsion in pubertal and adult rats. J Urol. 2010;184:790–6. doi: 10.1016/j.juro.2010.03.109. [DOI] [PubMed] [Google Scholar]
- 7.Yildiz H, Durmus AS, Simsek H, Yaman M. Protective effect of sildenafil citrate on contralateral testis injury after unilateral testicular torsion/detorsion. Clinics. 2011;66:137–42. doi: 10.1590/S1807-59322011000100024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Visser AJ, Heyns CF. Testicular function after torsion of the spermatic cord. BJU Int. 2003;92:200–3. doi: 10.1046/j.1464-410x.2003.04307.x. [DOI] [PubMed] [Google Scholar]
- 9.Uz E, Sogut S, Sahin S, Var A, Ozyurt H, et al. The protective role of caffeic acid phenethyl ester (CAPE) on testicular torsion and detorsion. World J Urol. 2002;20:264–70. doi: 10.1007/s00345-002-0259-2. [DOI] [PubMed] [Google Scholar]
- 10.Yurtcu M, Abasiyanik A, Avunduk MC, Muhtaroglu S. Effects of melatonin on spermatogenesis and testicular ischemia-reperfusion injury after unilateral testicular torsion-detorsion. J Ped Surg. 2008;43:1873–8. doi: 10.1016/j.jpedsurg.2008.01.065. [DOI] [PubMed] [Google Scholar]
- 11.Can C, Tore F, Tuncel N, Uysal O, Gurer F, et al. Protective effect of vasoactive intestinal peptide on testicular torsion-detorsion injury: association with heparin-containing mast cells. Urology. 2004;63:195–200. doi: 10.1016/j.urology.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 12.Koc A, Narci A, Duru M, Gergerlioglu HS, Akaydin Y, et al. The protective role of erdosteine on testicular tissue after testicular torsion and detorsion. Mol Cell Biochem. 2005;280:193–9. doi: 10.1007/s11010-005-8911-y. [DOI] [PubMed] [Google Scholar]
- 13.Ozturk A, Baltaci AK, Mogulkoc R, Ozturk B. The effect of prophylactic melatonin administration on reperfusion damage in experimental testis ischemia-reperfusion. Neuro Endocrinol Lett. 2003;24:170–2. [PubMed] [Google Scholar]
- 14.Reiter R, Tan DX. Melatonin: a novel protective agent against oxidative injury of the ischemic/reperfused heart. Cardiovasc Res. 2003;58:10–9. doi: 10.1016/s0008-6363(02)00827-1. [DOI] [PubMed] [Google Scholar]
- 15.Marshall KA, Reiter RJ, Poeggeler B, Aruoma OI, Halliwell B. Evaluation of the antioxidant activity of melatonin in vitro. Free Radic Biol Med. 1996;21:307–15. doi: 10.1016/0891-5849(96)00046-9. [DOI] [PubMed] [Google Scholar]
- 16.Duru FI, Noronha CC, Akinwande AI, Okanlawon AO. Effects of torsion, detorsion and melatonin on testicular malodialdehyde level. West Afr J Med. 2007;26:312–5. doi: 10.4314/wajm.v26i4.28333. [DOI] [PubMed] [Google Scholar]
- 17.Yurtcu M, Abasiyanik A, Bicer S, Avunduk MC. Efficacy of antioxidant treatment in the prevention of testicular atrophy in experimental testicular torsion. J Ped Surg. 2009;44:1754–8. doi: 10.1016/j.jpedsurg.2008.11.043. [DOI] [PubMed] [Google Scholar]
- 18.Adivarekar PK, Bhagwat SS, Raghavan V, Bandivdekar AH. Effect of lomodex-MgSO4 in the prevention of reperfusion injury following unilateral testicular torsion: an experimental study in rats. Pediatr Surg Int. 2005;21:184–90. doi: 10.1007/s00383-004-1317-1. [DOI] [PubMed] [Google Scholar]
- 19.Beheshtian A, Salmasi AH, Payabvash S, Kiumehr S, Ghazinezami B, et al. Protective effects of sildenafil administration on testicular torsion/detorsion damage in rats. World J Urol. 2008;26:197–202. doi: 10.1007/s00345-008-0243-6. [DOI] [PubMed] [Google Scholar]
- 20.Barlas M, Hatiboglu C. The effect of nitric oxide in testicular ischemia-reperfusion injury. Int Urol Nephrol. 2002;34:81–6. doi: 10.1023/a:1021311029572. [DOI] [PubMed] [Google Scholar]
- 21.Erol B, Tokgoz H, Hanci V, Bektas S, Akduman B, et al. Vardenafil reduces testicular damage following ischemia/reperfusion injury in rats. Kaohsiung J Med Sci. 2009;25:374–80. doi: 10.1016/S1607-551X(09)70530-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oztürk A, Baltaci AK, Bediz CS, Mogulkoc R, Güngör S. Effects of zinc and melatonin deficiency on testicular tissue of rats. Biol Trace Elem Res. 2003;96:255–62. doi: 10.1385/BTER:96:1-3:255. [DOI] [PubMed] [Google Scholar]
- 23.Mogulkoc R, Baltaci AK, Oztekin E, Aydin L, Tuncer I. Hyperthyroidism causes lipid peroxidation in kidney and testis tissues of rats: protective role of melatonin. Neuro Endocrinol Lett. 2005;26:806–10. [PubMed] [Google Scholar]
- 24.Koltuksuz U, Irmak MK, Karaman A, Uz E, Var A, et al. Testicular nitric oxide levels after unilateral testicular torsion/detorsion in rats pretreated with caffeic acid phenethyl ester. Urol Res. 2000;28:360–3. doi: 10.1007/s002400000145. [DOI] [PubMed] [Google Scholar]
- 25.Akgur FM, Kilinç K, Aktuğ T, Olguner M. The effect of allopurinol pretreatment before detorting testicular torsion. J Urol. 1994;151:1715–7. doi: 10.1016/s0022-5347(17)35351-x. [DOI] [PubMed] [Google Scholar]
- 26.Turan C, Küçükaydin N, Bekerecioğlu A, Kazez A, Doðan P, et al. The effect of vitamin E on ipsilateral and contralateral testis following unilateral testicular torsion in rats. Res Exp Med. 1996;196:243–6. doi: 10.1007/BF02576847. [DOI] [PubMed] [Google Scholar]
- 27.Sarica K, Küpeli B, Budak M, Koşar A, Kavukçu M, et al. Inluence of experimental spermatic cord torsion on the contralateral testis in rats. Evaluation of tissue free oxygen radical scavenger enzyme levels. Urol Int. 1997;58:208–12. doi: 10.1159/000282985. [DOI] [PubMed] [Google Scholar]
- 28.Reiter RJ. Functional diversity of the pineal hormone melatonin: its role as an antioxidant. Exp Clin Endocrinol. 1996;104:10–6. doi: 10.1055/s-0029-1211415. [DOI] [PubMed] [Google Scholar]


