Abstract
In 2010, 3.5 million people were living with HIV in the World Health Organization (WHO) Southeast Asia Region (SEAR), giving this region the greatest burden of HIV after Africa. Scale-up of antiretroviral therapy (ART) has resulted in over 717,000 benefitting from it at the end of 2010. A systematic review of studies of HIV drug resistance (HIVDR) in SEAR published between 2000 and 2011 was performed. Of 10 studies of transmitted HIVDR in recently infected patients, all but two reported low levels (<5%) of transmitted HIVDR. Of 23 studies of HIVDR in pre-treatment populations initiating ART, three reported moderate levels (5–15%) of HIVDR and 20 reported low levels. Amongst 17 studies of acquired HIVDR, levels of nucleoside reverse transcriptase inhibitor (NRTI) and non-nucleoside reverse transcriptase inhibitor (NNRTI) resistance ranged from 52%–92% and 43%–100%, respectively amongst those with virological failure. Overall, data included in this review suggest that currently recommended first- and second-line regimens are appropriate for the cohorts studied. However, data were only available from two of 11 SEAR countries and studies largely examined urban populations. Results are unlikely to be representative of the region. Studies lacked standardized methods which greatly limit comparability of data and their use for public health and ART program planning. Routine, standardized and nationally representative HIVDR surveillance should be strongly encouraged in SEAR to best characterize population-level HIVDR. National-level HIVDR surveillance data may be used to optimize delivery of HIV care and treatment and minimize emergence of population-level HIVDR, thus promoting the long-term efficacy and durability of available first- and second-line ART regimens.
Keywords: HIV, Drug Resistance, Genotype, Southeast Asia
Introduction
In 2010, an estimated 3.5 million people were living with HIV (PLHIV) in the World Health Organization (WHO) Southeast Asia Region (SEAR)1 (Figure 1), giving this region the greatest burden of HIV after Africa. Five of 11 SEAR countries shoulder the region’s HIV burden (India, Indonesia, Myanmar, Nepal and Thailand), five countries carry less than 1% of the HIV burden (Bangladesh, Bhutan, Maldives, Sri Lanka and Timor-Leste) and data are unavailable from the Democratic People’s Republic of Korea (DPR Korea). In SEAR, the number of children living with HIV increased from 89,000 in 2001 to 140,000 in 2010 suggesting that mother to child transmission remains a significant mode of transmission.1 Although the prevalence of HIV in the general adult population is low (estimated at 0.3% in 2010), most-at-risk populations (MARPs) including sex workers and their clients, injecting drug users (IDU), men who have sex with men (MSM) and transgender (TG) populations carry a disproportionate HIV burden.1 Across 269 sentinel sites in nine SEAR countries (excluding Bhutan and DPR Korea) for 2007–2010, HIV prevalence in female sex workers (FSWs) was <1% in 33% of sites, 1–5% in 38% of sites and 5–20% in 25% of sites, with the highest prevalence observed in south India. National HIV prevalence estimates in MSM in SEAR range from 5.2%–28.8%.1 However, these national estimates mask higher local estimates i.e., 31% and 41% in MSM populations in Bangkok, Thailand and Hyderabad, India, respectively. Data regarding HIV prevalence in TG populations is limited but where data are available in MSM and corresponding TG populations in the same geographic area, estimates in TG populations are generally higher than in MSM populations. Five SEAR countries have significant HIV epidemics in IDU populations including Myanmar, Indonesia and Thailand where HIV prevalence was 26.5% (2010), 27% (2007) and 46% (2010), respectively.1
Figure 1.
Southeast Asia Region (SEAR) of the World Health Organization (WHO). Countries represented in the SEAR are shown in orange. Dotted and dashed lines represent approximate borders which may not yet be in full agreement.
Southeast Asia Region Antiretroviral Treatment Scale-up and Guidelines
Globally, over 8 million people were receiving antiretroviral therapy (ART) in low and middle -income countries as of the end of 2010, representing a 26-fold increase since 2003.2 As in other regions of the world, SEAR has experience rapid expansion of ART with over 717,000 individuals benefitting from it at the end of 2010.1 Successful ART scale-up in SEAR has been largely due to the use of a public health approach to ART delivery supported by standardized protocols and simplified patient monitoring.3 ART guidelines from all 10 SEAR countries recommend use of non-nucleoside reverse transcriptase inhibitor (NNRTI) based first-line regimens in combination with two nucleoside reverse transcriptase inhibitors (NRTI). 4–13 In eight of 10 countries (Sri Lanka, Temor-Leste, Nepal, Myanmar, Indonesia, Bangladesh, India and Thailand), tenofovir is a preferred component of first-line regimens.4–10, 13 In all SEAR countries, ritonavir-boosted protease inhibitors (PI) in combination with two NRTIs are reserved as second-line agents for patients with virological failure or toxicity to NNRTIs.4–13 Treatment guidelines from the DPR Korea were unavailable.
HIV Drug Resistance
In the presence of drug selective pressure, emergence of some HIVDR is inevitable due to the error-prone replication of HIV, its high mutation rate, and the need for lifelong treatment.14, 15 Given the inevitability of some HIVDR, it is not surprising that a 2012 analysis showed that higher levels of HIVDR were observed in areas with greater ART coverage16 (defined as number of people on ART divided by the number of people with HIV). Moreover, increased levels were observed with increasing time since treatment roll-out, a finding particularly notable in southern and east Africa, where HIVDR was estimated to increase at a rate of almost 15% and 30% per year, respectively, since ART roll-out. This increase in HIVDR was driven almost exclusively by NNRTIs. Although similar increases were not observed in other regions, this increase may reflect lack of data rather than differences in levels of HIVDR.17 As in most resource limited settings, access to patient-level HIVDR testing, viral load monitoring and second-line and salvage regimens is often restricted in SEAR. Therefore, reliable information about HIVDR which can inform public health and ART program decision making is required in the region to support choice of treatment regimens and the optimization of patient care.18
Broadly, population-level HIVDR may be divided into three main categories: transmitted HIVDR, acquired HIVDR, and HIVDR in pre-treatment populations. 1) Transmitted HIVDR is HIVDR detected in recently infected populations who are unexposed to antiretroviral (ARV) drugs. In the absence of drug selective pressure, certain drug resistance mutations will revert to “wild type”, at varying rates after initial infection.19 When patients with transmitted HIVDR initiate ART, drug selective pressure may result in rapid re-emergence of clinically relevant mutations leading to rapid virological failure. 2) Acquired HIVDR is HIVDR which emerges in response to drug selective pressure. Acquired HIVDR may emerge even when optimal regimens are provided and adherence is supported. 3) Pre-treatment HIVDR is HIVDR detected in populations initiating ART for the first time. Pre-treatment HIVDR may have been acquired due to ARV drug exposures including prevention of mother to child transmission (PMTCT), pre-exposure prophylaxis (PrEP) or post exposure prophylaxis (PEP), previous combination ART, or may have been present since time of infection (transmitted).18, 19
The purpose of this review is to summarize the published literature assessing transmitted, acquired, and pre-treatment HIVDR in the SEAR region and identify gaps in knowledge required for public health decision making and suggest directions for future research.
Selection of Studies
We performed a systematic review of the literature to identify reports published in English between January 2000 and August 2011 which documented transmitted, pre-treatment and acquired HIVDR in SEAR countries. The following databases were used: PubMed, GlobalHealthLibrary (WHO database), National Library of Medicine Gate Way and EMBASE. In addition, abstracts presented at the International AIDS Society (IAS) Conference on HIV Pathogenesis Treatment and Prevention from 2001–2011, Conference on Retroviruses and Opportunistic Infections (CROI) from 2002–2011 and the International AIDS Conference (AIDS) from 2002–2010 were included.
The following search terms were used: “HIV” OR “AIDS” OR “human immunodeficiency virus” OR “acquired immunodeficiency syndrome” AND “resistance” OR “drug resistance” OR “genotype” AND “India” OR “Maldives” OR “Sri Lanka” OR “Nepal” OR “Bangladesh” OR “Bhutan” OR “Myanmar” OR “Thailand” OR “Indonesia” OR “Timor Leste” OR “Korea” OR “Asia” or “Southeast Asia”. All eleven SEAR countries were included in the literature search.1 In cases where HIVDR was evaluated in SEAR countries as part of a multicenter international study, country level data were abstracted and evaluated independently. Bibliographies were reviewed to supplement the literature search. When two cohorts are described in the same study, such as a pre-treatment cohort and cohort on ART, each cohort is reported separately in the pre-treatment section and acquired HIVDR section. Thus, two cohorts in different HIVDR sections may represent the same study.
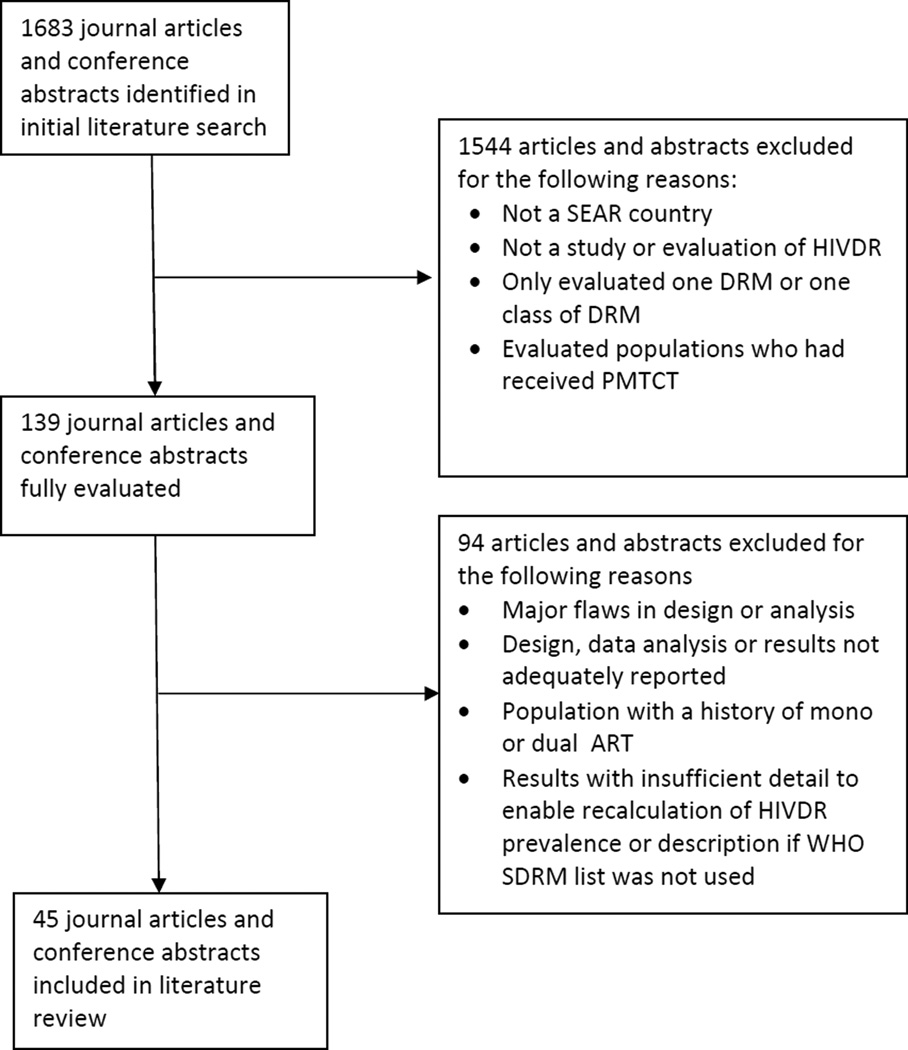
All relevant articles in English were reviewed independently by two authors (Trotter and Jordan). Studies were included in the analysis if they reported on acquired or transmitted HIVDR or described HIVDR in pre-treatment populations. Studies were excluded if they evaluated patients with histories of mono-or dual NRTI therapy, PI monotherapy, had major epidemiological or design flaws, documented PMTCT exposures, only reported HIVDR to a single ARV, described HIVDR in a SEAR country as part of a larger multinational study without reporting individual country data, or acknowledged partial reporting of detected HIVDR mutations. To ensure comparability across reports, reanalysis of data using the 2009 WHO Surveillance Drug Resistance Mutations (SDRM) list was performed if study authors had used different mutations lists.20 A flow diagram illustrating the inclusion and exclusion of publications and abstracts is presented in Figure 2. Due to marked heterogeneity of study designs, a meta-analysis was not performed.
Figure 2.
Description of journal articles and conference abstracts included and excluded from selection
ART: antiretroviral therapy; DRM: drug resistance mutation; SEAR: World Health Organization Southeast Asian Region; SDRM: 2009 WHO Surveillance Drug Resistance Mutation list
Definition of categories of drug resistance
Between 2005 and 2012, WHO recommended a method to categorize transmitted HIVDR into three prevalence classifications: low (≤5%), moderate (5–15%) or high (≥15%).15 For the purpose of this review, these prevalence classifications were applied to results from pre-treatment populations to facilitate comparison between studies and discussion. Accurate estimation of levels of transmitted HIVDR requires genotyping of specimens from populations likely to be recently infected and ARV naïve. Commonly used WHO criteria to maximize inclusion of individuals likely to be recently infected include age < 24 years, no previous pregnancy (if female), CD4 cell count > 500 cells/mm3 and first HIV-risk defining event within past three years, if available. Recent data suggest that under certain circumstances, and if resources permit, LAg-Avidity EIA or Multi-Assay Algorithm assay (MAA) may be used to identify individuals likely to be recently infected.21, 22 Studies evaluating the rate of CD4 cell count decay from time of seroconversion facilitate estimation of duration of infection.23, 24 Lodi et al document that the median time from seroconversion to a CD4 count of less than 350 cells/mm3 is 4.19 years. In this review, to standardize results and facilitate discussion, cohorts with median CD4 cell counts of greater than 350 cells/mm3 were classified as describing transmitted HIVDR and cohorts with median CD4 cell counts less than 350 cell/mm3, were classified as describing pre-treatment populations.
Summary data tables
Table 1 summarizes cohorts from journal articles and conference abstracts which reported on transmitted HIVDR, acquired HIVDR and HIVDR in pre-treatment populations. Results from cohorts described in studies of acquired HIVDR are subdivided into two groups: 1.) results from cohorts described in studies evaluating HIVDR in patients on ART using a cross-sectional method (“cross- sectional studies”) at various time periods after ART initiation and 2.) results from cohorts described in studies evaluating HIVDR in patients determined to be failing ART by clinical, immunological and or virological criteria after variable durations on ART. Table 2 summarizes HIVDR prevalence estimates by drug class as reported in journal articles and conference abstracts evaluating transmitted HIVDR, acquired HIVDR and HIVDR in pre-treatment populations. Tables 1 and 2 summarize data from 45 studies which include 50 cohorts that fulfill the inclusion criteria and were not excluded. Five of 45 studies reported findings from two cohorts: one cohort describing acquired HIVDR and one cohort describing HIVDR in a pre-treatment population.25–29 Each cohort is reported separately in the appropriate section of Table 1 or Table 2. HIVDR in seventy-eight percent of cohorts was defined by the WHO SDRM 2009 list.20 Results standardized using the 2009 WHO SDRM list are denoted by (†) in Table 1 and (‖) in Table 2. All studies included in this review are from India or Thailand. No study from Maldives, Sri Lanka, Nepal, Bangladesh, Bhutan, Myanmar, Indonesia, Democratic People’s Republic of Korea or Timor-Leste met inclusion criteria.
Table 1.
Studies of HIV Drug Resistance in the South East Asia Region
| Authors | Location | Year(s) of Cohort |
Study Design, Population Description, Inclusion or Exclusion Criteria, if available |
Results and Detected Drug Resistance |
Reviewers’ Comments |
|---|---|---|---|---|---|
| Studies of Transmitted HIVDR | |||||
| Apisarnthanarak et al 200874 | Thailand (Pathum Thani) | 2003– 2006 | 305 ARV naïve patients enrolled from 12 provinces in central Thailand. Median age 24 years, median duration of known HIV infection 3 months, 55% women of which 77% were pregnant. Median CD4 545 cells/mm3; (range 356–901). Inclusion criteria – age >15 years, CD4 >200 cells/mm3, no AIDS defining illness.* |
Detected HIVDR: 2003 0% (0/75), 2004 1.2% (1/78), 2005 2.6% (2/76), 2006 5.2% (4/76). Across all years: 7/305 (2%) had HIVDR who fulfilled their inclusion criteria for recent infection. Annual HIVDR increased from 0 to 5.2% (P = 0.06) between 2003 and 2006. | Low overall detected HIVDR. Interpretation limited by mixed risk groups and by grouping of multiple years. |
| Ayouba et al 200933 | Thailand (Chiang Mai) | Not stated | Pregnant women, median CD4 508 cells/mm3 (range 394–599). Median number of pregnancies 1.5. Median age 22.1 years (IQR: 20.8–25.8). Included pregnant women with CD4>500 cells/mm3, ARV naïve. | No detected HIVDR. | Low detected HIVDR. |
| Chonwattana et al 200736 | Thailand (Bangkok) | 2001–2004 | 178 ARV naive HIV-infected injection drug users screened every 6 months and genotyped at HIV diagnosis; sub-analysis of vaccine trial; Median time between first positive serology and genotype was 0.7 months. Median CD4 count not reported. | 94% (165/178) of specimens with detectable VL were successfully genotyped. No HIVDR was detected in 56 specimens from 2001. Three sequences with DRMs were observed: Y181C 1/62 (2%) from 2002, M41L 1/32 (3%) from 2003; M41L 1/15 (7%) from 2004. | Low detected HIVDR. |
| Iqbal et al 201030 | India (Chennai) | 2006 | 18 patients identified as recently infected by BED-CEIA. All were reported ARV naive. CD4 count not reported. | K103 N and V106M/V were detected in 2 (11%) isolates. | Moderate levels of HIVDR. Small sample size and mixed population s limit generalizability and interpretation of results. |
| Leelawiwat et al 201135 | Thailand (Bangkok) | Not stated | 91 HIV-1 MSM seroconvertors in Bangkok over a three year period. Specimens collected on day of documented HIV-1 seropositivity (71%), or shortly thereafter (mean 50 days, range 3–258 days). Median CD4 count not reported. | In 2007, 1/22 (4.5%) found to have G190E. In 2008, 1/40 (2.5%) found to have K219R. In 2009, 1/29 (3.5%) found to have T69D, K70R, V75M, F77L, Q151M, M184V, Y181C within one specimen. | Low detected HIVDR. |
| Sirivichayakul et al 200637 | Thailand (Bangkok) | 2005 | 47 consecutive recently seroconverted blood donors were genotyped for resistance to NRTI/NNRTI and protease inhibitors. Seroconversion was defined by having negative HIV antibody test at an earlier blood donation within the last 12 months. 66% were male. Mean age was 30.9. CD4 counts not reported. | The mean interval of their earlier HIV negative donation was 6.0+2.5 months (range: 2–11 months). All specimens wild-type and HIV-1 CRF01_AE. | Low detected HIVDR. |
| Sirivichayakul et al 200834 | Thailand (Bangkok) | Population 1: 2005–2006 Population 2: 2005 |
Two populations:
|
Population 1: In the first 34 specimens, no major HIV DRMs detected. Population 2: Of 552/3338 clients found to be HIV infected, 50 had positive BED tests. 46/50 genotyped. 38/46 had RT and PR genotyped, 7/46 PR genotype only and 1/46 with RT only. No major DRMs reported. |
Low detected HIVDR. |
| Soundararajan et al 200771 | India (Chennai), single tertiary center | Not stated | 48 ARV naïve children reported as newly diagnosed; mean age 5.7 years, median CD4 470 cells/mm3. Previous PMTCT exposures not reported. | 32/48 with RT and 25/48 with protease gene. HIVDR: None. | No detected HIVDR. Limited by high rates of amplification failure. |
| Thorat et al 201132 | India (Kakinada) | 2007–2009 | 56 primigravida women. CD4 cell count not reported. Inclusion criteria: 15–24 years, first time attendees, asymptomatic, no previous ART exposure, no history of 'HIV related illness’ |
56 consecutive specimens collected, 51 were processed and 47 were able to be amplified. HIVDR: NNRTI: 1/47 K101E (2%). NRTI, PI: None. |
Low detected HIVDR |
| Truong et al 200931 | India (Mumbai) | 2002–2004 | Prospective cohort of men presenting at two public STI clinics were enrolled (n=3,403). 303 were found to be newly HIV positive and had BED-CEIA performed. | Genotypes performed in 62/303. Transmitted drug resistance prevalence was 5.7%. Major resistance mutations detected were M184V and K101E. | Overall low detected HIVDR. However, low number of genotypes performed limits interpretation. |
| Studies of Acquired HIVDR – Cross-sectional Evaluation of Populations on ART | |||||
| Chasombat et al 2010†,25 | Thailand (multiple sites) | 2006 | 304 ART naïve patients enrolled at initiation of ART during February to September 2006 and followed with VL monitoring every 6m; genotyping if VL >1,000 copies/ml. ARV regimens not described. Median age 38 (range: 21–74). Male: female ratio was 1.2:1. | Baseline median CD4 cell count 56 cells/mm3 (1–334). Of patients retained at 1 year, 15/264 (5.7%) had virological failure. At 1 year, 7/264 (3%) had any RT resistance. Cumulatively 33 patients had died. Death rates at 6months, 1 year and 2 years were 7.9%, 8.9% and 10.8%, respectively. | Most patients would likely suppress on a boosted PI-based second-line regimen. |
| Ekstrand et al 201152 | India (Bangalore) | Not stated | Cohort of 551 outpatients receiving first-line ART. VL testing was conducted in all patients. ARV regimens included NRTI or NNRTI based (547/551), PI-based (3/551) and AZT monotherapy (1/551) | 101/552 (18%) had VL>1000 copies/mL and genotype testing performed. Mean duration of ART in those with virological failure was 30 months (1–175) compared to 18 months (1–52) in those with virological suppression (p<0.001). 69% (63/92) had 1 or more NRTI mutation, with M184V being the most common at 62% and 44% having one or more TAM. 72% had 1 or more NNRTI mutation and 23% had three or more NNRTI mutations | Most patients would likely suppress on a boosted PI-based second-line regimen. Lack of reporting on TAMs tempers this conclusion. |
| Gupta et al 2010‡,50 | India (Mumbai) - 3 private clinics | 2005 | 200 HIV infected ARV experienced patients enrolled from January to April 2005. Regimens included 2 NRTI+1 NNRTI (53%), 2 NRTIs (37%), 2 NRTIs+2PIs (6%), 1 NRTI (2%) and 2NRTI+ T-20 (2%). Median duration of ART was 24 months, median time on current ART regimen 12 months, median CD4 cell count 217 cells/mm3, median VL 28,2000 copies/mL. Excluded those with acute illness, patients not on ART, patients whose ART was started/changed within 3 months of study enrollment, patients who could not be consented, <18 years old. |
61/200 (30.5%) with detectable VL (VL>1000 c/mL). 51/61 genotyped. 49/51 resistance to at least 1 ARV. 47/51 (92%) with NRTI resistance, 32/51 (62%) with NNRTI resistance, 4/51 (8%) with PI resistance, 30/51 (59%) with two class resistance, 3/51 (6%) with three class resistance. NRTI: M184V 46/51 (90%), K65R 1/51 (2%). TAMs: M41L 13/51 (26%), T215F/Y 16/51 (31%), D67N 18/51 (35%), K70R 10/51 (20%). NNRTI: K103N/S 14/51 (28%), K101E/P 11/51 (22%), G190A/S/E 11/51 (22%), Y181C/I 10/51 (20%). PI: I54V 3/51 (6%), V82A/F 2/51 (4%), L90M 2/51 (4%). | Majority of patients likely to suppress on recommended second-line therapy. Interpretation limited by heterogeneity of time on ART and use of multiple regimens. Exclusion of patients with acute illness may have influenced detected levels of HIVDR. |
| Karnkawinpong et al 200675 | Thailand (Ubon Rachatani) | 2005 | Cross-sectional study of 864 patients on d4T/3TC/NVP between January – March 2005. VL testing was performed and patients whose VL>1,000 copies/ ml had genotyping. 77% (644/864) of patients consented to VL testing. | The proportion with VL > 1,000 copies/ ml at 6–12 months was 7.4% (17/229), 12–24 months 9.2% (34/369) and at >24 months 15.2% (7/46) (p = 0.24). All 58 cases with viral load > 1,000 copies/ ml had genotypes performed, but only 42 were reported. 32/42 had HIVDR. Overall, 32/644 (4.9%) had HIVDR. The proportions of reported HIVDR: NVP 31/32 (97%), EFV 31/32 (97%), 3TC 30/32 (94%), d4T 10/32 (31%), AZT 8/32 (25%). | Most patients would likely suppress on a boosted PI-based second-line regimen. Low rates of virological failure. |
| Puthanakit et al 201176 | Thailand | Not stated | Cohort of 303 children receiving 2NRTI+1NNRTI first-line ART at 2 sites. HIV VL was assessed every 6 months. Genotype performed if VL >1000 copies/mL. Median age 7 years (IQR: 4– 9), CD4 5% (IQR: 1–13), VL at ART start 5.33 log10 copies/mL (IQR: 4.98–5.76). Regimens contained: NVP (66%), EFV (34%), NRTI backbone d4T/3TC (78%) or AZT/3TC (21%). | Median follow-up 4.6 years (2.7 to 5.1). 232/303 children (77%) maintained viral suppression and 71/303 (23%) had virological failure (viral load >1000 copies/mL). At first genotype testing: 20% prevalence of any TAM, 8% resistance to multi-NRTI (≥4 TAM or Q151M or T69 insertion complex), 83% resistance to 3TC, 100% resistance to any NNRTI, and 8% resistance to ETR. At last genotype testing: 58% prevalence of any TAM, 23% resistance to multi-NRTI, 95% resistance to 3TC, 100% resistance to any NNRTI, and 20% resistance to ETR. The crude rate of new TAM accumulation was 0.69/patient-year of follow-up (95%CI 0.50 to 0.93). | Majority of patients identified with virological failure likely to suppress on second-line ART. |
| Sen et al 2007†,‡,28 | India (Pune) | 2004–2005 | 50 chronically infected patients on NNRTI or NRTI based ART regimens enrolled between June 2004 and December 2005. Median duration of ART exposure was 1.6 years (0.3–7 years). Used peripheral blood mononuclear cell proviral DNA. |
36/50 (72%) had VL>1000. 31/50 (62%) had detected HIVDR. 81% had at least one resistance mutation, 67% with NRTI mutations and 72% with NNRTI mutations. NRTI: M41L 8/31 (26%), D67N 9/31 (29%), T69 insertion complex 3/31 (10%), K70R 6/31 (19%), V75M 1/31 (3%), Q151M 1/31 (3%), M184V 20/31 (65%), L210W 1/31 (3%), T215Y/F 9/31(29%), K219E/Q 7/31 (23%). NNRTI: K101E 4/31 (13%), K103N 8/31 (26%), Y181C 9/31 (29%), Y188L 2/31 (6%), G190A 7/31 (23%). | Small sample size makes interpretation limited. Most patients likely to suppress on second-line therapy. |
| Sen et al 2007†,‡,27 | India (Pune) | Not stated | Prospectively followed 34 ARV experienced chronically infected patients. Median duration of ART therapy 2.15 years (0.5–10 years). ARV regimens included NRTI based (30/34) and PI-based (4/34). | 33/34 (97%) had HIV VL>1000. One or more HIVDR mutations were seen in 27/33 (82%) of those failing. NRTI DRMs were seen in 26/33 (79%) and NNRTI DRMs were seen in 25/31 (81%). NRTI DRMs: M41L 8/34 (24%), D67N 11/34 (32%), T69 insertion complex 4/34 (12%), K70R 11/34 (32%), L74V 2/34 (6%), L75M 2/34 (6%), F77L 1/34 (3%), F116Y 2/34 (6%), Q151M 2/34 (6%), M184V 23/34 (68%), L210W 3/34 (9%), T215Y/F 10/34 (29%), K219E/Q 7/34 (21%). NNRTI DRMs: L100I 1/34 (3%), K101E 2/34 (6%), K103N 6/34 (8%), V106A/M 3/34 (9%), Y181C 10/34 (29%), Y188L 2/34 (6%), G190A 11/34 (32%). PI DRMs: M46I 2/34 (6%), V82A 1/34 (3%), L90M 1/34 (3%). | Those failing on PI regimens may be at risk for failure to suppress on standard lopinavir/ritonavir regimen but may suppress on second generation boosted PIs. Lack of patient level genotypes and data on TAMs tempers interpretation. |
| Solomon et al 200777 | India (Chennai) | 2005 | 95 consecutive patients on ART for >1 year, no history of switching ART in last 6 months. ART regimens not described. All specimens with detectable VL underwent genotypic testing. Mean age was 33 years; 68% were male. Mean duration of ART was 32 months. | Prevalence of detectable VL (>400 copies/mL) was 54% overall, and was 82% in those on therapy for >4 years. The most common NNRTI mutations were K103N (44%), Y181C (25%), G190A (14%), and V106M (11%). M184V/I detected in 82%. Intermediate or high level resistance to NRTI was as follows: any TAM (18%), TDF (4.5%), ABC (20%), ddI (25%). | High rates of virological failure and high rates of detected HIVDR. However most should suppress on available second-line ART. |
| Sukasem et al 2007†,29 | Thailand (multicenter) | 2002–2005 | 1,709 adult patients with virological failure (VL>1000 on two or more visits) in large urban centers across Thailand. Median CD4 487 cells/mm3 (148–1024) 25.9% had CD4<200 cells/mm3. Median duration of failure not described. 46.1% on NNRTI-based regimens, 41.8% on PI-based regimens and 12% on NRTI-based regimens. Inclusion - ≥ 18 years, uninterrupted ARV therapy for at least 12 months |
1,709 (100%) RT regions amplified, 1166/1709 (68.2%) of PR genes were amplified. NRTI DRMs: M184V/I 857/1709 (50%). TAMs: M41L 492/1709 (29%), L210W 342/1709 (20%), T215Y/F/S 693/1709 (41%), D67N 720/1709 (42%), K70R 452/1709 (27%), K219Q/E 440/1709 (26%). NNRTI DRMs: K103N 404/1709 (24%), Y181C/I 391/1709 (23%), G190A/S/E 375/1709 (22%). PI DRMs: L90M 54/1166 (5%), M46I/L 63/1166 (5%), V82A/F/T 61/1166 (5%), I54V 55/1166 (5%), G48V 37/1166 (3%), N88S/D 24/1166 (2%), I84V 22/1166 (2%), D30N 8/1166 (0.7%), V32I 6/1166 (0.5%), I47V 6/1166 (0.5%). | Significant levels of NRTI resistance. Interpretation limited by the heterogeneous population. Most will likely suppress on a boosted PI-based second-line regimen. |
| Studies of Acquired HIVDR – Evaluation of Populations with Known Failure (Clinical, Immunological and/or Virological Failure) | |||||
| Bhrushundi et al 201149 | India (Nagpur) | 2009–2010 | 30 individuals identified as failing ART by WHO guidelines between January 1, 2009 to December 31, 2010. ART regimens not described. | 27/30 isolates showed one or more NRTI mutation, 28/30 isolates had NNRTI mutations and 6/30 isolates had either major or minor PI mutations. 25/30 isolates had (83.3%) M184V, 90% had at least one TAM. VL suppression rates not reported. | Majority should suppress on second-line ART. |
| Deshpande et al 2007‡,44 | India (Mumbai) | Not stated | 223 patients on ART (6months-6years) with clinical/immunological failure. 81% had known previous ARV exposure. All on NRTI based regimens. | 112/223 amplified. 109/112 HIV-1 subtype C. NRTI HIVDR: M184V 80/112 (71%), K65R 2/112 (2%), Q151M 2/112 (2%), K65R 2/112 (2%), T69D/N/S complex 16/112 (14%), L74V 7/112 (6%), V75I 3/112 (3%), Q151M 2/112 (2%). TAMs: M41L 40/112 (36%), D67N 40/112 (36%), K70R 21/112 (19%), L210W 9/112 (8%), T215Y/F 37/112 (33%), K219QE 12/112 (11%), NNRTI HIVDR: K103N/S 28/112 (25%), V106M/A 8/112 (7%), Y181C 30/112 (27%), G190A 30/112 (27%), Y188H 1/112 (1%), V106M 8/112 (7%), Y188L 5/112 (4%). | Significant NNRTI HIVDR detected. Interpretation is limited due to heterogeneity of duration of ART (6 months to 6 years). Most should suppress on a boosted PI-based second-line regimens. |
| Deshpande et al 2010†,26 | India (Mumbai) - 4 urban teaching hospitals | 2008 | 132 ART experienced failing patients (by WHO clinical or immunologic criteria) between January 2008 to December 2008, only sequenced RT. All on NNRTI based regimens | 128/132 had VL performed, 90/128 (70.3%) found to have VL>1000 and the first 27 specimens had genotyping performed. Detected HIVDR: NRTI: M184V/I 23/27 (85%), T215Y/F 17/27 (63%), D67N 17/27 (63%), M41L 15/27 (56%), K70R 12/27 (44%), K219Q/E 8/27 (30%), L210W 5/27 (19%), V75 T/M 6/27 (22%), Q151M 5/27 (19%), L74I/V 4/27 (15%), K65R 3/27 (11%), T69del 3/27 (11%). NNRTI: K103N 13/27(48%), Y181C 11/27 (41%), G190A 6/27 (22%), Y188L 4/27(15%), V106M 3/27 (11%), K101E 3/27(11%). | 11% K65R and 15% Q151M are higher than reported in other studies in the region. However, only specimens from the first 27 of 90 virologically failing patients were genotyped. This sampling method and small size makes assessment of acquired HIVDR difficult. |
| Dettrairat et al 200672 | Thailand | 2005 | 337 patients receiving ARVs for > 1 year with clinical or immunological failure had genotype testing performed if VL>2,000 copies/ml. 62/98 patients (63.3%) were treated with d4T/3TC/NVP. | 98/337 patients (29.1%) with VL>2,000 copies/ml. Genotype performed in 66/98 patients and 54/66 (81.8%) had detected HIVDR. Resistance to 3TC, d4T and AZT were found in 46 (85.2%), 13 (24.1%) and 10 (18.5%) patients, respectively. Resistance to NVP and EFV were also found in 49 (90.7%) and 29 (53.7%) patients, respectively. | Limited interpretability due to lack of reported data on TAMs. |
| Kumarasamy et al 200945 | India (Chennai) | 1996–2008 | 138 patients with clinical or immunological failure of first-line ART (3TC+d4T+NVP or EFV; AZT+d4T+NVP or EFV) consecutively enrolled. Excluded - those who were known to be ART exposed before initiation of ART at study site |
Median CD4 at initial start of ART 69 cells/mm3, median CD4 at failure 144 cells/mm3 (90–199). 46% had clinical failure with development of a new WHO defined OI. VL suppression not described. 90% had > 1 major DRM to NRTI, 65% had >1 major DRM to NNRTI, 88% had ≥ 2 major NNRTI and/or NRTI DRM, 5% had no NRTI or NNRTI DRM. NRTI: M184V 79%, Q151M 11%, L74V 7%, K65R 5%, T69D 4%. TAM: M41L 40%, T215Y/F 39%, D67N 32%, K70R 18%, K219E/Q 12%, L210W 7%, 60% TAMS. NNRTI: Y181C 33%, K103N 27%, G190A 26%. | 11% Q151M conferring broad resistance to NRTIs, but majority would likely suppress on second-line ART. |
| Sungkanuparph et al 200746 | Thailand (tertiary center) | 2003–2005 | 98 patients with virological failure after D4T/3TC/NVP regimen January 2003–December 2005; Failure defined as inadequate virological response, virological failure (at least 2 VL>1000). No known prior ART exposure | Median CD4 155 cells/mm3. Ten (10.2%) had TDF resistance. 6 /98 with K65R and 4/98 with 3 or more TAMs. All 10 TDF resistant patients had NNRTI resistance (Y181C [7/10], G190A [5/10], K103N [3/10], V106A [1/10] and Y188L [1/10]). Those with TDF resistance had a significantly longer duration of ART before failure. Multivariate analysis showed VL at failure and duration of ART prior to failure were associated with TDF resistance. | 10% TDF resistance concerning due to introduction of TDF as component of second-line ART. |
| Sungkanuparph et al 2008‡,51 | Thailand (tertiary center) | 2000–2008 | 21 children (<15 years) with virological failure (VL>1000). 57% on NVP-based regimens, all others on EFV based regimens. Recruited between January 2000–December 2007. No prior known ART exposure. | Median CD4 647 cells/mm3. >1 DRM to NRTI 52%, to NNRTI 43%; NRTI: 38% with M184V, 33% M184I, 5% Q151M; NNRTI mutations K103N (33%), Y181C (10%), G190A (10%). | Limited by small sample size but majority likely to suppress on boosted PI-based second-line ART. |
| Vidya et al 200978 | India (Chennai) | 2004–2007 | 126 patients with immunological failure on first-line ART between March 2004 and June 2007. 75% male. 96% acquired by heterosexual transmission. Regimens: 62% d4T/3TC/NVP/EFV, 38% AZT/3TC/NVP/EFV. Inclusion criteria: Greater than 18 years old, been on first-line ART for >6 months, had immunological failure as defined by WHO criteria. Excluded those with drug holiday for >4 weeks before genotypic testing. |
Median CD4 count at initiation of ART 148 cells/mm3 (12–998), median CD4 count at failure 197 cells/mm3 (24–757). 100/126 specimens could be amplified. RT DRMs were found in 92%. 63% had TAMs. NRTI: M184V 75/100 (75%), D67N 40/100 (40%), M41L 27/100 (27%), K70R 26/100 (26%), T215Y 16/100 (16%), K219E/Q 15/100 (15%), T69N/D 14/100 (14%), K65R 5/100 (5%). NNRTI: Y181C 37/100 (37%), G190A 28/100 (28%), 27/100 K103N (27%), Y188L/H/C 9/100 (9%), V106M 8/100 (8%), K101E/P 7/100 (7%). | Significant NRTI and NNRTI HIVDR but pattern indicates majority would suppress on second-line boosted PI-based regimens. |
| Studies of HIVDR in Pre-treatment Populations | |||||
| Apisarnthanarak et al 200861 | Thailand, university tertiary care hospital but patients represent 12 provinces | 2005–2007 | 151 ARV naïve patients with HIV of unknown duration, median CD4 120 cells/mm3 (56–180); 75% heterosexual, 9% MSM, 11% IVDU | 6/151 (4%) cases with HIVDR. Among the 6 individuals with HIVDR, DRMs included: 5 K103N, 1 Y181C/I, 2 G190S/A, 1 P225H, 6 M184V, 5 M41L, 5 T215F/Y, 4 L210W, 1 K65R, no PI HIVDR. | Low detected HIVDR. Mixed risk groups limits public health interpretation. |
| Arora et al 2008‡,79 | India (Chandigarh) | Not stated | 52 newly diagnosed HIV 1 infected ARV naïve individuals tested at VCT center; median CD4 cell count 215 cells/mm3 (IQR 95–320). | 49/52 isolates analyzed. No NRTI or NNRTI DRMs. PI: 2/49 D30N, 1/49 M46L, 1/49 F53L. | Low detected HIVDR. |
| Auwanit et al 200959 | Thailand (nationwide) | 2006 | 50 specimens from ARV naïve patients. Median CD4 count not reported. | 43/50 PR amplified and 50/50 RT amplified. 39/46 specimens HIV-1 CRF01AE. No significant DRMs found. | Low detected HIVDR. |
| Balakrishnan et al 2005‡,68 | India (Chennai), tertiary center | 2002–2003 | Cohort of 50 ARV naïve patients; Median CD4 208 cells/mm3 (6–790); 88% from heterosexual transmission, 12% by blood transfusion. | 3 DRMs found in 50 isolates tested. NRTI: One D67E. NNRTI: One M230L. PI: One M46I. | Low to moderate detected HIVDR but no confidence intervals provided limits interpretation. |
| Chasombat et al 2010†,25 | Thailand (multiple sites) | 2006 | 304 ARV naïve patients from six ART sites in four provinces in Thailand. Median CD4 was 56 cells/ mm3 (1–334). Median age was 38 (21–74). Male: female ratio was 1.2:1. | NNRTI DRMs were found in 5 patients (1.6%). | Low detected HIVDR. |
| Chaturbhuj et al 201041 | India (Mumbai) | 2007 | 50 specimens collected among VCTC patients. Median CD4 count not reported. Inclusion criteria: asymptomatic, no history of HIV/AIDS related illness, ART naïve. |
Genotyping of a consecutive sample of 34 specimens did not reveal any detected HIVDR. | No detected HIVDR. |
| Deshpande 2010†,‡,26 | India (Mumbai) - 4 urban teaching hospitals | 2008 | 117 ARV naïve patients. Median CD4 count not reported. | Detected HIVDR: 2% had M184V. | Low levels of detected HIVDR. |
| Deshpande et al 200469 | India (Mumbai) | 2003 | 128 HIV positive ARV naïve patients at single tertiary center in Mumbai, all with CD4>400 cells/mm3 (median CD4 594 cells/mm3) | RT gene: M184V (2/128); Protease gene: M46I (1/128). | Low detected HIVDR |
| Iqbal et al 2009‡,62 | India (Chennai) | 2005–2006 | Cohort of 272 HIV positive IDU, 55 randomly selected for inclusion. Retrospectively used stored specimens from a longitudinal cohort. Median CD4 count 328 cells/mm3 (IQR 268–473). Inclusion: able to provide consent, at least 18 years of age, active IDU in last 6 months, male gender |
RT was amplified in all cases, PR was amplified in 37/55. Overall prevalence of any HIVDR in an individual was 2/55. Prevalence of NRTI DRM: 1/55 (2%): M41L 1/55 (2%). No NNRTI or PI DRM found. | Interpretation limited by high rate of amplification failure of PR> overall low detected NRTI and NNRTI HIVDR. |
| Kandathil et al 2009‡,67 | India (Vellore) | Not stated | 93 ARV naïve patients. Mean VL 5.81 log copies (3.04–7.0), median CD4 cell count 221cells/mm3 (10–1037). 70% male. Average age 37.4 years (12–61) | Detected HIVDR: 1/93 (1%) M41L, 1/93 (1%) Y181C. | Low detected HIVDR. |
| Lall et al 2008‡,63 | India (Pune) | Not stated | 40 ARV naïve patients at a VCT at military college; primarily heterosexual transmission; Median CD4 263 cells/mm3(14–906), median VL 68,967 copies/ml (4584–872,251) Excluded patients with history of previous ARV exposure. |
Overall 4/40 (10%) had detected HIVDR. NRTI: 3/40 (8%) T69N/S, 1/40 (3%) M41L, 1/40 (3%) M184V, 1/40 (3%) T215Y, 1/40 (3%) D67N. NNRTI: none. PI: 1/40 (3%) V82A | Low levels of PI HIVDR, no detected NNRTI HIVDR, and moderate levels of NRTI HIVDR. |
| Neogi et al 201038 | India (Bangalore) | 2009–2010 | 21 ARV naïve patients from one center. Median CD4 200 (13–538), 48% WHO stage 1, 19% stage 2, 19% stage 3, 14% stage 4. Heterosexual route most common (20/21). | No major DRM found. | No major HIVDR found. |
| Nuntachit et al 2011‡,64 | Thailand (Chiang Ma) | 2007–2010 | Retrospective analysis of 271 ARV naïve patients. Transmission primarily heterosexual contact (80%).HIV-1 CRF01_AE most common subtype (238/271, 88%). Median CD4 count 79 cells/mm3 (IQR 29–174). | Median HIV VL 84,701 copies/ml (IQR 40,273–100,001). NNRTI DRMs: V179D/F 11/271 (4%), V106M/I/A 9/271 (3%), K103N 1/271 (0.4%), and G190A/S 1/271 (0.4%). NRTI associated mutations include T69 13/271 (5%), D67N 2/271 (0.7%). | Low detected HIVDR. No PI resistance, low NNRTI and low NRTI resistance. Interpretation limited by convenience sampling. |
| Potdar et al 2011‡,65 | India (Mumbai) | 2008–2010 | 50 ARV naïve HIV positive patients attending a single center. Median CD4 cell count not reported. | 32/50 had a detectable VL (>1000). Median VL 3.6×105 copies. Successful genotyping in 15/32 with detectable VL. Detected HIVDR: PI: V32I 1/15 (7%), V82A/D/G 6/15 (40%), N88S 3/15 (22%), I50V 1/15 (7%), M46L 5/15 (33%). NRTI: M41L 1/15 (7%), M184V 2/15 (14%), D67N 1/15 (7%), K219E/R 3/15 (20%). NNRTI: Y181C 5/15 (33%), P225H 3/15 (20%), G190A 1/15 (7%). | Interpretation limited by high rates of genotyping amplification failure. Detected HIVDR is higher than in other reports but amplification failure rate limits interpretation. |
| Rajesh et al 200970 | India (Chennai) | Not stated | 107 adult HIV/TB co-infected patients prospectively followed for 6 months after being randomized to ddI/3TC/NVP or ddI/3TC/EFV. Baseline HIVDR testing performed. Median CD4 87 cells/mm3. Inclusion - newly diagnosed pulmonary or extra-pulmonary TB, CD4<250 cells/mm3. Excluded – history of TB/ HIV treatment, pregnant/lactating women, those with 'major' complications of HIV |
104/107 patients with RT successfully amplified, 99/107 patients with PR successfully amplified. Detected HIVDR: NNRTI: 1/104 G190A, 1/104 V108I. NRTI: 2/104 T215I/S. PI: 1/99 L90M | Low detected HIVDR. |
| Saini et al 201080 | India (Delhi), single center | Not stated | 70 ARV naïve patients with subtype C HIV. No median CD4 count reported. | The median HIV-1 RNA levels were log10 4.6 copies/ml (IQR; 4.2–5.3). 1/70 with M184I mutation. No protease mutations detected. | Low detected HIVDR. |
| Sen et al 2007†,27 | India (Pune) | Not stated | 22 ARV naïve patients initiating ART. Median CD4 count not reported | No HIVDR detected. | No detected HIVDR. |
| Sen et al 2007†,28 | India (Pune) | 2004–2005 | 25 ARV naïve patients enrolled. 73/75 (97.3%) HIV-1 subtype C. Mostly heterosexual transmission. Median CD4 count not reported. | No HIVDR detected. | Low levels of detected HIVDR. |
| Shet et al 2011‡,66 | India (Bangalore) | 2007–2010 | ARV-naïve perinatally-acquired HIV-infected children aged 1–12yrs attending 2 clinics (tertiary hospital and community clinic) were enrolled. A total of 50 specimens with viral load >1000 copies/ml were genotyped. No median CD4 count reported. | 49/50 specimens HIV-1 subtype C; one specimen HIV-1 subtype A1. No major NRTI DRMs. 1/50 showed a K103N mutation (patient with known prior ART exposure). | Low detected HIVDR. |
| Sinha et al 201060 | India (Delhi) – single tertiary center | Not stated | Cohort of 53 ARV naïve individuals enrolled. Median CD4 count not reported. | 4/53 (7.5%) individuals had NRTI mutations and one subject (1.9%) had a PI mutation. Mutations found include M184V, V118I, T69 Insertion complex and D30 N. | Overall moderate NRTI HIVDR but no confidence intervals reported limiting interpretation of results. |
| Sirivichayakul et al 201139 | Thailand (Bangkok) | 2008–2010 | Patients routinely screened for HIV by the Thai Red Cross Anonymous Clinic in 2008 (N=130), 2009 (N=89), and 2010 (N=80) with total of 299 recruited over three years. Median age 23 years (range 17–47 years), 65/299 (89%) MSM. Eligibility requirements: age 18 to 24 years old with newly positive HIV antibody tests or age >25 years old, newly positive HIV antibody test with a negative HIV antibody test within the last 12 months. After 2008, only males who self-identified as MSM were included. | Median viral load 36,860 copies/ml (range 39–10,000,000), median CD4 348 cells/mm3 (range 9–1007). Prevalence of HIVDR was 5% in 2008, 3.9% in 2009, and 6.8% in 2010 (p=0.71). Detected resistance to NRTI, NNRTI and PI was 0.8%, 0.8%, 4.3% in 2008; 2.6%, 1.3%, 1.3% in 2009; and 5.1%, 3.4%, 3.4% in 2010. 86% (255/299) were CRF01_AE, 10% were subtype B. | Low detected HIVDR. |
| Sukasem et al 2007†,29 | Thailand (multicenter) | 2002–2005 | 113 newly diagnosed (defined as asymptomatic) ARV naïve patients recruited. Median CD4 count was 439 cells/mm3 (201–995), 22% CD4 <200 cells/mm3, majority heterosexual transmission. Inclusion criteria: no history of exposure to ARVs by patient report and medical records, VL>1000. |
14/113 (12.4%) had HIVDR. No detected NNRTI or PI DRMs. 8/113 (7%) M41L, 5/113 (4%) D67N, 4/113 (3%) K70R, 3/113 (3%) L74V, 4/113 (3%) M184V, 2/113 (2%) K219Q/E. 3/113 (3%) had at least 3 TAMs. | Moderate levels of detected HIVDR. |
| Tovanabutra et al 200440 | Thailand (Chiang Mai) | 1999–2002 | 34 HIV-1 reported recent seroconverters from a prospective cohort. Genotype performed on peripheral blood mononuclear cells. Median CD4 count not reported. | 30/34 (88.2) HIV-1 CRF01_AE and 4/34 (11.8%) HIV-1 B/CRF01_AE recombinant. No detected HIVDR. | Low detected HIVDR. Duration of infection unclear. |
Eligibility - (1) detectable viral load and negative or intermediate Western blot with subsequent antibody seroconversion (2) positive EIA with Western blot confirmation within 12 months of a documented negative HIV1 antibody result (3) an optical density signal to cut off ratio of <0.75 according to a less sensitive or standard dual EIA testing system (4) detection of HIV RNA in the absence of a positive antibody test or an incident detection of low affinity antibody in a detuned assay if there is a history compatible with acute HIV infection
This study described two cohorts: one cohort on ART and one cohort initiating ART for the first time. Therefore, results from each of the cohorts described in this study are reported separately in studies of acquired HIVDR and studies of HIVDR in pre-treatment populations.
Reported data on detected HIVDR from the primary source was edited to conform to the 2009 World Health Organization Surveillance Drug Resistance Mutation (SDRM) list.
3TC=Lamivudine; ABC=Abacavir; ART=Antiretroviral Therapy; ARV=Antiretroviral; d4T=Stavudine; ddI=Didanosine; DRM=Drug Resistance Mutation; EFV=Efavirenz; HIVDR=Human Immunodeficiency Virus Drug Resistance; IDU= Intravenous Drug User; MSM=Men who have Sex with Men; NAM=Nucleoside Analogue Mutation; NNRTI=Non Nucleoside Reverse Transcriptase Inhibitor; NRTI=Nucleoside Reverse Transcriptase Inhibitor; NVP=Nevirapine; OI=Opportunistic Infection; PI=Protease Inhibitor; PMTCT=Prevention of Mother to Child Transmission; PR=Protease Region; RT=Reverse Transcriptase; T-20=enfuvirtide; TB=tuberculosis; VCT=Volunteer Counseling and Testing; VL=Viral Load
Table 2.
Prevalence of HIV Drug Resistance in South East Asia Region By Drug Class*
| Study | Prevalence of Nucleoside Reverse Transcriptase Inhibitor HIVDR† |
Prevalence of Non Nucleoside Reverse Transcriptase Inhibitor HIVDR† |
Prevalence of Protease Inhibitor HIVDR† |
|---|---|---|---|
| Studies of Transmitted HIVDR | |||
| Apisarnthanarak et al 2008 (All years)74 | 6/305 (2%) | 7/305 (2%) | None |
| Ayouba et al 200933 | None | None | None |
| Chonwattana et al 200736 | 2/165 (1%) | 1/165 (1%) | None |
| Iqbal et al 201030 | None | 2/18 (11%) | None |
| Leelawiwat et al 201135 | 2/91 (2%) | 2/91 (2%) | None |
| Sirivichayakul et al 200637 | None | None | None |
| Sirivichayakul et al 200834 – Population 1: – Population 2: |
None None |
None None |
None None |
| Soundararajan et al 200771 | None | None | None |
| Thorat et al 201132 | None | 1/47 (2%) | None |
| Truong et al 2009‡,31 | - | - | - |
| Studies of Acquired HIVDR | |||
| Bhrushundi et al 201149 | 27/30 (90%) | 28/30 (93%) | 6/30 (20%)§ |
| Chasombat et al 2010‡,‖,25 | - | - | - |
| Deshpande et al 2007‡,44 | - | - | - |
| Deshpande et al 2010‖,26 | 26/27 (96%) | 27/27 (100%) | None |
| Dettrairat et al 2006‡,72 | - | - | - |
| Ekstrand et al 201152 | 63/92 (68%) | 66/92 (72%) | None |
| Gupta et al 201050 | 47/51 (92%) | 32/51 (62%) | 4/51 (8%) |
| Karnkawinpong et al 2006‡,75 | - | - | - |
| Kumarasamy et al 200945 | 124/138 (90%) | 90/138 (65%) | None |
| Puthanakit et al 2011‡,76 | - | - | - |
| Sen et al 2007‡,‖,27 | - | - | - |
| Sen et al 2007‖,28 | 24/50 (67%) | 26/50 (72%) | None |
| Solomon et al 2007‡,77 | - | - | - |
| Sukasem et al 2007‡,‖,29 | - | - | - |
| Sungkanuparph et al 2007‡,46 | - | - | - |
| Sungkanuparph et al 200851 | 11/21 (52%) | 9/21 (43%) | None |
| Vidya et al 2009‡,78 | - | - | - |
| Studies of HIVDR in Pre-treatment Populations | |||
| Apisarnthanarak et al 2008‡,61 | - | - | None |
| Arora et al 2008‡,79 | - | - | - |
| Auwanit et al 200959 | None | None | None |
| Balakrishnan et al 200568 | 1/50 (2%) | 1/50 (2%) | 1/50 (2%) |
| Chasombat et al 2010‖,25 | None | 5/304 (2%) | None |
| Chaturbhuj et al 201041 | None | None | None |
| Deshpande 2010‖,26 | 2% | None | None |
| Deshpande et al 200469 | 2/128 (2%) | None | 1/128 (1%) |
| Iqbal et al 200962 | 1/55 (2%) | None | None |
| Kandathil et al 200967 | 1/93 (1%) | 1/93 (1%) | None |
| Lall et al 200863 | 3/40 (8%) | None | 1/40 (3%) |
| Neogi et al 201038 | None | None | None |
| Nuntachit et al 2011‡,64 | - | - | - |
| Potdar et al 2011‡,65 | - | - | - |
| Rajesh et al 200970 | 2/104 (2%) | 2/104 (2%) | 1/99 (1%) |
| Saini et al 201080 | 1/70 (1%) | None | None |
| Sen et al 2007‖,27 | None | None | None |
| Sen et al 2007‖,28 | None | None | None |
| Shet et al 201166 | None | 1/50 (2%) | None |
| Sinha et al 201060 | 4/53 (8%) | None | 1/53 (2%) |
| Sirivichayakul et al 201139 | 2008: 0.8% 2009: 2.6% 2010: 5.1% |
2008: 0.8% 2009: 1.3% 2010: 3.4% |
2008: 4.3% 2009: 1.3% 2010: 3.4% |
| Sukasem et al 2007‡,‖,29 | - | None | None |
| Tovanabutra et al 200440 | None | None | None |
The prevalence of HIV drug resistance in this table was calculated by dividing the total number of specimens with a ≥ 1 drug resistance mutation conferring resistance in the appropriate drug class divided by the total number of specimens from patients in the cohort with virological failure
Class HIVDR defined using the WHO Surveillance Drug Resistance Mutations 2009 list
Data presented in journal article or abstract did not have sufficient detail which might include specific DRMs detected or individual sample-level information to allow calculation of prevalence of drug class HIVDR
PI resistance described as ‘major or minor mutations’ without description of specific DRMs which did not allow exclusion of minor PI mutations from the calculation of the mean prevalence estimate
This study described two cohorts: one cohort on ART and one cohort about to start ART. Therefore, results from each of the cohorts described in this study are reported separately in studies of acquired HIVDR and studies of HIVDR in pre-treatment populations.
Transmitted HIVDR
A total of 10 studies of transmitted HIVDR were included (Table 1). Nine of 10 studies reported low (<5%) levels of transmitted HIVDR when WHO classifications were applied. Two studies documented moderate levels (5–15%) of transmitted NNRTI resistance. One study described a cohort of 18 voluntary counseling and testing (VCT) attendees in Chennai, India and reported 11% NNRTI HIVDR.30 The other described a cohort of 303 sexually transmitted infection (STI) clinic attendees in Mumbai, India of which 62 of 303 had a genotype performed and reported 5.7% HIVDR.31 Overall, findings suggest that resistance transmission remains minimal in the cohorts assessed and that current recommendations for first-line ART are likely to be appropriate for the majority of patients included in these studies when they require therapy in the future.
The accurate estimation of transmitted HIVDR requires assessment of recently infected individuals. As the time period between seroconversion and HIVDR testing increases, the likelihood that transmitted HIVDR is underestimated due to reversion to wild type virus increases.19 In addition, undisclosed ARV exposure between seroconversion and the time of HIVDR testing may result in acquired HIVDR, which can lead to falsely elevated estimates of transmitted HIVDR.
In the studies included in this review, various criteria were used to define recently infected populations including: women with no previous pregnancy,32 BED capture enzyme immunoassay analysis,30, 31, 34 or serial HIV testing to document seroconversion.34–37
Four studies were reclassified reporting studies of HIVDR in pre-treatment populations.38–41 Two38, 39 were reclassified because the median CD4 cell counts of the study cohort was less than 350 cells/mm3 suggesting the majority were chronically rather that recently infected with HIV. 23, 24 One was reclassified because the frequency of serial testing was inadequately described and the median CD4 cell count of the cohort was not reported.40 The remaining study evaluated a VCT population of which 71% were female but no information was provided concerning previous or current pregnancy or median CD4 cell count of the study population.41 For these reasons, this publication was reclassified as a study in a pre-treatment population. The overall few studies successfully achieving their stated aim of assessing transmitted HIVDR underscores challenges associated with identifying recently infected populations. To facilitate surveillance of transmitted HIVDR, the WHO has recently developed new methods and expanded and updated epidemiological criteria used to define recently infected populations in resource limited settings.15, 42, 43
Acquired HIVDR
Nine cross-sectional studies of acquired HIVDR and eight studies of HIVDR in populations identified as failing ART by clinical, immunological, and or virological criteria were included. Levels of NRTI and NNRTI resistance amongst those failing with detectable viral load across all studies of acquired drug resistance ranged from 52%–92% and 43%–100%, respectively (Table 2).
In cross-sectional studies of acquired HIVDR, the duration on ART at time of viral load and genotyping ranged from 6 to 50 months. Overall eight of nine cross-sectional studies reported low levels of PI resistance in patients failing PI-based first-line ART. However, in one study, 2 of 4 (50%) patients on PI-based ART were failing with major PI mutations.27 Specific ART regimens were not described and use of un-boosted PIs cannot be excluded. Moreover, this study’s small sample size renders uncertain any interpretation. In all nine cross-sectional studies, NNRTI and NRTI resistance predominated amongst patients with virological failure and detected HIVDR. The levels of HIVDR and complex NRTI patterns observed in some reports suggest a long duration of virological failure in the setting of ongoing drug selective pressure prior to detection of treatment failure.
Eight studies of acquired HIVDR evaluating populations failing first-line ART by clinical, immunologic or virological criteria were included (Table 1). Amongst these eight studies, the prevalence of Q151M, which confers broad NRTI cross resistance, ranged from 0–15%. One study reported 11% K65R which was considerably higher when compared to other reports in this review (range 0–6%).26 In 50% of studies of acquired HIVDR performed in populations detected as failing ART by clinical, immunological, or virological criteria, higher levels of thymidine analogue mutations (TAMs),44 Q151M,45 K65R in combination with Q151M26 or any mutations conferring resistance to tenofovir (TDF)46 were reported. Q151M confers broad high level resistance to most NRTIs and low level resistance to TDF; K65R confers high level resistance to TDF47 an important component of recommended ART regimens.48 Studies reporting higher levels of Q151M suggest prolonged duration of virological failure on NRTI containing regimens to detection.26, 45
Due to marked heterogeneity of data, it was not feasible to assess the proportion of patients with NRTI-only resistance or the proportion with one or more TAMs (Table 2).
Although results indicate that the majority of those assessed who were failing a NNRTI-based first-line regimen in these studies would achieve virological suppression on PI-based second-line regimens, anticipated levels of resistance to second-line NRTI components of commonly used regimens supports scale-up of routine viral load testing. Specifically, routine viral load testing would permit early detection of virological failure, thus allowing for reinforcement of patient adherence to ART and timely switch to PI-based ART, if required.
In most cases, patient-level data were unavailable thus precluding assessment of the anticipated clinical relevance of TAMs to second-line ART regimens used in the region. One study of acquired HIVDR in patients with clinical, immunological or virological failure reported a 20% prevalence of PI resistance49 and one cross-sectional study of acquired HIVDR reported an eight percent prevalence of PI resistance.50 The remaining studies of acquired HIVDR in patients with clinical, immunological or virological failure26, 45, 51 and cross-sectional studies of acquired HIVDR28, 52 found little or no PI resistance. Possible explanations for this difference include variable previous PI exposures, concurrent NRTI resistance, use of un-boosted PIs or differences in levels of adherence.
In India, the private sector provides health care for up to 70% of the population50 and differences in ART delivery and HIV care in the private sector may be related to the country’s higher reported levels of acquired HIVDR. For example, Shet et al found that patients receiving ART in the private sector in south India had a lower level of self-reported adherence, a lower level of virological suppression (defined as viral load <100 copies/ml) and a higher prevalence of HIVDR when compared to patients in the public-private and public sectors.53 Another study from India reported that patients receiving ART in the private sector were 2.7 times more likely to experience a treatment interruption when compared to those in the public sector.54 Poor adherence and treatment interruptions are well documented to increase the likelihood of treatment failure and selection of HIVDR.55, 56 Other barriers to adherence described in India’s private sector include the cost of ART, drug side effects or toxicities, lack of prescriber knowledge about ART and inability to mitigate ART side effects/toxicities, and drug stock outs.54, 57
Pre-Treatment HIVDR
Twenty-three studies of HIVDR in pre-treatment populations were included (Table 1). Twenty studies reported low levels of HIVDR and three studies reported moderate levels of NRTI HIVDR. Among the studies of HIVDR in pre-treatment populations, the prevalence of NRTI, NNRTI and PI HIVDR ranged from 0–8%, 0–8% and 0–4.3%, respectively (Table 2).
The finding that the majority of studies report low levels of HIVDR suggests that amongst the cohorts studied, levels of HIVDR would not preclude successful virological suppression when currently recommended first-line regimens are used in populations studied.
Patterns of HIVDR Detected
Across all studies of transmitted HIVDR, a total of 17 drug resistance mutations (DRMs) were detected. Eight DRMs conferring resistance to NRTIs were reported: M41L, T69D, K70R, V75M, F77L, Q151M, M184V and K219R, of which M41L occurred twice and was the only NRTI DRM reported more than once in more than one sequence in one or more studies. Five DRMs conferring resistance to NNRTIs were reported: K101E, K103N, V106M/V, Y181C, G190E, of which Y181C, K103N and V106M/V occurred twice and were the only NNRTI DRMs reported more than once in more than one sequence in one or more studies. One study detected K101E and M184V but did not report their frequency; therefore, they are not included in this summary.31 No PI DRMs were reported.
In cross-sectional studies of acquired HIVDR, most detected resistance was to NRTIs or NNRTIs. Among NRTI mutations, M184V was most common, occurring in 50 to 90% of genotypes with any HIVDR. Of the studies which reported on the prevalence of TAMs, any TAM was described in 3–42% of specimens and included M41L, D67N, K70R, T215F/Y and L210W.27–29, 50, 58 The most commonly detected NNRTI DRMs included K103N, K101E, G190A, and Y181C which were detected in 24–44%, 14–22%, 18–35% and 23–37% of studies, respectively.27–29, 50, 58 Only three studies detected PI DRMs with the most commonly observed mutations being I54M (0–6%), V82A (3–5%) and L90M (3–5%).17, 27, 29
All studies of acquired HIVDR in patients with known clinical, immunological or virological failures reported NRTI and NNRTI resistance. Among NRTI DRMs, TAMs were found in up to 65% of patients failing with HIVDR within any single study. Frequently observed non-TAMs included M184V detected in 33–85% of genotypes and Q151M detected in 5–11% of genotypes with HIVDR. Among NNRTI DRMs, K103N, Y181C and Y181C were detected in 25–48%, 10–41% and 10–28% of genotypes, respectively.
In studies of HIVDR in pre-treatment populations, six reported no HIVDR27, 28, 38, 40, 41, 59 and three did not report which DRMs had been detected.25, 39, 60 Of the remaining 14 studies, the most commonly observed DRM was the M184V reported in seven of 14 studies. TAMs were present in six29, 61–65 of 14 studies and accounted for up to 47% of all DRMs in an individual study.61 K103N was present in three of 14 studies61, 64, 66 while Y181C was reported in three of 14 studies.61, 65, 67 Five of 14 studies reported PI DRMs,62, 65, 68–70 the most common being M46I which was present in three of the five studies reporting PI DRMs65, 68, 69 and which represented up to 33% of all DRMs described in any individual study.68, 69
Discussion
At the end of 2010, the WHO SEAR accounted for 10% of the global population of PLHIV.1 In the setting of ongoing ART scale-up in SEAR, emergence of HIVDR is inevitable and necessitates population-based HIVDR surveillance to guide ART programs in the selection of appropriate and effective regimens for first- and second-line ART, PMTCT, PrEP, and PEP. Moreover, when combined with data obtained from routine ART program monitoring and evaluation activities, HIVDR surveillance data supports identification of ART program and clinic-level factors requiring optimization in order to minimize the preventable emergence and transmission of HIVDR.
In this review of HIVDR studies published in SEAR, overall reported levels of HIVDR were low. Based on results from studies assessing transmitted and pre-treatment HIVDR, currently recommended first-line ART regimens appear appropriate for the majority of study participants. Moreover, the frequency and pattern of DRMs described in studies of acquired HIVDR supports currently recommended PI-based second-line ART regimens for the majority participating in these studies. However, relatively high levels of acquired resistance to one or more of the NRTIs used in second-line regimens support use of routine viral load testing to detect virological failure early and support interventions to improve adherence or earlier switch to second-line. Finally, HIVDR described in studies of pre-treatment populations support currently recommended first-line ART regimens for those assessed in the studies included in this review.
This review has several important limitations. The interpretation of four of ten studies of transmitted HIVDR is limited due to the studies’ small sample sizes, wide confidence intervals, mixed populations (VCT and ANC), and multiple years of pooled data.30, 31, 61, 71 In addition, a wide range of genotyping amplification rates (52–94%) raises concerns regarding specimen quality, reproducibility and the sensitivity/specificity of genotyping assays used.
Amongst studies of acquired HIVDR, interpretation of eight studies was limited due to their small sample size, limited description of epidemiological methods, HIVDR prevalence estimates with wide confidence intervals, heterogeneity of previous ARV experience, or partially missing genotypic information, specifically no information about TAMs.26, 28, 29, 44, 50, 52, 72, 73 These study characteristics greatly limit the generalizability and interpretation of results beyond the cohorts assessed. Finally, interpretation of two of twenty-three studies of HIVDR in pre-treatment populations was limited due to high rates of amplification failure.62, 65 In almost all studies, small conveniently chosen samples limit generalization of results; thus, greatly limiting utility of results for public health planners in the region. Finally, very few studies reported prevalence estimates with corresponding confidence intervals, greatly limiting data interpretation.
The absence of reports from nine of 11 SEAR countries combined with the fact that all data available were obtained from urban areas further highlights important gaps in our knowledge about HIVDR in the SEAR region. The magnitude and possible impact of HIVDR on ART treatment outcomes in the remaining nine SEAR countries and in non-urban areas remains unknown. The paucity of data and the limited applicability of available data for public health planning underscore the urgent need for routine national HIVDR surveillance in SEAR countries. To support population-level statements about HIVDR and provide needed information to ART program planners and Ministries of Health, WHO recommends standardized nationally representative methods to assess acquired, transmitted and pre-treatment HIVDR within defined populations.15
Conclusion
In this systematic review of HIVDR in the WHO SEAR region, most studies reported low levels of HIVDR which is reassuring. However, limited generalizability of results, heterogeneity of study designs, and biases introduced by high and possibly non-random rates of genotyping failure diminish the strength of findings to support public health and ART program recommendations and actions.
Routine, standardized and nationally representative HIVDR surveillance should be strongly encouraged in SEAR to best characterize population-level HIVDR. In countries with low-prevalence and concentrated epidemics, surveillance activities should be extended to poorly characterized most at risk populations, as well as to geographic areas where information is limited or nonexistent. Results of HIVDR surveillance activities should be actively used to optimize delivery of HIV care and treatment and promote the long term efficacy and durability of available first- and second-line ART regimens in the region.
Acknowledgements
This work was supported by T32 AI007438-18 (ABT), NIH K23 AIO74423-05 (MRJ), NIH L30 AI080268-02 (SYH), NIH 1K23AI097010-01A1 (SYH), CFAR P30AI42853 and by a gift from Christine E. Driscoll O’Neill and James M. Driscoll, Driscoll-O’Neill Charitable Foundation.
Footnotes
Disclosures: All authors report no conflict of interests. Some authors are employees of the World Health Organization. Views expressed in this manuscript are the views of the authors and not the World Health Organization.
References
- 1.World Health Organization: Regional Office for South-East Asia. HIV/AIDS in the south-east region: Progress report 2011. 2011 http://www.searo.who.int/entity/hiv/documents/hiv-aids_in_south-east_asia.pdf.
- 2.Joint United Nations Programme on HIV/AIDS. Together we will end AIDS. 2012 [Google Scholar]
- 3.World Health Organizatiossn. The treatment 2.0 framework for action: Catalysing the next phase of treatment, care and support. 2011 http://whqlibdoc.who.int/publications/2011/9789241501934_eng.pdf.
- 4.National AIDS Control Organization, Ministry of Health and Family Welfare. India. National guidelines on second-line ART for HIV-infected adults and adolescents. 2013 [Google Scholar]
- 5.National AIDS Program; Ministry of Health, Myanmar. Guidelines for the clinical management of HIV infection in adults and adolescents in Myanmar. 2011 [Google Scholar]
- 6.National AIDS/STD Program, Directorate General of Health Services, Ministry of Health and Family Welfare, Bangledesh. National guidelines of antiretroviral therapy Bangladesh. 2011 [Google Scholar]
- 7.National Centre for AIDS and STD Control, Ministry of Health and Population, Government of Nepal. National anti-retroviral therapy guidelines. 2012
- 8.National STD/AIDS Control Programme, Ministry of Healthcare, Nutrition and UVA-Wellassa Development, Government of Sri Lanka. A guide to antiretroviral therapy. 2005
- 9.Sungkanuparph S, Techasathit W, Utaipiboon C, Chasombat S, Bhakeecheep S, Leechawengwongs M, et al. Thai national guidelines for antiretroviral therapy in HIV-1 infected adults and adolescents 2010. Asian Biomedicine. 2010;4(4):515–528. [Google Scholar]
- 10.Indonesia. Kementerian Kesehatan RI. Direktorat Jenderal Pengendalian Penyakit dan Penyehatan Lingkungan. Pedoman Nasional Tatalaksana Klinis Infeksi HIV dan Terapi Antiretroviral pada orang Dewasa dan Remaja. 2012 [Google Scholar]
- 11.World Health Organization. World Health Organization AIDS medicines and diagnostic service survey on ARV use, laboratory use and implementation of WHO related guidelines: Maldives. 2011
- 12.World Health Organization. World Health Organization AIDS medicines and diagnostic service survey on ARV use, laboratory use and implementation of WHO related guidelines: Bhutan. 2011
- 13.IV Constitutional Government Ministerio Da Saude, Republica Democratica De Timor-Leste. Opportunistic infections and antiretrovrial therapy guidelines for HIV-infected adults, adolescents, children and infants in Timor-Leste. 2011
- 14.Coffin J. HIV population dynamics in vivo: implications for genetic variation, pathogenesis, and therapy. Science (Wash.) 1995;267(5197):483. doi: 10.1126/science.7824947. [DOI] [PubMed] [Google Scholar]
- 15.World Health Organization. World Health Organization global strategy for the surveillance and monitoring of HIV drug resistance. 2012 http://apps.who.int/iris/bitstream/10665/77349/1/9789241504768_eng.pdf.
- 16.World Health Organization. WHO HIV drug resistance report. 2012 http://apps.who.int/iris/bitstream/10665/75183/1/9789241503938_eng.pdf.
- 17.Gupta RK, Jordan MR, Sultan BJ, Hill A, Davis DH, Gregson J, et al. Global trends in antiretroviral resistance in treatment-naive individuals with HIV after rollout of antiretroviral treatment in resource-limited settings: a global collaborative study and meta-regression analysis. Lancet. 2012 Jul 20;380:1250–1258. doi: 10.1016/S0140-6736(12)61038-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jordan MR. Assessments of HIV drug resistance mutations in resource-limited settings. Clin Infect Dis. 2011 Apr 15;52(8):1058–1060. doi: 10.1093/cid/cir093. [DOI] [PubMed] [Google Scholar]
- 19.Pingen M, Nijhuis M, de Bruijn JA, Boucher CA, Wensing AM. Evolutionary pathways of transmitted drug-resistant HIV-1. J Antimicrob Chemother. 2011 Jul;66(7):1467–1480. doi: 10.1093/jac/dkr157. [DOI] [PubMed] [Google Scholar]
- 20.Bennett DE, Camacho RJ, Otelea D, Kuritzkes DR, Fleury H, Kiuchi M, et al. Drug resistance mutations for surveillance of transmitted HIV-1 drug-resistance: 2009 update. PLoS One. 2009;4(3):e4724. doi: 10.1371/journal.pone.0004724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Duong YT, Qiu M, De AK, Jackson K, Dobbs T, Kim AA, et al. Detection of recent HIV-1 infection using a new limiting-antigen avidity assay: potential for HIV-1 incidence estimates and avidity maturation studies. PLoS One. 2012;7(3):e33328. doi: 10.1371/journal.pone.0033328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Laeyendecker O, Brookmeyer R, Cousins MM, Mullis CE, Konikoff J, Donnell D, et al. HIV incidence determination in the United States: a multiassay approach. J Infect Dis. 2013 Jan 15;207(2):232–239. doi: 10.1093/infdis/jis659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lodi S, Phillips A, Touloumi G, Geskus R, Meyer L, Thiebaut R, et al. Time from human immunodeficiency virus seroconversion to reaching CD4+ cell count thresholds <200, <350, and <500 Cells/mm(3): assessment of need following changes in treatment guidelines. Clin Infect Dis. 2011 Oct;53(8):817–825. doi: 10.1093/cid/cir494. [DOI] [PubMed] [Google Scholar]
- 24.eligibility for ART in lower income countries (eART-linc) collaboration. Wandel S, Egger M, Rangsin R, Nelson KE, Costello C, et al. Duration from seroconversion to eligibility for antiretroviral therapy and from ART eligibility to death in adult HIV-infected patients from low and middle-income countries: collaborative analysis of prospective studies. Sex Transm Infect. 2008 Aug;84(Suppl 1):i31–i36. doi: 10.1136/sti.2008.029793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chasombat S, Kantipong P, Pathipvanich P, Luakamlung N, Malai S, Kohreanudom S, et al. Thailand national surveillance system to determine the development of HIV drug resistance among ARV treated patients. Presented at: XVIII International AIDS Conference; 2010 July 18–23; Vienna, Austria. Abstract THPE0427. [Google Scholar]
- 26.Deshpande A, Jeannot AC, Schrive MH, Wittkop L, Pinson P, Fleury HJ. Analysis of RT sequences of subtype C HIV-type 1 isolates from Indian patients at failure of a first-line treatment according to clinical and/or immunological WHO guidelines. AIDS Res Hum Retroviruses. 2010 Mar;26(3):343–350. doi: 10.1089/aid.2009.0217. [DOI] [PubMed] [Google Scholar]
- 27.Sen S, Tripathy SP, Patil AA, Chimanpure VM, Paranjape RS. High prevalence of human immunodeficiency virus type 1 drug resistance mutations in antiretroviral treatment-experienced patients from Pune, India. AIDS Res Hum Retroviruses. 2007 Oct;23(10):1303–1308. doi: 10.1089/aid.2007.0090. [DOI] [PubMed] [Google Scholar]
- 28.Sen S, Tripathy SP, Chimanpure VM, Patil AA, Bagul RD, Paranjape RS. Human immunodeficiency virus type 1 drug resistance mutations in peripheral blood mononuclear cell proviral DNA among antiretroviral treatment-naive and treatment-experienced patients from Pune, India. AIDS Res Hum Retroviruses. 2007 Apr;23(4):489–497. doi: 10.1089/aid.2006.0221. [DOI] [PubMed] [Google Scholar]
- 29.Sukasem C, Churdboonchart V, Sirisidthi K, Riengrojpitak S, Chasomba S, Watitpun C, et al. Genotypic resistance mutations in treatment-naive and treatment-experienced patients under widespread use of antiretroviral drugs in Thailand: implications for further epidemiologic surveillance. Jpn J Infect Dis. 2007;60(5):284–289. [PubMed] [Google Scholar]
- 30.Iqbal HS, Sunil SS, Saravanan S, Vidya M, Kumarasamy N, Murugavel KG, et al. High-level resistance to certain NNRTIs in association with aminoacid substitutions and K122E-K49R/KR polymorphisms among recently infected HIV patients in India. Presented at: XVIII International AIDS Conference; 2010 July 18–23; Vienna, Austria. Abstract CDB0056. [Google Scholar]
- 31.Truong HM, Lindan C, Kellogg T, Mathur M, Mungekar S, Jerajani H, et al. HIV-1 primary drug resistance and recent infection in a prospective cohort of men attending public STI clinics in Mumbai. Presented at: 18th International HIV Drug Resistance Workshop; 2009 June 9–13; Fort Myers, United States. Abstract 141. [Google Scholar]
- 32.Thorat SR, Chaturbhuj DN, Hingankar NK, Chandrasekhar V, Koppada R, Datkar SR, et al. Surveillance of transmitted HIV type 1 drug resistance among HIV type 1-positive women attending an antenatal clinic in Kakinada, India. AIDS Res Hum Retroviruses. 2011 Dec;27(12):1291–1297. doi: 10.1089/AID.2011.0036. [DOI] [PubMed] [Google Scholar]
- 33.Ayouba A, Lien TTX, Nouhin J, Vergne L, Aghokeng AF, Ngo Giang Huong N, et al. Low prevalence of HIV type 1 drug resistance mutations in untreated, recently infected patients from Burkina Faso, Cote d'Ivoire, Senegal, Thailand, and Vietnam: the ANRS 12134 study. AIDS Res Hum Retroviruses. 2009;25(11):1193–1196. doi: 10.1089/aid.2009.0142. [DOI] [PubMed] [Google Scholar]
- 34.Sirivichayakul S, Phanuphak P, Pankam T, O-Charoen R, Sutherland D, Ruxrungtham K. HIV drug resistance transmission threshold survey in Bangkok, Thailand. Antivir Ther (Lond) 2008;13(Suppl 2):109–113. [PubMed] [Google Scholar]
- 35.Leelawiwat W, McNicholl J, Kongpechsatit O, Mueanpai F, Chonwattana W, Reangsakulrach B, et al. Prevalence of HIV-1 drug resistance in recently HIV-infected men who have sex with men (MSM) in Thailand, 2007–2009: implications for PrEP. Presented at: 6th International AIDS Society Conference on HIV Pathogenesis and Treatment and Prevention; 2011 July 17–20; Rome, Italy. Abstract CDB114. [Google Scholar]
- 36.Chonwattana W, Leelawiwat W, Kittinunvorakoon C, Choopanya K, Vanichseni S, Martin MT, et al. The prevalence of transmitted HIV-1 drug resistance in recently HIV-1 infected injecting drug users in Bangkok, Thailand 2001–2004. Presented at: 4th International AIDS Society Conference on HIV Pathogenesis and Treatment and Prevention; 2007 July 22–25; Sydney, Australia. Abstract TUPEB050. [Google Scholar]
- 37.Sirivichayakul S, O-Charoen R, Ruxrungtham K, Sutherland D, Phanuphak P. Low rate of transmission of drug resistance HIV among recently-infected blood donors in Bangkok, Thailand. Presented at: XVI International AIDS Conference; 2006 August 13–18; Toronto, Canada. Abstract WEPE0289. [Google Scholar]
- 38.Neogi U, Prarthana BS, Gupta S, D'souza G, De Costa A, Kuttiatt VS, et al. Naturally occurring polymorphisms and primary drug resistance profile among antiretroviral-naive individuals in Bangalore, India. AIDS Res Hum Retroviruses. 2010 Oct;26(10):1097–1101. doi: 10.1089/aid.2010.0092. [DOI] [PubMed] [Google Scholar]
- 39.Sirivichayakul S, DeLong A, Wongkunya R, Mekprasan S, Sohn A, Ruxrungtham K, et al. Increasing HIV drug resistance among recently infected treatment-naïve MSM in Thailand: results from three years of annual surveillance. Presented at: 6th International AIDS Society Conference on HIV Pathogenesis and Treatment and Prevention; 2011 July 17–20; Rome, Italy. Abstract MOPE053. [Google Scholar]
- 40.Tovanabutra S, Kijak GH, Razak MH, Wongworapat K, Vongchak T, Jittiwutikarn J, et al. First report on antiretroviral-drug resistance profile among recent HIV-1 seroconverter Thai intravenous drug users. Presented at: XV International AIDS Conference; 2004 July 11–16; Bangkok, Thailand. Abstract WEPEB5721. [Google Scholar]
- 41.Chaturbhuj DN, Hingankar NK, Padmini Srikantiah, Renu Garg, Sandhya Kabra, Deshmukh PS, et al. Transmitted HIV drug resistance among HIV-infected voluntary counseling and testing centers (VCTC) clients in Mumbai, India. AIDS Res Hum Retroviruses. 2010;26(8):927–932. doi: 10.1089/aid.2010.0032. [DOI] [PubMed] [Google Scholar]
- 42.Bertagnolio S, De Luca A, Vitoria M, Essaje S, Penazzato M, Hong SY, et al. Determinants of HIV Drug Resistance and Public Health Implications in Low- and Middle-Income Countries. Antiviral Therapy. 2012 doi: 10.3851/IMP2320. in press. [DOI] [PubMed] [Google Scholar]
- 43.HIV drug resistance [Internet] Available from: http://www.who.int/hiv/topics/drugresistance/en/index.html.
- 44.Deshpande A, Jauvin V, Magnin N, Pinson P, Faure M, Masquelier B, et al. Resistance mutations in subtype C HIV type 1 isolates from Indian patients of Mumbai receiving NRTIs plus NNRTIs and experiencing a treatment failure: resistance to AR. AIDS Res Hum Retroviruses. 2007 Feb;23(2):335–340. doi: 10.1089/aid.2006.0183. [DOI] [PubMed] [Google Scholar]
- 45.Kumarasamy N, Vidya Madhavan, Venkatesh KK, Saravanan S, Kantor R, Balakrishnan P, et al. High frequency of clinically significant mutations after first-line generic highly active antiretroviral therapy failure: implications for second-line options in resource-limited settings. Clin Infect Dis. 2009;49(2):306–309. doi: 10.1086/600044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sungkanuparph S, Manosuthi W, Kiertiburanakul S, Chantratitra W. Tenofovir resistance among HIV-infected patients failing a fixed-dose combination of stavudine, lamivudine, and nevirapine in a resource-limited setting. AIDS Patient Care & STDs. 2007 Oct;21(10):711–714. doi: 10.1089/apc.2007.0011. [DOI] [PubMed] [Google Scholar]
- 47.Wainberg MA, Zaharatos GJ, Brenner BG. Development of antiretroviral drug resistance. N Engl J Med. 2011 Aug 18;365(7):637–646. doi: 10.1056/NEJMra1004180. [DOI] [PubMed] [Google Scholar]
- 48.World Health Organization. Antiretroviral therapy for HIV infection in adults and adolescents: Recommendations for a public health approach. 2010 [PubMed]
- 49.Bhrushundi M, Mukhopadhyaya PN, Pradhan R, Acharya A, Chavan Y, Mishra S, et al. Pattern of drug resistance mutations in patients failing first line therapy in HIV-1 infected individuals in resource limited settings. Presented at: 6th International AIDS Society Conference on HIV Pathogenesis and Treatment and Prevention; 2011 July 17–20; Rome, Italy. Abstract CDB381. [Google Scholar]
- 50.Gupta A, Saple DG, Nadkarni G, Shah B, Satish Vaidya, Nitin Hingankar, et al. One-, two-, and three-class resistance among HIV-infected patients on antiretroviral therapy in private care clinics: Mumbai, India. AIDS Res Hum Retroviruses. 2010;26(1):25–31. doi: 10.1089/aid.2009.0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sungkanuparph S, Apiwattanakul N, Thititanyanont A, Chantratita W, Sirinavin S. HIV-1 Drug Resistance Mutations in Children Failing an Initial NRTI-based Regimen and Options for a Second-line Antiretroviral Regimen. Presented at: 15th Conference of Retroviruses and Opportunistic Infections; 2008 February 3–6; Boston, United States. Abstract 588. [Google Scholar]
- 52.Ekstrand ML, Shet A, Chandy S, Singh G, Shamsundar R, Madhavan V, et al. Suboptimal adherence associated with virological failure and resistance mutations to first-line highly active antiretroviral therapy (HAART) in Bangalore, India. Int Health. 2011 Mar 1;3(1):27–34. doi: 10.1016/j.inhe.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shet A, DeCosta A, Heylen E, Shastri S, Chandy S, Ekstrand M. High rates of adherence and treatment success in a public and public-private HIV clinic in India: potential benefits of standardized national care delivery systems. BMC Health Serv Res. 2011 Oct 17;11:277. doi: 10.1186/1472-6963-11-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vallabhaneni S, Chandy S, Heylen E, Ekstrand M. Reasons for and correlates of antiretroviral treatment interruptions in a cohort of patients from public and private clinics in southern India. AIDS Care. 2012;24(6):687–694. doi: 10.1080/09540121.2011.630370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bangsberg DR, Acosta EP, Gupta R, Guzman D, Riley ED, Harrigan PR, et al. Adherence-resistance relationships for protease and non-nucleoside reverse transcriptase inhibitors explained by virological fitness. AIDS. 2006 Jan 9;20(2):223–231. doi: 10.1097/01.aids.0000199825.34241.49. [DOI] [PubMed] [Google Scholar]
- 56.Harrigan PR, Hogg RS, Dong WW, Yip B, Wynhoven B, Woodward J, et al. Predictors of HIV drug-resistance mutations in a large antiretroviral-naive cohort initiating triple antiretroviral therapy. J Infect Dis. 2005 Feb 1;191(3):339–347. doi: 10.1086/427192. [DOI] [PubMed] [Google Scholar]
- 57.Ramchandani SR, Mehta SH, Saple DG, Vaidya SB, Pandey VP, Vadrevu R, et al. Knowledge, attitudes, and practices of antiretroviral therapy among HIV-infected adults attending private and public clinics in India. AIDS Patient Care STDs. 2007 Feb;21(2):129–142. doi: 10.1089/apc.2006.0045. [DOI] [PubMed] [Google Scholar]
- 58.Ekstrand ML, Chandy S, Gandhi M, Srikanth N, Balakrishnan P, Singh G, et al. Adherence, virologic failure and major mutations - need for 2nd line HAART in India. Presented at: XVII International AIDS Conference; 2008 August 3–8; Mexico City, Mexico; Abstract WEPE0040. [Google Scholar]
- 59.Auwanit W, Isarangkura-Na-Ayuthaya P, Kasornpikul D, Ikuta K, Sawanpanyalert P, Kameoka M. Detection of drug resistance-associated and background mutations in human immunodeficiency virus type 1 CRF01_AE protease and reverse transcriptase derived from drug treatment-naive patients residing in central Thailand. AIDS Res Hum Retroviruses. 2009 Jun;25(6):625–631. doi: 10.1089/aid.2008.0298. [DOI] [PubMed] [Google Scholar]
- 60.Sinha S, Ahmad H, Kumar H, Dar L, Samantaray JC, Bhargava A, et al. Study of prevalence and types of baseline HIV drug resistance in the antiretroviral treatment-naive HIV infected Northern India population. Presented at: XVIII International AIDS Conference; 2010 July 18–23; Vienna, Austria; Abstract THPE0360. [Google Scholar]
- 61.Apisarnthanarak A, Mundy LM. Antiretroviral drug resistance among antiretroviral-naive individuals with HIV infection of unknown duration in Thailand. Clin Infect Dis. 2008;46(10):1630–1631. doi: 10.1086/587753. [DOI] [PubMed] [Google Scholar]
- 62.Iqbal HS, Solomon SS, Vidya Madhavan, Suniti Solomon, Balakrishnan P. Primary HIV-1 drug resistance and polymorphic patterns among injecting drug users (IDUs) in Chennai, Southern India. J Int Assoc Physicians AIDS Care. 2009;8(5):323–327. doi: 10.1177/1545109709341854. [DOI] [PubMed] [Google Scholar]
- 63.Lall M, Gupta RM, Sen S, Kapila K, Tripathy SP, Paranjape RS. Profile of primary resistance in HIV-1-infected treatment-naive individuals from Western India. AIDS Res Hum Retroviruses. 2008 Jul;24(7):987–990. doi: 10.1089/aid.2008.0079. [DOI] [PubMed] [Google Scholar]
- 64.Nuntachit N, Chaiwarith R, Praparattanapan J, Supparatpinyo K, Sirisanthana T. Prevalence of primary HIV drug resistance in Chiang Mai University Hospital Cohort. Presented at: 6th International AIDS Society Conference on HIV Pathogenesis and Treatment and Prevention; 2011 July 17–20; Rome, Italy; Abstract CDB008. [Google Scholar]
- 65.Potdar P, Daswani B, Rane N. Drug Resistance Mutations to Protease and Reverse Transcriptase Inhibitors in Treatment Naive HIV-1 Clade C Infected Individuals from Mumbai, India. Int J of Virology. 2011;7(1):13. [Google Scholar]
- 66.Shet A, Neogi U, Arumugam K, Sahoo PN, Rajagopalan N, Krishnamurthy S, et al. Genetic variation and primary drug resistance mutations among children with perinatally-acquired HIV infection. Presented at: 6th International AIDS Society Conference on HIV Pathogenesis and Treatment and Prevention; 2011 July 17–20; Rome, Italy. Abstract TUPE094. [Google Scholar]
- 67.Kandathil AJ, Kannangai R, Abraham OC, Rupali P, Pulimood SA, Verghese VP, et al. The frequency of HIV-I drug resistance mutations among treatment-naive individuals at a tertiary care centre in south India. Int J STD AIDS. 2009;20(8):522–526. doi: 10.1258/ijsa.2008.008403. [DOI] [PubMed] [Google Scholar]
- 68.Balakrishnan P, Kumarasamy N, Kantor R, Solomon S, Vidya S, Mayer KH, et al. HIV type 1 genotypic variation in an antiretroviral treatment-naive population in southern India. AIDS Res Hum Retroviruses. 2005 Apr;21(4):301–305. doi: 10.1089/aid.2005.21.301. [DOI] [PubMed] [Google Scholar]
- 69.Deshpande A, Recordon-Pinson P, Deshmukh R, Faure M, Jauvin V, Garrigue I, et al. Molecular characterization of HIV type 1 isolates from untreated patients of Mumbai (Bombay), India, and detection of rare resistance mutations. AIDS Res Hum Retroviruses. 2004 Sep;20(9):1032–1035. doi: 10.1089/aid.2004.20.1032. [DOI] [PubMed] [Google Scholar]
- 70.Rajesh L, Karunaianantham R, Narayanan PR, Swaminathan S. Antiretroviral drug-resistant mutations at baseline and at time of failure of antiretroviral therapy in HIV type 1-coinfected TB patients. AIDS Res Hum Retroviruses. Nov;200925(11):1179–1185. doi: 10.1089/aid.2009.0110. [DOI] [PubMed] [Google Scholar]
- 71.Soundararajan L, Karunaianandham R, Jauvin V, Schrive MH, Ramachandran R, Narayanan PR, et al. Characterization of HIV-1 isolates from antiretroviral drug-naive children in southern India. AIDS Res Hum Retroviruses. 2007;23(9):1119–1126. doi: 10.1089/aid.2007.0012. [DOI] [PubMed] [Google Scholar]
- 72.Dettrairat S, Promchim C, Kunachiwa W, Kohreanudom S. HIV drug resistance testing of national access to antiretroviral program in upper Northern Thailand. Presented at: XVI International AIDS Conference; 2006 August 13–18; Toronto, Canada. Abstract CDB0188. [Google Scholar]
- 73.Sungkanuparph S, Apiwattanakul N, Thitithanyanont A, Chantratita W, Sirinavin S. HIV-1 drug resistance mutations in children who failed non-nucleoside reverse transcriptase inhibitor-based antiretroviral therapy. Southeast Asian Journal of Tropical Medicine & Public Health. 2009 Jan;40(1):83–88. [PubMed] [Google Scholar]
- 74.Apisarnthanarak A, Jirayasethpong T, Sa-nguansilp C, Thongprapai H, Kittihanukul C, Kamudamas A, et al. Antiretroviral drug resistance among antiretroviral-naive persons with recent HIV infection in Thailand. HIV Med. 2008 May;9(5):322–325. doi: 10.1111/j.1468-1293.2008.00562.x. [DOI] [PubMed] [Google Scholar]
- 75.Karnkawinpong O, Akksilp S, Korianudom S. Viral load, genotypic drug resistance assay in AIDS patients treated with d4T+3TC +NVP regimen: from routine to research. Presented at: XVI International AIDS Conference; 2006 August 13–18; Toronto, Canada. Abstract MOPE0306. [Google Scholar]
- 76.Puthanakit T, Kerr S, Bunupuradah T, Aurpibul L, Taecharoenkul S, Sirisanthana V, et al. Slow accumulation of thymidine analog and ETR-associated mutations in NNRTI-treated children with virologic failure. Presented at: 18th Conference of Retroviruses and Opportunistic Infections; 2011 27 Febuary–2 March; Boston, United States. Abstract A720. [Google Scholar]
- 77.Solomon S, Balakrishnan P, Shetty N, Cecelia A, Madhavan V, Ganesh A, et al. Prevalence of treatment failure and drug resistance among treatment-experienced HIV-1-infected individuals at a tertiary HIV referral center in south India. Presented at: 14th Conference of Retroviruses and Opportunistic Infections; 2007 February 25–28; Los Angeles, United States. Abstract 647. [Google Scholar]
- 78.Vidya M, Saravanan S, Uma S, Kumarasamy N, Sunil SS, Kantor R, et al. Genotypic HIV type-1 drug resistance among patients with immunological failure to first-line antiretroviral therapy in south India. Antivir Ther (Lond) 2009;14(7):1005–1009. doi: 10.3851/IMP1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Arora SK, Sachin Gupta, Toor JS, Anuj Singla. Drug resistance-associated genotypic alterations in the pol gene of HIV type 1 isolates in ART-naive individuals in North India. AIDS Res Hum Retroviruses. 2008;24(2):125–130. doi: 10.1089/aid.2007.0156. [DOI] [PubMed] [Google Scholar]
- 80.Saini S, Bhalla P, Baveja UK, Pasha ST, Dewan R. Antiretroviral resistance-associated mutations and genomic diversity in reverse transcriptase and protease gene among drug-naive patients. Presented at: 14th International Congress of Infectious Disease; 2010 March 9–12; Miami, United States. [Google Scholar]