Abstract
IMPORTANCE
Obsessive-compulsive disorder (OCD) is one of the world’s most disabling illnesses according to the World Health Organization. Serotonin reuptake inhibitors (SRIs) are the only medications approved by the Food and Drug Administration to treat OCD, but few patients achieve minimal symptoms from an SRI alone. In such cases, practice guidelines recommend adding antipsychotics or cognitive-behavioral therapy consisting of exposure and ritual prevention (EX/RP).
OBJECTIVE
To compare the effects of these 2 SRI augmentation strategies vs pill placebo for the first time, to our knowledge, in adults with OCD.
DESIGN, SETTING, AND PARTICIPANTS
A randomized clinical trial (conducted January 2007–August 2012) at 2 academic outpatient research clinics that specialize in OCD and anxiety disorders. Patients (aged 18–70 years) were eligible if they had OCD of at least moderate severity despite a therapeutic SRI dose for at least 12 weeks prior to entry. Of 163 who were eligible, 100 were randomized (risperidone, n = 40; EX/RP, n = 40; and placebo, n = 20), and 86 completed the trial.
INTERVENTIONS
While continuing their SRI at the same dose, patients were randomized to the addition of 8 weeks of risperidone (up to 4 mg/d), EX/RP (17 sessions delivered twice weekly), or pill placebo. Independent assessments were conducted every 4 weeks.
MAIN OUTCOME AND MEASURE
The Yale-Brown Obsessive Compulsive Scale (Y-BOCS) to measure OCD severity.
RESULTS
Patients randomized to EX/RP had significantly greater reduction in week 8 Y-BOCS scores based on mixed-effects models (vs risperidone: mean [SE], −9.72 [1.38]; P<.001 vs placebo: mean [SE], −10.10 [1.68]; P < .001). Patients receiving risperidone did not significantly differ from those receiving placebo (mean [SE], −0.38 [1.72]; P=.83). More patients receiving EX/RP responded (Y-BOCS score decrease ≥25%: 80% for EX/RP, 23% for risperidone, and 15% for placebo; P < .001). More patients receiving EX/RP achieved minimal symptoms (Y-BOCS score ≤12: 43% for EX/RP, 13% for risperidone, and 5% for placebo; P = .001). Adding EX/RP was also superior to risperidone and placebo in improving insight, functioning, and quality of life.
CONCLUSIONS AND RELEVANCE
Adding EX/RP to SRIs was superior to both risperidone and pill placebo. Patients with OCD receiving SRIs who continue to have clinically significant symptoms should be offered EX/RP before antipsychotics given its superior efficacy and less negative adverse effect profile.
TRIAL REGISTRATION
clinicaltrials.gov Identifier: NCT00389493.
Serotonin reuptake inhibitors (SRIs) (ie, clomipramine hydrochloride and selective SRIs) are the only medications approved by the Food and Drug Administration to treat obsessive-compulsive disorder (OCD). Although many patients respond, few achieve minimal symptoms from an SRI alone.2 In those with some SRI response, practice guidelines1 recommend adding either antipsychotics or cognitive-behavioral therapy. This article describes the first study, to our knowledge, to compare these 2 strategies.
Adding antipsychotics (eg, haloperidol, risperidone, olanzapine, quetiapine fumarate, or aripiprazole) has improved SRI response in patients with OCD in some randomized clinical trials. Meta-analyses3,4 estimate that about one-third of patients with OCD receiving SRIs will respond. Among second-generation antipsychotics, risperidone appears to have the strongest effects. However, these effects are based on 3 small studies.5–7
Adding cognitive-behavioral therapy consisting of exposure and ritual prevention (EX/RP) has also improved SRI response in 2 randomized clinical trials. Tenneij et al8 randomized patients with OCD who had responded to 3 months of paroxetine hydrochloride or venlafaxine hydrochloride to 6 months of continued medication alone or augmented with EX/ RP. We randomized patients with OCD receiving SRIs to 8 weeks of augmentation with EX/RP or stress management therapy.9 In both studies, patients who received EX/RP were significantly more likely to benefit than the comparison group.
Given the importance of SRI augmentation strategies for treatment of OCD, more data are needed on the effects of risperidone and EX/RP and their relative efficacy. Thus, we conducted a randomized clinical trial comparing risperidone, EX/ RP, and pill placebo augmentation of SRIs in 100 adults with OCD. We recruited patients with at least moderate OCD severity despite an adequate SRI dose, allowed comorbid depressive and anxiety disorders as long as OCD was the principal diagnosis, and used a twice-weekly EX/RP format with proven efficacy.9,10 Our risperidone protocol was similar to that of McDougle et al,6 although our starting dose was lower and dose escalation slower to minimize adverse effects. Based on prior studies, we hypothesized that risperidone and EX/RP would each reduce OCD symptoms more than placebo and that risperidone and EX/RP would not differ in efficacy.
Methods
Setting
This randomized clinical study was conducted at 2 academic outpatient clinics in New York City, New York, and Philadelphia, Pennsylvania. Subjects were recruited by clinical referral and advertisements, and data were collected (January 2007–August 2012). Each site’s institutional review board approved the study. Patients provided written informed consent.
Participants
Eligible patients were adults (aged 18–70 years) with a principal diagnosis of OCD (≥1 year), who were receiving an SRI at a stable dose (≥12 weeks), and who remained at least moderately symptomatic (Yale-Brown Obsessive Compulsive Scale11,12 [Y-BOCS] score ≥16). Consistent with the literature at the time this study was initiated,1,9 an optimal SRI dose was defined as the following: clomipramine hydrochloride, at least 225 mg/d; fluoxetine, at least 60 mg/d; paroxetine hydrochloride, at least 60 mg/d; sertraline hydrochloride, at least 200 mg/d; fluvoxamine, at least 250 mg/d; citalopram hydrobromide, at least 60 mg/d; and escitalopram oxalate, at least 30 mg/d. However, patients unable to tolerate doses this high because of adverse effects were also eligible if at their maximally tolerated dose. At least 12 weeks at this dose was required so that patients had likely experienced maximal SRI benefit.1 Except for antipsychotics, concomitant psychotropics were permitted if the dose was stable (≥4 weeks), remained stable during the study, and was not contraindicated with risperidone.
Patients were excluded for bipolar and psychotic disorders, substance abuse or dependence in the past 3 months, prominent suicidal ideation, a17-item Hamilton Depression Scale(HAM-D)13 score indicating severe depression (>25), or hoarding as the only OCD symptom. Other Axis I diagnoses were permitted if OCD was the most severe and impairing. Patients were excluded if they had an unstable medical or neurological condition that increased the risk of participation (eg, dementia), were pregnant or nursing, or had previously received risperidone (≥0.5 mg/d for 8 weeks) or EX/RP (≥8 sessions within 2 months) while receiving an SRI as described earlier. Patients were also excluded if receiving their first SRI with no response because practice guidelines1 suggest switching to another SRI first.
Eligibility was determined by trained clinicians with expertise in OCD and related disorders. Psychiatric diagnoses were confirmed by trained raters prior to study entry using the Structured Clinical Interview for DSM-IV Axis I Disorders–Patient Edition.14 Treatment history was confirmed with the referring clinician and/or medical record review.
Randomization
Patients were randomized to risperidone, EX/RP, or pill placebo(allocated 2:2:1) using a computer-generated stratified block randomization procedure that balanced the treatments for every 5 entrants at each site.15 Subjects were told their randomization (pill or therapy) by the study coordinator at week 0.
Treatments
Maintenance SRI
Patients entered receiving a stable SRI dose and the dose was maintained by their study psychiatrist. For those receiving EX/ RP augmentation, psychiatrist visits were every 4 weeks (weeks 0, 4, and 8). For those receiving pill augmentation (either risperidone or pill placebo), psychiatrist visits were weekly for the first 4 weeks and then every other week (weeks 0, 1, 2, 3, 4, 6, and 8). The first visit was 60 minutes; others were 30 minutes. Psychiatrists conducted standard medication management.
EX/RP Augmentation
Patients randomized to EX/RP received 17 twice-weekly 90-minute sessions delivered over 8 weeks by a study therapist. Treatment included 2 introductory sessions, 15 exposure sessions (during which patients faced their obsessional fears for a prolonged period without ritualizing), daily homework (at least 1 hour of self-directed exposures daily), and between-session telephone checkins.16 At least 2 sessions occurred outside the clinic to promote generalization to daily life. The goal was for patients to stop their rituals as early in treatment as possible; patients were asked to try refraining from ritualizing after the first exposure session. Formal cognitive therapy procedures were not used, but dysfunctional cognitions were discussed within the context of exposure.
Pill Augmentation
Patients randomized to pill augmentation met with their study psychiatrist as noted earlier. The risperidone and placebo capsules appeared identical and the dosing schedule was identical (first week: 0.25 mg/d for 3 days and then 0.5 mg/d; subsequent weeks: increases of 0.5 mg per week to a maximum of 4.0 mg/d if clinically indicated). Dose increases could be withheld for adverse events or marked improvement. Although our starting dose was lower and the dose escalation slower than McDougle et al,6 our study was longer (8 weeks, not 6 weeks), and our maximum dose (4.0 mg/d) was the maximum dose received by patients in the McDougle et al study.
Assessments
Independent evaluators, blind to treatment, evaluated patients at baseline (week 0), midway through treatment (week 4), and posttreatment (week 8). They used the Y-BOCS11,12 to assess OCD severity, the 17-item HAM-D13 for depressive severity, and the Brown Assessment of Beliefs Scale (BABS)17 to assess the degree of insight/delusionality about the main OCD belief. Patients also completed the Quality of Life Enjoyment and Satisfaction Questionnaire–Short Form(QLESQ-SF)18 and the Social Adjustment Scale–Self-report19 (SAS-SR). Study psychiatrists assessed adverse effects at each visit (using a modified version of the Systemic Assessment for Treatment Emergent Events that included 26 items, each of which were rated as absent, mild, moderate, or severe).20 Vital signs and weight were measured at each visit; height was measured at baseline and used to calculate the body mass index (calculated as weight in kilograms divided by height in meters squared). Psychiatrists assessed for tics at baseline using the Yale Global Tic Severity Scale21 and for extra pyramidal symptoms using the Simpson-Angus Scale22 and Barnes Akathisia Scale23 at each visit and the Abnormal Involuntary Movement Scale24,25 at weeks 0 and 8.
Protocol Adherence
Training and supervision of study therapists, psychiatrists, and independent evaluators are described in the eAppendix in the Supplement; data there document their protocol adherence. Serotonin reuptake inhibitor adherence was confirmed by patient report and SRI blood levels26–28 at weeks 0 and 8 (or dropout). Blood levels of risperidone and its active metabolite (9-hydroxy risperidone) were measured (13–15 hours) after the last risperidone/placebo dose at week 8 (or dropout) using high-performance liquid chromatography and mass spectroscopy.
Statistical Methods
The Y-BOCS total score was the primary outcome. Mixed-effects linear regression was used to model each continuous outcome (using SuperMix software [http://www.ssicentral.com/supermix/index.html]) as a function of 2 time dummy variables (t4 = 1 for week 4 and 0 for week 0 or week 8; t8 = 1 for week 8 and 0 for week 0 or week 4), 2 group dummy variables (EXRP = 1 for EX/RP and 0 for risperidone or placebo; RIS = 1 for risperidone and 0 for EX/RP or placebo), and 4 time × group interactions (EXRP × t4, EXRP × t8, RIS × t4, and RIS × t8). We examined other models and chose this one based on the lowest Akaike information criterion. The model accounts for clustering of the 3 repeated measures (baseline, week 4, and week 8) within each individual29 and allows use of data from patients with missing observations by using maximum likelihood estimation to provide valid inferences in the presence of ignorable nonresponse.30 Pairwise contrasts examined the magnitude and significance of effect sizes for each 2-group comparison at weeks 4 and 8. Site was initially included but then dropped for lack of significant main effect or interactions. Proportions of patients responding(Y-BOCS score decrease ≥ 25%2) and achieving minimal symptoms (Y-BOCS score ≤122) were compared across groups using the Pearson χ2 (χ2) test of association. All tests were 2-sided with a significance level of α = .05.
In addition, we used analysis of variance or χ2 tests to examine whether the treatment groups differed at baseline with respect to demographic or clinical characteristics. Further, we did sensitivity analyses to assess whether baseline differences could explain, at least in part, group differences in Y-BOCS outcome. Specifically, we added each of the following terms separately to our final regression model (baseline Y-BOCS scores, proportion with current OCD only, proportion with current comorbid depressive disorder, proportion with current comorbid other anxiety disorder, proportion receiving SRIs only, dose of clomipramine or paroxetine, proportion with prior antipsychotic exposure, and proportion with prior exposure sessions) and all possible 2-way and 3-way interactions with time, group, and time × group. We looked for a significant 3-way interaction (P < .05) as evidence that the group differences in week 8 Y-BOCS score could be explained in part by baseline differences in these clinical variables.31
Results
Sample
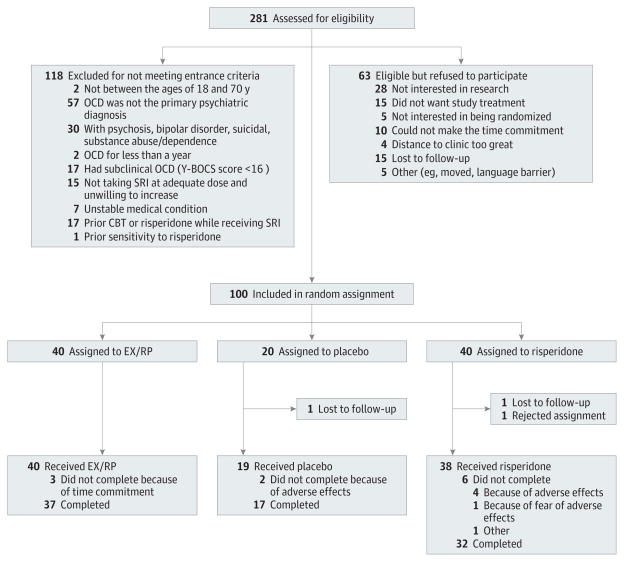
As shown in Figure 1, 281 patients were assessed for eligibility, 163 were eligible, 100 were randomized (risperidone, n = 40; EX/RP, n = 40; and placebo, n = 20), and 86 completed the trial. Dropout did not significantly differ by treatment group (risperidone: 8 of 40 [20%]; EX/RP: 3 of 40 [7.5%]; placebo: 3 of 20 [15%]; ; P = .27).
Figure 1. Consolidated Standards of Reporting Trials Diagram.
Flow of patients through the study. CBT indicates cognitive-behavioral therapy; EX/RP, cognitive-behavioral therapy consisting of exposure and ritual prevention; OCD, obsessive-compulsive disorder; SRI, serotonin reuptake inhibitor; and Y-BOCS, Yale-Brown Obsessive Compulsive Scale.
Demographics and clinical characteristics are presented in Table 1. The only one that differed significantly by group was the proportion with current OCD only ( ; P = .04). At randomization, most patients had received a stable SRI dose for longer than the required 12-week minimum (≥16 weeks, 77%; ≥24 weeks, 48%). All but 2 reported at least minimal improvement while receiving the SRI, which is why they continued receiving the SRI despite clinically significant residual OCD symptoms.
Table 1.
Demographic and Clinical Characteristics of the Sample
| Characteristic | Risperidone (n = 40) | EX/RP (n = 40) | Pill Placebo (n = 20) | All (n = 100) |
|---|---|---|---|---|
| Age, y, mean (SD) | 33.8 (10.8) | 34.3 (12.7) | 33.4 (10.4) | 33.9 (11.4) |
| Age at OCD onset, y, mean (SD)a | 17.3 (9.0) | 17.7 (9.3) | 18.3 (7.7) | 17.7 (8.8) |
| Duration of OCD, y, mean (SD)a | 16.2 (11.4) | 16.2 (11.1) | 15.8 (9.9) | 16.1 (10.9) |
| Education, y, mean (SD) | 15.9 (2.1) | 15.4 (3.1) | 15.7 (1.9) | 15.63 (2.5) |
| Female, No. (%) | 21 (52.5) | 21 (52.5) | 6 (30.0) | 48 (48.0) |
| Non-Hispanic white, No. (%) | 35 (87.5) | 36 (90.0) | 19 (95.0) | 90 (90.0) |
| Marital status, No. (%) | ||||
| Single | 27 (67.5) | 25 (62.5) | 16 (80.0) | 68 (68.0) |
| Married/partnered | 10 (25.0) | 12 (30.0) | 4 (20.0) | 26 (26.0) |
| Divorced/separated | 3 (7.5) | 3 (7.5) | 0 | 6 (6.0) |
| Employed, No. (%)b | 30 (75.0) | 29 (72.5) | 15 (75.0) | 74 (74.0) |
| Current Axis I diagnoses, No. (%) | ||||
| OCD only | 17 (42.5) | 27 (67.5) | 8 (40.0) | 52 (52.0) |
| Depressive disorderc | 15 (37.5) | 9 (22.5) | 7 (35.0) | 31 (31.0) |
| Other anxiety disorderd | 16 (40.0) | 9 (22.5) | 9 (45.0) | 34 (34.0) |
| Othere | 0 | 1 (2.5) | 2 (10.0) | 3 (3.0) |
| Lifetime Axis I diagnoses, No. (%) | ||||
| OCD only | 11 (27.5) | 11 (27.5) | 3 (15) | 25 (25.0) |
| Depressive disorderc | 22 (55.0) | 22 (55.0) | 15 (75.0) | 59 (59.0) |
| Other anxiety disorderd | 11 (27.5) | 8 (20.0) | 6 (30.0) | 25 (25.0) |
| Otherf | 10 (25.0) | 10 (25.0) | 7 (35.0) | 27 (27.0) |
| Chronic tic disorder, No. (%) | 1 (0.03) | 2 (0.05) | 2 (0.10) | 5 (5.0) |
| Current SRI dose, mg/d, mean (SD) | ||||
| Citalopram hydrobromide (n = 9) | 35.0 (19.2) | 40.0 (…) | 50.0 (14.1) | 40.0 (14.1) |
| Clomipramine hydrochloride (n = 6) | 133.3 (28.9) | 225.0 (…) | 187.5 (88.4) | 166.7 (58.5) |
| Escitalopram oxalate (n = 11) | 34.0 (27.0) | 24.0 (11.4) | 40.0 (…) | 30.0 (19.5) |
| Fluoxetine (n = 28) | 66.4 (18.0) | 64.0 (8.4) | 57.1 (18.0) | 63.2 (15.2) |
| Fluvoxamine (n = 17) | 271.1 (75.6) | 222.2 (66.7) | 300.0 (…) | 247.1 (71.7) |
| Paroxetine hydrochloride (n = 10) | 34.0 (24.1) | 70.0 (11.5) | 10.0 (…) | 46.0 (28.0) |
| Sertraline hydrochloride (n = 19) | 170.0 (67.1) | 184.4 (64.0) | 125.0 (61.2) | 161.8 (65.8) |
| Weeks receiving SRI dose, mean (SD) | 50.4 (88.1) | 65.7 (101.6) | 98.9 (124.9) | 66.2 (102.2) |
| Participants receiving first SRI, No. (%) | 9 (23.0) | 7 (18.0) | 4 (20.0) | 20 (20.0) |
| Current adjunctive psychiatric medication, No. (%) | ||||
| SRI only | 27 (67.5) | 20 (50.0) | 10 (50.0) | 57 (57.0) |
| Benzodiazepines | 10 (25.0) | 14 (35.0) | 6 (30.0) | 30 (30.0) |
| Mood stabilizers | 1 (2.5) | 2 (5.0) | 2 (10.0) | 5 (5.0) |
| Stimulants | 1 (2.5) | 3 (7.5) | 0 | 4 (4.0) |
| Otherg | 5 (12.5) | 3 (7.5) | 4 (20.0) | 12 (12.0) |
| History of any prior EX/RP sessions, No. (%) | 4 (10.0) | 2 (5.0) | 1 (5.0) | 7 (7.0) |
| History of prior EX/RP sessions while receiving SRI | 3 (7.5) | 0 | 1 (5.0) | 4 (4.0) |
| History of any prior antipsychotic exposure, No. (%)h | 8 (20.0) | 11 (28.0) | 5 (25.0) | 24 (24.0) |
| Any antipsychotic exposure for >1 wk | 6 (15.0) | 8 (20.0) | 3 (15.0) | 17 (17.0) |
Abbreviations: ellipses, no SD is provided in those cells where n = 1; EX/RP, exposure and ritual prevention; OCD, obsessive-compulsive disorder; SRI, serotonin reuptake inhibitor.
Age at onset of OCD unknown for 5 subjects.
Working at least part-time.
Major depressive disorder, dysthymia, and major depressive disorder not otherwise specified.
Generalized anxiety disorder, panic disorder, agoraphobia, and social and specific phobias.
Binge eating, bulimia, and bipolar II disorder.
For example, anorexia, pathological gambling, substance or alcohol abuse/dependence, or substance-induced mood disorder.
For example, bupropion, buspirone, or zolpidem.
At least 1 dose.
Treatment Adherence
Patients reported that they adhered to their SRI regimen, and SRI blood levels showed little change between weeks 0 and 8 in those with both values (intraclass correlation = 0.95; 95% CI, 0.92 to 0.97). For those receiving risperidone, the week 6 and week 8 mean (SD) dose was 1.8 (0.8) mg/d (median, 2.0 mg/d) and 1.9 (1.1) mg/d (median, 2.0 mg/d), respectively; the mean (SD) maximum dose was 2.2 (0.9) mg/d. For those receiving placebo, the week 6 and week 8 mean (SD) dose equivalent was 2.0 (0.8) mg/d (median, 2.0 mg/d) and 2.2 (1.0) mg/d (median, 2.0 mg/d); the mean (SD) maximum dose was 2.3 (0.9) mg/d. All receiving risperidone had blood levels of risperidone and/or its active metabolite at week 8 (or dropout). Based on the Patient EX/RP Adherence Scale,32 which rates patient adherence to EX/RP procedures on a scale from 1 (no adherence) to 7 (excellent adherence), those receiving EX/RP exhibited good between-session adherence and very good within-session adherence (mean [SD] Patient EX/RP Adherence Scale score: between-session = 5.4 [0.9]; within-session = 6.3 [0.7]).
Efficacy of Augmentation
Primary Outcome
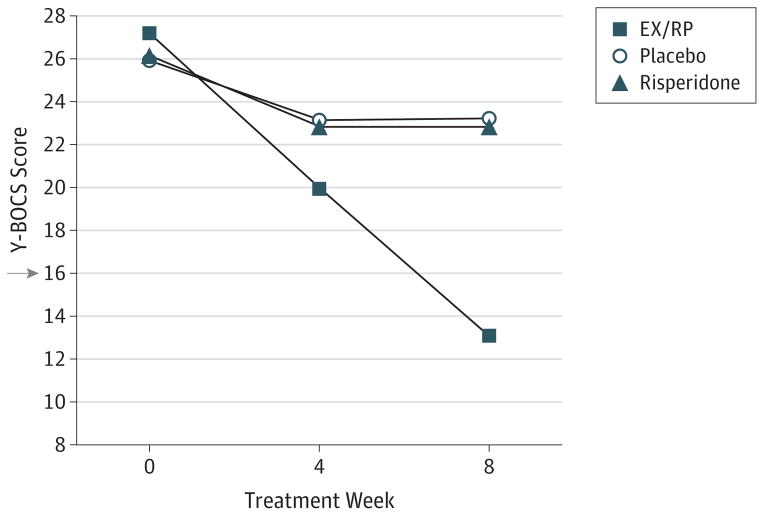
Observed outcomes are presented in Table 2. As Figure 2 illustrates, patients receiving EX/RP had significantly greater reduction in week 8 Y-BOCS scores than those receiving risperidone or placebo based on mixed-effects models (vs risperidone: mean [SE], −9.72 [1.38]; 95% CI, −7.00 to −12.43; z = −7.02; P < .001; vs placebo: mean [SE], −10.10 [1.68]; 95% CI, −6.80 to −13.39; z = −5.99; P < .001). Patients randomized to risperidone did not significantly differ from those randomized to placebo (mean [SE], −0.38 [1.72]; 95% CI, 2.99 to −3.75; z = −0.22; P = .83). Sensitivity analyses indicated that these group differences in outcome could not be attributed to differences in baseline clinical characteristics (ie, baseline Y-BOCS scores, proportion with current OCD only, proportion with current comorbid depressive disorder, proportion with current comorbid other anxiety disorder, proportion receiving SRIs only, dose of clomipramine or paroxetine, proportion with prior antipsychotic exposure, and proportion with prior exposure sessions).
Table 2.
Observed Outcomesa
| Risperidone
|
EX/RP
|
Pill Placebo
|
||||
|---|---|---|---|---|---|---|
| Week 0 (n = 40) | Week 8 (n = 32) | Week 0 (n = 40) | Week 8 (n = 37) | Week 0 (n = 20) | Week 8 (n = 17) | |
| Primary outcome, mean (SD) | ||||||
|
| ||||||
| Y-BOCS | 26.1 (4.3) | 22.6 (8.8) | 27.2 (3.9) | 13.0 (6.1) | 25.9 (4.6) | 23.1 (6.9) |
|
| ||||||
| Secondary outcome, mean (SD) | ||||||
|
| ||||||
| SAS-SR | 2.3 (0.4) | 2.2 (0.4) | 2.3 (0.5) | 1.9 (0.4)b | 2.2 (0.7) | 2.1 (0.5) |
|
| ||||||
| QLESQ-SF | 52.3 (14.1) | 55.1 (13.9) | 57.8 (16.2) | 70.2 (14.2) | 56.1 (16.2) | 62.6 (16.7) |
|
| ||||||
| HAM-D | 9.8 (5.6)c | 8.0 (5.7) | 7.8 (6.1) | 4.7 (4.0) | 7.7 (5.9) | 7.8 (5.9) |
|
| ||||||
| BABS | 5.7 (4.0) | 4.5 (4.2) | 6.1 (4.6) | 2.4 (2.9) | 5.3 (3.8) | 4.3 (3.3) |
Abbreviations: BABS, Brown Assessment of Beliefs; EX/RP, exposure and ritual prevention; HAM-D, Hamilton Depression Rating Scale; QLESQ-SF, Quality of Life Enjoyment and Satisfaction Questionnaire–Short Form; SAS-SR, Social Adjustment Scale–Self-report; Y-BOCS, Yale-Brown Obsessive Compulsive Scale.
At week 0, there were no significant group differences in any of these measures.
n = 36.
n = 39.
Figure 2. Change in Symptom Severity During Augmentation.
Mixed-effects linear regression was used to model change in severity of obsessive-compulsive disorder (OCD) as measured by the Yale-Brown Obsessive Compulsive Scale (Y-BOCS) in 100 adults who were receiving a stable dose of a serotonin reuptake inhibitor and who were randomized to the addition of 8 weeks of risperidone (n = 40), exposure and ritual prevention (EX/RP) therapy (n = 40), or pill placebo (n = 20). Patients randomized to EX/RP had significantly greater reduction in Y-BOCS scores at week 8 than those randomized to risperidone or placebo (see text for details). The x-axis is weeks of augmentation. The y-axis is OCD severity as measured by the Y-BOCS. A score of 16 (arrow) or higher is considered clinically meaningful OCD symptoms warranting treatment.
Secondary Outcomes
Observed outcomes are presented in Table 2. Compared with those receiving risperidone at week 8 and based on mixed-effects models, those receiving EX/RP had lower week 8 scores on the HAM-D (mean [SE], −3.35 [1.25]; 95% CI, −0.90 to −5.80; z = −2.68; P = .007), BABS (mean [SE], −2.01 [0.95]; 95% CI, −0.15 to −3.86; z = −2.12; P = .03), and SAS-SR (mean [SE], −0.29 [0.11]; 95% CI, −0.07 to −0.50; z = −2.59; P = .009) and higher QLESQ-SF scores (mean [SE], +15.98 [3.49]; 95% CI, 22.83 to 9.13; z = 4.57; P < .001). Compared with those receiving placebo at week 8, those receiving EX/RP had lower scores on the HAM-D (mean [SE], −3.25 [1.52]; 95% CI, −0.27 to −6.23; z = −2.14; P = .03) and SAS-SR (mean [SE], −0.32 [0.14]; 95% CI, −0.05 to −0.58; z = −2.34; P = .02), did not differ on BABS scores (mean [SE], −1.55 [1.15]; 95% CI, 0.72 to −3.81; z = −1.3398; P = .18), and had higher QLESQ-SF scores (mean [SE], +9.49 [4.26]; 95% CI, 17.83 to 1.15; z = 2.23; P = .03). At week 8, those receiving risperidone did not differ from those receiving placebo on any measure (HAM-D score: mean [SE], 0.10 [1.55]; z = 0.07; P = .95; BABS score: mean [SE], 0.45 [1.17]; z = 0.39; P = .70; SAS-SR score: mean [SE], −0.03 [0.14]; z = −0.21; P = .84; and QLESQ-SF score: mean [SE], −6.49 [4.32]; z = −1.50; P = .13).
Significantly more patients receiving EX/RP (32 of 40 [80%]) than those receiving risperidone (9 of 40 [22.5%]) or placebo (3 of 20 [15%]) responded to treatment (≥25% Y-BOCS score decrease: ; P < .001). Significantly more patients receiving EX/RP achieved minimal symptoms (Y-BOCS score ≤12; 17 of 40 [42.5%] vs 5 of 40 [12.5%] vs 1 of 20 [5%]; ; P = .001). For EX/RP, the number needed to treat using placebo as the comparator was 1.5 to achieve response and 2.7 to achieve minimal symptoms; for risperidone, the number needed to treat (using placebo as the comparator) was 13.3 for both response and minimal symptoms.
Adverse Effects
As shown in Table 3, some patients reported moderate or severe physical complaints at baseline; across the whole sample, anorgasmia/erectile dysfunction and fatigue were the most common complaints (each 13%), consistent with known SRI adverse effects. Patients on average were also overweight (ie, mean body mass index at baseline ≥25).
Table 3.
Observed Adverse Effectsa
| Risperidone | EX/RP | Pill Placebo | |
|---|---|---|---|
| Moderate or severe adverse effects at week 0, No./Total No. (%)b | 14/40 (35) | 18/40 (45) | 5/20 (25) |
| Most frequent (≥10%) in at least 1 treatment group, % | |||
| Anorgasmia/erective dysfunction | 13 | 15 | 10 |
| Decreased libido | 13 | 8 | 5 |
| Dry mouth | 5 | 0 | 10 |
| Fatigue | 13 | 18 | 5 |
| Insomnia | 13 | 10 | 0 |
| Nervousness | 18 | 13 | 0 |
| Somnolence | 5 | 10 | 5 |
| Treatment-emergent adverse effects at week 4 or 8, No./Total No. (%)b,c | 30/34 (88) | 22/38 (58) | 13/18 (72) |
| Most frequent (≥20%) in at least 1 group, % | |||
| Decreased libido | 27 | 16 | 6 |
| Dry mouth | 29 | 5 | 28 |
| Fatigue | 27 | 21 | 22 |
| Headache | 21 | 24 | 22 |
| Insomnia | 21 | 13 | 17 |
| Nervousness | 24 | 16 | 22 |
| Somnolence | 21 | 18 | 22 |
| BMI | |||
| Week 0, mean (SD) | 26.6 (5.5) | 25.5 (4.7) | 26.8 (3.6) |
| Increase of ≥0.5 kg/m2 over their baseline BMI, No./Total No. (%) | 18/29 (62) | 13/31 (42) | 1/12 (8) |
| Increase of ≥1.0 kg/m2 over their baseline BMI, No./Total No. (%) | 13/29 (45) | 6/31 (19) | 0/12 (0) |
| Barnes Akathisia Scale | |||
| Global score of at least “mild” at week 4 or 8, No./Total No. (%) | 1/34 (3) | 0/38 (0) | 1/18 (6) |
| Simpson-Angus Scale | |||
| Clear symptom (≥2 on any item) at week 4 or 8, No./Total No. (%) | 0/34 (0) | 0/38 (0) | 2/18 (11) |
| Abnormal Involuntary Movement Scale | |||
| Score of at least mild on any item at week 8, No./Total No. (%) | 0/32 (0) | 0/37 (0) | 0/17 (0) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); EX/RP, exposure and ritual prevention.
Because of dropout and missing data, the sample size varied across these different measures. In particular, 9 patients did not reach week 4 (risperidone, n = 5; EX/RP, n = 2; and pill placebo, n = 2) and another 5 dropped out before week 8 (risperidone, n = 3; EX/RP, n = 1; and pill placebo, n = 1). Of those who dropped out, 4 patients receiving risperidone and 2 receiving pill placebo dropped out because of intolerable adverse effects.
Assessed using a modified version of the Systemic Assessment for Treatment Emergent Events (see text for details).
Any increase in severity above week 0.
During augmentation, many patients reported increased physical complaints; this limited maximal dose titration in both pill groups (Table 3). Those receiving risperidone reported the most treatment-emergent complaints, with dry mouth, fatigue, and decreased libido being the most common. More patients receiving risperidone and EX/RP than patients receiving pill placebo had increases in mean body mass index; the proportion of patients with an increase of at least 1.0 kg/m2 differed significantly by group ( ; P = .006). One patient receiving risperidone and 1 patient receiving placebo reported akathisia; 2 patients receiving placebo had extrapyramidal symptoms on the Simpson-Angus Scale. Intolerable adverse effects caused 4 patients receiving risperidone, 2 patients receiving placebo, and no patients receiving EX/RP to leave the study.
Discussion
To our knowledge, this is the first randomized clinical trial to compare 2 recommended SRI augmentation strategies for adults with OCD. Adding EX/RP to SRIs was superior to risperidone and pill placebo in reducing OCD symptoms and improving insight, functioning, and quality of life. Risperidone was not superior to placebo on any outcome.
That EX/RP was superior to placebo corroborates Tenneij et al,8 who found that adding EX/RP to paroxetine or venlafaxine was superior to continued medication alone in 96 adults with OCD. The findings also confirm our prior study,9 which found augmentation with EX/RP superior to stress management therapy(a control for time and attention) in 108 adults with OCD. That our 2 studies, conducted more than 5 years apart, yielded such similar effects underscores the efficacy of EX/RP as an SRI augmentation strategy for adults with OCD: across both studies, 33% to 43% who began EX/RP and 38% to 46% who completed it achieved minimal symptoms (Y-BOCS score ≤12), which is associated with good quality of life and adaptive functioning.33 Our study also affirms the low placebo response seen in other OCD SRI augmentation studies3,9: of our patients randomized to pill placebo augmentation, only 15% responded, and 5% achieved minimal symptoms after 8 weeks.
Contrary to our expectations, adding EX/RP to SRIs was superior to adding risperidone on every outcome. These findings are important because antipsychotics are increasingly prescribed to outpatients with OCD,34 and risperidone is recommended as the medication of first choice to augment SRI response.3,4 Our results call for increased use of EX/RP for augmenting unsatisfactory SRI effects.
Adding risperidone to SRIs was not significantly better than placebo on any outcome measure, even though risperidone’s response rates were numerically higher. These findings are at odds with 3 smaller trials.5–7 The most positive6 found risperidone (n = 18) superior to placebo (n = 15), with a mean (SD) Y-BOCS score decrease from 27.4 (5.4) to 18.7 (8.3) and a 50% response rate in treatment completers; their mean (SD) daily risperidone dose (2.2 [0.7] mg/d; median, 2.0 mg/d) was similar to ours (1.9 [1.1] mg/d; median, 2.0 mg/d). That study used a different response definition and analyzed only treatment completers, potentially explaining some of the discrepancy. However, applying a response definition used in meta-analyses (Y-BOCS score decrease ≥ 35%3,4) to all randomized subjects (assuming drop-outs are non responders), the difference between our risperidone and placebo response rates (17.5% vs 15%) leads to an absolute risk difference of only 0.025. The 3 other controlled trials had absolute risk differences of 0.356 and 0.305,7; patients in 1 of these studies5 received only 0.5 mg/d of risperidone.
Several differences between our study and these prior trials may explain the different outcomes. First, the samples differed. We randomized patients with clinically significant symptoms (Y-BOCS score ≥16) despite receiving an SRI at a maximally tolerated dose for 12 weeks or more. All but 2 reported at least minimal improvement while receiving an SRI, which is why they had continued receiving their SRI. The other studies focused on patients with no more than minimal response to an SRI. Thus, the other studies may have had a more SRI-refractory sample. For example, McDougle et al6 treated patients for 8 weeks with an SRI and then randomized only those who met all of the following criteria: (1) less than 35% Y-BOCS score decrease or Y-BOCS score more than 16; (2) no more than minimal improvement; and (3) consensus of 3 clinicians that the patient was unimproved. Importantly, Erzegovesi et al5 found that only SRI nonresponders (not responders) benefit from risperidone augmentation. In contrast, those with and without an SRI response have been shown to benefit from EX/RP augmentation.8,9,35–37 Second, given the increasing use of second-generation antipsychotics in OCD,34 our sample might have had greater prior antipsychotic exposure and therefore be more antipsychotic resistant. Prior antipsychotic exposure is not described in prior studies; 17% of our sample had previously been exposed to an antipsychotic for atleast a week. However, our sensitivity analysis indicated that baseline differences in prior antipsychotic exposure did not explain differences in outcome. Third, patients with OCD have treatment preferences.38 Our study randomized patients to medication or EX/RP; it likely attracted either those without preferences or those with preferences but the willingness to chance being randomized to the other. The other studies randomized patients only to medication, likely attracting those who preferred medication. Treatment preferences like these can affect treatment outcome.39 Finally, to our knowledge, ours is the largest sample (risperidone, n = 40; placebo, n = 20); the absolute risk difference for the other studies was based on only 6 to 20 patients per group.
Risperidone’s adverse effects were expected. However, there were 2 surprises. First, 37% of patients had moderate to severe adverse effects at baseline; assuming these are due to SRIs, this suggests that long-term SRI treatment is not benign. Second, while most patients receiving risperidone (88%) reported treatment-emergent adverse effects, many receiving placebo (72%) or EX/RP (58%) did too. Moreover, both patients receiving risperidone and EX/RP gained weight, although those receiving risperidone were more likely to do so.
Limitations
Several limitations deserve consideration. First, we recruited patients already receiving SRIs. Consequently, we do not have measures of OCD severity prior to SRI treatment to quantify degree of SRI response. Second, patients receiving EX/RP knew they were receiving therapy, whereas those taking a pill understood they had a one-third chance of receiving placebo; thus, factors like differential expectancy could have contributed to the outcome. In addition, the groups differed in clinician contact: those receiving EX/RP had 2 hours of contact with their psychiatrist and about 26 contact hours with their therapist, whereas those receiving risperidone or placebo had 4 hours of contact with their psychiatrist. However, prior studies have shown that attentional one has minimal effects on OCD symptoms.9 Third, some data suggest that antipsychotic augmentation is most helpful in patients with OCD and tic disorders.3 Because only 5% of our patients reported a lifetime tic disorder, our data cannot address this question. Finally, the study design could not address whether patients with OCD receiving SRIs who fail to respond to EX/RP (or are unwilling to try it) might benefit from risperidone augmentation.
Generalizability
Our study recruited patients like those commonly seen in out-patient practice. We minimized exclusions and permitted co-morbid anxiety and depressive disorders. Thus, our findings likely apply to patients with OCD receiving SRIs who are willing to try medication or psychotherapy and who can adhere to these treatments. We have previously shown that patient adherence to EX/RP is strongly associated with outcome.40,41 With regard to EX/RP’s effectiveness outside academic settings like ours, EX/RP has been successfully disseminated to specialty fee-for-service practices42,43 and nonacademic community clinics44; it has even been successfully delivered by the Internet.45
Clinical Implications
Patients with OCD receiving SRIs should be offered EX/RP before antipsychotics given EX/RP’s superior efficacy and less negative adverse effect profile. Identifying who achieves minimal OCD symptoms from adding EX/RP to SRIs and whether such patients can then successfully discontinue their SRI warrants future research. Whether patients with OCD receiving SRIs who fail to respond to EX/RP (or are unwilling to try it) can benefit from risperidone augmentation remains an unanswered question. Alternative medication augmentation strategies for patients with OCD receiving SRIs are needed.
Supplementary Material
Acknowledgments
Funding/Support: This study was funded by National Institute of Mental Health grants R01 MH045436 (Dr Simpson) and R01 MH45404 (Dr Foa). Medication was provided at no cost by Janssen Scientific Affairs LLC.
Role of the Sponsor: The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Author Contributions: Drs Simpson and Foa had full access to all of the data in the study and take responsibility for the integrity of the data and, with Dr Marcus (the senior statistician), the accuracy of the data analysis. Drs Simpson and Foa made equal contributions.
Study concept and design: Simpson, Foa, Liebowitz, Huppert, Marcus, Campeas.
Acquisition of data: Simpson, Huppert, Cahill, Maher, McLean, Bender, Williams, Vermes, Van Meter, Rodriguez, Powers, Pinto, Imms, Hahn, Campeas.
Analysis and interpretation of data: Simpson, Foa, Liebowitz, Huppert, Cahill, Marcus, Weaver, Van Meter.
Drafting of the manuscript: Simpson, Foa, Marcus.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Simpson, Marcus, Weaver, Van Meter.
Obtained funding: Simpson, Foa, Liebowitz.
Administrative, technical, or material support: Simpson, Foa, Maher, McLean, Bender, Williams, Vermes, Van Meter, Rodriguez, Powers, Imms, Hahn.
Study supervision: Simpson, Foa, Huppert, Cahill, Maher, McLean, Bender, Williams, Powers, Pinto, Imms, Campeas.
Conflict of Interest Disclosures: During this study, in addition to medication at no cost from Janssen Scientific Affairs LLC, Dr Simpson received research funds from Transcept Pharmaceuticals (2011–2013) and Neuropharm Ltd (2009), served on a scientific advisory board for Pfizer (for Lyrica, 2009–2010) and Jazz Pharmaceuticals (for Luvox CR [controlled release], 2007–2008), and received royalties from Cambridge University Press and Up To Date Inc. Dr Foa was a consultant to Jazz Pharmaceuticals (for Acetelion), and she receives royalties from Bantam and Oxford University Press for book sales, including a manual of cognitive-behavioral therapy for OCD. Dr Liebowitz received research funds from pharmaceutical companies (Abbott, Allergan, AstraZeneca, Avera, Forest, Cephalon, Endo, Gruenthal, GlaxoSmithKline, Horizon, Indevus, Jazz Pharmaceuticals, Johnson & Johnson, Lilly, Lundbeck, MAP, Novartis, Ortho-McNeil, Pfizer, PGX Health, Purdue Pharma, Sepracor, Takeda, Tikvah, and Wyeth), consulted (to AstraZeneca, Avera, Eisai, Lilly, Otsuka, Pfizer, Pherin Pharmaceutical, Takeda, Tikvah, and Wyeth), presented talks or posters (for Pherin Pharmaceutical, Pfizer, and Wyeth), has equity ownership in Pherin Pharmaceutical, ChiMatrix LLC (ended 2011), and the Liebowitz Social Anxiety Scale, and has licensing software for the Liebowitz Social Anxiety Scale for Avera, Endo, GlaxoSmithKline, Indevus, Lilly, Pfizer, Servier, and Tikvah. Dr Hahn received research funds from Pfizer, GlaxoSmithKline, and AstraZeneca. No other disclosures were reported.
Additional Contributions: We thank staff of the Anxiety Disorders Clinic and Center for the Treatment and Study of Anxiety for help conducting this trial, including the expert research assistance of Samantha Farris, BA, Jessica McCarthy, BA, Rena Staub, BA, Liane Hunter, BA, and Josephine Curry, BA. We also thank John Markowitz, MD, and Franklin Schneier, MD, for commenting on a prior draft of the manuscript. We thank the study subjects for participating.
Additional Information: This study was conducted at 2 sites: the Anxiety Disorders Clinic at Columbia University/the New York State Psychiatric Institute (1051 Riverside Dr, Unit 69, New York, NY 10032) and the Center for the Treatment and Study of Anxiety in the Department of Psychiatry, University of Pennsylvania (3600 Market St, Philadelphia, PA 19104).
References
- 1.Koran LM, Hanna GL, Hollander E, Nestadt G, Simpson HB. American Psychiatric Association. Practice guideline for the treatment of patients with obsessive-compulsive disorder. Am J Psychiatry. 2007;164(7 suppl):5–53. [PubMed] [Google Scholar]
- 2.Simpson HB, Huppert JD, Petkova E, Foa EB, Liebowitz MR. Response versus remission in obsessive-compulsive disorder. J Clin Psychiatry. 2006;67(2):269–276. doi: 10.4088/jcp.v67n0214. [DOI] [PubMed] [Google Scholar]
- 3.Bloch MH, Landeros-Weisenberger A, Kelmendi B, Coric V, Bracken MB, Leckman JF. A systematic review: antipsychotic augmentation with treatment refractory obsessive-compulsive disorder. Mol Psychiatry. 2006;11(7):622–632. doi: 10.1038/sj.mp.4001823. [DOI] [PubMed] [Google Scholar]
- 4.Dold M, Aigner M, Lanzenberger R, Kasper S. Antipsychotic augmentation of serotonin reuptake inhibitors in treatment-resistant obsessive-compulsive disorder: a meta-analysis of double-blind, randomized, placebo-controlled trials. Int J Neuropsychopharmacol. 2013;16(3):557–574. doi: 10.1017/S1461145712000740. [DOI] [PubMed] [Google Scholar]
- 5.Erzegovesi S, Guglielmo E, Siliprandi F, Bellodi L. Low-dose risperidone augmentation of fluvoxamine treatment in obsessive-compulsive disorder: a double-blind, placebo-controlled study. Eur Neuropsychopharmacol. 2005;15(1):69–74. doi: 10.1016/j.euroneuro.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 6.McDougle CJ, Epperson CN, Pelton GH, Wasylink S, Price LH. A double-blind, placebo-controlled study of risperidone addition in serotonin reuptake inhibitor-refractory obsessive-compulsive disorder. Arch Gen Psychiatry. 2000;57(8):794–801. doi: 10.1001/archpsyc.57.8.794. [DOI] [PubMed] [Google Scholar]
- 7.Hollander E, Baldini Rossi N, Sood E, Pallanti S. Risperidone augmentation in treatment-resistant obsessive-compulsive disorder: a double-blind, placebo-controlled study. Int J Neuropsychopharmacol. 2003;6(4):397–401. doi: 10.1017/S1461145703003730. [DOI] [PubMed] [Google Scholar]
- 8.Tenneij NH, van Megen HJ, Denys DA, Westenberg HG. Behavior therapy augments response of patients with obsessive-compulsive disorder responding to drug treatment. J Clin Psychiatry. 2005;66(9):1169–1175. doi: 10.4088/jcp.v66n0913. [DOI] [PubMed] [Google Scholar]
- 9.Simpson HB, Foa EB, Liebowitz MR, et al. A randomized, controlled trial of cognitive-behavioral therapy for augmenting pharmacotherapy in obsessive-compulsive disorder. Am J Psychiatry. 2008;165(5):621–630. doi: 10.1176/appi.ajp.2007.07091440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abramowitz JS, Foa EB, Franklin ME. Exposure and ritual prevention for obsessive-compulsive disorder: effects of intensive versus twice-weekly sessions. J Consult Clin Psychol. 2003;71(2):394–398. doi: 10.1037/0022-006x.71.2.394. [DOI] [PubMed] [Google Scholar]
- 11.Goodman WK, Price LH, Rasmussen SA, et al. The Yale-Brown Obsessive Compulsive Scale, I: development, use, and reliability. Arch Gen Psychiatry. 1989;46(11):1006–1011. doi: 10.1001/archpsyc.1989.01810110048007. [DOI] [PubMed] [Google Scholar]
- 12.Goodman WK, Price LH, Rasmussen SA, et al. The Yale-Brown Obsessive Compulsive Scale, II: validity. Arch Gen Psychiatry. 1989;46(11):1012–1016. doi: 10.1001/archpsyc.1989.01810110054008. [DOI] [PubMed] [Google Scholar]
- 13.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for DSM-IV Axis I Disorders-Patient Edition. New York: Biometrics Research Department, New York State Psychiatric Institute; 1996. [Google Scholar]
- 15.Lee ET. Statistical Methods for Survival Data Analysis. New York, NY: Wiley & Sons; 1992. [Google Scholar]
- 16.Kozak MJ, Foa EB. Mastery of Obsessive-Compulsive Disorder: A Cognitive-Behavioral Approach. San Antonio, TX: The Psychological Corporation; 1997. [Google Scholar]
- 17.Eisen JL, Phillips KA, Baer L, Beer DA, Atala KD, Rasmussen SA. The Brown Assessment of Beliefs Scale: reliability and validity. Am J Psychiatry. 1998;155(1):102–108. doi: 10.1176/ajp.155.1.102. [DOI] [PubMed] [Google Scholar]
- 18.Endicott J, Nee J, Harrison W, Blumenthal R. Quality of Life Enjoyment and Satisfaction Questionnaire: a new measure. Psychopharmacol Bull. 1993;29(2):321–326. [PubMed] [Google Scholar]
- 19.Weissman MM, Bothwell S. Assessment of social adjustment by patient self-report. Arch Gen Psychiatry. 1976;33(9):1111–1115. doi: 10.1001/archpsyc.1976.01770090101010. [DOI] [PubMed] [Google Scholar]
- 20.Levine J, Schooler NR. SAFTEE: a technique for the systematic assessment of side effects in clinical trials. Psychopharmacol Bull. 1986;22(2):343–381. [PubMed] [Google Scholar]
- 21.Leckman JF, Riddle MA, Hardin MT, et al. The Yale Global Tic Severity Scale: initial testing of a clinician-rated scale of tic severity. J Am Acad Child Adolesc Psychiatry. 1989;28(4):566–573. doi: 10.1097/00004583-198907000-00015. [DOI] [PubMed] [Google Scholar]
- 22.Simpson GM, Angus JW. A rating scale for extrapyramidal side effects. Acta Psychiatr Scand Suppl. 1970;212:11–19. doi: 10.1111/j.1600-0447.1970.tb02066.x. [DOI] [PubMed] [Google Scholar]
- 23.Barnes TR. The Barnes Akathisia Rating Scale—revisited. J Psychopharmacol. 2003;17(4):365–370. doi: 10.1177/0269881103174013. [DOI] [PubMed] [Google Scholar]
- 24.Guy W. ECDEU Assessment Manual for Psychopharmacology. Rockville, MD: US Department of Health, Education, and Welfare; 1976. [Google Scholar]
- 25.Munetz MR, Benjamin S. How to examine patients using the Abnormal Involuntary Movement Scale. Hosp Community Psychiatry. 1988;39(11):1172–1177. doi: 10.1176/ps.39.11.1172. [DOI] [PubMed] [Google Scholar]
- 26.Suckow RF, Zhang MF, Cooper TB. Sensitive and selective liquid-chromatographic assay of fluoxetine and norfluoxetine in plasma with fluorescence detection after precolumn derivatization. Clin Chem. 1992;38(9):1756–1761. [PubMed] [Google Scholar]
- 27.Oyehaug E, Ostensen ET, Salvesen B. Determination of the antidepressant agent citalopram and metabolites in plasma by liquid chromatography with fluorescence detection. J Chromatogr. 1982;227(1):129–135. doi: 10.1016/s0378-4347(00)80362-x. [DOI] [PubMed] [Google Scholar]
- 28.Foglia JP, Birder LA, Perel JM. Determination of fluvoxamine in human plasma by high-performance liquid chromatography with ultraviolet detection. J Chromatogr. 1989;495:295–302. doi: 10.1016/s0378-4347(00)82635-3. [DOI] [PubMed] [Google Scholar]
- 29.Gibbons RD, Hedeker D, Elkin I, et al. Some conceptual and statistical issues in analysis of longitudinal psychiatric data: application to the NIMH treatment of Depression Collaborative Research Program dataset. Arch Gen Psychiatry. 1993;50(9):739–750. doi: 10.1001/archpsyc.1993.01820210073009. [DOI] [PubMed] [Google Scholar]
- 30.Laird NM. Missing data in longitudinal studies. Stat Med. 1988;7(1–2):305–315. doi: 10.1002/sim.4780070131. [DOI] [PubMed] [Google Scholar]
- 31.Gibbons RD, Hur K, Brown CH, Davis JM, Mann JJ. Benefits from antidepressants: synthesis of 6-week patient-level outcomes from double-blind placebo-controlled randomized trials of fluoxetine and venlafaxine. Arch Gen Psychiatry. 2012;69(6):572–579. doi: 10.1001/archgenpsychiatry.2011.2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Simpson HB, Maher M, Page JR, Gibbons CJ, Franklin ME, Foa EB. Development of a patient adherence scale for exposure and response prevention therapy. Behav Ther. 2010;41(1):30–37. doi: 10.1016/j.beth.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Farris SG, McLean CP, Van Meter PE, Simpson HB, Foa EB. Treatment response, symptom remission and wellness in obsessive-compulsive disorder. J Clin Psychiatry. 2013;74(7):685–690. doi: 10.4088/JCP.12m07789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Comer JS, Mojtabai R, Olfson M. National trends in the antipsychotic treatment of psychiatric outpatients with anxiety disorders. Am J Psychiatry. 2011;168(10):1057–1065. doi: 10.1176/appi.ajp.2011.11010087. [DOI] [PubMed] [Google Scholar]
- 35.Tolin DF, Maltby N, Diefenbach GJ, Hannan SE, Worhunsky P. Cognitive-behavioral therapy for medication nonresponders with obsessive-compulsive disorder: a wait-list-controlled open trial. J Clin Psychiatry. 2004;65(7):922–931. doi: 10.4088/jcp.v65n0708. [DOI] [PubMed] [Google Scholar]
- 36.Kampman M, Keijsers GP, Hoogduin CA, Verbraak MJ. Addition of cognitive-behaviour therapy for obsessive-compulsive disorder patients non-responding to fluoxetine. Acta Psychiatr Scand. 2002;106(4):314–319. doi: 10.1034/j.1600-0447.2002.01261.x. [DOI] [PubMed] [Google Scholar]
- 37.Tundo A, Salvati L, Busto G, Di Spigno D, Falcini R. Addition of cognitive-behavioral therapy for nonresponders to medication for obsessive-compulsive disorder: a naturalistic study. J Clin Psychiatry. 2007;68(10):1552–1556. doi: 10.4088/jcp.v68n1013. [DOI] [PubMed] [Google Scholar]
- 38.Patel SR, Simpson HB. Patient preferences for obsessive-compulsive disorder treatment. J Clin Psychiatry. 2010;71(11):1434–1439. doi: 10.4088/JCP.09m05537blu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Swift JK, Callahan JL, Vollmer BM. Preferences. J Clin Psychol. 2011;67(2):155–165. doi: 10.1002/jclp.20759. [DOI] [PubMed] [Google Scholar]
- 40.Simpson HB, Maher MJ, Wang Y, Bao Y, Foa EB, Franklin M. Patient adherence predicts outcome from cognitive behavioral therapy in obsessive-compulsive disorder. J Consult Clin Psychol. 2011;79(2):247–252. doi: 10.1037/a0022659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Simpson HB, Marcus SM, Zuckoff A, Franklin M, Foa EB. Patient adherence to cognitive-behavioral therapy predicts long-term outcome in obsessive-compulsive disorder. J Clin Psychiatry. 2012;73(9):1265–1266. doi: 10.4088/JCP.12l07879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rothbaum BO, Shahar F. Behavioral treatment of obsessive-compulsive disorder in a naturalistic setting. Cogn Behav Pract. 2000;7(3):262–270. doi: 10.1016/S1077-7229(00)80082-6. [DOI] [Google Scholar]
- 43.Warren R, Thomas JC. Cognitive-behavior therapy of obsessive-compulsive disorder in private practice: an effectiveness study. J Anxiety Disord. 2001;15(4):277–285. doi: 10.1016/s0887-6185(01)00063-9. [DOI] [PubMed] [Google Scholar]
- 44.Valderhaug R, Larsson B, Götestam KG, Piacentini J. An open clinical trial of cognitive-behaviour therapy in children and adolescents with obsessive-compulsive disorder administered in regular outpatient clinics. Behav Res Ther. 2007;45(3):577–589. doi: 10.1016/j.brat.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 45.Andersson E, Enander J, Andrén P, et al. Internet-based cognitive behaviour therapy for obsessive-compulsive disorder: a randomized controlled trial. Psychol Med. 2012;42(10):2193–2203. doi: 10.1017/S0033291712000244. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.